Abstract
Our knowledge of the hearing abilities of frogs and toads is largely defined by work with a few well-studied species. One way to further advance comparative work on anuran hearing would be greater use of minimally invasive electrophysiological measures, such as the auditory brainstem response (ABR). This study used the ABR evoked by tones and clicks to investigate hearing in Cope's gray treefrog (Hyla chrysoscelis). The objectives were to characterize the effects of sound frequency, sound pressure level, and subject sex and body size on ABRs. The ABR in gray treefrogs bore striking resemblance to ABRs measured in other animals. As stimulus level increased, ABR amplitude increased and latency decreased, and for responses to tones, these effects depended on stimulus frequency. Frequency-dependent differences in ABRs were correlated with expected differences in the tuning of two sensory end organs in the anuran inner ear (the amphibian and basilar papillae). The ABR audiogram indicated two frequency regions of increased sensitivity corresponding to the expected tuning of the two papillae. Overall, there was no effect of subject size and only small effects related to subject sex. Together, these results indicate the ABR is an effective method to study audition in anurans.
Keywords: Auditory brainstem response, Audiogram, Communication, Grey treefrog, Hearing
Introduction
The ability of relatively simple, species-specific vocal signals to elicit stereotyped behaviors in noisy environments makes the anuran auditory system an important model in neuroethology and sensory biology (Gerhardt and Huber 2002; Kelley 2004; Narins et al. 2007; Wilczynski and Ryan 2010; Bee 2012). However, we still lack a well-developed understanding of both species differences in anuran hearing and the influences such differences potentially have on the species specificity of vocalizations and behaviors in a broad, comparative framework. One reason for this is because the vast majority of anatomical, biomechanical, and electrophysiological studies of anuran hearing have been conducted using a relatively small number of model species, such as northern leopard frogs, Rana pipiens (e.g., Fuzessery and Feng 1981, 1982; Simmons et al. 1992; Ratnam and Feng 1998; Ho and Narins 2006), North American bullfrogs, R. catesbeiana (e.g., Feng and Capranica 1976; Schwartz and Simmons 1990; Simmons and Ferragamo 1993; Simmons et al. 1993, 2000), grass frogs, R. temporaria (e.g., Christensen-Dalsgaard and Jørgensen 1996; Christensen-Dalsgaard et al. 1998; Christensen-Dalsgaard and Walkowiak 1999; Bibikov 2002), African clawed frogs, Xenopus laevis (e.g., Christensen-Dalsgaard et al. 1990; Edwards and Kelley 2001; Bibikov and Elepfandt 2005; Elliott et al. 2007, 2011), and green treefrogs, Hyla cinerea (e.g., Feng and Capranica 1978; Ehret and Capranica 1980; Mudry and Capranica 1987; Klump et al. 2004; Miranda and Wilczynski 2009a, b). Efforts to assess audition in frogs and toads using relatively fast, minimally invasive procedures, such as dermal or subdermal recordings of auditory evoked potentials (AEPs) (reviewed in Hall 2007), could significantly enhance experimental neuroethological research on this group by facilitating comparisons among a much greater diversity of species.
AEPs have been widely used to study hearing and sound communication in a broad diversity of nonhuman vertebrates, including mammals (Walsh et al. 1986; Katbamna et al. 1992; Supin et al. 1993; Aitkin et al. 1996; McFadden et al. 1996; Uetake et al. 1996; Uzuka et al. 1996; Szymanski et al. 1998; Popov and Supin 2001; Song et al. 2006; D'Angelo et al. 2007; Nachtigall et al. 2007; Nachtigall and Supin 2008; Ramsier and Dominy 2010), birds (Brittan-Powell et al. 2002, 2005, 2010a; Brittan-Powell and Dooling 2004; Lucas et al. 2002; Henry and Lucas 2008, 2009, 2010a, 2010b; Caras et al. 2010; Gall et al. 2011; Noirot et al. 2011; Lohr et al. 2013), reptiles (Bartol et al. 1999; Higgs et al. 2002; Brittan-Powell et al. 2010b; Martin et al. 2012), and fish (Kenyon et al. 1998; Ladich and Yan 1998; Wysocki and Ladich 2001, 2003; Lugli et al. 2003; Smith et al. 2004; Amoser and Ladich 2005; Horodysky et al. 2008), as well as a few invertebrates (Lovell et al. 2005; Hu et al. 2009; Mooney et al. 2010). While a few previous studies used AEPs to investigate the auditory systems of frogs, these studies used invasive recording procedures requiring surgery (Corwin et al. 1982; Hillery 1984a; Seaman 1991; Carey and Zelick 1993; Bibikov and Elepfandt 2005; Katbamna et al. 2006b; Yu et al. 2006). To our knowledge, only two previous studies have recorded AEPs in frogs using less invasive subdermal procedures (Katbamna et al. 2006a; Zhang et al. 2012).
In the present study, we used subdermal recordings of the auditory brainstem response (ABR) to investigate auditory sensitivity in Cope's gray treefrog (Hyla chrysoscelis). The ABR is a form of AEP that represents the summed output of synchronized neural activity in the auditory nerve and brainstem. ABR waveforms are typically characterized by a series of positive and negative deflections, whose presence or absence can be used to determine auditory thresholds (reviewed in Hall 2007). Neither of the two previous studies that used minimally invasive methods to record ABRs in frogs (Katbamna et al. 2006a; Zhang et al. 2012) systematically investigated the effects on the ABR of stimulus properties, such as frequency and sound pressure level, or subject characteristics, such as sex and size. We had three objectives in this study. First, we sought to characterize the ABR in gray treefrogs and describe its dependence on sound pressure level and different sound frequencies. Typically, ABR amplitude and latency are directly and inversely related, respectively, to sound pressure level, whereas changes in tone frequency can have complex effects on the waveform (reviewed in Hall 2007). The directional effects of sound level on amplitude and latency are quite consistent across species, but the effects of frequency can vary (e.g., Popov and Supin 1990; Kenyon et al. 1998; Higgs et al. 2002; Song et al. 2006). Second, we investigated the extent to which the ABR in gray treefrogs differs according to sex and body size. There is some evidence for sex-differences in ABRs from some animals (Jerger and Hall 1980; Church et al. 1984; Hall 2007; Gall et al. 2011), and previous invasive studies of frogs have revealed differences in auditory processing related to sex (Narins and Capranica 1976; Wilczynski and Capranica 1984; Wilczynski et al. 1992; Zakon and Wilczynski 1988; Keddy-Hector et al. 1992) and body size (Shofner and Feng 1981; Zakon and Wilczynski 1988; Keddy-Hector et al. 1992). Third, we generated an ABR audiogram to quantify variation in auditory sensitivity across frequency, and we assessed the influence of sex and body size on sensitivity. Furthermore, we compared our ABR audiogram to one previously derived for this species from invasive multiunit recordings from the auditory midbrain (Hillery 1984b) to verify the utility of ABRs as a method for assessing auditory sensitivity in frogs. Taking into account results from the first three objectives, we discuss the possibility that the ABR might be useful for describing the frequency ranges of sensitivity of the different auditory end organs in the frog inner ear (Zakon and Wilczynski 1988; Simmons et al. 2007), which vary by species and are unknown for most anurans.
Materials and methods
Subjects
Thirty-five adult Cope's gray treefrogs (17 females, 18 males) of the western mitochondrial DNA lineage (Ptacek et al. 1994) were used as subjects. We collected pairs of frogs in amplexus between 15 May and 30 June, 2011, from the Carver Park Reserve (Carver County, MN), the Crow-Hassan Park Reserve (Hennepin County, MN), and the Lake Maria State Park (Wright County, MN). All of our ABR recordings were made during the annual breeding season (mid-May to early-July) and within five days of the animal's collection. Collected frogs were brought to the lab at the University of Minnesota, placed in small containers with conditioned tap water, and kept at 2 °C until they were used in behavioral tests not described here. After behavioral testing, we maintained the frogs at ambient room temperature (near 20 °C) until ABR experiments began. We waited until females had released their eggs prior to recording so that we could administer a correct size-dependent dose of paralytic (see below). Subject body mass ranged between 2.8 g and 8.3 g (X̄ ± s.d.; females: X̄ = 5.5 ± 1.1 g; males: X̄ = 4.4 ± 0.9 g). Body temperatures during ABR recordings were measured by placing a Miller & Weber quick-reading thermometer against the abdominal body wall; temperatures ranged between 17.0 and 20.0 °C, which is within the range of wet-bulb air temperatures at which this species breeds. After the completion of recordings, we released animals at their location of capture. All animals were collected with permission from the Minnesota Department of Natural Resources (permit #17031) and treated according to protocols approved by the Institutional Animal Care and Use Committee of the University of Minnesota (#1103A97192, last approved 04/16/2013).
ABR recordings
All ABR recordings were made inside a radio-shielded mini-acoustical chamber (MAC-3, Industrial Acoustics Corporation, Bronx, NY; inside dimensions: 81.3 cm × 61 cm × 61 cm, W × H × D). The sound chamber was equipped with a breadboard floor, and the ceiling and walls were covered with acoustic foam to reduce reverberations. The sound pressure level (SPL re. 20 μPa) of the ambient noise in the chamber was measured with a Larson Davis System 831 sound-level meter (Larson Davis Inc., Depew, NY) and ranged between 10 and 13 dB SPL (fast RMS, flat weighting) in the 1/3-octave bands between 250 and 5000 Hz, which span the frequency range of our test stimuli.
For recordings, subjects were immobilized by an intramuscular injection of d-tubocurarine chloride (2.5 to 4 μg/g body mass). Subjects were able to regulate their lung volume as the paralytic took effect over several minutes and maintained what appeared upon visual inspection to be a normal lung volume throughout neural recordings. We did not manually manipulate lung volume. During recordings, subjects were draped with moist surgical gauze to facilitate cutaneous respiration and placed on an acoustically transparent pedestal made of plastic mesh (2-cm height, 4-mm mesh grid) positioned on the breadboard floor of the sound chamber. We positioned subjects in a natural sitting posture directly facing an Orb Mod 1 speaker (Orb Audio, New York, NY) also located on the breadboard so that the rostral edges of both tympanic membranes were 30 cm from the sound source. Note that for frequencies below about 1.1 kHz, this distance was less than one wavelength from the sound source; thus there was also some potential for particle motion to influence responses to these sounds. Prior to electrode placement, the subject's head was treated with liberal application of a topical anesthetic (lidocaine HCl 2.5%). Platinum alloy, subdermal needle electrodes (Grass F-E2; West Warwick, RI) were inserted 2-3 mm under the skin of the subject's head between the eyes (non-inverting) and adjacent to the right (ground) and left (inverting) tympanic membranes (Fig. 1a). Electrode impedance ranged from 1 to 5 kΩ. The electrode wires were twisted together to reduce electrical noise, connected to a TDT (Tucker-Davis Technologies, Alachua, FL) RA4LI low-impedance headstage and TDT RA4PA pre-amp that amplified (20×) and digitized the biological signal before sending it via fiber optic cable to a TDT RZ5 digital signal processor located outside the chamber. We used a computer running TDT BioSig software to sample the biological signal at a sampling rate of 25 kHz (16 bit). Responses were notch filtered at 60 Hz, bandpass filtered between 0.03 kHz and 3 kHz, and stored on hard disk for offline analyses using MATLAB v2010b (Mathworks, Natick, MA). Recording sessions lasted approximately 1.5 hrs.
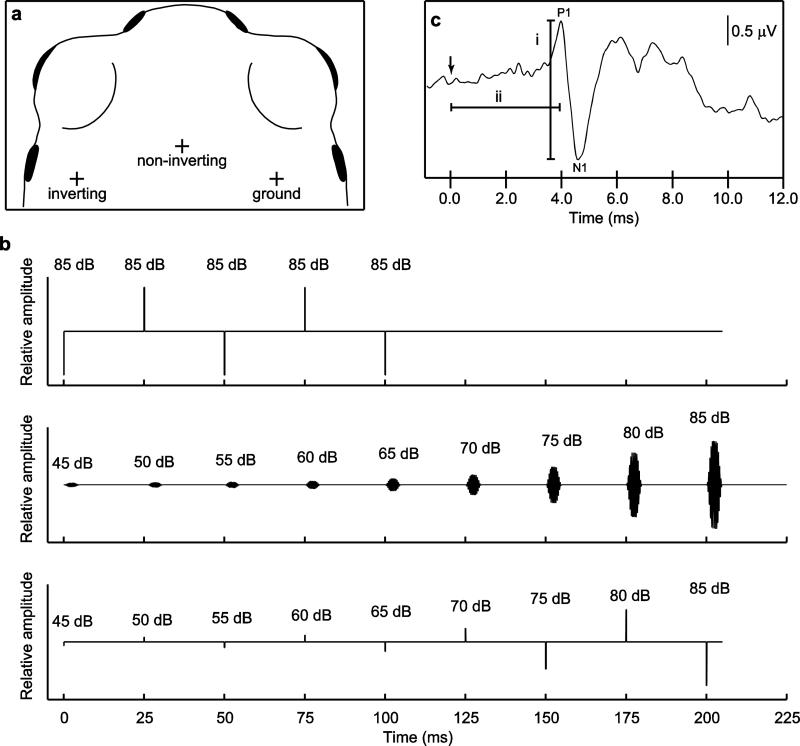
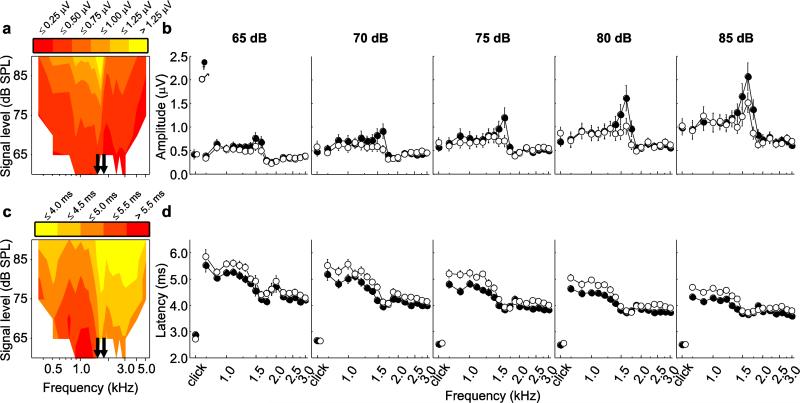
Fig. 1.
a Placement of recording electrodes depicted by “+”s. b Schematics of stimulus trains used in experiments. Depicted are examples of (top) a train of 5 clicks (0.1 ms, 24.9 ms inter-click interval) followed by a 100-ms period of silence broadcast at the beginning and end of a session to verify the presence of a signal (during clicks) and to obtain a recording in the absence of stimulus (during silence), (middle) a train consisting of 9 tones (5 ms in duration, 20-ms inter-tone interval) of increasing intensity, and (bottom) a train of 9 clicks of increasing intensity. Stimulus level is indicated above each sound in the train, in dB SPL (for tones) or dB pSPL (for clicks). c A typical ABR waveform indicating the three measures quantified in this study, including (i) amplitude, measured from maximum of the first positive deflection (P1) to minimum of the subsequent negative deflection (N1) and (ii) latency, measured from the time sound impinged on the tympanic membranes, indicated by the arrow, to the time of P1.
We synthesized digital sound files (50 kHz sampling rate, 16-bit) using TDT SigGen software. Stimuli comprising short trains of clicks or tones were output via a TDT RM2 signal processor, attenuated by a TDT PA5 programmable attenuator, amplified by a Crown XLS 202 amplifier (Crown Audio, Inc., Elkhart, IN) and broadcast through the Orb Mod 1 speaker located 30 cm in front of the frog. Examples of click stimuli and tone stimuli broadcast through this setup are shown in Online Resource 1 Fig 1.
Each recording session began and ended by verifying the presence of a biological signal in response to sound. To do this, we presented a stimulus train consisting of five 0.1-ms rectangular-pulse, broadband clicks (24.9-ms inter-click intervals) at either 85 dB or 90 dB pSPL (peak equivalent SPL re. 1 kHz tone), followed by a 100-ms silent interval, as illustrated in Fig. 1b (top). When output through our setup, the spectrum of the clicks was broadband, with a center frequency of approximately 1.6 kHz and 6-dB down points of approximately 0.345 and 2.8 kHz. Together, these procedures allowed us to verify the presence of an ABR in response to a suprathreshold stimulus (the clicks), to measure the biological signal in the absence of a stimulus (during the 100-ms silent interval), and to verify that neural responses to sound did not change during a recording session. If signals were very small or noisy, electrodes were repositioned until a robust, repeatable signal was acquired. Visual inspection of these click-evoked ABRs indicated no change in responses over the duration of recording sessions.
After initially verifying the presence of a robust ABR, we next investigated the effects of stimulus frequency and intensity on the ABR by presenting subjects with stimulus trains that consisted either of nine tone bursts or nine clicks, examples of which are depicted in Fig. 1b (middle and bottom, respectively). Tone trains consisted of 5-ms pure tones (1-ms rise/fall cos2) separated by 20-ms inter-stimulus intervals. The frequency of tones was held constant within a tone train. In different tone trains, frequency was varied across 21 values ranging from 0.35 kHz to 5.0 kHz. These frequencies included (in kHz) 0.35, 0.5. 0.75, 0.875, 1.0 to 1.5 (in 0.1 kHz steps), 1.625, 1.75, 1.875, 2.0 to 2.8 (in 0.2 kHz steps), 3.0, 4.0, and 5.0. Click trains consisted of clicks (as described above) that were 0.1 ms in duration and separated by 24.9 ms inter-click intervals.
The nine consecutive tones or clicks in the train increased in 5-dB steps over a 40-dB range (see Fig. 1b), starting at either 45, 50, or 55 dB and ending at 85, 90, or 95 dB (SPL re 20 μPa, fast RMS, c-weighting for tones; pSPL for clicks). Because of variation in sensitivity across both subjects and frequencies, we selected an absolute range for each stimulus train that included values above and below the visually detected ABR threshold (see below). For all stimuli, we obtained two replicate averages of the ABR, each based on averaging responses to 400 consecutive presentations of each stimulus train (800 presentations total across both replicates). Stimulus trains were presented at a rate of 4 train/s. All consecutive sounds within a stimulus train and between each repeated presentation of the train alternated in phase (tones) or polarity (clicks) to reduce the microphonic potential. During acquisition of the first set of replicate averages of the ABR, presentations of click trains preceded presentations of tone trains; this order was reversed for acquiring the second replicates. Within each replicate, the order of tone trains was randomized for each subject. On rare occasions, a subject would produce an isolated buccal pumping movement that produced a large artifact in the biological signal. Recordings disrupted by such artifacts were immediately rejected and repeated.
The sound levels of tone trains were calibrated by placing the 1/2″ condenser microphone of a Larson Davis System 831 sound-level meter 30 cm from the speaker at the approximate position of a subject's head during a recording session. The tone of highest intensity in each train was calibrated by matching its peak-to-peak voltage to that of a 1-s tone of the same frequency calibrated to the highest level in the train (85, 90, or 95 dB SPL). The voltage of subsequent tones in each train was digitally adjusted to achieve the appropriate nominal sound level. The frequency response of our system was ± 2 - 2.5 dB, based on recordings of the tone trains in the chamber. We used the peak-to-peak voltage of the calibrated 1 kHz tone to calibrate the pSPL of clicks.
ABR characterization
We characterized the morphology of the ABR waveform across stimuli using descriptive cross-correlation analyses and standard quantitative measurements of ABR amplitude and latency. The cross-correlation analysis was designed to assess how the general shape of the ABR varied between responses to clicks and tones and across different tone frequencies. We focused these cross-correlation analyses on tones with frequencies of 1.3, 1.625, and 2.6 kHz. We chose frequencies of 1.3 and 2.6 kHz for two reasons. First, they are close to the average frequencies present in the bimodal spectrum of male advertisement calls in our study populations (Schrode et al. 2012). Second, according to the “matched filter hypothesis” of anuran hearing (Capranica and Moffat 1983), they are presumed to be encoded primarily by the amphibian papilla (AP) and the basilar papilla (BP), respectively (Hillery 1984b; Gerhardt 2005). We chose 1.625 kHz as an additional frequency for further investigation because it was intermediate between the expected ranges encoded by each papilla. For cross-correlation analyses, we removed the DC offset from each response by subtracting its baseline mean amplitude and then positioned a 10-ms analysis window over the response extending from 2 ms before the peak of the first positive deflection (P1) to 8 ms after this peak (i.e., from −2 ms to +8 ms relative to P1 at 0 ms). We then averaged windowed responses across both replicates obtained for each individual before determining the average response across all individuals. We used MATLAB's xcorr function to compute the maximum cross-correlation coefficient comparing the average responses to tones of 1.3, 1.625, and 2.6 kHz and clicks to the average responses to tones at each frequency tested. These analyses were replicated at stimulus levels of 70, 75, 80, and 85 dB SPL. We selected these particular levels for analysis because they reliably elicited robust responses from most individuals at most frequencies. To rule out the possibility that differences in cross correlations resulted from frequency-dependent differences in sensation level (SL), we performed additional cross-correlation analyses using the average responses recorded at a common sensation level of approximately 10 dB SL. We defined 10 dB SL as the stimulus level nearest to 10 dB above the average visually detected threshold across all individuals for that stimulus (see below).
We measured ABR amplitude and latency in responses to all combinations of stimulus and level at which a visually detectable ABR waveform was present. The first peak (P1) and the subsequent trough (N1) constituted the only visible, event-related deflection of the ABR waveform that were observed consistently in responses to both clicks and tones across all subjects. An example of these measurements is illustrated in Fig. 1c. We measured ABR amplitude (hereafter “amplitude”) as the absolute voltage difference (in μV) between P1 and N1 (i.e., the peak-to-trough voltage; Fig. 1c). We measured ABR latency (hereafter “latency”) as the time from when the stimulus arrived at the frogs’ ears to P1 (Fig. 1c). We calculated 0.88 ms as the time required for sound to travel the 30-cm distance between the speaker and the frogs’ ears given the range of temperatures recorded in the test chamber. Values of amplitude and latency in response to each stimulus were averaged across the two replicates for statistical analysis.
Threshold determination and ABR audiograms
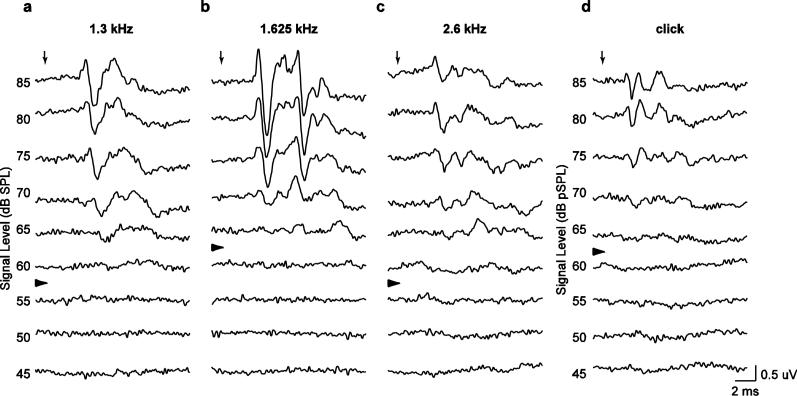
We assessed auditory sensitivity using two methods of threshold estimation. First, two experienced observers independently determined ABR thresholds based on visual detection (e.g., Brittan-Powell et al. 2002, 2005, 2010b; Brittan-Powell and Dooling 2004; Lohr et al. 2013). We plotted the responses to each tone or click within a stimulus train in order of descending stimulus level, as illustrated for a single individual in Fig. 2. We operationally defined threshold as the arithmetic mean of the lowest stimulus level at which an ABR waveform was visible and the next lowest intensity (Fig. 2, arrowheads). Since stimulus level varied in 5-dB steps, threshold was effectively the sound pressure level 2.5 dB (one-half step) below the lowest stimulus level at which a response could be visually detected. We used a 10-ms window beginning 2 ms after stimulus onset for visual detection of responses. Threshold differences between the two observers were small (across all estimates: mode = 0.0 dB, median = 0.0 dB, and mean = 0.9 ± 3.8 dB), and the agreement between observers was quite high (intraclass correlation: r = 0.89). Below, we report thresholds that were averaged over the two stimulus replicates and across both observers.
Fig. 2.
Representative recordings of ABRs from a single individual in response to tones at a 1.3 kHz, b 1.625 kHz, c 2.6 kHz, and in response to d clicks presented at different sound pressure levels. Downward pointing arrows depict arrival times of sound at the tympanic membranes. The right-pointing arrowheads depict the visually detected thresholds for each frequency or for clicks
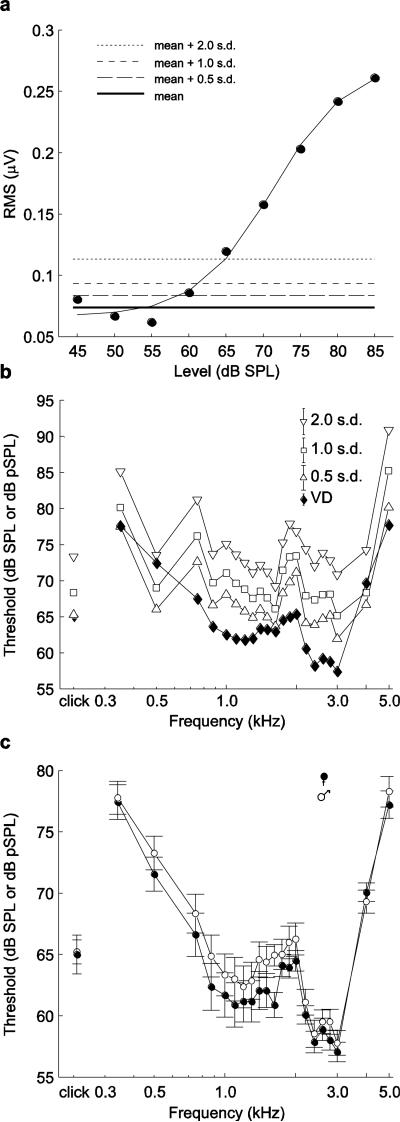
As a second method of threshold estimation, we performed an automated, objective analysis in which we compared the predicted RMS amplitude of the ABR in response to a stimulus to the RMS amplitude of the biological signal determined when no stimulus was presented. We separately computed predicted RMS amplitudes for each frog and each stimulus as follows. Using MATLAB's fminsearch optimization function, we minimized the sum of squares to find the best-fit sigmoid curve fitting the actual RMS amplitudes of the biological signal computed over a 10-ms window between 2-12 ms after stimulus onset, averaged between replicates, and plotted as a function of stimulus level (see Fig. 5a in the Results section for an example). These fits had a mean (± s.d.) R2 of 0.94 ± 0.13 across subjects (N = 35). We determined the ABR threshold as the lowest stimulus level at which the fitted curve of predicted RMS amplitudes first exceeded a fixed threshold criterion. This criterion was based on the mean and standard deviation of the RMS amplitudes of the biological signal recorded from the same animal when no acoustic stimulus was broadcast. We estimated these two statistical parameters separately for each individual by computing the RMS amplitudes of the biological signal in six 10-ms analysis windows (60 ms total) that were recorded in the absence of a stimulus at the beginning (three windows) and ending (three windows) of each recording session. This procedure allowed us to estimate for each subject a mean and standard deviation for the “baseline” RMS amplitude of the neural recording in the absence of acoustic stimulation by tones or clicks. We explored three criterion values corresponding to 0.5, 1.0, and 2.0 standard deviations above the mean baseline RMS amplitude and calculated the threshold for each as the minimum predicted RMS amplitude value exceeding each criterion value.
Fig. 5.
a A representative example of threshold detection based on predicted values of RMS amplitudes of ABRs in response to stimuli presented at different levels. RMS amplitudes of responses were computed over a 10 ms analysis window that began when the stimulus arrived at the tympanic membranes. Depicted here is the best-fit sigmoid curve fit to RMS data for responses from one frog to tones of 2.6 kHz. Thresholds determined from the fits for each stimulus for each frog were averaged to construct the audiogram based on automatically detected thresholds plotted in b. Different threshold criteria based on the mean (solid bold line) and s.d. of the RMS amplitude of the biological signal recorded in the absence of a stimulus are indicated by dashed lines. b Comparison of audiograms and click-evoked response thresholds based on visually detected (VD) thresholds (filled diamonds) and automatically determined thresholds based on different criteria (open triangles and squares). To improve clarity of the plot, error bars are not shown for individual data. Error bars in the legend depict the s.e.m. averaged across frequencies and clicks for each threshold determination method. c Comparisons of mean ± s.e.m. visually detected thresholds for males (open circles) and females (filled circles) for responses to tones of different frequencies and to clicks
Statistical analyses
We used repeated measures analysis of covariance (ANCOVA) to investigate the effects of frequency, level, sex, and size on tone-evoked and click-evoked responses. Estimates of threshold were available for all subjects in response to clicks and to tones at all 21 frequencies tested. This was not the case for our quantitative measures of ABR amplitude and latency, for which measures were only available for stimuli that produced a visually detectable ABR. Hence, sub-threshold stimulus levels resulted in missing values of amplitude and latency, which we dealt with using a two-step procedure. First, we limited our statistical analyses of ABR amplitude and latency to reduced datasets that included stimuli with which all subjects were tested and that elicited visually detectable responses from a majority of subjects. For tone-evoked responses, we included only the 17 frequencies between 0.75 kHz and 3.0 kHz (inclusive). For both tone-evoked and click-evoked responses, we included only the five stimulus levels between 65 dB and 85dB SPL (inclusive). This procedure reduced the proportion of missing values of amplitude and latency to 10% for tone-evoked responses and 14% for click-evoked responses. Second, we used multiple imputation, in which Monte Carlo methods are used to simulate the remaining missing values m times (Rubin 1976). A small number of imputations (e.g. m = 3-5) is generally used, as increasing this number does not significantly increase the accuracy of the estimated values (Rubin 1976; Schafer and Olsen 1998; Schafer 1999). However, a larger number of imputations is associated with greater statistical power (Graham et al. 2007), and so we used 20 imputations (m = 20) for each of our four reduced datasets.
We performed separate factorial ANCOVAs for amplitude and latency and for each imputed dataset. For tone-evoked responses, each analysis consisted of a 17 frequency (within) × 5 level (within) × 2 sex (between) ANCOVA with subject size included as the covariate. For click-evoked responses each analysis consisted of a 5 level (within) × 2 sex (between) ANCOVA, with size as the covariate. Values of amplitudes and latencies were log-transformed to achieve normality prior to analysis. For each response variable, we report the mean and range of the resulting F statistics, effect sizes (partial η2), and P values calculated over the 20 imputed datasets for that variable. We compared visually detected ABR thresholds in response to tones and clicks using separate repeated measures ANCOVAs. Our analysis of tone-evoked responses consisted of a 21 frequency (within) × 2 sex (between) ANCOVA with subject size as the covariate. We compared thresholds for click-evoked responses between sexes with a univariate ANCOVA having sex as the single between-subjects factor (2 levels) and subject size as the covariate.
For all repeated measures analyses, we report P-values for omnibus tests having more than a single numerator degree of freedom based on the Greenhouse and Geisser (1959) correction method. In all ANCOVAs, the covariate of size was based on subject mass and was centered around the mean mass by subtracting the mean from each subject's mass prior to analysis. We employed a significance criterion of α = 0.05 for all ANCOVAs. For multiply imputed data, an effect was considered significant if the mean P-value was below 0.05.
Results
ABR characterization
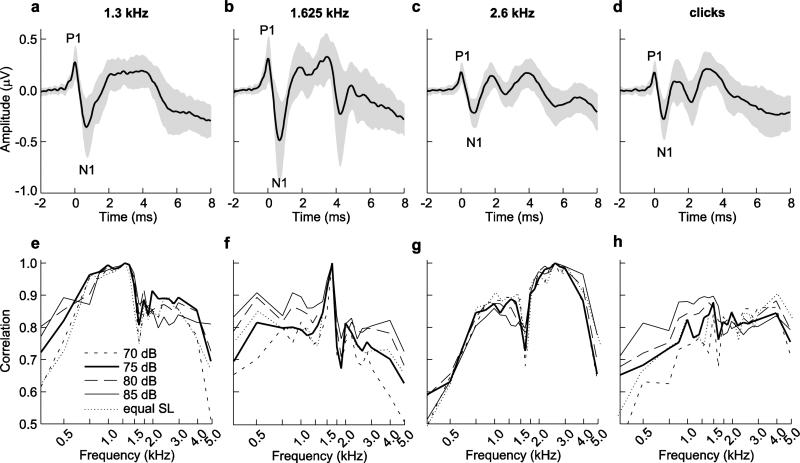
Average ABR waveforms evoked by 75-dB broadcasts of tones (1.3, 1.625, and 2.6 kHz) and clicks are illustrated in Fig. 3a-d. In many cases, the N1 deflection was much larger (relative to baseline) than that of the preceding P1 deflection (Fig. 3a-d; see also Fig. 2). The presence of additional peaks after P1-N1 was variable, both across animals and across stimuli (Fig. 3a-d). For example, responses to the 1.3 kHz tone (Fig. 3a) commonly had one prominent peak (P1) followed by a broader peak or plateau, while responses to the 2.6 kHz tone (Fig. 3c) typically included P1 and two additional prominent peaks. Responses at the intermediate frequency of 1.625 kHz (Fig. 3b) had a prominent P1 followed by a multi-peaked plateau and resembled a combination of the responses observed at 1.3 and 2.6 kHz. Responses to clicks were generally more similar to those elicited by the 2.6 kHz tones in having a pronounced P1 followed by two or more additional peaks (cf. Fig. 3c, 3d).
Fig. 3.
a-d Average traces temporally aligned to P1 (time = 0 ms) in response to tones of 75 dB SPL at frequencies of a 1.3 kHz, b 1.625 kHz, and c 2.6 kHz and to d clicks of 75 dB pSPL. Shaded areas depict ± 1 s.d. e-h Cross correlation coefficients between the average response to tones presented at frequencies of e 1.3 kHz, f 1.625 kHz, and g 2.6 kHz or to h clicks and average responses to all tone frequencies. Legend in e applies to e-h and is in units of dB SPL (for tones) or dB pSPL (for clicks)
The results of the cross-correlation analyses are depicted in Fig. 3e-h. The patterns of cross-correlations were broadly similar across the range of 70 dB to 85 dB signal levels and at ~10-dB SLs. As depicted in Fig. 3e-h, comparisons of tone-evoked responses revealed the presence of two different waveform shapes, one characteristic of responses to lower tone frequencies (between 0.75 kHz and 1.5 kHz) and a second characteristic of responses to higher frequencies (between 1.75 kHz and 4 kHz), with a sharp transition between these two shapes (between 1.5 kHz and 1.75 kHz). Consider first responses to the 1.3-kHz tone (Fig. 3e). The ABRs evoked by the 1.3-kHz tone were most similar to those elicited by tones with frequencies ranging from 0.75 kHz to about 1.5 kHz, as indicated by cross-correlation coefficients near 1.0. Correlations were markedly weaker at frequencies below 0.75 kHz (Fig. 3e). Between 1.5 and 1.75 kHz, there was a sharp transition to somewhat lower correlation coefficients that remained similar up to about 4.0 kHz, above which correlations became even weaker (Fig. 3e). A near mirror image of this general pattern was found in correlations with responses to the 2.6-kHz tone (cf. Fig. 3e and 3g). Responses to the 2.6-kHz tone (Fig. 3g) were most similar to those across a range of frequencies extending between about 1.75 and 4.0 kHz. Cross-correlation coefficients dropped off sharply above 4.0 kHz, and there was, again, a distinctive transition to lower correlation coefficients below 1.5 kHz. Coefficients decreased even further at frequencies below about 0.75 kHz (Fig. 3g). The ABR waveforms evoked by the intermediate tone frequency of 1.625 kHz (Fig. 3f) were most similar to those at 1.5 kHz, with somewhat lower coefficients at frequencies below 1.5 kHz. Correlations were generally even weaker at frequencies of 1.75 kHz and above (Fig. 3f). Coefficients for the cross-correlations between click-evoked responses and the responses to tones at different frequencies (Fig. 3h) were somewhat more variable as a function of stimulus level than the correlations observed between tones (Fig. 3e-g). Generally, correlations between click-evoked and tone-evoked responses (Fig. 3h) tended to be somewhat higher and more consistent across stimulus levels for responses to higher tone frequencies. Effects of frequency, level, sex, and size
ABR amplitude
Repeated measures ANCOVAs revealed significant differences in the amplitude of tone-evoked responses due to the main effects of frequency and level (Table 1). The main effect of frequency was moderately large (partial η2 = 0.45, Table 1), while the main effect of stimulus level had the largest effect size in the ANCOVA model (partial η2 = 0.84, Table 1). The two-way interactions of frequency × level and frequency × sex were also significant, but were associated with smaller effect sizes (partial η2 ≤ 0.12, Table 1).
Table 1.
Repeated measures analysis of covariance (ANCOVA) for ABR amplitude in response to tones and clicks. Shown are means (and ranges) for F statistics, P values, and effect sizes (partial η2) from analysis of the 20 imputed datasets. Bold values indicate variables in which the mean P-value ≤ 0.05
| Stimulus | Effect | df | F | P | Partial η2 |
|---|---|---|---|---|---|
| Tones | frequency | 16, 512 | 26.6 (22.7 - 29.8) | <0.001 (all <0.001) | 0.45 (0.42 - 0.48) |
| level | 4, 128 | 169.8 (137.6 - 204.4) | <0.001 (all <0.001) | 0.84 (0.81 - 0.86) | |
| sex | 1, 32 | 1.1 (0.8 - 1.4) | 0.306 (0.243 - 0.370) | 0.03 (0.03 - 0.04) | |
| size | 1, 32 | 0.1 (<0.1 - 0.2) | 0.752 (0.653 - 0.825) | <0.01 (<0.01 - 0.01) | |
| frequency × level | 64, 2048 | 4.3 (3.7 – 5.0) | <0.001 (all <0.001) | 0.12 (0.10 - 0.14) | |
| frequency × sex | 16, 512 | 3.8 (2.9 - 4.6) | 0.015 (0.006 - 0.042) | 0.11 (0.08 - 0.13) | |
| frequency × size | 16, 512 | 0.9 (0.6 - 1.4) | 0.440 (0.245 - 0.603) | 0.03 (0.02 - 0.04) | |
| level × sex | 4, 128 | 0.2 (<0.1 - 0.6) | 0.745 (0.496 - 0.962) | 0.01 (<0.01 - 0.02) | |
| level × size | 4, 128 | 0.6 (0.3 - 1.2) | 0.531 (0.299 - 0.692) | 0.02 (0.01 - 0.04) | |
| frequency × level × sex | 64, 2048 | 1.4 (1.1 - 1.8) | 0.156 (0.040 - 0.362) | 0.04 (0.03 - 0.05) | |
| frequency × level × size | 64, 2048 | 1.0 (0.7 - 1.3) | 0.473 (0.194 - 0.767) | 0.03 (0.02 - 0.04) | |
| Clicks | level | 4, 128 | 86.3 (31.6 - 155.9) | <0.001 (all <0.001) | 0.70 (0.50 - 0.83) |
| sex | 1, 32 | 1.3 (0.2 - 2.6) | 0.291 (0.117 - 0.651) | 0.04 (0.01 - 0.08) | |
| size | 1, 32 | 0.2 (<0.1 - 1.0) | 0.723 (0.321 - 0.972) | 0.01 (<0.01 - 0.03) | |
| level × sex | 4, 128 | 1.9 (0.3 - 5.5) | 0.253 (0.011 - 0.658) | 0.05 (0.01 - 0.15) | |
| level × size | 4, 128 | 1.1 (0.1 - 6.6) | 0.534 (0.005 - 0.898) | 0.03 (<0.01 - 0.17) |
The effects of frequency, level, and sex on ABR amplitudes are illustrated in Figure 4 for responses averaged over all subjects in contour plots (Fig. 4a) and for each sex separately as iso-intensity plots (Fig. 4b). For responses to tones across levels, ABR amplitudes varied between 0.4 and 2.1 μV. There was a sharp discontinuity in ABR amplitude in an intermediate frequency range from about 1.5 kHz to 1.75 kHz (indicated by the solid arrows in Fig. 4a, 4c). At a given stimulus level, amplitudes tended to be larger for frequencies below this frequency range (< 1.5 kHz) compared with frequencies above it (> 1.75 kHz) and highest within this intermediate frequency range (Fig. 4a, 4b). As illustrated in Figure 4a-b, ABR amplitudes increased as a function of increasing stimulus level, but the rate of increase in ABR amplitude as a function of stimulus level varied across frequencies, which accounts for the significant two-way interaction between frequency and level (Table 1). There was generally little difference between the ABR amplitudes of males and females in response to tones at most frequencies, though a more notable difference occurred at intermediate frequencies (Fig. 4b). This trend accounts for the relatively weak (partial η2 = 0.11) but significant two-way interaction between frequency and sex (Table 1). We found no indication that the amplitudes of tone-evoked responses varied as a function of subject body size (Table 1).
Fig. 4.
Characterization of the ABR in terms of a-b amplitude (absolute voltage difference between P1 and N1) and c-d latency (time to P1 from sound arrival at tympanic membranes). a & c Mean amplitude and latency (averaged over all individuals) depicted in the form of a contour plot across all frequencies and all levels. Arrows indicate the range of frequencies (1.5 – 1.75 kHz) within which there is a sharp discontinuity in the values of the response measure. b & d Mean (±s.e.m.) amplitude and latency of click-evoked responses and tone-evoked responses across frequencies at five stimulus levels. Data are shown separately for males (open circles) and females (filled circles). The data depicted in b and d represent reduced datasets that included responses to clicks at levels of 65 to 85 dB pSPL and tones of frequencies from 0.75 kHz to 3.0 kHz presented at levels of 65 dB to 85 dB SPL. Plotted data were pooled across multiple imputations of the reduced datasets (see text). Values for some click-evoked responses are slightly displaced along the x-axis to reveal symbols otherwise hidden
Repeated measures ANCOVAs for the amplitudes of click-evoked responses revealed a large and significant effect of stimulus level (Table 1). No other effects or interactions in the ANCOVA models were significant. On average, click-evoked responses were typically smaller than tone-evoked responses. At a given stimulus level, the amplitudes of click-evoked responses were similar to the amplitudes of the smallest tone-evoked responses to tones (e.g. tone frequencies > 2.0 kHz). Mean amplitudes increased as a function of stimulus level from 0.5 μV at 65 dB to 1.0 μV at 85 dB (Fig. 4a, 4b). The effects of subject sex and the covariate of body size were quite small compared with the effect of stimulus level (Table 1).
ABR latency
In our ANCOVAs for the latency for tone-evoked responses, there were significant main effects of frequency, level, and sex, and significant two-way interactions of frequency × level and frequency × sex (Table 2). Evaluation of effect sizes, however, indicated that the main effects of frequency (partial η2 = 0.77) and level (partial η2 = 0.89) were much more important than other effects in the model (partial η2 ≤ 0.18) in determining the latency of tone-evoked responses (Table 2).
Table 2.
Repeated measures analysis of covariance (ANCOVA) for ABR latency in response to tones and clicks. Shown are means (and ranges) for F statistics, P values, and effect sizes (partial η2) from analysis of the 20 imputed datasets. Bold values indicate variables in which the mean P-value ≤ 0.05
| Stimulus | Effect | df | F | P | Partial η2 |
|---|---|---|---|---|---|
| Tones | frequency | 16, 512 | 106.3 (96.4 - 115.7) | <0.001 (all <0.001) | 0.77 (0.75 - 0.78) |
| level | 4, 128 | 258.6 (192.7 - 365.1) | <0.001 (all <0.001) | 0.89 (0.86 - 0.92) | |
| sex | 1, 32 | 6.9 (5.1 - 8.3) | 0.014 (0.007 - 0.031) | 0.18 (0.14 - 0.21) | |
| size | 1, 32 | <0.1 (<0.1 - 0.1) | 0.904 (0.792 - 0.997) | <0.01 (all <0.01) | |
| frequency × level | 64, 2048 | 2.7 (1.7 - 3.9) | 0.012 (<0.001 - 0.079) | 0.08 (0.05 - 0.11) | |
| frequency × sex | 16, 512 | 2.3 (1.4 - 3.1) | 0.041 (0.004 - 0.201) | 0.07 (0.04 - 0.09) | |
| frequency × size | 16, 512 | 0.8 (0.4 - 1.2) | 0.569 (0.316 - 0.897) | 0.03 (0.01 - 0.04) | |
| level × sex | 4, 128 | 0.7 (0.1 - 2.5) | 0.569 (0.087 - 0.948) | 0.02 (<0.01 - 0.07) | |
| level × size | 4, 128 | 1.1 (0.2 - 2.7) | 0.417 (0.079 - 0.868) | 0.03 (0.01 - 0.08) | |
| frequency × level × sex | 64, 2048 | 1.2 (0.8 - 1.8) | 0.345 (0.062 - 0.649) | 0.04 (0.02 - 0.05) | |
| frequency × level × size | 64, 2048 | 1.1 (0.7 - 1.8) | 0.365 (0.056 - 0.713) | 0.03 (0.02 - 0.05) | |
| Clicks | level | 4, 128 | 23.2 (6.7 - 34.9) | <0.001 (<0.001 - 0.006) | 0.41 (0.17 - 0.52) |
| sex | 1, 32 | 0.1 (<0.1 - 0.7) | 0.768 (0.410 - 0.991) | <0.01 (<0.01 - 0.02) | |
| size | 1, 32 | 3.6 (1.4 - 6.5) | 0.087 (0.016 - 0.238) | 0.10 (0.04 - 0.17) | |
| level × sex | 4, 128 | 3.2 (0.8 - 6.7) | 0.114 (0.002 - 0.474) | 0.09 (0.02 - 0.17) | |
| level × size | 4, 128 | 2.2 (0.1 - 5.7) | 0.265 (0.011 - 0.941) | 0.06 (<0.01 - 0.15) |
Contour plots and iso-intensity plots of ABR latency are depicted in Figure 4c and 4d, respectively. In response to tones, latencies typically ranged between 3 and 7 ms and decreased as a function of increasing frequency, particularly in the range of frequencies between 0.35 and 1.5 kHz. As with amplitudes, a discontinuity in the latencies of tone-evoked responses occurred in the frequency region between 1.5 and 1.75 kHz (Fig. 4c, 4d). For a given stimulus level, latencies below 1.5 kHz were generally longer than those above 1.75 kHz. Latencies decreased as stimulus level increased (Fig. 4c, 4d). Latencies were slightly shorter for females compared to males, particularly at tone frequencies below ~1.75 kHz (Fig. 4d), resulting in the significant main effect of sex and its interaction with frequency. There was no indication that ABR latencies varied as a function of subject body size.
In the ANCOVA models for the latency of click-evoked responses, only the effect of stimulus level was significant (Table 2). Latencies for click-evoked responses were shorter than those for tone-evoked responses and were in the range of 2 to 3 ms (Fig. 4c, 4d). As with tone-evoked responses, the latencies of click-evoked responses decreased with increasing stimulus level (Fig. 4c, 4d). Neither sex nor body size influenced latencies in response to clicks.
ABR thresholds
General patterns of threshold differences across most frequencies were broadly similar between the different methods of threshold estimation examined here; that is, all resulting audiograms had the same general shape (Fig. 5b). An exception to this generalization occurred at 0.5 kHz, which corresponded with a “dip” in automated thresholds that was not apparent in the visually detected thresholds. At present, it is not clear what is responsible for this difference between detection methods. As would be expected, increasing the threshold criterion for automated detection of responses from 0.5 s.d. to 2.0 s.d. resulted in progressively higher threshold estimates (Fig. 5a, 5b). The audiogram generated using a threshold criterion of 0.5 s.d. most closely matched that generated using visual threshold detection, with thresholds averaging about 5 dB higher across frequencies for automated thresholds (Fig. 5b). The mean (± s.e.m. here and elsewhere) threshold in response to clicks using a criterion of 0.5 s.d. was 65.3 ± 0.9, which was close to the average visually detected threshold of 65.1 ± 0.9 (Fig. 5b).
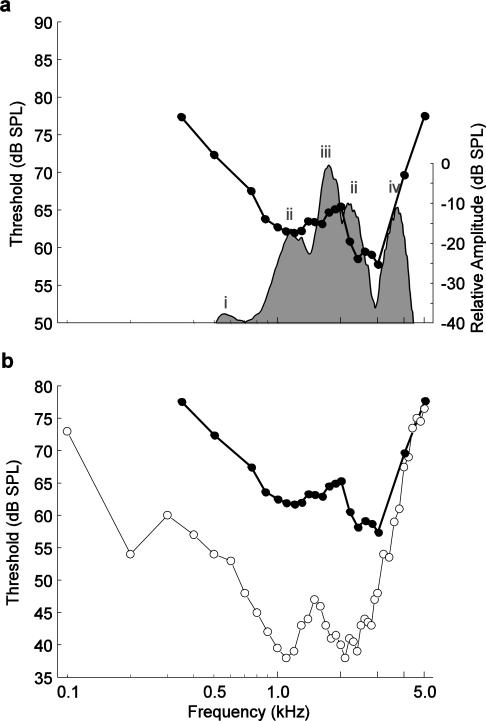
For clarity and brevity, and because of general similarities in shape, we focus here on interpreting the audiogram based on visually detected thresholds. One region of best sensitivity was broadly centered around 1.2 kHz (between 0.875 and 1.5 kHz), and a second was centered around 2.6 kHz (between 2.2 and 3.0 kHz). Visually detected thresholds in the lower frequency region ranged between 61 to 63 dB and were generally 2.5 to 4.4 dB higher than thresholds for the higher frequency region. Visually detected thresholds in a mid-frequency region between 1.5 and 2.0 kHz were higher than the most sensitive frequencies by about 5 to 8 dB. The slopes of the changes in thresholds between this less sensitive mid-frequency region and the adjacent, more sensitive regions at lower and higher frequencies were, respectively, 3.9 dB/octave (computed between 1.1 to 2.0 kHz) and −13.6 dB/octave (computed between 2.0 to 3.0 kHz). At the extreme low (< 0.875 kHz) and high (> 3.0 kHz) frequencies tested, visually detected thresholds increased to 15 to 20 dB over peak sensitivity. The changes in threshold occurred with a slope of −10.6 dB/octave below 0.875 kHz and 27.6 dB/octave above 3.0 kHz.
For tone-evoked ABRs, a repeated measures ANCOVA revealed significant differences in threshold related to differences in frequency (F20, 640 = 87.8, P < 0.001, partial η2 = 0.73), but not sex (F1, 32 = 1.0, P = 0.338, partial η2 = 0.03) or size (F1, 32 = 0.2, P = 0.701, partial η2 = 0.01). The frequency × sex (F20, 640 = 0.7, P = 0.595, partial η2 = 0.02) and frequency × size (F20, 640 = 0.9, P = 0.447, partial η2 = 0.03) interactions were also not significant. Averaged across frequencies, females had slightly lower visually detected thresholds compared with males (Fig. 5c; females: 63.8 ± 0.4 dB; males: 64.9 ± 0.4 dB). This non-significant trend for a sex difference was slightly more pronounced at frequencies below 2.2 kHz (Fig. 5c). For click-evoked responses, average thresholds for males and females differed by less than 1.5 dB and there was no significant effect of sex (Fig. 5c; F1, 32 < 0.1, P = 0.898, partial η2 < 0.01) or the covariate of size (F1, 32 = 0.1, P = 0.821, partial η2 < 0.01).
Discussion
ABR characterization
The ABRs we recorded in Cope's gray treefrogs were characterized by a series of positive and negative deflections in amplitude. This general morphology, which is consistent with ABRs recorded across a wide range of taxa (Walsh et al. 1986; Popov and Supin 1990; Boettcher et al. 1993; Brittan-Powell et al. 2002, 2010b; Higgs et al. 2002; Lucas et al. 2002), is also similar to that described from invasive studies of evoked potentials in frogs (Corwin et al. 1982; Seaman 1991; Carey and Zelick 1993; Katbamna et al. 2006b). While all ABRs in gray treefrogs shared the features of a distinctive P1 and N1, cross-correlation analyses suggested two discrete classes of tone-evoked waveforms, typified by responses to 1.3 kHz tones and 2.6 kHz tones. A low-frequency class included responses to frequencies below about 1.5 kHz, while a high frequency class included responses to frequencies above about 1.75 kHz. To rule out the possibility that differences in waveforms resulted from frequency-dependent differences in sensation level (SL), we compared the results for responses at a constant stimulus level to those at a common sensation level (~10 dB SL). The general patterns were still evident in the analysis of responses at a common sensation level. This result confirms that frequency-dependent differences in shape of the ABR waveform were not merely a reflection of frequency-dependent differences in sensitivity. Quantitative analyses of the effects of stimulus frequency and level on ABR amplitudes and latencies revealed discontinuities at intermediate frequencies that also support a division of responses into two classes. We suggest these two classes reflect differences in responses evoked by frequencies encoded primarily by the separate sensory papillae in the frogs’ inner ear most sensitive to airborne sounds, the amphibian papilla (AP) and basilar papilla (BP). In most anuran species studied thus far, the AP tends to be sensitive to frequencies up to 1.0-2.0 kHz and the BP tends to be sensitive to frequencies higher than 1.0-2.0 kHz (Gerhardt and Schwartz 2001; Zakon and Wilczynski 1988). Waveforms for click-evoked responses were somewhat more similar to the 2.6 kHz frequency class. This result is consistent with reports from human studies that higher frequencies (1.0 to 4.0 kHz) are responsible for the generation of the click-evoked ABR (Hall 2007). The waveforms of the responses to the intermediate frequency of 1.625 kHz were not as easily classified as belonging to the low-frequency or high-frequency class, appearing instead to be intermediate in shape between waveforms of these two classes. We suggest suprathreshold tones at this intermediate frequency were able to excite both inner ear papillae simultaneously. Such an interpretation is consistent with previous behavioral data examining the preferences of female gray treefrogs for spectral call properties (Gerhardt 2005). From these ABR waveform data, we deduce the gray treefrog AP is sensitive to frequencies less than approximately 1.75 kHz and the BP is sensitive to frequencies above about 1.5 kHz.
ABR latencies are the main evidence used in determining the generators of ABR waves. The generator for P1 of the ABR in all animals is generally considered to be the VIIIth nerve (Seaman 1991; Carey and Zelick 1993; Brittan-Powell et al. 2002; Lucas et al. 2002). Consistent with this view, the ABR latencies we recorded in gray treefrogs ranged from 3 to 7 ms for tone-evoked responses and 2 to 3 ms for click-evoked responses. These latency values are similar to latencies reported from single-unit recordings of VIIIth nerve fibers in other frog species (Zakon and Capranica 1981; Feng 1982; Hillery and Narins 1984; Stiebler and Narins 1990). ABR latencies in this study were also similar to those reported in more invasive studies of evoked potentials of other frogs, including R. catesbeiana (2.5 ms to 4 ms; Seaman 1991), R. pipiens (2 ms to 4 ms; Carey and Zelick 1993), and X. laevis (5 ms to 8 ms; Katbamna et al. 2006b). The ABR latencies reported here are also within the range of those reported for fish and reptiles, but perhaps slightly longer, on average, than those reported for mammals and birds. For example, our range of tone-evoked latencies (3 to 7 ms) is similar to the 2 to 7 ms range reported for fish (Kenyon et al. 1998) and overlaps the 6 to 10 ms range reported for alligators (Higgs et al. 2002), while latencies were 1.5 to 3 ms in gerbils (Boettcher et al. 1993) and cats (Walsh et al. 1986) and 1.5 to 4 ms in birds (Brittan-Powell et al. 2002; Henry and Lucas 2009). Our click-evoked latencies of 2 to 3 ms in gray treefrogs were similar to the 2 to 4 ms latencies reported for Tokay geckos and green anoles (Brittan-Powell et al. 2010b), but were generally longer than those reported for mammals (Walsh et al. 1986; Klishin et al. 1990) and birds (Brittan-Powell et al. 2002; Lucas et al. 2002). Previous studies showed a negative correlation between ABR latency and body temperature, which suggests that the shorter latencies of birds and mammals might, in part, be attributable to endothermy (Higgs et al. 2002).
Effects of frequency, level, sex, and size
ABR amplitude and latency
For responses to tones at a given frequency and to clicks, amplitude increased with increasing level while latency decreased, consistent with ABR recordings in many other animals (Kenyon et al. 1998; Brittan-Powell et al. 2002, 2005; Nachtigall et al. 2007; Zhang et al. 2012) and with invasive recordings of brainstem evoked potentials in other frogs (Seaman 1991; Carey and Zelick 1993; Katbamna et al. 2006b). At a given signal level, ABR amplitudes tended to be higher and latencies longer for responses to frequencies within the putative range of the AP (< 1.75 kHz) compared with those within the putative range of the BP (> 1.5 kHz). These differences were highlighted by sharp discontinuities in both measures between the values for frequencies below about 1.5 kHz and those above 1.75 kHz. The largest amplitudes were found within this intermediate frequency range (1.5 – 1.75 kHz). ABR amplitude should depend, in part, on the number of units that respond to a stimulus. Frogs have a larger number of fibers innervating the AP than BP (Will and Fritzsch 1988), which might lead one to speculate that this difference in numbers of fibers could account for the differences in amplitudes. However, only a subset of the fibers innervating the tonotopically-organized AP is sensitive to any given frequency. Hence the total number of AP fibers that respond to each stimulus, while unknown, is certainly less than the total number of fibers innervating the AP. Thus, the contribution of fiber number to ABR amplitude for frequencies within the range of the AP and BP in this species is unclear. However, since the intermediate frequencies of 1.5 and 1.75 kHz probably excite fibers from both auditory papillae, the exceptionally large amplitudes measured at these frequencies likely result from summation of responses of fibers from each papilla. The differences in latency between the two frequency ranges were consistent with previous work showing that fibers arising from the AP tend to have slower responses than those innervating the BP (Zakon and Capranica 1981; Feng 1982; Hillery and Narins 1984; Stiebler and Narins 1990). Within the range of the AP, latencies decreased as a function of increasing frequency, a result that is consistent with reports from mammals (Gorga et al. 1988; Ramsier and Dominy 2010) and birds (Brittan-Powell et al. 2002; Henry and Lucas 2008; Caras et al. 2010). In humans, it is assumed that this relationship reflects the traveling wave in the cochlea (Hall 2007). The dependence of latency on frequency in the ABRs of gray treefrogs is consistent with previous evidence for a traveling wave within the AP of anurans (Hillery and Narins 1984).
Sex differences in amplitudes and latencies were generally quite small and inconsistent across frequencies in responses to tones and negligible in response to clicks. When there was a sex difference, the trend was for females to have slightly larger ABR amplitudes and slightly shorter latencies. Overall, the effect sizes associated with sex differences in amplitude and latency were much smaller than those associated with stimulus frequency and level. While in the human ABR literature it has long been established that responses in women have larger amplitudes and shorter latencies than those in men (Jerger and Hall 1980), our results are more consistent with the lack of evidence for strong and consistent effects of sex on ABRs in nonhuman animals (Munro et al. 1997; Zhou et al. 2006; Caras et al. 2010, but see Gall et al. 2011).
We found no evidence to suggest body size influenced either the absolute magnitudes of ABR amplitude or latency or how these properties changed in response to different stimuli. Because sex and body size are correlated in treefrogs, with females being slightly larger than males on average, this lack of a significant size effect suggests any apparent effects of sex on ABR characteristics were independent of sex-dependent size differences.
ABR thresholds
Differences in ABR thresholds were influenced by frequency for tone-evoked responses, but there were no effects of sex or size on ABR thresholds. The ABR audiogram had peaks in sensitivity around 1.2 kHz and 2.6 kHz. These frequencies correspond to the tuning of the AP and BP (Hillery 1984b; Gerhardt 2005), respectively, and are also close to the average frequencies in male advertisement calls (Schrode et al. 2012). Thresholds increased above and below these frequencies. One might expect increased sensitivity to frequencies between the two peaks noted here, because ABR amplitudes were highest in this frequency region. However, neither of the papillae is tuned to these intermediate frequencies; rather, the large amplitudes observed to occur at intermediate frequencies are likely attributable to summation of the responses of the two auditory papillae at supra-threshold levels. Thus, at lower stimulus levels, intermediate frequencies do not stimulate either papilla, rendering these stimuli undetectable. These results are broadly consistent with predictions of the matched-filter hypothesis (Capranica and Moffat 1983), which suggests that frogs’ inner ear organs are maximally sensitive to the frequencies emphasized in conspecific calls. For example, gray treefrogs often communicate in spectrally complex, mixed-species choruses, such as the typical Minnesota chorus depicted in Figure 6a (shaded area). In this example, in addition to the spectral energy in gray treefrog calls (1.25 and 2.5 kHz), leopard frogs calls contribute energy at around 0.6 kHz, American toad (Bufo americanus) calls correspond to the spectral peak around 1.8 kHz in the chorus, and boreal chorus frog (Pseudacris maculata) calls have a dominant frequency of about 3.5 kHz. Peaks in sensitivity in the ABR audiogram overlap parts of the chorus that correspond to conspecific calls, while somewhat higher thresholds occur at the frequencies emphasized in heterospecific calls (e.g. 1.6-1.8 kHz and ~3.5 kHz). This matched filtering has long been considered a mechanism to improve the detectability of conspecific calls in mixed-species choruses by increasing the signal-to-noise ratio between conspecific and heterospecific signals.
Fig. 6.
a Visually detected ABR thresholds from this study (unweighted means averaged across all individuals; filled circles) compared with the frequency spectrum of a mixed-species chorus recorded in central Minnesota during the peak of the gray treefrog breeding season (shaded area). Peaks of the chorus spectrum are contributed by i) northern leopard frogs (Rana pipiens), ii) Cope's gray treefrogs (Hyla chrysoscelis), iii) American toads (Bufo americanus), and iv) boreal chorus frogs (Pseudacris maculata). b Visually detected ABR thresholds from this study (filled circles) compared with average multiunit thresholds from invasive recordings from the midbrain of Cope's gray treefrogs (open circles) reported by Hillery (1984b)
Recordings from the VIIIth nerve of frogs have not generally detected sex or size differences in the thresholds of auditory nerve fibers (Frishkopf et al. 1968; Elliott et al. 2007). Consistent with these results, we found no influence of sex or size on ABR thresholds. Previous studies of VIIIth nerve fibers found that the BP of female frogs tend to have a best frequency that is lower than the corresponding best frequency of males in many (but not all) species, while AP tuning is not different between the sexes (Narins and Capranica 1976; Zakon and Wilczynski 1988; Wilczynski et al. 1992, but see Elliott et al. 2007). However, we saw little evidence of an effect of sex on tuning in our audiograms, suggesting that H. chrysoscelis is not a species in which BP tuning varies strongly between the sexes.
Comparison of ABR and midbrain audiograms
In Fig. 6b, we compare our ABR audiogram (based on visual detection) to an audiogram derived from multiunit recordings in the midbrain of gray treefrogs (Hillery 1984b). The most striking difference between the ABR and midbrain audiograms is the overall difference in threshold. ABR thresholds were, on average, 15 to 25 dB higher than thresholds derived from midbrain recordings. It is common for ABR thresholds to be higher than those derived from more invasive recording methods or behavioral methods (Gorga et al. 1988; Brittan-Powell et al. 2002, 2010a, 2010b, but see Henry and Lucas 2009). As an onset response, the ABR is not affected by the ability of the auditory system to integrate sound over time, as are these other methods of threshold determination, which likely accounts for the difference between thresholds (Gorga et al. 1984; Szymanski et al. 1999).
In terms of general shape, the ABR audiogram resembles Hillery's (1984b) midbrain audiogram. That is, frequency tuning (i.e., the differences in thresholds across frequencies) was broadly similar between the ABR audiogram and midbrain audiogram. In both audiograms, sensitivity peaked around the average spectral peaks present in male calls. The low frequency peak in sensitivity for both audiograms was near 1.2 kHz; however, the frequency of the second sensitivity peak was about 400 Hz lower in the midbrain audiogram than the ABR audiogram. While the peaks in the midbrain audiogram had equivalent sensitivity, there was a 2.5 – 4.4 dB difference between thresholds at the two peaks in the ABR audiogram. Thresholds increased similarly for both audiograms in responses to frequencies between the two peaks of greatest sensitivity (Fig. 6b). For example, the difference between thresholds at the most sensitive frequencies and the intermediate frequencies was about 5 to 8 dB in the ABR audiogram, compared with approximately 10 dB in the midbrain audiogram. Thresholds in both audiograms increased sharply at frequencies below the low-frequency peak and above the high-frequency peak (Fig. 6b). The changes in threshold for frequencies from 0.3 kHz up to the ~1.2 kHz peak of sensitivity were comparable, with slopes of −10.6 dB/octave in the ABR audiogram and approximately −11.5 dB/octave in the midbrain audiogram (Fig. 6b). For frequencies above the second (higher frequency) peak in sensitivity, the slopes differed somewhat more, with rates of 27.6 dB/octave and 35.4 dB/octave in the ABR and midbrain audiograms, respectively (Fig. 6b).
We believe the small differences in high-frequency tuning between our ABR audiogram and Hillery's (1984b) midbrain audiogram could reflect evolutionary differences, size differences, or both, between the frogs tested in each study. Regarding evolutionary differences, we note that H. chrysoscelis consists of two distinct genetic lineages (Ptacek et al. 1994). The frogs used in the current study were of the western mitochondrial DNA lineage, while those used for midbrain recordings were collected from Tennessee (Hillery 1984b) and belonged to the eastern lineage. Lineage differences in female preferences for spectral properties of advertisement calls have been reported previously (Schrode et al. 2012). Comparative ABR studies of the two lineages might shed considerable light on possible mechanisms underlying the apparent variation in female preferences for spectral call properties. Although we did not see an effect of size on frequency tuning across the range of sizes in our sample of Minnesota frogs, some previous research has indicated that larger individuals can have BPs tuned to lower frequencies (Zakon and Wilczynski 1988). In the study by Hillery (1984b), subject mass ranged from 4.1 to 11.2 g. This is a wider range shifted to larger body sizes compared to those of the frogs tested in our study (2.8 to 8.3 g). Therefore, we cannot rule out the possibility that population differences in body size contributed to physiological differences in tuning for higher frequencies reflected in the ABR and midbrain audiograms. To determine definitively what differences in the audiograms result from population differences, the best test would be to conduct both types of recording in the same individuals.
Fibers in the anuran auditory nerve tend to cluster into three distinct populations that are sensitive to different frequency ranges (reviewed in Zakon and Wilczynski 1988). The absolute frequency ranges vary by species, but there is generally a group of fibers sensitive to low-frequencies and one sensitive to mid-frequencies, both arising from the AP, and a third group sensitive to high-frequencies, which arises from the BP. Midbrain audiograms from some other hylid treefrogs have peaks near 0.5 kHz that are thought to arise from the low-frequency AP fibers (Hubl and Schneider 1979; Penna et al. 1992; Wilczynski et al. 1993; Miranda and Wilczynski 2009a). Although the audiogram described by Hillery (1984b) based on multiunit recordings in the midbrain of Cope's gray treefrogs shows no increased sensitivity near 0.5 kHz, single unit recordings in the midbrain of the closely related eastern gray treefrog (H. versicolor) suggest that there is a distinct low-frequency population of neurons sensitive to frequencies around 0.5 kHz in the latter species (Diekamp and Gerhardt 1995). Our ABR audiogram showed no increased sensitivity at 0.5 kHz relative to nearby frequencies, which is similar to the audiogram of Hillery (1984b). However, we would point out that the use of ABRs often results in overestimation of thresholds to low-frequency tones. This overestimation stems from the use of relatively short tone pips with fast rise/fall times (see discussion in Brittan-Powell et al. 2010b). We chose these temporal stimulus properties to be consistent with several previous studies of the ABR (e.g., Brittan-Powell et al. 2002, 2010b; Katbamna et al. 2006a; Lohr et al. 2013) and invasive studies of evoked potentials in frogs ((Katbamna et al. 2006b; Zhang et al. 2012). In addition, longer tone durations and slower rise/fall times can increase ABR latencies (Hecox et al. 1976) and alter waveform morphology (Popov and Supin 1990; Hall 2007). In general, ABRs evoked by short tone pips are probably most useful for assessing sensitivity to middle and high frequencies in frogs, but may be more limited in assessing low-frequency sensitivity.
Utility of ABRs
Our results indicate that recordings of ABRs via minimally invasive procedures represent a useful method for characterizing and investigating the physiology of hearing in frogs. There are some advantages of using ABRs over more traditional physiological methods. Recording the ABR is quick, allowing for acquisition of data from large sample sizes in a short amount of time (e.g., during a species’ breeding season). Because surgery is not required to record subdermal ABRs, animals must be held captive and housed for shorter periods of time and there is dramatically lower risks of infection and death. Many of the more under-studied species are in remote locations, making the potential portability and relatively low cost of the ABR technique ideal for studying these taxa. Additionally, individuals can be used in both behavioral tests and ABR studies, facilitating direct comparison of physiological and behavioral responses in the same animal, as well as within-subject longitudinal studies (e.g., Zhang et al. 2012).
An obvious application for the ABR is in comparative studies of auditory sensitivity across species (e.g., Kenyon et al. 1998; Lucas et al. 2002; Brittan-Powell et al. 2005, 2010b;). A particularly interesting comparison would be between closely related species of frogs that exhibit behavioral differences in male calls, female preferences, or both. A considerable focus of previous research in anurans has compared the spectral tuning of the auditory system to the spectral content of advertisements calls and to female frequency preferences (Gerhardt and Schwartz 2001). Recordings of the ABR might provide a useful method for furthering this research using a broader range of species, lineages, or populations and could complement or serve as an alternative to other minimally invasive methods (e.g., Meenderink et al. 2010).
Supplementary Material
Acknowledgements
We thank Alejandro Vélez for helpful feedback on an earlier version of the manuscript, Justin Becknell for the illustration in Figure 1, Madeleine Linck, Don Pereira, and Ed Quinn for access to study sites and collection permissions, and many undergraduate students for help collecting frogs. We also thank Ed Smith for programming. This work was supported by the National Institutes Health in the form of R01 DC009582 to author M. A. Bee at the University of Minnesota, P30 DC004664 to R. J. Dooling at the University of Maryland and T32 NS048944 to T. J. Ebner at the University of Minnesota.
References
- Aitkin L, Nelson J, Shepherd R. Development of hearing and vocalization in a marsupial, the Northern Quoll, Dasyurus hallucatus. J Exp Zool. 1996;276:394–402. doi: 10.1002/(SICI)1097-010X(19961215)276:6<394::AID-JEZ3>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Amoser S, Ladich F. Are hearing sensitivities of freshwater fish adapted to the ambient noise in their habitats? J Exp Biol. 2005;208:3533–3542. doi: 10.1242/jeb.01809. [DOI] [PubMed] [Google Scholar]
- Bartol SM, Musick JA, Lenhardt ML. Auditory evoked potentials of the loggerhead sea turtle (Caretta caretta). Copeia. 1999;1999:836–840. [Google Scholar]
- Bee MA. Sound source perception in anuran amphibians. Curr Opin Neurobiol. 2012;22:301–310. doi: 10.1016/j.conb.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibikov NG. Addition of noise enhances neural synchrony to amplitude-modulated sounds in the frog's midbrain. Hear Res. 2002;173:21–28. doi: 10.1016/s0378-5955(02)00456-2. [DOI] [PubMed] [Google Scholar]
- Bibikov NG, Elepfandt A. Auditory evoked potentials from medulla and midbrain in the clawed frog, Xenopus laevis laevis. Hear Res. 2005;204:29–36. doi: 10.1016/j.heares.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Boettcher FA, Mills JH, Norton BL, Schmiedt RA. Age-related changes in auditory evoked potentials of gerbils. II. Response latencies. Hear Res. 1993;71:146–156. doi: 10.1016/0378-5955(93)90030-5. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EE, Dooling RJ, Ryals B, Gleich O. Electrophysiological and morphological development of the inner ear in Belgian Waterslager canaries. Hear Res. 2010a;269:56–69. doi: 10.1016/j.heares.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittan-Powell EF, Christensen-Dalsgaard J, Tang YZ, Carr C, Dooling RJ. The auditory brainstem response in two lizard species. J Acoust Soc Am. 2010b;128:787–794. doi: 10.1121/1.3458813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ. Development of auditory sensitivity in budgerigars (Melopsittacus undulatus). J Acoust Soc Am. 2004;115:3092–3102. doi: 10.1121/1.1739479. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Dooling RJ, Gleich O. Auditory brainstem responses in adult budgerigars (Melopsittacus undulatus). J Acoust Soc Am. 2002;112:999–1008. doi: 10.1121/1.1494807. [DOI] [PubMed] [Google Scholar]
- Brittan-Powell EF, Lohr B, Hahn DC, Dooling RJ. Auditory brainstem responses in the Eastern Screech Owl: An estimate of auditory thresholds. J Acoust Soc Am. 2005;118:314–321. doi: 10.1121/1.1928767. [DOI] [PubMed] [Google Scholar]
- Capranica RR, Moffat JM. Neurobehavioral correlates of sound communication in anurans. In: Ewert JP, Capranica RR, Ingle DJ, editors. Advances in vertebrate neuroethology. Plenum Press; New York: 1983. pp. 701–730. [Google Scholar]
- Caras ML, Brenowitz E, Rubel EW. Peripheral auditory processing changes seasonally in Gambel's white-crowned sparrow. J Comp Physiol A. 2010;196:581–599. doi: 10.1007/s00359-010-0545-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carey MB, Zelick R. The effect of sound level, temperature and dehydration on the brain-stem auditory-evoked potential in anuran amphibians. Hear Res. 1993;70:216–228. doi: 10.1016/0378-5955(93)90160-3. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Breithaupt T, Elepfandt A. Underwater hearing in the clawed frog, Xenopus laevis: Tympanic motion studied with laser vibrometry. Naturwissenschaften. 1990;77:135–137. doi: 10.1007/BF01134478. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Jørgensen MB. Sound and vibration sensitivity of VIIIth nerve fibers in the grassfrog, Rana temporaria. J Comp Physiol A. 1996;179:437–445. doi: 10.1007/BF00192311. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Jørgensen MB, Kanneworff M. Basic response characteristics of auditory nerve fibers in the grassfrog (Rana temporaria). Hear Res. 1998;119:155–163. doi: 10.1016/s0378-5955(98)00047-1. [DOI] [PubMed] [Google Scholar]
- Christensen-Dalsgaard J, Walkowiak W. In vitro and in vivo responses of saccular and caudal nucleus neurons in the grassfrog (Rana temporaria). European J Morphol. 1999;37:206–210. doi: 10.1076/ejom.37.2.206.4745. [DOI] [PubMed] [Google Scholar]
- Church MW, Williams HL, Holloway JA. Brain-stem auditory evoked-potentials in the rat: Effects of gender, stimulus characteristics and ethanol sedation. Electroencephalogr Clin NeuroPhysiol. 1984;59:328–339. doi: 10.1016/0168-5597(84)90050-9. [DOI] [PubMed] [Google Scholar]
- Corwin JT, Bullock TH, Schweitzer J. The auditory brainstem response in five vertebrate classes. Electroencephalogr Clin NeuroPhysiol. 1982;54:629–641. doi: 10.1016/0013-4694(82)90117-1. [DOI] [PubMed] [Google Scholar]
- D'Angelo GJ, De Chicchis AR, Osborn DA, Gallagher GR, Warren RJ, Miller KV. Hear range of white-tailed deer as determined by auditory brainstem response. J Wildl Manag. 2007;71:1238–1242. [Google Scholar]
- Diekamp B, Gerhardt HC. Selective phonotaxis to advertisement calls in the gray treefrog Hyla versicolor: Behavioral experiments and neurophysiological correlates. J Comp Physiol A. 1995;177:173–190. doi: 10.1007/BF00225097. [DOI] [PubMed] [Google Scholar]
- Edwards CJ, Kelley DB. Auditory and lateral line inputs to the midbrain of an aquatic anuran; Neuroanatomic studies in Xenopus laevis. J Comp Neurol. 2001;438:148–162. doi: 10.1002/cne.1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehret G, Capranica RR. Masking patterns and filter characteristics of auditory nerve fibers in the green treefrog (Hyla cinerea). J Comp Physiol A. 1980;141:1–12. [Google Scholar]
- Elliott TM, Christensen-Dalsgaard J, Kelley DB. Tone and call responses of units in the auditory nerve and dorsal medullary nucleus of Xenopus laevis. J Comp Physiol A. 2007;193:1243–1257. doi: 10.1007/s00359-007-0285-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott TM, Christensen-Dalsgaard J, Kelley DB. Temporally selective processing of communication signals by auditory midbrain neurons. J Neurophysiol. 2011;105:1620–1632. doi: 10.1152/jn.00261.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng AS. Quantitative analysis of intensity-rate and intensity-latency functions in peripheral auditory nerve fibers of northern leopard frogs (Rana pipiens). Hear Res. 1982;6:241–246. doi: 10.1016/0378-5955(82)90057-0. [DOI] [PubMed] [Google Scholar]
- Feng AS, Capranica RR. Sound localization in anurans. I. Evidence of binaural interaction in dorsal medullary nucleus of bullfrogs (Rana catesbeiana). J Neurophysiol. 1976;39:871–881. doi: 10.1152/jn.1976.39.4.871. [DOI] [PubMed] [Google Scholar]
- Feng AS, Capranica RR. Sound localization in anurans II. Binaural interaction in superior olivary nucleus of the green tree frog (Hyla cinerea). J Neurophysiol. 1978;41:43–54. doi: 10.1152/jn.1978.41.1.43. [DOI] [PubMed] [Google Scholar]
- Frishkopf LS, Capranica RR, Goldstein MH., Jr Neural coding in the bullfrog's auditory system a teleological approach. Proc IEEE. 1968;56:969–980. [Google Scholar]
- Fuzessery ZM, Feng AS. Frequency representation in the dorsal medullary nucleus of the leopard frog, Rana pipiens. J Comp Physiol A. 1981;143:339–347. [Google Scholar]
- Fuzessery ZM, Feng AS. Frequency selectivity in the anuran auditory midbrain: Single unit responses to single and multiple tone stimulation. J Comp Physiol A. 1982;146:471–484. [Google Scholar]
- Gall MD, Brierley LE, Lucas JR. Species and sex effects on auditory processing in brown-headed cowbirds and red-winged blackbirds. Anim Behav. 2011;81:973–982. [Google Scholar]
- Gerhardt HC. Acoustic spectral preferences in two cryptic species of grey treefrogs: Implications for mate choice and sensory mechanisms. Anim Behav. 2005;70:39–48. [Google Scholar]
- Gerhardt HC, Huber F. Acoustic communication in insects and anurans: Common problems and diverse solutions. University of Chicago Press; Chicago: 2002. [Google Scholar]
- Gerhardt HC, Schwartz JJ. Auditory tuning, frequency preferences and mate choice in anurans. In: Ryan MJ, editor. Anuran Communication. Smithsonian Institution Press; Washington DC: 2001. pp. 73–85. [Google Scholar]
- Gorga MP, Beauchaine KA, Reiland JK, Worthington DW, Javel E. The effects of stimulus duration on ABR and behavioral thresholds. J Acoust Soc Am. 1984;76:616–619. doi: 10.1121/1.391158. [DOI] [PubMed] [Google Scholar]
- Gorga MP, Kaminski JR, Beauchaine KA, Jesteadt W. Auditory brainstem responses to tone bursts in normally hearing subjects. J Speech Hear Res. 1988;31:87–97. doi: 10.1044/jshr.3101.87. [DOI] [PubMed] [Google Scholar]
- Graham JW, Olchowski AE, Gilreath TD. How many imputations are really needed? - Some practical clarifications of multiple imputation theory. Prev Sci. 2007;8:206–213. doi: 10.1007/s11121-007-0070-9. [DOI] [PubMed] [Google Scholar]
- Greenhouse SW, Geisser S. On methods in the analysis of profile data. Psychom. 1959;24:95–112. [Google Scholar]
- Hall JW. New handbook of auditory evoked responses. Allyn & Bacon; Boston: 2007. [Google Scholar]
- Hecox K, Squires N, Galambos R. Brainstem auditory evoked responses in man. I. Effect of stimulus rise–fall time and duration. J Acoust Soc Am. 1976;60:1187–1192. doi: 10.1121/1.381194. [DOI] [PubMed] [Google Scholar]
- Henry KS, Lucas JR. Coevolution of auditory sensitivity and temporal resolution with acoustic signal space in three songbirds. Anim Behav. 2008;76:1659–1671. [Google Scholar]
- Henry KS, Lucas JR. Vocally correlated seasonal auditory variation in the house sparrow (Passer domesticus). J Exp Biol. 2009;212:3817–3822. doi: 10.1242/jeb.033035. [DOI] [PubMed] [Google Scholar]
- Henry KS, Lucas JR. Auditory sensitivity and the frequency selectivity of auditory filters in the Carolina chickadee, Poecile carolinensis. Anim Behav. 2010a;80:497–507. [Google Scholar]
- Henry KS, Lucas JR. Habitat-related differences in the frequency selectivity of auditory filters in songbirds. Func Ecol. 2010b;24:614–624. [Google Scholar]
- Higgs DM, Brittan-Powell EF, Soares D, Souza MJ, Carr CE, Dooling RJ, Popper AN. Amphibious auditory responses of the Amn alligator (Alligator mississipiensis). J Comp Physiol A. 2002;188:217–223. doi: 10.1007/s00359-002-0296-8. [DOI] [PubMed] [Google Scholar]
- Hillery CM. Detection of amplitude-modulated tones by frogs: Implications for temporal processing mechanisms. Hear Res. 1984a;14:129–143. doi: 10.1016/0378-5955(84)90012-1. [DOI] [PubMed] [Google Scholar]
- Hillery CM. Seasonality of two midbrain auditory responses in the treefrog, Hyla chrysoscelis. Copeia. 1984b;1984:844–852. [Google Scholar]
- Hillery CM, Narins PM. Neurophysiological evidence for a traveling wave in the amphibian inner ear. Science. 1984;225:1037–1039. doi: 10.1126/science.6474164. [DOI] [PubMed] [Google Scholar]
- Ho CCK, Narins PM. Directionality of the pressure-difference receiver ears in the northern leopard frog, Rana pipiens pipiens. J Comp Physiol A. 2006;192:417–429. doi: 10.1007/s00359-005-0080-7. [DOI] [PubMed] [Google Scholar]
- Horodysky AZ, Brill RW, Fine ML, Musick JA, Latour RJ. Acoustic pressure and particle motion thresholds in six sciaenid fishes. J Exp Biol. 2008;211:1504–1511. doi: 10.1242/jeb.016196. [DOI] [PubMed] [Google Scholar]
- Hu MY, Yan HY, Chung WS, Shiao JC, Hwang PP. Acoustically evoked potentials in two cephalopods inferred using the auditory brainstem response (ABR) approach. Comp Biochem and Physiol A. 2009;153:278–283. doi: 10.1016/j.cbpa.2009.02.040. [DOI] [PubMed] [Google Scholar]
- Hubl L, Schneider H. Temperature and auditory thresholds: Bioacoustic studies of the frogs Rana r. ridibunda, Hyla a. arborea and Hyla a. savignyi (Anura, amphibia). J Comp Physiol A. 1979;130:17–27. [Google Scholar]
- Jerger J, Hall J. Effects of age and sex on auditory brainstem response. Arch Otolaryngol. 1980;106:387–391. doi: 10.1001/archotol.1980.00790310011003. [DOI] [PubMed] [Google Scholar]
- Katbamna B, Brown JA, Collard M, Ide CF. Auditory brainstem responses to airborne sounds in the aquatic frog Xenopus laevis: correlation with middle ear characteristics. J Comp Physiol A. 2006a;192:381–387. doi: 10.1007/s00359-005-0076-3. [DOI] [PubMed] [Google Scholar]
- Katbamna B, Langerveld AJ, Ide CF. Aroclor 1254 impairs the hearing ability of Xenopus laevis. J Comp Physiol A. 2006b;192:971–983. doi: 10.1007/s00359-006-0134-5. [DOI] [PubMed] [Google Scholar]
- Katbamna B, Thodi C, Senturia JB, Metz DA. Auditory evoked brainstem responses in the hibernating woodchuck Marmota monax. Comp Biochem Physiol A. 1992;102:513–517. doi: 10.1016/0300-9629(92)90203-3. [DOI] [PubMed] [Google Scholar]
- Keddy-Hector AC, Wilczynski W, Ryan MJ. Call patterns and basilar papilla tuning in cricket frogs. II. Intrapopulation variation and allometry. Brain Behav Evol. 1992;39:238–246. doi: 10.1159/000114121. [DOI] [PubMed] [Google Scholar]
- Kelley DB. Vocal communication in frogs. Curr Opin Neurobiol. 2004;14:751–757. doi: 10.1016/j.conb.2004.10.015. [DOI] [PubMed] [Google Scholar]
- Kenyon TN, Ladich F, Yan HY. A comparative study of hearing ability in fishes: the auditory brainstem response approach. J Comp Physiol A. 1998;182:307–318. doi: 10.1007/s003590050181. [DOI] [PubMed] [Google Scholar]
- Klishin VO, Diaz RP, Popov VV, Supin AY. Some characteristics of hearing of the Brazilian manatee, Trichechus inunguis. Aquat Mamm. 1990;16:139–144. [Google Scholar]
- Klump GM, Benedix JH, Gerhardt HC, Narins PM. AM representation in green treefrog auditory nerve fibers: Neuroethological implications for pattern recognition and sound localization. J Comp Physiol A. 2004;190:1011–1021. doi: 10.1007/s00359-004-0558-8. [DOI] [PubMed] [Google Scholar]
- Ladich F, Yan HY. Correlation between auditory sensitivity and vocalization in anabantoid fishes. J Comp Physiol A. 1998;182:737–746. doi: 10.1007/s003590050218. [DOI] [PubMed] [Google Scholar]
- Lohr B, Brittan-Powell EF, Dooling RJ. Auditory brainstem responses and auditory thresholds in woodpeckers. J Acoust Soc Am. 2013;133:337–342. doi: 10.1121/1.4770255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JM, Findlay MM, Moate RM, Yan HY. The hearing abilities of the prawn Palaemon serratus. Comp Biochem Physiol A. 2005;140:89–100. doi: 10.1016/j.cbpb.2004.11.003. [DOI] [PubMed] [Google Scholar]
- Lucas JR, Freeberg TM, Krishnan A, Long GR. A comparative study of avian auditory brainstem responses: correlations with phylogeny and vocal complexity, and seasonal effects. J Comp Physiol A. 2002;188:981–992. doi: 10.1007/s00359-002-0359-x. [DOI] [PubMed] [Google Scholar]
- Lugli M, Yan HY, Fine ML. Acoustic communication in two freshwater gobies: the relationship between ambient noise, hearing thresholds and sound spectrum. J Comp Physiol A. 2003;189:309–320. doi: 10.1007/s00359-003-0404-4. [DOI] [PubMed] [Google Scholar]
- Martin KJ, Alessi SC, Gaspard JC, Tucker AD, Bauer GB, Mann DA. Underwater hearing in the loggerhead turtle (Caretta caretta): a comparison of behavioral and auditory evoked potential audiograms. J Exp Biol. 2012;215:3001–3009. doi: 10.1242/jeb.066324. [DOI] [PubMed] [Google Scholar]
- McFadden SL, Walsh EJ, McGee J. Onset and development of auditory brainstem responses in the Mongolian gerbil (Meriones unguiculatus). Hear Res. 1996;100:68–79. doi: 10.1016/0378-5955(96)00108-6. [DOI] [PubMed] [Google Scholar]
- Meenderink SWF, Kits M, Narins PM. Frequency matching of vocalizations to inner-ear sensitivity along an altitudinal gradient in the coqui frog. Biol Lett. 2010;6:278–281. doi: 10.1098/rsbl.2009.0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Female reproductive state influences the auditory midbrain response. J Comp Physiol A. 2009a;195:341–349. doi: 10.1007/s00359-008-0410-7. [DOI] [PubMed] [Google Scholar]
- Miranda JA, Wilczynski W. Sex differences and androgen influences on midbrain auditory thresholds in the green treefrog, Hyla cinerea. Hear Res. 2009b;252:79–88. doi: 10.1016/j.heares.2009.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mooney TA, Hanlon RT, Christensen-Dalsgaard J, Madsen PT, Ketten DR, Nachtigall PE. Sound detection by the longfin squid (Loligo pealeii) studied with auditory evoked potentials: sensitivity to low-frequency particle motion and not pressure. J Exp Biol. 2010;213:3748–3759. doi: 10.1242/jeb.048348. [DOI] [PubMed] [Google Scholar]
- Mudry KM, Capranica RR. Correlation between auditory thalamic area evoked responses and species-specific call characteristics II. Hyla cinerea (Anura: Hylidae). J Comp Physiol A. 1987;161:407–416. doi: 10.1007/BF00603966. [DOI] [PubMed] [Google Scholar]
- Munro KJ, Shiu JN, Cox CL. The effect of head size on the auditory brainstem response for two breeds of dog. Brit J of Audiol. 1997;31:309–314. doi: 10.3109/03005364000000025. [DOI] [PubMed] [Google Scholar]
- Nachtigall PE, Supin AY. A false killer whale adjusts its hearing when it echolocates. J Exp Biol. 2008;211:1714–1718. doi: 10.1242/jeb.013862. [DOI] [PubMed] [Google Scholar]
- Nachtigall PE, Supin AY, Amundin M, Roken B, Moller T, Mooney TA, Taylor KA, Yuen M. Polar bear Ursus maritimus hearing measured with auditory evoked potentials. J Exp Biol. 2007;210:1116–1122. doi: 10.1242/jeb.02734. [DOI] [PubMed] [Google Scholar]
- Narins PM, Capranica RR. Sexual differences in the auditory system of the tree frog Eleutherodactylus coqui. Science. 1976;192:378–380. doi: 10.1126/science.1257772. [DOI] [PubMed] [Google Scholar]
- Narins PM, Feng AS, Fay RR, Popper AN. Hearing and Sound Communication in Amphibians. Springer; New York: 2007. [Google Scholar]
- Noirot IC, Brittan-Powell EF, Dooling RJ. Masked auditory thresholds in three species of birds, as measured by the auditory brainstem response (L). J Acoust Soc Am. 2011;129:3445–3448. doi: 10.1121/1.3578452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penna M, Capranica RR, Somers J. Hormone-induced vocal behavior and midbrain auditory sensitivity in the green treefrog, Hyla cinerea. J Comp Physiol A. 1992;170:73–82. doi: 10.1007/BF00190402. [DOI] [PubMed] [Google Scholar]
- Popov VV, Supin AY. Auditory brain stem responses in characterization of dolphin hearing. J Comp Physiol A. 1990;166:385–393. doi: 10.1007/BF00204811. [DOI] [PubMed] [Google Scholar]
- Popov VV, Supin AY. Contribution of various frequency bands to ABR in dolphins. Hear Res. 2001;151:250–260. doi: 10.1016/s0378-5955(00)00234-3. [DOI] [PubMed] [Google Scholar]
- Ptacek MB, Gerhardt HC, Sage RD. Speciation by polyploidy in treefrogs: Multiple origins of the tetraploid, Hyla versicolor. Evol. 1994;48:898–908. doi: 10.1111/j.1558-5646.1994.tb01370.x. [DOI] [PubMed] [Google Scholar]
- Ramsier MA, Dominy NJ. A comparison of auditory brainstem responses and behavioral estimates of hearing sensitivity in Lemur catta and Nycticebus coucang. Am J Primatol. 2010;72:217–233. doi: 10.1002/ajp.20780. [DOI] [PubMed] [Google Scholar]
- Ratnam R, Feng AS. Detection of auditory signals by frog inferior collicular neurons in the presence of spatially separated noise. J NeuroPhysiol. 1998;80:2848–2859. doi: 10.1152/jn.1998.80.6.2848. [DOI] [PubMed] [Google Scholar]
- Rubin DB. Inference and missing data. Biom. 1976;63:581–590. [Google Scholar]
- Schafer JL. Multiple imputation: a primer. Stat Methods Med Res. 1999;8:3–15. doi: 10.1177/096228029900800102. [DOI] [PubMed] [Google Scholar]
- Schafer JL, Olsen MK. Multiple imputation for multivariate missing-data problems: A data analyst's perspective. Multivar Behav Res. 1998;33:545–571. doi: 10.1207/s15327906mbr3304_5. [DOI] [PubMed] [Google Scholar]
- Schrode K, Ward JL, Vélez A, Bee MA. Female preferences for spectral call properties in the western genetic lineage of Cope's gray treefrog (Hyla chrysoscelis). Behav Ecol Sociobiol. 2012;66:1595–1606. doi: 10.1007/s00265-012-1413-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz JJ, Simmons AM. Encoding of a spectrally complex natural call in the bullfrog's auditory nerve. J Comp Physiol A. 1990;166:489–499. doi: 10.1007/BF00192019. [DOI] [PubMed] [Google Scholar]
- Seaman RL. Method to record evoked-potentials from the frog 8th nerve. Hear Res. 1991;51:301–305. doi: 10.1016/0378-5955(91)90046-c. [DOI] [PubMed] [Google Scholar]
- Shofner WP, Feng AS. Post-metamorphic development of the frequency selectivities and sensitivities of the peripheral auditory system of the bullfrog, Rana catesbeiana. J Exp Biol. 1981;93:181–196. [Google Scholar]
- Simmons AM, Ferragamo M. Periodicity extraction in the anuran auditory nerve. I. “Pitch-shift” effects. J Comp Physiol A. 1993;172:57–69. doi: 10.1007/BF00214715. [DOI] [PubMed] [Google Scholar]
- Simmons AM, Reese G, Ferragamo M. Periodicity extraction in the anuran auditory nerve. II. Phase and temporal fine-structure. J Acoust Soc Am. 1993;93:3374–3389. doi: 10.1121/1.405693. [DOI] [PubMed] [Google Scholar]
- Simmons AM, Sanderson MI, Garabedian CE. Representation of waveform periodicity in the auditory midbrain of the bullfrog, Rana catesbeiana. J Assoc Res Otolaryngol. 2000;1:2–24. doi: 10.1007/s101620010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons DD, Bertolotto C, Narins PM. Innervation of the amphibian and basilar papillae in the leopard frog: Reconstructions of single labeled fibers. J Comp Neurol. 1992;322:191–200. doi: 10.1002/cne.903220205. [DOI] [PubMed] [Google Scholar]
- Simmons DD, Meenderink SWF, Vassilakis PN. Anatomy, physiology, and function of the auditory end-organs in the frog inner ear. In: Narins PA, Feng AS, Fay RR, Popper AN, editors. Hearing and Sound Communication in Amphibians. Springer; New York: 2007. pp. 184–220. [Google Scholar]
- Smith ME, Kane AS, Popper AN. Noise-induced stress response and hearing loss in goldfish (Carassius auratus). J Exp Biol. 2004;207:427–435. doi: 10.1242/jeb.00755. [DOI] [PubMed] [Google Scholar]
- Song L, McGee J, Walsh EJ. Frequency- and level-dependent changes in auditory brainstem responses (ABRs) in developing mice. J Acoust Soc Am. 2006;119:2242–2257. doi: 10.1121/1.2180533. [DOI] [PubMed] [Google Scholar]
- Stiebler IB, Narins PM. Temperature-dependence of auditory nerve response properties in the frog. Hear Res. 1990;46:63–81. doi: 10.1016/0378-5955(90)90140-k. [DOI] [PubMed] [Google Scholar]
- Supin AY, Popov VV, Klishin VO. ABR frequency tuning curves in dolphins. J Comp Physiol A. 1993;173:649–656. doi: 10.1007/BF00197772. [DOI] [PubMed] [Google Scholar]
- Szymanski MD, Bain DE, Kiehl K, Pennington S, Wong S, Henry KR. Killer whale (Orcinus orca) hearing: Auditory brainstem response and behavioral audiograms. J Acoust Soc Am. 1999;106:1134–1141. doi: 10.1121/1.427121. [DOI] [PubMed] [Google Scholar]
- Szymanski MD, Supin AY, Bain DE, Henry KR. Killer whale (Orcinus orca) auditory evoked potentials to rhythmic clicks. Mar Mamm Sci. 1998;14:676–691. [Google Scholar]
- Uetake K, Yayou KI, Okamoto T. Auditory brainstem response and objective assessment of hearing thresholds in cowshed calves. J Ethol. 1996;14:73–75. [Google Scholar]
- Uzuka Y, Furuta T, Yamaoka M, Ohnishi T, Tsubone H, Sugano S. Threshold changes in auditory brainstem response (ABR) due to the administration of kanamycin in dogs. Exp Anim. 1996;45:325–331. doi: 10.1538/expanim.45.325. [DOI] [PubMed] [Google Scholar]
- Walsh EJ, McGee J, Javel E. Development of auditory-evoked potentials in the cat. II. Wave latencies. J Acoust Soc Am. 1986;79:725–744. doi: 10.1121/1.393462. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Capranica RR. The auditory system of anuran amphibians. Prog Neurobiol. 1984;22:1–38. doi: 10.1016/0301-0082(84)90016-9. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Keddy-Hector AC, Ryan MJ. Call patterns and basilar papilla tuning in cricket frogs. I. Differences among populations and between sexes. Brain Behav and Evol. 1992;39:229–237. doi: 10.1159/000114120. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, McClelland BE, Rand AS. Acoustic, auditory, and morphological divergence in three species of neotropical frog. J Comp Physiol A. 1993;172:425–438. doi: 10.1007/BF00213524. [DOI] [PubMed] [Google Scholar]
- Wilczynski W, Ryan MJ. The behavioral neuroscience of anuran social signal processing. Curr Opin Neurobiol. 2010;20:754–763. doi: 10.1016/j.conb.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will U, Fritzsch B. The eighth nerve of amphibians: peripheral and central distribution. In: Fritzsch B, Wolkowiak W, Ryan MJ, Wilczynski W, Hetheringon T, editors. The Evolution of the amphibian auditory system. Wiley; New York: 1988. pp. 159–183. [Google Scholar]
- Wysocki LE, Ladich F. The ontogenetic development of auditory sensitivity, vocalization and acoustic communication in the labyrinth fish Trichopsis vittata. J Comp Physiol A. 2001;187:177–187. doi: 10.1007/s003590100186. [DOI] [PubMed] [Google Scholar]
- Wysocki LE, Ladich F. The representation of conspecific sounds in the auditory brainstem of teleost fishes. J Exp Biol. 2003;206:2229–2240. doi: 10.1242/jeb.00417. [DOI] [PubMed] [Google Scholar]
- Yu ZL, Qiu Q, Xu ZM, Shen JX. Auditory response characteristics of the piebald odorous frog and their implications. J Comp Physiol A. 2006;192:801–806. doi: 10.1007/s00359-006-0125-6. [DOI] [PubMed] [Google Scholar]
- Zakon H, Capranica RR. An anatomical and physiological study of regeneration of the eighth nerve in the leopard frog. Brain Res. 1981;209:325–338. doi: 10.1016/0006-8993(81)90157-8. [DOI] [PubMed] [Google Scholar]
- Zakon HH, Wilczynski W. The physiology of the anuran eighth nerve. In: Fritzsch B, Wolkowiak W, Ryan MJ, Wilczynski W, Hetherington T, editors. The Evolution of the Amphibian Auditory System. Wiley; New York: 1988. pp. 125–155. [Google Scholar]
- Zhang D, Cui JG, Tang YZ. Plasticity of peripheral auditory frequency sensitivity in Emei music frog. PLoS ONE. 2012;7:e45792. doi: 10.1371/journal.pone.0045792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jen PHS, Seburn KL, Frankel WN, Zheng QY. Auditory brainstem responses in 10 inbred strains of mice. Brain Res. 2006;1091:16–26. doi: 10.1016/j.brainres.2006.01.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.