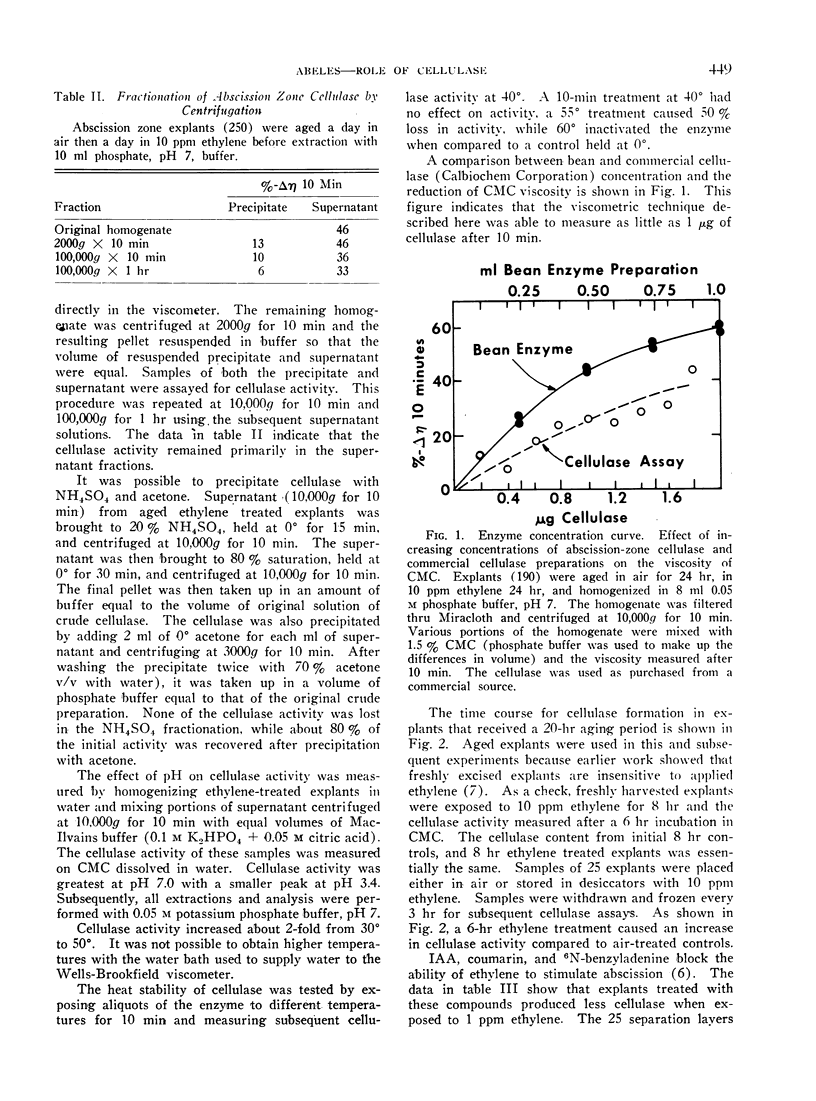
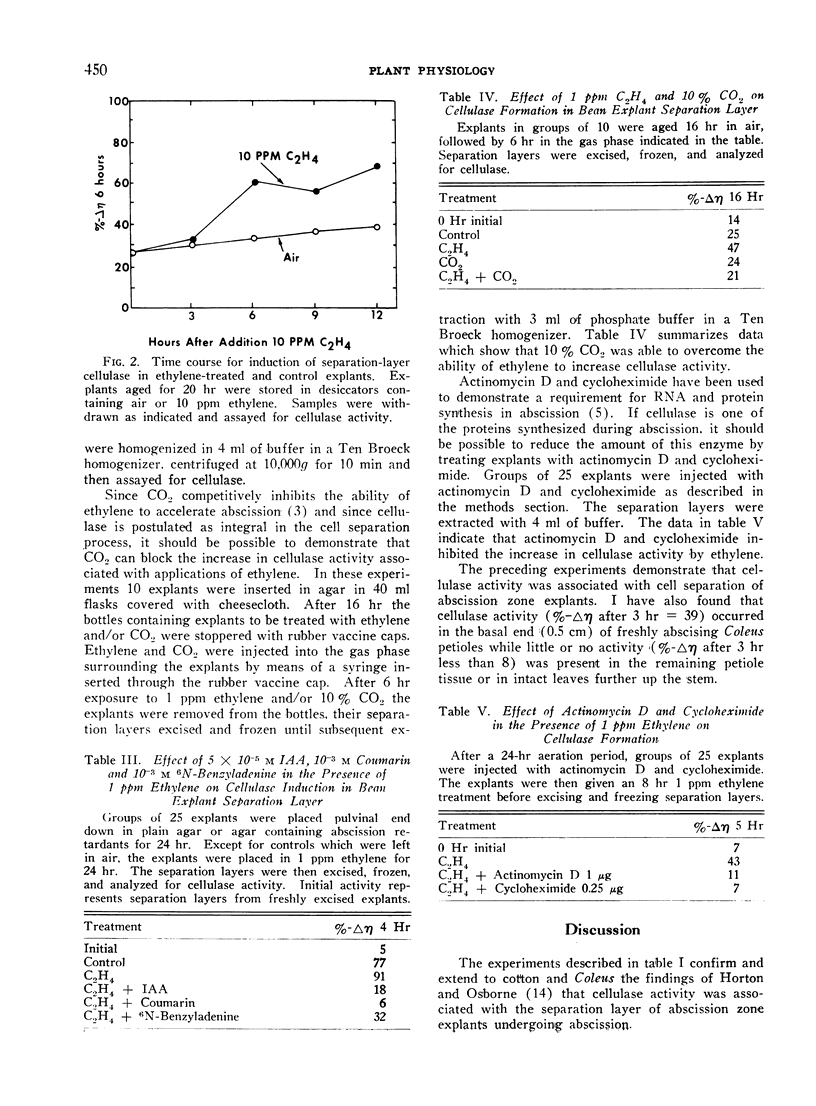
Abstract
Cellulase (β-1,4-glucan-glucanohydrolase EC 3.2.1.4) activity increased during abscission and was localized in the cell separation layer of Phaseolus vulgaris L. cv. Red Kidney (bean), Gossypium hirsutum L. cv. Acala 4-42 (Cotton) and Coleus blumei Benth. Princeton strain (Coleus) abscission zone explants. Cellulase activity was optimum at pH 7, was reduced by one-half after heating to 55° for 10 min, and was associated with the soluble components of the cell. Explants treated with aging retardants (indoleacetic acid, 6N-benzyladenine, and coumarin), CO2, actinomycin D or cycloheximide had less cellulase activity than untreated controls. Ethylene increased cellulase activity of aged explants after a 3-hr lag period but had no effect on cellulase activity of freshly excised explants. It was concluded that 1 of the roles of ethylene in abscission is to regulate the production of cellulase which in turn is required for cell separation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abeles F. B., Gahagan H. E. Abscission: the role of ethylene, ethylene analogues, carbon dioxide, and oxygen. Plant Physiol. 1968 Aug;43(8):1255–1258. doi: 10.1104/pp.43.8.1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Holm R. E. Enhancement of RNA synthesis, protein synthesis, and abscission by ethylene. Plant Physiol. 1966 Oct;41(8):1337–1342. doi: 10.1104/pp.41.8.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Holm R. E., Gahagan H. E. Abscission: the role of aging. Plant Physiol. 1967 Oct;42(10):1351–1356. doi: 10.1104/pp.42.10.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B. Role of RNA and protein synthesis in abscission. Plant Physiol. 1968 Sep;43(9 Pt B):1577–1586. [PMC free article] [PubMed] [Google Scholar]
- Abeles F. B., Rubinstein B. Regulation of Ethylene Evolution and Leaf Abscission by Auxin. Plant Physiol. 1964 Nov;39(6):963–969. doi: 10.1104/pp.39.6.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almin K. E., Eriksson K. E. Enzymic degradation of polymers. I. Viscometric method for the determination of enzymic activity. Biochim Biophys Acta. 1967 Jul 11;139(2):238–247. doi: 10.1016/0005-2744(67)90028-9. [DOI] [PubMed] [Google Scholar]
- Almin K. E., Eriksson K. E., Jansson C. Enzymic degradation of polymers. II. Viscometric determination of cellulase activity in absolute terms. Biochim Biophys Acta. 1967 Jul 11;139(2):248–253. doi: 10.1016/0005-2744(67)90029-0. [DOI] [PubMed] [Google Scholar]
- DICKINSON D. B., MCCOLLUM J. P. CELLULASE IN TOMATO FRUITS. Nature. 1964 Aug 1;203:525–526. doi: 10.1038/203525a0. [DOI] [PubMed] [Google Scholar]
- HALLIWELL G., BRYANT M. P. THE CELLULOLYTIC ACTIVITY OF PURE STRAINS OF BACTERIA FROM THE RUMEN OF CATTLE. J Gen Microbiol. 1963 Sep;32:441–448. doi: 10.1099/00221287-32-3-441. [DOI] [PubMed] [Google Scholar]
- SISON B. C., Jr, SCHUBERT W. J., NORD F. F. On the mechanism of enzyme action. LXV. A cellulolytic enzyme from the mold Poria vaillantii. Arch Biochem Biophys. 1958 May;75(1):260–272. doi: 10.1016/0003-9861(58)90415-6. [DOI] [PubMed] [Google Scholar]
- WELLS R. E., Jr, DENTON R., MERRILL E. W. Measurement of viscosity of biologic fluids by cone plate viscometer. J Lab Clin Med. 1961 Apr;57:646–656. [PubMed] [Google Scholar]
- Yager R. E. Possible Role of Pectic Enzymes in Abscission. Plant Physiol. 1960 Mar;35(2):157–162. doi: 10.1104/pp.35.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]



