Abstract
Low-dose 5-fluorouracil (5-FU), a widely used chemotherapeutic, has been reported to have immunomodulatory effects. This study aimed to evaluate the optimal dose of 5-FU that produces antitumor and immunomodulatory effects. In a hepatoma 22 tumor-bearing mouse model, 0, 10, 20 and 40 mg/kg 5-FU (i.p.) was administered for 10 days. Tumor weight and volume were measured, thymus index (TI) and spleen index (SI) were calculated, and the number of white blood cells (WBCs) and lymphocytes (LYs) were counted following treatment. The percentages of CD3+, CD4+, CD8+ and natural killer (NK) cells were measured by flow cytometry. In addition, the body weights of the mice were measured and the average diet consumption was calculated. Administration of 5-FU produced a potent antitumor effect in a dose-dependent manner (P<0.01). At 20 and 40 mg/kg, a significant reduction of body weight and food consumption was observed. TI and SI decreased in the 20- and 40-mg/kg groups (P<0.01) for 10 days. The number of WBCs significantly decreased in each group (P<0.01); however, the number of LYs only decreased in the 40-mg/kg group (P<0.01). Percentages of CD3+ and CD4+ cells were increased in the 10- and 20-mg/kg groups (P<0.01). Thus, 5-FU at 10 mg/kg inhibits tumor growth while maintaining the immune function of the mice. 5-FU may exert its antitumor effect at a low dose with low toxicity and stimulate the host immune system. Future clinical trials taking into account the immunostimulatory capacity of chemotherapeutic agents are desirable for certain patients.
Keywords: 5-FU, immunomodulatory, antitumor, mice
Introduction
Cancer patients are frequently immunologically suppressed during treatment. Chemotherapy is usually considered immunosuppressive due to its toxicity to bone marrow cells (1). However, certain chemotherapeutic agents, including leucovorin, cyclophosphamide, methotrexate and cisplatin, have been shown to activate an anticancer immune response at a low dose (2–4).
5-Fluorouracil (5-FU) is one of the most commonly used chemotherapeutics in the treatment of malignancies arising from the breast, gastrointestinal tract, head and neck (5). Over the last decades a variety of doses of 5-FU have been developed in clinical trials for the treatment of gastrointestinal tumors. These include the Mayo regimen (600 mg/m2 bolus) (6), FOLFOX4 regimen (400 mg/m2 bolus plus 600 mg/m2 continuous infusion) (7) and Arbeitsgemeinschaft Internistische Onkologie schedule (2,000–2,600 mg/m2) (8). These regimens have been shown to produce different toxicity patterns (9). For example, single-agent bolus 5-FU administered weekly, which was in the past the standard schedule and route of administration for this drug in gastrointestinal cancer, is associated with marked myelosuppression. The commonly used 5-FU/calcium folinate regimens, depending on the doses and schedules, may produce a combination of mucositis, diarrhea and myelosuppression (10). However, low-dose (250–300 mg/m2/day) continuous infusion of 5-FU is associated with little myelosuppression (11). In general, the incidence of grade 3–4 toxicity (mainly neutropenia, diarrhea, mucositis and hand-foot syndrome) increases with higher systemic exposure to 5-FU (12).
However, several studies have shown that patients receiving low-dose 5-FU have favorable survival rates without toxicity (13,14). These studies suggest that the favorable outcome in patients receiving low-dose 5-FU may be due to its immunomodulatory effects and enhancement of the immunologic function of the host. However, whether 5-FU exerts its antitumor action at a low dose that enhances the host’s immune function, as well as having minimal side effects, has not been reported.
In this study, we evaluated whether there was an optimal dose of 5-FU with antitumor and immunomodulatory effects, without evident side effects in hepatoma 22 (H22) tumor-bearing mice.
Materials and methods
Reagents
5-FU (25 mg/ml) was purchased from Shanghai Amino Acids Company (Shanghai, China) and diluted to 10 mg/ml with normal saline (NS). Peridinin chlorophyll protein complex-conjugated hamster anti-mouse CD3, fluorescein isothiocyanate-conjugated rat anti-mouse CD4, phycoerythrin (PE)-conjugated rat anti-mouse CD8 and PE-conjugated rat anti-mouse CD49 were purchased from BD Biosciences (San Jose, CA, USA).
Mice
Male Institute of Cancer Research mice (body weight, 18–22 g) were purchased from the School of Basic Medical Science, Peking University (Beijing, China). The animals were maintained in a pathogen-free facility (23±2°C, 55±5% humidity). All procedures on treating mice were performed according to the law on Animal Care Guidelines, and the Animal Care Committee of Fujian University of Traditional Chinese Medicine (Fuzhou, China) approved the study protocols. The murine H22 cell line was from the School of Basic Medical Science, Peking University. Cells were cultured in Dulbecco’s modified Eagle’s medium (Gibco Laboratories, Grand Island, NY, USA) supplemented with 10% fetal bovine serum (Gibco Laboratories), penicillin and streptomycin (10,000 U/ml; Gibco Laboratories) in a humidified atmosphere with 5% CO2 at 37°C.
Tumor xenograft
The hepatoma model was established by subcutaneous inoculation of H22 cells (1×106 cells per mouse) into the right flank of mice. Then, 48 h after inoculation, mice were randomly divided into four groups (n=6) as follows: Control group (NS, i.p.), 10-mg/kg group (i.p.), 20-mg/kg group (i.p.) and 40-mg/kg group (i.p.). On day 10, peripheral blood was collected from the orbital plexus and tumors were excised. The tumor volume (TV) was calculated according to the following formula: , where d and D were the shortest and longest diameters, respectively.
Food intake and body weight
Each cage of mice was provided with daily food. The following day, the remaining food was collected and weighed. The daily food intake was calculated by subtracting this value from the amount of diet provided the previous day. The well-being of the mice was monitored daily and the body weight was measured at baseline and after 10 days of treatment.
Biochemical assay
A capillary pipette containing anticoagulant [ethylene diamine tetraacetic acid (EDTA) for cell counting and heparin for flow cytometry] was inserted in the lateral canthus and blood was collected from the retroorbital sinus. A 20-μl whole blood sample was collected and blood cells were counted using an automated Blood Cell Counter (Abbott Laboratories, Abbott Park, IL, USA).
Thymus index (TI) and spleen index (SI)
The thymus and spleen were collected from the mice, washed with phosphate-buffered saline (PBS) and weighed. TI and SI were calculated according to the following formulae: .
Assay for percentage of immune cells
Heparin-coated blood (100 μl) was added to a tube and incubated with fluorochrome-conjugated antibodies, respectively, in the dark for 10 min. The 2.5 μl antibodies used included a combination of CD3/CD4, CD3/CD8 and CD3/CD49. Erythrocytes were lysed by red blood cell lysis buffer (0.155 mol/l ammonium chloride, 0.01 mol/l potassium bicarbonate, 0.1 mmol/l EDTA and 1% paraformaldehyde in PBS) for 10 min. After washing with PBS, the samples were resuspended with 500 μl PBS and analyzed using a FACSCalibur™ flow cytometer with CellQuest™ software (BD Biosciences).
Statistical analyses
Statistical analysis was performed using SPSS 16.0 (SPSS, Inc., Chicago, IL, USA). Data are presented as the mean ± standard deviation. One-way analysis of variance was used and significant differences were analyzed by Dunnett’s multiple comparison test to compare with the control group. P<0.05 was considered to indicate a statistically significant difference.
Results
Effects of 5-FU on tumor growth in H22-bearing mice
Tumor-bearing mice were randomly divided into four groups as described in Materials and methods and treated with 5-FU for 10 days. After 10 days, tumor weights were 0.39±0.05, 0.17±0.02, 0.12±0.02 and 0.07±0.01 g in the control, 10-, 20- and 40-mg/kg groups, respectively, which exhibited a reduction by 56 (P<0.01), 69 (P<0.01) and 82% (P<0.01), respectively. TV demonstrated the same tendency as tumor weight among the four groups (Fig. 1).
Figure 1.
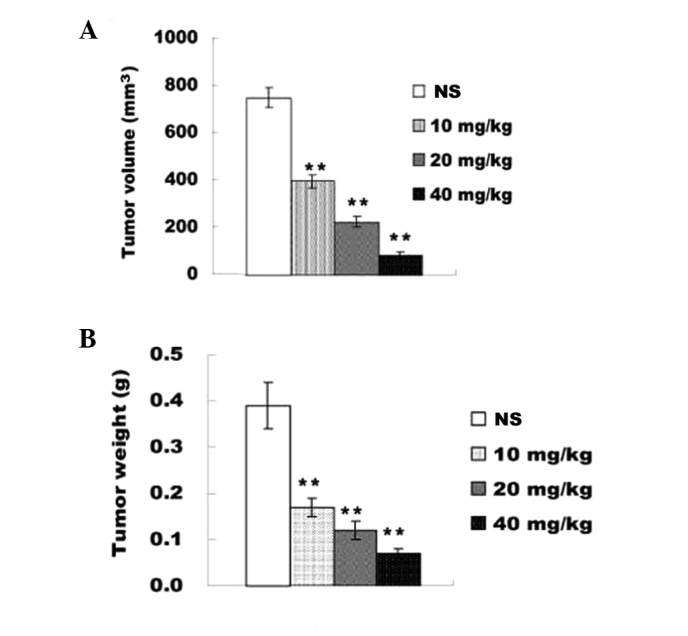
Effects of different doses of 5-FU on H22 solid tumor growth. H22 tumor-bearing mice were treated with NS (control group) or 5-FU (10, 20 and 40 mg/kg). The antitumor effect of each regimen was evaluated by measuring (A) tumor volume and (B) tumor weight. The results presented are the mean ± standard deviation (n=6). **P<0.01, compared with the NS group. 5-FU, 5-fluorouracil; NS, normal saline.
Food intake and body weight
We monitored the body weight at baseline and after treatment, as well as diet consumption every day, which are widely used to assess gross toxicity of a test compound. There was no statistically significant difference in body weight among the groups at baseline. Following treatment with 5-FU for 10 days, the body weight was similar between the control group and the 10-mg/kg group. However, body weight was significantly lower in the 20- and 40-mg/kg groups compared with the control group (P<0.01; Fig. 2). The average diet consumption of the control and 10-mg/kg groups were similar; however, it was smaller in the 20- and 40-mg/kg groups (P<0.01 vs. control group; Fig. 3).
Figure 2.
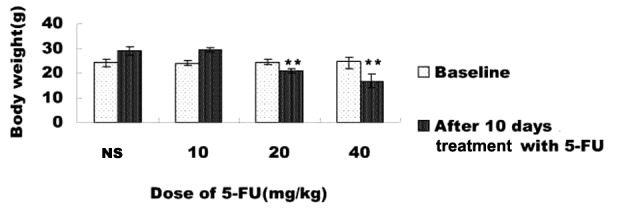
Effects of different doses of 5-FU on body weight. H22 tumor-bearing mice were established and treated for 10 days with different doses of 5-FU. The body weight gain was calculated after treatment for 10 days. The results presented are the mean ± standard deviation (n=6). **P<0.01, compared with the NS group. 5-FU, 5-fluorouracil; NS, normal saline.
Figure 3.
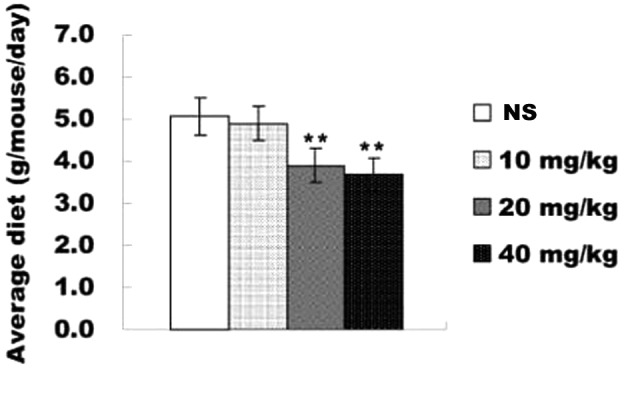
Effects of different doses of 5-FU on average diet. H22 tumor-bearing mice were established and treated for 10 days with different doses of 5-FU. The diet weight was measured and the average consumption per mouse is shown. The results presented are the mean ± standard deviation (n=6). **P<0.01, compared with the NS group. 5-FU, 5-fluorouracil; NS normal saline.
Effects of 5-FU on the number of WBCs
To investigate the role of 5-FU on the distribution of circulating WBCs, whole blood WBC and LY levels were counted at day 10. As shown in Table I, WBC number was decreased significantly in the 10- (P<0.01), 20- (P<0.01) and 40-mg/kg (P<0.01) groups compared with the control group. LY number was only decreased in the 40-mg/kg group (P<0.01).
Table I.
Number of WBCs and LYs in peripheral blood of H22-bearing mice.
| Dose (mg/kg) | WBC (×109/l) | LY (×109/l) |
|---|---|---|
| NS | 8.0±1.2 | 3.5±1.1 |
| 10 | 4.6±0.5a | 3.6±0.3 |
| 20 | 4.4±0.4a | 3.5±0.4 |
| 40 | 1.1±0.4a | 0.9±0.4a |
Dose was administered over a 10-day period. Results are presented as the mean ± standard deviation, n-=6.
P<0.01, compared with the NS group.
NS, normal saline; WBC, white blood cell, LY, lymphocyte.
Effects of 5-FU on percentage of LYs in peripheral blood
To evaluate the effects of 5-FU on host immune system, percentages of LYs [CD3+, CD4+, CD8+, natural killer (NK) and B cells] were measured after mice were treated with different doses of 5-FU for 10 days. As shown in Table II, the percentages of CD3+ and CD4+ cells were higher in the 10- (P<0.01) and 20-mg/kg (P<0.01) groups compared with the control group. Mice in the 40 mg/kg group had the lowest percentages of CD3+ and CD4+ cells (P<0.05 vs. control group). The percentage of CD8+ cells was only decreased in the 40-mg/kg group (P<0.01). However, the number of NK cells was decreased in the 10- (P<0.01) and 20-mg/kg (P<0.05) groups compared with the control group.
Table II.
Percentages of CD3+, CD4+, CD8+ and NK cells in peripheral blood of H22-bearing mice.
| Dose (mg/kg) | CD3+ (%) | CD4+ (%) | CD8+ (%) | NK (%) |
|---|---|---|---|---|
| NS | 48.5±3.5 | 36.4±3.9 | 12.1±1.6 | 14.0±3.3 |
| 10 | 82.7±1.8a | 71.7±0.4a | 11.0±1.3 | 6.4±2.9a |
| 20 | 81.0±8.5a | 71.3±8.2a | 9.7±6.9 | 9.9±11.2b |
| 40 | 38.8±6.6a | 34.4±7.4 | 4.4±1.8a | 12.0±3.7 |
Dose was administered over a 10-day period. Results are presented as the mean ± standard deviation (n=6).
P<0.01 and
P<0.05, compared with the NS group.
NK, natural killer; NS, normal saline.
Effects of 5-FU on TI and SI in H22-bearing mice
We also examined the effect of 5-FU on TI and SI in H22 tumor-bearing mice. After 10 days, TI and SI decreased in the 20- (P<0.01) and 40-mg/kg (P<0.01) groups compared with the control group (Fig. 4).
Figure 4.
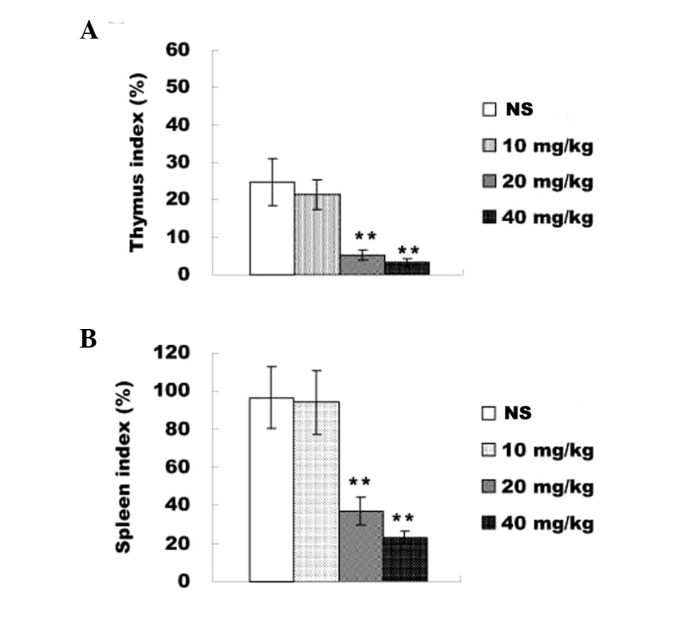
Effects of different doses of 5-FU on TI and SI. The thymus and spleen were washed with phosphate-buffered saline and weighed. (A) TI was calculated according to the following formula: . (B) SI was calculated according to the following formula: . The results presented are the mean ± standard deviation (n=6). **P<0.01, compared with the NS group. 5-FU, 5-fluorouracil; TI, thymus index; SI, spleen index.
Discussion
The adverse effects of 5-FU include severe immunosuppression due to inhibition of hemotopoietic cell proliferation (13). A previous study demonstrated that low-dose chemotherapeutic drugs have a positive effect on various solid tumors. Kobayashi et al reported that low dose leucovorin plus 5-FU treated seven colorectal cancer patients for a long duration without toxicity (14). The authors further reported that low dose leucovorin plus 5-FU may improve host immunity in certain patients (15).
Our results showed that 20 and 40 mg/kg 5-FU induced a significant loss of body weight and reduction in diet consumption. Codacci-Pisanelli et al reported that 15 and 20 mg/kg caused ~25% loss of weight in mice (16). Vichaya et al also reported that injection with 20 and 40 mg/kg 5-FU every other day in C57BL/6J mice resulted in weight loss and suppressed food consumption (17). Our results are consistent with these studies.
Certain chemotherapeutics have been reported to have benefit for patients at a low dose, including cyclophosphamide (CY), methotrexate (MTX) and cisplatin (Cis). Daily oral administration of low dose CY (50 or 100 mg/day) is effective in patients with advanced solid tumors, since CY is capable of not only selectively ablating circulating T regulatory (Treg) cells, but also recovering the function of conventional T and NK cells. T and Treg cells coregulate the activity of each other, which leads to the restoration of peripheral T-cell proliferation and innate killing activities (2,18–20). Shen et al demonstrated that low-dose, metronomic chemotherapy with Cis (0.6 mg/kg/day) dramatically inhibits tumor growth without apparent body weight loss and significant upregulation of Fas (CD95) mRNA and protein in SW480 colon cancer cells and oral cancer cell lines. It is possible for low-dose Cis to attenuate inflammatory responses by inducing Fas expression on effector T cells (21–23). We found that although 5-FU at 10 mg/kg demonstrated moderate antitumor effects, no severe side effects were observed. In addition, 10 mg/kg 5-FU may enhance host immune function as indicated by increased percentages of CD3+ and CD4+ cells. A dose of 10 mg/kg for tumor-bearing mice is approximately equal to 0.83 mg/kg for cancer patients. In clinical settings, 5-FU is usually administered at 12 mg/kg/day for 4 days (max, 800 mg/day) followed by 6 mg/kg/day every other day for 4 days. Kobayashi et al reported improved immune function in cancer patients receiving low-dose 5-FU (14,15).
In conclusion, our study demonstrated that improved immune function achieved by low dose 5-FU may translate into an antitumor effect. Future clinical trials are required to confirm this finding.
Acknowledgements
This study was funded by the CHEN Ke-ji Integrative Medicine Development Fund (CKJ2010020) and the International Science Joint Project of the Ministry of Science and Technology of the People’s Republic of China (2008DFA32200).
Abbreviations
- 5-FU
5-fluorouracil
- H22
hepatoma 22
- WBC
white blood cell
- LY
lymphocyte
- TI
thymus index
- SI
spleen index
- TV
tumor volume
- PBS
phosphate-buffered saline
References
- 1.Mackall CL. T-cell immunodeficiency following cytotoxic antineoplastic therapy: a review. Stem cells. 2000;18:10–18. doi: 10.1634/stemcells.18-1-10. [DOI] [PubMed] [Google Scholar]
- 2.Cao Y, Zhao J, Yang Z, et al. CD4+FOXP3+ regulatory T cell depletion by low-dose cyclophosphamide prevents recurrence in patients with large condylomata acuminata after laser therapy. Clin Immunol. 2010;136:21–29. doi: 10.1016/j.clim.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 3.Boehm IB, Boehm GA, Bauer R. Management of cutaneous lupus erythematosus with low-dose methotrexate: indication for modulation of inflammatory mechanisms. Rheumatol Int. 1998;18:59–62. doi: 10.1007/s002960050058. [DOI] [PubMed] [Google Scholar]
- 4.Castano AP, Mroz P, Wu MX, Hamblin MR. Photodynamic therapy plus low-dose cyclophosphamide generates antitumor immunity in a mouse model. Proc Natl Acad Sci USA. 2008;105:5495–5500. doi: 10.1073/pnas.0709256105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casale F, Canaparo R, Serpe L, et al. Plasma concentrations of 5-fluorouracil and its metabolites in colon cancer patients. Pharmacol Res. 2004;50:173–179. doi: 10.1016/j.phrs.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 6.Machover D, Goldschmidt E, Chollet P, et al. Treatment of advanced colorectal and gastric adenocarcinomas with 5-fluorouracil and high-dose folinic acid. NCI Monogr. 1987;5:193–198. [PubMed] [Google Scholar]
- 7.de Gramont A, Bosset JF, Milan C, et al. Randomized trial comparing monthly low-dose leucovorin and flourouracil bolus with bimonthly high-dose leucovorin and fluorouracil bolus plus continuous infusion for advanced colorectal cancer: a French intergroup study. J Clin Oncol. 1997;15:808–815. doi: 10.1200/JCO.1997.15.2.808. [DOI] [PubMed] [Google Scholar]
- 8.Schmoll HJ, Köhne CH, Lorenz M. Weekly 24h infusion of high dose (HD) 5-fluorouracil (FU24h) with or without folinic acid (FA) vs. bolus FU/FA (NCCTG/Mayo) in advanced colorectal cancer (CRC): a randomized Phase III study of the a randomized Phase III study of the EORTC GUTCCG and the AIO. Proc Am Soc Clin Oncol. 2000;19:241a. [Google Scholar]
- 9.Grem JL, McAtee N, Steinberg SM, et al. A phase I study of continuous infusion 5-fluoruuracil plus calcium leucovorin in combination with N-(phosphonoacetyl)-L aspartate in metastatic gastrointestinal adenocarcinoma. Cancer Res. 1993;53:4828–4836. [PubMed] [Google Scholar]
- 10.Hodi FS, Catalano P, Macdonald JS, et al. Age as a factor influencing the toxicity of adjuvant chemotherapy for colorectal cancer. Pending submission, 2nd International Conference on Gastrointestinal Oncology; Cologne, Germany. 1996. [Google Scholar]
- 11.Toxicity of fluorouracil in patients with advanced colorectal cancer: effect of administration schedule and prognostic factors. Meta-Analysis Group In Cancer. J Clin Oncol. 1998;16:3537–3541. doi: 10.1200/JCO.1998.16.11.3537. No authors listed. [DOI] [PubMed] [Google Scholar]
- 12.Garg MB, Sevester JC, Sakoff JA, Ackland SP. Simple liquid chromatographic method for the determination of uracil and dihydrouracil plasma levels: a potential pretreatment predictor of 5-flurouracil toxicity. J Chromatogr B Analyt Technol Biomed Life Sci. 2002;774:223–230. doi: 10.1016/s1570-0232(02)00239-8. [DOI] [PubMed] [Google Scholar]
- 13.Vetvicka V, Kincade PW, Witte PL. Effects of 5-fluorouracil on B lymphocyte lineage cells. J Immunol. 1986;137:2405–2410. [PubMed] [Google Scholar]
- 14.Kobayashi R, Yoshimatsu K, Ishibashi K, Yokomizo H, Umehara A, Yoshida K, Fujimoto T, Watanabe K, Ogawa K. Host immunity in colorectal cancer patients treated with low-dose Leucovorin plus 5-fluorouracil. Gan To Kagaku Ryoho. 2004;31:1783–1785. (In Japanese) [PubMed] [Google Scholar]
- 15.Kobayashi R, Yoshimatsu K, Yokomizo H, Katsube T, Ogawa K. Low-dose chemotherapy with leucovorin plus 5-fluorouracil for colorectal cancer can maintain host immunity. Anticancer Res. 2007;27:675–679. [PubMed] [Google Scholar]
- 16.Codacci-Pisanelli G, van der Wilt CL, Pinedo HM, Franchi F, Noordhuis P, Braakhuis B, van Laar JA, Peters GJ. Antitumour activity, toxicity and inhibition of thymidylate synthase of prolonged administration of 5-fluorouracil in mice. Eur J Cancer. 1995;31A:1517–1525. doi: 10.1016/0959-8049(95)00218-8. [DOI] [PubMed] [Google Scholar]
- 17.Vichaya EG, Cook JL, Frazier MA, Young EE, Meagher MW. Modeling symptoms of chemotherapy: Bortezomib and 5-fluorouracil induce sickness in mice. Brain Behav Immun. 2011;24:6–7. [Google Scholar]
- 18.Young SD, Whissell M, Noble JC, Cano PO, Lopez PG, Germond CJ. Phase II clinical trial results involving treatment with low-dose daily oral cyclophosphamide, weekly vinblastine, and rofecoxib in patients with advanced solid tumors. Clin Cancer Res. 2006;12:3092–3098. doi: 10.1158/1078-0432.CCR-05-2255. [DOI] [PubMed] [Google Scholar]
- 19.Awwad M, North RJ. Cyclophosphamide-induced immunologically mediated regression of a cyclophosphamide-resistant murine tumor: a consequence of eliminating precursor L3T4+ suppressor T-cells. Cancer Res. 1989;49:1649–1654. [PubMed] [Google Scholar]
- 20.Berd D, Mastrangelo MJ. Effect of low dose cyclophosphamide on the immune system of cancer patients: reduction of T-suppressor function without depletion of the CD8+ subset. Cancer Res. 1987;47:3317–3321. [PubMed] [Google Scholar]
- 21.Shen FZ, Wang J, Liang J, Mu K, Hou JY, Wang YT. Low-dose metronomic chemotherapy with cisplatin: can it suppress angiogenesis in H22 hepatocarcinoma cells? Int J Exp Pathol. 2010;91:10–16. doi: 10.1111/j.1365-2613.2009.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pasquier E, Kavallaris M, André N. Metronomic chemotherapy: new rationale for new directions. Nat Rev Clin Oncol. 2010;7:455–465. doi: 10.1038/nrclinonc.2010.82. [DOI] [PubMed] [Google Scholar]
- 23.Zhu Q, Liu JY, Yang CM, Xu HW, Zhang AZ, Cui Y, Wang HB, Qin CY, Li YQ. Influence of antitumor drugs on the expression of Fas systemin SW480 colon cancer cells. Eur J Gastroenterol Hepatol. 2006;18:1071–1077. doi: 10.1097/01.meg.0000231750.68513.6c. [DOI] [PubMed] [Google Scholar]


