Abstract
NOS1AP gene (nitric oxide synthase 1-adaptor protein) is strongly associated with abnormalities in the QT interval of the electrocardiogram and with sudden cardiac death. To determine the role of NOS1AP in the physiology of the cardiac myocyte, we assessed the impact of silencing NOS1AP, using siRNA, on [Ca2+]i transients in neonatal cardiomyocytes. In addition, we examined the co-localization of NOS1AP with cardiac ion channels, and finally, evaluated the expression of NOS1AP in a mouse model of dystrophic cardiomyopathy. Using siRNA, NOS1AP levels were reduced to ~30% of the control levels (p<0.05). NOS1AP silencing in cardiac myocytes reduced significantly the amplitude of electrically evoked calcium transients (p<0.05) and the degree of S-nitrosylation of the cells (p<0.05). Using confocal microscopy, we evaluated NOS1AP subcellular location and interactions with other proteins by co-localization analysis. NOS1AP showed a high degree of co-localization with the L-type calcium channel and the inwardly rectifying potassium channel Kir3.1, a low degree of co-localization with the ryanodine receptor (RyR2) and alfa-sarcomeric actin and no co-localization with connexin 43, suggesting functionally relevant interactions with the ion channels that regulate the action potential duration. Finally, using immunofluorescence and Western blotting, we observed that in mice with dystrophic cardiomyopathy, NOS1AP was significantly up-regulated (p<0.05). These results suggest for a role of NOS1AP on cardiac arrhythmias, acting on the L-type calcium channel, and potassium channels, probably through S-nitrosylation.
Keywords: Capon, S-nitrosylation, NOS, heart, Duchenne
Introduction
Sudden cardiac death (SCD) and cardiac arrhythmias remain a daunting public health problem. It is estimated that between 250,000 and 400,000 SCD occur in the United States each year [1]. Altered ventricular repolarization, reflected in abnormalities of the electrocardiogram QT interval, is a phenotype associated with an increased risk of SCD. Using genome-wide association studies, it has been demonstrated by independent groups and in different populations that allelic variants of the NOS1AP gene (nitric oxide synthase 1 adaptor protein) are strongly associated with abnormalities of the QT interval of the ECG [2,3] and with sudden cardiac death [4]. This suggests that NOS1AP may be involved in the regulation of the cardiac action potential and repolarization. Furthermore, one of the single polymorphism of NOS1AP increases even more the QT duration and mortality among users of calcium channels blockers [5,6]. The mechanisms for these effects are unknown. The NOS1AP gene encodes for a ligand of neuronal nitric oxide synthase (NOS1). Since both NOS1 [7] and calcium channel blockers suppress calcium influx, it is speculated that this could be the underlying mechanism, although in the cardiomyocyte NOS1 also regulates global and sarcoplasmic reticulum [Ca2+]i [8].
NOS1AP (also known as CAPON) was first identified in the brain as a binding partner of neuronal nitric oxide synthase (NOS1) [9]. Later studies have revealed that there are at least two alternatively expressed forms of NOS1AP [10]. Transcription of all 10 NOS1AP exons gives rise to a cDNA that can be translated into a protein, designated as NOS1AP long (NOS1AP-L). When only the last two exons are transcribed, a protein of ~30 kDa, designated as NOS1AP-short (NOS1AP-S), is produced. NOS1AP-S has unique N-terminal aminoacids, followed by 113 aminoacids that are shared with the C-terminus of NOS1AP-L. NOS1AP-L contains two distinguishable domains, the C-terminal PDZ binding domain, which is responsible for the interaction with NOS1 and a phosphotyrosine-binding domain, responsible for Dexras-1 S-nitrosylation [11]. The regulation of these spliced variants remains unknown.
Consistent with the idea that variations in NOS1AP would alter the electrophysiological properties of the heart, recent reports indicate unequivocally that the channels involved in the generation of the cardiac action potential and repolarization (Na+, K+ and Ca2+ channels) are regulated by S-nitrosylation [12], a post-translational modification that involves the addition of NO to the thiol moiety of cysteines [13]. This raises the possibility that under certain pathological conditions, malfunctioning of the systems that control the level of S-nitrosylation of these channels would lead to electrical instability of the heart.
The information regarding the physiological role of NOS1AP in the heart is scarce. NOS1AP over expression shortens the action potential duration in guinea-pig myocytes [14], while NOS1AP silencing in zebrafish with morpholinos also leads to action potential duration shortening [15]. The mechanisms remain unknown, but in the case of the guinea pig, apparently involve alterations in the calcium influx and potassium currents.
The aim of this work was to evaluate the influence of NOS1AP on the cardiac myocyte calcium handling, to evaluate ion channels that may interact with it and to assess the expression of NOS1AP in dystrophic cardiomyopathy.
Materials and methods
Reagents
NOS1AP siRNA, siRNA Transfection Reagent, siRNA Transfection Medium, siRNA Dilution Buffer, Control siRNAs, NOS1AP antibody, inward rectifying potassium channel (Kir), connexin 43 (Cx43) and nitric oxide synthase 1 (NOS1) antibodies were obtained from Santa Cruz (Santa Cruz Biotechnology, CA). The rest of antibodies were obtained from the following vendors: RyR2 from Pierce (Pierce Biotech-nology, IL), LTCa2+ from Abcam (Abcam Inc., MA), nitrosocysteine and α-sarcomeric actin from Sigma (Sigma-Aldrich, MO, USA) and mouse monoclonal anti-GAPDH antibody (Research Diagnostic Inc., Flanders, NJ).
Neonatal cardiac myocytes culture
Experiments were conducted in accordance with the Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, 1996), on cultured NRVM isolated from 1-3 days-old Sprague Dawley rats as described [16]. Briefly, 10-15 hearts were digested in phosphate buffer saline (PBS) with 55 mmol/L glucose, 740 units of collagenase II, 370 units of trypsin I and 2880 units of DNAse I (all from Whortington, NJ). Cells were filtered through 100 μm mesh, spun at 2000 rpm for 5 min and were then run through a Percoll gradient (Amersham Bioscience, NJ) to separate cardiomyocytes from other cell types. Cardiomyocytes were washed with F-10 media and incubated for 24 h under humidified air with 5% CO2 at 37°C, in F-10 media containing 10% fetal bovine serum (FBS), 10% horse serum, 10 μmol/L cytosine β-D-arabinofuranoside, 100 units/mL penicillin and 10 μg/mL streptomycin (all from Invitrogen Life Technologies, NY). The next day, the cell medium was replaced by 1x NRVM medium: DMEM (Gibco Life Technologies, NY) containing 1% FBS, 20 nmol/L selenium, 10 μg/mL insulin, 10 μg/mL transferrin, 2 mg/mL bovine serum albumin, 10 μmol/L cytosine β-D-arabinofuranoside and 20 μg/mL ascorbic acid. Following this, the medium was replaced with serum-free DMEM and cardiomyocytes were incubated for an additional 24 h before being for experiments. NRVM were plated at a density of 105/cm2.
siRNA gene silencing
NOS1AP gene silencing was performed using a modified protocol from the manufacturer (Santa Cruz Biotechnology). Briefly, 1.5x106 NRVM cells were plated in 60 mm dishes with 1x NRVM medium and incubated for 24 h at 37°C in a CO2 incubator until 60-80% of confluence. Transfection mixture containing duplex NOS1AP siRNA or scrambled (scr) RNA was added, and cells were incubated for 6 h at 37°C in 5% CO2. Next, normal growth medium containing 2 times each compound (2X NRVM medium) was added, without removing the transfection mixture, and cells were incubated for another 24 hrs. Finally, medium was replaced with fresh 1X NRVM medium for an additional 24 hours and then used for experiments.
Isolation of adult cardiomyocytes
MDX mice (C57BL/10ScSn-DMDmdx/J) and their corresponding control (C57BL/10SnJ) were anesthetized with sodium pentobarbital (100 mg/kg i.p. with 4000 U/kg heparin). After suppression of spinal cord reflexes, the heart was exposed via a midline thoracotomy, and removed. For isolation of myocytes, the hearts were cannulated and perfused through the aorta with Ca2+-free bicarbonate buffer containing (in mmol/L) 120 NaCl, 5.4 KCl, 1.2 MgSO4, 1.2 NaH2PO4, 5.6 glucose, 20 NaHCO3, 10 2,3-butanedione monoxime, and 5 taurine, gassed with 95% O2-5% CO2, followed by enzymatic digestion with collagenase II (1 mg/ml; Worthington, NJ) and protease type XIV. Ventricular myocytes were obtained by mechanical disruption of digested hearts, filtration, centrifugation, and re-suspension in a Tyrode solution.
Western blotting
Total NRVM proteins were quantified using the Micro BCA protein assay (Pierce Biotechnology, Rockford, IL). SDS-PAGE was performed using 60 μg of protein on NuPAGE gels (Invitrogen Life Technologies, Carlsbad, CA). Proteins were transferred to a PVDF membrane (Bio-Rad, Hercules, CA) and then blocked overnight by incubation with 5% nonfat dry milk in Tris-buffered saline containing 0.1% Tween 20 (TBS). After washing with TBS, the blots were incubated with rabbit anti-NOSA1P (Santa Cruz, CA) or monoclonal anti-GAPDH antibody used as load control.
Determination of intracellular [Ca2+]i levels
NRVMs treated with NOS1AP siRNA or scrRNA were grown on collagen-coated coverslips. NRVMs were loaded with fura-2 AM (Invitrogen) for 20 min, followed by 3 washes with dye-free solution and incubation for another 20 min to allow for dye de-esterification. The fura-2 loaded myocytes were transferred to a Lucite chamber, on the stage of an inverted microscope (Eclipse-TiS Ni-kon). Myocytes were field-stimulated (0.5 Hz) and [Ca2+]i was monitored as the rate of fluorescence at 360/380 nm, in real time using an IonOptix iCCD camera and specialized data acquisition software (IonWizard Acquisition System, IonOptix, Inc). Cells were continuously superfused with Tyrode containing 1.8 mM Ca2+.
Immunostaing and confocal microscopy
NRMV were prepared as described above, fixed in paraformaldehyde 2%, and incubated overnight at 4°C with antibodies to NOS1AP, Kir1.3, Cx43, LTCa2+, α-sarcomeric actin and nitrosocysteine. Secondary antibodies incubations were performed at 37°C for 1 hr using anti-mouse or anti-rabbit TRITC and anti-rabbit or anti-mouse FITC (Jackson Immunoresearch, West Grove PA). Nuclei were stained with Dapi (Invitrogen), and samples were mounted with Prolong Gold (Invitrogen). Digital images were obtained using a Zeiss LSM-700 confocal microscope.
Co-localization analysis
Quantitative co-localization analysis was performed after the images were deconvolved, using Huygens Essential software (Scientific Volume Imaging, Hilversum, The Netherlands) computing the Pearson correlation coefficient, as recommended [17,18].
Statistical analysis
Data are expressed as mean ± SEM. For comparisons of two groups, Student’s t test was used. A P value of less than 0.05 was considered significant.
Results
Expression and abundance of NOS1AP in neonataland adult cardiomyocytes
The expression of NOS1AP was verified in our models. For this, we processed NRVM for Western blot analysis (Figure 1A). NRVM expressed both the long (~50 KDa) and short (~25 KDa) isoforms of NOS1AP, in a similar fashion of what is observed a mouse brain lysate, a positive control. In the brain lysate, other bands were observed, which could be due to NOS1AP posttranslational modifications (i.e., phosphorylation) [10]. We confirmed this expression in NRVMS using immunofluorescence with confocal microscopy (Figure 1B). In these cells, NOS1AP presents a punctuate pattern and also nuclear localization, particularly at the nuclear membrane. Immunofluorescence for NOS1AP was also positive in adult mouse cardiomyocytes (Figure 1C), were the distribution pattern is more associated with the T-tubules.
Figure 1.
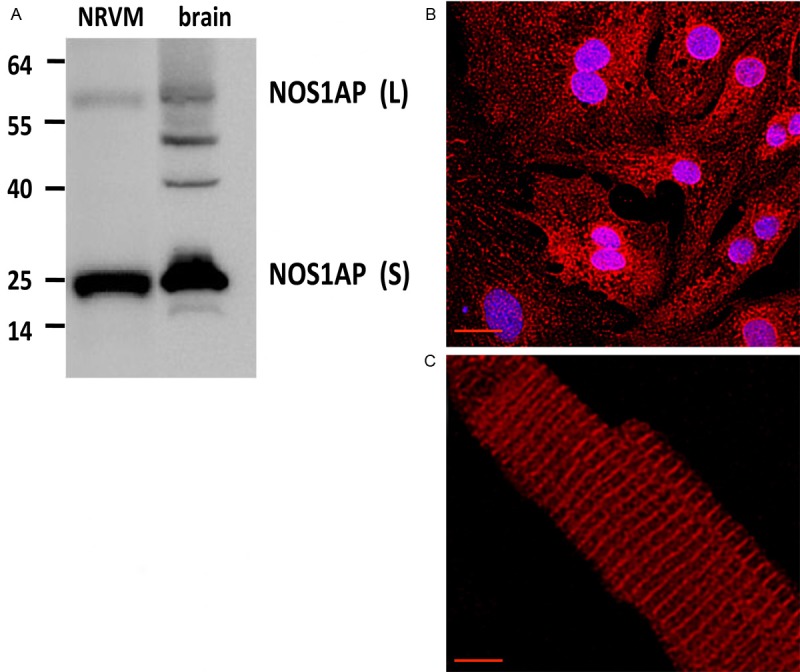
Expression and abundance of NOS1AP in neonatal and adult cardiomyocytes. A. Representative Western blot of NOS1AP, long and short isoforms, from neonatal rat cardiomyocytes (NRVMS) lysate and rat brain lysate (brain) as positive control. NOS1AP-L corresponds to NOS1AP long isoform (~50 KDa), NOS1AP-S corresponds to NOS1AP short isoform (~30 KDa). B. Representative immunostaining for NOS1AP (red) in NRVMS. Nuclei appear in blue. C. Representative immunostaining for NOS1AP in adult mouse cardiomyocyte. The bar indicates 20 μm.
NOS1AP co-localizations in neonatal cardiac myocytes
Next, we analyzed possible interactions of NOS1AP to define subcellular location and physiologically relevant interactions. For this, we analyzed the degree of co-localization in situ of NOS1AP with the L type calcium channel, the inward rectifying potassium channel Kir3.1, the ryanodine receptor (RyR2), alpha sarcomeric actin and connexin 43 (Figure 2A-E). The analysis (Figure 2F) showed a high degree of co-localization of NOS1AP with the Kir3.1 potassium channel (Pearson coefficient 0.7 ± 0.03), and the L-type calcium channel (0.68 ± 0.018). Much lower was the degree of co-localization with RyR2, a sarcoplasmic reticulum protein (0.4 ± 0.011) and alpha-sarcomeric actin, a myofilament protein (0.35 ± 0.032) and minimal in the case of connexin 43 (Cx43), an intercalated-discs protein (0.15 ± 0.01). These results suggest possible functional interactions with ion channels that influence the cardiac action potential and less relevant interactions with the sarcoplasmic reticulum, sarcomeres and intercalated discs.
Figure 2.
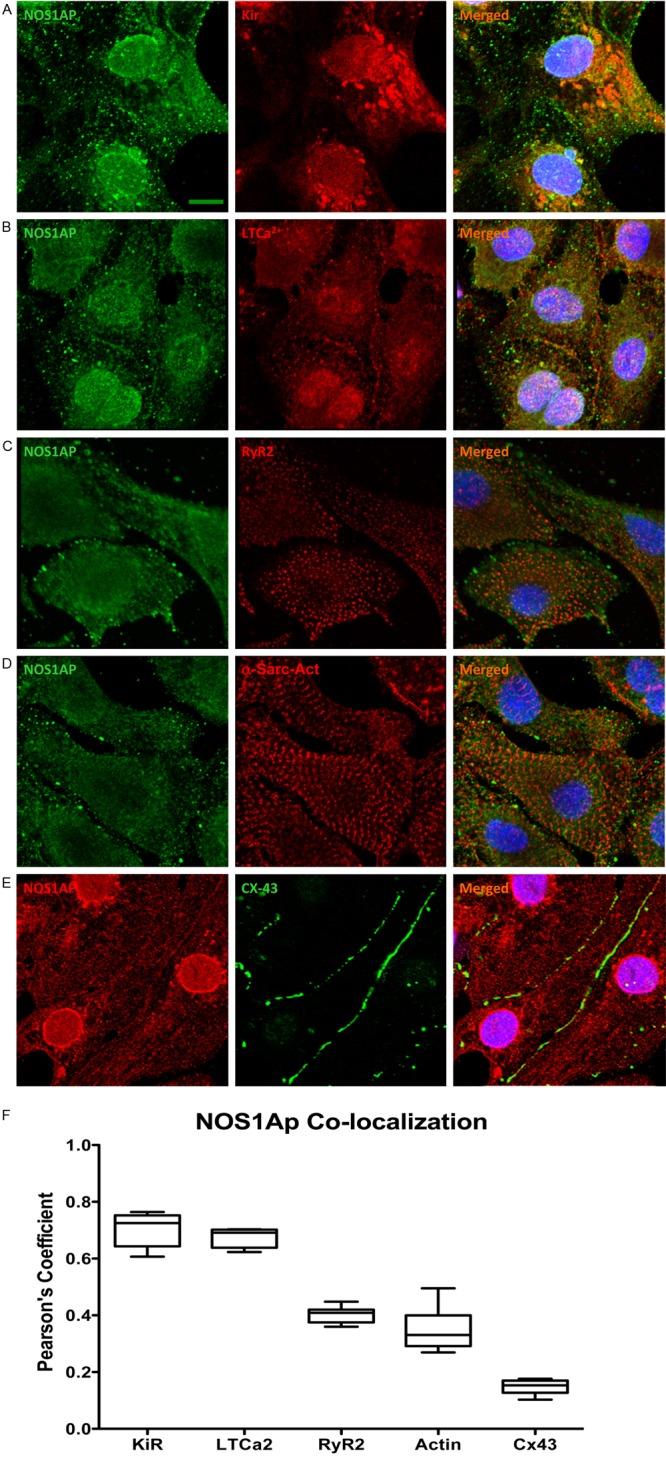
NOS1AP co-localizations in neonatal cardiac myocytes. (A-D) Representative confocal microscopy images of neonatal rat cardiomyocytes immunostained for NOS1AP (green, left panels), and its co-localization with the potassium channel Kir1.3 (Kir), L-type calcium channel (LTCa2+), ryanodine receptor (RyR2), α-sarcomeric-actin (actin9, red) (middle panels), and (E) NOS1AP (red) and connexin 43 (Cx43, green) and the merged images (right panles). Nuclei appear in blue. The bars indicate 20 μm. (F) The images were deconvolved, and the degree of co-localization was analyzed. The box and whiskers graph depicts the Pearson’s coefficients for co-localization, n=7 cells in each group.
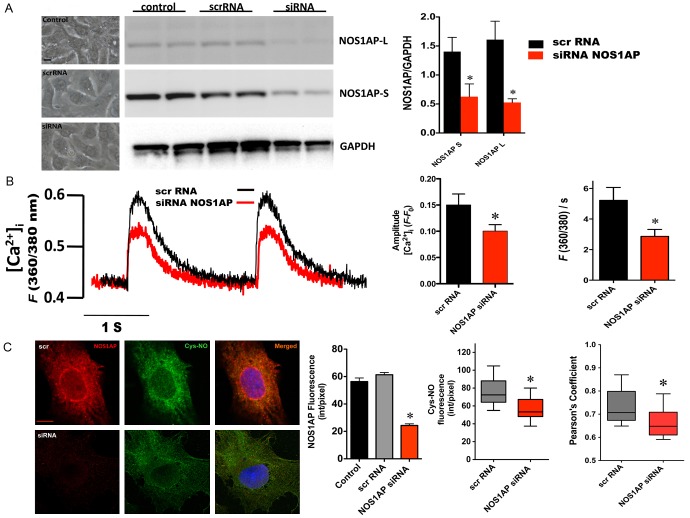
Impact of NOS1AP gene silencing on [Ca2+]i transients and S-nitrosylation
Since NOS1AP co-localized with the L-type calcium channel, we investigated the influence of this protein on calcium handing in NRVMs. For this purpose, using small interfering RNA, we silenced NOS1AP expression. In this way, NOS1AP levels drop to ~30% of control levels (Figure 3A and 3C). These treatment neither altered viability nor morphology of the cells (Figure 3A, left panel). In these conditions we evaluated [Ca2+]i transients in myocytes electrically stimulated at 0.5 Hz (Figure 3B). Compared to the cells treated with scrambled RNA, cell treated with NOS1AP small interfering RNA showed reduced amplitude of the [Ca2+]i transients (fluorescence at 360/380 nm (F 360/380): 0.15 ± 0.02 scrRNA vs. 0.94 ± 0.01 NOS1AP siRNA, p=0.008) along with decreased [Ca2+]i rise velocity (F 360/380/s 5.3 ± 0.84 scrRNA vs. 2.9 ± 0.46 NOS1AP siRNA, p=0.007).
Figure 3.
Impact of NOS1AP gene silencing on [Ca2+]i transients and S-nitrosylation. A. Left panel, representative images of NRVM after transfections. Middle panel, representative Western blots of cell lysates from control NRVM, NRVM transfected with scramble RNA (scrRNA) or NOS1AP siRNA (siRNA), probed with anti-NOS1AP antibody, showing the expression of NOS1AP- L and S isoforms. GAPDH was used as loading control. The graph depicts densitometric analysis (n=4). B. Assessment of [Ca2+]i transients. Representative [Ca2+]i transients of myocytes transfected with scrRNA (black line) or NOS1AP siRNA (red line). The graph shows the analysis for amplitude and departure velocity (F 360/380/s) of the electrically evoked [Ca2+]i transients in NOS1AP siRNA (n=15, red bars) and scrRNA-treated cells (n=9, black bars). C. Representative immunostaining for NOS1AP and S-nitroso-cysteine (Cys-NO) in myocytes after transfection with scrRNA or NOS1AP siRNA. The bar indicates 20 μm. The graphs depicts immunofluorescence intensity for NOS1AP and CySNO and the degree of co-localization for NOS1AP/Cys-NO. N=8 cells for each treatment. Asterisks indicate P<0.05 siRNA vs. scrambled RNA.
Since the effects of NOS1 and nitric oxide in the heart are, in part, mediated by S-nitrosylation [12], we evaluated the degree of S-nitrosylation in transfected cells. For this, we used an anti S-nitrosylated cysteine antibody, as previously described [19], to detect nitrosylated proteins (Figure 3C). In NOS1AP siRNA-treated cells, the intensity for CysNO (75.8 ± 5.2 scrRNA vs. 56.2 ± 4.8 siRNA, p=0.0145) and degree of co-localization for NOS1AP and CysNO were significantly diminished (0.73 ± 0.02 scrRNA vs. 0.66 ± 0.02 siRNA, p<0.05). This suggests that the effect of NOS1AP on [Ca2+]i transients could be due to redox modifications on ion channels.
NOS1AP expression in dystrophic cardiomyopathy
Finally, we analyzed the expression of NOS1AP in the heart of mdx mice, which is a model of dystrophic cardiomyopathy that presents arrhythmias [20]. Using Western blotting (Figure 4A), we found increased expression of NOS1AP, nearly double (considered as both long and short isoforms) in mdx hearts compared to control mice (p<0.05). This was also observed using immunofluorescence in isolated myocytes (Figure 4B): NOS1AP fluorescence was increased significantly in mdx myocytes compared to control (97.0 ± 10.1 in mdx cells vs. 70 ± 11 in control cells, p<0.05). In addition, the degree of co-localization between NOS1 and NOS1AP was increased in mdx cells (Pearson coefficient 0.8 ± 0.02 control vs. 0.85 ± 0.008 mdx, p<0.05). These results suggest that NOS1AP up-regulation may play a role in dystrophic cardiomyopathy.
Figure 4.
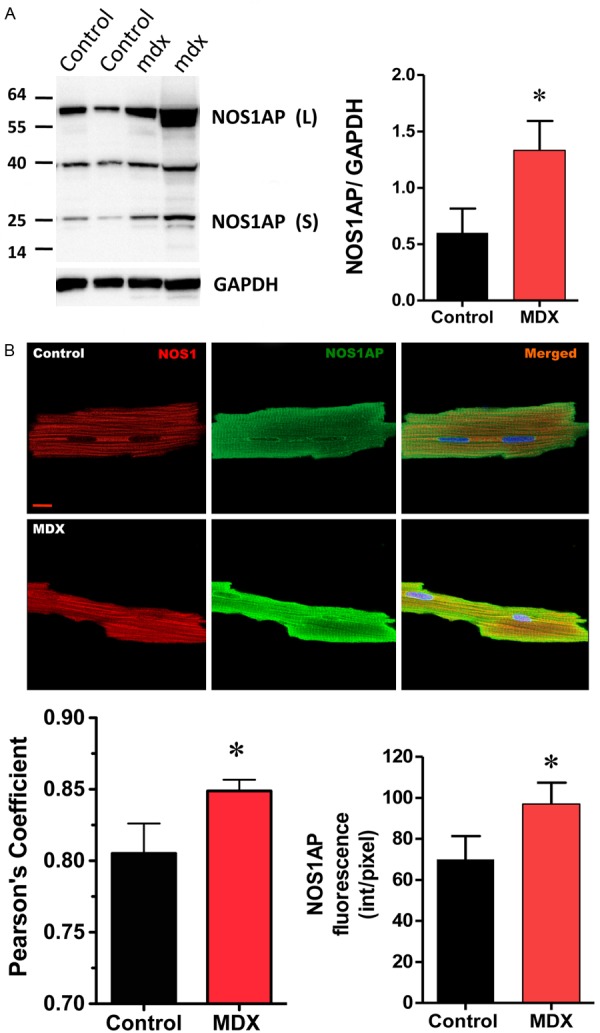
NOS1AP expression in dystrophic cardiomyopathy. A. Left, Representative Western blot for NOS1AP, long (L) and short (S) isoforms in cardiac homogenates from control (first and second lanes from left to right) and dystrophic (mdx) mice (third and forth lanes, from left to right). GAPDH was used as load control. Right, graph depicting the analysis of NOS1AP expression, normalized to GAPDH expression, n=3 hearts of each strain. B. Representative immunofluorescence for NOS1 (red) and its co-localization with NOS1AP (green, both short and long isoforms) in control and mdx myocytes. The graphs show the quantification of NOS1AP immunofluorescence (intensity/pixels) and the co-localization (Pearson coefficient) with NOS1. N=6 cells from each strain for co-localization analysis and n=18 of each strain for fluorescence intensity analysis. Asterisk indicates P<0.05 control vs. mdx.
Discussion
Multiple studies have shown a strong association between genetic variants of NOS1AP gene and prolonged/shortened QT [2,21,22] and sudden cardiac death [3,4,23], though the mechanisms remain unknown. NOS1AP is a chaperone for neuronal nitric oxide synthase (NOS1) in the brain [9], as well in the heart [14,24], where NOS1 regulates intracellular calcium handling [7,8]. Chang et al overexpressed NOS1AP (CAPON) in guinea pig ventricular myocytes, resulting in shortening of the action potential, through inhibition of the L-type calcium channel and enhancement of the rapidly-activating component of the delayed-rectifier potassium current (I Kr). On the contrary, using morpholinos to knock-down NOS1AP in zebrafish, Milan et al found shortening of the action potential. Since the presence of NOS1 in zebrafish was not reported, it is possible that NOS1AP may exert effects independently of the binding to NOS1.
We have found that NOS1AP silencing attenuates the amplitude and kinetic of the [Ca2+]i transients, which could be related to the effects of genetic variants of NOS1AP. It has been shown that these variants are associated with QT prolongation and increased mortality in patients using calcium channel blockers [5,6]. Consistent with this, we have found that in neonatal ventricular myocytes, NOS1AP co-localizes with the L-type calcium channel.
The co-localization of NOS1AP with Kir3.1 is of interest. Kir3.1 is the pore forming unit of the acetylcholine-activated potassium channel (KAch) [25]. It has a role in atrial fibrillation [26,27] and is a target for anti-arrhythmic drugs [28,29]. Interestingly, NOS1AP mRNA levels are decreased in patients using pacemaker and defibrillators, presenting the rs10494366 and rs10918594 NOS1AP polymorphisms [30].
The mechanism by which NOS1AP exerts its effects on ion channels may include S-nitrosylation. Indeed, we observed a decrease in S-nitrosylation in the myocytes treated with the NOS1AP siRNA. Voltage-gated Na+ and Ca2+ channels [12,31] and the ryanodine receptor RyR2 [8,19] are regulated by S-nitrosylation.
In addition, we observed increased expression of NOS1AP in the heart of mdx mice, a mouse model of dystrophic cardiomyopathy, consistent with the up-regulation of NOS1AP in the skeletal muscle of these mice [32]. The impact of this up-regulation remains to be determined, but may play a role in calcium handling, NOS1 subcellular location and the arrhythmias present in this model [33,34].
The differential role for NOS1AP short and long isoforms in the heart is not known. In brain cells, it has been shown that NOS1AP-L but not NOS1AP-S interacts with carboxipeptidase E, promoting dendrite growth [35].
In conclusion, in neonatal cardiomyocytes, NOS1AP silencing reduces calcium transients and S-nitrosylation. NOS1AP co-localizes with the L-type calcium channel and the potassium channel Kir3.1 and is up-regulated in dystrophic hearts. Further studies are needed to clarify the complex role of NOS1AP in the heart, and its pathophysiological role in arrhythmias.
Acknowledgements
This work was funded by Proyecto Fondecyt 1120595, Chilean National Fund for Scientific and Technological Development.
Disclosure of conflict of interest
None.
References
- 1.Zheng ZJ, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation. 2001;104:2158–2163. doi: 10.1161/hc4301.098254. [DOI] [PubMed] [Google Scholar]
- 2.Arking DE, Pfeufer A, Post W, Kao WH, Newton-Cheh C, Ikeda M, West K, Kashuk C, Akyol M, Perz S, Jalilzadeh S, Illig T, Gieger C, Guo CY, Larson MG, Wichmann HE, Marban E, O’Donnell CJ, Hirschhorn JN, Kaab S, Spooner PM, Meitinger T, Chakravarti A. A common genetic variant in the NOS1 regulator NOS1AP modulates cardiac repolarization. Nat Genet. 2006;38:644–651. doi: 10.1038/ng1790. [DOI] [PubMed] [Google Scholar]
- 3.Eijgelsheim M, Newton-Cheh C, Aarnoudse AL, van NC, Witteman JC, Hofman A, Uitterlinden AG, Stricker BH. Genetic variation in NOS1AP is associated with sudden cardiac death: evidence from the Rotterdam Study. Hum Mol Genet. 2009;18:4213–4218. doi: 10.1093/hmg/ddp356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kao WH, Arking DE, Post W, Rea TD, Sotoodehnia N, Prineas RJ, Bishe B, Doan BQ, Boerwinkle E, Psaty BM, Tomaselli GF, Coresh J, Siscovick DS, Marban E, Spooner PM, Burke GL, Chakravarti A. Genetic variations in nitric oxide synthase 1 adaptor protein are associated with sudden cardiac death in US white community-based populations. Circulation. 2009;119:940–951. doi: 10.1161/CIRCULATIONAHA.108.791723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker ML, Visser LE, Newton-Cheh C, Hofman A, Uitterlinden AG, Witteman JC, Stricker BH. A common NOS1AP genetic polymorphism is associated with increased cardiovascular mortality in users of dihydropyridine calcium channel blockers. Br J Clin Pharmacol. 2009;67:61–67. doi: 10.1111/j.1365-2125.2008.03325.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van NC, Aarnoudse AJ, Eijgelsheim M, Sturkenboom MC, Straus SM, Hofman A, Kors JA, Newton-Cheh C, Witteman JC, Stricker BH. Calcium channel blockers, NOS1AP, and heart-rate-corrected QT prolongation. Pharmacogenet Genomics. 2009;19:260–266. doi: 10.1097/FPC.0b013e328324e556. [DOI] [PubMed] [Google Scholar]
- 7.Sears CE, Bryant SM, Ashley EA, Lygate CA, Rakovic S, Wallis HL, Neubauer S, Terrar DA, Casadei B. Cardiac neuronal nitric oxide synthase isoform regulates myocardial contraction and calcium handling. Circ Res. 2003;92:e52–e59. doi: 10.1161/01.RES.0000064585.95749.6D. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez DR, Beigi F, Treuer AV, Hare JM. Deficient ryanodine receptor S-nitrosylation increases sarcoplasmic reticulum calcium leak and arrhythmogenesis in cardiomyocytes. Proc Natl Acad Sci U S A. 2007;104:20612–20617. doi: 10.1073/pnas.0706796104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jaffrey SR, Snowman AM, Eliasson MJ, Cohen NA, Snyder SH. CAPON: a protein associated with neuronal nitric oxide synthase that regulates its interactions with PSD95. Neuron. 1998;20:115–124. doi: 10.1016/s0896-6273(00)80439-0. [DOI] [PubMed] [Google Scholar]
- 10.Xu B, Wratten N, Charych EI, Buyske S, Firestein BL, Brzustowicz LM. Increased expression in dorsolateral prefrontal cortex of CAPON in schizophrenia and bipolar disorder. PLoS Med. 2005;2:e263. doi: 10.1371/journal.pmed.0020263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fang M, Jaffrey SR, Sawa A, Ye K, Luo X, Snyder SH. Dexras1: a G protein specifically coupled to neuronal nitric oxide synthase via CAPON. Neuron. 2000;28:183–193. doi: 10.1016/s0896-6273(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez DR, Treuer A, Sun QA, Stamler JS, Hare JM. S-Nitrosylation of cardiac ion channels. J Cardiovasc Pharmacol. 2009;54:188–195. doi: 10.1097/FJC.0b013e3181b72c9f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schulman IH, Hare JM. Regulation of cardiovascular cellular processes by S-nitrosylation. Biochim Biophys Acta. 2012;1820:752–762. doi: 10.1016/j.bbagen.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang KC, Barth AS, Sasano T, Kizana E, Kashiwakura Y, Zhang Y, Foster DB, Marban E. CAPON modulates cardiac repolarization via neuronal nitric oxide synthase signaling in the heart. Proc Natl Acad Sci U S A. 2008;105:4477–4482. doi: 10.1073/pnas.0709118105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Milan DJ, Kim AM, Winterfield JR, Jones IL, Pfeufer A, Sanna S, Arking DE, Amsterdam AH, Sabeh KM, Mably JD, Rosenbaum DS, Peterson RT, Chakravarti A, Kaab S, Roden DM, MacRae CA. Drug-sensitized zebrafish screen identifies multiple genes, including GINS3, as regulators of myocardial repolarization. Circulation. 2009;120:553–559. doi: 10.1161/CIRCULATIONAHA.108.821082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dulce RA, Hurtado C, Ennis IL, Garciarena CD, Alvarez MC, Caldiz C, Pierce GN, Portiansky EL, Chiappe de Cingolani GE, Camilion de Hurtado MC. Endothelin-1 induced hypertrophic effect in neonatal rat cardiomyocytes: involvement of Na+/H+ and Na+/Ca2+ exchangers. J Mol Cell Cardiol. 2006;41:807–815. doi: 10.1016/j.yjmcc.2006.05.016. [DOI] [PubMed] [Google Scholar]
- 17.Scriven DR, Lynch RM, Moore ED. Image acquisition for colocalization using optical microscopy. Am J Physiol Cell Physiol. 2008;294:C1119–C1122. doi: 10.1152/ajpcell.00133.2008. [DOI] [PubMed] [Google Scholar]
- 18.Zinchuk V, Zinchuk O, Okada T. Quantitative colocalization analysis of multicolor confocal immunofluorescence microscopy images: pushing pixels to explore biological phenomena. Acta Histochem Cytochem. 2007;40:101–111. doi: 10.1267/ahc.07002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gonzalez DR, Treuer AV, Castellanos J, Dulce RA, Hare JM. Impaired S-nitrosylation of the ryanodine receptor caused by xanthine oxidase activity contributes to calcium leak in heart failure. J Biol Chem. 2010;285:28938–28945. doi: 10.1074/jbc.M110.154948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chu V, Otero JM, Lopez O, Sullivan MF, Morgan JP, Amende I, Hampton TG. Electrocardiographic findings in mdx mice: a cardiac phenotype of Duchenne muscular dystrophy. Muscle Nerve. 2002;26:513–519. doi: 10.1002/mus.10223. [DOI] [PubMed] [Google Scholar]
- 21.Aarnoudse AJ, Newton-Cheh C, de Bakker PI, Straus SM, Kors JA, Hofman A, Uitterlinden AG, Witteman JC, Stricker BH. Common NOS1AP variants are associated with a prolonged QTc interval in the Rotterdam Study. Circulation. 2007;116:10–16. doi: 10.1161/CIRCULATIONAHA.106.676783. [DOI] [PubMed] [Google Scholar]
- 22.Tomas M, Napolitano C, De GL, Bloise R, Subirana I, Malovini A, Bellazzi R, Arking DE, Marban E, Chakravarti A, Spooner PM, Priori SG. Polymorphisms in the NOS1AP gene modulate QT interval duration and risk of arrhythmias in the long QT syndrome. J Am Coll Cardiol. 2010;55:2745–2752. doi: 10.1016/j.jacc.2009.12.065. [DOI] [PubMed] [Google Scholar]
- 23.Westaway SK, Reinier K, Huertas-Vazquez A, Evanado A, Teodorescu C, Navarro J, Sinner MF, Gunson K, Jui J, Spooner P, Kaab S, Chugh SS. Common variants in CASQ2, GPD1L, and NOS1AP are significantly associated with risk of sudden death in patients with coronary artery disease. Circ Cardiovasc Genet. 2011;4:397–402. doi: 10.1161/CIRCGENETICS.111.959916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beigi F, Oskouei BN, Zheng M, Cooke CA, Lamirault G, Hare JM. Cardiac nitric oxide synthase-1 localization within the cardiomyocyte is accompanied by the adaptor protein, CAPON. Nitric Oxide. 2009;21:226–233. doi: 10.1016/j.niox.2009.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hibino H, Inanobe A, Furutani K, Murakami S, Findlay I, Kurachi Y. Inwardly rectifying potassium channels: their structure, function, and physiological roles. Physiol Rev. 2010;90:291–366. doi: 10.1152/physrev.00021.2009. [DOI] [PubMed] [Google Scholar]
- 26.Dobrev D, Friedrich A, Voigt N, Jost N, Wettwer E, Christ T, Knaut M, Ravens U. The G protein-gated potassium current I(K,ACh) is constitutively active in patients with chronic atrial fibrillation. Circulation. 2005;112:3697–3706. doi: 10.1161/CIRCULATIONAHA.105.575332. [DOI] [PubMed] [Google Scholar]
- 27.Voigt N, Trausch A, Knaut M, Matschke K, Varro A, Van Wagoner DR, Nattel S, Ravens U, Dobrev D. Left-to-right atrial inward rectifier potassium current gradients in patients with paroxysmal versus chronic atrial fibrillation. Circ Arrhythm Electrophysiol. 2010;3:472–480. doi: 10.1161/CIRCEP.110.954636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noujaim SF, Stuckey JA, Ponce-Balbuena D, Ferrer-Villada T, Lopez-Izquierdo A, Pandit S, Calvo CJ, Grzeda KR, Berenfeld O, Chapula JA, Jalife J. Specific residues of the cytoplasmic domains of cardiac inward rectifier potassium channels are effective antifibrillatory targets. FASEB J. 2010;24:4302–4312. doi: 10.1096/fj.10-163246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Voigt N, Rozmaritsa N, Trausch A, Zimniak T, Christ T, Wettwer E, Matschke K, Dobrev D, Ravens U. Inhibition of IK,ACh current may contribute to clinical efficacy of class I and class III antiarrhythmic drugs in patients with atrial fibrillation. Naunyn Schmiedebergs Arch Pharmacol. 2010;381:251–259. doi: 10.1007/s00210-009-0452-6. [DOI] [PubMed] [Google Scholar]
- 30.Saba S, Mehdi H, Shah H, Islam Z, Aoun E, Termanini S, Mahjoub R, Aleong R, McTiernan CF, London B. Cardiac levels of NOS1AP RNA from right ventricular tissue recovered during lead extraction. Heart Rhythm. 2012;9:399–404. doi: 10.1016/j.hrthm.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 31.Tamargo J, Caballero R, Gomez R, Delpon E. Cardiac electrophysiological effects of nitric oxide. Cardiovasc Res. 2010;87:593–600. doi: 10.1093/cvr/cvq214. [DOI] [PubMed] [Google Scholar]
- 32.Segalat L, Grisoni K, Archer J, Vargas C, Bertrand A, Anderson JE. CAPON expression in skeletal muscle is regulated by position, repair, NOS activity, and dystrophy. Exp Cell Res. 2005;302:170–179. doi: 10.1016/j.yexcr.2004.09.007. [DOI] [PubMed] [Google Scholar]
- 33.Bia BL, Cassidy PJ, Young ME, Rafael JA, Leighton B, Davies KE, Radda GK, Clarke K. Decreased myocardial nNOS, increased iNOS and abnormal ECGs in mouse models of Duchenne muscular dystrophy. J Mol Cell Cardiol. 1999;31:1857–1862. doi: 10.1006/jmcc.1999.1018. [DOI] [PubMed] [Google Scholar]
- 34.Branco DM, Wolf CM, Sherwood M, Hammer PE, Kang PB, Berul CI. Cardiac electrophysiological characteristics of the mdx (5cv) mouse model of Duchenne muscular dystrophy. J Interv Card Electrophysiol. 2007;20:1–7. doi: 10.1007/s10840-007-9168-z. [DOI] [PubMed] [Google Scholar]
- 35.Carrel D, Du Y, Komlos D, Hadzimichalis NM, Kwon M, Wang B, Brzustowicz LM, Firestein BL. NOS1AP regulates dendrite patterning of hippocampal neurons through a carboxypeptidase E-mediated pathway. J Neurosci. 2009;29:8248–8258. doi: 10.1523/JNEUROSCI.5287-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]