Abstract
Background
Hemodynamic stability and blood loss reduction are subjects to further consideration in patients undergoing percutaneous nephrolithotomy (PNCL).
Objectives
This study compared the preference of spinal anaesthesia (SA) or general anaesthesia (GA) in respect to mentioned concerns.
Patients and Methods
In this randomized clinical trial, 59 patients who underwent PCNL divided into SA and GA groups. 15-20 mg from intra-thecal bupivacaine 0.5%, and premedication of 0.01-0.02 mg from midazolam, were given to patients in SA group (n = 29). Patients in GA group (n = 30) received premedication of 1-2 µg/kg from fentanyl and 0.01-0.02 mg/kg from midazolam, and intravenously anaesthetized with 100 µg/kg/min of propofol and 0.5 mg/kg of atracurium, given by continuous infusion and N2O/O2 50%. Mean arterial pressure (MAP) and heart rate were recorded intra-operatively and during recovery.
Results
MAP and heart rate show no significant differences at designated time points between two groups (P > 0.05). Surgery time, anesthesia time, bleeding volume, and analgesic intake were significantly reduced in SA group (P < 0.05).
Conclusions
It seems that, in patients undergoing PNCL, SA is as effective and safe as GA. Patients who undergo PNCL under SA require smaller amounts of analgesic dose and show hemodynamic stability during surgery and recovery time. Also, SA technique provides decreased blood loss and shortened surgery as well as anesthesia times compared to GA.
Keywords: Nephrostomy, Percutaneous; Hemodynamics; Analgesia; Hemorrhage
1. Background
Nowadays, percutaneous nephrolithotomy (PNCL) is a common method for extracting renal and urinary stones, and a choice modality in large, multiple, and stag-horn stones. Furthermore, PNCL can be used in patients with failed shock and endoscopic trials (1-3). In about 20% of cases, urologic procedures are undertaken with general anesthesia (GA) or regional anesthesia such as spinal anesthesia (SA). Despite good results of PNCL with GA, it may cause atelectasis, drug reactions, nausea, and vomiting (4, 5). In abdominal and lower extremities surgeries, SA is mainly employed by a single drug and comprises some advantages such as less bleeding, and reduces venous pressure in the surgery field (6, 7). However, there are recent reports regarding the use of SA in PNCL demonstrating lower post-operation pain, less drug intake, and reduced adverse effects. Some studies have also shown that surgeries with SA had better outcomes in spinal surgeries (4, 5, 8).
There are controversies among researchers regarding the use of SA in PNCL due to the most important issue which is acute hypotension, resulting from sympathetic block (9-12). Therefore, BP and pulse rate (PR) can be helpful to monitor sympathetic drive in these patients. There are many studies comparing GA and SA in several surgeries (13-17); however, there is no definite comparison made by BP and PR in PNCL during surgery and in recovery room.
2. Objectives
Considering the type of anesthesia as well as patients' hemodynamics that can influence on surgery outcomes and relevant morbidity and mortality of the intervention, and that these factors directly reflect on regional health-care, we aimed this study to compare mean BP and PR among PNCL patients underwent GA and SA.
3. Patients and Methods
3.1. Subjects
In this randomized clinical trial, all patients referred to Shahid Hasheminejad hospital in 2011 as PNCL candidates were included sequentially if they met these inclusion criteria: age between 18-65 years with physical status I or II of American Society of Anesthesiologists (ASA). All patients with spinal deformity, local infection at injection site, pregnancy, history of any neuromuscular or psychiatric disorder or chronic pain, who were suffering from hypertension, diabetes and coagulation disorders, patients with hypersensitivity to any anesthesia drugs, substance abusers, and patients who needed anesthesia higher than T4 and lower than T10 levels were excluded. The included patients were divided into SA and GA groups using randomized number table. Standard monitoring included continuous electrocardiogram, pulse oximetry, and end-tidal carbon dioxide. Noninvasive BP measurements were performed at 5-min intervals. All patients were routed with a green (18-gauge) catheter and infused with 3-4 cc/kg isotonic crystalloids. Maintenance venous liquid during surgery was based on 4/2/1 rule. For blood loss limited to "maximum allowable blood loss", 3 mL of Ringer solution was injected for every 1 mL of blood loss, and equal volume of matched iso-group packed cell for more blood losses. Both types of anesthesia were performed by a 4th year resident of anesthesiology.
3.2. GA Group
Premedication of 1-2 µg/kg from fentanyl and 0.01-0.02 mg/kg from midazolam was administered. Oxygen with an inspired fraction of 1.0 was administered for 3 min before intubation. Then, GA was induced by 3-5 mg/kg thiopental-Na, and to obtain desired anesthesia, 0.5 mg/kg of atracurium was injected intravenously for easier intubation; then, all patients were intubated by a suitable endotracheal tube. For maintaining GA, an intravenous 100 µg/kg/min of propofol with 50% O2 and 50% N2O were induced. The ventilation protocol consisted of an inspired oxygen fraction of 1.0, inspiratory to expiratory ratio of 1:2, and a respiratory rate adjusted to normocapnia (end-tidal carbon dioxide partial pressure between 30 and 40 mmHg). Mechanical ventilation has been set with a tidal volume of 10 ml/kg ideal body weight (IBW) and ZEEP (zero-positive end expiratory pressure). Atracurium and fentanyl re-administration was based on train-of-four (TOF) and every 45 minutes, respectively.
3.3. SA Group
Premedication of 0.01-0.02 mg/kg from midazolam was administered. The patients were placed in a sitting position. The drug was administered by a 25-gauge Quincke needle in midline of L3-L4 or L4-L5 level by a physician. For inducing SA, isobar intra-thecal 15-20 mg of bupivacaine 0.5% without any additives was administered. Then, the patients' positions were changed to prone and intranasal 100% oxygen was administered. Sensory blockade was evaluated by a cotton peak (for heat perception) or a needle (for touching sense) every 15-20 seconds; then, motor blockade was tested by Bromage scale with following score: 0 = no paralysis; 1 = inability to raise extended leg; 2 = inability to flex knee; 3 = inability to move leg joints. Blood pressure below 100 mmHg of 30% from the baseline was corrected by 6 mg ephedrine and crystalloids, and all PR descents (less than 60/min) were treated by intravenous Atropine. All mentioned anesthetic drugs were provided by a regional pharmaceutical company (Darupakhsh, Iran).
3.4. Anesthesia Assessment
Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP), and PR were recorded every 20 minutes during surgery from the beginning of anesthesia. Intraoperative blood loss was calculated by blood volume of suction devices, and estimated volume of blood in sponges and drapes already were weighted before operation.
SBP, DBP, MAP, and PR were recorded in the PACU, every 10 min from entering PACU. Fifty mg from Meperidine was administered in patients suffered from additional pain. All patients were positioned in supine. MAP and PR were evaluated every 10 minutes for 1 hour. Other information were extracted from medical files and inserted into a pre-prepared checklist.
3.5. Ethical Issues
The patients were not charged by additional fees for the drugs used in any step of this study. The local ethics review committee of Iran University of medical sciences approved the study protocol. All participants gave written informed consent before participating.
3.6. Statistical Analysis
Based on a pilot study in 12 patients (six from each group), we determined that a sample size of 26 in each group would be sufficient to detect the differences between mean of blood loss and analgesic demand, estimate a standard deviation of 10, a power of 95%, and a significance level of 5%; this number was increased to 30 per group, to allow a predicted drop-out of around 10% from the study.
The data were evaluated and analyzed by SPSS version 19 (SPSS Inc., Illinois, USA). All quantitative data were expressed as mean ± SD, and qualitative data as No. (%). For comparing the groups, t-test and Mann-Whitney-U test were used for parametric and non-parametric data, evaluated by Kolmogorov-Smirnov test, respectively. P less than 0.05 were considered as significant.
4. Results
4.1. Demographic Data
Fifty nine patients were enrolled in the study consisting of 38 males and 21 females. The patients were randomly divided into SA (n = 29) and GA (n = 30) groups. Table 1 demonstrates all demographic data. Surgery duration (P = 0.016) and anesthesia duration (P = 0.044) were significantly lower in SA (Table 2). According to Bromage scale, motor block level was zero in all patients in SA group.
Table 1. Comparison of Demographics Between Two Groups .
| Variable | General Anesthesia | Spinal Anesthesia | P value |
|---|---|---|---|
| Gender | |||
| Male, No. (%) | 19 (63.3) | 19 (65.5) | 0.86 |
| Female, No. (%) | 11 (36.7) | 10 (34.5) | |
| ASAaClass | 22 (72.3) | 23 (79.3) | 0. 590 |
| I | |||
| II | 8 (26.7) | 6 (20.7) | |
| Age, Mean ± SD, y | 46.9 ± 13.6 | 39.6 ± 9.7 | 0.022 |
| BMIa, Mean ± SD, kg/m2 | 28.1 ± 4.6 | 26.4 ± 3.8 | 0.129 |
aAbbreviations: ASA, American Society of Anesthesiologists; BMI, body mass index
Table 2. Duration of Surgery, Anesthesia, Recovery time, Blood Loss, Analgesic Demand, and Blood Transfusion Amount in Both Groups .
| Variable | General Anesthesia | Spinal Anesthesia | P value |
|---|---|---|---|
| Surgery Duration, Mean ± SD, min | 112.2 ± 18.3 | 99.3 ± 21.1 | 0.016 |
| Anesthesia Duration, Mean ± SD, min | 112.2 ± 18.3 | 101.3 ± 22.03 | 0.044 |
| Recovery Duration, Mean ± SD, min | 42.2 ± 12.8 | 41.5 ± 19.1 | 0.878 |
| Blood Loss, Mean ± SD, ml | 331.7 ± 151.1 | 211.03 ± 89.6 | 0.001 |
| Analgesicdemand, Mean ± SD | 6.3 ± 8.9 | 2.03 ± 6.3 | 0.038 |
| Blood Transfusion, No. (%) | 0.321 | ||
| Positive | 1 (3.3) | 0 (0) | |
| Negative | 29 (96.7) | 29 (100) |
4.2. Endpoint Results
In operation time-to-time analysis, SBP was significantly lower in GA group only in 120th minute; DBP in 60th, 90th, and 120th minutes, and MAP in 90th and 120th minutes (P < 0.05). The trend was not significantly different in none of 4 items (Figure 1; P > 0.05).
Figure 1. Trends of Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP), and Pulse Rate (PR) in Operation Room. None of the factors differed significantly (P = 0.990, P = 0.568, P = 0.710, P = 0.934, respectively- from Repeated measurements).
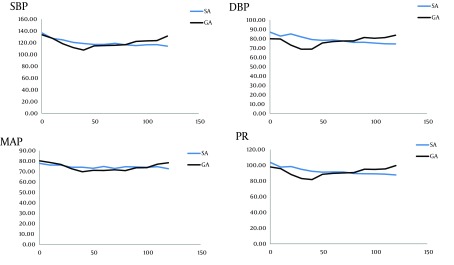
Table 2 demonstrates blood loss, analgesic demand, and blood transfusion amount in both groups. As seen, blood loss (P = 0.001) and analgesic demand (P = 0.038) were significantly higher in GA group.
5. Discussion
Using SA in PNCL surgery is acceptable and more secure. By faster discharge and reduced recovery time, the patients’ quality of life can be improved using SA, which can be a good choice for urologist (18).
Overall, our study demonstrated that SBP, DBP, MAP, and PR in the whole surgery and recovery times did not have any significant difference between 2 groups, and that the trend was also somewhat similar in SA and GA; however, patients’ hemodynamics were more stable in SA group. Furthermore, bleeding and analgesic demand were significantly higher in GA group. None of the patients needed blood transfusion. These results were similar to other studies demonstrating that SA group had better hemodynamics and lower bleeding during and after the surgery (19-26).
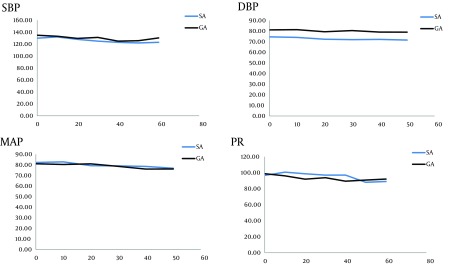
In PACU, SBP was significantly lower in 10th, 20th, 30th, 40th minute; DBP and MAP in all evaluations and PR only in the 20th minutes were lower (P < 0.05). The trend was not significantly different in none of 4 items (Figure 2 ; P > 0.05).
Figure 2. Trends of Systolic Blood Pressure (SBP), Diastolic Blood Pressure (DBP), Mean Arterial Pressure (MAP), and Pulse Rate (PR) in Recovery Room. None of the factors differed significantly (P = 0.844, P = 0.122, P = 0.863, P = 0.855, respectively- from Repeated measurements).
It seems that SA can result in vasodilation and hypotension following sympathetic block. On the other hand, reduced intra-thoracic pressure and epidural vein distension, due to spontaneous ventilation, result in reduced bleeding. Therefore, the results do not seem to be irrational because SA can inhibit stress hormone secretion better than GA (27-30).
SA blocks preganglionic sympathetic nerves with many advantages compared to GA, such as redistribution of blood flow to musculoskeletal system, skin, and subcutaneous tissues, as well as reducing SBP, DBP, MAP, and PAP, and better hemostasis. Furthermore, other studies demonstrated better PNCL surgery results, lower blood loss, and lesser side effects (such as nausea, vomiting, and post-op pain) in SA (19, 31). Among these advantages of SA, decreasing blood loss is a main issue of SA in PCNL surgery. Recent studies investigated the effects of a 200-μg of oral clonidine tablet 60 - 90 minutes before anesthesia, which reduced blood loss significantly in several kinds of surgeries under GA that could be a future choice along with SA in PCNL (32, 33)
In McClain et al. study, SA could reduce the amount of anesthesia drugs, length of surgery time, and other side effects in discus decompression surgery (34). Tetzlaff et al. have also shown that in spinal surgeries, SA was a better choice for anesthesia compared to GA resulting in lower side effects (35). In an observational study, Mehrabi et al. evaluated 160 patients who underwent PCNL under spinal anesthesia in prone position. Blood transfusion was performed for ten patients (6.3%), and six patients complained of mild to moderate headache, dizziness, and mild postoperative low back pain for 2 to 4 days. Complete clearance of calculus or no significant residual calculi larger than 5 mm was achieved in 70% of patients (36). In another prospective randomized study on PCNL, 52 patients underwent general anesthesia and 58 patients received spinal anesthesia. PCNL was performed by standard technique. Intraoperative hypotension, postoperative headache, and low back pain were significantly higher in spinal group, but, compared to SA, the cost of anesthetic drugs was more than five times , and post-operative analgesic consumption about two times in GA group. Finally, authors suggested SA as a safe, effective, and cost-effective method in adult PCNL, the same as our results (37). Moreover , in other studies, additional analgesic consumption was reduced in SA group compared to GA group. This may be due to afferent nociceptive block of the spinal cord and faster block of sensory than that of motor nerves (13, 19).
In this study, patients with stone in upper pole of kidney, tolerated efficiently, but our sample size was designated for a whole kidney and not solely for upper pole; so because of general concerns about this subtype of kidney stones, future studies are needed with a study population designated for upper pole stones to compare competency and efficacy of SA versus GA.
In view of the results of our study, SA is a faster and safer method of anesthesia in PNCL surgeries. Using this method can help surgeons to maintain patient in a better hemodynamic and hemostatic state, reduce the GA complications, decrease the need of analgesics, and duration of surgery.
Acknowledgments
The authors are grateful to urologists of Shahid Hamsheminejad hospital for their cooperation.
Footnotes
Implication for health policy/practice/research/medical education
Considering that the type of anesthesia as well as patients' hemodynamics can influence on surgery outcomes and relevant morbidity and mortality of the intervention, and that these factors directly reflect on regional health-care, we aimed this study to compare mean BP and PR among PNCL patients underwent general and spinal anesthesia.
Authors’ Contribution
Conception and design, collection of data, critical revision of the article, and administrative technical revision of the article, scientifically: Gholamreza Movassaghi. Conception and design, obtaining funding, data interpretation and writing the article: Valiollah Hassani. Conception and design, clinical analysis: Mahmood Reza Mohaghegh. Literature search, clinical analysis and scientifically revision of the article: Saeid Safari and Mohammad Mahdi Zamani. Conception and design, data collection, critical revision of the article: Roya Nabizadeh
Financial Disclosure
We certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding/Support
The research presented in this manuscript has been funded by Iran University of Medical Sciences Thesis grants.
References
- 1.Stening SG, Bourne S. Supracostal percutaneous nephrolithotomy for upper pole caliceal calculi. J Endourol. 1998;12(4):359–62. doi: 10.1089/end.1998.12.359. [DOI] [PubMed] [Google Scholar]
- 2.Lojanapiwat B, Prasopsuk S. Upper-pole access for percutaneous nephrolithotomy: comparison of supracostal and infracostal approaches. J Endourol. 2006;20(7):491–4. doi: 10.1089/end.2006.20.491. [DOI] [PubMed] [Google Scholar]
- 3.Jun-Ou J, Lojanapiwat B. Supracostal access: does it affect tubeless percutaneous nephrolithotomy efficacy and safety? Int Braz J Urol. 2010;36(2):171–6. doi: 10.1590/s1677-55382010000200006. [DOI] [PubMed] [Google Scholar]
- 4.Kuzgunbay B, Turunc T, Akin S, Ergenoglu P, Aribogan A, Ozkardes H. Percutaneous nephrolithotomy under general versus combined spinal-epidural anesthesia. J Endourol. 2009;23(11):1835–8. doi: 10.1089/end.2009.0261. [DOI] [PubMed] [Google Scholar]
- 5.Karacalar S, Bilen CY, Sarihasan B, Sarikaya S. Spinal-epidural anesthesia versus general anesthesia in the management of percutaneous nephrolithotripsy. J Endourol. 2009;23(10):1591–7. doi: 10.1089/end.2009.0224. [DOI] [PubMed] [Google Scholar]
- 6.Wong MY. Evolving technique of percutaneous nephrolithotomy in a developing country: Singapore General Hospital experience. J Endourol. 1998;12(5):397–401. doi: 10.1089/end.1998.12.397. [DOI] [PubMed] [Google Scholar]
- 7.Singh I, Kumar A, Kumar P. "Ambulatory PCNL" (tubeless PCNL under regional anesthesia) -- a preliminary report of 10 cases. Int Urol Nephrol. 2005;37(1):35–7. doi: 10.1007/s11255-004-6706-9. [DOI] [PubMed] [Google Scholar]
- 8.Singh V, Sinha RJ, Sankhwar SN, Malik A. A prospective randomized study comparing percutaneous nephrolithotomy under combined spinal-epidural anesthesia with percutaneous nephrolithotomy under general anesthesia. Urol Int. 2011;87(3):293–8. doi: 10.1159/000329796. [DOI] [PubMed] [Google Scholar]
- 9.Urwin SC, Parker MJ, Griffiths R. General versus regional anaesthesia for hip fracture surgery: a meta-analysis of randomized trials. Br J Anaesth. 2000;84(4):450–5. doi: 10.1093/oxfordjournals.bja.a013468. [DOI] [PubMed] [Google Scholar]
- 10.Indelli PF, Grant SA, Nielsen K, Vail TP. Regional anesthesia in hip surgery. Clin Orthop Relat Res. 2005;441:250–5. doi: 10.1097/01.blo.0000192355.71966.8e. [DOI] [PubMed] [Google Scholar]
- 11.Sakura S. [Epidural anesthesia and spinal anesthesia in the elderly]. Masui. 2007;56(2):130–8. [PubMed] [Google Scholar]
- 12.Ditzler JW, Dumke PR, Harrington JJ, Fox JD. Should spinal anesthesia be used in surgery for herniated intervertebral disk. Anesth Analg. 1959;38(2):118–24. [PubMed] [Google Scholar]
- 13.Hassi N, Badaoui R, Cagny-Bellet A, Sifeddine S, Ossart M. [Spinal anesthesia for disk herniation and lumbar laminectomy. Apropos of 77 cases]. Cah Anesthesiol. 1995;43(1):21–5. [PubMed] [Google Scholar]
- 14.Sadrolsadat SH, Mahdavi AR, Moharari RS, Khajavi MR, Khashayar P, Najafi A, et al. A prospective randomized trial comparing the technique of spinal and general anesthesia for lumbar disk surgery: a study of 100 cases. Surg Neurol. 2009;71(1):60–5. doi: 10.1016/j.surneu.2008.08.003. discussion 65. [DOI] [PubMed] [Google Scholar]
- 15.Hartmann B, Junger A, Klasen J, Benson M, Jost A, Banzhaf A, et al. The incidence and risk factors for hypotension after spinal anesthesia induction: an analysis with automated data collection. Anesth Analg. 2002;94(6):1521-9. doi: 10.1097/00000539-200206000-00027. [DOI] [PubMed] [Google Scholar]
- 16.Frolich MA, Caton D. Baseline heart rate may predict hypotension after spinal anesthesia in prehydrated obstetrical patients. Can J Anaesth. 2002;49(2):185–9. doi: 10.1007/BF03020493. [DOI] [PubMed] [Google Scholar]
- 17.Kawase M, Komatsu T, Nishiwaki K, Kobayashi M, Kimura T, Shimada Y. Heart rate variability and arterial blood pressure variability show different characteristic changes during hemorrhage in isoflurane-anesthetized, mechanically ventilated dogs. Anesth Analg. 2002;94(1):16-21. doi: 10.1097/00000539-200201000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Rozentsveig V, Neulander EZ, Roussabrov E, Schwartz A, Lismer L, Gurevich B, et al. Anesthetic considerations during percutaneous nephrolithotomy. J Clin Anesth. 2007;19(5):351–5. doi: 10.1016/j.jclinane.2007.02.010. [DOI] [PubMed] [Google Scholar]
- 19.Covino BG. Rationale for spinal anesthesia. Int Anesthesiol Clin. 1989;27(1):8–12. doi: 10.1097/00004311-198902710-00003. [DOI] [PubMed] [Google Scholar]
- 20.Davis S, Erskine R, James MF. A comparison of spinal and epidural anaesthesia for hip arthroplasty. Can J Anaesth. 1992;39(6):551–4. doi: 10.1007/BF03008316. [DOI] [PubMed] [Google Scholar]
- 21.Guedj P, Eldor J, Gozal Y. [Comparative study of conventional spinal anesthesia and combined spinal-epidural anesthesia in gynecological surgery]. Ann Fr Anesth Reanim. 1992;11(4):399–404. doi: 10.1016/s0750-7658(05)80338-7. [DOI] [PubMed] [Google Scholar]
- 22.Holmstrom B, Laugaland K, Rawal N, Hallberg S. Combined spinal epidural block versus spinal and epidural block for orthopaedic surgery. Can J Anaesth. 1993;40(7):601–6. doi: 10.1007/BF03009695. [DOI] [PubMed] [Google Scholar]
- 23.Niemi TT, Pitkanen M, Syrjala M, Rosenberg PH. Comparison of hypotensive epidural anaesthesia and spinal anaesthesia on blood loss and coagulation during and after total hip arthroplasty. Acta Anaesthesiol Scand. 2000;44(4):457–64. doi: 10.1034/j.1399-6576.2000.440418.x. [DOI] [PubMed] [Google Scholar]
- 24.Seeberger MD, Lang ML, Drewe J, Schneider M, Hauser E, Hruby J. Comparison of spinal and epidural anesthesia for patients younger than 50 years of age. Anesth Analg. 1994;78(4):667–73. doi: 10.1213/00000539-199404000-00009. [DOI] [PubMed] [Google Scholar]
- 25.Stober HD, Mencke T. [General anesthesia or spinal anesthesia for hip prosthesis replacement? Studies of acceptance of both procedures by patients]. Anaesthesiol Reanim. 1999;24(6):151–6. [PubMed] [Google Scholar]
- 26.Sutter PA, Gamulin Z, Forster A. Comparison of continuous spinal and continuous epidural anaesthesia for lower limb surgery in elderly patients. A retrospective study. Anaesthesia. 1989;44(1):47–50. doi: 10.1111/j.1365-2044.1989.tb11098.x. [DOI] [PubMed] [Google Scholar]
- 27.Thorburn J, Louden JR, Vallance R. Spinal and general anaesthesia in total hip replacement: frequency of deep vein thrombosis. Br J Anaesth. 1980;52(11):1117–21. doi: 10.1093/bja/52.11.1117. [DOI] [PubMed] [Google Scholar]
- 28.Davis FM, McDermott E, Hickton C, Wells E, Heaton DC, Laurenson VG, et al. Influence of spinal and general anaesthesia on haemostasis during total hip arthroplasty. Br J Anaesth. 1987;59(5):561–71. doi: 10.1093/bja/59.5.561. [DOI] [PubMed] [Google Scholar]
- 29.Cook PT, Davies MJ, Cronin KD, Moran P. A prospective randomised trial comparing spinal anaesthesia using hyperbaric cinchocaine with general anaesthesia for lower limb vascular surgery. Anaesth Intensive Care. 1986;14(4):373–80. doi: 10.1177/0310057X8601400409. [DOI] [PubMed] [Google Scholar]
- 30.Greenbarg PE, Brown MD, Pallares VS, Tompkins JS, Mann NH. Epidural anesthesia for lumbar spine surgery. J Spinal Disord. 1988;1(2):139–43. [PubMed] [Google Scholar]
- 31.Kehlet H. The stress response to surgery: release mechanisms and the modifying effect of pain relief. Acta Chir Scand Suppl. 1989;550:22–8. [PubMed] [Google Scholar]
- 32.McLain RF, Kalfas I, Bell GR, Tetzlaff JE, Yoon HJ, Rana M. Comparison of spinal and general anesthesia in lumbar laminectomy surgery: a case-controlled analysis of 400 patients. J Neurosurg Spine. 2005;2(1):17–22. doi: 10.3171/spi.2005.2.1.0017. [DOI] [PubMed] [Google Scholar]
- 33.Ebneshahidi A, Mohseni M. Premedication with oral clonidine decreases intraoperative bleeding and provides hemodynamic stability in cesarean section. Anesth Pain. 2011;1(1):30–3. doi: 10.5812/kowsar.22287523.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taghipour Anvari Z, Afshar-Fereydouniyan N, Imani F, Sakhaei M, Alijani B, Mohseni M. Effect of Clonidine Premedication on Blood Loss in Spine Surgery. Anesth Pain. 2011;1(4):252–6. doi: 10.5812/aapm.2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tetzlaff JE, Dilger JA, Kodsy M, al-Bataineh J, Yoon HJ, Bell GR. Spinal anesthesia for elective lumbar spine surgery. J Clin Anesth. 1998;10(8):666–9. doi: 10.1016/s0952-8180(98)00112-3. [DOI] [PubMed] [Google Scholar]
- 36.Mehrabi S, Karimzadeh Shirazi K. Results and complications of spinal anesthesia in percutaneous nephrolithotomy. Urol J. 2010;7(1):22–5. [PubMed] [Google Scholar]
- 37.Mehrabi S, Mousavi Zadeh A, Akbartabar Toori M, Mehrabi F. General versus spinal anesthesia in percutaneous nephrolithotomy. Urol J. 2013;10(1):756–61. [PubMed] [Google Scholar]


