Abstract
Nonhuman primate species spend a conspicuous amount of time grooming during social interactions, a behavior that probably serves both social and health-related functions. While the social implications of grooming have been relatively well studied, less attention has been paid to the health benefits, especially the removal of ectoparasites, which may act as vectors in disease transmission. In this study, we examined the relationship between grooming behavior, tick load (number of ticks), and haemoprotozoan infection status in a population of wild free-ranging baboons (Papio cynocephalus). We found that the amount of grooming received was influenced by an individual’s age, sex and dominance rank. The amount of grooming received, in turn, affected the tick load of an individual. Baboons with higher tick loads had lower packed red cell volume (PCV or haematocrit), one general measure of health status. We detected a tick-borne haemoprotozoan, Babesia microti, but its low prevalence in the population precluded identifying sources of variance in infection.
Keywords: grooming, ticks, haemoparasitic infections, baboons
INTRODUCTION
Grooming behavior encompasses all forms of care and attention to the body surfaces (Saunders 1988). It constitutes a major social activity in many species of social mammals including ungulates ( Mooring et al. 1996; Hart 2006; Heitor et al 2006), rodents (Ferron and Lefebvre 1982), bats (Wilkinson 1986) and primates (Schino 2007) among others. Grooming is a frequent and conspicuous behavior; indeed, some nonhuman primates invest at least one fifth of their time engaged in grooming (Dunbar 1991; Shutt et al. 2007). This time investment in grooming suggests that it serves important functions. The social functions of grooming include the establishment and maintenance of affiliative relationships and the reduction of tension and aggression between individuals (Terry 1970; Saunders 1988; Kimura 1998; Kutsukake and Clutton-Brock 2006). Grooming has also been used as a quantitative measure of the strength of dyadic social relationships (Lazaro-Perea et al. 2004).
Grooming varies with many factors. For example, in most primates, grooming patterns are highly kin-biased and kinship explains a large fraction of the variance in grooming patterns, as predicted by kin selection models of social evolution (Schino 2001; Chapais and Berman 2004). Grooming also has potential value in relationships among non-relatives, and Seyfarth (1977) first suggested that grooming directed up a dominance hierarchy (i.e., preferential grooming of high ranking animals) represents an exchange of grooming services for coalitionary support (see also discussions in Schino 2001; Lazaro-Perea et al. 2004). Other studies have documented increased grooming down the hierarchy (Obrien 1993; Parr et al. 1997; Lazaro-Perea et al. 2004). These conflicting findings may result from differences in social and ecological context, which influence how resources are distributed in a social group. This in turn might affect the “market” for grooming (Barrett et al. 1999; Henzi and Barrett 1999), although support for this idea is mixed (see e.g., discussions in Lazaro-Perea et al. 2004; Silk 2004; Silk et al. 2006a).
In many primates, participation in grooming bouts differs between the sexes and with life history stage. In several studies on captive baboons, vervets and macaques, grooming was shown to be a female-biased behavior established during the first year of life, with females grooming almost twice as often as males (Simonds 1974; Young et al. 1982). In these species, females are philopatric while males are the dispersing sex; as a correlate of female philopatry, females tend to form strong social bonds with other females (Wrangham 1980) and grooming is a major contributor to these social bonds (Silk et al. 2003, 2006a, 2010). Further, Silk et al. (2006b) reported that female baboons that had the most equitable grooming relationships also had the strongest and most enduring social bonds. The strength, stability and quality of social bonds contribute, in turn, to offspring survival, enhanced longevity, and to the probability of receiving coalitionary support during within-group contests (Silk et al. 2003, 2006a, 2006b, 2010). The age of an individual also plays a role in the amount of grooming received or given. Saunders (1988) showed that more than half of the grooming bouts between adult female and juvenile baboons were initiated by adult females. Adult male baboons groomed less often than adult females (Saunders 1988). Because males can provide important services, such as protection against infanticidal attacks and harassment (Smuts 1985; Saunders 1988; Silk et al. 2003; Nguyen et al. 2012), females may be motivated to groom males in return for these services.
Grooming may also have important indirect or direct health consequences. With respect to indirect consequences, previous work in rhesus macaques (Macaca mulatta) has shown that receiving grooming reduces heart rate and thus improves well being ( Boccia et al. 1989; Aureli et al. 1999). Additionally, a study on free ranging Barbary macaques has shown that grooming others is correlated with a reduction in the stress hormone cortisol in the groomer (Shutt et al. 2007). Finally, work in captive Talapoin monkeys (Miopithecus spp) has shown that participation in grooming (receiving or giving) increases the production of endorphins, a biomarker of increased psychological well being (Keverne et al. 1989).
In most mammal species in which it has been investigated, grooming also has direct effects on well being via removal of ectoparasites such as lice, fleas and ticks (e.g. (Saunders and Hausfater 1988; Tanaka and Takefushi 1993; Eckstein and Hart 2000; Hart 2006; Kutsukake and Clutton-Brock 2006). Among ectoparasites, ticks are of particular medical and economic importance. They are prevalent in many environments, are resistant to many environmental stressors, and have relatively long life cycles and high reproductive potential (Ginsberg 2005). Ticks are vectors for transmission of infectious and toxic disease and are able to transmit pathogens to a wide range of hosts, including humans and nonhuman primates (Edlow 1999).
The tick family of greatest veterinary and medical importance is Ixodidae. Saunders (1988) found in the habitat of Amboseli, Kenya, several genera and multiple species of Ixodidae (hard) ticks (Rhipicephalus, Ixodes, Hyalomma and Amblyomma spp), most of which were also associated with domestic animals (e.g., cattle, sheep and goats) and with wild ungulates (e.g. wildebeests, zebras, gazelle). Direct detrimental effects of ticks to these hosts include inflammation and irritation from tick bites, itching, allergic reactions to protein in tick saliva, secondary anemia (sometimes fatal), and paralysis. Ticks also transmit parasites such as bacteria, protozoa, viruses and nematodes, some of which cause diseases that generally affect the blood or the lymphatic system (Ginsberg 2005). For example, Ixodid ticks are known to carry Babesia, a haemoprotozoan parasite capable of infecting nonhuman primates in captivity and also present in nonhuman primate populations in the wild (Moore and Kuntz 1981; Jeneby et al. 2008; Maamun et al. 2011). Indeed, (Maamun et al. 2011) found B. microti parasites (a species known to present health risks to humans and nonhuman primates: (Cogswell 2000; Ginsberg 2005)) in both free ranging primates and in R. simus ticks. Parasites of the genus Babesia were of particular interest in this study. We also screened for Entopolypoides macaci, which is closely related to Babesia and is suspected to be transmitted by ectoparasites, although evidence of such transmission is yet to be demonstrated (Hawking 1972).
In wild primates, few studies have investigated the role of health benefits of grooming with regards to reducing ectoparasite load (Saunders 1988; Sanchez-Villagra et al. 1988; Tanaka and Takefushi 1993) or in reducing downstream infection by vector-borne parasites. Here, we investigated the association between grooming behavior and tick load in wild baboons in the Amboseli basin in southern Kenya. We also carried out a survey of tick-borne haemoparasite infection status in these baboons. Our study took advantage of three different data sets to examine these associations. The first data set included demographic data on individual age, sex, and group membership, as well as behavioral data on grooming and dominance rank. These data were used to investigate the factors that predict grooming and whether these factors had any association with tick presence. Both types of data were mined from the long-term database of the Amboseli Baboon Research Project (Babase). The second data set included tick identification and tick counts from baboons darted in Amboseli during darting and immobilization projects in 2007 – 2008. The third data set focused on health and haemoparasite screening data, which included packed cell volume (PCV) analysis and PCR screening of samples obtained during immobilization. We used these three data sets to test the hypotheses that grooming reduces tick presence and that tick presence, in turn, affects health.
MATERIAL AND METHODS
Study subjects
Individuals in this study were adult members of a wild baboon population resident in the area immediately north and west of Mount Kilimanjaro in southern Kenya, part of the Amboseli region of East Africa. This population has been studied by the Amboseli Baboon Research Project since 1971 (Alberts and Altmann 1995; Altmann J. 1980; Charpentier et al. 2008). The baboons in this area are yellow baboons (Papio cynocephalus) that experience some admixture with neighboring populations of anubis baboons (Papio anubis), (Alberts and Altmann 2001; Tung et al. 2008).
Darting and sample collection
Using an anaesthetic-bearing dart delivered from a handheld blowgun (Altmann J. et al. 1996; Tung et al. 2011), we darted thirty-two individuals in June and July 2007 and an additional thirty-three individuals in June and July 2008, no more than two on any day. These 65 dartings were done under the authority of the Kenya Wildlife Service, using the anaesthetic Telazol, a Tiletamine-Zolazepam combination (Altmann et al. 1996; Tung et al. 2011). From each individual, we collected blood samples from the saphenous vein and ticks as described below. Each study subject was placed singly into a covered holding cage until fully recovered from the effects of the anesthetic (~ 3 – 4 hours). Subjects were then released in the vicinity of their social group. All subjects rejoined their social groups quickly upon release and without incident.
Ticks were collected from each darted baboon using forceps and preserved in 70% ethanol for subsequent manual counting in a laboratory. Immature ticks were not plucked from the animal but were counted on the baboon’s body because they were too small to carefully retrieve. For adult ticks, we identified species by microscopic morphological characterization using the International Livestock Research Institute (ILRI) Tick Identification Training Manual (ILRI 2004).
All protocols complied with regulations in Kenya (Republic of Kenya Research Permits NCST/5/002/R/776 to J.A. and NCST/5/002/R/777 to S.C.A.) and in the United States (Duke University IACUC A028-12-02), and adhered to the Animal Behavior Society’s Guidelines for the Treatment of Animals in Behavioral Research and Teaching.
Grooming data
Behavioral data on grooming were collected on all members of five different study groups, using both ad libitum and focal sampling methods (Altmann 1974). Ad libitum grooming data were collected throughout the day by observers who monitored the group members’ behavior; focal data were collected as all occurrences of grooming during 10-minute focal samples on juveniles and on adult females. These data were entered in the long-term relational database for the Amboseli Baboon Research Project, Babase. We subsequently extracted grooming data from these datasets for 62 of the 65 individuals darted; the remaining three individuals in the darting project were members of non-study groups, for which we lacked detailed grooming data. The data of interest were the counts of grooming received by each study animal in the six months prior to their darting date. This 6-month cumulative measure is referred to hereafter as the frequency of grooming received.
Social and demographic data
Data on age and dominance rank for individual study subjects were extracted from Babase. The age of each individual was calculated based on a known birth date for individuals born within study groups (accurate within several days; N = 54), or estimated upon first entry into a study group based on external characteristics, such as body carriage, teeth and pelage condition in the case of immigrant males not born within study groups (N = 11). Dominance rank for each individual was determined based on field observations of dyadic agonistic interactions (N = 62). As with grooming data, we lacked dominance rank data for the 3 individuals that were darted in non-study groups; Table 1). These interactions were then used to compute social dominance rank for each animal on a monthly basis (Hausfater 1975; Alberts et al. 2003). The dominance ranks used in this study represented the dominance rank for each animal in the month that it was darted. Dominance rank varies little over the lifetime of females. Although ranks in males are more dynamic (Altmann et al. 1988; Alberts et al. 2003), during the six months prior to darting (the period for which grooming data were examined) male dominance ranks changed little (Table S1 in Supplementary Material).
TABLE 1.
Summary of sample sizes in models used for statistical analysis
| Analysis | Sample size (number of individuals) | Missing individuals | Explanation |
|---|---|---|---|
| Grooming received | 62 | 3 | Three individuals in non study groups lacked grooming data and dominance rank data |
| Tick load (using total number of ticks) | 62 | 3 | Three individuals in non study groups lacked grooming data |
| PCV (using total number of ticks) | 61 | 4 | Haematocrit was not measured for one individual, and three individuals in non study groups lacked dominance rank data |
| PCV (using number of adult ticks) | 61 | 4 | Haematocrit measure was not taken for one individual and three individuals in non study groups lacked dominance rank data |
| Haemoparasite screening | 63 | 2 | Parasite screening was not completed for two individuals |
Packed Cell Volume (PCV)
Packed cell volume (PCV or haematocrit) is the volume percentage of red blood cells in a blood sample; it is a component of the total blood count and functions as an indicator of anemia, which could arise from tick infestations either directly from ticks ingesting blood, or from haemoparasite infection. For field measurements of PCV, heparinized capillary tubes were filled with a sample of venous blood and spun for five minutes in a portable microhaematocrit centrifuge (Ziprocrit, LW Scientific, Inc, Model ZO-1). PCV was measured with a standardized hematocrit card reader. Two to three replicate PCV measurements were obtained per animal, and the mean of the measurements was used in subsequent analyses.
Parasite screening
Total genomic DNA was extracted from 20 μl of each blood sample using a commercial Dneasy kit (Qiagen blood and tissue DNA extraction kit, Hilden, Germany). We then used PCR-based screening to detect the presence or absence of haemoparasitic infections of two genera, Babesia and Entopolypoides, which have been reported to cause infection in some non-human primates such as baboons (Papio spp) and vervet monkeys (Chlorocebus aethiops) (Maamun 2011). As noted earlier, Babesia is known to be transmitted by ticks, but such evidence is currently lacking for Entopolyploides.
Parasite-specific DNA was amplified using four different primer sets (Table S2 in supplementary material). Primer set BmicF1/BmicR1 specifically targeted the 18s rRNA of Babesia microti, based on existing sequence in GenBank (accession number AB219802). Primer set EmacF1/EmacR1 specifically targeted the 18s rRNA of Entopolypoides macaci. Two additional primer sets (F34/R323 and F79/R206, GenBank accession numbers AJ289244 to AJ289252) for nested PCR (n-PCR), were used to non-specifically amplify a portion of the b-tubulin gene of tick-borne piroplasms of the genera Babesia and Theileria as described by Caccio et al. (2000). Theileria is a tickborne parasite closely related to Babesia which has been identified in livestock that share the range of the baboons we studied.
PCR amplifications were done on a PTC-200 Peltier Thermocycler and the resulting PCR products were visualized on a 1.5 % agarose gel stained with ethidium bromide. Purification of PCR products was done using a Qiagen Minelute 96UF PCR purification kit and retrieved products were sequenced with an ABI 3730 DNA Analyzer in the Duke’s Genome Sequencing and Analysis Core Resource. See Table S3 supplementary material for PCR amplification and sequencing preparation conditions. The resulting sequences were then compared to existing sequences in the GenBank sequence database by carrying out a BLAST search.
Statistical analysis
Our statistical analyses focused on three main individual-level response variables: (1) the frequency of grooming received by an individual, (2) the individual’s tick load (specifically the number of ticks present on an individual) and (3) its PCV. In the analyses of grooming received and of PCV, we used multivariate analyses to control for the effects of multiple predictor variables, using the R statistical software package (R version 2.14.0). The sample size varied slightly for different components of the analysis (see Table 1 for explanations).
Because our grooming data were count data, we used a Poisson mixed effects regression model to determine the factors that influenced the frequency of grooming received. The fixed predictor variables in our model were the age, sex, and dominance rank of each subject. The social group to which the individual belonged was treated as a random effect.
In examining the relationship between grooming and tick load, we assumed that any relationship between grooming received during the six months prior to darting and tick load would reflect a causal relationship, such that grooming resulted in ectoparasite removal (see Discussion). To determine whether the amount of grooming received influenced tick load we carried out a zero-inflated Poisson regression. We used the zero-inflated Poisson regression because the distribution of the dependent variable (tick load) was highly skewed with many zero values. The predictor variable for the Poisson parts of our model was the number of times we observed the individual to receive grooming in the six month period prior to darting. We used the year of darting as the predictor variable on the logistic parts of the model.
To assess the predictors of PCV, we used a multivariate general linear regression model. Our predictor variables were dominance rank, age, sex and the total number of ticks (adult ticks and larvae combined). We also modeled PCV in a multivariate analysis replacing the total number of ticks with only the number of adult ticks; the number of adult ticks was much greater in 2007 than in 2008 (Table S4 in Supplementary Material), and we reasoned that adult ticks (because of their size) were more likely than larvae to impact the host’s PCV.
RESULTS
Factors influencing grooming received
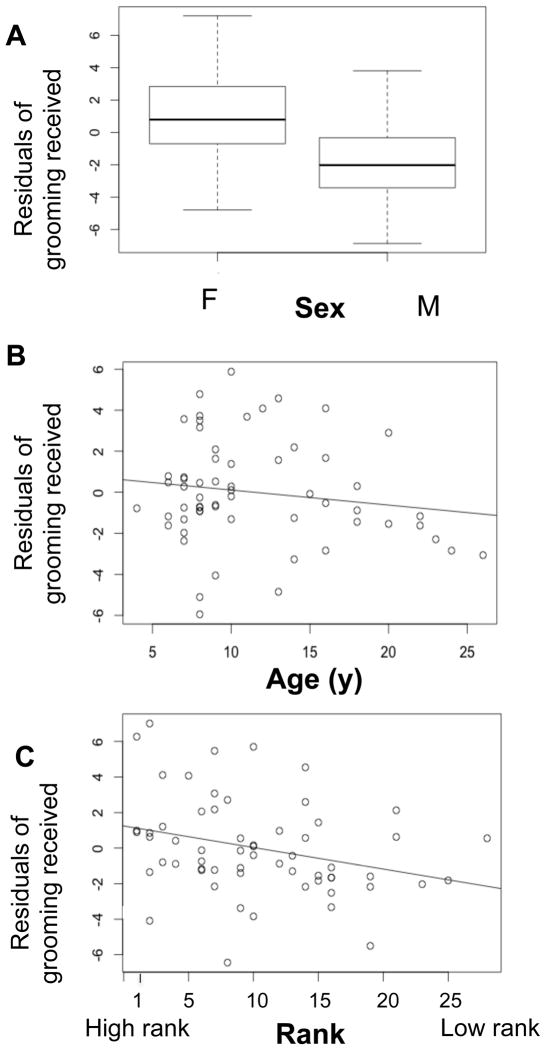
Our mixed effects Poisson regression model indicated that the frequency of grooming received was influenced by age (β = −0.013, P = < 0.001, N = 62), dominance rank (β =−0.0277, P = < 0.0001, N = 62), and sex (β = −0.6050, P = < 0.001, N = 62). Younger and higher ranking adults were groomed more often than older, low ranking adults, and females were groomed more often than males (Figure 1). The social group to which an individual animal belonged, which we included as a random variable in the model, was associated with 7% of the variance in the sample (Table 2).
FIGURE 1.
Results of the mixed effects regression model of grooming received. Residual values calculated from the multivariate model (Table 2) have been used to illustrate the effects of sex, group, dominance rank, and age on amount of grooming received. A) Females receive more grooming than males after controlling for other variables in the model. B) )Younger animals receive more grooming that older individuals after controlling for other variables. C) Higher ranking animals receive more grooming than the lower ranking animals after controlling for other variables. See Table 2 for parameter estimates for each independent variable.
Table 2.
Results of mixed model analysis of frequency of grooming received (combined model: N=62)
| Predictor variable | Significance | β | Z value | SE | Direction |
|---|---|---|---|---|---|
| Sex | <0.0001 | −0.605 | −12.50 | 0.048 | Adult females received more grooming than adult males |
| Age | <0.001 | −0.013 | −2.94 | 0.005 | Younger adults received more grooming than older adults. |
| Dominance rank | <0.0001 | −0.0277 | −6.89 | 0.0040 | High ranking adults received more grooming than low ranking adults |
| Social group | - | - | - | - | Random variable |
| Intercept | <0.0001 | 31.62 |
β - regression coefficient, Z – z-statistic value, SE – standard error, Direction – direction of the observed effect
Tick species and the effect of grooming on tick load
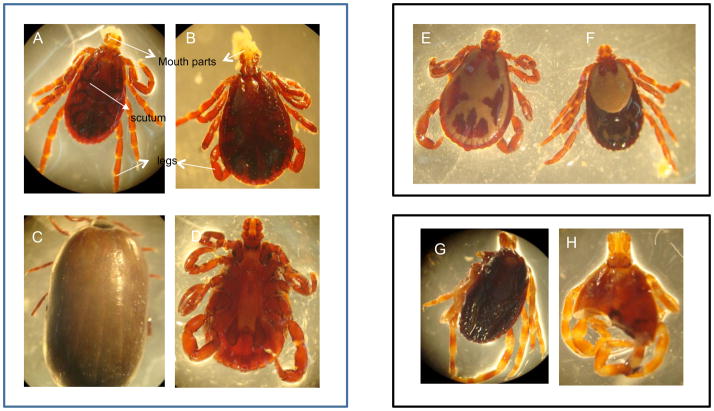
The tick species identified in our samples were Rhipicephalus simus, Rhipicephalus pulchellus and Hyalomma truncatum (Figure 2). The dominant tick species collected was Rhipicephalus simus (98% of 951 total ticks). R. pulchellus and Hyalomma were relatively rare 1.8% (17 ticks) and 0.2% (2 ticks), respectively. Baboons darted in 2007 had higher adult tick infestation than those darted in 2008 (see Table S3).
FIGURE 2.
Representative tick specimens obtained from Amboseli baboons. (A) Rhipicephalus simus simus female, (B) R. s.simus male, (C) R. s.simus, engorged female and (D) R. s.simus, ventral view, (E) R. pulchellus male and (F) R. pulchellus female, (G) Hyalomma truncatum female and (H) H. truncatum unknown sex
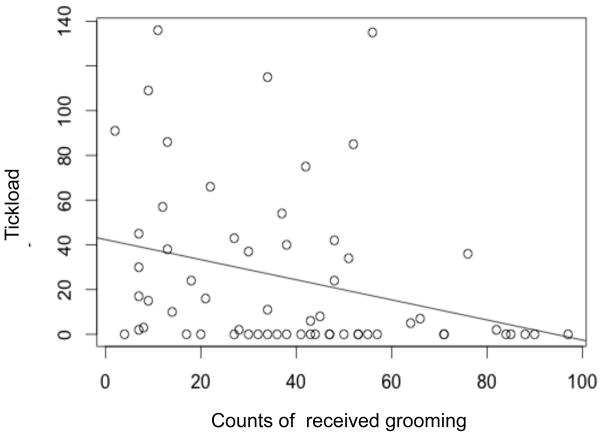
We found that the frequency of grooming received by an individual significantly predicted tick load, such that individuals that had received more grooming in the six months prior to darting had fewer ticks (β =−0.0076, P = < 0.0001, N = 62; Table 3 and Figure 3). The logistic part of our model strongly suggests that the year of darting contributed to probability of an individual having no ticks (zero number of ticks), (β = −0.9725, P = <0.068, N = 62; Table 3).
Table 3.
Results of zero inflated Poisson regression model of tick load (using total number of ticks, both adult ticks and larvae; N = 62)
| Predictor variable | Significance | β | Z | SE | Direction | |
|---|---|---|---|---|---|---|
| Effects of grooming on presence of ticks (Poisson part of the model) | Grooming received | 0.0001 | −0.0076 | −6.105 | 0.0012 | More grooming was associated with fewer ticks |
| Effect of year on darting (logistic part of the model) | Year of darting | 0.068 | −0.9725 | −1.824 | 0.0533 | Animals darted in 2008 were more likely to have 0 ticks than animals darted in 2007 |
N – number of animals sampled, Z – z-statistic value SE – standard error, Direction – direction of the observed effect
FIGURE 3.
Individuals receiving higher grooming counts had lower tick loads.
Direct detrimental effects of ticks
a) Wounds
In a number of cases, especially in 2007 when adult ticks were quite abundant, we saw skin wounds caused by the clustering of the ticks on various parts of the body (e.g., under the armpits, ears, neck and back region; Figure 4). This clustering was observed somewhat more frequently in males, with 11 males and 4 females being extensively affected. These wounds were generally minor (Figures 4C and 4D), and were often characterized by areas of scar formation indicating onset of healing. But in some cases the wounds seemed severe enough that they could increase susceptibility to bacterial infection; such wounds were often characterized by signs of underlying inflammation such as redness (Figure 4A).
FIGURE 4.
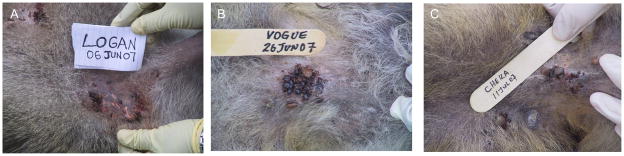
Clustering of ticks on various body parts of three individuals. A) Tick clusters on the chest; B) on the neck; and C) on the side of the body near the armpit.
b) Packed cell volume (PCV)
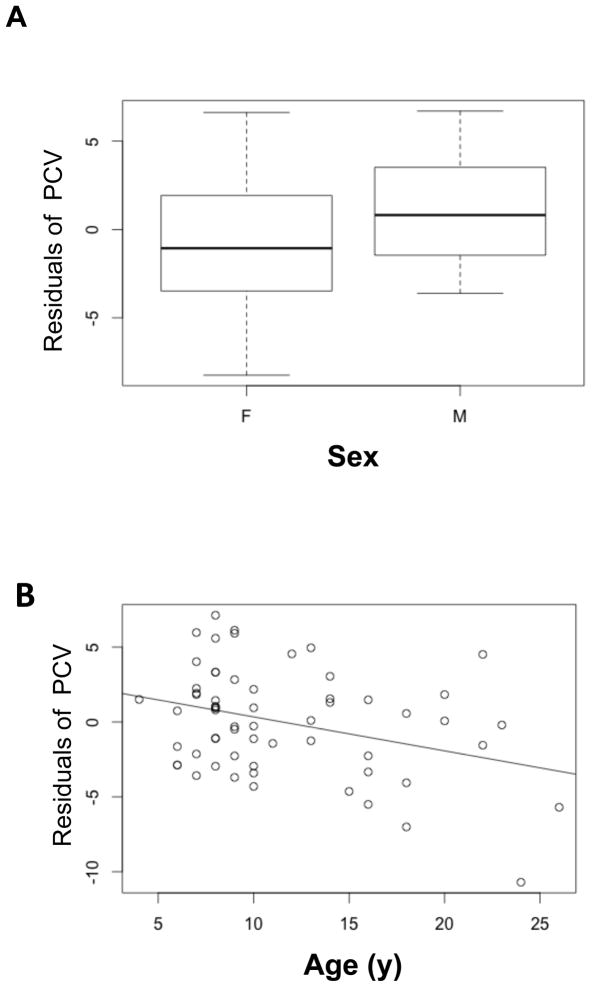
The average packed cell volume among the study subjects was 41.91% +/− 4 (SD) (n = 64). Individuals with fewer ticks exhibited higher PCV than those with more ticks (β = −0.037, P = 0.010, N = 61), males exhibited higher mean PCV than females (β = 2.32, P = 0.03, N = 61), and younger adults exhibited higher PCV than older adults (β = −0.29, P = <0.003, N = 61), Dominance rank did not have a significant effect on PCV (β=−0.095, P =0.212) (Table 4 and Figure 5).
Table 4.
Results of multivariate model of PCV (using total number of ticks, both adult ticks and larvae; N = 61)
| Predictor variable | Adjusted R2(%) | T | β | Significance | Direction |
|---|---|---|---|---|---|
| Age | - | −3.085 | −0.29 | <0.003 | Younger animals had higher PCV than older ones |
| Sex | - | 2.236 | 2.32 | 0.03 | Females had lower PCV than males |
| Dominance Rank | - | −1.262 | −0.095 | 0.212 | NS |
| Number of ticks | - | −2.655 | −0.037 | 0.010 | Individuals with fewer ticks had higher PCV than those with more ticks |
| Combined model | 35 |
N – number of animals sampled, Adjusted R2(%) - percentage of variance explained by the model, T – t-statistic value, β - regression coefficient,, Direction – direction of the observed effect, NS - not significant.
FIGURE 5.
Results of the multivariate general regression model. Residual values calculated from the model (Table 4) have been used to illustrate the effects of sex and age on PCV (Packed Cell Volume/haematocrit). A) Males had higher PCV than females after controlling for other variables. B) Younger animals exhibited higher PCV than older animals after controlling for other variables.
When only adult ticks were included in the model, the effect of number of ticks on PCV was larger than in the total tick count model (Table 5). Age and sex still contributed significantly to PCV. Bivariate comparisons of PCV by tick stages using t-tests also showed that individuals with adult ticks had lower mean PCVs (38.73%) than those with immature ticks (43.83%), (p=0.004, n=30; the sample size reflects the number of animals that had ticks of only one kind or the other and excludes those that had no ticks). These results led us to conclude that adult ticks had a larger effect on PCV than larvae.
Table 5.
Results of multivariate models of PCV (using adult tick stages only; N = 61)
| Predictor variable | Adjusted R2(%) | T | β | Significance | Direction |
|---|---|---|---|---|---|
| Age | - | −3.095 | −0.27 | <0.003 | Younger animals had higher PCV than older ones |
| Sex | - | 2.606 | 2.450 | 0.012 | Females had lower PCV than males |
| Dominance Rank | - | −1.353 | −0.095 | 0.181 | NS |
| Number of adult ticks | - | −4.058 | −0.053 | <0.0001 | Individuals with fewer ticks had higher PCV than those with more ticks |
| Combined model | 43 |
N – number of animals sampled, Adjusted R2(%) - percentage of variance explained by the model, T – t-statistic value, β - regression co-efficient, Direction – direction of the observed effect, NS - not significant
Tick-borne haemoparasite screening
We screened blood samples from 63 of 65 individuals (refer to Table 1 for information on sample sizes) for various tickborne parasitic infections using the primer sets described in the methods (Table S2). None of the samples tested positive for E. macaci (i.e., no amplification occurred in the PCRs using E. macaci primers). However, amplification with B. microti primers produced bands at the expected base pair size of 505 bp for two blood samples. Sequencing and BLAST search confirmed the presence B. microti in these two blood samples (BLAST e-value of 5e-59, with a maximum identity of 98%). These results provide strong evidence that Babesia microti occurs in this baboon population, although at a low frequency (3.2% of tested individuals). This low frequency of infection made it impossible to carry out a statistical analysis of the relationship between tick presence and probability of infection or to analyze the effect of grooming on the infection status of individuals.
Fifty-four of 63 samples were amplified by the two-stage nested PCR designed to detect the β-tubulin gene in any of two Theileria and six Babesia species (F34 and R323 primer set followed by the F79 and R206 primer set). However, the nested, non-specific PCR product, when sequenced, BLASTed not only to multiple species of Theileria and Babesia, and also to the Papio hamadryas b-tubulin gene; in other words, it was impossible to differentiate the source of the PCR product in any of our samples, suggesting that b-tubulin is too highly conserved between baboons and these parasites to be useful in determining parasite infections.
DISCUSSION
Studies of the relationship between tick burden and grooming behavior are relatively uncommon, and most have been done in wild ungulates (Hart 2000; Mooring et al. 2004). Observations of grooming in many different nonhuman primate species suggest its importance for ectoparasite removal (Tanaka and Takefushi 1993; Sanchez-Villagra et al. 1988, see also and Norval et al. (1989) and Hart (2006) for similar discussions about wild ungulates). We are aware of only three studies that examined the relationship between tick burden and grooming in primates, and all three provided only qualitative assessments of this relationship (Saunders 1988; Saunders and Hausfater 1988; Brain and Bohrmann 1992; Sanchez-Villagra et al. 1998). Our study is unusual among primate studies of grooming in using quantitative measures of both tick burdens and grooming to evaluate the relationship between grooming and ectoparasite loads. We also examined the health consequences of tick infestations, specifically, the effects of ticks on blood composition (PCV) and observations of tick-induced wounds.
Grooming and ticks
Several experimental studies of rodents and viverrids have demonstrated that when ectoparasites are experimentally removed, grooming rates decrease within social groups; these experiments provide several pieces of evidence to support the ideas that the presence of ectoparasites stimulates grooming behavior and that grooming functions to reduce ectoparasite load (Hawlena et al. 2008; Madden and Clutton-Brock 2009; Hillegass et al. 2010). Further, experimental studies in several vertebrate species in which individuals were prevented from grooming themselves showed that grooming reduces ectoparasite loads (Eckstein and Hart 2000; Mooring et al. 1996). In our study, as predicted, we observed that individuals that received more grooming in the six months prior to darting had lower ectoparasite loads than those that received less grooming, suggesting that grooming influenced tick load.
Detrimental effects of ticks
The main beneficiaries of grooming in this study were younger individuals, females and high ranking individuals. Because these individuals received higher rates of grooming, we predict that, all other things being equal, these classes of individuals will generally be in better health, at least with regards to the consequences of ectoparasite infection, than other classes of individuals. Specifically, our data indicate that individuals that received more grooming were likely to suffer from the detrimental effects of tick infestation.
We found several detrimental health effects of ticks in this baboon population. First, we saw, in some cases, skin wounds caused by the clustering of the ticks on various parts of the body. An individual would have difficulty grooming itself in the areas where these clusters tended to occur (e.g., under the armpits, and on the ears, neck and back), unlike the legs and genitals, which are listed by Saunders (1988) as body parts that individuals can effectively self-groom. Previous studies in baboons in the Namib Desert showed that ticks caused direct harmful effects to the extent that some animals with heavier tick infestations died (Brain 1992; Brain and Bohrmann 1992). Similarly, in wild bovids, ticks are implicated as a cause of detrimental effects such as reduced weight gain in animals with ticks, as well as blood loss and increased incidence of screwworms (Norval et al. 1989; Hart 2006).
Second, individuals with more ticks had lower PCV (an indicator of anaemia) perhaps because they lost blood to ticks. These PCV ranges were generally within the range observed in wild-caught captive Papio anubis baboons (33% – 57%, (IPR 2011) except for two females who had PCV levels of 30%. Low PCV levels may contribute to reduced blood oxygen levels. However, we note that anaemia can only be completely evaluated by measuring not only PCV, but red blood cell counts and haemoglobin levels as well, neither of which we were able to measure in this study.
Our results also indicated a stronger effect of adult ticks than of larvae on PCV, which probably is a simple consequence of the size differences between adult and larval ticks. Compared to small sized larvae, adult ticks, especially adult female Ixodid ticks, can undergo a 10 to 30-fold increase in size after ingesting blood (Figure 2C). Our results suggest that grooming may play a particularly important role in maintaining a normal PCV during seasons of high tick infestation, as adult ticks are probably easier to remove than larvae, because of their size and visibility (Figure 4). It is also well known that increased duration of tick attachment on a host is important in predicting tickborne infection risks. For example, a study by (Homer et al. 2000) on hamsters and white foot mice has shown that the length of time that a tick is attached to the vertebrate host directly increases the efficacy of haemoparasite transmission from the tick to the host. Tick removal during grooming in nonhuman primates may reduce the time the tick is attached to the baboon, thus reducing downstream transmission of haemoparasitic infection.
As in other primates, sex and age influenced the PCV (Harewood et al. 2000; Howell et al. 2003; Setchell et al. 2006). PCV was lower in older adults than in younger adults, and adult females had lower PCV values than adult males. Consequently, older animals and females may be more vulnerable to tick-induced anaemia because of their higher tick loads.
Presence of Babesia microti
We observed a low prevalence (3.2%) of Babesia microti infections in this population of baboons. Our study complements a recent study carried out by (Maamun et al. 2011), who reported the first case of Babesia microti parasites in free ranging non-human primates (baboons and vervets). They detected the presence of B. microti and Theileiria in R. simus ticks, suggesting the possibility of transmission of these by ticks to nonhuman primates. Our results suggest that factors affecting tick presence likely predispose individuals to downstream haemoparasite infection, such that older individuals, males and low ranking individuals will be more prone to infection. Unfortunately, the low prevalence of haemoparasite infection did not enable us to directly test our second hypothesis, which posited a link between increased tick presence and increased transmission of tick-borne haemoparasite infection.
CONCLUSION
Our study supports the idea that grooming, in addition to having social value, is a behavioral means by which baboons reduce tick loads, and thus grooming may protect baboons from the detrimental effects of ticks. These findings point to potentially important benefits of group living in primates. Having social partners confers multiple potential advantages including protection from detrimental effects of ticks through grooming. Further, our data provide clear evidence that individual differences in social behavior, age and sex may result in differences in vulnerability to tick infestations. Whether grooming also reduces the transmission of tick-borne parasites remains unclear and addressing this question will likely benefit from larger sample sizes.
Supplementary Material
Acknowledgments
This work was supported by the National Science Foundation (BCS-0323553, IOS 0919200, BCS-0323596, DEB-0846286, DEB-0846532) and the National Institute of Aging (R01AG034513-01 and P01-AG031719). We thank the Office of the President, Republic of Kenya, the Kenya Wildlife Service, its Amboseli staff and Wardens, the National Museums of Kenya, and the members of the Amboseli-Longido pastoralist communities. Particular thanks go the Amboseli fieldworkers who contributed to behavioral and physiological sampling, especially Raphael Mututua, Serah Sayialel, and Kinyua Warutere. We thank Tim Wango for invaluable support during sample collection efforts, Lacey Roerish and Niki Learn for database expertise and support, and the research staff and administration of the Institute of Primate Research, especially Dr. Tom Kariuki, for support during all phases of the research. We also thank the International Livestock Research Institute especially Dr. Rob Skilton and Sam Mwaura for their assistance in molecular screening of haemoparasites and tick identification.
References
- Alberts SC, Altmann J. Balancing Costs and Opportunities - Dispersal in Male Baboons. American Naturalist. 1995;145:279–306. [Google Scholar]
- Alberts SC, Altmann J. Immigration and hybridization patterns of yellow and anubis baboons in and around Amboseli, Kenya. American Journal of Primatology. 2001;53:139–154. doi: 10.1002/ajp.1. [DOI] [PubMed] [Google Scholar]
- Alberts SC, Watts HE, Altmann J. Queuing and queue-jumping: long-term patterns of reproductive skew in male savannah baboons, Papio cynocephalus. Animal Behaviour. 2003;65:821–840. [Google Scholar]
- Altmann J. Observational Study of Behavior - Sampling Methods. Behaviour. 1974;49:227–267. doi: 10.1163/156853974x00534. [DOI] [PubMed] [Google Scholar]
- Altmann J. Baboon Mothers and Infants. Cambridge: Harvard University Press; 1980. [Google Scholar]
- Altmann J, Glenn H, Stuart A. Determinants of Reproductive Success in Savannah BaboonsPapio cynocephalus. In: Clutton-Brock TH, editor. Reproductive success - Studies of Individual Variation in Contrasting Breeding Systems. Chicago: The University of Chicago Press; 1988. pp. 403–418. [Google Scholar]
- Altmann J, et al. Behavior predicts genetic structure in a wild primate group. Proceedings of the National Academy of Sciences of the United States of America. 1996;93:5797–5801. doi: 10.1073/pnas.93.12.5797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aureli F, Preston SD, de Waal FBM. Heart rate responses to social interactions in free-moving rhesus macaques (Macaca mulatta): A pilot study. Journal of comparative psychology. 1999;113:59–65. doi: 10.1037/0735-7036.113.1.59. [DOI] [PubMed] [Google Scholar]
- Barrett L, Henzi SP, Weingrill T, Lycett JE, Hill RA. Market forces predict grooming reciprocity in female baboons. Proceedings of the Royal Society of London Series B-Biological Sciences. 1999;266:665–670. [Google Scholar]
- Boccia ML, Reite M, Laudenslager M. On the Physiology of Grooming in a Pigtail Macaque. Physiology & Behavior. 1989;45:667–670. doi: 10.1016/0031-9384(89)90089-9. [DOI] [PubMed] [Google Scholar]
- Brain C. Deaths in a Desert Baboon Troop. International Journal of Primatology. 1992;13:593–599. [Google Scholar]
- Brain C, Bohrmann R. Tick Infestation of Baboons (Papio ursinus) in the Namib Desert. Journal of Wildlife Diseases. 1992;28:188–191. doi: 10.7589/0090-3558-28.2.188. [DOI] [PubMed] [Google Scholar]
- Caccio C, Camma C, Onuma M, Severini C. The beta-tubulin gene of Babesia and Theileria parasites is an informative marker for species discrimination. International Journal for Parasitology. 2000;30:1181–1185. doi: 10.1016/s0020-7519(00)00105-3. [DOI] [PubMed] [Google Scholar]
- Chapais B, Berman C. Kinship and Behavior in Primates. New York: Oxford University Press; 2004. [Google Scholar]
- Charpentier MJE, Tung J, Altmann J, Alberts SC. Age at maturity in wild baboons: genetic, environmental and demographic influences. Molecular Ecology. 2008;17:2026–2040. doi: 10.1111/j.1365-294X.2008.03724.x. [DOI] [PubMed] [Google Scholar]
- Cogswell FB. Malaria and Piroplasms of Non-Human Primates. Companion and Exotic Animal Parasitology, Publisher: International Veterinary Information Service. 2000 ( www.ivs.org) Url: http://www.ivis.org/advances/parasit_bowman/cogswell_primate/ivis.pdf.
- Dunbar RIM. Functional-Significance of Social Grooming in Primates. Folia Primatologica. 1991;57:121–131. [Google Scholar]
- Eckstein RA, Hart BL. Grooming and control of fleas in cats. Applied Animal Behaviour Science. 2000;68:141–150. doi: 10.1016/s0168-1591(00)00095-2. [DOI] [PubMed] [Google Scholar]
- Edlow JA. Lyme disease and related tick-borne illnesses. Annals of Emergency Medicine. 1999;33:680–693. [PubMed] [Google Scholar]
- Ferron J, Lefebvre L. Comparative Organization of Grooming Sequences in Adult and Young Sciurid Rodents. Behaviour. 1982;81:110–127. [Google Scholar]
- Ginsberg HS, Stafford KC. Book Chapter: Tick-Borne Diseases of Humans. Washington D.C: ASM press; 2005. Management of ticks and Tick-borne Disease; pp. 65–86. [Google Scholar]
- Harewood WJ, Gillin A, Hennessy A, Armitstead J, Horvath JS, Tiller DJ. The effects of the menstrual cycle, pregnancy and early lactation on haematology and plasma biochemistry in the baboon (Papio hamadryas) Journal of Medical Primatology. 2000;29:415–420. doi: 10.1111/j.1600-0684.2000.290606.x. [DOI] [PubMed] [Google Scholar]
- Hart B. Role of Grooming in Biological Control of Ticks. Annals of the New York Academy of Sciences. 2000:565–569. doi: 10.1111/j.1749-6632.2000.tb05337.x. [DOI] [PubMed] [Google Scholar]
- Hausfater G. Book Chapter: Contribution to Primatology. New York: S Karger; 1975. Dominance and Reproduction in Baboons: A Quantitative Analysis; pp. 145–150. [PubMed] [Google Scholar]
- Hawking Frank. Entopolypoides macaci, a Babesia-like parasite in Cercopithecus monkeys. Parasitology. 1972;65(01):89–109. doi: 10.1017/s0031182000044267. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villagra Marcelo R, Pope Theresa R, Salas Viviana. Relation of Intergroup Variation in Allogrooming to Group Social Structure and Ectoparasite Loads in Red Howlers (Alouatta seniculus) International Journal of Primatology. 1998;19(3) [Google Scholar]
- Hawlena H, Bashary D, Abramsky Z, Khokhlova IS, Krasnov BR. Programmed versus stimulus-driven antiparasitic grooming in a desert rodent. Behavioral Ecology. 2008;19:929–935. [Google Scholar]
- Heitor F, Oom MD, Vicente L. Social relationships in a herd of Sorraia horses - Part II. Factors affecting affiliative relationships and sexual behaviours. Behavioural Processes. 2006;73:231–239. doi: 10.1016/j.beproc.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Henzi SP, Barrett L. The value of grooming to female primates. Primates. 1999;40:47–59. doi: 10.1007/BF02557701. [DOI] [PubMed] [Google Scholar]
- Hillegass MA, Waterman JM, Roth JD. Parasite removal increases reproductive success in a social African ground squirrel. Behavioral Ecology. 2010;21:696–700. [Google Scholar]
- Homer MJ, Aguilar-Delfin I, Telford SR, Krause PJ, Persing DH. Babesiosis. Clinical Microbiology Reviews. 2000;13:451. doi: 10.1128/cmr.13.3.451-469.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell S, Hoffman K, Bartel L, Schwandt M, Morris J, Jo F. Normal Hematologic and Serum Clinical Chemistry Values for Captive Chimpanzees (Pan troglodytes) Comparative Medicine. 2003;53(411):413–423. [PubMed] [Google Scholar]
- ILRI. Tick identification laboratory manual. Nairobi: International Livestock Research Institute; 2004. [Google Scholar]
- IPR. Institute of Primate Research, Quality systems procedures manual, document reference: NMK/IPR002. 99. Nairobi: National Museums of Kenya; 2011. [Google Scholar]
- Jeneby MM, Ngeiywa M, Yole DS, Mwenda JM, Suleman MA, Carlson HE. Enzootic simian piroplasm (Entopolypoides macaci ) in wild-caught Kenyan non-human primates. Journal of Medical Primatology. 2008;37:329–336. doi: 10.1111/j.1600-0684.2008.00294.x. [DOI] [PubMed] [Google Scholar]
- Keverne EB, Martensz ND, Tuite B. Beta-Endorphin Concentrations in Cerebrospinal-Fluid of Monkeys Are Influenced by Grooming Relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- Kimura R. Mutual grooming and preferred associate relationships in a band of free-ranging horses. Applied Animal Behaviour Science. 1998;59:265–276. [Google Scholar]
- Kutsukake N, Clutton-Brock T. Aggression and submission reflect reproductive conflict between females in cooperatively breeding meerkats Suricata suricatta. Behavioral Ecology and Sociobiology. 2006;59:541–548. [Google Scholar]
- Lazaro-Perea C, De Fatima M, Snowdon CT. Grooming as a reward? Social function of grooming between females in cooperatively breeding marmosets. Animal Behaviour. 2004;67:627–636. doi: 10.1016/j.anbehav.2003.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maamun JM, Sulemant MA, Akinyi M, Ozwara H, Kariuki T, Carlsson HE. Prevalence of Babesia Microti in Free-Ranging Baboons and African Green Monkeys. Journal of Parasitology. 2011;97:63–67. doi: 10.1645/GE-2391.1. [DOI] [PubMed] [Google Scholar]
- Madden JR, Clutton-Brock TH. Manipulating grooming by decreasing ectoparasite load causes unpredicted changes in antagonism. Proceedings of the Royal Society B-Biological Sciences. 2009;276:1263–1268. doi: 10.1098/rspb.2008.1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore JA, Kuntz RE. Babesia microti Infections in Non-Human Primates. Journal of Parasitology. 1981;67:454–456. [PubMed] [Google Scholar]
- Mooring MS, McKenzie AA, Hart BL. Grooming in impala: Role of oral grooming in removal of ticks and effects of ticks in increasing grooming rate. Physiology & Behavior. 1996;59:965–971. doi: 10.1016/0031-9384(95)02186-8. [DOI] [PubMed] [Google Scholar]
- Mooring MS, Blumstein DT, Stoner CJ. The evolution of parasite-defence grooming in ungulates. Biological Journal of the Linnean Society. 2004;81:17–37. [Google Scholar]
- Nguyen N, Gesquiere L, Alberts SC, Altmann J. Sex differences in the mother-neonate relationship in wild baboons: social, experiential and hormonal correlates. Animal Behaviour. 2012;83:891–903. [Google Scholar]
- Norval RAI, Sutherst RW, Jorgensen OG, Gibson JD, Kerr JD. The Effect of the Bont Tick (Amblyomma hebraeum) on the Weight-Gain of Africander Steers. Veterinary Parasitology. 1989;33:329–341. doi: 10.1016/0304-4017(89)90142-8. [DOI] [PubMed] [Google Scholar]
- Obrien TG. Allogrooming Behavior among Adult Female Wedge-Capped Capuchin Monkeys. Animal Behaviour. 1993;46:499–510. [Google Scholar]
- Parr LA, Matheson MD, Bernstein IS, DeWaal FBM. Grooming down the hierarchy: allogrooming in captive brown capuchin monkeys, Cebus apella. Animal Behaviour. 1997;54:361–367. doi: 10.1006/anbe.1996.0419. [DOI] [PubMed] [Google Scholar]
- Sanchez-Villagra Marcelo R, Pope Theresa R, Salas Viviana. Relation of Intergroup Variation in Allogrooming to Group Social Structure and Ectoparasite Loads in Red Howlers (Alouatta seniculus) International Journal of Primatology. 1998;19(3) [Google Scholar]
- Saunders CD. PhD Thesis. Cornell University; 1988. Ecological, Social, and Evolutionary Aspects of Baboon Grooming Behavior. [Google Scholar]
- Saunders CD, Hausfater G. The Functional-Significance of Baboon Grooming Behavior. Annals of the New York Academy of Sciences. 1988;525:430–432. [Google Scholar]
- Schino G. Grooming, competition and social rank among female primates: a meta-analysis. Animal Behaviour. 2001;62:265–271. [Google Scholar]
- Schino G. Grooming and agonistic support: a meta-analysis of primate reciprocal altruism. Behavioral Ecology. 2007;18:115–120. [Google Scholar]
- Setchell JM, Tshipamba P, Bourry O, Rouquet P, Wickings EJ, Knapp LA. Hematology of a Semi-Free-Ranging Colony of Mandrills (Mandrillus sphinx) International Journal of Primatology. 2006;27:1709–1729. [Google Scholar]
- Seyfarth RM. Model of Social Grooming among Adult Female Monkeys. Journal of Theoretical Biology. 1977;65:671–698. doi: 10.1016/0022-5193(77)90015-7. [DOI] [PubMed] [Google Scholar]
- Shutt K, MacLarnon A, Heistermann M, Semple S. Grooming in Barbary macaques: better to give than to receive? Biology Letters. 2007;3:231–233. doi: 10.1098/rsbl.2007.0052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silk J. The adaptive value of social bonds. American Journal of Primatology. 2004;62:32–32. [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social bonds of female baboons enhance infant survival. Science. 2003;302:1231–1234. doi: 10.1126/science.1088580. [DOI] [PubMed] [Google Scholar]
- Silk JB, Alberts SC, Altmann J. Social relationships among adult female baboons (Papio cynocephalus) II. Variation in the quality and stability of social bonds. Behavioral Ecology and Sociobiology. 2006a;61:197–204. [Google Scholar]
- Silk JB, Altmann J, Alberts SC. Social relationships among adult female baboons (Papio cynocephalus) I. Variation in the strength of social bonds. Behavioral Ecology and Sociobiology. 2006b;61:183–195. [Google Scholar]
- Silk JB, Beehner JC, Bergman TJ, Crockford C, Engh AL, Moscovice LR, Wittig RM, Seyfarth RM, Cheney DL. Strong and Consistent Social Bonds Enhance the Longevity of Female Baboons. Current Biology. 2010;20:1359–1361. doi: 10.1016/j.cub.2010.05.067. [DOI] [PubMed] [Google Scholar]
- Simonds PE. Sex Differences in Bonnet Macaque Networks and Social Structure. Archives of Sexual Behavior. 1974;3:151–166. doi: 10.1007/BF01540999. [DOI] [PubMed] [Google Scholar]
- Smuts B. Sex and Friendships in baboons. New York: Aldine Publishing Company; 1985. [Google Scholar]
- Tanaka I, Takefushi H. Elimination of External Parasites (Lice) Is the Primary Function of Grooming in Free-Ranging Japanese Macaques. Anthropological Science. 1993;101:187–193. [Google Scholar]
- Terry RL. Primate Grooming as a Tension Reduction Mechanism. Journal of Psychology. 1970;76:129. doi: 10.1080/00223980.1970.9916830. [DOI] [PubMed] [Google Scholar]
- Tung J, Charpentier MJE, Garfield DA, Altmann J, Alberts SC. Genetic evidence reveals temporal change in hybridization patterns in a wild baboon population. Molecular Ecology. 2008;17:1998–2011. doi: 10.1111/j.1365-294X.2008.03723.x. [DOI] [PubMed] [Google Scholar]
- Tung J, Akinyi MY, Mutura S, Altmann J, Wray GA, Alberts SC. Allele-specific gene expression in a wild nonhuman primate population. Molecular Ecology. 2011;20:725–739. doi: 10.1111/j.1365-294X.2010.04970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson GS. Social Grooming in the Common Vampire Bat, Desmodus rotundus. Animal Behaviour. 1986;34:1880–1889. [Google Scholar]
- Wrangham RW. An Ecological Model of Female-Bonded Primate Groups. Behaviour. 1980;75:262–300. [Google Scholar]
- Young G, Coelho AM, Bramblett CA. The development of grooming, sociosexual behavior, play and aggression in captive baboons in their first two years. Primates. 1982;23:511–519. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.