Abstract
Purines induce transient contraction and prolonged relaxation of detrusor muscles. Transient contraction could be due to activation of inward currents in smooth muscle cells, but the mechanism of purinergic relaxation has not been determined. We recently reported a new class of interstitial cells in detrusor muscles and showed that these cells could be identified with antibodies against platelet-derived growth factor receptor-α (PDGFRα+ cells). The current density of small conductance Ca2+-activated K+ (SK) channels in these cells is far higher (∼100 times) than in smooth muscle cells. Thus, we examined purinergic receptor (P2Y) mediated SK channel activation as a mechanism for purinergic relaxation. P2Y receptors (mainly P2ry1 gene) were highly expressed in PDGFRα+ cells. Under voltage clamp conditions, ATP activated large outward currents in PDGFRα+ cells that were inhibited by blockers of SK channels. ATP also induced significant hyperpolarization under current clamp conditions. A P2Y1 agonist, MRS2365, mimicked the effects of ATP, and a P2Y1 antagonist, MRS2500, inhibited ATP-activated SK currents. Responses to ATP were largely abolished in PDGFRα+ cells of P2ry1−/− mice, and no response was elicited by MRS2365 in these cells. A P2X receptor agonist had no effect on PDGFRα+ cells but, like ATP, activated transient inward currents in smooth muscle cells (SMCs). A P2Y1 antagonist decreased nerve-evoked relaxation. These data suggest that purines activate SK currents via mainly P2Y1 receptors in PDGFRα+ cells. Our findings provide an explanation for purinergic relaxation in detrusor muscles and show that there are no discrete inhibitory nerve fibres. A dual receptive field for purines provides the basis for inhibitory neural regulation of excitability.
Introduction
Overactive bladder is a common condition, affecting approximately 33 million adults in the United States. Symptoms include frequency, urgency, urge incontinence, or nocturia (Gajewski, 2011). Involuntary contractions of the detrusor muscle during bladder filling often occur in overactive bladder. The mechanisms underlying maintenance of relaxation (i.e. suppression of contraction) during bladder filling have not yet been fully elucidated.
Most investigators view purines as excitatory neurotransmitters in bladder muscles (Hoyle, 1994; Fry et al. 2004); however, studies of detrusor muscles have also reported purinergic relaxation responses: (i) exogenous ATP causes transient contraction followed by prolonged relaxation of detrusor muscles (Burnstock et al. 1972; Boland et al. 1993; Palea et al. 1993; Bolego et al. 1995; McMurray et al. 1998; Giglio et al. 2001); (ii) P2Y receptor-mediated SK channel activation is hypothesized to be the mechanism for purinergic relaxation (Obara et al. 1998; King et al. 2004); (iii) apamin (SK channel blocker) increases spontaneous contractility of bladder muscles suggesting ongoing or tonic inhibition of bladder muscles (Thorneloe et al. 2008); and (iv) SK knockout mice display bladder overactivity (Herrera et al. 2003; Thorneloe et al. 2008). Purinergic responses may stabilize the excitability of detrusor muscles during bladder filling and defects in this response could contribute to bladder overactivity. The bladder stabilizing effects of SK conductances have been attributed to responses in smooth muscle cells (SMCs), but the current density of SK channels in SMCs is extremely low (Herrera et al. 2003; Parajuli et al. 2012). Thus, outward currents due to SK channels in SMCs would be neglible at the negative resting potentials of bladder SMCs.
Recently we discovered a new class of interstitial cell in the bladder and identified these cells with antibodies against platelet-derived growth factor receptor-α (PDGFRα; Koh et al. 2012). PDGFRα+ cells have been identified in human, guinea pig and murine detrusor muscles (Koh et al. 2012; Monaghan et al. 2012). PDGFRα+ cells are associated with varicose nerve processes in detrusor muscles, suggesting the cells may be innervated and receive and transduce neurotransmitters. However, little is known about the molecular apparatus of PDGFRα+ cells that might facilitate transduction of neurotransmitters. Previously we demonstrated that the current density of SK channels is at least 100 times higher in PDGFRα+ cells than in SMCs (Lee et al. 2013), suggesting these cells might contribute to maintenance of relaxation during bladder filling and possibly mediate purinergic relaxation responses. In the present study, we investigated the effects of purines on PDGFRα+ cells, the receptors mediating the effects of purines, and the coupling of purine receptor binding to activation of SK channels.
Methods
Preparation of detrusor PDGFRα+ cells and SMCs
Maintenance of animals and all experimental protocols involving animals were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee at the University of Nevada. Briefly, mice were anaesthetized by isofluorane and killed by cervical dislocation before harvesting bladder tissues. C57BL/6, Pdgfratm11(EGFP)Sor/J (both purchased from Jackson Laboratory, Bar Harbor, ME, USA), smMHC/Cre/eGFP (donated by Dr Michael Kotlikoff, Cornell University) and Pdgfratm11(EGFP)Sor/J bred with P2ry1−/− (called P2ry1−/−/eGFP) mice, 3–6 weeks of age, were used for this study. Smooth muscle cells (SMCs) and PDGFRα+ cells were prepared as previously described (Lee et al. 2013). Briefly, dissected bladder detrusor muscles (with urothelial layer peeled away) were incubated in Ca2+-free solution containing 1 mg ml−1 papain and 1 mg ml−1 dithioerythritol (both from Sigma, St Louis, MO, USA) at 37°C for 20–25 min, then rinsed briefly and further digested in Ca2+-free solution including 3–5 mg ml−1 collagenase type II (Sigma) and 100 μm CaCl2 at 37°C for 25–30 min. Single SMCs or PDGFRα+ cells were dissociated by trituration with a fire-polished glass Pasteur pipette. For SMCs, aliquots of the resulting cell suspensions were transferred to a 0.5 ml chamber and allowed to adhere for 10–15 min before experiments. For PDGFRα+ cells, the resulting cell suspensions were plated onto glass coverslips, previously coated with murine collagen (2.5 μg ml−1, Falcon/BD, San Jose, CA). PDGFRα+ cells were maintained in a humidified atmosphere of 95% O2/5% CO2 at 37°C and were used for recordings within 6–8 h after plating.
Electrophysiological recordings
Patch pipettes were pulled from borosilicate capillaries (Sutter Instrument Co., Novato, CA, USA). When filled with pipette solutions, tip resistances were 2–3 MΩ for SMCs and 4–6 MΩ for PDGFRα+ cells. Whole-cell configuration was achieved in Ca2+-containing physiological saline bath solution (mm): NaCl 135, KCl 5, MgCl2 1.2, CaCl2 2, glucose 10, Hepes 10, pH 7.4 with Tris-base. The pipette solution contained (mm): KCl 135, CaCl2 0.012, MgATP 3, Na2GTP 0.1, creatine phosphate disodium 2.5, EGTA 0.1, glucose 10, Hepes 10, pH 7.2 with Tris-base. Whole-cell voltage-and current-clamp techniques were performed. Cells were placed in a 0.5 ml chamber mounted on an inverted microscope (Nikon, Japan). PDGFRα+ cells were identified by the fluorescence of enhanced green fluorescent protein (eGFP) in nuclei. SMCs were easily identified by their rod-shaped morphology. An Axopatch 200B amplifier with a CV–4 headstage (Molecular Devices, Sunnyvale, CA, USA) was used. All data were analysed using pCLAMP software (Axon Instruments, USA) and Graphpad Prism (v. 3.0, Graphpad Software Inc., San Diego, CA, USA). All recordings were made at a room temperature of ∼23°C. The dose–response relationship for ATP was characterized in Graphpad Prism by fitting a Hill equation to calculate the half-maximal response (EC50) with the Hill coefficient.
Isometric force measurements
Standard organ bath techniques were employed to measure the changes in force generated by murine detrusor muscles. One end of the muscle strips was attached to a fixed mount and the other to an isometric force transducer (Fort 10, WPI, Sarasota, FL, USA). Muscles were immersed in organ baths perfused with oxygenated (95% O2 and 5% CO2) Krebs–Ringer bicarbonate buffer (KRB) solution of the following composition (mm): NaCl 118.5, KCl 4.5, MgCl2 1.2, NaHCO3 23.8, KH2PO4 1.2, dextrose 11.0, and CaCl2 2.4. The bath temperature was maintained at 37.5 ± 0.5°C. A resting force of 10 mN was applied to set the muscles at optimum length, and the muscles were allowed to equilibrate for 1–2 h with constant perfusion with KRB solution. Electrical field stimulation (EFS) was applied at frequencies of 0.5, 5, 10 and 20 Hz with 0.5 ms and 150 V of pulse duration and voltage, respectively. Tetrodotoxin (1 μm) completely abolished the responses evoked by EFS. Stimulation at each frequency was maintained for 30 s. This is not a typical protocol for bladder studies, but we were interested in the sustained responses to EFS, not only the peak responses. Mechanical responses were recorded on a computer running LabChart (ADInstruments, Colorado Springs, CO, USA) and measurements of peak amplitude and sustained amplitude during EFS were obtained.
Drugs
All drugs and reagents, including ATP, α,β-methylene ATP (α,β-meATP), MRS2365, MRS2500, apamin and UCL1684, were purchased from Sigma-Aldrich (St Louis, MO, USA) and solubilized in the bath solution for whole-cell recordings. ATP (for SMCs only) and α,β-meATP were applied via a glass pipette (∼1 μm tip diameter, 10 p.s.i.) using a Picospritzer (General Valve Corporation, Picospritzer II, Frankfurt, Germany). The tip of the pipette was located down-stream from the cell with respect to the direction of flow of the superfusing solution, and temporarily repositioned to a point 20 μm up-stream from the cell for the period of application only.
Cell purification, RNA isolation, reverse-transcription PCR and quantitative PCR
PDGFRα+ cells and eGFP/SMCs were purified by fluorescence-activated cell sorting (FACS; Becton Dickinson FACSAria using the blue laser (488 nm) and the GFP emission detector (530 nm/30 nm)). Cells were further purified by the hand collecting of fluorescent cells to maximize purity for molecular tests. Total RNA was isolated from PDGFRα+ cells and eGFP/SMCs using illustra RNAspin Mini RNA Isolation kit (GE Healthcare, Little Chalfont, UK), and first-strand cDNA was synthesized using SuperScript III (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. PCR was performed with specific primers using Go-Taq Green Master Mix (Promega Corp., Madison, WI, USA). The following PCR primers designed against murine sequences were used (GenBank accession number is given in parentheses for the reference nucleotide sequence used): P2ry1 (NM_008772), P2ry2 (NM_008773), P2ry4 (NM_020621), P2ry6 (NM_183168), P2ry12 (NM_027571), P2ry13 (NM_028808) and P2ry14 (NM_133200). PCR products were analysed on 2% agarose gels and visualized by ethidium bromide. Quantitative PCR (qPCR) was performed with the same primers as PCR using Fast SYBR Green chemistry (Applied Biosystems, Foster City, CA, USA) on the 7900HT Real Time PCR System (Applied Biosystems). Regression analysis of the mean values of three multiplex qPCRs for the log10-diluted cDNA was used to generate standard curves. Unknown amounts of messenger RNA (mRNA) were plotted relative to the standard curve for each set of primers and graphically plotted using Microsoft Excel. This gave transcriptional quantification of each gene relative to the endogenous glyceraldehyde 3-phosphate dehydrogenase (Gapdh) standard after log transformation of the corresponding raw data.
Statistical analyses
All data are expressed as means ± SEM. “n” represents the number of experiments. All statistical analyses were performed using Graphpad Prism. A paired Student's t test and one-way analysis of variance (ANOVA) were used to compare groups of data and differences were considered to be significant at P < 0.05.
Results
Purinergic responses of PDGFRα+ cells
We compared transcriptional expression of the seven members of the P2Y receptor family (P2ry1–P2ry14, Abbracchio et al. 2006) in unsorted cells, sorted smooth muscle cells (SMCs) and sorted PDGFRα+ cells. Sorted PDGFRα+ cells showed minimal expression of Kit (marker for interstitial cells of Cajal), Myh11 (SMC marker) and Uchl1 (neuronal marker), and Pdgfra was highly enriched (12-fold vs. unsorted cells) in these cells. Detrusor PDGFRα+ cells expressed P2ry1 dominantly, but P2ry2, P2ry4 and P2ry14 were also enriched relative to unsorted cells and SMCs (Fig. 1).
Figure 1.
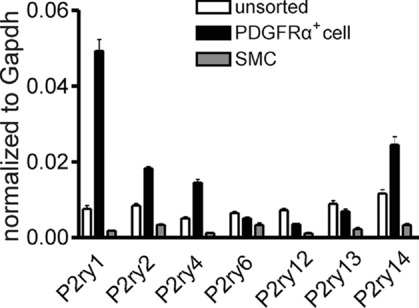
Summary graph showing that P2ry1, P2ry2, P2ry4 and P2ry14 were enriched in PDGFRα+ cells vs. SMCs or unsorted cells. Relative expression of all transcripts was normalized to Gapdh.
Patch clamp experiments using whole-cell configuration were performed to test the effects of ATP (1–1000 μm) on membrane currents in PDGFRα+ cells. ATP activated outward current from a holding potential of −40 mV (Fig. 2A). Ramp depolarizations were applied to cells from −80 mV to +80 mV. Evoked currents during ATP application displayed voltage-independent properties (Fig. 2B). The EC50 for the effects of ATP were obtained by normalizing peak currents evoked at each ATP concentration (1 μm to 1 mm) to the maximal effect of ATP (1 mm) at a holding potential of −40 mV (see Fig. 2A). The EC50 for ATP at −40 mV was 26 μm with a Hill slope of 0.44 ± 0.05 (Fig. 2C, n = 4). Long exposures to ATP (i.e. >5 min, 1 mm) generated sustained outward current responses (Fig. 2D, n = 4). Ramp depolarizations (arrowheads in Fig. 2D) were applied during some of the currents to demonstrate the current–voltage (I–V) properties of the evoked currents. ATP (100 μm) achieved an 80% maximal response, so we utilized this concentration throughout the rest of the experiments. When PDGFRα+ cells were held at −40 mV, ATP (100 μm) induced large outward currents (Fig. 2A). The currents evoked by ATP at −40 mV averaged 5.0 ± 0.9 pA pF–1 (n = 8). When cells were ramped from −80 mV to +80 mV (lower inset in Fig. 2B), a delayed rectifying K+ current was evoked under control conditions (Fig. 2Ba). ATP activated a large voltage-independent outward current and caused a negative shift in reversal potential (towards the K+ equilibrium potential) in response to ramp depolarizations (Fig. 2Bc–e). Under current clamp (I = 0; Fig. 2E), ATP induced hyperpolarization from −31.0 ± 2.4 mV to −74.0 ± 2.4 mV (n = 9, P < 0.0001). ATP activated outward currents at a holding potential of −40 mV in the same cells (dotted line in Fig. 2E).
Figure 2.
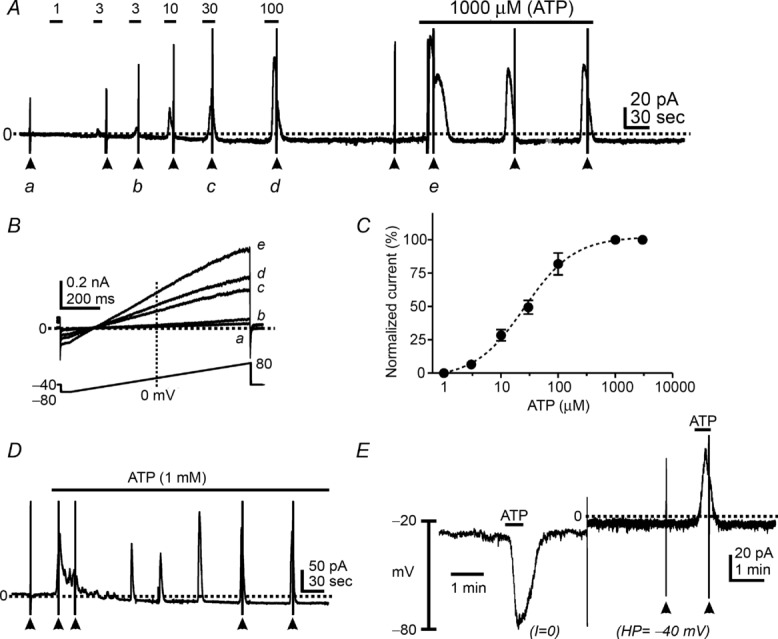
A, ATP (1 μm to 1 mm) activated outward currents in a dose-dependent manner in PDGFRα+ cells at a holding potential of −40 mV. These responses were reproducible upon repetitive or continuous application of ATP. B, expanded time scales of current responses to ramp protocols from −80 mV to +80 mV denoted by a–e in A. C, concentration–response curve of ATP. Calculated EC50 was 26 μm. D, continuous application of ATP generated reproducible outward currents. E, ATP (100 μm) induced hyperpolarization under current-clamp mode (I = 0) and activated outward currents in the same cell after switching to voltage-clamp mode (holding potential: HP = −40 mV). Dotted line denotes 0 pA under voltage clamp. Filled arrowheads denote ramp depolarizations before and during ATP application.
Activation of SK channels by ATP in PDGFRα+ cells
SK3 channels are highly expressed in detrusor PDGFRα+ cells, as shown previously (Lee et al. 2013). Outward currents activated by ATP showed I–V relationships and voltage independence as typical for SK currents (Lee et al. 2013; see Figs 2B, and 3B and D). Thus, we tested the effects of SK channel blockers on the outward currents activated by ATP. Pre-exposure (5–7 min) to apamin (1 μm, SK channel blocker, Herrera et al. 2003; Thorneloe et al. 2008; Parajuli et al. 2012) completely blocked the outward current responses to ATP (n = 7, P < 0.0001). Ramp depolarizations showed the voltage-independent outward currents activated by ATP (Fig. 3Ba), and these currents were completely abolished by apamin. Only a small, voltage-dependent delayed rectifier K+ current was resolved in PDGFRα+ cells after apamin (Fig. 3Bb). A second SK channel blocker (UCL1684) was also tested on the outward currents activated by ATP. Pre-exposure (5–7 min) to UCL1684 (1 μm, Gluais et al. 2005; Diness et al. 2010) inhibited current responses to ATP (Fig. 3C and D, n = 5). These pharmacological data and the I–V properties suggest that SK channels are responsible for the currents activated by ATP.
Figure 3.
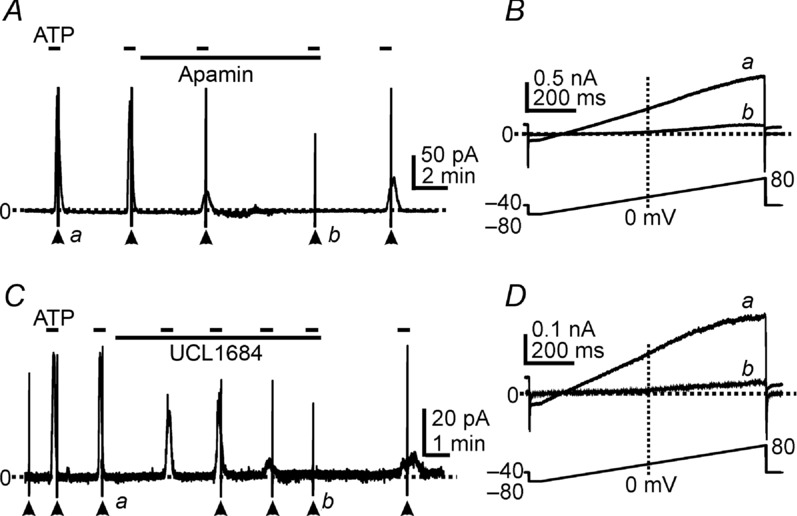
A and C, cells were held at −40 mV. ATP (100 μm) activated outward currents and these responses were inhibited by apamin (1 μm, in A) or UCL1684 (1 μm, in C). Filled arrowheads denote ramp depolarizations applied before and during the period of ATP exposure (see ramp protocol in lower panels in B and D). B and D, expanded time scales of current responses to ramp protocols denoted by a and b in A and C, respectively. ATP (a) activated voltage-independent outward currents, and apamin (Bb) or UCL1684 (Db) inhibited the currents in responses to ATP. Dotted lines denote 0 pA. Short dash lines in pane A & C denote ATP applications.
Activation of SK channels in PDGFRα+ cells by purines is linked to P2Y receptors
P2ry1 transcripts are highly expressed in PDGFRα+ cells (see Fig. 1). We tested the role of P2Y1 receptors in ATP responses in PDGFRα+ cells. We investigated the effects of a P2Y1 agonist (MRS2365, Ravi et al. 2002; Gil et al. 2013) on PDGFRα+ cells. MRS2365 (100 nm) activated outward currents averaging 5.79 ± 0.79 pA pF–1 (n = 8) under voltage-clamp (Fig. 4A) and induced hyperpolarization from −32 ± 2.2 mV to −75 ± 2.8 mV (n = 5) in the same cells under current clamp (I = 0; Fig. 4A). Ramp depolarizations revealed that MRS2365 activated currents with properties similar to the currents activated by ATP (Fig. 4B).
Figure 4.
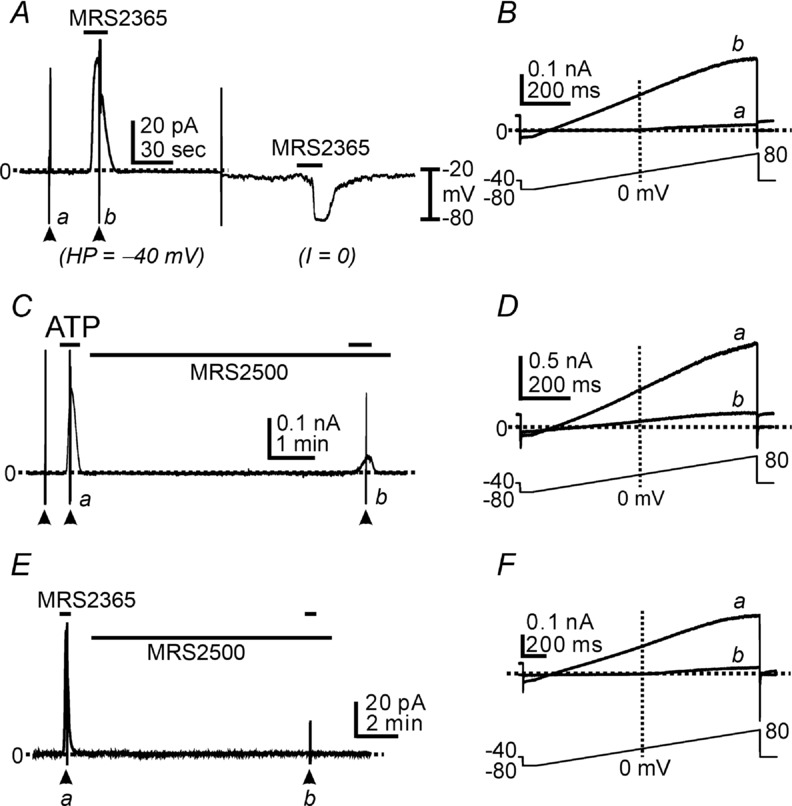
A, P2Y1 agonist (MRS2365, 100 nm) activated outward currents under voltage clamp (HP = −40 mV) and induced hyperpolarization in the same cells in current clamp mode (I = 0). B, expanded time scale of ramp depolarizations indicated by a (control) and b (during MRS2365) in A. C, cells were held at −40 mV. ATP (100 μm) activated the outward currents. MRS2500 (1 μm) significantly inhibited the outward current activated by ATP. D, current traces at an expanded time scale during ramp depolarizations indicated by a (during ATP application) and b (ATP effect in the presence of MRS2500) in C. E, MRS2365 (100 nm) activated the outward currents. MRS2500 (1 μm) completely abolished MRS2365 responses. F, current traces at an expanded time scale during ramp depolarizations indicated by a (MRS2365) and b (MRS2365 effect in the presence of MRS2500) in E. Filled arrowheads denote ramp depolarizations. Dotted lines denote 0 pA.
We also tested the effects of a P2Y1 antagonist, MRS2500 (Kim et al. 2003; Cattaneo et al. 2004; Grasa et al. 2009; Gallego et al. 2012; Hwang et al. 2012). Outward currents activated by ATP at a holding potential of −40 mV were inhibited by MRS2500 (1 μm) by 91.9 ± 3.2% (n = 7; Fig. 4C). Ramp depolarizations revealed the substantial decrease in SK current in the presence of MRS2500, but also showed remaining voltage-independent outward currents after MRS2500 (Fig. 4D). We also tested the effects of MRS2500 on MRS2365-evoked currents. In the presence of MRS2500, MRS2365 (100 nm) failed to activate outward current at −40 mV after pretreatment with MRS2500 (Fig. 4E and F, n = 4).
P2ry1−/− mice bred with the PDGFRα+ cell reporter strain (P2ry1−/−/eGFP mice) were used to further test the involvement of P2Y1 receptors in responses of PDGFRα+ cells to ATP. PDGFRα+ cells from these mice were held under voltage clamp or current clamp and exposed to ATP or the P2Y1 agonist MRS2365. The higher concentration of ATP (0.5 mm) activated small outward currents averaging 0.54 ± 0.11 pA pF–1 (n = 9) in PDGFRα+ cells from P2ry1−/−/eGFP mice (Fig. 5A and C). MRS2365 (200 nm) failed to activate outward currents in PDGFRα+ cells from P2ry1−/−/eGFP mice (n = 8, Fig. 5B and C). These findings are consistent with our pharmacological findings and demonstrate that the dominant current responses to ATP in PDGFRα+ cells are mediated through P2Y1 receptors.
Figure 5.
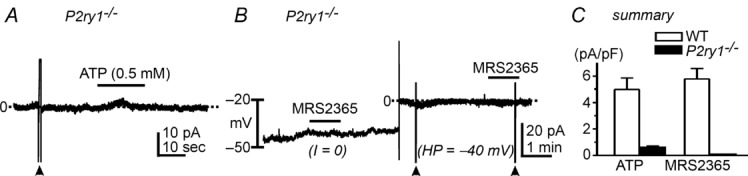
A, cells were held at −40 mV. ATP induced only a small outward current. B, P2Y1 agonist (MRS2365, 200 nm) failed to induce hyperpolarization in current clamp mode (I = 0) or activate the outward currents under voltage clamp (HP = −40 mV). C, summary of the effects of ATP and MRS2365 on membrane currents in PDGFRα+ cells of wild-type (WT) and P2ry1−/− mice. Filled arrowheads denote ramp depolarizations. Dotted lines denote 0 pA.
PDGFRα+ cells do not display responses mediated by P2X receptors
We also tested the effects of α,β-meATP (P2X receptor agonist) and ATP on PDGFRα+ cells held at −60 mV to examine whether these cells also manifest P2X-like responses. α,β-meATP (10 μm) failed to evoke inward current in PDGFRα+ cells (n = 9, Fig. 6A). Both α,β-meATP (1 μm) and ATP (100 μm) evoked transient inward currents in SMCs, but these responses rapidly desensitized such that only minor responses were elicited by 2nd applications of either drug (n = 5 each, see filled circles in Fig. 6B and C).
Figure 6.
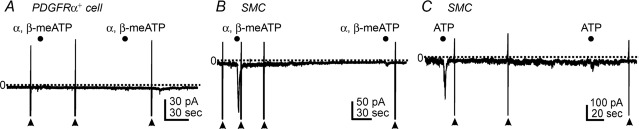
A, α,β-meATP (10 μm, filled circles) had no effect in PDGFRα+ cells at holding potential of −60 mV. B, α,β-meATP (1 μm, filled circles) transiently activated inward current in smooth muscle cells (SMC) held at −60 mV. Responses to 2nd application of α,β-meATP were minimal due to significant desensitization. C, ATP (100 μm, filled circles) also activated transient inward currents in SMCs held at −60 mV. Responses to 2nd application of ATP were minimal due to significant desensitization. Filled arrowheads denote ramp depolarizations. Dotted lines denote 0 pA. Filled circles denote drug applications by Picospritzer (10 p.s.i., 1 s).
Role of detrusor P2Y1 receptor on EFS-induced contractions
We examined the role of P2Y1-mediated relaxations in bladder smooth muscle by testing the effects of the P2Y1 antagonist MRS2500 on responses to electrical field stimulation (EFS). Responses were evoked by 30 s of EFS at various frequencies (0.5–20 Hz) (Fig. 7A and B). The responses evoked by EFS were reproducible upon repetitive stimulation. EFS evoked an initial contraction followed by relaxation from the maximal peak contraction at frequencies of 5 Hz and above. After a 20 min pre-incubation with MRS2500 (1 μm), the peak contractile responses had no effect at all frequencies tested (n = 8, Fig. 7E). We reasoned that the major impact of purinergic (P2Y1-dependent) relaxation would be on the sustained component of the response which might have sustained effects of bladder compliance in vivo. Thus, we tabulated the effects of MRS2500 on EFS-induced responses at the end of the stimulus train (i.e. at 30 s). MRS2500 increased the amplitude of the sustained response to EFS 5–20 Hz significantly (one-way ANOVA; F = 4.288, P = 0.042, n = 8, Fig. 7C, D and F). The effects of MRS2500 were reversible after washout of this compound. These data demonstrate an inhibitory component in nerve-evoked responses that is superimposed upon the contractile responses. The inhibitory component was mediated by P2Y1 receptors.
Figure 7.
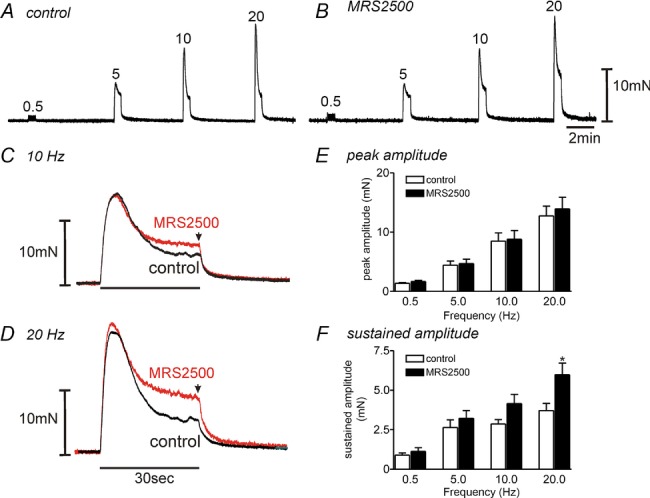
A and B, frequency-dependent responses showed that MRS2500 (1 μm, B) decreased EFS-induced relaxation in comparison to control responses (A) in murine detrusor muscles. C and D, expanded time scale of responses at 10 Hz (C) and 20 Hz (D). EFS applied for 30 s in control and after addition of MRS2500 (1 μm). Arrow denotes sustained amplitude of EFS-induced contraction at 30 s. E and F, summary of peak amplitude (E) and sustained amplitude (F) in response to EFS at several frequencies before and in the presence of MRS2500 (n = 8). *P < 0.05 by one-way ANOVA between control and after MRS2500.
Discussion
We recently reported a new class of interstitial cell in the bladder (Koh et al. 2012; Lee et al. 2013), and others have confirmed the existence of these cells in other species including humans (Monaghan et al. 2012). These cells are identifiable by immunolabelling with antibodies against platelet-derived growth factor receptor-α (PDGFRα+). PDGFRα+ cells are distributed throughout the detrusor muscle, and lie in close apposition to intramural nerve fibres. PDGFRα+ cells express a high current density from SK3 channels, whereas SK current density is very low in SMCs (Herrera et al. 2003; Parajuli et al. 2012). Therefore, we considered the possibility that PDGFRα+ cells might mediate the purinergic relaxation that has been observed in bladder muscles, but not explained, for several decades (Burnstock et al. 1972; Boland et al. 1993; Palea et al. 1993; Bolego et al. 1995; McMurray et al. 1998; Giglio et al. 2001).
ATP activated large amplitude, outward currents in PDGFRα+ cells that were inhibited by blockers of SK channels. ATP also induced significant hyperpolarization in PDGFRα+ cells. A P2Y1 agonist MRS2365 mimicked the responses to ATP, and a potent and selective P2Y1 antagonist, MRS2500, inhibited most of the response to ATP and all of the response to MRS2365. The selectivity of the pharmacological agents used was confirmed by showing that responses to ATP were also greatly diminished in PDGFRα+ cells of P2ry1−/− mice and MRS2365 failed to elicit outward currents and membrane hyperpolarization in these cells. P2Y1 receptors were expressed dominantly in PDGFRα+ cells, but a lesser expression of P2ry2 and P2ry4 receptor transcripts may explain small residual responses to ATP in the presence of MRS2500 and in cells of P2ry1−/− mice. No P2X-like receptor effects were resolved in PDGFRα+ cells (Fig. 6A), but purinergic agonists activated transient P2X-like inward currents in SMCs (Fig. 6B and C). Taken together, our findings suggest that purines activate SK currents in PDGFRα+ cells by binding mainly to P2Y1 receptors.
ATP activated an apamin-and UCL1684-sensitive conductance with voltage-independent properties consistent with SK channels in PDGFRα+ cells. Previous studies showed that SK3 channels are highly expressed in these cells (Lee et al. 2013). The cellular apparatus for activation of SK3 channels in PDGFRα+ cells has not been clarified at present, but several additional observations and common modes of receptor/effector coupling make it likely that the appropriate apparatus is functional in these cells. SK3 channels are activated by enhanced intracellular [Ca2+], and a source of Ca2+ might be release from intracellular stores. P2Y1–P2Y11 receptors are coupled to responses via the G protein Gq/11 (Abbracchio et al. 2006), and the dominant response of PDGFRα+ cells was mediated by P2Y1 receptors. Gq/11 is typically coupled to the activation of phospholipase Cβ and the generation of inositol 1,4,5-trisphosphate (IP3) and diacylglycerol. Thus, release of Ca2+ from IP3 receptors is the likely mechanism coupling P2Y1 receptor binding to the activation of SK3 channels in PDGFRα+ cells. A recent study shows such Ca2+ dynamics in PDGFRα+ cells of the gastrointestinal (GI) tract (Baker et al. 2013).
PDGFRα+ cells in the GI tract are implicated in purinergic inhibitory neurotransmission (Kurahashi et al. 2011, 2012; Hwang et al. 2012). GI muscles are innervated by enteric motor neurons that fall into specific classes of excitatory and inhibitory motor neurons. The latter releases both nitric oxide and a purine neurotransmitter and is responsible for relaxing GI organs during filling and storage and providing relaxation of gut regions immediately preceding propulsive peristaltic contractions (receptive relaxation; Olsson & Holmgren, 2001). There appears to be no distinct population of inhibitory neurons innervating bladder muscles, and it has been rather a mystery as to how bladder compliance increases during bladder filling. Mechanisms intrinsic to smooth muscle cells, such as those involving stretch-activated K+ channels or SK channels, have been proposed as processes by which bladder excitability is restrained during filling (Baker et al. 2010). Apamin treatment and/or gene deactivation of SK channels leads to increased non-voiding contractions, a hallmark of bladder overactivity (Herrera et al. 2003; Thorneloe et al. 2008). Previous studies, however, showed that the expression of SK channels in SMCs results in extremely low current densities in these cells (Herrera et al. 2003; Thorneloe et al. 2008). We tested responses of SMCs at holding potentials simulating resting membrane potentials in detrusor muscles and found little or no outward current attributable to SK channel activation in SMCs (Lee et al. 2013). Thus, the availability of SK channels appears to be dependent upon expression in cells other than SMCs, and PDGFRα+ cells are likely candidates. These cells form gap junctions with SMCs in GI muscles (Komuro, 2006), and if this occurs in the bladder, then conductance changes in either SMCs or PDGFRα+ cells would contribute to the regulation of excitability in the greater syncytium of cells. Experiments have not been performed on animals or bladder muscles lacking stretch-activated K+ channels; however, pharmacological manipulations of these channels increased non-voiding contractions in cystometric tests (Baker et al. 2010).
The question of whether stabilization of excitability during filling occurs via neural regulation of the bladder remains undetermined. Data in the present study suggest that purines released by motor neurons in the vicinity of SMCs and PDGFRα+ cells would have dual effects. Initially there may be a contractile response due to activation of transient inward currents in SMCs carried by P2X receptors (Burnstock & Kennedy, 1985). P2X receptors (predominantly P2X1) mediate nerve-evoked excitatory responses (Vial & Evans, 2000). However, desensitization of these receptors makes it unlikely that inward current and contractions would persist for more than a few seconds. Binding of P2Y1 receptors on PDGFRα+ cells would convert purinergic responses to net inhibitory effects due to activation of SK currents in these cells. As we showed, the generation of outward currents due to the binding of P2Y1 receptors is quite sustainable, and net purinergic relaxation responses therefore may persist for many minutes. Outward currents activated by ATP were not tonic, but activated in transients, as might occur from periodic Ca2+ release events. Summation of outward currents, however, in hundreds or thousands of PDGFRα+ cells in detrusor muscles would yield net hyperpolarization and membrane stabilization.
Previous studies have described biphasic contractile responses to exogenous ATP and to sustained nerve stimulation, characterized by initial contraction followed by sustained relaxation (Boland et al. 1993; Bolego et al. 1995; McMurray et al. 1998; Giglio et al. 2001). These responses were suggested to be due to superposition of excitatory and inhibitory responses to purines in bladder muscles. Relaxation responses were enhanced with multiple applications of purines as the P2X-driven excitatory phase desensitized or by pre-desensitization by α,β-meATP (Boland et al. 1993; Bolego et al. 1995; McMurray et al. 1998). Our findings, localizing purinergic inhibitory effects to PDGFRα+ cells, may provide the basis for a novel neural regulatory mechanism for the stabilization of bladder excitability during the filling phase. We observed that during extended neural stimulation of bladder muscles, the sustained component of contraction is significantly reduced by P2Y1-mediated purinergic regulation. The detrusor muscle is also innervated by adrenergic nerves that act via adrenergic β (mainly β3) receptors and cause bladder relaxation (Brown et al. 2008; Afeli et al. 2012). However, we found that these neurons were exceedingly rare in detrusor muscles of mice (data not shown).
In conclusion, this study shows a novel role for PDGFRα+ cells in the detrusor muscles of the bladder. These cells express P2Y receptors and develop large amplitude, outward currents due to the activation of SK channels in response to purines. Purines released from autonomic neurons may have dual actions in micturition: activation of contraction during voiding contractions and stabilization of membrane potential and excitability during bladder filling. While discrete populations of inhibitory neurons do not appear to innervate the bladder, there may be bimodal purinergic responses due to two contradistinctive receptive fields provided by P2X-receptor-predominant SMCs and P2Y1-predominant PDGFRα+ cells. Temporal differences in the responses activated by these receptors suggest, on the basis of duration, that neurogenic purinergic responses are predominantly inhibitory. Purinergic inhibitory input, mediated by P2Y1 receptors, may be an important factor in stabilizing bladder excitability during filling. This realization may open important and novel areas of investigation in urodynamics, new understanding of the pathophysiology of bladder dysfunction, and new opportunities for an effective treatment for bladder overactivity.
Key points
Platelet-derived growth factor receptor-α-positive (PDGFRα+) interstitial cells in detrusor muscles may participate in post-junctional responses to neurotransmitters.
PDGFRα+ interstitial cells express purinergic receptors (P2Y) and small conductance Ca2+-activated K+ channels (mainly SK3). ATP elicited large amplitude outward currents and hyperpolarization in PDGFRα+ cells. SK channel blockers and a P2Y1 receptor antagonist blocked responses to ATP.
ATP elicited only minor responses in PDGFRα+ cells of P2ry1−/− mice. ATP elicited transient inward currents in smooth muscle cells and purinergic receptor (P2X) agonists had no effect on PDGFRα+ cells.
A specific P2Y1 receptor blocker decreased electrical field stimulation-induced relaxation.
Our findings provide an explanation for the purinergic relaxation of detrusor muscles and describe a novel mechanism for inhibitory regulation of bladder muscles that may control detrusor excitability during the filling phase.
Acknowledgments
We are grateful to Nancy Horowitz for technical assistance, and Huili Zheng for help with breeding and maintenance of the transgenic animals.
Glossary
- α,β-meATP
α,β-methylene ATP
- EFS
electrical field stimulation
- eGFP
enhanced green fluorescent protein
- GI
gastrointestinal
- KRB
Krebs–Ringer bicarbonate buffer
- SK
small conductance Ca2+-activated K+ channel
- PDGFRα
platelet-derived growth factor receptor-α
- SMCs
smooth muscle cells
Additional information
Competing interests
None declared.
Author contributions
All experiments were performed in the laboratories of the Department of Physiology and Cell Biology at the University of Nevada, Reno. Most of the data were collected and analysed by H.L. with the assistance of other authors. Immunohistochemistry and molecular study were performed by B.H.K. and L.E.P. K.M.S., S.D.K. and H.L. shared in the design of experiments, interpretation of the data and the writing of the manuscript. All authors approved the final version of the manuscript.
Funding
This project was supported by NIH/NIDDK grant R01 DK098388.
References
- Abbracchio MP, Burnstock G, Boeynaems JM, Barnard EA, Boyer JL, Kennedy C, Knight GE, Fumagalli M, Gachet C, Jacobson KA, Weisman GA. International Union of Pharmacology LVIII: update on the P2Y G protein-coupled nucleotide receptors: from molecular mechanisms and pathophysiology to therapy. Pharmacol Rev. 2006;58:281–341. doi: 10.1124/pr.58.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afeli SA, Hristov KL, Petkov GV. Do β3-adrenergic receptors play a role in guinea pig detrusor smooth muscle excitability and contractility. Am J Physiol Renal Physiol. 2012;302:F251–F263. doi: 10.1152/ajprenal.00378.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker SA, Hatton WJ, Han J, Hennig GW, Britton FC, Koh SD. Role of TREK–1 potassium channel in bladder overactivity after partial bladder outlet obstruction in mouse. J Urol. 2010;183:793–800. doi: 10.1016/j.juro.2009.09.079. [DOI] [PubMed] [Google Scholar]
- Baker SA, Hennig GW, Salter AK, Kurahashi M, Ward SM, Sanders KM. Distribution and Ca2+ signalling in fibroblast-like (PDGFRα+) cells in the murine gastric fundus. J Physiol. 2013;591:6193–6208. doi: 10.1113/jphysiol.2013.264747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boland B, Himpens B, Paques C, Casteels R, Gillis JM. ATP induced-relaxation in the mouse bladder smooth muscle. Br J Pharmacol. 1993;108:749–753. doi: 10.1111/j.1476-5381.1993.tb12872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolego C, Pinna C, Abbracchio MP, Cattabeni F, Puglisi L. The biphasic response of rat vesical smooth muscle to ATP. Br J Pharmacol. 1995;114:1557–1562. doi: 10.1111/j.1476-5381.1995.tb14939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown SM, Bentcheva-Petkova LM, Liu L, Hristov KL, Chen M, Kellett WF, Meredith AL, Aldrich RW, Nelson MT, Petkov GV. β–Adrenergic relaxation of mouse urinary bladder smooth muscle in the absence of large-conductance Ca2+-activated K+ channel. Am J Physiol Renal Physiol. 2008;295:F1149–F1157. doi: 10.1152/ajprenal.00440.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Kennedy C. Is there a basis for distinguishing two types of P2-purinoceptor. Gen Pharmacol. 1985;16:433–440. doi: 10.1016/0306-3623(85)90001-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Satchell DG, Smythe A. A comparison of the excitatory and inhibitory effects of non-adrenergic, non-cholinergic nerve stimulation and exogenously applied ATP on a variety of smooth muscle preparations from different vertebrate species. Br J Pharmacol. 1972;46:234–242. doi: 10.1111/j.1476-5381.1972.tb06868.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo M, Lecchi A, Ohno M, Joshi BV, Besada P, Tchilibon S, Lombardi R, Bischofberger N, Harden TK, Jacobson KA. Antiaggregatory activity in human platelets of potent antagonists of the P2Y1 receptor. Biochem Pharmacol. 2004;68:1995–2002. doi: 10.1016/j.bcp.2004.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diness JG, Sørensen US, Nissen JD, Al-Shahib B, Jespersen T, Grunnet M, Hansen RS. Inhibition of small-conductance Ca2+-activated K+ channels terminates and protects against atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:380–390. doi: 10.1161/CIRCEP.110.957407. [DOI] [PubMed] [Google Scholar]
- Fry CH, Ikeda Y, Harvey R, Wu C, Sui GP. Control of bladder function by peripheral nerves: avenues for novel drug targets. Urology. 2004;63:24–31. doi: 10.1016/j.urology.2003.10.031. [DOI] [PubMed] [Google Scholar]
- Gajewski JB. Patients with medication-refractory OAB symptoms should be further treated with neuromodulation. Can Urol Assoc J. 2011;5:283–284. doi: 10.5489/cuaj.11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallego D, Gil V, Martínez-Cutillas M, Mañé N, Martín MT, Jiménez M. Purinergic neuromuscular transmission is absent in the colon of P2Y1 knocked out mice. J Physiol. 2012;590:1943–1956. doi: 10.1113/jphysiol.2011.224345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglio D, Delbro DS, Tobin G. On the functional role of muscarinic M2 receptors in cholinergic and purinergic responses in the rat urinary bladder. Eur J Pharmacol. 2001;428:357–364. doi: 10.1016/s0014-2999(01)01286-9. [DOI] [PubMed] [Google Scholar]
- Gil V, Martínez-Cutillas M, Mañé N, Martín MT, Jiménez M, Gallego D. P2Y1 knockout mice lack purinergic neuromuscular transmission in the antrum and cecum. Neurogastroenterol Motil. 2013;25:e170–182. doi: 10.1111/nmo.12060. [DOI] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Feletou M. Role of SKCa and IKCa in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery. Br J Pharmacol. 2005;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grasa L, Gil V, Gallego D, Martín MT, Jiménez M. P2Y1 receptors mediate inhibitory neuromuscular transmission in the rat colon. Br J Pharmacol. 2009;158:1641–1652. doi: 10.1111/j.1476-5381.2009.00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera GM, Pozo MJ, Zvara P, Petkov GV, Bond CT, Adelman JP, Nelson MT. Urinary bladder instability induced by selective suppression of the murine small conductance calcium-activated potassium (SK3) channel. J Physiol. 2003;551:893–903. doi: 10.1113/jphysiol.2003.045914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoyle CH( Non-adrenergic, non-cholinergic control of the urinary bladder. World J Urol. 1994;12:233–244. doi: 10.1007/BF00191202. [DOI] [PubMed] [Google Scholar]
- Hwang SJ, Blair PJ, Durnin L, Mutafova-Yambolieva V, Sanders KM, Ward SM. P2Y1 purinoreceptors are fundamental to inhibitory motor control of murine colonic excitability and transit. J Physiol. 2012;590:1957–1972. doi: 10.1113/jphysiol.2011.224634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HS, Ohno M, Xu B, Kim HO, Choi Y, Ji XD, Maddileti S, Marquez VE, Harden TK, Jacobson KA. 2-Substitution of adenine nucleotide analogues containing a bicyclo[3.1.0]hexane ring system locked in a northern conformation: enhanced potency as P2Y1 receptor antagonists. J Med Chem. 2003;46:4974–4987. doi: 10.1021/jm030127+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Knowles ID, Burnstock G, Ramage AG. Investigation of the effects of P2 purinoceptor ligands on the micturition reflex in female urethane-anaesthetized rats. Br J Pharmacol. 2004;142:519–530. doi: 10.1038/sj.bjp.0705790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM, Koh SD. Platelet-derived growth factor receptor–α cells in mouse urinary bladder: a new class of interstitial cells. J Cell Mol Med. 2012;16:691–700. doi: 10.1111/j.1582-4934.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro T. Structure and organization of interstitial cells of Cajal in the gastrointestinal tract. J Physiol. 2006;576:653–658. doi: 10.1113/jphysiol.2006.116624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J Cell Mol Med. 2012;16:1397–1404. doi: 10.1111/j.1582-4934.2011.01510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurahashi M, Zheng H, Dwyer L, Ward SM, Don KS, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol. 2011;589:697–710. doi: 10.1113/jphysiol.2010.201129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Koh BH, Peri LE, Sanders KM, Koh SD. Functional expression of SK channels in murine detrusor PDGFRα+ cells. J Physiol. 2013;591:503–513. doi: 10.1113/jphysiol.2012.241505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray G, Dass N, Brading AF. Purinoceptor subtypes mediating contraction and relaxation of marmoset urinary bladder smooth muscle. Br J Pharmacol. 1998;123:1579–1586. doi: 10.1038/sj.bjp.0701774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monaghan KP, Johnston L, McCloskey KD. Identification of PDGFRα positive populations of interstitial cells in human and guinea pig bladders. J Urol. 2012;188:639–647. doi: 10.1016/j.juro.2012.03.117. [DOI] [PubMed] [Google Scholar]
- Obara K, Lepor H, Walden PD. Localization of P2Y1 purinoceptor transcripts in the rat penis and urinary bladder. J Urol. 1998;160:587–591. [PubMed] [Google Scholar]
- Olsson C, Holmgren S. The control of gut motility. Comp Biochem Physiol A Mol Integr Physiol. 2001;128:481–503. doi: 10.1016/s1095-6433(00)00330-5. [DOI] [PubMed] [Google Scholar]
- Palea S, Artibani W, Ostardo E, Trist DG, Pietra C. Evidence for purinergic neurotransmission in human urinary bladder affected by interstitial cystitis. J Urol. 1993;150:2007–2012. doi: 10.1016/s0022-5347(17)35955-4. [DOI] [PubMed] [Google Scholar]
- Parajuli SP, Soder RP, Hristov KL, Petkov GV. Pharmacological activation of small conductance calcium-activated potassium channels with naphtho[1,2-d]thiazol-2-ylamine decreases guinea pig detrusor smooth muscle excitability and contractility. J Pharmacol Exp Ther. 2012;340:114–123. doi: 10.1124/jpet.111.186213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravi RG, Kim HS, Servos J, Zimmermann H, Lee K, Maddileti S, Boyer JL, Harden TK, Jacobson KA. Adenine nucleotide analogues locked in a Northern methanocarba conformation: enhanced stability and potency as P2Y1 receptor agonists. J Med Chem. 2002;45:2090–2100. doi: 10.1021/jm010538v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorneloe KS, Knorn AM, Doetsch PE, Lashinger ES, Liu AX, Bond CT, Adelman JP, Nelson MT. Small-conductance, Ca2+-activated K+ channel 2 is the key functional component of SK channels in mouse urinary bladder. Am J Physiol Regul Integr Comp Physiol. 2008;294:R1737–R1743. doi: 10.1152/ajpregu.00840.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vial C, Evans RJ. P2X receptor expression in mouse urinary bladder and the requirement of P2X1 receptors for functional P2X receptor responses in the mouse urinary bladder smooth muscle. Br J Pharmacol. 2000;131:1489–1495. doi: 10.1038/sj.bjp.0703720. [DOI] [PMC free article] [PubMed] [Google Scholar]


