Introduction
Drugs acting on μ-opioid receptors (MOR) are widely used as analgesics but present serious side-effects such as addiction and respiratory depression. The latter is critical considering its potential lethality and the current absence of treatments to prevent it. The development of therapies to reduce respiratory depression is limited because the critical neural sites and mechanisms of action of opioids in causing respiratory depression are unclear. Here we discuss evidence highlighting the importance of the preBötzinger complex (preBötC), a critical site in the medulla for respiratory rhythm generation, in mediating respiratory rate depression by MOR drugs. This is of significance given that the isolated preBötC in vitro is widely utilized to develop and test new therapies to prevent respiratory depression (Manzke et al. 2003; Ren et al. 2006).
Sensitivity of preBötC neurons to MOR agonists
Opioid-induced impairment of breathing encompasses central depression of respiratory rate, amplitude and reflex responses, reduced brain arousability, as well as upper airway dysfunction. This paper focuses on opioid-induced depression of respiratory rate. MOR are expressed in various brain structures regulating breathing including, but not limited to, the medullary raphé (Zhang et al. 2007), pontine nuclei (Prkic et al. 2012), nucleus tractus solitarii (Zhang et al. 2011), rostral ventromedial medulla (Phillips et al. 2012), peripheral chemoreceptors (Pokorski & Lahiri, 1981), and the preBötC (Gray et al. 1999; Manzke et al. 2003). Among all the opioid-sensitive neural sites, the preBötC is unique as it constitutes a cluster of neurons that has the property to generate rhythm by itself (Smith et al. 1991). In the intact and mature organism, however, the capacity of preBötC MOR to affect respiratory rhythm has been controversial. To determine whether preBötC MOR activation can substantially decrease respiratory rate in intact animals, a number of criteria need consideration.
(i) Identification of preBötC neurons
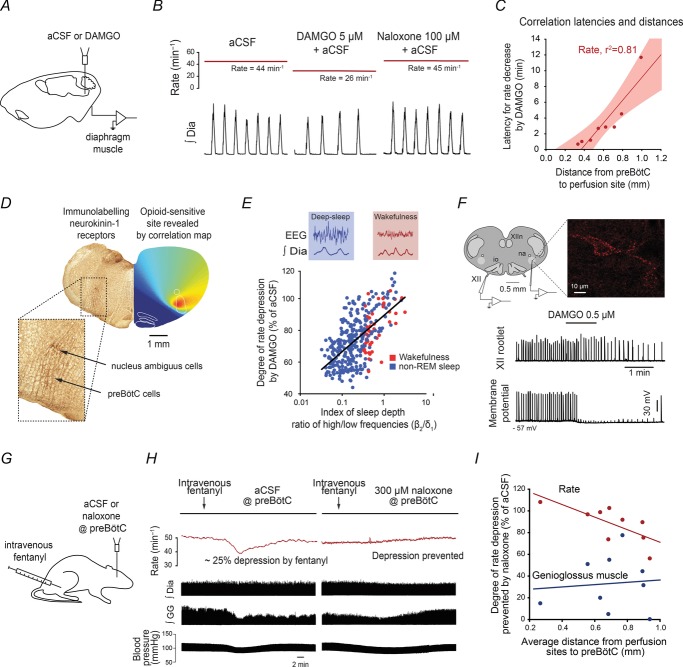
The preBötC needs to be identified for accurate targeting using anatomical markers, expression of known proteins, and/or functional markers. PreBötC neurons express neurokinin-1 receptors (Gray et al. 2001; Montandon & Horner, 2013), the peptide somatostatin (Stornetta et al. 2003; Tan et al. 2008) and its cognate somatostatin 2A receptor (Gray et al. 2010) which can be used to locate the preBötC. Respiratory responses to agonists for neurokinin-1 (Gray et al. 1999), N-methyl-d-aspartic acid and somatostatin 2A receptors (Gray et al. 2010) may be appropriate functional markers, but variations in drug diffusion rates and specificity of ligands limit their validity. For instance, the use of the N-methyl-d-aspartic acid receptor agonist d-homocysteic acid, which has a smaller molecular weight than the MOR agonist [d-Ala2, N-MePhe4, Gly-ol]-enkephalin (DAMGO) and can activate various respiratory nuclei, is potentially problematic (Mustapic et al. 2010) as it may reach and diffuse within and outside the targeted neuronal population while ligands with different diffusion capacities may not. To circumvent these issues, one approach is to use the capacity of drugs to diffuse and progressively affect respiratory function as a tool to identify opioid-sensitive brain regions (Montandon et al. 2011). For instance, perfusion of MOR agonists (Fig. 1A and B) close to the preBötC (identified by neurokinin-1 receptors) induced a faster respiratory rate depression than perfusion further away (Fig. 1C). Using the property of opioid drugs to diffuse and progressively reduce respiratory rate, it is possible to create a correlation map (Fig. 1D) that identifies a region of the brainstem that is statistically highly sensitive to MOR agonists. This ‘hotspot’ for MOR agonists corresponds to the preBötC identified by a high expression of neurokinin-1 receptors (Fig. 1D). However, when the MOR agonist endomorphin-1 was perfused in the preBötC region of anaesthetized rats without using such anatomical markers, it had variable effects as it increased, decreased, or had no effects on respiratory rhythm (Lonergan et al. 2003). It is plausible that endomorphin-1 was perfused into the Bötzinger complex, a structure rostral to the preBötC, which would increase respiratory rate. Similarly in decerebrate dogs, perfusion of MOR agonist in the preBötC region (without using neurokinin-1 receptors as a marker) increased respiratory rate rather than decreasing it (Mustapic et al. 2010), which shows that identification of the preBötC with appropriate anatomical markers is essential to accurately study its function.
Figure 1.
Microperfusion of the μ-opioid receptor agonist DAMGO (5 μm) into the preBötC (A) elicits a significant respiratory rate depression (B), with this depression reversed by the μ-opioid receptor antagonist naloxone (100 μm). The latency for a 10% decrease in respiratory rate following microperfusion of DAMGO depends on the distance from preBötC to perfusion site (C) suggesting that microperfusion close to the preBötC elicits a faster decrease than microperfusion further away from it. A correlation map based on latencies and microperfusion sites shows that a region of the medulla highly sensitive to DAMGO (D, red area) corresponds to neurokinin-1-receptor-expressing preBötC neurons. The degree of rate depression induced by DAMGO in freely behaving rats is more pronounced in a state of non-REM sleep (blue circles, E) compared to wakefulness (red circles). In rhythmically active brainstem sections containing the preBötC, only neurokinin-1 receptor-expressing preBötC neurons (F, immunolabelled in red) are hyperpolarized by DAMGO (F, lower traces). Systemic injection of the MOR drug fentanyl (G) lowers respiratory rate whereas no reduction is observed when both preBötCs are locally blocked by naloxone (H). The degree of prevention of respiratory rate depression by naloxone is dependent on the proximity of the perfusion sites to preBötCs (I). aCSF, artificial cerebro-spinal fluid; Non-REM, non-rapid-eye-movement sleep; XII, hypoglossal rootlet; XIIn, hypoglossal motor nucleus; na, nucleus ambiguus; io, inferior olive. Figure adapted from Montandon et al. (2011).
(ii) State-dependent sensitivity to opioids
The state of the respiratory network needs to be clearly assessed as inputs from brain areas active in wakefulness may influence the role of preBötC neurons and their sensitivity to MOR agonists. For instance, states of consciousness strongly impact the role of the preBötC in generating respiratory rhythm (McKay et al. 2005) as the mechanisms and ion channels involved differ between states of active wakefulness and sleep (Montandon & Horner, 2013). In awake goats for instance, preBötC MOR activation had no effects on respiratory rate (Krause et al. 2009). Similarly in freely behaving rats, MOR agonists had little effects in wakefulness, but depressed respiratory rhythm in sleeping (Fig. 1E) or anaesthetized animals (Montandon et al. 2011). In reduced in vitro preparations, where inputs from other brain structures are absent, the preBötC is highly sensitive to MOR agonists (Gray et al. 1999; Manzke et al. 2003).
(iii) Heterogeneity of preBötC neurons
The mechanism mediating inhibition of preBötC neurons by MOR is currently unclear. In cats, MOR agonists did not alter membrane conductance nor did they have postsynaptic actions on neurons of the ventral respiratory group, despite overall slowing of respiratory rhythm (Lalley, 2003). Neuronal responses to MOR agonists, however, vary considerably within the preBötC, not to mention the ventral respiratory group. Only neurokinin-1-receptor-expressing neurons are hyperpolarized by MOR activation (Fig. 1F). Such heterogeneity in MOR sensitivity within the preBötC therefore requires adequate identification of preBötC neuron phenotype before assessing its electrophysiological properties.
Respiratory rate depression following systemic administration of opioid analgesics
The decisive test to identify the neural sites mediating respiratory rate depression consists of blocking MOR locally while administering doses of opioid analgesics systemically. This test, however, may be misinterpreted if local blocking of MOR alone stimulates breathing. The Kölliker–Fuse and parabrachial nuclei, for instance, are important pontine hubs through which nociceptive signals stimulate breathing (Jiang et al. 2004). MOR activation by endogenous opioids in these pontine nuclei inhibits pain circuits and consequently lowers breathing, while MOR inactivation increases respiratory rate. When respiratory depression was induced by systemic opioids, unilateral MOR blockade in the pons increased respiratory rate, which can be interpreted as a reversal of the depressant effects of opioids (Prkic et al. 2012). However, the fact that a unilateral blockade reversed respiratory depression despite having the other side of the pons still inhibited by opioids rather supports the idea that MOR blockade stimulates respiratory rate by blocking endogenous opioids. Similarly, following systemic administration of opioids, MOR blockade in the rostral ventromedial medulla, a structure important in pain modulation, increased breathing (by augmenting amplitude and rate; Phillips et al. 2012). This increase can be interpreted as a reversal of respiratory depression by opioids or stimulation of breathing by blockade of endogenous opioids.
A bilateral approach has been used (Montandon et al. 2011) to determine whether preBötC mediates respiratory rate depression with systemically administered opioids (Fig. 1G). Local administration of naloxone at both preBötCs did not induce a significant increase in respiratory rate, but completely blocked rate depression by a subsequent dose of opioid analgesics (Fig. 1H). Naloxone did not reverse the suppressant effects of opioids on upper airway muscle activity and blood pressure (Fig. 1H and I) as these effects are due to the actions of opioids on other brain areas, as revealed by correlation maps of regions responsive to MOR ligands. Although it is plausible that naloxone diffused beyond the preBötCs and therefore blocked MOR of other respiratory nuclei, this hypothesis is unlikely since the capacity of naloxone to prevent respiratory rate depression depends on the proximity of the probes to the preBötC (Fig. 1I). In addition, when only one side of the medulla was accurately targeted only a partial reversal was observed, unlike what was observed while blocking the pontine nuclei unilaterally (Prkic et al. 2012).
Concluding remarks
There is compelling in vitro and in vivo evidence showing that the preBötC is highly sensitive to MOR agonists and that it mediates respiratory rate depression by opioids. However, despite the clear role of preBötCs in mediating rate depression, it cannot be excluded that the underlying mechanisms may differ between mammals, at various concentrations of opioids and/or with opioids of different affinities for MOR. Here we conclude that of all the possible sites in the central nervous system that mediate respiratory depression by systemically administered opioids, a parsimonious examination of in vivo and in vitro data indicate that: (i) the preBötC is the most sensitive site mediating opioid-induced respiratory rate depression, and (ii) the effects are state dependent, being more pronounced in states of non-rapid-eye-movement sleep and anaesthesia, and less in wakefulness.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief comment. Comments may be posted up to 6 weeks after publication of the article, at which point the discussion will close and authors will be invited to submit a ‘final word’. To submit a comment, go to http://jp.physoc.org/letters/submit/jphysiol;592/6/1159
Biographies
Gaspard Montandon is a Parker B. Francis Fellow in Respiratory Research at the University of Toronto, Departments of Medicine and Physiology. He investigates the mechanisms mediating the generation of rhythmic breathing and how common drugs, such as opioid analgesics, disrupt breathing. Dr Montandon's research aims to understand how G-protein-coupled receptors modulate the brainstem circuitry mediating rhythmic breathing in vivo and the ion channels modulated by neurodepressive drugs.
Richard Horner is Professor of Medicine and Physiology at the University of Toronto and a Canada Research Chair in Sleep and Respiratory Neurobiology. His laboratory investigates the neural networks controlling the brain states of wakefulness and sleep, breathing and motor control, and the mechanisms underlying the effects of sedatives and anaesthetics. Dr Horner is Director of Sleep and Biological Rhythms Toronto, a research and training programme funded by the Canadian Institutes of Health Research. He has published over 100 peer-reviewed articles. 
Additional information
Competing interests
None declared.
References
- Gray PA, Hayes JA, Ling GY, Llona I, Tupal S, Picardo MC, Ross SE, Hirata T, Corbin JG, Eugenin J, Del Negro CA. Developmental origin of preBötzinger complex respiratory neurons. J Neurosci. 2010;30:14883–14895. doi: 10.1523/JNEUROSCI.4031-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Janczewski WA, Mellen N, McCrimmon DR, Feldman JL. Normal breathing requires preBötzinger complex neurokinin-1 receptor-expressing neurons. Nat Neurosci. 2001;4:927–930. doi: 10.1038/nn0901-927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Alheid GF, Calandriello T, McCrimmon DR. Parabrachial-lateral pontine neurons link nociception and breathing. Respir Physiol Neurobiol. 2004;143:215–233. doi: 10.1016/j.resp.2004.07.019. [DOI] [PubMed] [Google Scholar]
- Krause KL, Neumueller SE, Marshall BD, Kiner T, Bonis JM, Pan LG, Qian B, Forster HV. μ-Opioid receptor agonist injections into the presumed pre-Bötzinger complex and the surrounding region of awake goats do not alter eupneic breathing. J Appl Physiol. 2009;107:1591–1599. doi: 10.1152/japplphysiol.90548.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalley PM. μ-Opioid receptor agonist effects on medullary respiratory neurons in the cat: evidence for involvement in certain types of ventilatory disturbances. Am J Physiol Regul Integr Comp Physiol. 2003;285:R1287–R1304. doi: 10.1152/ajpregu.00199.2003. [DOI] [PubMed] [Google Scholar]
- Lonergan T, Goodchild AK, Christie MJ, Pilowsky PM. Mu opioid receptors in rat ventral medulla: effects of endomorphin-1 on phrenic nerve activity. Respir Physiol Neurobiol. 2003;138:165–178. doi: 10.1016/s1569-9048(03)00173-3. [DOI] [PubMed] [Google Scholar]
- McKay LC, Janczewski WA, Feldman JL. Sleep-disordered breathing after targeted ablation of preBötzinger complex neurons. Nat Neurosci. 2005;8:1142–1144. doi: 10.1038/nn1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzke T, Guenther U, Ponimaskin EG, Haller M, Dutschmann M, Schwarzacher S, Richter DW. 5-HT4(a) receptors avert opioid-induced breathing depression without loss of analgesia. Science. 2003;301:226–229. doi: 10.1126/science.1084674. [DOI] [PubMed] [Google Scholar]
- Montandon G, Horner RL. State-dependent contribution of the hyperpolarization-activated Na+/K+ and persistent Na+ currents to respiratory rhythmogenesis in vivo. J Neurosci. 2013;33:8716–8728. doi: 10.1523/JNEUROSCI.5066-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montandon G, Qin W, Liu H, Ren J, Greer JJ, Horner RL. PreBötzinger complex neurokinin-1 receptor-expressing neurons mediate opioid-induced respiratory depression. J Neurosci. 2011;31:1292–1301. doi: 10.1523/JNEUROSCI.4611-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mustapic S, Radocaj T, Sanchez A, Dogas Z, Stucke AG, Hopp FA, Stuth EA, Zuperku EJ. Clinically relevant infusion rates of μ-opioid agonist remifentanil cause bradypnea in decerebrate dogs but not via direct effects in the pre-Bötzinger complex region. J Neurophysiol. 2010;103:409–418. doi: 10.1152/jn.00188.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RS, Cleary DR, Nalwalk JW, Arttamangkul S, Hough LB, Heinricher MM. Pain-facilitating medullary neurons contribute to opioid-induced respiratory depression. J Neurophysiol. 2012;108:2393–2404. doi: 10.1152/jn.00563.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorski M, Lahiri S. Effects of naloxone on carotid body chemoreception and ventilation in the cat. J Appl Physiol. 1981;51:1533–1538. doi: 10.1152/jappl.1981.51.6.1533. [DOI] [PubMed] [Google Scholar]
- Prkic I, Mustapic S, Radocaj T, Stucke AG, Stuth EA, Hopp FA, Dean C, Zuperku EJ. Pontine μ-opioid receptors mediate bradypnea caused by intravenous remifentanil infusions at clinically relevant concentrations in dogs. J Neurophysiol. 2012;108:2430–2441. doi: 10.1152/jn.00185.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Poon BY, Tang Y, Funk GD, Greer JJ. Ampakines alleviate respiratory depression in rats. Am J Respir Crit Care Med. 2006;174:1384–1391. doi: 10.1164/rccm.200606-778OC. [DOI] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stornetta RL, Rosin DL, Wang H, Sevigny CP, Weston MC, Guyenet PG. A group of glutamatergic interneurons expressing high levels of both neurokinin-1 receptors and somatostatin identifies the region of the pre-Bötzinger complex. J Comp Neurol. 2003;455:499–512. doi: 10.1002/cne.10504. [DOI] [PubMed] [Google Scholar]
- Tan W, Janczewski WA, Yang P, Shao XM, Callaway EM, Feldman JL. Silencing preBötzinger complex somatostatin-expressing neurons induces persistent apnea in awake rat. Nat Neurosci. 2008;11:538–540. doi: 10.1038/nn.2104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Xu F, Zhang C, Liang X. Activation of opioid μ receptors in caudal medullary raphe region inhibits the ventilatory response to hypercapnia in anaesthetized rats. Anesthesiology. 2007;107:288–297. doi: 10.1097/01.anes.0000270760.46821.67. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Zhuang J, Zhang C, Xu F. Activation of opioid μ-receptors in the commissural subdivision of the nucleus tractus solitarius abolishes the ventilatory response to hypoxia in anaesthetized rats. Anesthesiology. 2011;115:353–363. doi: 10.1097/ALN.0b013e318224cc1f. [DOI] [PubMed] [Google Scholar]