Abstract
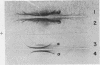
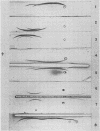
Fourteen antigenic constituents have been detected in Arachis hypogaea seeds. The major proteins of the classic arachin and conarachin fractions have been identified. Arachin contains 4 antigens (the major one called α-arachin) and conarachin contains 2 which have been called α1, and α2-conarachin. Structural differences between α-arachin, α1 and α2-conarachin are indicated by their different antigenic specificities. α-Arachin precipitates as a separate entity at low temperature. The action of trypsin on this protein induces an increase in electrophoretic mobility and prevents precipitation at low temperature. This enzyme has no detectable effect on α1 and α2-conarachin.
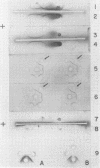
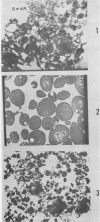
The proteins of the cotyledon and of the entire axis are quite similar in their immunoelectrophoretic patterns. However, quantitative and qualitative differences occur between the proteins found in the cotyledon and those of the 1-millimeter tip of the axis. In the latter, there is a 4-fold smaller proportion of α-arachin than in the cotyledon. Immunoelectrophoretic analysis of subcellular preparations from cotyledons confirms that α-arachin is an aleurin and that α1-conarachin is a typical cytoplasmic protein. Large and small aleurone grains appear very similar in their qualitative antigenic composition.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALTSCHUL A. M., SNOWDEN J. E., Jr, MANCHON D. D., Jr, DECHARY J. M. Intracellular distribution of seed proteins. Arch Biochem Biophys. 1961 Nov;95:402–404. doi: 10.1016/0003-9861(61)90166-7. [DOI] [PubMed] [Google Scholar]
- ERLANDSON R. A. A NEW MAGAGLAS, D.E.R.(R) 732, EMBEDMENT FOR ELECTRON MICROSCOPY. J Cell Biol. 1964 Sep;22:704–709. doi: 10.1083/jcb.22.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EVANS W. J., CARNEY W. B., DECHARY J. M., ALTSCHUL A. M. Zone electrophoresis of conarachin, alpha-conarachin and bovine serum albumin on polyacrylamide gel. Arch Biochem Biophys. 1962 Feb;96:233–239. doi: 10.1016/0003-9861(62)90403-4. [DOI] [PubMed] [Google Scholar]
- GRABAR P., WILLIAMS C. A. Méthode permettant l'étude conjuguée des proprietés électrophorétiques et immunochimiques d'un mélange de protéines; application au sérum sanguin. Biochim Biophys Acta. 1953 Jan;10(1):193–194. doi: 10.1016/0006-3002(53)90233-9. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., SHOOTER E. M., RIDEAL E. K. The globulins of the ground nut. (Arachis hypogaea) II. Electrophoretic examination of the arachin system. Biochim Biophys Acta. 1950 Jun;5(3/4):376–396. doi: 10.1016/0006-3002(50)90184-3. [DOI] [PubMed] [Google Scholar]
- JOHNSON P., SHOOTER E. M. The globulins of the ground nut. (Arachis hypogaea) I. Investigation of arachin as a dissociation system. Biochim Biophys Acta. 1950 Jun;5(3/4):361–375. doi: 10.1016/0006-3002(50)90183-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- STEWARD F. C., LYNDON R. F., BARBER J. T. ACRYLAMIDE GEL ELECTROPHORESIS OF SOLUBLE PLANT PROTEINS: A STUDY ON PEA SEEDLINGS IN RELATION TO DEVELOPMENT. Am J Bot. 1965 Feb;52:155–164. [PubMed] [Google Scholar]
- VENABLE J. H., COGGESHALL R. A SIMPLIFIED LEAD CITRATE STAIN FOR USE IN ELECTRON MICROSCOPY. J Cell Biol. 1965 May;25:407–408. doi: 10.1083/jcb.25.2.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON M. L. Staining of tissue sections for electron microscopy with heavy metals. J Biophys Biochem Cytol. 1958 Jul 25;4(4):475–478. doi: 10.1083/jcb.4.4.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOLF W. J., BABCOCK G. E., SMITH A. K. Purification and stability studies of the 11 S component of soybean proteins. Arch Biochem Biophys. 1962 Nov;99:265–274. doi: 10.1016/0003-9861(62)90009-7. [DOI] [PubMed] [Google Scholar]
- Wright S. T. Cellular differentiation at the molecular level with special reference to proteins. Symp Soc Exp Biol. 1963;17:18–39. [PubMed] [Google Scholar]