Abstract
Studies have shown increased incorporation of omega-3 fatty acids into whole skeletal muscle following supplementation, although little has been done to investigate the potential impact on the fatty acid composition of mitochondrial membranes and the functional consequences on mitochondrial bioenergetics. Therefore, we supplemented young healthy male subjects (n = 18) with fish oils [2 g eicosapentaenoic acid (EPA) and 1 g docosahexanoic acid (DHA) per day] for 12 weeks and skeletal muscle biopsies were taken prior to (Pre) and following (Post) supplementation for the analysis of mitochondrial membrane phospholipid composition and various assessments of mitochondrial bioenergetics. Total EPA and DHA content in mitochondrial membranes increased (P < 0.05) ∼450 and ∼320%, respectively, and displaced some omega-6 species in several phospholipid populations. Mitochondrial respiration, determined in permeabilized muscle fibres, demonstrated no change in maximal substrate-supported respiration, or in the sensitivity (apparent Km) and maximal capacity for pyruvate-supported respiration. In contrast, mitochondrial responses during ADP titrations demonstrated an enhanced ADP sensitivity (decreased apparent Km) that was independent of the creatine kinase shuttle. As the content of ANT1, ANT2, and subunits of the electron transport chain were unaltered by supplementation, these data suggest that prolonged omega-3 intake improves ADP kinetics in human skeletal muscle mitochondria through alterations in membrane structure and/or post-translational modification of ATP synthase and ANT isoforms. Omega-3 supplementation also increased the capacity for mitochondrial reactive oxygen species emission without altering the content of oxidative products, suggesting the absence of oxidative damage. The current data strongly emphasize a role for omega-3s in reorganizing the composition of mitochondrial membranes while promoting improvements in ADP sensitivity.
Introduction
Skeletal muscle is a key tissue influencing metabolic homeostasis, as it represents ∼40% of body weight, is highly adaptable and has a variable metabolic rate. Within muscle, the transport of both carbohydrate and fatty acids across membrane bilayers represents a key regulatory step influencing fuel selection and overall metabolism. Omega-3 supplementation, particularly with fish oils enriched with eicosapentaenoic acid (EPA; 20:5n-3) and DHA (docosahexanoic acid; 22:6n-3), results in significant incorporation of polyunsaturated fatty acids (PUFAs) into numerous membrane phospholipid species within whole skeletal muscle (Yamaoka et al. 1988; Andersson et al. 2002; Owen et al. 2004; Dangardt et al. 2012).
Alterations in dietary fatty acid composition are therefore thought to alter the thickness, stiffness and fluidity of the lipid bilayer, impacting metabolism. To date, studies examining the effect of EPA and DHA supplementation on skeletal muscle membrane composition have been limited to whole muscle measurements. Nonetheless, DHA represents a primary fatty acid in skeletal muscle mitochondrial phospholipids (Fiehn et al. 1971; Tsalouhidou et al. 2006) and it has been suggested that DHA is required for the organization and function of membrane proteins (Infante & Huszagh, 2000). This may be particularly important in mitochondria, where electron transfer is tightly coupled between the complexes of the embedded electron transport chain. Therefore, accumulation of omega-3 PUFAs within mitochondrial membranes may affect mitochondrial bioenergetics, a suggestion supported by the recent observation that the unsaturation index of mitochondrial membranes in rodents is positively associated with rates of palmitate oxidation (Holloway et al. 2012). However, it remains to be determined if mitochondrial membrane composition is altered in human skeletal muscle following omega-3 PUFA supplementation.
Direct evidence suggesting that mitochondrial oxidative phosphorylation is improved following omega-3 PUFA supplementation does not exist in human skeletal muscle. However, it has been shown that rats fed omega-3 PUFAs utilized less oxygen for a given twitch force both during and recovering from multiple bouts of prolonged contraction (Peoples & McLennan, 2010). This indirectly suggests improved mitochondrial efficiency with omega-3 intake, resulting either from greater content of the electron transport complexes, or from enhanced kinetics of existing proteins. Although mitochondrial changes have not been investigated in detail in skeletal muscle following omega-3 PUFA supplementation, initial work in rat heart mitochondria demonstrated increased in vitro ATP synthase activity consistent with an improvement in mitochondrial respiratory function following supplementation. However, direct assessments of maximal mitochondrial respiration suggested that omega-3 supplementation decreases (Yamaoka et al. 1988) or has no effect (Khairallah et al. 2012; Lanza et al. 2013) on mitochondrial respiration in either heart or skeletal muscle, complicating interpretations. One limitation of these studies is that mitochondrial function has been exclusively determined in the presence of saturating ADP concentrations. As such, these studies reflect the in vitro capacity for oxidative phosphorylation and probably not the in vivo situation, as ADP transport appears to be highly regulated (Perry et al. 2012). From a physiological standpoint, it may be more appropriate to examine mitochondrial function in the presence of submaximal concentrations of ADP, although this has yet to be considered following omega-3 PUFA supplementation. The metabolic consequence of altering omega-3 PUFA availability therefore remains ambiguous.
The primary purpose of the current study was to investigate the effects of prolonged fish oil supplementation on mitochondrial content, mitochondrial bioenergetics and submaximal respiratory substrate kinetics in human vastus lateralis muscle. Specifically, we aimed to determine if omega-3 PUFA supplementation altered (1) mitochondrial membrane composition, (2) maximal ADP and pyruvate-supported respiration, (3) the dynamic response and/or sensitivity of the electron transport chain to ADP and pyruvate, and (4) the propensity for mitochondrial reactive oxygen species (ROS) production. We found that omega-3 PUFA supplementation resulted in pronounced omega-3 incorporation into mitochondrial membranes without altering maximal ADP-supported respiration. In contrast, mitochondrial ADP sensitivity, submaximal ADP-stimulated respiration and maximal mitochondrial ROS emission were all increased following fish oil supplementation. The improved sensitivity of mitochondria to ADP was independent of creatine kinase involvement, changes in the protein content of ATP synthase or ADP transporters. The current data therefore provide direct evidence that omega-3 PUFA supplementation improves mitochondrial respiratory function in human skeletal muscle.
Methods
Subjects and supplementation
Eighteen healthy, recreationally active men (22.7 ± 0.8 years, 82.1 ± 2.2 kg and 182.8 ± 1.5 cm) were recruited in two rounds for this study. Mitochondria were isolated for phospholipid analysis from the first nine participants, while ADP respiratory kinetics in the presence of creatine were determined in the last nine participants. All other assessments of mitochondrial bioenergetics were determined in all 18 participants. The two groups of participants (n = 9 per group) completed the study separated by 12 months. Importantly, mitochondrial ROS, ADP respiratory kinetics and pyruvate respiratory kinetics, were measured in both groups, and showed the same statistical responses. Therefore, we have reported data for these measurements on all individuals (n = 18).
All participants were free of disease and were not on prescription medication or supplements. Subjects were placed on Omega-3 Complete (Jamieson Laboratories Ltd, Windsor, Canada) on a dose of five pills per day (omega-3 intake: 2 g EPA, 1 g DHA per day) for a 12 week period. Prior to (Pre) and following (Post) the intervention two biopsies were taken from the vastus lateralis. A small portion of the first biopsy was immediately used for the preparation of permeabilized fibres (described below) with the remaining portion flash-frozen for Western blotting. The second biopsy was used for the isolation of mitochondria. This study was approved by the Research Ethics Boards of the University of Guelph (Guelph, Ontario) and McMaster University (Hamilton, Ontario), and conforms to the Declaration of Helsinki.
Mitochondrial isolation
Mitochondrial subsarcolemmal (SS) and intermyofibrillar (IMF) populations were isolated by differential centrifugation as described previously (Campbell et al. 2004) with minor modifications. Muscle was quickly minced and diluted in 1 ml mg−1 isolation buffer [100 mm sucrose, 100 mm KCl, 50 mm Tris-HCl, 1 mm KH2PO4, 0.1 mm EGTA, 0.2% bovine serum albumin (BSA), 1 mm ATP; pH 7.4] and homogenized using a polytron with a teflon head. SS mitochondria underwent centrifugation at 800 g for 10 min and the supernatant was pelleted at 10,000 g for 10 min before being washed in MiR05, (detailed below) and spun again at 10,000 g for 10 min. After the initial 800 g spin, the IMF mitochondrial pellet was resuspended 10-fold in isolation buffer and treated with 0.25 μg protease/mg muscle for 5 min before being diluted 10-fold in isolation buffer and spun again at 5000 g for 5 min. The IMF pellet was resuspended in isolation buffer and pelleted at 10,000 g for 10 min before being washed in MiR05, and spun again for 10 min at 10,000 g. The final pellets for both SS and IMF isolations were combined and resuspended in 100 μl S&M solution (225 mm mannitol, 75 mm sucrose, 10 mm Tris-HCl, 0.1 mm EDTA; pH 7.4). Mitochondria were further purified using a percoll gradient and pooled for analysis.
Mitochondrial lipid analysis
The membrane phospholipid composition of isolated mitochondria was determined using gas chromatography compared to an internal 15:0 standard as previously described (Bonen et al. 2004). Briefly, the phospholipid fraction was separated by thin-layer chromatography. The gel bands corresponding to the standards were scrapped off the plates, transferred into fresh tubes and then transmethylated in 14% methanolic boron trifluoride (Sigma) at 100°C for 30 min. Individual fatty acid methyl esters were identified and quantified according to the retention times of standards by gas liquid chromatography [Hewlett-Packard 5890 Series II gas chromatograph, HP Varian CP-SIL capillary column (50 m × 0.25 mm internal diameter) and flame-ionization detector (Agilent Technologies, CA, USA]. Total phospholipid content was estimated as the sum of the particular fatty acid species of the assessed fraction and expressed in nanomoles per gram of tissue.
Preparation of permeabilized fibres
A small portion of each biopsy was placed in ice-cold BIOPS (50 mm MES, 7.23 mm K2EGTA, 2.77 mm CaK2EGTA, 20 mm imidazole, 0.5 mm dithiothreitol, 20 mm taurine, 5.77 mm ATP, 15 mm PCr and 6.56 mm MgCl2.H2O; pH 7.1) and separated under a microscope into bundles using fine-tipped forceps as described previously (Perry et al. 2012). Fibre bundles were then treated with 30 μg ml−1 saponin for 30 min at 4°C, then either washed for 15 min in MiR05 respiration buffer (0.5 mm EGTA, 10 mm HH2PO4, 110 mm sucrose and 1 mg ml−1 fatty acid-free BSA; pH 7.1) for respiration analysis, or buffer Z (105 mm K-MES, 30 mm KCl, 1 mm EGTA, 10 mm KH2PO4, 5 mm MgCl2.H2O, 0.005 mm pyruvate, 0.002 mm malate with 5 mg ml−1 BSA; pH 7.4) for measurements of H2O2 emission as described below.
Mitochondrial respiration
Measurements of O2 consumption were performed in MiR05 respiration medium on prepared permeabilized fibres using an Oxygraph-2K (Oroboros Instruments, Innsbruck, Austria) at 37°C in the presence of 25 μm blebbistatin as previously described (Perry et al. 2011). Mitochondrial kinetics were analysed using three separate titration protocols. ADP kinetics were analysed in the presence and absence of 20 mm creatine, separately. Both ADP titrations were initiated with 5 mm malate and 10 mm pyruvate, and ADP was titrated at various concentrations. Pyruvate kinetics were determined in the presence of 5 mm ADP, 5 mm malate and 20 mm creatine, while pyruvate was titrated at various concentrations. Then, 10 mm glutamate and 10 mm succinate were added following the pyruvate protocol to determine maximum mitochondrial respiration. All fibres were recovered from the respirometer, freeze-dried and weighed for normalization to muscle bundle weight. The apparent Km for pyruvate and ADP was determined as previously described (Perry et al. 2012).
H2O2 emission
Mitochondrial H2O2 emission was measured in buffer Z fluorometrically (Lumina, Thermo Scientific, Waltham, MA, USA) at 37°C in the presence of 25 μm blebbistatin. Cuvettes contained 10 μg ml−1 oligomycin, 10 μm Amplex Red (Invitrogen, Carlsbad, CA, USA), 0.5 U ml−1 horseradisch peroxidase and 40 U ml−1 superoxide dismutase. The reaction was initiated by the addition of 10 mm succinate and H2O2 emission was calculated from the slope (absorbance min–1) and made relative to fibre dry weight as performed previously (Anderson et al. 2009).
Western blotting
Whole muscle was homogenized in lysis buffer, diluted to 1 μg μl−1, and loaded equally for α-tubulin (Abcam, Cambridge, MA, USA), ANT1 (MitoSciences, Eugene, OR, USA), ANT2 (Abcam), 4HNE (Alpha Diagnostics, San Antonio, TX, USA) and OXPHOS (MitoSciences), and for use of the Oxyblot protein oxidation detection kit (Millipore, Billerica, MA, USA). Proteins were separated by SDS-PAGE, transferred to polyvinylidene difluoride membranes, and incubated in blocking solution, primary antibody, and the corresponding secondary antibody as specified by the supplier. Membrane proteins were detected by enhanced chemiluminesence (ChemiGenius2 Bioimaging system, SynGene, Cambridge, UK).
To confirm homogeneity in the OXPHOS content between fibre bundles, Western blotting was performed on recovered fibres as previously described (Murphy, 2011) and modified (Lally et al. 2013). Briefly, permeabilized fibres were digested in 5 μl μg−1 digestion buffer [10% glycerol (w/v), 5% β-mercapoethanol (v/v), 2.3% SDS (w/v) in 62.5 mm Tris-HCl, pH 6.8] for 15 min at 65°C with gentle shaking. For determination of OXPHOS protein content, 10 μl was loaded onto a 12% SDS-polyacrylamide gel and run and detected as described above. Due to the sensitive nature of complex IV, the heating preparation made this protein undetectable with this method.
Statistics
All values are presented as mean ± SEM. The apparent Km values for pyruvate and ADP were determined using Prism (GraphPad Software, Inc., La Jolla, CA, USA), as published previously (Smith et al. 2013). All pre-and post-supplementation measures were analysed for significance using a paired t test with the α-value set to P < 0.05.
Results
Mitochondrial phospholipid composition
The purity of our mitochondrial isolations was first confirmed by the presence of mitochondrial proteins as well as the absence of sarcoplasmic reticulum (SERCA) and plasma membrane (caveolin-1) proteins (Fig. 1). The effect of n-3 fish oil supplementation on mitochondrial membrane phospholipid composition was thus determined in highly purified mitochondrial samples. Specifically, we examined the total abundance of α-linolenic acid (ALA, 18:3n-3), EPA (20:5n-3) and DHA (22:6n-3), as well as their incorporation into phosphatidylcholine (PC), phosphatidylethanolamine (PE), cardiolipin (CL), sphingomielin (SM), phosphatidylinositol (PI) and phosphatidylserine (PS). Although the omega-3 intervention did not alter total mitochondrial membrane phospholipid content (Table 1), the EPA and DHA present in the fish oil supplements increased total membrane incorporation of these PUFAs ∼4.5-and ∼3-fold, respectively, without altering the content of ALA (Fig. 2A). EPA increased ∼8.8-and ∼3.9-fold in PC and PE fractions, respectively, while either remaining unchanged or undetectable in other phospholipid fractions (Fig. 2A). DHA incorporation increased ∼6.4-and ∼2.9-fold in PC and PE fractions, respectively, while also increasing ∼2.9-fold in PS.
Figure 1.
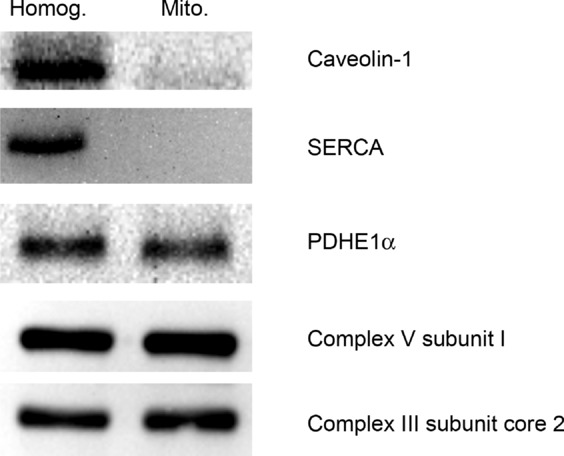
Representative Western blots of whole muscle lysates (Homog.) and isolated mitochondria (Mito.) showing the presence of known mitochondrial proteins and the absence of plasma membrane (caveolin-1) and sarcoplasmic reticulum (SERCA) proteins. Mitochondrial proteins included the E1α subunit pyruvate dehydrogenase (PDHE1α) and subunits of complex 3 and 4 of the electron transport chain. These were loaded as 30 μg of whole muscle and 5 μg of mitochondria.
Table 1.
Absolute quantification of phospholipid species in isolated mitochondria before and after fish oil supplementation
| 18:3 (ALA) | 20:5 (EPA) | 22:6 (DHA) | 18:2 (LA) | 20:4 (ALA) | Total phospholipids | ||
|---|---|---|---|---|---|---|---|
| PC | Pre | 0.98 ± 0.07 | 1.06 ± 0.07 | 0.70 ± 0.14 | 98.43 ± 8.18 | 12.68 ± 1.36 | 242.20 ± 15.78 |
| Post | 0.82 ± 0.06 | 9.35 ± 4.51 | 4.51 ± 0.46* | 83.95 ± 4.69* | 16.78 ± 1.29* | 243.27 ± 8.30 | |
| PE | Pre | 0.44 ± 0.07 | 2.72 ± 0.45 | 5.49 ± 1.14 | 19.43 ± 3.94 | 63.65 ± 7.45 | 188.40 ± 18.54 |
| Post | 0.36 ± 0.12 | 10.61 ± 2.52* | 15.85 ± 2.27* | 17.62 ± 2.30 | 51.15 ± 5.05* | 178.69 ± 15.96 | |
| CL | Pre | 0.68 ± 0.13 | 0.57 ± 0.05 | 0.11 ± 0.02 | 81.10 ± 13.53 | 0.73 ± 0.10 | 101.53 ± 13.84 |
| Post | 0.56 ± 0.06 | 0.50 ± 0.03 | 0.12 ± 0.03 | 63.30 ± 9.36 | 0.54 ± 0.04* | 81.71 ± 6.18 | |
| SM | Pre | 0.18 ± 0.04 | ND | 0.066 ± 0.023 | 1.71 ± 0.37 | 0.27 ± 0.04 | 21.51 ± 1.99 |
| Post | 0.15 ± 0.04 | ND | 0.029 ± 0.010 | 1.43 ± 0.41 | 0.22 ± 0.03 | 12.30 ± 1.05* | |
| PI | Pre | 0.20 ± 0.02 | 0.33 ± 0.08 | 0.15 ± 0.05 | 2.55 ± 0.17 | 9.62 ± 1.02 | 49.91 ± 3.75 |
| Post | 0.21 ± 0.01 | 0.36 ± 0.06 | 0.19 ± 0.04 | 2.38 ± 0.24 | 8.79 ± 0.76 | 48.95 ± 3.29 | |
| PS | Pre | 0.24 ± 0.04 | ND | 0.45 ± 0.06 | 2.78 ± 0.44 | 0.75 ± 0.13 | 33.15 ± 6.39 |
| Post | 0.22 ± 0.04 | ND | 1.32 ± 0.23* | 2.07 ± 0.21 | 0.63 ± 0.11 | 34.41 ± 4.57 |
Mitochondrial membrane phospholipid species before (Pre) and after (Post) 12 weeks of fish oil supplementation. Alpha linoleic acid (ALA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), linoleic acid (LA) and arachidonic acid are reported in various phospholipid species, including: phosphatidylcholine (PC), phosphatidylethanolamine (PE), cardiolipin (CL), sphingomielin (SM), phosphatidylinositol (PI) and phosphotidylserine (PS). Values represent means ± SEM, and are expressed as nmol mg−1 protein. n = 9.
Significantly (P < 0.05) different from pre-supplementation.
Figure 2.

Fish oil supplementation results in changes in omega-3 (A) and omega-6 (B) PUFA content in isolated mitochondrial membranes of human skeletal muscle. The content of α-linolenic acid (ALA), eicosapentaenoic acid (EPA), docosahexaenoic acid (DHA), linoleic acid (LA) and arachidonic acid (AA) are reported in various phospholipid species, including: phosphatidylcholine (PC), phosphatidylethanolamine (PE), cardiolipin (CL), sphingomielin (SM), phosphatidylinositol (PI) and phosphatidylserine (PS). Values represent means ± SEM, and are expressed as a percentage of pre-supplementation. n = 9. *Significantly (P < 0.05) different from pre-supplementation. ND; Not detected.
We also analysed mitochondrial membranes for the presence of linoleic acid (LA, 18:2n-6) and arachidonic acid (AA, 20:4n-6) phospholipid species. Total LA content was reduced to 83% of Pre and decreased to ∼85% in the PC fraction (Fig. 2B). While total AA content was not altered, AA accumulation in PE decreased to ∼80% of Pre, and, by contrast, increased ∼1.3-fold in the PC fraction.
Mitochondrial respiration and kinetics
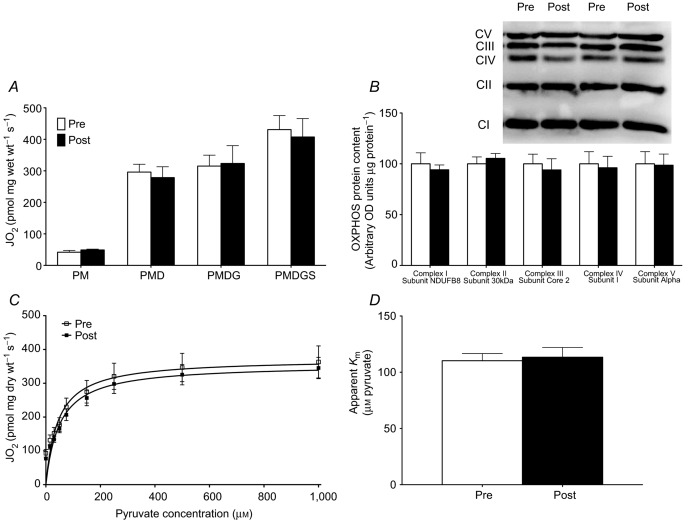
Given that omega-3 supplementation resulted in incorporation of omega-3 PUFAs into the mitochondrial membrane, we aimed to determine if this impacted mitochondrial respiratory function. In coupled mitochondria (respiratory control ratio ∼6), we first analysed maximal pyruvate-stimulated respiration, as well as maximal complex I (pyruvate+glutamate)-and complex I+II (pyruvate+glutamate+succinate)-stimulated respiration, and found no differences following omega-3 supplementation (Fig. 3A). These data suggest that omega-3 fish oil supplementation does not alter the capacity of the electron transport chain. This observation was further confirmed by Western blotting for subunits of the electron transport chain in whole muscle (Fig. 3B), which showed no difference in OXPHOS protein content between time-points.
Figure 3.
Omega-3 incorporation into mitochondrial membranes does not alter maximal mitochondrial respiration (A), the expression of oxidative phosphorylation (OXPHOS) proteins (B), mitochondrial pyruvate kinetics, (C) or the apparent Km for pyruvate (D). Values were compared before (Pre) and after (Post) 12 weeks of omega-3 supplementation. Non-ADP-stimulated (pyruvate + malate; PM), ADP-stimulated (PMD), maximal complex I supported respiration (PMDG) and maximal coupled respiration (PMDGS) were not different after supplementation. Values represent means ± SEM. n = 18.
Given the absence of changes in maximal substrate-supported respiration, we next examined the dynamic response of mitochondria to small changes in pyruvate availability. Similar to maximal substrate-supported respiration, omega-3 supplementation did not alter the kinetics of pyruvate, as the apparent sensitivity (Km) (Fig. 3D) and maximal respiration (Fig. 3C) induced by pyruvate were not altered.
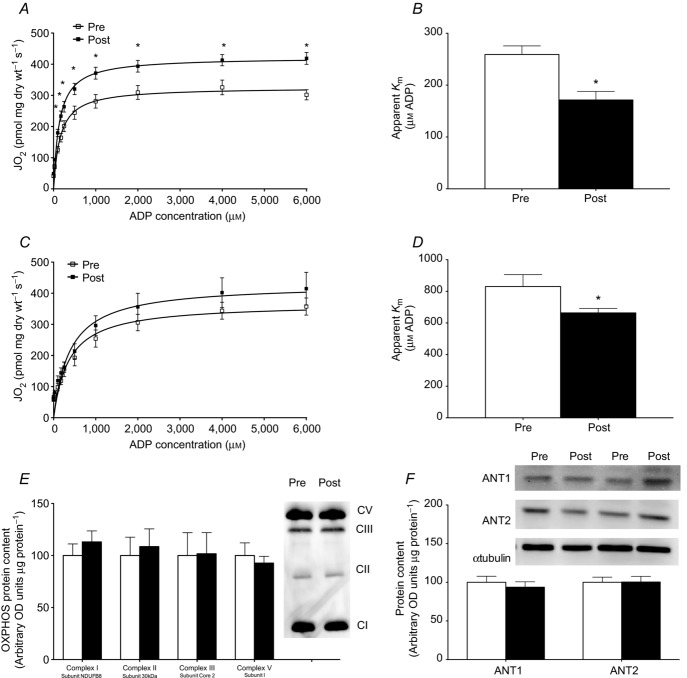
We next determined if omega-3 supplementation altered ADP kinetics, by performing ADP titrations both in the presence and in the absence of creatine. While titrating ADP produced the same maximal respiration as observed in the pyruvate titrations before supplementation, fish oil consumption increased maximal respiration in the presence of creatine (Fig. 4A), but not in the absence of creatine (Fig. 4C). Given the unexpected finding of increased maximal respiration, we digested the actual fibre bundles used to determine ADP sensitivity for Western blotting of OXPHOS subunits to ensure mitochondrial content was not higher. Similar to the whole muscle (Fig. 2D), various markers of OXPHOS were not increased in fibre bundles used for ADP titrations (Fig. 4E). In addition to changes in maximal respiration, ADP titrations revealed an increased sensitivity to ADP, as shown by significant decreases of 34 and 20% in the apparent Km following supplementation in both the presence (Fig. 4A and B) and absence (Fig. 4C and D) of creatine, respectively. These data suggest that the increased ADP sensitivity following supplementation is probably mitochondrial creatine kinase (miCK) independent, potentially involving ATP synthase or adenine nucleotide translocase (ANT). However, the content of ATP synthase was not altered at the whole muscle level or in fibre bundles (Figs 3B and 4E). Furthermore, we also determined the protein content of ANT1 and ANT2 (Fig. 4F) and found no differences following supplementation. Therefore, these data suggest that the improvement in ADP sensitivity is probably a result of post-translational modification of these proteins.
Figure 4.
Omega-3 incorporation into mitochondrial membranes altered ADP respiratory kinetics in the presence (A, B) and absence (C, D) of creatine. Michaelis–Menten kinetic curves were generated in the presence (A) and absence (C) of creatine. The ADP concentration required to elicit half-maximal respiration (apparent Km) was determined in the presence (B) and absence (D) of creatine. Values were compared before (Pre) and after (Post) 12 weeks of omega-3 supplementation. Fibre bundles were recovered from the respiration chamber and used to determine the expression of oxidative phosphorylation (OXPHOS) proteins (E). The protein content of adenine nucleotide translocase (ANT1, ANT2) was determined by Western blotting (F). Values represent means ± SEM. n = 9–18. *Significantly (P < 0.05) different from Pre.
H2O2 emission and oxidative stress
Omega-3 incorporation into the mitochondria probably alters membrane fluidity and the ease by which electron slippage and superoxide production occurs. Given the observation of increased respiration at submaximal ADP concentrations, we speculated that this could occur as a result of increased coupling efficiency, which may augment the propensity for mitochondria to produce ROS. We therefore determined mitochondrial ROS emission following fish oil supplementation and showed that the propensity for succinate-induced mitochondrial H2O2 emission was increased ∼1.3-fold following supplementation (Fig. 5A). As this supports a larger capacity for ROS generation with omega-3 consumption, we also examined products of oxidative stress. The presence of lipid peroxidation as determined by 4HNE immunoblotting (Fig. 5B) and the abundance of protein carbonyl groups (Fig. 5C) were not different after supplementation in muscle homogenates. Therefore, the enhanced capacity for H2O2 formation does not result in increased oxidative damage.
Figure 5.
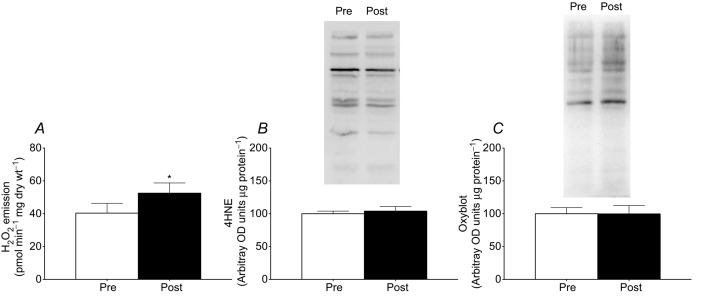
Omega-3 incorporation into mitochondrial membranes increased maximal succinate-induced reactive oxygen species (ROS) emission (A) without altering markers of oxidative stress. Specifically, 4-hydroxynonenal (4HNE; B) and protein carbonylation (Oxyblot; C) were not different before (Pre) or after (Post) 12 weeks of omega-3 supplementation. Values represent means ± SEM. n = 18. *Significantly (P < 0.05) different from Pre.
Discussion
The present study investigated the impact of fish oil supplementation on mitochondrial phospholipid composition, bioenergetics and respiratory function in human skeletal muscle. Our novel findings demonstrate that omega-3s are robustly incorporated into mitochondrial membranes following supplementation, but without altering maximal mitochondrial function. By contrast, mitochondrial ADP sensitivity, submaximal ADP-stimulated respiration, and maximal mitochondrial ROS emission were all increased following fish oil supplementation.
It is well established that altering fatty acid composition of the diet can effect membrane composition in both rodent heart (Charnock et al. 1986) and skeletal muscle (Owen et al. 2004), and utilizing various dosages and time frames, omega-3 PUFA supplementation has also been shown to result in omega-3 accumulation in whole muscle membranes from human tissue (Andersson et al. 2002; Stark et al. 2002; Dangardt et al. 2012; Smith et al. 2013). Here we show that fish oil supplementation results in robust incorporation of omega-3 PUFAs into human muscle mitochondrial membranes and displaces omega-6 PUFAs from some phospholipid species, supporting previous work conducted in rat heart (Khairallah et al. 2012) and liver (Pehowich, 1999). Specifically, in mitochondria, we observed EPA and DHA content increases of 3-to 9-fold in PC and PE fractions, which coincided with a decrease in LA abundance. The increased incorporation of omega-3 PUFAs into the mitochondrial membranes is remarkably similar to previous reports on whole muscle samples (Stark et al. 2002). However, in contrast to previous work in rodents suggesting that DHA is efficiently incorporated into CL (Khairallah et al. 2012), omega-3 supplementation did not alter the composition of CL in this study. At the plasma membrane, increased AA has been hypothesized to contribute to inflammatory eicosanoids. Therefore, while it is possible that a similar relationship exists at the mitochondria, currently how mitochondria are impacted by changes in omega-6 content in individual phospholipid species is unknown. In addition, the present data represent the phospholipid species contained within both the inner and the outer mitochondrial membrane, and therefore it remains unknown what changes specifically occur in the more biologically relevant inner mitochondrial membrane. CL is a mitochondrial phospholipid composed primarily of LA (Minkler & Hoppel, 2010). CL is unique to the inner membrane and is vital to the integrity of the membrane proteins, as it aids in the formation of contact sites on the inner and outer membranes and is closely associated with the proteins of the electron transport chain (Sparagna & Lesnefsky, 2009). Although we observed no changes in omega-3 incorporation into CL, there were strong trends for reductions in both LA (P = 0.058) and AA (P = 0.10) content in CL following supplementation. The consequence of this is unknown, although previous work supports an important role for CL in the failing heart, as LA supplementation aids in attenuating losses in CL content and improving mitochondrial dysfunction in cardiomyopathies (Sparagna et al. 2007; Mulligan et al. 2012). Therefore, small reductions in CL omega-6 PUFA content may have biological relevance, although the metabolic consequences of alterations in CL content presently remain ambiguous.
In the current study we provide evidence that maximal ADP-stimulated respiration in permeabilized muscle fibres was not altered by omega-3 intake, corroborating previous work in rodents (Lanza et al. 2013). We also examined the mitochondrial response to small changes in substrate availability. Following the titration of pyruvate, no change was detected in either maximal respiration (Vmax) or the apparent affinity (Km) under conditions of saturating ADP. This suggests that following omega-3 supplementation, electron acceptance through complex I, flow to complex IV, and ultimately electron acceptance by oxygen were not altered. Where complex I substrates are seldom limiting physiologically, rapid changes in free ADP concentrations probably dictate fluctuations in mitochondrial respiration in vivo. While ATP and ADP are thought to freely diffuse across concentration gradients through ANT, the majority of the energy transfer from the matrix to the cytoplasm occurs through miCK-dependent phosphate shuttling in oxidative muscle (Aliev et al. 2011; Guzun et al. 2012). We unexpectedly found that titrating ADP in the presence of creatine resulted in a robust increase in Vmax, which was independent of changes in OXPHOS protein expression. In addition, although not significantly different, the same basic trend is visually depicted in the absence of creatine. No explanation currently exists for this observation, but it probably does not reflect changes in ADP transport directly, given that ADP concentrations are saturating. Interestingly, in both the presence and the absence of creatine, the apparent Km for ADP decreased following omega-3 supplementation. This occurred in the absence of altered ANT1, ANT2, or ATP synthase protein content. These data suggest that increased omega-3 PUFA incorporation into mitochondrial membranes directly alters ADP sensitivity, or perhaps indirectly affects post-translational modifications of ANT isoforms or ATP synthase. In combination with the unaltered pyruvate kinetics, these data suggest that the mitochondria respond differently following fish oil supplementation to situations of low membrane potential (i.e. ADP added before pyruvate titration) and high membrane potential (i.e. pyruvate added before ADP titrations). The apparent fish oil-induced improvement in ADP sensitivity may be speculated to improve the efficiency with which ATP resynthesis occurs during exercise. This may explain previous reports demonstrating omega-3-induced improvements in skeletal muscle contraction efficiency in rats (Peoples & McLennan, 2010), lower steady-state submaximal whole body oxygen consumption in exercising humans (Peoples et al. 2008), potential fuel shifts during exercise in humans (i.e. strong trends for increased fat oxidation and glucose/glycogen sparing) (Delarue et al. 2003) and possibly the improvements seen in cardiac contractile function (Pepe & McLennan, 2002). However, in the current study we have not determined rates of ATP synthesis directly, and any beneficial consequence of increasing ADP sensitivity is based on the assumption of unaltered ATP/oxygen coupling. While rates of ‘leak respiration’ (absence of ADP) were not altered in the current study, uncoupling mitochondria could also be speculated to increase the apparent ADP sensitivity. However, a general uncoupling of mitochondria would also be expected to increase the Vmax of all conditions and alter the apparent Km for pyruvate, which did not occur. In addition, uncoupling mitochondria would be expected to decrease the capacity for mitochondrial ROS emission, although in the current study we observed an increased ROS emission. Therefore, the alteration in ADP sensitivity appears to be specific to the ADP protocols, and therefore may reflect an increased propensity for uncoupling during a metabolic challenge (i.e. the provision of ADP). However, omega-3 supplementation during a high-fat challenge in rodents does not alter the ADP/oxygen ratios in isolated mitochondria, challenging the notion that omega-3 supplementation increases the susceptibility of mitochondria to uncoupling (Lanza et al. 2013). Together, the current data suggest that ADP sensitivity is improved, probably through allosteric or covalent modifications of either ANT isoforms or ATP synthase.
The current data may also have implications to understanding the mechanisms of action by which omega-3 fish oils improve insulin sensitivity. The insulin-sensitizing effects of omega-3 fish oils have been proposed to involve remodelling of mitochondrial membrane phospholipid composition and a subsequent increase in substrate oxidation, particularly fatty acids (Borkman et al. 1993). However, it was recently reported in mice that the improvement of insulin sensitivity by omega-3 fish oils occurs independently of improvements in mitochondrial content or maximal respiratory function, challenging the ‘mitochondria-centric’ view of how omega-3 fatty acids improve insulin sensitivity (Lanza et al. 2013). Therefore, the mechanistic basis for how omega-3 fish oils improve insulin sensitivity remains ambiguous. However, we have recently reported decreased ADP sensitivity within the skeletal muscle (Smith et al. 2013) of Zucker diabetic fatty rats, suggesting impaired ADP kinetics is a potential cause of insulin resistance. Therefore, it is tempting to speculate that an improvement in ADP sensitivity observed in the current study following fish oil supplementation represents a mechanism by which fish oils improve insulin sensitivity. The improvement in insulin sensitivity may also involve ROS-induced gene transcription of antioxidant enzymes, as recently proposed (Lanza et al. 2013). In the current study, mitochondrial ROS emission was increased, supporting this potential interpretation. It remains unknown mechanistically how omega-3 fish oils increase the propensity of mitochondria for ROS emission. However, the expected increase in mitochondrial membrane fluidity that would be expected following EPA and DHA incorporation into the mitochondrial membranes may create a ‘tighter’ interface between the phospholipid bilayer and integral membrane proteins, decreasing proton ‘leak’ and increasing mitochondrial ROS emission. While this mechanism is plausible, direct support for this supposition remains to be determined.
Clearly additional work examining the metabolic role of fish oil supplementation in various pathological conditions/models is warranted, especially in the context of ADP sensitivity. Supplementation with fish oils has long been recognized to have beneficial effects on the cardiovascular system, although the direct benefits to muscle bioenergetics have received less attention. The present findings strongly emphasize a role for omega-3s in reorganizing the composition of the mitochondrial membrane, and through an unknown mechanism, promoting beneficial adaptations to situations where ADP is progressively increased.
Key points
Following fish oil supplementation, omega-3 fatty acids are incorporated into cellular membranes, which may affect lipid–protein interactions and therefore the function of embedded proteins.
As the components of the electron transport chain required for oxidative phosphorylation are contained in the mitochondrial membrane, omega-3 supplementation may alter metabolic function.
We supplemented male participants for 12 weeks with fish oil [eicosapentaenoic acid (EPA) and docosahexanoic acid (DHA)] and analysed mitochondrial function and reactive oxygen species (ROS) emissions in permeabilized muscle fibres from the vastus lateralis muscle.
Supplementation incorporated EPA and DHA into mitochondrial membranes, but did not result in changes in maximal mitochondrial respiratory function or pyruvate respiration kinetics.
However, the apparent Km for ADP was decreased following supplementation, and was independent of creatine, changes in the protein content of ADP synthase or ANT transporters.
The propensity for ROS emissions increased with omega-3 supplementation, although there were no changes in markers of lipid or protein oxidative damage.
These results demonstrate that omega-3 supplementation improves mitochondrial ADP kinetics, suggesting post-translational modification of existing proteins.
Acknowledgments
The authors would like to acknowledge the participants for their time and effort in completing this study.
Glossary
- AA
arachidonic acid
- ALA
α-linoleic acid
- ANT
adenine nucleotide translocase
- CL
cardiolipin
- DHA
docosahexanoic acid
- EPA
eicosapentaenoic acid
- IMF
intermyofibrillar
- LA
linoleic acid
- miCK
mitochondrial creatine kinase
- PC
phosphatidylcholine
- PE
phosphatidylethanolamine
- PI
phosphatidylinositol
- PS
phosphatidylserine
- PUFA
polyunsaturated fatty acid
- ROS
reactive oxygen species
- SM
sphingomielin
- SS
subsarcolemmal
Additional information
Competing interests
None declared.
Author Contributions
E.A.F.H., S.P., C.G., J.W., L.L.S. and G.P.H. designed experiments, performed experiments, analysed and interpreted data, and wrote the manuscript. K.M., A.C. and G.J.F. performed experiments, interpreted data and edited the manuscript.
Funding
This work was funded by The Natural Sciences and Engineering Research Council of Canada (NSERC; G.P.H., L.L.S.) and an operating grant to A.C. (N N401 292739). Infrastructure was purchased with the assistance of the Canadian Foundation for Innovation as well as the Ontario Research Fund. E.A.F.H. is supported by an NSERC graduate scholarship.
References
- Aliev M, Guzun R, Karu-Varikmaa M, Kaambre T, Wallimann T, Saks V. Molecular system bioenergics of the heart: experimental studies of metabolic compartmentation and energy fluxes versus computer modelling. Int J Mol Sci. 2011;12:9296–9331. doi: 10.3390/ijms12129296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, 3rd, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH, Neufer PD. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest. 2009;119:573–581. doi: 10.1172/JCI37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson A, Nalsen C, Tengblad S, Vessby B. Fatty acid composition of skeletal muscle reflects dietary fat composition in humans. Am J Clin Nutr. 2002;76:1222–1229. doi: 10.1093/ajcn/76.6.1222. [DOI] [PubMed] [Google Scholar]
- Bonen A, Parolin ML, Steinberg GR, Calles-Escandon J, Tandon NN, Glatz JF, Luiken JJ, Heigenhauser GJ, Dyck DJ. Triacylglycerol accumulation in human obesity and type 2 diabetes is associated with increased rates of skeletal muscle fatty acid transport and increased sarcolemmal FAT/CD36. FASEB J. 2004;18:1144–1146. doi: 10.1096/fj.03-1065fje. [DOI] [PubMed] [Google Scholar]
- Borkman M, Storlien LH, Pan DA, Jenkins AB, Chisholm DJ, Campbell LV. The relation between insulin sensitivity and the fatty-acid composition of skeletal-muscle phospholipids. N Engl J Med. 1993;328:238–244. doi: 10.1056/NEJM199301283280404. [DOI] [PubMed] [Google Scholar]
- Campbell SE, Tandon NN, Woldegiorgis G, Luiken JJ, Glatz JF, Bonen A. A novel function for fatty acid translocase (FAT)/CD36: involvement in long chain fatty acid transfer into the mitochondria. J Biol Chem. 2004;279:36235–36241. doi: 10.1074/jbc.M400566200. [DOI] [PubMed] [Google Scholar]
- Charnock JS, Abeywardena MY, McLennan PL. Comparative changes in the fatty-acid composition of rat cardiac phospholipids after long-term feeding of sunflower seed oil-or tuna fish oil-supplemented diets. Ann Nutr Metab. 1986;30:393–406. doi: 10.1159/000177221. [DOI] [PubMed] [Google Scholar]
- Dangardt F, Chen Y, Gronowitz E, Dahlgren J, Friberg P, Strandvik B. High physiological omega-3 fatty acid supplementation affects muscle fatty acid composition and glucose and insulin homeostasis in obese adolescents. J Nutr Metab. 2012;2012:395757. doi: 10.1155/2012/395757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delarue J, Labarthe F, Cohen R. Fish-oil supplementation reduces stimulation of plasma glucose fluxes during exercise in untrained males. Br J Nutr. 2003;90:777–786. doi: 10.1079/bjn2003964. [DOI] [PubMed] [Google Scholar]
- Fiehn W, Peter JB, Mead JF, Gan-Elepano M. Lipids and fatty acids of sarcolemma, sarcoplasmic reticulum, and mitochondria from rat skeletal muscle. J Biol Chem. 1971;246:5617–5620. [PubMed] [Google Scholar]
- Guzun R, Gonzalez-Granillo M, Karu-Varikmaa M, Grichine A, Usson Y, Kaambre T, Guerrero-Roesch K, Kuznetsov A, Schlattner U, Saks V. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within mitochondrial interactosome. Biochim Biophys Acta. 2012;1818:1545–1554. doi: 10.1016/j.bbamem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- Holloway GP, Fajardo VA, McMeekin L, LeBlanc PJ. Unsaturation of mitochondrial membrane lipids is related to palmitate oxidation in subsarcolemmal and intermyofibrillar mitochondria. J Membr Biol. 2012;245:165–176. doi: 10.1007/s00232-012-9426-6. [DOI] [PubMed] [Google Scholar]
- Infante JP, Huszagh VA. Secondary carnitine deficiency and impaired docosahexaenoic (22:6n-3) acid synthesis: a common denominator in the pathophysiology of diseases of oxidative phosphorylation and β-oxidation. FEBS Lett. 2000;468:1–5. doi: 10.1016/s0014-5793(00)01083-8. [DOI] [PubMed] [Google Scholar]
- Khairallah RJ, Kim J, O'Shea KM, O'Connell KA, Brown BH, Galvao T, Daneault C, Des Rosiers C, Polster BM, Hoppel CL, Stanley WC. Improved mitochondrial function with diet-induced increase in either docosahexaenoic acid or arachidonic acid in membrane phospholipids. PLoS One. 2012;7:e34402. doi: 10.1371/journal.pone.0034402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally JS, Herbst EA, Matravadia S, Maher AC, Perry CG, Ventura-Clapier R, Holloway GP. Over-expressing mitofusin-2 in healthy mature mammalian skeletal muscle does not alter mitochondrial bioenergetics. PLoS One. 2013;8:e55660. doi: 10.1371/journal.pone.0055660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza IR, Blachnio-Zabielska A, Johnson ML, Schimke JM, Jakaitis DR, Lebrasseur NK, Jensen MD, Sreekumaran Nair K, Zabielski P. Influence of fish oil on skeletal muscle mitochondrial energetics and lipid metabolites during high-fat diet. Am J Physiol Endocrinol Metab. 2013;304:E1391–1403. doi: 10.1152/ajpendo.00584.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minkler PE, Hoppel CL. Separation and characterization of cardiolipin molecular species by reverse-phase ion pair high-performance liquid chromatography-mass spectrometry. J Lipid Res. 2010;51:856–865. doi: 10.1194/jlr.D002857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan CM, Sparagna GC, Le CH, De Mooy AB, Routh MA, Holmes MG, Hickson-Bick DL, Zarini S, Murphy RC, Xu FY, Hatch GM, McCune SA, Moore RL, Chicco AJ. Dietary linoleate preserves cardiolipin and attenuates mitochondrial dysfunction in the failing rat heart. Cardiovasc Res. 2012;94:460–468. doi: 10.1093/cvr/cvs118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fibre-type dependence of AMPK-α1 and-β1. J Appl Physiol. 2011;110:820–825. doi: 10.1152/japplphysiol.01082.2010. [DOI] [PubMed] [Google Scholar]
- Owen AJ, Peter-Przyborowska BA, Hoy AJ, McLennan PL. Dietary fish oil dose-and time-response effects on cardiac phospholipid fatty acid composition. Lipids. 2004;39:955–961. doi: 10.1007/s11745-004-1317-0. [DOI] [PubMed] [Google Scholar]
- Pehowich DJ. Thyroid hormone status and membrane n-3 fatty acid content influence mitochondrial proton leak. Biochim Biophys Acta. 1999;1411:192–200. doi: 10.1016/s0005-2728(99)00041-9. [DOI] [PubMed] [Google Scholar]
- Peoples GE, McLennan PL. Dietary fish oil reduces skeletal muscle oxygen consumption, provides fatigue resistance and improves contractile recovery in the rat in vivo hindlimb. Br J Nutr. 2010;104:1771–1779. doi: 10.1017/S0007114510002928. [DOI] [PubMed] [Google Scholar]
- Peoples GE, McLennan PL, Howe PR, Groeller H. Fish oil reduces heart rate and oxygen consumption during exercise. J Cardiovasc Pharmacol. 2008;52:540–547. doi: 10.1097/FJC.0b013e3181911913. [DOI] [PubMed] [Google Scholar]
- Pepe S, McLennan PL. Cardiac membrane fatty acid composition modulates myocardial oxygen consumption and postischemic recovery of contractile function. Circulation. 2002;105:2303–2308. doi: 10.1161/01.cir.0000015604.88808.74. [DOI] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Herbst EA, Mukai K, Lark DS, Wright DC, Heigenhauser GJ, Neufer PD, Spriet LL, Holloway GP. Mitochondrial creatine kinase activity and phosphate shuttling are acutely regulated by exercise in human skeletal muscle. J Physiol. 2012;590:5475–5486. doi: 10.1113/jphysiol.2012.234682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry CG, Kane DA, Lin CT, Kozy R, Cathey BL, Lark DS, Kane CL, Brophy PM, Gavin TP, Anderson EJ, Neufer PD. Inhibiting myosin-ATPase reveals a dynamic range of mitochondrial respiratory control in skeletal muscle. Biochem J. 2011;437:215–222. doi: 10.1042/BJ20110366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith BK, Perry CG, Herbst EA, Ritchie IR, Beaudoin MS, Smith JC, Neufer PD, Wright DC, Holloway GP. Submaximal ADP-stimulated respiration is impaired in ZDF rats and recovered by resveratrol. J Physiol. 2013;591:6089–6101. doi: 10.1113/jphysiol.2013.259226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparagna GC, Chicco AJ, Murphy RC, Bristow MR, Johnson CA, Rees ML, Maxey ML, McCune SA, Moore RL. Loss of cardiac tetralinoleoyl cardiolipin in human and experimental heart failure. J Lipid Res. 2007;48:1559–1570. doi: 10.1194/jlr.M600551-JLR200. [DOI] [PubMed] [Google Scholar]
- Sparagna GC, Lesnefsky EJ. Cardiolipin remodeling in the heart. J Cardiovasc Pharmacol. 2009;53:290–301. doi: 10.1097/FJC.0b013e31819b5461. [DOI] [PubMed] [Google Scholar]
- Stark KD, Mulvad G, Pedersen HS, Park EJ, Dewailly E, Holub BJ. Fatty acid compositions of serum phospholipids of postmenopausal women: a comparison between Greenland Inuit and Canadians before and after supplementation with fish oil. Nutrition. 2002;18:627–630. doi: 10.1016/s0899-9007(02)00812-2. [DOI] [PubMed] [Google Scholar]
- Tsalouhidou S, Argyrou C, Theofilidis G, Karaoglanidis D, Orfanidou E, Nikolaidis MG, Petridou A, Mougios V. Mitochondrial phospholipids of rat skeletal muscle are less polyunsaturated than whole tissue phospholipids: implications for protection against oxidative stress. J Anim Sci. 2006;84:2818–2825. doi: 10.2527/jas.2006-031. [DOI] [PubMed] [Google Scholar]
- Yamaoka S, Urade R, Kito M. Mitochondrial function in rats is affected by modification of membrane phospholipids with dietary sardine oil. J Nutr. 1988;118:290–296. doi: 10.1093/jn/118.3.290. [DOI] [PubMed] [Google Scholar]