Abstract
The Ca2+ uptake properties of the sarcoplasmic reticulum (SR) were compared between type I and type II fibres of vastus lateralis muscle of young healthy adults. Individual mechanically skinned muscle fibres were exposed to solutions with the free [Ca2+] heavily buffered in the pCa range (–log10[Ca2+]) 7.3–6.0 for set times and the amount of net SR Ca2+ accumulation determined from the force response elicited upon emptying the SR of all Ca2+. Western blotting was used to determine fibre type and the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) isoform present in every fibre examined. Type I fibres contained only SERCA2 and displayed half-maximal Ca2+ uptake rate at ∼pCa 6.8, whereas type II fibres contained only SERCA1 and displayed half-maximal Ca2+ uptake rate at ∼pCa 6.6. Maximal Ca2+ uptake rate was ∼0.18 and ∼0.21 mmol Ca2+ (l fibre)–1 s–1 in type I and type II fibres, respectively, in good accord with previously measured SR ATPase activity. Increasing free [Mg2+] from 1 to 3 mm had no significant effect on the net Ca2+ uptake rate at pCa 6.0, indicating that there was little or no calcium-induced calcium release occurring through the Ca2+ release channels during uptake in either fibre type. Ca2+ leakage from the SR at pCa 8.5, which is thought to occur at least in part through the SERCA, was ∼2-fold lower in type II fibres than in type I fibres, and was little affected by the presence of ADP, in marked contrast to the larger SR Ca2+ leak observed in rat muscle fibres under the same conditions. The higher affinity of Ca2+ uptake in the type I human fibres can account for the higher relative level of SR Ca2+ loading observed in type I compared to type II fibres, and the SR Ca2+ leakage characteristics of the human fibres suggest that the SERCAs are regulated differently from those in rat and contribute comparatively less to resting metabolic rate.
Introduction
In skeletal muscle the release and reuptake of Ca2+ by the sarcoplasmic reticulum (SR) governs force production by the contractile apparatus, and hence the SR properties are major determinants of muscle function and performance. The Ca2+ in the SR is predominantly bound to calsequestrin (CSQ), a high capacity, low affinity Ca2+-binding protein (MacLennan & Wong, 1971; Beard et al. 2004; Murphy et al. 2009a) and can be released extremely rapidly into the cytoplasm through the ryanodine receptor–Ca2+ release channels (RyRs) in the SR terminal cisternae (Franzini-Armstrong & Protasi, 1997). The Ca2+ is subsequently sequestered back into the SR by the sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA), with SERCA1 being the predominant or sole isoform present in adult mammalian type II fibres and SERCA2a the predominant or sole isoform in type I fibres (Lytton et al. 1992; Wu & Lytton, 1993; Murphy et al. 2009a), including in human muscle (Talmadge et al. 2002; Lamboley et al. 2013).
Little is known about the Ca2+ uptake characteristics and other SR properties of human skeletal muscle fibres (Bottinelli & Reggiani, 2000). In rat and rabbit the SR resequesters Ca2+ very much more rapidly in type II fibres than in type I fibres, due in large part to the ∼5-to 7-fold higher density of the SERCA in the type II fibres (Leberer & Pette, 1986; Wu & Lytton, 1993), and in rat the Ca2+ affinity of the uptake was found to be substantially higher in type I fibres than in type II fibres (Fryer & Stephenson, 1996). Experiments involving SERCA expression in COS-1 cells concluded that the quantitative properties of SERCA1 and SERCA2a were virtually identical in all respects (Lytton et al. 1992), with similar findings when expressing either rabbit or human SERCA. In those experiments Ca2+ uptake occurred with half-maximal rate at ∼0.4 μm free Ca2+ (i.e. pCa ∼6.4) and a Hill coefficient (h) ∼2.1 for both SERCA isoforms. The study also found similar Ca2+ uptake characteristics in SR vesicles from rabbit skeletal muscle but an approximately 2–fold lower uptake affinity in dog cardiac SR. In the latter the SERCA isoform present is SERCA2a, just as in type I skeletal muscle fibres, and the investigators concluded that the relatively low Ca2+ affinity was probably due to reversible modulation of the SERCA by the associated inhibitory protein phospholamban (Lytton et al. 1992). In contrast to the above, another study expressing rabbit SERCA1 and SERCA2a in COS-1 cells concluded that whilst the [Ca2+] for half-maximal uptake was similar for the two isoforms (both ∼pCa 6.7) the maximum uptake rate of SERCA1 was twice that of SERCA2a (Sumbilla et al. 1999). On the other hand another study expressing rabbit SERCA1 and human SERCA2a in HEK293 cells concluded that their maximal uptake rate was the same but that SERCA2a took up Ca2+ with significantly higher affinity than SERCA1 (half-maximal uptake at ∼pCa 6.7 and 6.5, respectively; Dode et al. 2003).
Given the above discrepancies, and the absence of the key SERCA modulating proteins phospholamban and sarcolipin (Periasamy & Kalyanasundaram, 2007) in the expression systems, there is a clear need to characterize SR Ca2+ uptake properties directly in human skeletal muscle fibres. The only such study to date, which measured 45Ca2+ uptake in chemically skinned human fibres, concluded that uptake occurred with similar affinity in type I and type II fibres (Salviati et al. 1982). However, the relatively low maximal rate of Ca2+ uptake determined in that study (∼30–60 μmol (l fibre)–1 s–1) is not readily reconcilable with the ability of fast motor performance in humans nor with the SR Ca2+-ATPase activity measured in human muscle fibres (Szentesi et al. 2001; see Discussion).
A further key SR property of human muscle fibres that needs to be defined is the rate of SR Ca2+ leakage. Ca2+ can leak out of the SR not only through the RyRs, but also through the SERCA (Inesi & de Meis, 1989; Macdonald & Stephenson, 2001; Murphy et al. 2009a). In rat muscle, Ca2+ leakage through the SERCA was found to increase with cytoplasmic [ADP] in both type I and type II fibres (Macdonald & Stephenson, 2001, 2006), which could be expected to affect not only SR and overall muscle performance during strenuous activity, but also possibly Ca2+ movements between the SR and mitochondria (Rossi et al. 2011). In the steady state, any Ca2+ leaking out of the SR by any pathway ultimately has to be recovered by the SERCA, which by necessity involves ATP expenditure. It has been proposed that the SERCA in skeletal muscle plays a significant role in heat production and thermogenesis (de Meis, 2001), and ATP consumption by SERCA has been reported to be responsible for 40–50% of resting metabolic rate in mouse fast and slow twitch muscles (Smith et al. 2013). The presence of sarcolipin increases the Ca2+ leak through the SERCA (Smith et al. 2002) and this putatively plays an important role in non-shivering thermogenesis and whole body energy metabolism (Bal et al. 2012).
The present study measured the Ca2+ dependence and absolute rate of Ca2+ uptake and SR Ca2+ leak properties in individual human muscle fibres of known type, freshly obtained by needle biopsy from the vastus lateralis muscle of young adults. Fibres were mechanically skinned, preserving normal SR function, and Ca2+ uptake assessed using solutions mimicking the normal intracellular ionic conditions, with free [Ca2+] heavily buffered at set levels in the range pCa 7.3–6.0. Additional experiments gauged the rate of Ca2+ leakage out of the SR and the effect of ADP. The findings provide novel quantitative information about SR Ca2+ uptake in human muscle and reveal significant differences in SR leak properties between human and rat muscle.
Methods
Ethical approvals, muscle biopsies and dissection
All protocols and procedures for human experiments were approved by the Human Research Ethics Committees at Victoria University and La Trobe University. All subjects gave informed consent in writing and the studies conformed to the standards set by the Declaration of Helsinki. Skinned muscle fibres were obtained from vastus lateralis muscle biopsies from 15 rested subjects, comprising 10 males and 5 females (age 23 ± 3 years; height, 176 ± 11 cm; body mass, 72 ± 10 kg, mean ± SD); further fibres from seven of these subjects had been used in our previous study examining SR Ca2+ content and CSQ in type I and II fibres (Lamboley et al. 2013). All subjects were healthy and most participated in regular physical activity but were not specifically trained in any sport. After injection of local anaesthetic (1% lidocaine (lignocaine)) into the skin and fascia, an experienced medical practitioner made a small incision in the middle third of the vastus lateralis muscle of each subject and took a muscle sample using a Bergstrom biopsy needle (McKenna et al. 2006). The excised muscle sample was rapidly blotted on filter paper to remove excess blood and placed in paraffin oil (Ajax Chemicals, Sydney, Australia) and then brought down to ∼10°C for ∼45 min before individual muscle fibres were dissected. The remainders of the muscle samples from the biopsies were frozen and stored in liquid nitrogen for later analyses.
Animal experiments were carried out in accordance with the ‘Australian code of practice for care and use of animals for scientific purposes’ of the National Health and Medical Research Council of Australia, and with approval of the Animal Ethics Committee of La Trobe University. Male Long–Evans hooded rats (three rats, ∼10–12 months old) were killed by isoflurane overdose (4% v/v) in a glass chamber and the extensor digitorum longus (EDL) and soleus muscles removed.
Preparations and force recording
Preparations (human muscle biopsies or rat muscles) were pinned at resting length in a Petri dish containing paraffin oil and kept cool (∼10°C) on an icepack. As described previously (Murphy et al. 2009b; Dutka et al. 2012), individual fibre segments were mechanically skinned and mounted at 120% of resting length on a force transducer (AME801, SensoNor, Horten, Norway), and then placed in a Perspex bath containing 2 ml of the standard K+-based solution broadly mimicking the intracellular milieu (see below). Force responses were recorded using a Bio Amp pod and PowerLab 4/20 series hardware (ADInstruments, Sydney, Australia). All experiments were performed at room temperature (∼23 ± 2°C).
Skinned fibre solutions
All chemicals were purchased from Sigma-Aldrich (St Louis, MO, USA) unless specified otherwise. The standard K-HDTA solution contained (in mm): hexa-methylene-diamine-tetraacetate (HDTA2−), 50 (Fluka, Buchs, Switzerland); total ATP, 8; Na+, 36; K+, 126; total Mg2+, 8.5 (giving 1 mm free [Mg2+]); creatine phosphate (CP), 10; total EGTA, 0.05; Hepes, 90; pH 7.1 and pCa (−log10[Ca2+]) ∼7.3, except where stated. Where required, the SR of a skinned fibre was depleted of all releasable Ca2+ by exposure to the ‘full release solution’, which was similar to the K-HDTA solution but with 30 mm caffeine, 0.05 mm free Mg2+ (total Mg2+ of 2.1 mm) and 0.5 mm free EGTA (pCa 8.5).
The SR was reloaded with Ca2+ as required using one of two types of solutions, either (i) K-HDTA solution with 5 mm CaEGTA and various free EGTA concentrations (1–15 mm) so as to strongly buffer the free [Ca2+] at values in the range pCa 6.0–7.3, or (ii) K-HDTA solution with free [Ca2+] buffered at pCa 6.7 with only 0.5 mm CaEGTA/0.5 mm EGTA, in which case ∼3 min was needed to load the SR to close to its maximum capacity (Lamboley et al. 2013). These load solutions were made from the standard K-HDTA solution by adding appropriate amounts of the EGTA-based ‘relaxing’ and ‘maximum Ca2+-activating’ solutions (containing 50 mm EGTA and 49.5 mm CaEGTA–0.5 mm EGTA, respectively, see below). Further load solutions with 5 mm CaEGTA at pCa 6.0 were made with 3 mm or 10 mm free Mg2+, by appropriate mixture with a K-HDTA solution with higher total magnesium (22.7 mm total; see Lamb & Stephenson, 1994).
Mechanically skinned skeletal muscle fibres retain high levels of bound creatine kinase activity (Macdonald & Stephenson, 2001; Dutka & Lamb, 2004; Maughan et al. 2005) and consequently when the fibre was in standard K-HDTA solution (with 8 mm ATP, 10 mm CP, no added creatine) the ADP concentration within the skinned fibre was maintained by the creatine kinase reaction at ∼0.1 μm or lower (based on an equilibrium constant of 260 for the reaction ADP + CP ↔ ATP + creatine, and with creatine <30 μm in these solutions; see Macdonald & Stephenson, 2001). Where required, skinned fibres were placed in a K-HDTA-based ‘leak solution’ with 2 mm EGTA (pCa ∼8.5) to chelate Ca2+ and with the [ADP] set at ∼0.1 μm, ∼31 μm or 1 mm. The latter two solutions were similar to standard K-HDTA solution but with 10 mm added creatine (∼31 μm ADP) or with 1 mm added ADP and all CP replaced by 10 mm extra HDTA.
Contractile apparatus properties were examined using solutions with the [Ca2+] very strongly buffered by the presence of 50 mm EGTA–CaEGTA; these solutions were very similar to the standard K-HDTA solution (same ionic strength, ATP, CP and free Mg2+ etc.) but with all the HDTA replaced with EGTA. The ‘relaxing’ solution had 50 mm EGTA with no added Ca2+ (pCa >9) and the ‘maximum Ca2+-activating’ solution had 49.5 mm CaEGTA and 0.5 mm free EGTA (giving pCa 4.7), with the total added Mg2+ being 10.3 and 8.1 mm, respectively, to maintain the free [Mg2+] at 1 mm (see Lamb & Stephenson, 1994). A strontium-based solution (at pSr (–log10[Sr2+]) 5.2) was made by mixing appropriate proportions of the relaxing solution (50 mm EGTA) and a similar solution containing 40 mm SrEGTA and 10 mm free EGTA (see Trinh & Lamb, 2006).
SR Ca2+ content and Ca2+ uptake rate assay
Fibres were skinned under paraffin oil and initially still contained their endogenous SR Ca2+ content. As described previously (Trinh & Lamb, 2006; Lamboley et al. 2013), following skinning each fibre was first equilibrated for 2 min in standard HDTA solution with very little contaminating Ca2+ and Ca2+ buffering (∼pCa 7.3 with 50 μm total EGTA); the SR is unable to take up any appreciable Ca2+ in this solution and any Ca2+ leaking from the SR is resequestered before being lost to the bathing solution. Following this equilibration period, the SR was emptied of all its releasable Ca2+ by exposure to a caffeine-low [Mg2+] solution (full release solution) containing 0.5 mm free EGTA to chelate the released Ca2+. The skinned fibre was then washed for 1 min in standard K-HDTA solution (with 0.5 mm free EGTA present to prevent any Ca2+ reuptake), and subjected to repeated load–release cycles as follows:
Step 1. Load SR for a set time in load solution with either strong Ca2+ buffering (5 mm CaEGTA at various pCa) or moderate buffering (0.5 mm CaEGTA at pCa 6.7).
Step 2. Stop uptake by 5 s immersion in K-HDTA solution with 10 mm free EGTA (for 5 mm CaEGTA load cases only) and then pre-equilibrate fibre for 15 s in K-HDTA solution with 0.5 mm free EGTA.
Step 3. Empty SR of all releasable Ca2+ by exposing fibre for 60 s to full release solution.
Step 4. Wash skinned fibre for 1 min in K-HDTA solution with 0.5 mm free EGTA.
The time integral (area) of the force response to the initial exposure to the full release solution was indicative of the endogenous SR Ca2+ content initially present in the fibre, and the responses to the subsequent exposures indicated the SR content for the respective load time and condition. As described previously (Lamboley et al. 2013), when loading in the moderately Ca2+-buffered solution at pCa 6.7 the relationship between the time integral of the force response and load time is well fitted by an exponential fit approaching saturation after ∼180 s. When examining Ca2+ uptake with the heavily Ca2+-buffered load solutions (i.e those with 5 mm CaEGTA), the load times were kept short so as to only load the SR to less than ∼50% of maximum capacity, that is, in the range where the Ca2+ loading increased approximately linearly with load time (see Fig. 4 in Lamboley et al. 2013). The load analysis took into account the amount of SR Ca2+ that had to be released to elicit any detectable force in the presence of 0.5 mm EGTA by back-extrapolation of the response area–load time curve to zero load time (see Fig. 2 and also Murphy et al. 2009a).
Figure 4.
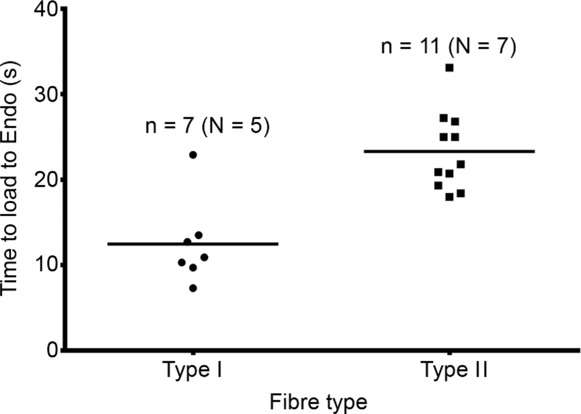
Time required in load solution at pCa 7.0 to reload SR from empty back to original endogenous level in human type I fibres (•) and in type II fibres (▪). Heavily Ca2+-buffered load solution with 5 mm CaEGTA. Amount of endogenous Ca2+ initially present in SR ascertained in each fibre as in Fig. 1. n denotes number of fibres and N the number of subjects. Same fibres as in Fig. 3. Horizontal bars indicate mean.
Figure 2.
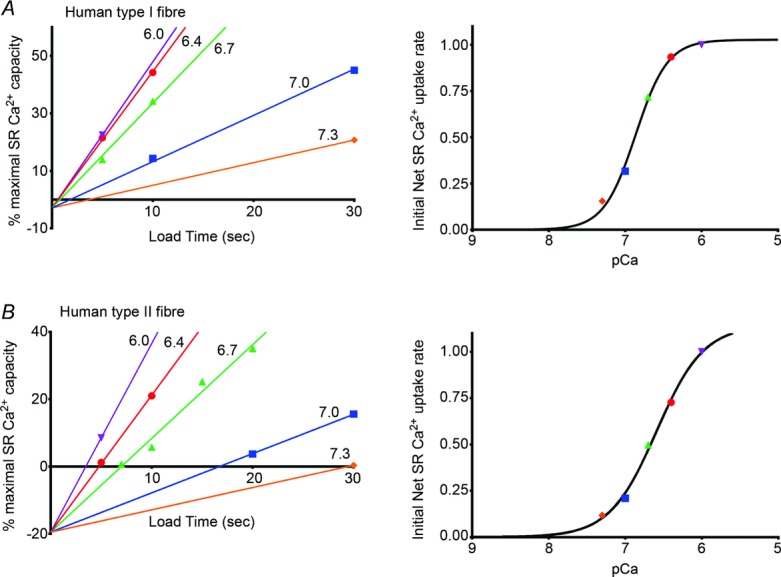
Analysis of data for two fibres shown in Fig. 1. Left-hand panels plot the relative amount of Ca2+ found in SR after loading for the specified time in a solution with 5 mm CaEGTA at indicated pCa (see Methods). Values represent the time integral of the force responses expressed relative to that obtained after maximal loading of the SR (at pCa 6.7 for 180 s, see Fig. 1). Back-extrapolation of the line of best fit for the data sets obtained when loading at pCa 7.0, 6.7 and 6.4 all gave a similar intercept value on the ordinate axis (mean ± SEM: −2.9 ± 1.8% and −19.5 ± 0.4% in A and B, respectively); all data then refitted with lines constrained to this mean intercept value. This negative intercept in effect indicates the true origin and hence how much Ca2+ has to be loaded into the SR and released in order to elicit any measurable force (see text). Right-hand panels plot the relative slope of the SR Ca2+ content versus load time fits shown in left-hand panels, with values expressed relative to that found at pCa 6.0. Best-fit Hill curves to these data gave the pCa for half-maximal uptake rate and Hill coefficient (h) as 6.86 and 2.1 for the type I fibre (A), and 6.59 and 1.4 for the type II fibre (B).
SR Ca2+ leak experiments assay with various myoplasmic [ADP]
SR Ca2+ leakage was assessed by loading the SR with Ca2+ for 1 min using the load solution with moderate Ca2+ buffering (0.5 mm CaEGTA–0.5 mm EGTA, pCa 6.7) and then placing the skinned fibre for 1 min in a leak solution (2 mm EGTA, pCa 8.5) with the appropriate [ADP], and then finally releasing the remaining SR Ca2+ as in steps 2–4 above. Such measurements were performed with bracketing examination of SR loading with no leakage period (see Fig. 6).
Figure 6.
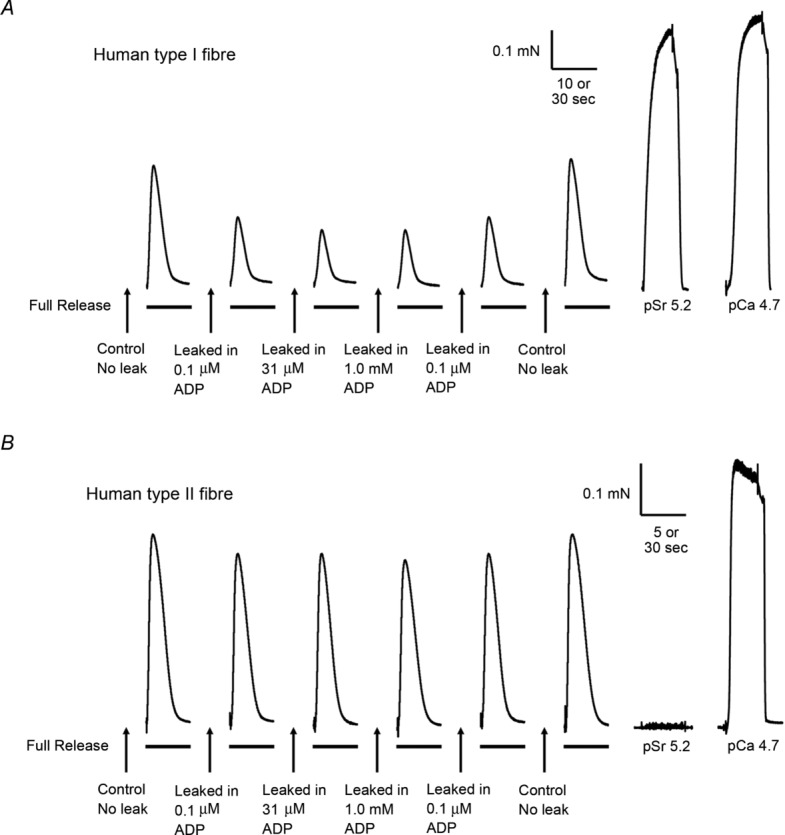
Representative force responses in a type I (A) and in a type II (B) human muscle fibre when emptying the SR of all releasable Ca2+ with the full release solution, with or without a preceding 1 min leakage period in leak solution at pCa 8.5 and various [ADP] (see Methods). Before each leak–release cycle the SR was loaded for 1 min in solution at pCa 6.7 (0.5 mm CaEGTA). Maximum Ca2+-activated force (at pCa 4.7) and response of contractile apparatus to Sr2+ (at pSr 5.2) determined at end of experiment, as in Fig. 1. Time scale: 5 or 10 s during SR Ca2+ release, and 30 s for maximum and Sr2+-activated force.
Western blotting
Individual skinned fibre segments were placed in a small volume (10 μl) of 1x solubilizing buffer which contained 0.125 m Tris-HCl, 10% glycerol, 4% SDS, 4 m urea, 10% mercaptoethanol and ∼0.001% bromophenol blue (pH 6.8) diluted (2:1 v/v) with double distilled water. Fibres were stored at −80°C until analysed by Western blotting. Total protein in the single fibres was separated on 4–15% Criterion Stain Free gels (Bio-Rad, Hercules, CA, USA), as previously described (Murphy, 2011), and wet-transferred to nitrocellulose for 30 min at 100 V in a circulating ice-cooled bath with transfer buffer containing 25 mm Tris and 192 mm glycine at pH 8.3 and 20% methanol. A range of muscle homogenate samples was also loaded on every gel to generate a standard curve for signal calibration (Murphy & Lamb, 2013). After appropriate washes and blocking, membranes were probed with the required primary antibody and constantly rocked overnight at 4°C and for 2 h at room temperature (RT), and then incubated with the appropriate secondary antibody (2 h, RT). Chemiluminescence images were captured and densitometry performed using Chemidoc MP and ImageLab software (BioRad). For each fibre the density of the total protein in the relevant lane on the Stain Free gel image was used as the measure of the relative amount of protein loaded. Fibre type was defined by the predominant/sole myosin heavy chain (MHC) isoform present (e.g. Fig. 1), as detected with mouse monoclonal antibodies to MHC I and MHC II (Developmental Studies Hybridoma Bank (DSHB), Iowa City, IA, USA; A4.840 and A4.74, respectively, 1:200). SERCA was detected with mouse monoclonal anti-SERCA1 (DSHB CA F2-5D, 1:1000) and anti-SERCA2 (Badrilla, Leeds, UK A010–2, 1:5000). Secondary antibodies were goat anti-mouse IgG-horseradish peroxidase (HRP; Pierce, Rockford, Il, USA 31430) or goat anti-mouse IgM-HRP (Invitrogen, Sydney, Australia 62 6820) at 1:20,000. To assess the variation in SERCA density between individual fibres of the same type, the density of the SERCA bands in a given fibre were normalized by the measure of total protein for that fibre segment (see above), and the resulting values then re-normalized to the mean obtained in all type I fibres (SERCA2) or in all type II fibres (SERCA1) run on the same gel, enabling comparison of data across different gels; this is analogous to the procedure used previously for quantifying the relative amounts of CSQ in different fibres (Lamboley et al. 2013). Normalizing SERCA bands to the common standard run on all gels gave similar results.
Figure 1.

Force responses elicited in type I fibre (A) and type II fibre (B) upon emptying the SR of all releasable Ca2+ after loading under indicated conditions. First response (‘Endo’) produced when releasing the endogenous SR Ca2+. Subsequent responses elicited after loading SR (for 5–20 s) in solutions with free [Ca2+] heavily buffered at indicated pCa and with amount of CaEGTA set at 5 mm to ensure a large constant rate of Ca2+ diffusion into the skinned fibre (see Methods and text). Last full release response elicited after loading for 180 s in solution at pCa 6.7 with moderate level of buffering (0.5 mm CaEGTA–0.5 mm EGTA) (labelled with *); time integral of response indicative of maximal SR Ca2+ capacity. Last two responses in each panel are the maximum Ca2+-activated force elicited by directly activating the contractile apparatus (with solution at pCa 4.7 with 50 mm CaEGTA) and the force response to a solution with pSr 5.2. Time scale in B: 5 s during SR Ca2+ release responses and 10 s during maximum activation and pSr 5.2 exposure. Inset in A: western blots for MHC and SERCA isoforms in same two fibres.
Statistics
Values are presented individually, or as mean ± SEM or mean ± SD as indicated, with n denoting the number of fibres examined and N the number of subjects. Statistical significance (P < 0.05) was determined with Student's t test or non-parametric Mann–Whitney rank test as appropriate.
Results
Fibre-type determination and SERCA isoforms
SR Ca2+ uptake and leak properties were examined in a total of 14 type I fibres and 22 type II fibres from 15 young adult subjects. Fibres containing only MHC I were classified as type I and those containing only MHC II as type II (e.g. inset in Fig. 1). In every case, the type I fibres gave near maximal force when exposed to the pSr 5.2 solution and the type II fibres gave very little or no force response (Fig. 1), consistent with the presence of slow and fast isoforms of troponin C, respectively, similar to our previous findings with other human skinned fibres (Lamboley et al. 2013). Every type I fibre examined contained SERCA2 and little or no detectable SERCA1, and conversely every type II fibre examined contained SERCA1 and little or no detectable SERCA2 (e.g. inset in Fig. 1), again similar to our previous findings. One further fibre examined contained both MHC I and MHC II, and both SERCA1 and SERCA2, and was classified as a mixed fibre and not included in the study. The relative amount of SERCA1 present in the type II fibres was broadly similar in every fibre examined (≤2-fold range in measured density, see Methods), and likewise the relative amount of SERCA2 present in type I fibres was also similar in all but one of the fibres examined. Interestingly, it was also found that with the procedures and gels used here SERCA1 migrated with a higher apparent molecular weight than SERCA2, similar to the findings of a previous study with SERCA expressed in COS-1 cells which used 7.5% SDS-PAGE (Lytton et al. 1992).
Calcium dependence and maximum rate of SR Ca2+ uptake
The calcium dependence and maximum rate of SR Ca2+ uptake in type I and type II fibres were determined by assessing the net amount of Ca2+ accumulated by the SR when exposing individual skinned fibres to heavily Ca2+-buffered load solutions for set times. The amount of Ca2+ accumulated in each instance was assayed by releasing all the accumulated SR Ca2+ by exposing the fibre to the caffeine-low [Mg2+] full release solution (e.g. Fig. 1) and determining the amount of Ca2+ released from the time-integral of the resulting force response (see Methods), similarly to that described previously (Lamboley et al. 2013). The load solutions all contained the same high concentration of CaEGTA (5 mm), so that the rate of diffusion of total calcium into the skinned fibre was (i) the same at each pCa, and (ii) considerably exceeded the maximum rate at which the SR could sequester Ca2+, thereby ensuring that the free [Ca2+] within the fibre space closely corresponded to that of the applied load solution (Moisescu & Thieleczek, 1978). At a given pCa the net amount of Ca2+ accumulated by the SR, as indicated by the time integral of the force response upon release, increased in an approximately linear manner with load time (e.g. see Fig. 2B, green triangles for loading at pCa 6.7 in a type II fibre) when load levels were kept <50% of maximal (see Methods). As described previously (Murphy et al. 2009a; Lamboley et al. 2013), the analysis of SR Ca2+ uptake needs to take into account the fact that a finite amount of Ca2+ has to be loaded into and released from the SR in order to evoke any measurable force response in the full release solution, which contained 0.5 mm free EGTA to chelate and remove any released Ca2+ and keep the force response within a non-saturating range. This Ca2+ amount was derived by back-extrapolation of the loading curves to zero load time. In a given fibre such back-extrapolation of the loading at pCa 7.0, 6.7 and 6.4 all yielded a similar intercept on the ordinate axis (e.g. Fig. 2, mean of SD in intercept being 1.5% and 2.1% across all type I and type II fibres, respectively), with the mean intercept value (±SEM) being −4.8 ± 0.8% in the 7 type I fibres examined and −15.4 ± 1.9% in the 12 type II fibres. The less negative value in the type I fibres primarily reflects that the contractile apparatus is more sensitive to Ca2+ in such fibres (see Fig. 1 in Lamboley et al. 2013), and hence that less Ca2+ had to be loaded into and released from the SR to elicit just measurable force. Taking this below-threshold SR Ca2+ content value into account in each fibre, the relative rate of Ca2+ loading at each pCa could be derived simply from the slopes of the lines describing Ca2+ accumulation versus time (see left-hand panels in Fig. 2); values for the fibres shown in Fig. 1 are plotted in the right-hand panels in Fig. 2. Using this procedure the relative rate of Ca2+ uptake at each pCa could be measured with reasonable accuracy because in effect the values primarily depended on measuring the loading time at each pCa required to yield a just measurable force response (e.g. Fig. 1), and such values should be directly comparable irrespective of almost all methodological assumptions.
The plot of relative Ca2+ uptake rate versus pCa for each fibre was fitted with a Hill curve (e.g. Fig. 2). The individual fits found in the 7 type I fibres and 12 type II fibres examined are shown in Fig. 3 (pale dashed lines). The pCa at which the SR Ca2+ uptake rate was half-maximal (pCa50(SR)) was significantly higher in the type I fibres (mean ± SEM: 6.79 ± 0.02) than in the type II fibres (6.60 ± 0.02) (P < 0.05), with the Hill coefficient (h) being similar in the two cases (1.73 ± 0.09 and 1.68 ± 0.08, respectively). In other words, the SR Ca2+ uptake process in human type I fibres shows higher Ca2+ sensitivity than in type II fibres. The average Ca2+ uptake behaviour in the two fibre types is illustrated in Fig. 3 by the superimposed continuous lines, which are Hill curves plotted using the mean values for pCa50(SR) and h found for each fibre type.
Figure 3.
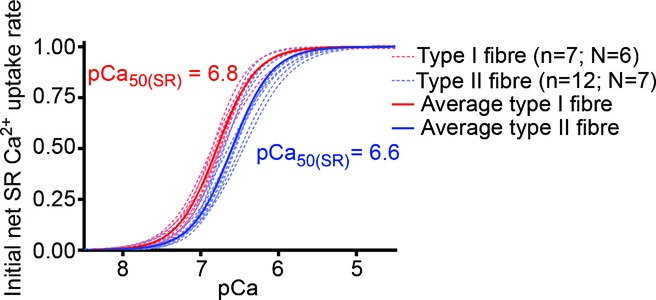
Superposition of average (continuous lines) and individual single fibre cases (dashed lines) of Hill curve fits to SR Ca2+ uptake data for all type I (red) and type II (blue) muscle fibres examined, determined as in Fig. 2. The mean values for the fits to the individual fibre data gave pCa50(SR), the pCa for half-maximal SR Ca2+ uptake rate, as 6.79 ± 0.02 and 6.60 ± 0.02 in type I and type II fibres, respectively, with similar coefficients in the two cases (1.73 ± 0.09 and 1.68 ± 0.08, respectively). n denotes number of fibres and N the number of subjects.
The procedure used here also allowed assessment of the relative amount of Ca2+ present in the SR endogenously in each fibre, indicated by the response elicited when first emptying the SR of Ca2+ (‘Endo’ in Fig. 1; see Methods). It was found that when using a load solution with the free [Ca2+] set at pCa 7.0, which is probably close to the normal resting cytoplasmic levels prevailing in the muscle fibres, the time taken to reload the depleted SR back to its endogenous level was considerably shorter in type I fibres (12.5 ± 1.9 s) than in type II fibres (23.3 ± 1.4 s) (P < 0.05); the data for the individual fibres are presented in Fig. 4. In contrast, when operating at their maximal Ca2+ uptake rate (derived for each fibre from the Hill fit to the data of Ca2+ uptake rate versus pCa) the calculated time taken to reload the SR to its endogenous level was quite similar in the two fibre types (∼3.9 ± 0.4 s and ∼3.8 ± 0.3 s in type I and type II fibres, respectively). As the absolute amount of Ca2+ present in the SR endogenously is on average only slightly lower in type I fibres than in type II fibres (∼0.7 and 0.8 mmol per litre fibre volume, respectively; Lamboley et al. 2013), these data indicate that the maximum absolute rate of SR Ca2+ uptake is quite similar in type I and type II fibres, and also are further evidence of the relatively higher Ca2+ affinity of the uptake in the type I fibres (see Discussion).
Calcium-induced calcium release
The above experiments did not measure SR Ca2+ accumulation per se, but rather the net amount of Ca2+ accumulation by the SR, and this would be considerably smaller if there were any substantial amount of calcium-induced calcium release (CICR) through the Ca2+ release channels/ryanodine receptors (Endo, 2009) occurring in parallel with the Ca2+ uptake. This was further explored by examining whether the net amount of Ca2+ accumulated by the SR was increased when the load solution had a higher free [Mg2+], which should block or strongly reduce such CICR (Meissner et al. 1986; Lamb et al. 2001; Endo, 2009). Ca2+ uptake was examined in each of four type I and four type II fibres using load solutions with the Ca2+ heavily buffered at pCa 6.0 and with the free [Mg2+] set at 1 mm (i.e. the standard conditions in this study) or 3 mm or 10 mm. As seen in Fig. 5, net Ca2+ uptake was not significantly changed when the [Mg2+] was raised from 1 to 3 mm, but was reduced appreciably at 10 mm Mg2+. These data indicate that there was very little if any CICR occurring in either the type I or type II human muscle fibres. The reduction in uptake apparent at 10 mm Mg2+ is consistent with previous findings in mammalian muscle indicating that in the presence of 1 μm free Ca2+ (i.e. at pCa 6.0) the inhibitory action of Mg2+ competing with Ca2+ binding at the calcium pumps becomes appreciable at above 3 mm Mg2+ (Yamamoto & Tonomura, 1967; Kabbara & Stephenson, 1994).
Figure 5.
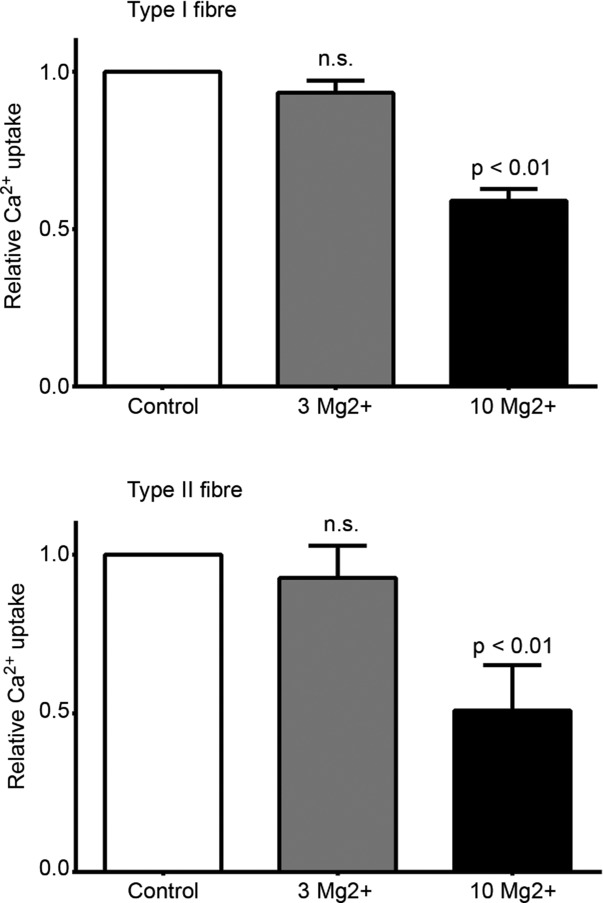
Mean (+SEM) of relative amount of SR Ca2+ uptake in human type I and type II muscle fibres when loading at pCa 6.0 in presence of 1 mm (control), 3 mm or 10 mm free Mg2+. Loading examined at each [Mg2+] in each of four type I and four type II fibres (N = 2 subjects in both cases). Experiments performed similarly to that shown in Fig. 1 using 5 s loading in pCa 6.0 solution with 5 mm CaEGTA present. Amount of Ca2+ uptake normalized to that found with 1 mm Mg2+ (i.e. standard load conditions) in same fibre. No significant difference between Ca2+ uptake with 1 and 3 Mg2+.
SR Ca2+ leak and effect of ADP
The amount of Ca2+ leakage out of the SR in human muscle fibres at pCa 8.5, and the effect of ADP on the leakage, was examined as in Fig. 6. Similar experiments were also carried out on type I fibres (from soleus muscle) and type II fibres (from EDL muscle) of rat, using exactly the same procedures and solutions. The mean data from these experiments are presented in Fig. 7. It was found with the human fibres that the SR Ca2+ leakage at pCa 8.5 was significantly greater in type I fibres than in type II fibres, but in both cases the leakage was considerably less than that occurring in rat fibres. Furthermore, the amount of Ca2+ leakage in rat fibres increased progressively as the [ADP] was increased from 0.1 μm to 1 mm, similar to previous findings (Macdonald & Stephenson, 2001, 2006), whereas the [ADP] had comparatively little effect on the Ca2+ leakage in the human fibres (Fig. 7).
Figure 7.

Mean (±SEM) of relative amount of Ca2+ remaining in SR following a 1 min leak period at pCa 8.5 in presence of indicated [ADP] (∼0.1 μm, 31 μm or 1 mm) in type I and type II fibres from human vastus lateralis muscle and from rat soleus and EDL muscles. Experiments carried out as in Fig. 6. Content values expressed relative to that found with no leakage period, determined from bracketing responses in each fibre. n denotes number of fibres and N the number of human subjects or rats. *Significantly different from data for 0.1 μm ADP. #Significantly different from data for 31 μm ADP.
Discussion
SR Ca2+ uptake in human muscle fibres
This study characterized SR Ca2+ uptake and leakage properties in functional fibres from vastus lateralis muscle of young healthy adults. One major finding was that the Ca2+ sensitivity of uptake is substantially higher in type I fibres than in type II fibres (half-maximal uptake at ∼pCa 6.8 and ∼pCa 6.6, i.e. at ∼0.16 μm and ∼0.25 μm Ca2+, respectively), with the Hill coefficient being similar in the two cases (∼1.7; Fig. 3). Western blotting showed that SERCA2a was the predominant or sole SERCA isoform present in the type I fibres, and SERCA1 in the type II fibres. Previous studies expressing SERCA1 and SERCA2a in COS-1 cells found the Ca2+ sensitivity of uptake to be the same for both isoforms (Lytton et al. 1992; Sumbilla et al. 1999; see Introduction), whereas another study expressing the SERCA in HEK293 cells found results very similar to those found here in human muscle fibres (half-maximal uptake at ∼pCa 6.7 and 6.5 for SERCA2a and SERCA1, respectively; Dode et al. 2003). Importantly, the Ca2+ uptake properties found here reflect the overall functioning of the SERCA in situ in the sarcoplasmic reticulum, with any association with endogenous auxiliary proteins, in particular any phospholamban and sarcolipin, preserved. The higher Ca2+ sensitivity of Ca2+ uptake in type I fibres probably reflects the functional requirements of such fibres, with the contractile apparatus being more sensitive to Ca2+ than in type II fibres and full relaxation occurring only when the SERCA have lowered the cytoplasmic [Ca2+] to below ∼0.4 μm (>pCa 6.4; see Fig. 1A in Lamboley et al. 2013). In other words, one reason why type I fibres are likely to express SERCA2a rather than SERCA1 is probably because the higher Ca2+ sensitivity of SERCA2a means that it can always decrease the cytoplasmic [Ca2+] sufficiently to ensure that the fibre can fully relax in all circumstances, despite the relatively high sensitivity of the contractile apparatus to Ca2+.
The maximum rate of SR Ca2+ uptake in the type I and type II fibres was calculated as being ∼0.18 and 0.21 mmol Ca2+ per litre fibre volume per second, respectively; these values were derived simply from the time taken to load the SR from empty to its endogenous level in the two fibre types (mean ∼3.9 and 3.8 s, respectively) and the previously measured values for average endogenous SR Ca2+ content (∼0.68 and 0.78 mmol Ca2+ per litre, respectively; Lamboley et al. 2013). Given these maximum uptake rates and endogenous content values, the fact that the SR could be loaded at pCa 7.0 back to the endogenous level much faster in type I fibres than in type II fibres (Fig. 4) is fully consistent with the Ca2+ sensitivity of the uptake being substantially higher in the former (see Fig. 3; compare relative loading rates at pCa 7.0).
This higher Ca2+ sensitivity of Ca2+ uptake in the type I fibres also helps explain our previous observation that the SR is loaded endogenously to a greater proportion of maximum in type I fibres than in type II fibres (Lamboley et al. 2013). The resting cytoplasmic [Ca2+] in human muscle fibres appears to be ∼100 nm (Lopez et al. 1992; i.e. pCa 7.0), though it is unknown whether the value differs between type I and type II fibres. In rat and mouse muscle, resting cytoplasmic [Ca2+] is reported to be about 2–fold higher in type I fibres than in type II fibres (estimates ∼60–90 nm versus ∼30–50 nm, respectively; Gailly et al. 1993; Carroll et al. 1997; Fraysse et al. 2006), whereas in rabbit muscle the cytoplasmic levels were reported to be similar in type I and type II fibres (∼120 and ∼110 nm, respectively; Sreter et al. 1987). Assuming that, as in other mammalian muscle, the resting cytoplasmic [Ca2+] in human type I fibres is similar or higher than in type II fibres, the amount of Ca2+ uptake into the SR in resting conditions would be considerably greater in the type I fibres (see Fig. 3), and hence the Ca2+ content of the SR would be expected to increase to a relatively higher amount, that being the equilibrium point where the higher uptake was matched by equal leakage out of the SR back into the cytoplasm (see further discussion in ‘SR Ca2+ leak properties’ below).
The maximum rate of SR Ca2+ uptake found here appears in good accord with the maximum SR ATPase rate, which in type I and type IIA fibres of adult human vastus lateralis muscle were found at 20°C to be ∼0.051 and 0.069 mmol ATP per litre fibre volume per second (Szentesi et al. 2001). Taking into account that the fibre dimensions in that study were measured after fibre swelling in solution (unlike calculations here, see Lamboley et al. 2013), and hence that the apparent fibre volume was increased by a factor of ∼1.44 (see Szentesi et al. 2001), those values equate in the units used here to maximum SR ATPase rates of ∼0.073 and ∼0.10 mmol ATP l−1 s−1 in type I and type IIA fibres, respectively. These values match quite well with the maximum Ca2+ uptake rates found here (∼0.18 and 0.21 mmol Ca2+ l−1 s−1 in type I and type II fibres, respectively) if the SERCA transported two Ca2+ ions per ATP hydrolysed, particularly given that the Ca2+ uptake experiments here were performed at slightly higher temperature (∼23°C) than were the ATPase experiments. Type IIX and IIA/X fibres have substantially higher SR ATPase rates but the proportion of such fibres was found to be relatively low in vastus lateralis muscle of healthy adults (Bottinelli & Reggiani, 2000; Szentesi et al. 2001; Talmadge et al. 2002), and it seems likely that most of the type II fibres examined here were type IIA.
A previous study examining Ca2+ uptake in human muscle fibres (Salviati et al. 1982) found the maximum uptake rate at 25°C to be only ∼0.03 and 0.06 mmol Ca2+ l−1 s−1 in type I and type II fibres, respectively, from quadriceps muscle, and even lower in fibres from pectoralis muscle. That study was performed using muscle fibres that had been chemically skinned in a glycerol–EGTA solution for 24 h, and measured oxalate-supported Ca2+ uptake. The maximum SR Ca2+ content achievable in those fibres in the absence of oxalate was much less than that measured in fresh mechanically skinned fibres (Lamboley et al. 2013), probably owing to increased SR Ca2+ leakage in the chemically skinned fibres due to disruption of the normal coupling between the transverse tubular dihydropyridine receptors and the RyRs and also the low free [Mg2+] used (<0.1 mm). Thus, the lower maximal Ca2+ uptake rates observed by Salviati et al. (1982) could be the result of both increased SR Ca2+ leakage and possible limitations in Ca2+ uptake related to the associated requirement for oxalate transport. Significantly, studies measuring oxalate-supported Ca2+ uptake in isolated SR vesicles and COS-1 microsomes (Lytton et al. 1992) in general find much less efficient net Ca2+ uptake (i.e. only 0.2–0.4 Ca2+ per ATP hydrolysed) than found in the present study using mechanically skinned fibres with functional coupling and solutions closely mimicking the ionic conditions prevailing in the normal cytosol (including low [Cl−] and 1 mm Mg2+; see also Lamb & Stephenson, 1994; Lamb et al. 2001).
The Ca2+ uptake rate in the human muscle fibres here was measured at ∼23°C. It has been estimated that the SR ATPase rate increases by a factor of ∼3 times per 10°C (Stienen et al. 1995; Szentesi et al. 2001), which would imply that the maximal Ca2+ uptake rate at normal body temperature could be as much as ∼0.9 mmol Ca2+ l−1 s−1. As the amount of Ca2+ that needs to come off troponin C and be taken up by the SR to fully relax a maximally activated fibre is probably only ∼0.14 and 0.19 mmol l−1 in type I and type II fibres, respectively (see Lamboley et al. 2013), and given that SR Ca2+ uptake occurs at >85% of the maximal rate at the relevant [Ca2+] (i.e. at pCa <6.4 in type I fibres and <6.1 in type II fibres; Fig. 3), the uptake activity found here would seemingly be able to account for full muscle relaxation in <0.25 s, which is compatible with the relaxation rate observed in humans (e.g. see Fig. 6 in Bigland-Ritchie et al. 1983). Furthermore, even though human fibres completely lack any cytoplasmic Ca2+ buffering by parvalbumin, Ca2+ binding to ATP could be expected to aid the lowering and removal of free Ca2+ from the cytoplasm (Baylor & Hollingworth, 2012).
SR Ca2+ leak properties
The rate of Ca2+ leakage out of the SR at pCa 8.5 observed in rat muscle fibres was similar to that found previously with the same protocol (Macdonald & Stephenson, 2001, 2006), whereas the leakage rate in the human muscle fibres was substantially lower (Fig. 7). Much of this leakage in rat muscle fibres evidently occurs through the SERCAs themselves (Inesi & de Meis, 1989), because it can be blocked by SERCA inhibitors (Macdonald & Stephenson, 2001, 2006; Murphy et al. 2009a). With the loading protocol used (0.5 mm CaEGTA at pCa 6.7 for 1 min), the SR loads to ∼85–90% of maximum in both the human and rat type I fibres, and to ∼70% and ∼50% of maximum in the human and rat type II fibres, respectively, which equate to ∼1.1–1.2 mmol Ca2+ per litre fibre in the type I fibres and human type II fibres and to ∼1.8 mmol Ca2+ in the rat type II fibres (Murphy et al. 2009a; Lamboley et al. 2013). Thus, the data in Fig. 7 showing the percentage of the SR Ca2+ content remaining after a 60 s leak period indicate that the absolute rates of SR leakage were ∼10 and 15 μmol Ca2+ l−1 s−1 in the rat type I and type II fibres and ∼6 and 4 μmol Ca2+ l−1 s−1 in the human type I and type II fibres, respectively. In the rat fibres the absolute leakage rate in type II fibres was only ∼1.5-fold higher than in the type I fibres, despite there being ∼5-to 7-fold higher SERCA density (Wu & Lytton, 1993), most likely owing to the comparatively large amount of CSQ1 present in the type II fibres which meant that the free [Ca2+] in the SR was comparatively low (Murphy et al. 2009a). Similarly in the human fibres, the lower leakage rate observed in the type II fibres probably reflects the Ca2+ buffering effects of the relatively large amount of CSQ1 present compared to type I fibres (Lamboley et al. 2013).
The relatively lower leakage rate in human type I fibres compared to rat type I fibres (with the SR loaded to a similar percentage of maximum SR content in both, see above) is not readily explained simply in terms of the relative density of the SERCA2a present in the two cases (Everts et al. 1989; Wu & Lytton, 1993). Specifically, as ∼50% of the total SERCA density (∼6.4 μmol (kg muscle)−1) measured in rat soleus muscle by Everts et al. (1989) was probably attributable to SERCA1 present in type II fibres (Wu & Lytton, 1993; Murphy et al. 2009a), the density of the SERCA2a in the rat type I fibres was only ∼75% of the SERCA density observed in human vastus lateralis muscle homogenates (∼4.5 μmol kg−1; Everts et al. 1989); the latter probably consisted of similar proportions of type I and type II fibres and so, given that the maximal ATPase rate is only slightly lower for SERCA2a in human type I fibres than for SERCA1 in type II fibres (Szentesi et al. 2001), it can be inferred that the SERCA2a density in type I fibres of rat is similar or lower than that in type I fibres of humans. Thus, the relatively lower leakage rate in human type I fibres may reflect some difference in regulation of the SERCA between human and rats, which is also suggested by the apparent difference in the effects of ADP on SR Ca2+ leakage (Fig. 7). Ca2+ leaking from the SR ultimately has to be pumped back in again, at the cost of ATP, and consequently resting SR Ca2+ leakage in muscle can be expected to contribute to basal metabolic rate (Smith et al. 2013). The relatively lower SR Ca2+ leakage rate in human muscle might be an adaptation linked to the presumably lower relative rate of heat loss in humans arising from their much larger mass to surface area ratio compared to rats. It is worth bearing in mind that the SR Ca2+ leakage rate found in the human fibres (∼4–6 μmol l−1 s−1) is more than 30-fold lower than the maximal uptake rate. Furthermore, the leakage rates were measured at pCa 8.5 and leakage through the SERCA would be even less at resting cytoplasmic [Ca2+] (∼pCa 7.0) because there would be some level of occupancy of the cytoplasmic accessible Ca2+ binding sites on the SERCA, which would reduce the extent to which the SERCAs act as Ca2+–Ca2+ exchangers transferring luminal Ca2+ to the cytoplasm (Macdonald & Stephenson, 2001).
Although it is evident that SERCAs in muscle play a significant role in thermogenesis and basal metabolic rate (de Meis, 2001; Smith et al. 2013), the details of this regulation remain quite unclear, particularly in regard to SERCA modulation by sarcolipin and phospholamban and ADP, and also to possible species differences. Sarcolipin and phospholamban protein expression differs greatly between species and between muscle types within a species, with greater expression in larger animals such as rabbits and pigs, and no (Vangheluwe et al. 2005) or low expression (Babu et al. 2007) in rats and mice; a recent study has shown that sarcolipin is also present in human muscle (Fajardo et al. 2013) but the comparative level of expression is unknown. Phospholamban associates with SERCA2a and reduces its affinity for Ca2+ in cardiac muscle, but not in slow-twitch (type I) skeletal muscle, at least in dog (Briggs et al. 1992). Sarcolipin association with SERCA increases Ca2+ leak (Smith et al. 2002) and thermogenesis in mouse muscle (Bal et al. 2012), whereas phospholamban association reportedly does not (Sahoo et al. 2013). However, there are several major discrepancies between current reports on the comparative effects and mechanisms of action of sarcolipin and phospholamban (see Discussion in Akin et al. 2013). Furthermore, there is currently no information about whether the actions of sarcolipin and ADP in increasing SERCA leak are related, independent, or even mutually exclusive. Thus, whilst this study has provided important novel information about SR Ca2+ uptake and leak in human muscle, discrepancies and shortcomings in current knowledge make it difficult at present to relate the findings to specific mechanistic processes.
Concluding remarks
In summary, this study provides novel quantitative information about SR Ca2+ uptake and Ca2+ leak in human muscle fibres, showing that (i) the maximum SR Ca2+ uptake in functional muscle fibres with normal intracellular ionic conditions is seemingly in good accord with the measured SR Ca2+-ATPase activity and with translocation of two Ca2+ ions per ATP hydrolysed, (ii) the Ca2+ sensitivity of Ca2+ uptake is significantly higher in type I fibres than in type II fibres, consistent with the contractile apparatus requirements and also with the observed resting SR Ca2+ contents in the respective fibre types, (iii) the relative SR Ca2+ leak rates in human type I and type II fibres appear in accord with the known CSQ contents and presumed intra-SR free [Ca2+], and (iv) SR Ca2+ leak rates in human fibres are lower than in rat fibres and seemingly less affected by cytoplasmic [ADP], suggestive that SERCA regulation may differ appreciably in the two species, which could be of considerable significance in thermogenesis.
Key points
Release and uptake of Ca2+ ions by the sarcoplasmic reticulum (SR) regulates contraction in skeletal muscle. SR Ca2+ uptake and leak properties in human muscle are presently not well defined.
The surface membrane of individual human muscle fibres was removed by microdissection, and the rate of SR Ca2+ uptake at different applied [Ca2+] assessed from the amount of Ca2+ accumulated.
Ca2+ uptake occurred at lower [Ca2+] in type I fibres than in type II fibres, consistent with the contractile apparatus properties in the respective fibre types. Maximal uptake rate was slightly greater in type II fibres, and approximately two Ca2+ were taken up per ATP hydrolysed.
Ca2+ leaking out of the SR ultimately has to be pumped back in again at the cost of ATP usage. SR Ca2+ leakage in human muscle fibres was smaller and regulated differently to that in rat muscle fibres, probably reflecting different contributions to thermogenesis.
Acknowledgments
We thank Maria Cellini and Heidy Latchman for technical assistance, and Dr Noni Frankenberg for assistance in dissecting fibres. The monoclonal antibodies directed against adult human MHC isoforms (A4.84 and A4.74) were developed by Dr Blau and that against SERCA (CA F2-5D,) was developed by Dr Fambrough, and all were obtained from the Development Studies Hybridoma Bank, under the auspices of the NICHD and maintained by the University of Iowa, Department of Biological Sciences, Iowa City, IA 52242.
Glossary
- CICR
calcium-induced calcium release
- CP
creatine phosphate
- CSQ
calsequestrin
- EDL
extensor digitorum longus
- h
Hill coefficient
- HRP
horseradish peroxidase
- MHC
myosin heavy chain
- n
number of fibres
- N
number of subjects
- pCa
–log10[Ca2+]
- pCa50(SR)
the pCa at half-maximal SR Ca2+ uptake rate
- pSr
–log10[Sr2+]
- RyR
ryanodine receptor
- SERCA
sarco(endo)plasmic reticulum Ca2+-ATPase
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
Muscle biopsies were performed at Victoria University, and biochemical and physiological measurements on skinned fibres made at La Trobe University. C.R.L. and M.J.M. were responsible for subject care and obtaining muscle biopsies from human subjects. Skinned fibre experiments were designed and analysed by G.D.L. and C.R.L., and carried out by C.R.L., and R.M.M. and G.D.L. were responsible for Western blotting procedures and analysis. C.R.L. and G.D.L. drafted the manuscript. All authors were involved in the conception of the project and have reviewed the final version of the submitted manuscript.
Funding
This work was supported by the National Health & Medical Research Council of Australia (Grant number 1051460).
References
- Akin BL, Hurley TD, Chen Z, Jones LR. The structural basis for phospholamban inhibition of the calcium pump in sarcoplasmic reticulum. J Biol Chem. 2013;288:30181–30191. doi: 10.1074/jbc.M113.501585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu GJ, Bhupathy P, Carnes CA, Billman GE, Periasamy M. Differential expression of sarcolipin protein during muscle development and cardiac pathophysiology. J Mol Cell Cardiol. 2007;43:215–222. doi: 10.1016/j.yjmcc.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal NC, Maurya SK, Sopariwala DH, Sahoo SK, Gupta SC, Shaikh SA, Pant M, Rowland LA, Bombardier E, Goonasekera SA, Tupling AR, Molkentin JD, Periasamy M. Sarcolipin is a newly identified regulator of muscle-based thermogenesis in mammals. Nat Med. 2012;18:1575–1579. doi: 10.1038/nm.2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor SM, Hollingworth S. Intracellular calcium movements during excitation-contraction coupling in mammalian slow-twitch and fast-twitch muscle fibers. J Gen Physiol. 2012;139:261–272. doi: 10.1085/jgp.201210773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard NA, Laver DR, Dulhunty AF. Calsequestrin and the calcium release channel of skeletal and cardiac muscle. Prog Biophys Mol Biol. 2004;85:33–69. doi: 10.1016/j.pbiomolbio.2003.07.001. [DOI] [PubMed] [Google Scholar]
- Bigland-Ritchie B, Johansson R, Lippold OC, Woods JJ. Contractile speed and EMG changes during fatigue of sustained maximal voluntary contractions. J Neurophysiol. 1983;50:313–324. doi: 10.1152/jn.1983.50.1.313. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Reggiani C. Human skeletal muscle fibres: molecular and functional diversity. Prog Biophys Mol Biol. 2000;73:195–262. doi: 10.1016/s0079-6107(00)00006-7. [DOI] [PubMed] [Google Scholar]
- Briggs FN, Lee KF, Wechsler AW, Jones LR. Phospholamban expressed in slow-twitch and chronically stimulated fast-twitch muscles minimally affects calcium affinity of sarcoplasmic reticulum Ca2+-ATPase. J Biol Chem. 1992;267:26056–26061. [PubMed] [Google Scholar]
- Carroll SL, Klein MG, Schneider MF. Decay of calcium transients after electrical stimulation in rat fast-and slow-twitch skeletal muscle fibres. J Physiol. 1997;501:573–588. doi: 10.1111/j.1469-7793.1997.573bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Meis L. Role of the sarcoplasmic reticulum Ca2+-ATPase on heat production and thermogenesis. Biosci Rep. 2001;21:113–137. doi: 10.1023/a:1013640006611. [DOI] [PubMed] [Google Scholar]
- Dode L, Andersen JP, Leslie N, Dhitavat J, Vilsen B, Hovnanian A. Dissection of the functional differences between sarco(endo)plasmic reticulum Ca2+-ATPase (SERCA) 1 and 2 isoforms and characterization of Darier disease (SERCA2) mutants by steady-state and transient kinetic analyses. J Biol Chem. 2003;278:47877–47889. doi: 10.1074/jbc.M306784200. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Lamb GD. Effect of low cytoplasmic [ATP] on excitation–contraction coupling in fast-twitch muscle fibres of the rat. J Physiol. 2004;560:451–468. doi: 10.1113/jphysiol.2004.069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutka TL, Lamboley CR, McKenna MJ, Murphy RM, Lamb GD. Effects of carnosine on contractile apparatus Ca2+ sensitivity and sarcoplasmic reticulum Ca2+ release in human skeletal muscle fibers. J Appl Physiol. 2012;112:728–736. doi: 10.1152/japplphysiol.01331.2011. [DOI] [PubMed] [Google Scholar]
- Endo M. Calcium-induced calcium release in skeletal muscle. Physiol Rev. 2009;89:1153–1176. doi: 10.1152/physrev.00040.2008. [DOI] [PubMed] [Google Scholar]
- Everts ME, Andersen JP, Clausen T, Hansen O. Quantitative determination of Ca2+-dependent Mg2+-ATPase from sarcoplasmic reticulum in muscle biopsies. Biochem J. 1989;260:443–448. doi: 10.1042/bj2600443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fajardo VA, Bombardier E, Vigna C, Devji T, Bloemberg D, Gamu D, Gramolini AO, Quadrilatero J, Tupling AR. Co-expression of SERCA isoforms, phospholamban and sarcolipin in human skeletal muscle fibers. PloS One. 2013;8:e84304. doi: 10.1371/journal.pone.0084304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C, Protasi F. Ryanodine receptors of striated muscles: a complex channel capable of multiple interactions. Physiol Rev. 1997;77:699–729. doi: 10.1152/physrev.1997.77.3.699. [DOI] [PubMed] [Google Scholar]
- Fraysse B, Desaphy JF, Rolland JF, Pierno S, Liantonio A, Giannuzzi V, Camerino C, Didonna MP, Cocchi D, De Luca A, Conte Camerino D. Fiber type-related changes in rat skeletal muscle calcium homeostasis during aging and restoration by growth hormone. Neurobiol Dis. 2006;21:372–380. doi: 10.1016/j.nbd.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Fryer MW, Stephenson DG. Total and sarcoplasmic reticulum calcium contents of skinned fibres from rat skeletal muscle. J Physiol. 1996;493:357–370. doi: 10.1113/jphysiol.1996.sp021388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailly P, Boland B, Himpens B, Casteels R, Gillis JM. Critical evaluation of cytosolic calcium determination in resting muscle fibres from normal and dystrophic (mdx) mice. Cell Calcium. 1993;14:473–483. doi: 10.1016/0143-4160(93)90006-r. [DOI] [PubMed] [Google Scholar]
- Inesi G, de Meis L. Regulation of steady state filling in sarcoplasmic reticulum. Roles of back-inhibition, leakage, and slippage of the calcium pump. J Biol Chem. 1989;264:5929–5936. [PubMed] [Google Scholar]
- Kabbara AA, Stephenson DG. Effects of Mg2+ on Ca2+ handling by the sarcoplasmic reticulum in skinned skeletal and cardiac muscle fibres. Pflugers Arch. 1994;428:331–339. doi: 10.1007/BF00724515. [DOI] [PubMed] [Google Scholar]
- Lamb GD, Cellini MA, Stephenson DG. Different Ca2+ releasing action of caffeine and depolarisation in skeletal muscle fibres of the rat. J Physiol. 2001;531:715–728. doi: 10.1111/j.1469-7793.2001.0715h.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb GD, Stephenson DG. Effects of intracellular pH and [Mg2+] on excitation–contraction coupling in skeletal muscle fibres of the rat. J Physiol. 1994;478:331–339. doi: 10.1113/jphysiol.1994.sp020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamboley CR, Murphy RM, McKenna MJ, Lamb GD. Endogenous and maximal sarcoplasmic reticulum calcium content and calsequestrin expression in type I and type II human skeletal muscle fibres. J Physiol. 2013;591:6053–6068. doi: 10.1113/jphysiol.2013.265900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leberer E, Pette D. Immunochemical quantification of sarcoplasmic reticulum Ca-ATPase, of calsequestrin and of parvalbumin in rabbit skeletal muscles of defined fiber composition. Eur J Biochem. 1986;156:489–496. doi: 10.1111/j.1432-1033.1986.tb09607.x. [DOI] [PubMed] [Google Scholar]
- Lopez JR, Gerardi A, Lopez MJ, Allen PD. Effects of dantrolene on myoplasmic free [Ca2+] measured in vivo in patients susceptible to malignant hyperthermia. Anesthesiology. 1992;76:711–719. doi: 10.1097/00000542-199205000-00008. [DOI] [PubMed] [Google Scholar]
- Lytton J, Westlin M, Burk SE, Shull GE, MacLennan DH. Functional comparisons between isoforms of the sarcoplasmic or endoplasmic reticulum family of calcium pumps. J Biol Chem. 1992;267:14483–14489. [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effects of ADP on sarcoplasmic reticulum function in mechanically skinned skeletal muscle fibres of the rat. J Physiol. 2001;532:499–508. doi: 10.1111/j.1469-7793.2001.0499f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald WA, Stephenson DG. Effect of ADP on slow-twitch muscle fibres of the rat: implications for muscle fatigue. J Physiol. 2006;573:187–198. doi: 10.1113/jphysiol.2006.105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-Acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan DH, Wong PT. Isolation of a calcium-sequestering protein from sarcoplasmic reticulum. Proc Natl Acad Sci U S A. 1971;68:1231–1235. doi: 10.1073/pnas.68.6.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan DW, Henkin JA, Vigoreaux JO. Concentrations of glycolytic enzymes and other cytosolic proteins in the diffusible fraction of a vertebrate muscle proteome. Mol Cell Proteomics. 2005;4:1541–1549. doi: 10.1074/mcp.M500053-MCP200. [DOI] [PubMed] [Google Scholar]
- Meissner G, Darling E, Eveleth J. Kinetics of rapid Ca2+ release by sarcoplasmic reticulum. Effects of Ca2+, Mg2+, and adenine nucleotides. Biochemistry. 1986;25:236–244. doi: 10.1021/bi00349a033. [DOI] [PubMed] [Google Scholar]
- Moisescu DG, Thieleczek R. Calcium and strontium concentration changes within skinned muscle preparations following a change in the external bathing solution. J Physiol. 1978;275:241–262. doi: 10.1113/jphysiol.1978.sp012188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fiber-type dependence of AMPK-α1 and-β1. J Appl Physiol. 2011;110:820–825. doi: 10.1152/japplphysiol.01082.2010. [DOI] [PubMed] [Google Scholar]
- Murphy RM, Lamb GD. Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol. 2013;591:5823–5831. doi: 10.1113/jphysiol.2013.263251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Larkins NT, Mollica JP, Beard NA, Lamb GD. Calsequestrin content and SERCA determine normal and maximal Ca2+ storage levels in sarcoplasmic reticulum of fast-and slow-twitch fibres of rat. J Physiol. 2009a;587:443–460. doi: 10.1113/jphysiol.2008.163162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy RM, Mollica JP, Lamb GD. Plasma membrane removal in rat skeletal muscle fibers reveals caveolin–3 hot-spots at the necks of transverse tubules. Exp Cell Res. 2009b;315:1015–1028. doi: 10.1016/j.yexcr.2008.11.022. [DOI] [PubMed] [Google Scholar]
- Periasamy M, Kalyanasundaram A. SERCA pump isoforms: their role in calcium transport and disease. Muscle Nerve. 2007;35:430–442. doi: 10.1002/mus.20745. [DOI] [PubMed] [Google Scholar]
- Rossi AE, Boncompagni S, Wei L, Protasi F, Dirksen RT. Differential impact of mitochondrial positioning on mitochondrial Ca2+ uptake and Ca2+ spark suppression in skeletal muscle. Am J Physiol Cell Physiol. 2011;301:C1128–C1139. doi: 10.1152/ajpcell.00194.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahoo SK, Shaikh SA, Sopariwala DH, Bal NC, Periasamy M. Sarcolipin protein interaction with sarco(endo)plasmic reticulum Ca2+ ATPase (SERCA) is distinct from phospholamban protein, and only sarcolipin can promote uncoupling of the SERCA pump. J Biol Chem. 2013;288:6881–6889. doi: 10.1074/jbc.M112.436915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salviati G, Sorenson MM, Eastwood AB. Calcium accumulation by the sarcoplasmic reticulum in two populations of chemically skinned human muscle fibers. Effects of calcium and cyclic AMP. J Gen Physiol. 1982;79:603–632. doi: 10.1085/jgp.79.4.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith IC, Bombardier E, Vigna C, Tupling AR. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 40–50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. PloS One. 2013;8:e68924. doi: 10.1371/journal.pone.0068924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith WS, Broadbridge R, East JM, Lee AG. Sarcolipin uncouples hydrolysis of ATP from accumulation of Ca2+ by the Ca2+-ATPase of skeletal-muscle sarcoplasmic reticulum. Biochem J. 2002;361:277–286. doi: 10.1042/0264-6021:3610277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreter FA, Lopez JR, Alamo L, Mabuchi K, Gergely J. Changes in intracellular ionized Ca concentration associated with muscle fiber type transformation. Am J Physiol Cell Physiol. 1987;253:C296–C300. doi: 10.1152/ajpcell.1987.253.2.C296. [DOI] [PubMed] [Google Scholar]
- Stienen GJ, Zaremba R, Elzinga G. ATP utilization for calcium uptake and force production in skinned muscle fibres of Xenopus laevis. J Physiol. 1995;482:109–122. doi: 10.1113/jphysiol.1995.sp020503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumbilla C, Cavagna M, Zhong L, Ma H, Lewis D, Farrance I, Inesi G. Comparison of SERCA1 and SERCA2a expressed in COS–1 cells and cardiac myocytes. Am J Physiol Heart Circ Physiol. 1999;277:H2381–H2391. doi: 10.1152/ajpheart.1999.277.6.H2381. [DOI] [PubMed] [Google Scholar]
- Szentesi P, Zaremba R, van Mechelen W, Stienen GJ. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol. 2001;531:393–403. doi: 10.1111/j.1469-7793.2001.0393i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmadge RJ, Castro MJ, Apple DF, Jr, Dudley GA. Phenotypic adaptations in human muscle fibers 6 and 24 wk after spinal cord injury. J Appl Physiol. 2002;92:147–154. doi: 10.1152/japplphysiol.000247.2001. [DOI] [PubMed] [Google Scholar]
- Trinh HH, Lamb GD. Matching of sarcoplasmic reticulum and contractile properties in rat fast-and slow-twitch muscle fibres. Clin Exp Pharmacol Physiol. 2006;33:591–600. doi: 10.1111/j.1440-1681.2006.04412.x. [DOI] [PubMed] [Google Scholar]
- Vangheluwe P, Schuermans M, Zador E, Waelkens E, Raeymaekers L, Wuytack F. Sarcolipin and phospholamban mRNA and protein expression in cardiac and skeletal muscle of different species. Biochem J. 2005;389:151–159. doi: 10.1042/BJ20050068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu KD, Lytton J. Molecular cloning and quantification of sarcoplasmic reticulum Ca2+-ATPase isoforms in rat muscles. Am J Physiol Cell Physiol. 1993;264:C333–C341. doi: 10.1152/ajpcell.1993.264.2.C333. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Tonomura Y. Reaction mechanism of the Ca++-dependent ATPase of sarcoplasmic reticulum from skeletal muscle. I. Kinetic studies. J Biochem. 1967;62:558–575. doi: 10.1093/oxfordjournals.jbchem.a128706. [DOI] [PubMed] [Google Scholar]


