Abstract
Common carp (including ornamental koi carp) Cyprinus carpio L. are ecologically and economically important freshwater fish in Europe and Asia. C. carpio have recently been endangered by a third cyprinid herpesvirus, known as cyprinid herpesvirus-3 (CyHV-3), the etiological agent of koi herpesvirus disease (KHVD), which causes significant morbidity and mortality in koi and common carp. Clinical and pathological signs include epidermal abrasions, excess mucus production, necrosis of gill and internal organs, and lethargy. KHVD has decimated major carp populations in Israel, Indonesia, Taiwan, Japan, Germany, Canada, and the USA, and has been listed as a notifiable disease in Germany since 2005, and by the World Organisation for Animal Health since 2007. KHVD is exacerbated in aquaculture because of the relatively high host stocking density, and CyHV-3 may be concentrated by filter-feeding aquatic organisms. CyHV-3 is taxonomically grouped within the family Alloherpesviridae, can be propagated in a number of cell lines, and is active at a temperature range of 15 to 28°C. Three isolates originating from Japan (KHV-J), USA (KHV-U), and Israel (KHV-I) have been sequenced. CyHV-3 has a 295 kb genome with 156 unique open reading frames and replicates in the cell nucleus, and mature viral particles are 170 to 200 nm in diameter. CyHV-3 can be detected by multiple PCR-based methods and by enzyme-linked immunosorbent assay. Several modes of immunization have been developed for KHVD; however, fish immunized with either vaccine or wild-type virus may become carriers for CyHV-3. There is no current treatment for KHVD.
Keywords: Aquaculture, KHV, Koi herpesvirus disease, KHVD, Skin hemorrhages, Virus detection, Enzyme-linked immunosorbent assay, ELISA
INTRODUCTION
Fish protein makes up 20% or more of the total protein consumed in low-income, food-deficient countries, with aquaculture accounting for 46% of the global food fish supply in 2008; in addition to being a critical form of nutrition, foodfish production through aquaculture is also a major form of employment (FAO 2010). Carp (Cyprinidae) make up 71% of the global farmed freshwater fish production and are an important source of food in China and India where 70.7 and 15.7%, respectively, of farmed carp are produced. However, cyprinid herpesvirus (CyHV-3), which is the etiological agent of a highly contagious viral disease, koi herpesvirus disease (KHVD), is causing massive damage to the world production of koi and common carp Cyprinus carpio L. The disease has been listed since 2005 in Germany, and since 2007 in England and by the World Organisation of Animal Health (OIE) as a notifiable disease, and has spread to most regions of the world due to the global fish trade and international ornamental koi shows (Pokorova et al. 2005, Ilouze et al. 2008, FAO 2010). KHVD is characterized by white patches, skin hemorrhages, lethargy, lack of appetite, sunken eyes, enlargement of the spleen and kidney, and gill necrosis (Fig. 1) in infected fish (Hedrick et al. 2005). The virus replicates in gills, intestine, interstitium, liver, brain, and kidney tissues (Pikarsky et al. 2004), and was previously known as carp interstitial nephritis and gill necrosis virus. Because KHVD has become so detrimental to world foodfish production, this review will cover the discovery of KHVD, the spread of CyHV-3, progress in our understanding of the disease and host–pathogen relationship, tools developed to detect the virus, and methods developed to control the disease.
Fig. 1.
Cyprinus carpio. Clinical signs of koi herpesvirus disease. (A) Increased mucus production, petechia, enophthalmus. (B) Confluent bleedings in the skin. (C) Severe gill necrosis
The cyprinid herpesvirus family
Based on sequence alignment of conserved regions for the DNA polymerase and terminase genes, the cyprinid herpesviruses are closely related to anguillid herpesvirus 1 (AngHV-1) (Waltzek et al. 2009a). CyHV-3 shares 40 conserved genes with AngHV-1 (van Beurden et al. 2010), and according to the International Committee on Taxonomy of Viruses (http://ictvonline.org/), all 3 cyprinid herpesviruses and AngHV-1 are grouped within the genus Cyprinivirus. The first cyprinid herpesvirus, cyprinid herpesvirus-1 (CyHV-1), was isolated in Japan from the papillomatous skin growth on an infected koi and propagated in epithelioma papulosum cyprini (EPC) and fathead minnow (FHM) cell lines at 20°C (Sano et al. 1985a), and was described with the dimensions of 113 and 190 nm for the nucleocapsid and mature enveloped virion respectively (Sano et al. 1985b). Later, a cyprinid herpesvirus was isolated in North America by Hedrick et al. (1990), and was described with the dimensions of 109 and 157 nm for the nucleocapsid and mature enveloped virion. CyHV-1 is lethal in young fish, and can cause mortality rates up to 97% (Sano et al. 1991). Although CyHV-1 causes acute growth of papillomas, it is usually non-lethal to adult koi (Calle et al. 1999). Cyprinid herpesvirus-2 (CyHV-2) affects mainly goldfish Carassius auratus auratus and has been isolated in EPC and FHM cell lines grown at 20°C (Jung & Miyazaki 1995, Groff et al. 1998), but neither cell line is suitable for serially passaging the virus (Goodwin et al. 2006a). Jeffery et al. (2007) passaged CyHV-2 in a koi fin-1 (KF-1) cell line once, but no cytopathic effect (CPE) was observed on subsequent passages. CyHV-2 has been passaged in a goldfish fin cell line (GF-1) for studies in phylogenetic analysis and detection assays (Waltzek et al. 2005, 2009b). The clinical signs of CyHV-2 infection include lethargy, development of pale skin with white, mucoid, blister-like projections on tissue, and ultimately mortality (Jeffery et al. 2007). Postmortem inspection of CyHV-2 infected goldfish revealed severe necrosis of gills and deterioration of the kidney and liver (Jeffery et al. 2007). CyHV-2 causes herpesviral hematopoietic necrosis in goldfish (Jung & Miyazaki 1995), and can be detected by PCR (Goodwin et al. 2006a,b). CyHV-2 viral particles are hexagonal, and intranuclear particles have a diameter of 100 nm with a 50 nm core (Jeffery et al. 2007). Mature, enveloped virions range from 170 to 220 nm in size when observed in the cytoplasm and extracellular spaces (Jung & Miyazaki 1995). Random sampling in goldfish farms has shown that CyHV-2 is highly prevalent in the USA (Goodwin et al. 2009). There are 3 main isolates of the third cyprinid herpesvirus, CyHV-3, one originating in Japan, another in Israel, and another isolated in the US, denoted KHV-J, KHV-I, and KHV-U, respectively.
Taxonomy
CyHV-3 shares homology to Poxviridae, Iridoviridae, and Nimaviridae proteins involved in synthesis of deoxynucleotide tri-phosphates (dNTPs); however, CyHV-3 is genetically divergent from the aforementioned virus families and is more similar to CyHV-1 and CyHV-2; therefore, CyHV-3 is considered the third cyprinid herpesvirus (Waltzek et al. 2005, Ilouze et al. 2006). Protein sequence comparison of helicase, capsid triplex protein, DNA polymerase, and major capsid protein (open reading frame 39: ORF39) confirmed sufficient significant homology for CyHV-3 to be grouped with the other 2 cyprinid herpesviruses in a new order, the Herpesvirales (Waltzek et al. 2005). Based on the genes encoding viral DNA polymerase and the ATPase subunit of terminase, CyHV-3 is currently classified together with CyHV-1 and CyHV-2 in the genus Cyprinivirus within the family Alloherpesviridae (Waltzek et al. 2009a, Michel et al. 2010a). The isolates can be differentiated by duplex PCR to detect minor mutations in the coding region between ORF29 and ORF31 (Bigarré et al. 2009). The cloning and subsequent sequencing of the 3 glycoproteins ORF25, ORF65, and ORF 116 detected variation that include specific insertions and deletions among the 3 main KHV isolates (Han et al. 2013).
Early history and worldwide distribution
CyHV-3 was initially identified by Hedrick et al. (2000) as the causative agent for mass mortality outbreaks in the US and Israel, but may have been detected as early as 1996 in England (Haenen et al. 2004) and 1997 in Germany (Bretzinger et al. 1999). Three CyHV-3 isolates are completely sequenced: accession nos. in the NCBI nucleotide database NC_009127 (DQ177346 [strain I]; DQ657948 [strain U]; and AP008984 [strain J, TUMST1]). Each can be identified based on sequence disparities in different genes. Kurita et al. (2009) grouped the 3 sequenced CyHV-3 isolates as either US/I or J strains based on nucleotide polymorphism in the thymidine kinase (TK) gene; this grouping system was verified by Avarre et al. (2011) tracking disparities in tandem repeats in genetic sequences of 8 different CyHV-3 loci. Some European isolates are similar to isolates from the US and Israel (KHV-U and KHV-I, respectively). Other isolates that may be variants of CyHV-3, which are known as KHV-J, have been detected in Japan, Malaysia, Taiwan, and other East Asian countries (Kurita et al. 2009, Cheng et al. 2011). Recently, based on sequence comparison of glycoproteins, a new variant of KHV was detected in Korea (Han et al. 2013).
Europe
Researchers in Germany were among the earliest to describe KHVD in koi (Bretzinger et al. 1999). Since then, CyHV-3 has been detected in several farms in Poland, where carp is grown for food (Antychowicz et al. 2005, Bergmann et al. 2006). There are sporadic CyHV-3-positive locations of commercial koi and common carp farms in the Czech Republic (Pokorova et al. 2007). England and Wales have widespread KHVD in carp and koi fisheries, but there are separate carp and koi farms that are free of CyHV-3 (Taylor et al. 2010, 2011). Two cases of KHVD were reported in Ireland, one in 2005 and another in 2007, and each case involved imported koi (McCleary et al. 2011). As of today, most western European countries, including Austria, Belgium, Denmark, France, Italy, Luxemburg, Romania, Slovenia, Spain, Sweden, Switzerland, and the Netherlands have reported positive tests for CyHV-3 (Pokorova et al. 2005, OIE 2012).
Asia
An international taskforce was established in an attempt to determine the cause of a 2002 disease outbreak in Indonesia. Subsequently, a unique Indonesian isolate of CyHV-3 (a hybrid of the US/I and Japan isolate) was isolated and identified from regions of Indonesia (Bondad-Reantaso et al. 2007, Sunarto et al. 2011). KHVD was first detected in Japan in 2003 during a mass mortality event of farmed carp in Lake Kasumigaura, Ibaragi prefecture (Sano et al. 2004). Thereafter, CyHV-3 was detected by PCR- and ELISA-based assays in wild carp in Lake Biwa, and CyHV-3-DNA was detected in water samples from 4 sites along the Tamagawa River in Tokyo, Japan, during 2006; since then, CyHV-3 has been confirmed in 90% of the 109 national class-A natural rivers in Japan (Ishioka et al. 2005, Sano et al. 2005, Haramoto et al. 2009, Uchii et al. 2009, Minamoto et al. 2012). Mortality due to CyHV-3 was detected in northern Taiwan in 2002 (Tu et al. 2004), and mass mortality events were first observed in 2003 in southern Taiwan fisheries (Cheng et al. 2011). CyHV-3 has been detected in China (Dong et al. 2011) and in koi broodstock in South Korea (Gomez et al. 2011, Lee et al. 2012, Han et al. 2013).
North America
Since the initial detection of KHVD in the US, conventional and real-time PCR with pooled samples from liver, spleen, and kidney taken from carp during a 2004 mass mortality event of wild common carp in New York confirmed that the fish were infected by CyHV-3 (Grimmett et al. 2006). Subsequently, CyHV-3 was detected in Ontario, Canada, in 2007, and thereafter, multiple lakes in Ontario suffered gross carp mortality events due to KHVD (Garver et al. 2010). According to the Michigan Department of Natural Resources, there have been recent carp mass mortalities caused by CyHV-3 in the northern Midwest region of the US bordering Canada (Whalen 2011). It seems that the virus is spreading westward along the US/Canada border.
DETECTION
CyHV-3 was originally differentiated from channel catfish virus and cyprinid herpesviruses by polypeptide analysis that revealed novel peptides in purified viral extracts and by restriction analysis of purified DNA extracts. The latter technique led to the preliminary PCR-based method of detection (Gilad et al. 2002). Subsequently, a PCR-based method was developed based on the amplification of the TK gene (Bercovier et al. 2005). Loop-mediated isothermal amplification (LAMP) of the TK gene allows for detection of CyHV-3 in a 1-step process without requiring a thermal cycler (Gunimaladevi et al. 2004, Yoshino et al. 2006, 2009). Detection of CyHV-3 can be accomplished by nested PCR or by capturing viral particles with antibodies followed by LAMP to detect viral particles (El-Matbouli et al. 2007a, Soliman & El-Matbouli 2009). Real-time PCR, nested PCR, and semi-nested PCR are among the most sensitive tools for detection of CyHV-3 (Bergmann et al. 2010b). In one of the more elegant detection methods for CyHV-3 by Soliman & El-Matbouli (2005), the product of LAMP-PCR is visualized by mixing with SYBR-Green I to confirm positive results for CyHV-3 (Fig. 2). Differentiation of the CyHV-3 isolates from Israel, Japan, and the US can be accomplished by analysis of a variable number of tandem repeats in coding and non-coding regions (Avarre et al. 2011). In addition to the various molecular methods to detect CyHV-3, a monoclonal antibody produced against ORF68 has also been developed to be used for confirming CyHV-3 by immunohistochemistry (Akoi et al. 2011). A sensitive enzyme-linked immunosorbent assay (ELISA) that probes for captured carp anti-KHV antibodies was developed for the indirect detection of CyHV-3 by Adkison et al. (2005); another one by St-Hilaire et al. (2009). A further ELISA that uses anti-KHV antibody to probe for captured KHV particles was developed by Dishon et al. (2005) and is sold as a commercial kit. Fish suffering from KHVD are known to have secondary bacterial infections (Haenen et al. 2004), so a PCR-based method was coupled with DNA-array technology to rapidly detect CyHV-3 positive fish that are infected with Flavobacterium (Lievens et al. 2011) or other bacteria. A primer probe designed against an exonic mRNA coding sequence allows for the detection of CyHV-3 during the replication stage (Yuasa et al. 2012).
Fig. 2.
Detection methods for koi herpesvirus (KHV). (A) Agarose gel showing LAMP products of KHV DNA extracted by boiling. The reaction was carried out at 65°C using the 6 primers. Lanes: mar = 100 bp molecular weight marker, 1 = KHV DNA extracted by boiling, 2 = negative fish tissue, 3 = negative control. (B) Visual detection of KHV LAMP products using SYBR Green I stain. 1: Negative LAMP reaction remained orange; 2: positive LAMP reaction turned green. (Image from Soliman & El-Matbouli 2005)
MODE OF ENTRY
Bioluminescent assays using a luciferase-expressing recombinant CyHV-3 genome cloned into a bacterial artificial chromosome (BAC) revealed that CyHV-3 may enter through the skin in conjunction with the previously presumed route of entry, the gill (Costes et al. 2009). Epidermal abrasions provide sites susceptible to viral entry, and wound healing regions are also susceptible to viral entry (Raj et al. 2011). Feeding on CyHV-3-positive material provides an additional mode of entry for CyHV-3 through the pharyngeal periodontal mucosa during mastication, and it is known that CyHV-3 replicates profusely in the intestine (Fournier et al. 2012) rather than entering through the intestine (Ilouze et al. 2010). Inflammatory response and TK mRNAs are detectable in carp intestine 3 d post immersion with CyHV-3, and claudin genes (claudin-2, -3, -11, and -23) that participate in the maintenance of tight junctions in epithelial cells are modulated in the gut during CyHV-3 infection (Syakuri et al. 2013).
TRANSMISSION
Environmental factors
CyHV-3 induces high mortality for koi at temperatures between 18 and 28°C, but no mortality was observed for CyHV-3-exposed fish at 13°C (Gilad et al. 2003). CyHV-3 is transmitted horizontally via the excrement of diseased fish; therefore, having high densities of fish such as in aquaculture enterprises will exacerbate a CyHV-3 outbreak by facilitating the release of high levels of CyHV-3 via the excrement of diseased fish (Dishon et al. 2005). CyHV-3 titers in water of contaminated rivers and lakes can be measured by concentrating viral particles using ultracentrifugation or cationic-coated filters followed by quantitative real-time PCR (qPCR) (Honjo et al. 2010). Use of qPCR has demonstrated that CyHV-3 concentrations vary in nearby plankton deposit sites and are likely concentrated by filter-feeding Rotifera (Minamoto et al. 2011). CyHV-3 may be transmitted to carp when feeding on plankton directly, or on bivalves that have fed on plankton and have concentrated CyHV-3 in their digestive tubes (Minamoto et al. 2011). Other filter-feeding organisms, such as freshwater mollusks and crustaceans, can also test positive for CyHV-3 (Kielpinski et al. 2010). Mating may also increase the prevalence of CyHV-3 by aggregating infected fish and/or causing a reduction of the immune response (Uchii et al. 2011). Significant levels of CyHV-3 DNA were detected in a lagoon 1 mo before a mass mortality event, and similar concentrations were also detected in a lake that had been free of CyHV-3-associated mass mortality events for 3 yr (Honjo et al. 2010). Some strains of bacteria naturally clear water of CyHV-3 and thereby reduce the infectivity of CyHV-3 in natural environments within a few days (Shimizu et al. 2006).
Carriers
Survivors of KHVD have CyHV-3 DNA in liver, heart, gill, and eye, and CyHV-3 may persist in cells of the gastrointestinal epithelium or in leukocytes (Bergmann et al. 2009, Eide et al. 2011a). Surviving carp are carriers of CyHV-3 and can excrete virus especially following stress-related activities such as nesting (Bergmann & Kempter 2011). Goldfish have been shown to host CyHV-3 virus when co-habitated with infected koi (El-Matbouli et al. 2007b, Bergmann et al. 2010a). Goldfish have also been reported to act as carriers for CyHV-3 in the US (Sadler et al. 2008). Leukocytes from both koi and goldfish have tested positive for CyHV-3. In addition, in cohabitation studies, the infected goldfish transmitted virus to naïve koi as demonstrated by immunostaining with anti-CyHV-3 serum, in situ hybridization, and PCR (Bergmann et al. 2010a). In a similar study, goldfish were shown to act as a carrier for CyHV-3 in cohabitation experiments with naïve common carp (El-Matbouli & Soliman 2011). Nested PCR has revealed CyHV-3 DNA in samples of healthy fish including grass carp Ctenopharyngodon idella, blue back ide Leuciscus idus, and Ancistrus sp., suggesting that these fish species may be potential carriers of CyHV-3 (Bergmann et al. 2009). Similarly, Russian sturgeons Acipenser guel denstaedtii and Atlantic sturgeons A. oxyrinchus have also tested positive for CyHV-3 DNA by PCR-based methods (Kempter et al. 2009).
Factors affecting infection
Although young and adult common carp are susceptible to CyHV-3, carp larvae are impervious to infection by CyHV-3 (Ito et al. 2007). Single nucleotide polymorphisms in the innate immune response genes of carp, such as in Toll-like or in the IL-10a gene, may differentiate resistance to CyHV-3 (Kongchum et al. 2010, 2011). Upregulation of 8 immune-related genes, including interferon inducible protein gig-1 like and suppressor of cytokine signaling 1 were observed in survivors of KHVD, in addition to a more rapid cytokine reaction to KHV (Rakus et al. 2012). CyHV-3 may also be able to modulate the interferon response in different cell types (Adamek et al. 2012). CyHV-3 infection downregulates skin defense genes such as muc5B, a component of the mucus blanket, claudins (23, 30), which are important for maintenance of tight junctions, and anti microbial peptides such as β-defensin-1 and -2, involved in secondary bacterial infections (Adamek et al. 2013). However, mucus provides an innate protection against viral entry by providing fish with a viscous barrier against entry and as a storage medium for viral neutralizing factors (Raj et al. 2011). Therefore, although the skin may be susceptible to CyHV-3 entry (Costes et al. 2009), due to the protection that mucus provides, the digestive tract and or gill tissues are the more likely paths for CyHV-3 entry (S. M. Bergmann pers. obs.).
HISTOPATHOLOGICAL EFFECT OF CYHV-3
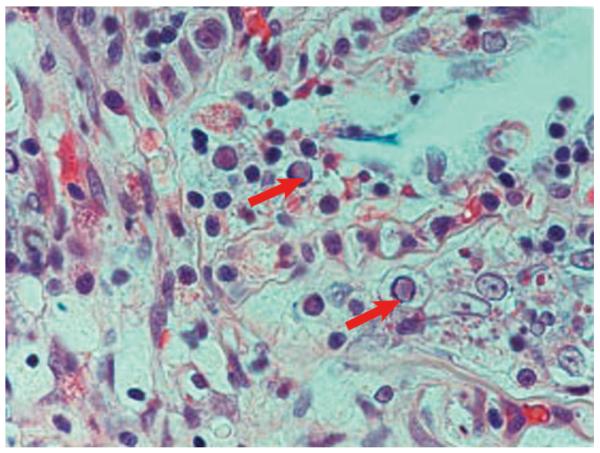
Characteristics of CyHV-3-infected fish include loss of appetite, erratic and uncoordinated movement, and gasping for air (Hutoran et al. 2005). CyHV-3 was detected in the mucus as early as 1 d post infection, and significant titers were also detectable in the brain, spleen, kidney, liver, and gut of infected koi (Gilad et al. 2004). The main clinical signs of CyHV-3 disease include: loss of epidermis, discoloration, erosion of fin extremities, and an increase in mucus production in gills (Bretzinger et al. 1999). Histopathological changes induced by CyHV-3 include: lesions and necrosis in gill and interstitial kidney tissues, and focal necrosis in the liver and nuclear inclusion bodies in gill tissue (Fig. 3) and in renal glomerulae (Perelberg et al. 2003). CyHV-3 infected common carp displayed inclusion bodies in splenocytes, nuclear degeneration in the myocardial cells, and congestion of capillaries in small veins of the brain (Miyazaki et al. 2008). Additionally, CyHV-3 causes hyperplasia of the epithelium lining of the gastric gland in the stomach and of the intestinal villi in the intestine forming cystic papillary projections, and hyperplasia of respiratory cells resulting in the fusion of lamella and hemorrhaging at the tips of lamella in the gills (El-Din 2011).
Fig. 3.
Cyprinus carpio. Koi herpesvirus forming inclusion bodies (arrows) in gill epithelium of common carp
CELL CULTURE AND VIRAL REPLICATION
CyHV-3-infected cells display a dense cytoplasm with organelle morphology that degrades as viral replication progresses (Miyazaki et al. 2008). CyHV-3 has been cultured in koi caudal fin (KF-1) and common carp brain (CCB) (Fig. 4) cell cultures, and has been shown to replicate in cell cultures derived from silver carp Hypophthalmichthys molitrix and goldfish (Neukirch & Kunz 2001, Davidovich et al. 2007, Bergmann et al. 2010a, Dong et al. 2011). CPE caused by infection with CyHV-3 has also been observed 15 d post inoculation of FHM (Grimmett et al. 2006). Optimal replication for CyHV-3 in the KF-1 cell-line occurs between 15 and 25°C (Gilad et al. 2003). Real-time PCR determined that a minimum of 6 × 103 genomic equivalents of CyHV-3 are needed to observe CPE in 106 KF-1 (koi fin) cells, and CyHV-3 titers range between 107 and 109 genomic equivalents per 106 KF-1 cells during peak infection in mucus, liver, kidney, spleen, gut, and brain (Gilad et al. 2004).
Fig. 4.
Cyprinus carpio. Koi herpesvirus (KHV) in common carp brain cell culture. Images were taken 13 d after passaging. (A) Non-infected cells. (B) Cells 11 d post KHV in fection. Arrows point to CyHV-3-infected cells and arrow heads point to CyHV-3-induced cell clearance. Cytoplasmic vacuolation is visible for both infected cells in the inset, and the lower cell shows signs of syncytia formation. Scale bar = (A, B) 50 μm, (Inset) 100 μm
MORPHOGENESIS
Electron microscope images of maturing virus showed that CyHV-3 replicates in the nucleus and forms capsids with characteristics similar to those of mammalian herpesviruses in terms of protrusions into and through the nuclear membrane (Miwa et al. 2007). Initially in the nucleus, immature CyHV-3 nucleocapsids that have low electron density and are 100 nm in diameter are formed. Subsequently, mature nucleocapsids with high electron density and 117 nm in diameter are formed (Miyazaki et al. 2008). Mature capsids with a diameter between 150 and 180 nm are located in the perinuclear region where they begin to bud off and take with them part of the nuclear envelope (Miyazaki et al. 2008). Transmission electron microscopic studies (TEM) of CyHV-3 show that the virus forms a symmetrical icosahedron; however, the viral core region is an asymmetrical electron-dense region where the genomic DNA and nucleoprotein complex exist (Hutoran et al. 2005). The electron-dense core of mature CyHV-3, as observed with TEM, shows the diameter to be between 170 and 230 nm and the protein core to be 110 nm (Cheng et al. 2011).
FUNCTIONAL GENES
The genome of each of the CyHV-3 isolates (Israel, Japan, and USA) consists of 295 kB with a 22 kB terminal repeat; therefore, the 164 potential ORFs consist of 8 repeated ORFs that flank both ends of the genome (Aoki et al. 2007). Of the 156 unique potential ORFs in the CyHV-3 genome, mass spectrometry studies identified 40 proteins in mature virions that include 3 capsid proteins, 13 envelope proteins, 2 tegument proteins, and 22 structural proteins that are yet to be classified (Michel et al. 2010b). A recent report by Ilouze et al. (2012a) demonstrated by reverse transcriptase-real-time PCR that all 156 ORFs of CyHV-3 are transcribed, and the ORFs have been annotated in terms of relative transcriptional timing. Three genes involved in the synthesis of dNTPs required for DNA synthesis, viz. thymidylate monophosphate kinase, ribonucleotide reductase (RNR), and TK, share homology with pox virus genes (Ilouze et al. 2006). However, TK, RNR, and another gene involved in DNA synthesis, deoxyuridine triphosphate pyrophosphatase, are non-essential for viral replication in CCB cell lines, but these genes are important virulence factors in affecting clinical signs and mortality (Fuchs et al. 2011). The protein encoded by ORF81 was shown to be incorporated into the viral envelope of intact CyHV-3 particles by immunogold microscopy (Rosenkranz et al. 2008). A majority of CyHV-3 genes involved in DNA synthesis are eliminated within 24 h in infected CCB cells that are transferred to 30°C (i.e. non-permissive temperatures); however, TK, B22Rh, ITP (intercapsomeric triplex protein), and clone Y genes persist for up 15 d after transfer (Dishon et al. 2007). In a parallel experiment in the aforementioned report, B22R homolog, Orf4, Orf5, and Gray Sph1hpi were the first 4 genes to be re-transcribed after infected cells were incubated for 22 d at 30°C to clear CyHV-3 transcripts and re-transferred to 22°C (i.e. permissive temperature). A BAC carrying the entire CyHV-3 genome with disruption to the TK gene displayed reduced virulence and caused 50% reduction in mortality in koi compared to a revertant BAC that had an additional gene producing TK (Costes et al. 2008). Protein screening with an antibody against glycoprotein ORF56 revealed that CyHV-3 interacts with a number of host defense proteins that include lysozymes and granulins, and with machinery involved in protein modification such as protease inhibitor, glutathione S-transferase rho, and members of the ubiquitin degradation pathway (Gotesman et al. 2013). ORF134 encodes a homolog of interleukin-10, expression of which is higher during acute and activation phases of CyHV-3 disease (Sunarto et al. 2012).
PROPHYLAXIS AND CONTROL
Immunization
Immunization against CyHV-3 is achieved by exposing carp for 2 to 3 d with infectious CyHV-3 and subsequently transferring the exposed carp to a non-permissive temperature (30°C), or by using a live attenuated virus produced by in vitro serial passages and treatment with ultraviolet (UV) irradiation (Ronen et al. 2003, Perelberg et al. 2005). Challenge with an attenuated or wild-type virus demonstrated that antibodies produced in the infected fish can neutralize CyHV-3 in vivo (Perelberg et al. 2008). Adkison et al. (2005) also demonstrated that treatment with a CyHV-3-induced antibody can attenuate CyHV-3 infection. Surviving koi that were inoculated with a BAC-derived strain carrying either full strength or attenuated CyHV-3 were resistant to CyHV-3 disease (Costes et al. 2008). A US patent has been filed based on the aforementioned method of immunization (Costes et al. 2011). Mathematical modeling predicts that inoculation of farmed fish with CyHV-3 during autumn will produce KHVD-resistant carp in readiness for the following permissive seasons (Omori & Adams 2011).
Immunized carp become carriers?
A potential flaw in immunizing carp against CyHV-3 by temperature shift is that immunized fish can become carriers for the virus. This threat was demonstrated by the re-emergence of CyHV-3 in cultures of the CCB cell line that were acutely exposed to CyHV-3 and later moved to a non-permissive temperature (30°C) for 30 d (Dishon et al. 2007). Furthermore, Bretzinger et al. (1999) initially reported that CyHV-3-affected koi maintained at 12°C (i.e. non-permissive temperature) were sub-clinical; however, they soon showed clinical signs and died when the temperature was raised to 21°C (i.e. permissive temperature). Sub-clinical fish that were immunized for CyHV-3 by incubation at 12°C (i.e. non-permissive temperature) suffered a 57% mortality rate, and naïve (non-infected) fish suffered a 100% mortality rate when they were cohabitated at 20°C (St-Hilaire et al. 2005). Furthermore, CyHV-3-infected fish carried detectable antibody specific for CyHV-3 for up to 65 wk post exposure, and developed CyHV-3 disease signs and mortality even when incubated at a non-permissive temperature (12°C) for 25 wk upon return to the permissive temperature (St-Hilaire et al. 2009). CyHV-3 remains latent in white blood cells and can be detected by real-time PCR in gill tissue and fecal deposits following heat stress (Bergmann et al. 2010b, Eide et al. 2011b). Cumulatively, the aforementioned reports suggest high potential for CyHV-3 to persist in sub-clinical carriers and infect naïve fish, and immunization will further propagate the virus.
Crossbreeding
Domestic Israeli carp strains, known as Dor-70 and Našice (from the former Yugoslavia), that were crossbred with a native Czech strain known as Sasson showed significant higher survival rates to KHVD (64 and 69%) compared with those of the parental strains Dor-70 and Nasice (28 and 9%, respectively; Shapira et al. 2005). Similar experiments in the UK showed that crossbreeding carp with a ‘wild’ strain originating from the Amor or Duna Rivers in Hungary produced carp that were more resistant to KHVD than domesticated carp (Dixon et al. 2009). Crossbreeds of koi × crucian carp Carassius carassius and koi × goldfish were analyzed for resistance to KHVD. Both crossbreeds showed pathological signs of KHVD; however, although the common koi × crucian carp hybrid showed similar mortality levels to a pure carp breed following CyHV-3-I infection (91 and 100%, respectively), hybrids between koi and goldfish showed reduced mortality (35%; Bergmann et al. 2010c).
CONCLUSION
CyHV-3 was initially described by Hedrick et al. (2000) as a serious virus affecting koi and common carp in the US and Israel. Currently, CyHV-3 is a serious epidemic threat to koi and common carp worldwide in terms of koi breeding, and for common carp production in natural environments and in aquaculture. CyHV-3 is a member of the family Alloherpesviridae, and is a double-stranded DNA virus that consists of a 295 kb genome which codes for 156 genes, all of which have been annotated and demonstrated to be transcribed (Ilouze et al. 2012a). The virus can replicate in a number of cell lines including CCB, KF-1, and EPC. KHVD is limited to koi and common carp; however, goldfish, blue back ide, and Russian and Atlantic sturgeons may act as carriers for the virus. Specific and validated diagnostic methods for detection and identification, especially for subclinical infections, were predicted to be the most important tool to contain the spread of CyHV-3 (Pearson 2004); however, the virus has spread in Europe, Asia, and North America.
A practical use for CyHV-3 has been described in Australia, where carp have been introduced and are considered a pest species that has recently been linked to the decline of native Australian fish species. CyHV-3 is considered as a potential control agent for eradicating carp in Australia (McColl et al. 2007). Fortunately, CyHV-3 has not been detected in India by using either the PCR primers developed by Ronen et al. (2003) or newly developed primers that specifically target the major capsid protein of CyHV-3 (Rathore et al. 2009). However, other major carp-producing countries, such as China, Indonesia, and Japan, have seen elevated incidence of KHVD (Dong et al. 2011, Avarre et al. 2012, Minamoto et al. 2012).
Several steps have been taken in recent years to control the spread of CyHV-3, such as recognition of the threat and development of rapid and sensitive tools for detection of the virus. Other methods include immunization of carp by infection with ‘wild-type’ infectious virus and subsequently transferring the fish to temperatures that are non-permissive for CyHV-3 replication or by infection of carp with attenuated virus. Selective breeding programs to breed KHVD-resistant carp have also been initiated. Any effort to contain CyHV-3 must recognize that survivors of CyHV-3 outbreaks can become carriers for the virus (St-Hilaire et al. 2005, Uchii et al. 2009, Bergmann et al. 2010b, Eide et al. 2011b, Ilouze et al. 2012b).
Acknowledgements
This study was funded by the Austrian Science Fund (FWF), Project no. P23550-B13.
LITERATURE CITED
- Adamek M, Rakus KL, Chyb J, Brogden G, Huebner A, Irnazarow I, Steinhagen D. Interferon type I responses to virus infections in carp cells: in vitro studies on cyprinid herpesvirus 3 and Rhabdovirus carpio infections. Fish Shellfish Immunol. 2012;33:482–493. doi: 10.1016/j.fsi.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Adamek M, Syakuri H, Harris S, Rakus KL, et al. Cyprinid herpesvirus 3 infection disrupts the skin barrier of common carp (Cyprinus carpio L.) Vet Microbiol. 2013;162:456–470. doi: 10.1016/j.vetmic.2012.10.033. [DOI] [PubMed] [Google Scholar]
- Adkison M, Gilad O, Hedrick RP. An enzyme linked immunosorbent assay (ELISA) for detection of antibodies to the koi herpesvirus (KHV) in the serum of koi Cyprinus carpio. Fish Pathol. 2005;40:53–62. [Google Scholar]
- Antychowicz J, Reichert M, Matras M, Bergmann SW, Haenan O. Epidemiology, pathogenicity and molecular biology of koi herpesvirus isolated in Poland. Bull Vet Inst Pulawy. 2005;49:367–373. [Google Scholar]
- Aoki T, Hirono I, Kurokawa K, Fukuda H, et al. Genome sequences of three koi herpesvirus isolates representing the expanding distribution of an emerging disease threatening koi and common carp worldwide. J Virol. 2007;81:5058–5065. doi: 10.1128/JVI.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Takano T, Unajak S, Takagi M, et al. Generation of monoclonal antibodies specific for ORF68 of koi herpesvirus. Comp Immunol Microbiol Infect Dis. 2011;34:209–216. doi: 10.1016/j.cimid.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Avarre JC, Madeira JP, Santika A, Zainun Z, et al. Investigation of cyprinid herpesvirus-3 genetic diversity by a multi-locus variable number of tandem repeats analysis. J Virol Methods. 2011;173:320–327. doi: 10.1016/j.jviromet.2011.03.002. [DOI] [PubMed] [Google Scholar]
- Avarre JC, Santika A, Bentenni A, Zainun Z, et al. Spatio-temporal analysis of cyprinid herpesvirus 3 genetic diversity at a local scale. J Fish Dis. 2012;35:767–774. doi: 10.1111/j.1365-2761.2012.01404.x. [DOI] [PubMed] [Google Scholar]
- Bercovier H, Fishman Y, Nahary R, Sinai S, et al. Cloning of the koi herpesvirus (KHV) gene encoding thymidine kinase and its use for a highly sensitive PCR-based diagnosis. BMC Microbiol. 2005;5:13–22. doi: 10.1186/1471-2180-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann SM, Kempter J. Detection of koi herpesvirus (KHV) after re-activation in persistently infected common carp (Cyprinus carpio L.) using non-lethal sampling methods. Bull Eur Assoc Fish Pathol. 2011;31:92–104. [Google Scholar]
- Bergmann SM, Kempter J, Sadowski J, Fichtner D. First detection, confirmation and isolation of koi herpesvirus (KHV) in cultured common carp (Cyprinus carpio L.) in Poland. Bull Eur Assoc Fish Pathol. 2006;26:97–104. [Google Scholar]
- Bergmann SM, Schütze H, Fischer U, Fichtner D, et al. Detection of koi herpes virus (KHV) genome in apparently healthy fish. Bull Eur Assoc Fish Pathol. 2009;29:145–152. [Google Scholar]
- Bergmann SM, Lutze P, Schütze H, Fischer U, Dauber M, Fichtner D, Kempter J. Goldfish (Carassius auratus auratus) is a susceptible species for koi herpesvirus (KHV) but not for KHV disease (KHVD) Bull Eur Assoc Fish Pathol. 2010a;30:74–83. [Google Scholar]
- Bergmann SM, Riechart M, Fichtner D, Leeb P, Kempter J. Investigation on the diagnostic sensitivity of molecular tools used for detection of koi herpesvirus. J Virol Methods. 2010b;163:229–233. doi: 10.1016/j.jviromet.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Bergmann SM, Sadowski J, Kielpinski M, Bartlomiejczyk M, Fichtner D, Riebe R. Susceptibility of koi × crucian carp and koi × goldfish hybrids to koi herpesvirus (KHV) and the development of KHV disease (KHVD) J Fish Dis. 2010c;33:267–272. doi: 10.1111/j.1365-2761.2009.01127.x. [DOI] [PubMed] [Google Scholar]
- Bigarré L, Baud M, Cabon J, Antychowicz J, et al. Differentiation between cyprinid herpesvirus type-3 lineages using duplex PCR. J Virol Methods. 2009;158:51–57. doi: 10.1016/j.jviromet.2009.01.023. [DOI] [PubMed] [Google Scholar]
- Bondad-Reantaso MG, Sunarto A, Subasinghe RP. Managing the koi herpesvirus disease outbreak in Indonesia and the lessons learned. Dev Biol. 2007;129:21–28. [PubMed] [Google Scholar]
- Bretzinger A, Fischer-Scherl T, Oumouna M, Hoffmann R, Truyen U. Mass mortality in koi carp, Cyprinus carpio, associated with gill and skin disease. Bull Eur Assoc Fish Pathol. 1999;19:182–185. [Google Scholar]
- Calle PP, McNamara T, Kress Y. Herpesvirus-associated papillomas in koi carp (Cyprinus carpio) J Zoo Wildl Med. 1999;30:165–169. [PubMed] [Google Scholar]
- Cheng L, Chen CY, Tsai MA, Wang PC, Hsu JP, Chern RS, Chen SC. Koi herpesvirus epizootic in cultured carp and koi, Cyprinus carpio L., in Taiwan. J Fish Dis. 2011;34:547–554. doi: 10.1111/j.1365-2761.2011.01266.x. [DOI] [PubMed] [Google Scholar]
- Costes B, Fournier G, Michel B, Delforge C, et al. Cloning of the koi herpesvirus genome as an infectious bacterial artificial chromosome demonstrates that disruption of the thymidine kinase locus induces partial attenuation in Cyprinus carpio koi. J Virol. 2008;82:4955–4964. doi: 10.1128/JVI.00211-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes B, Raj VS, Michel B, Fournier G, et al. The major portal of entry of koi herpesvirus in Cyprinus carpio is the skin. J Virol. 2009;83:2819–2830. doi: 10.1128/JVI.02305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costes B, Vanderplasschen AFC, Lieffrig F. Recombinant koi herpesvirus (KHV) or cyprinid herpesvirus 3 (CyHV-3) and a vaccine for the prevention of a disease caused by KHV/CyHV-3 in Cyprinus carpio carpio or Cyprinus carpio koi. Patent: US 2011/0287050 A1. 2011 www.google.com/patents/US20110287050
- Davidovich M, Dishon A, Ilouze M, Kotler M. Susceptibility of cyprinid cultured cells to cyprinid herpesvirus 3. Arch Virol. 2007;152:1541–1546. doi: 10.1007/s00705-007-0975-4. [DOI] [PubMed] [Google Scholar]
- Dishon A, Perelberg A, Bishara-Shieban J, Ilouze M, Davidovich M, Werker S, Kotler M. Detection of carp interstitial nephritis and gill necrosis virus in fish droppings. Appl Environ Microbiol. 2005;71:7285–7291. doi: 10.1128/AEM.71.11.7285-7291.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishon A, Davidovich M, Ilouze M, Kotler M. Persistence of cyprinid herpesvirus 3 in infected cultured carp cells. J Virol. 2007;81:4828–4836. doi: 10.1128/JVI.02188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon PF, Joiner CL, Way K, Reese RA, Jeney G, Jeney Z. Comparison of the resistance of selected families of common carp, Cyprinus carpio L., to koi herpesvirus: preliminary study. J Fish Dis. 2009;32:1035–1039. doi: 10.1111/j.1365-2761.2009.01081.x. [DOI] [PubMed] [Google Scholar]
- Dong C, Weng S, Li W, Li X, Yi Y, Liang Q, He J. Characterization of a new cell line from caudal fin of koi, Cyprinus carpio koi, and first isolation of cyprinid herpesvirus 3 in China. Virus Res. 2011;161:140–149. doi: 10.1016/j.virusres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Eide K, Miller-Morgan T, Heidel JR, Bildfell RJ, Jin L. Results of total DNA measurement in koi tissue by Koi Herpesvirus real-time PCR. J Virol Methods. 2011a;172:81–84. doi: 10.1016/j.jviromet.2010.12.012. [DOI] [PubMed] [Google Scholar]
- Eide KE, Miller-Morgan T, Heidel JR, Kent ML, et al. Investigation of Koi Herpesvirus latency in koi. J Virol. 2011b;85:4954–4962. doi: 10.1128/JVI.01384-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Din MMM. Histopathological studies in experimentally infected koi carp (Cyprinus carpio koi) with koi herpesvirus in Japan. World J Fish Mar Sci. 2011;3:252–259. [Google Scholar]
- El-Matbouli M, Soliman H. Transmission of cyprinid herpesvirus-3 (CyHV-3) from goldfish to naïve common carp by cohabitation. Res Vet Sci. 2011;90:536–539. doi: 10.1016/j.rvsc.2010.07.008. [DOI] [PubMed] [Google Scholar]
- El-Matbouli M, Rucker U, Soliman H. Detection of Cyprinid herpesvirus-3 (CyHV-3) DNA in infected fish tissues by nested polymerase chain reaction. Dis Aquat Org. 2007a;78:23–28. doi: 10.3354/dao01858. [DOI] [PubMed] [Google Scholar]
- El-Matbouli M, Saleh M, Soliman H. Detection of cyprinid herpesvirus type 3 in goldfish cohabiting with CyHV-3-infected koi carp (Cyprinus carpio koi) Vet Rec. 2007b;161:792–793. [PubMed] [Google Scholar]
- FAO (Food and Agriculture Organization of the United Nations) The state of world fisheries and aquaculture 2010. FAO; Rome: 2010. [Google Scholar]
- Fournier G, Boutier M, Raj VS, Mast J, et al. Feeding Cyprinus carpio with infectious materials mediates cyprinid herpesvirus 3 entry through infection of pharyngeal periodontal mucosa. Vet Res. 2012;43:6. doi: 10.1186/1297-9716-43-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W, Fichtner D, Bergmann SM, Mettenleiter TC. Generation and characterization of koi herpesvirus recombinants lacking viral enzymes of nucleotide metabolism. Arch Virol. 2011;156:1059–1063. doi: 10.1007/s00705-011-0953-8. [DOI] [PubMed] [Google Scholar]
- Garver KA, Al-Hussinee L, Hawley LM, Schroeder T, et al. Mass mortality associated with koi herpesvirus in wild common carp in Canada. J Wildl Dis. 2010;46:1242–1251. doi: 10.7589/0090-3558-46.4.1242. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Andree KB, Adkison MA, et al. Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Dis Aquat Org. 2002;48:101–108. doi: 10.3354/dao048101. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Adkison MA, Way K, Willits NH, Bercovier H, Hedrick RP. Molecular comparison of isolates of an emerging fish pathogen, the koi herpesvirus, and the effect of water temperature on mortality of experimentally infected koi. J Gen Virol. 2003;84:2661–2667. doi: 10.1099/vir.0.19323-0. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Zagmutt-Vergara FJ, Leutenegger CM, Bercovier H, Hedrick RP. Concentrations of a koi herpesvirus (KHV) in tissues of experimentally-infected Cyprinus carpio koi as assessed by real-time TaqMan PCR. Dis Aquat Org. 2004;60:179–187. doi: 10.3354/dao060179. [DOI] [PubMed] [Google Scholar]
- Gomez DK, Joh SJ, Jang H, Shin SP, et al. Detection of koi herpesvirus (KHV) from koi (Cyprinus carpio koi) broodstock in South Korea. Aquaculture. 2011;311:42–47. [Google Scholar]
- Goodwin AE, Khoo L, LaPatra SE, Bonar C, et al. Goldfish hematopoietic necrosis herpesvirus (cyprinid herpesvirus 2) in the USA: molecular confirmation of isolates from diseased fish. J Aquat Anim Health. 2006a;18:11–18. [Google Scholar]
- Goodwin AE, Merry GE, Sadler J. Detection of the herpesviral hematopoietic necrosis disease agent (Cyprinid herpesvirus 2) in moribund and healthy goldfish: validation of a quantitative PCR diagnostic method. Dis Aquat Org. 2006b;69:137–143. doi: 10.3354/dao069137. [DOI] [PubMed] [Google Scholar]
- Goodwin AE, Sadler J, Merry GE, Marecaux EN. Herpesviral haematopoietic necrosis virus (CyHV-2) infection: case studies from commercial goldfish farms. J Fish Dis. 2009;32:271–278. doi: 10.1111/j.1365-2761.2008.00988.x. [DOI] [PubMed] [Google Scholar]
- Gotesman M, Soliman H, El-Matbouli M. Antibody screening identifies 78 putative host proteins involved in cyprinid herpesvirus 3 infection or propagation in common carp, Cyprinus carpio L. J Fish Dis. 2013;36:721–733. doi: 10.1111/jfd.12073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimmett SG, Warg JV, Getchell RG, Johnson DJ, Bowser PR. An unusual koi herpesvirus associated with a mortality event of common carp Cyprinus carpio in New York State, USA. J Wildl Dis. 2006;42:658–662. doi: 10.7589/0090-3558-42.3.658. [DOI] [PubMed] [Google Scholar]
- Groff JM, LaPatra SE, Munn RJ, Zinkl JG. A viral epizootic in cultured populations of juvenile goldfish due to a putative herpesvirus etiology. J Vet Diagn Invest. 1998;10:375–378. doi: 10.1177/104063879801000415. [DOI] [PubMed] [Google Scholar]
- Gunimaladevi I, Kono T, Venugopal MN, Sakai M. Detection of koi herpesvirus in common carp, Cyprinus carpio L., by loop-mediated isothermal amplification. J Fish Dis. 2004;27:583–589. doi: 10.1111/j.1365-2761.2004.00578.x. [DOI] [PubMed] [Google Scholar]
- Haenen OLM, Way K, Bergmann SM, Ariel E. The emergence of koi herpesvirus and its significance to European aquaculture. Bull Eur Assoc Fish Pathol. 2004;24:293–307. [Google Scholar]
- Han JE, Kim JH, Renault T, Shin SP, Jun JW, Park SC. Identifying the viral genes encoding envelope glycoproteins for differentiation of cyprinid herpesvirus 3 isolates. Viruses. 2013;5:568–576. doi: 10.3390/v5020568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haramoto E, Kitajima M, Katayama H, Ito T, Ohgaki S. Development of virus concentration methods for detection of koi herpesvirus in water. J Fish Dis. 2009;32:297–300. doi: 10.1111/j.1365-2761.2008.00977.x. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, Groff JM, Okihiro MS, McDowell TS. Herpesviruses detected in papillomatous skin growths of koi carp (Cyprinus carpio) J Wildl Dis. 1990;26:578–581. doi: 10.7589/0090-3558-26.4.578. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, Gilad O, Yun SC, Spangenberg JV, et al. A herpesvirus associated with mass mortality of juvenile and adult koi, a strain of a common carp. J Aquat Anim Health. 2000;12:44–57. doi: 10.1577/1548-8667(2000)012<0044:AHAWMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, Gilad O, Yun SC, Mcdowell TS, Waltzek TB, Kelley GO, Adkison MA. Initial isolation and characterization of a herpes-like virus (KHV) from koi and common carp. Bull Fish Res Agency. 2005;(Suppl 2):1–7. [Google Scholar]
- Honjo MN, Minamoto T, Matsui K, Uchii K, et al. Quantification of cyprinid herpesvirus 3 in environmental water by using an external standard virus. Appl Environ Microbiol. 2010;76:161–168. doi: 10.1128/AEM.02011-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutoran M, Ronen A, Perelberg A, Ilouze M, et al. Description of an as yet unclassified DNA virus from diseased Cyprinus carpio species. J Virol. 2005;79:1983–1991. doi: 10.1128/JVI.79.4.1983-1991.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilouze M, Dishon A, Kahan T, Kotler M. Cyprinid herpes virus-3 (CyHV-3) bears genes of genetically distant large DNA viruses. FEBS Lett. 2006;580:4473–4478. doi: 10.1016/j.febslet.2006.07.013. [DOI] [PubMed] [Google Scholar]
- Ilouze M, Dishon A, Davidovich M, Perelberg A, Kotler M. KHV, CNGV or CyHV-3, which is the koi/carp killer? Dis Asian Aquacult. 2008;6:115–128. [Google Scholar]
- Ilouze M, Davidovich M, Diamant A, Kotler M, Dishon A. The outbreak of carp disease caused by CyHV-3 as a model for new emerging viral diseases in aquaculture: a review. Ecol Res. 2010;26:885–892. [Google Scholar]
- Ilouze M, Dishon A, Kotler M. Coordinated and sequential transcription of the cyprinid herpesvirus-3 annotated genes. Virus Res. 2012a;169:98–106. doi: 10.1016/j.virusres.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Ilouze M, Dishon A, Kotler M. Down-regulation of the cyprinid herpesvirus-3 annotated genes in cultured cells maintained at restrictive high temperature. Virus Res. 2012b;169:289–295. doi: 10.1016/j.virusres.2012.07.013. [DOI] [PubMed] [Google Scholar]
- Ishioka T, Yoshizumi M, Izumi S, Suzuki K, et al. Detection and sequence analysis of DNA polymerase and major envelope protein genes in koi herpesviruses derived from Cyprinus carpio in Gunma prefecture, Japan. Vet Microbiol. 2005;110:27–33. doi: 10.1016/j.vetmic.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Ito T, Sano M, Kurita J, Yuasa K, Iida T. Carp larvae are not susceptible to koi herpesvirus. Fish Pathol. 2007;42:107–109. [Google Scholar]
- Jeffery KR, Bateman K, Bayley A, Feist SW, et al. Isolation of a cyprinid herpesvirus 2 from goldfish, Carassius auratus (L.), in the UK. J Fish Dis. 2007;30:649–656. doi: 10.1111/j.1365-2761.2007.00847.x. [DOI] [PubMed] [Google Scholar]
- Jung SJ, Miyazaki T. Herpesviral haematopoietic necrosis of goldfish, Carassius auratus (L.) J Fish Dis. 1995;18:211–220. [Google Scholar]
- Kempter J, Sadowski J, Schütze H, Fischer U, et al. Koi herpes virus: Do acipenserid restitution programs pose a threat to carp farms in the disease-free zones? Acta Ichthyol Piscat. 2009;39:119–126. [Google Scholar]
- Kielpinski M, Kempter J, Panicz R, Sadowski J, Schütze H, Ohlemeyer S, Bergmann SM. Detection of KHV in freshwater mussels and crustaceans from ponds with KHV history in common carp (Cyprinus carpio) Isr J Aquacult. 2010;62:28–37. [Google Scholar]
- Kongchum P, Palti Y, Lutzky S, Hallerman EM, Hulata G, David L. SNP discovery and development of genetic markers for mapping innate immune response genes in common carp (Cyprinus carpio) Fish Shellfish Immunol. 2010;29:356–361. doi: 10.1016/j.fsi.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Kongchum P, Sandel E, Lutzky S, Hallerman EM, Hulata G, David L, Palti Y. Association between IL-10a single nucleotide polymorphism and resistance to cyprinid herpesvirus-3 infection in common carp (Cyprinus carpio) J Aquacult. 2011;315:417–421. [Google Scholar]
- Kurita J, Yuasa K, Ito T, Sano M, et al. Molecular epidemiology of koi herpesvirus. Fish Pathol. 2009;44:59–66. [Google Scholar]
- Lee NS, Jung SH, Park JW, Do JW. In situ hybridization detection of koi herpesvirus in paraffin-embedded tissues of common carp Cyprinus carpio collected in 1998 in Korea. Fish Pathol. 2012;47:100–103. [Google Scholar]
- Lievens B, Frans I, Heusdens C, Justé A, Jonstrup SP, Lieffrig F, Willems KA. Rapid detection and identification of viral and bacterial fish pathogens using a DNA array-based multiplex assay. J Fish Dis. 2011;34:861–875. doi: 10.1111/j.1365-2761.2011.01304.x. [DOI] [PubMed] [Google Scholar]
- McCleary S, Ruane NM, Cheslett D, Hickey C, Rodger HD, Geoghegan F, Henshilwood K. Detection of koi herpesvirus (KHV) in koi carp (Cyprinus carpio L.) imported into Ireland. Bull Eur Assoc Fish Pathol. 2011;31:124–128. [Google Scholar]
- McColl K, Sunarto A, Williams LM, Crane MSTJ. The koi herpesvirus: dreaded pathogen or white knight? Aquacult Health Int. 2007;9:4–6. [Google Scholar]
- Michel B, Fournier G, Lieffrig F, Costes B, Vanderplasschen A. Cyprinid herpesvirus 3. Emerg Infect Dis. 2010a:16. doi: 10.3201/eid1612.100593. doi:10.3201/eid1612.100593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B, Leroy B, Stalin Raj V, Lieffrig F, et al. The genome of cyprinid herpesvirus 3 encodes 40 proteins incorporated in mature virions. J Gen Virol. 2010b;91:452–462. doi: 10.1099/vir.0.015198-0. [DOI] [PubMed] [Google Scholar]
- Minamoto T, Honjo MN, Yamanaka H, Tanaka N, Itayama T, Kawabata Z. Detection of cyprinid herpesvirus-3 DNA in lake plankton. Res Vet Sci. 2011;90:530–532. doi: 10.1016/j.rvsc.2010.07.006. [DOI] [PubMed] [Google Scholar]
- Minamoto T, Honjo MN, Yamanaka H, Uchii K, Kawabata Z. Nationwide Cyprinid herpesvirus 3 contamination in natural rivers of Japan. Res Vet Sci. 2012;93:508–514. doi: 10.1016/j.rvsc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- Miwa S, Ito T, Sano M. Morphogenesis of koi herpesvirus observed by electron microscopy. J Fish Dis. 2007;30:715–722. doi: 10.1111/j.1365-2761.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kuzuya Y, Yasumoto S, Yasuda M, Kobayashi T. Histopathological and ultrastructural features of Koi herpesvirus (KHV)-infected carp Cyprinus carpio, and the morphology and morphogenesis of KHV. Dis Aquat Org. 2008;80:1–11. doi: 10.3354/dao01929. [DOI] [PubMed] [Google Scholar]
- Neukirch M, Kunz U. Isolation and preliminary characterization of several viruses from koi (Cyprinus carpio) suffering gill necrosis and mortality. Bull Eur Assoc Fish Pathol. 2001;21:125–134. [Google Scholar]
- OIE (World Organisation for Animal Health) Manual of diagnostic tests for aquatic animals. 7th edn. OIE; Paris: [accessed 25 April 2013]. 2012. Available at www.oie.int/fileadmin/Home/eng/Health_standards/aahm/2010/2.3.06_KHVD.pdf. [Google Scholar]
- Omori R, Adams B. Disrupting seasonality to control disease outbreaks: the case of koi herpes virus. J Theor Biol. 2011;271:159–165. doi: 10.1016/j.jtbi.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Pearson H. Carp virus crisis prompts moves to avert global spread. Nature. 2004;427:577. doi: 10.1038/427577a. [DOI] [PubMed] [Google Scholar]
- Perelberg A, Smirnov M, Hutoran M, Diamant A, Bejerano Y, Kotler M. Epidemiological description of a new viral disease afflicting cultured Cyprinus carpio in Israel. Isr J Aquacult Bamidgeh. 2003;55:5–12. [Google Scholar]
- Perelberg A, Ronen A, Hutoran M, Smith Y, Kotler M. Protection of cultured Cyprinus carpio against a lethal viral disease by an attenuated virus vaccine. Vaccine. 2005;23:3396–3403. doi: 10.1016/j.vaccine.2005.01.096. [DOI] [PubMed] [Google Scholar]
- Perelberg A, Ilouze M, Kotler M, Steinitz M. Antibody response and resistance of Cyprinus carpio immunized with cyprinid herpes virus 3 (CyHV-3) Vaccine. 2008;26:3750–3756. doi: 10.1016/j.vaccine.2008.04.057. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Ronen A, Abramowitz J, Levavi-Sivan B, et al. The pathogenesis of the acute viral disease in fish induced by the carp interstitial nephritis and gill necrosis virus. J Virol. 2004;78:9544–9551. doi: 10.1128/JVI.78.17.9544-9551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorova D, Vesely T, Piackova V, Reschova S, Hulova J. Current knowledge on koi herpesvirus (KHV): a review. Vet Med Czech. 2005;50:139–147. [Google Scholar]
- Pokorova D, Piackova V, Cizek A, Reschova S, Hulova J, Vicenova M, Vesely T. Tests for the presence of koi herpesvirus (KHV) in common carp (Cyprinus carpio carpio) and koi carp (Cyprinus carpio koi) in the Czech Republic. Vet Med (Praha) 2007;12:562–568. [Google Scholar]
- Raj VS, Fournier G, Rakus K, Ronsmans M, et al. A skin mucus of Cyprinus carpio inhibits cyprinid herpesvirus 3 binding to epidermal cells. Vet Res. 2011;42:92–100. doi: 10.1186/1297-9716-42-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakus KŁ, Irnazarow I, Adamek M, Palmeira L, et al. Gene expression analysis of common carp (Cyprinus carpio L.) lines during Cyprinid herpesvirus 3 infection yields insights into differential immune responses. Dev Comp Immunol. 2012;37:65–76. doi: 10.1016/j.dci.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Rathore G, Kumar G, Swain P, Lakra WS. Development of new PCR primers for detection of Koi Herpes Virus (KHV) Indian J Comp Microbiol Immunol Infect Dis. 2009;30:52–53. [Google Scholar]
- Ronen A, Perelberg A, Abramowitz J, Hutoran M, et al. Efficient vaccine against the virus causing a lethal disease in cultured Cyprinus carpio. Vaccine. 2003;21:4677–4684. doi: 10.1016/s0264-410x(03)00523-1. [DOI] [PubMed] [Google Scholar]
- Rosenkranz D, Klupp BG, Teifke JP, Granzow H, Fichtner D, Mettenleiter TC, Fuchs W. Identification of envelope protein pORF81 of koi herpesvirus. J Gen Virol. 2008;89:896–900. doi: 10.1099/vir.0.83565-0. [DOI] [PubMed] [Google Scholar]
- Sadler J, Marecaux E, Goodwin AE. Detection of koi herpes virus (CyHV-3) in goldfish, Carassius auratus (L.), exposed to infected koi. J Fish Dis. 2008;31:71–72. doi: 10.1111/j.1365-2761.2007.00830.x. [DOI] [PubMed] [Google Scholar]
- Sano T, Fukuda H, Furukawa M, Hosoya H, Moriya Y. A herpesvirus isolated from carp papilloma in Japan. In: Ellis AE, editor. Fish and shellfish pathology. Academic Press; London: 1985a. pp. 307–311. [Google Scholar]
- Sano T, Fukuda H, Furukawa M, Hosoya H, Moriya Y. Herpesvirus cyprini: biological and oncogenic properties. Fish Pathol. 1985b;20:381–388. [Google Scholar]
- Sano T, Morita N, Shima N, Akimoto M. Herpesvirus cyprini: lethality and oncogenicity. J Fish Dis. 1991;14:533–543. [Google Scholar]
- Sano M, Ito T, Kurita J, Yanai T, Watanabe N, Miwa S, Lida T. First detection of koi herpesvirus in cultured common carp Cyprinus carpio in Japan. Fish Pathol. 2004;39:165–167. [Google Scholar]
- Sano M, Ito T, Kurita J, Miwa S, Lida T. Diagnosis of Koi Herpesvirus (KHV) disease in Japan. Bull Fish Res Agency. 2005;2:59–64. [Google Scholar]
- Shapira Y, Magen Y, Zak T, Kotler M, Hulata G, Levavi-Sivan B. Differential resistance to koi herpes virus (KHV)/carp interstitial nephritis and gill necrosis virus (CNGV) among common carp (Cyprinus carpio L.) strains and crossbreds. Aquaculture. 2005;245:1–11. [Google Scholar]
- Shimizu T, Yoshida N, Kasai H, Yoshimizu M. Survival of koi herpesvirus (KHV) in environmental water. Fish Pathol. 2006;41:153–157. [Google Scholar]
- Soliman H, El-Matbouli M. An inexpensive and rapid diagnostic method of koi herpesvirus (KHV) infection by loop-mediated isothermal amplification. Virol J. 2005;2:83. doi: 10.1186/1743-422X-2-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman H, El-Matbouli M. Immunocapture and direct binding loop mediated isothermal amplification simplify molecular diagnosis of cyprinid herpesvirus-3. J Virol Methods. 2009;162:91–95. doi: 10.1016/j.jviromet.2009.07.021. [DOI] [PubMed] [Google Scholar]
- St-Hilaire S, Beevers N, Way K, Le Deuff RM, Martin P, Joiner C. Reactivation of koi herpesvirus infections in common carp Cyprinus carpio. Dis Aquat Org. 2005;67:15–23. doi: 10.3354/dao067015. [DOI] [PubMed] [Google Scholar]
- St-Hilaire S, Beevers N, Joiner C, Hedrick RP, Way K. Antibody response of two populations of common carp, Cyprinus carpio L., exposed to koi herpesvirus. J Fish Dis. 2009;32:311–320. doi: 10.1111/j.1365-2761.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- Sunarto A, McColl KA, Crane MS, Sumiati T, Hyatt AD, Barnes AC, Walker PJ. Isolation and characterization of koi herpesvirus (KHV) from Indonesia: identification of a new genetic lineage. J Fish Dis. 2011;34:87–101. doi: 10.1111/j.1365-2761.2010.01216.x. [DOI] [PubMed] [Google Scholar]
- Sunarto A, Liongue C, McColl KA, Adams MM, et al. Koi herpesvirus encodes and expresses a functional interleukin-10. J Virol. 2012;86:11512–11520. doi: 10.1128/JVI.00957-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Syakuri H, Adamek M, Brogden G, Rakus KŁ, Matras M, Irnazarow I, Steinhagen D. Intestinal barrier of carp (Cyprinus carpio L.) during a cyprinid herpesvirus 3-infection: molecular identification and regulation of the mRNA expression of claudin encoding genes. Fish Shellfish Immunol. 2013;34:305–314. doi: 10.1016/j.fsi.2012.11.010. [DOI] [PubMed] [Google Scholar]
- Taylor NGH, Dixon PF, Jeffery KR, Peeler EJ, Denway KL, Way K. Koi herpesvirus: distribution and prospects for control in England and Wales. J Fish Dis. 2010;33:221–230. doi: 10.1111/j.1365-2761.2009.01111.x. [DOI] [PubMed] [Google Scholar]
- Taylor NGH, Norman RA, Way K, Peeler EJ. Modelling the koi herpesvirus (KHV) epidemic highlights the importance of active surveillance within a national control policy. J Appl Ecol. 2011;48:348–355. [Google Scholar]
- Tu C, Weng MC, Shiau JR, Lin SY. Detection of koi herpesvirus in koi Cyprinus carpio in Taiwan. Fish Pathol. 2004;39:109–110. [Google Scholar]
- Uchii K, Matsui K, Iida T, Kawabata Z. Distribution of the introduced cyprinid herpesvirus 3 in a wild population of common carp, Cyprinus carpio L. J Fish Dis. 2009;32:857–864. doi: 10.1111/j.1365-2761.2009.01064.x. [DOI] [PubMed] [Google Scholar]
- Uchii K, Telschow A, Minamoto T, Yamanaka H, Honjo MN, Matsui K, Kawabata Z. Transmission dynamics of an emerging infectious disease in wildlife through host reproductive cycles. ISME J. 2011;5:244–251. doi: 10.1038/ismej.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Beurden SJ, Bossers A, Voorbergen-Laarman MH, Haenen OL, et al. Complete genome sequence and taxonomic position of anguillid herpesvirus 1. J Gen Virol. 2010;91:880–887. doi: 10.1099/vir.0.016261-0. [DOI] [PubMed] [Google Scholar]
- Waltzek TB, Kelley GO, Stone DM, Way K, et al. Koi herpesvirus represents a third cyprinid herpesvirus (CyHV-3) in the family Herpesviridae. J Gen Virol. 2005;86:1659–1667. doi: 10.1099/vir.0.80982-0. [DOI] [PubMed] [Google Scholar]
- Waltzek TB, Kelley GO, Alfaro ME, Kurobe T, Davison AJ, Hedrick RP. Phylogenetic relationships in the family Alloherpesviridae. Dis Aquat Org. 2009a;84:179–194. doi: 10.3354/dao02023. [DOI] [PubMed] [Google Scholar]
- Waltzek TB, Kurobe T, Goodwin AE, Hedrick RP. Development of a polymerase chain reaction assay to detect cyprinid herpesvirus 2 in goldfish. J Aquat Anim Health. 2009b;21:60–67. doi: 10.1577/H08-045.1. [DOI] [PubMed] [Google Scholar]
- Whalen G. DNR detects koi herpesvirus in Silver Lake fish kill. Michigan Department of Natural Resources; Newberry, MI: 2011. [Google Scholar]
- Yoshino M, Watari H, Kojima T, Ikedo M. Sensitive and rapid detection of Koi herpesvirus by LAMP method. Fish Pathol. 2006;41:19–27. [Google Scholar]
- Yoshino M, Watari H, Kojima T, Ikedo M, Kurita J. Rapid, sensitive and simple detection method for koi herpesvirus using loop mediated isothermal amplification. Microbiol Immunol. 2009;53:375–383. doi: 10.1111/j.1348-0421.2009.00145.x. [DOI] [PubMed] [Google Scholar]
- Yuasa K, Kurita J, Kawana M, Kiryu I, Oseko N, Sano M. Development of mRNA-specific RT-PCR for the detection of koi herpesvirus (KHV) replication stage. Dis Aquat Org. 2012;100:11–18. doi: 10.3354/dao02499. [DOI] [PubMed] [Google Scholar]