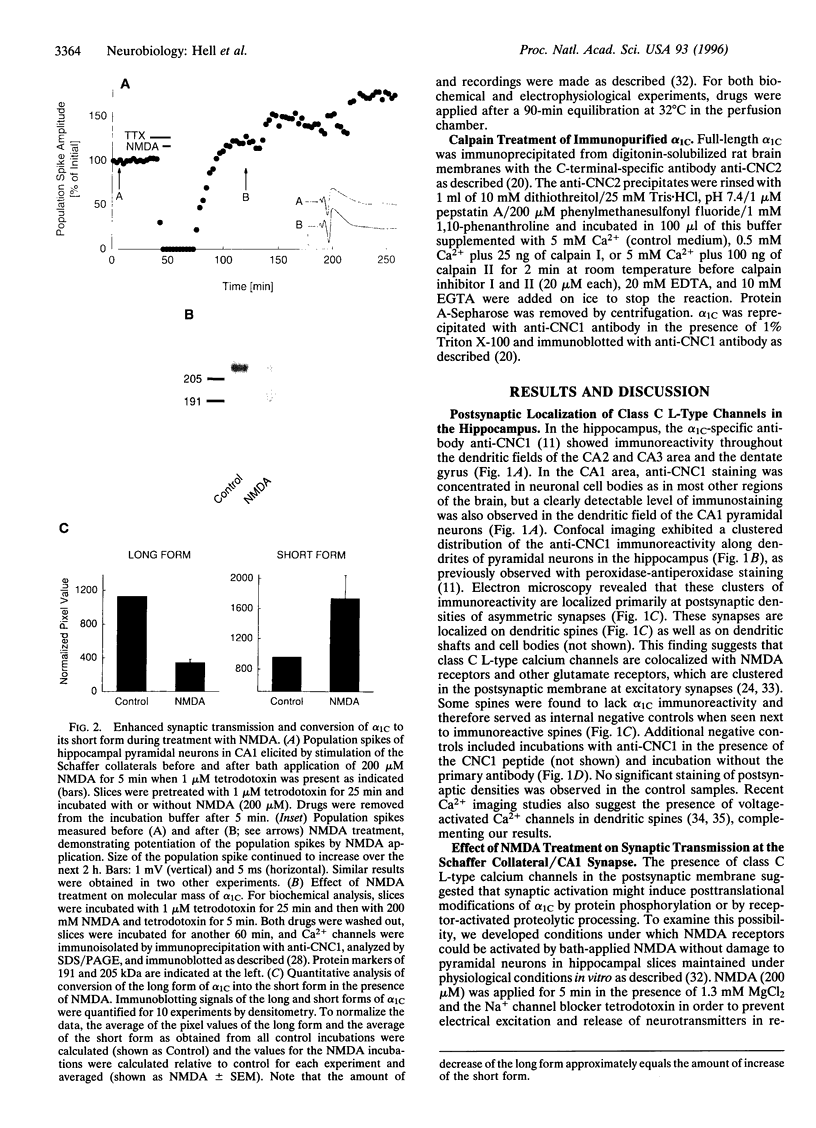
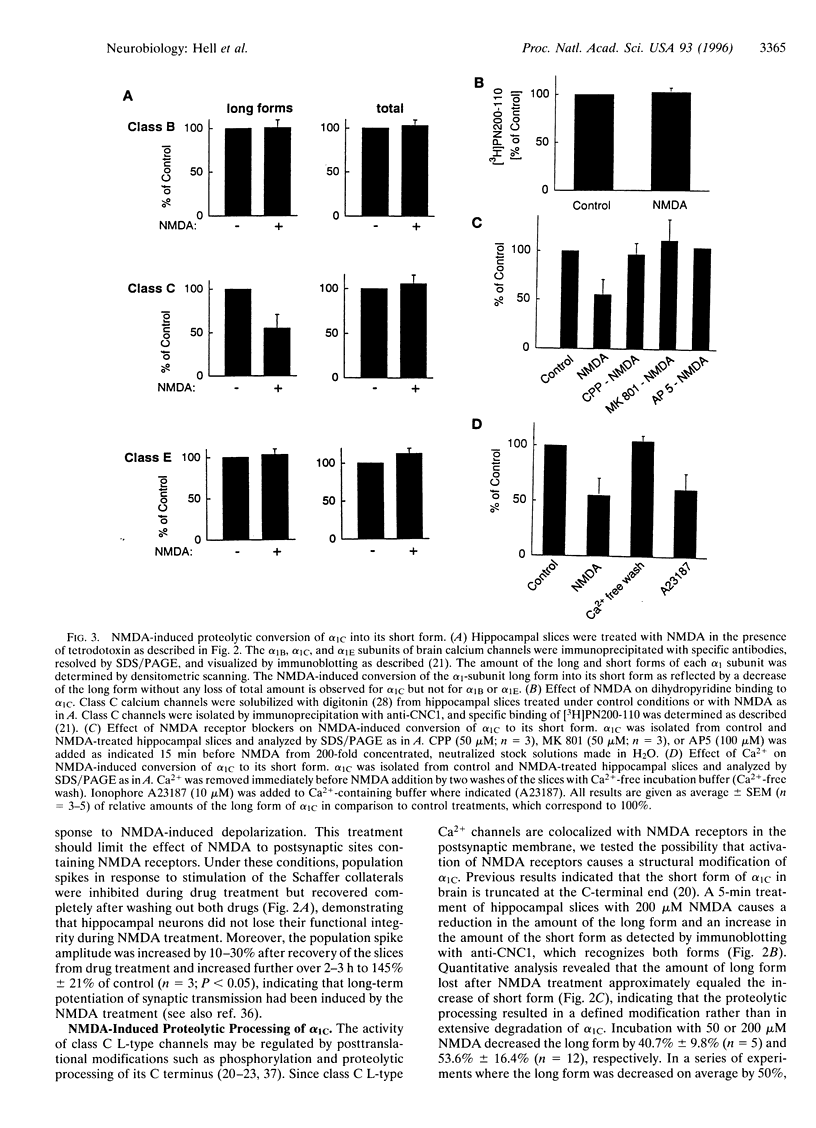
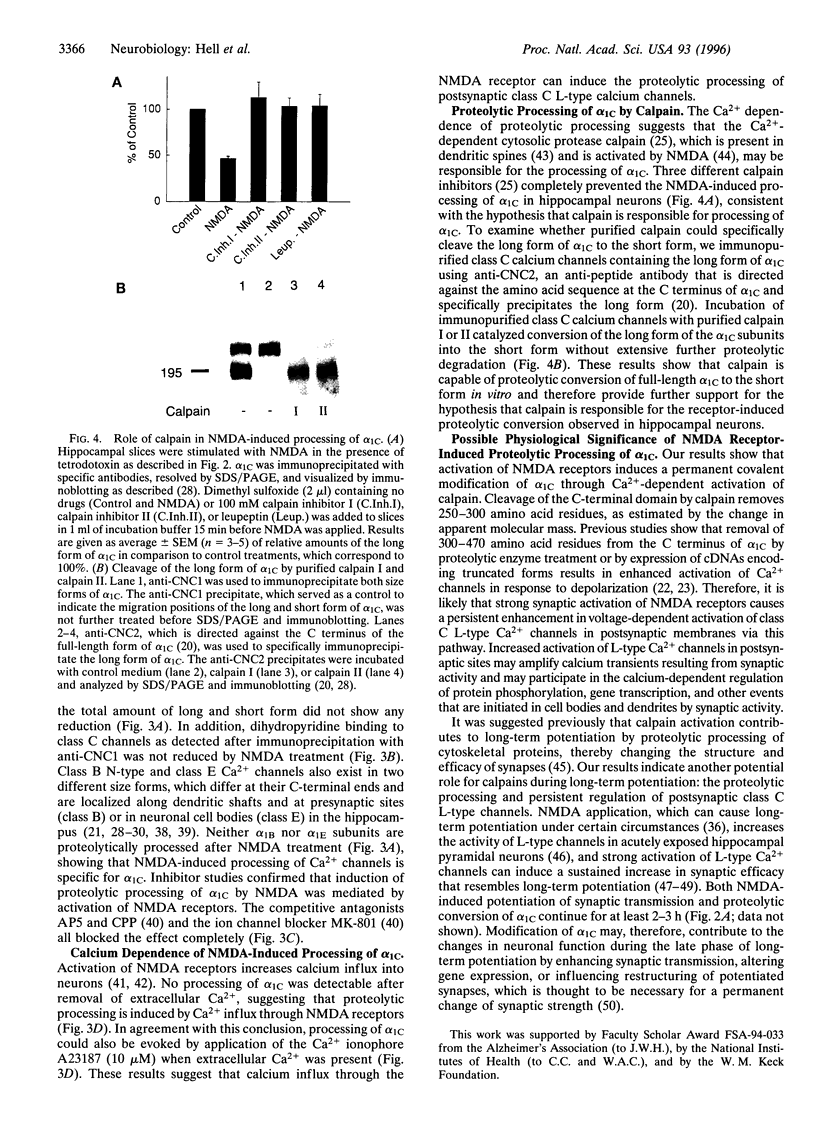
Abstract
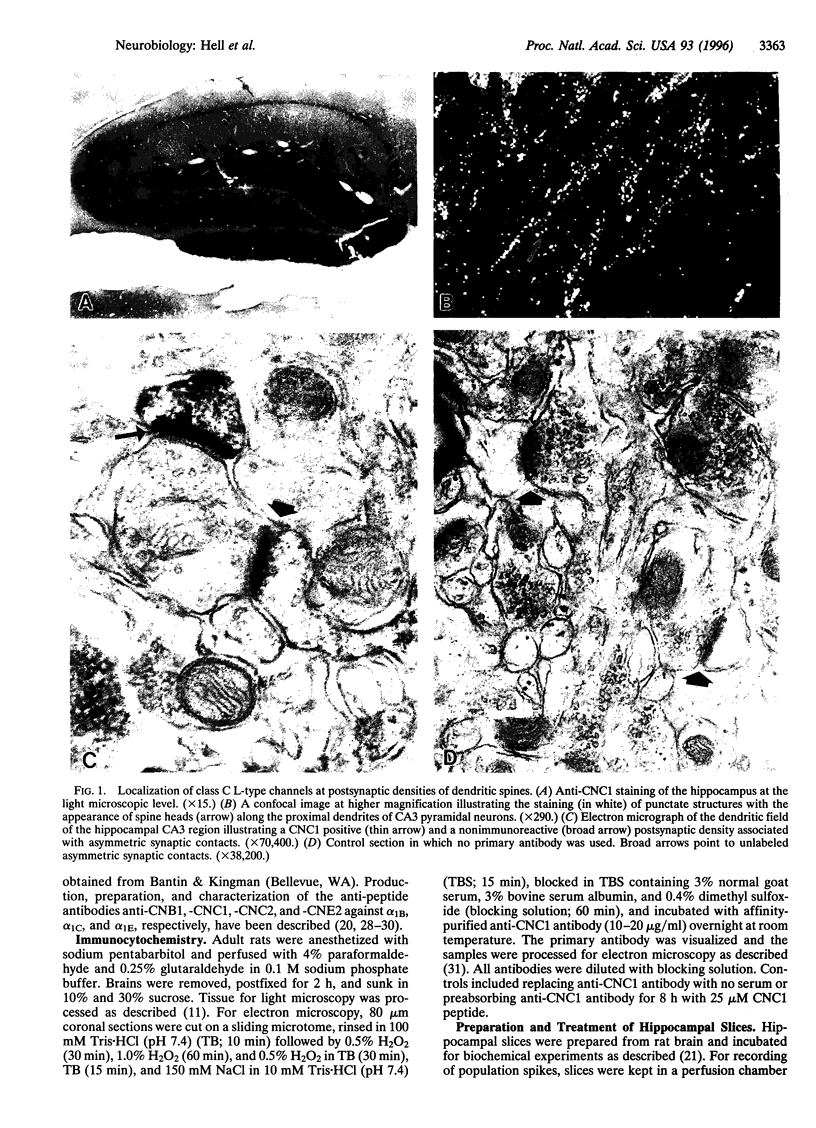
Ca2+ influx controls multiple neuronal functions including neurotransmitter release, protein phosphorylation, gene expression, and synaptic plasticity. Brain L-type Ca2+ channels, which contain either alpha 1C or alpha 1D as their pore-forming subunits, are an important source of calcium entry into neurons. Alpha 1C exists in long and short forms, which are differentially phosphorylated, and C-terminal truncation of alpha 1C increases its activity approximately 4-fold in heterologous expression systems. Although most L-type calcium channels in brain are localized in the cell body and proximal dendrites, alpha 1C subunits in the hippocampus are also present in clusters along the dendrites of neurons. Examination by electron microscopy shows that these clusters of alpha 1C are localized in the postsynaptic membrane of excitatory synapses, which are known to contain glutamate receptors. Activation of N-methyl-D-aspartate (NMDA)-specific glutamate receptors induced the conversion of the long form of alpha 1C into the short form by proteolytic removal of the C terminus. Other classes of Ca2+ channel alpha1 subunits were unaffected. This proteolytic processing reaction required extracellular calcium and was blocked by inhibitors of the calcium-activated protease calpain, indicating that calcium entry through NMDA receptors activated proteolysis of alpha1C by calpain. Purified calpain catalyzed conversion of the long form of immunopurified alpha 1C to the short form in vitro, consistent with the hypothesis that calpain is responsible for processing of alpha 1C in hippocampal neurons. Our results suggest that NMDA receptor-induced processing of the postsynaptic class C L-type Ca2+ channel may persistently increase Ca2+ influx following intense synaptic activity and may influence Ca2+-dependent processes such as protein phosphorylation, synaptic plasticity, and gene expression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlijanian M. K., Westenbroek R. E., Catterall W. A. Subunit structure and localization of dihydropyridine-sensitive calcium channels in mammalian brain, spinal cord, and retina. Neuron. 1990 Jun;4(6):819–832. doi: 10.1016/0896-6273(90)90135-3. [DOI] [PubMed] [Google Scholar]
- Aniksztejn L., Ben-Ari Y. Novel form of long-term potentiation produced by a K+ channel blocker in the hippocampus. Nature. 1991 Jan 3;349(6304):67–69. doi: 10.1038/349067a0. [DOI] [PubMed] [Google Scholar]
- Bailey C. H., Chen M., Keller F., Kandel E. R. Serotonin-mediated endocytosis of apCAM: an early step of learning-related synaptic growth in Aplysia. Science. 1992 May 1;256(5057):645–649. doi: 10.1126/science.1585177. [DOI] [PubMed] [Google Scholar]
- Bear M. F., Malenka R. C. Synaptic plasticity: LTP and LTD. Curr Opin Neurobiol. 1994 Jun;4(3):389–399. doi: 10.1016/0959-4388(94)90101-5. [DOI] [PubMed] [Google Scholar]
- Birnbaumer L., Campbell K. P., Catterall W. A., Harpold M. M., Hofmann F., Horne W. A., Mori Y., Schwartz A., Snutch T. P., Tanabe T. The naming of voltage-gated calcium channels. Neuron. 1994 Sep;13(3):505–506. doi: 10.1016/0896-6273(94)90021-3. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. doi: 10.1146/annurev.bi.64.070195.002425. [DOI] [PubMed] [Google Scholar]
- Chetkovich D. M., Gray R., Johnston D., Sweatt J. D. N-methyl-D-aspartate receptor activation increases cAMP levels and voltage-gated Ca2+ channel activity in area CA1 of hippocampus. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6467–6471. doi: 10.1073/pnas.88.15.6467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh K. S., Merrick D. K., Catterall W. A. Subunits of purified calcium channels: a 212-kDa form of alpha 1 and partial amino acid sequence of a phosphorylation site of an independent beta subunit. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8585–8589. doi: 10.1073/pnas.86.21.8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Jongh K. S., Warner C., Colvin A. A., Catterall W. A. Characterization of the two size forms of the alpha 1 subunit of skeletal muscle L-type calcium channels. Proc Natl Acad Sci U S A. 1991 Dec 1;88(23):10778–10782. doi: 10.1073/pnas.88.23.10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubel S. J., Starr T. V., Hell J., Ahlijanian M. K., Enyeart J. J., Catterall W. A., Snutch T. P. Molecular cloning of the alpha-1 subunit of an omega-conotoxin-sensitive calcium channel. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5058–5062. doi: 10.1073/pnas.89.11.5058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Luebke J. I., Turner T. J. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995 Feb;18(2):89–98. [PubMed] [Google Scholar]
- Elliott E. M., Malouf A. T., Catterall W. A. Role of calcium channel subtypes in calcium transients in hippocampal CA3 neurons. J Neurosci. 1995 Oct;15(10):6433–6444. doi: 10.1523/JNEUROSCI.15-10-06433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster A. C., Fagg G. E. Neurobiology. Taking apart NMDA receptors. Nature. 1987 Oct 1;329(6138):395–396. doi: 10.1038/329395a0. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Greenberg M. E. Calcium signaling in neurons: molecular mechanisms and cellular consequences. Science. 1995 Apr 14;268(5208):239–247. doi: 10.1126/science.7716515. [DOI] [PubMed] [Google Scholar]
- Grover L. M., Teyler T. J. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990 Oct 4;347(6292):477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- Hell J. W., Appleyard S. M., Yokoyama C. T., Warner C., Catterall W. A. Differential phosphorylation of two size forms of the N-type calcium channel alpha 1 subunit which have different COOH termini. J Biol Chem. 1994 Mar 11;269(10):7390–7396. [PubMed] [Google Scholar]
- Hell J. W., Westenbroek R. E., Warner C., Ahlijanian M. K., Prystay W., Gilbert M. M., Snutch T. P., Catterall W. A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J Cell Biol. 1993 Nov;123(4):949–962. doi: 10.1083/jcb.123.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell J. W., Yokoyama C. T., Breeze L. J., Chavkin C., Catterall W. A. Phosphorylation of presynaptic and postsynaptic calcium channels by cAMP-dependent protein kinase in hippocampal neurons. EMBO J. 1995 Jul 3;14(13):3036–3044. doi: 10.1002/j.1460-2075.1995.tb07306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell J. W., Yokoyama C. T., Wong S. T., Warner C., Snutch T. P., Catterall W. A. Differential phosphorylation of two size forms of the neuronal class C L-type calcium channel alpha 1 subunit. J Biol Chem. 1993 Sep 15;268(26):19451–19457. [PubMed] [Google Scholar]
- Hollmann M., Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Kawashima S., Nomoto M., Hayashi M., Inomata M., Nakamura M., Imahori K. Comparison of calcium-activated neutral proteases from skeletal muscle of rabbit and chicken. J Biochem. 1984 Jan;95(1):95–101. doi: 10.1093/oxfordjournals.jbchem.a134608. [DOI] [PubMed] [Google Scholar]
- Klöckner U., Mikala G., Varadi M., Varadi G., Schwartz A. Involvement of the carboxyl-terminal region of the alpha 1 subunit in voltage-dependent inactivation of cardiac calcium channels. J Biol Chem. 1995 Jul 21;270(29):17306–17310. doi: 10.1074/jbc.270.29.17306. [DOI] [PubMed] [Google Scholar]
- Kullmann D. M., Perkel D. J., Manabe T., Nicoll R. A. Ca2+ entry via postsynaptic voltage-sensitive Ca2+ channels can transiently potentiate excitatory synaptic transmission in the hippocampus. Neuron. 1992 Dec;9(6):1175–1183. doi: 10.1016/0896-6273(92)90075-o. [DOI] [PubMed] [Google Scholar]
- Lerea L. S., Butler L. S., McNamara J. O. NMDA and non-NMDA receptor-mediated increase of c-fos mRNA in dentate gyrus neurons involves calcium influx via different routes. J Neurosci. 1992 Aug;12(8):2973–2981. doi: 10.1523/JNEUROSCI.12-08-02973.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G., Baudry M. The biochemistry of memory: a new and specific hypothesis. Science. 1984 Jun 8;224(4653):1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- MacDermott A. B., Mayer M. L., Westbrook G. L., Smith S. J., Barker J. L. NMDA-receptor activation increases cytoplasmic calcium concentration in cultured spinal cord neurones. 1986 May 29-Jun 4Nature. 321(6069):519–522. doi: 10.1038/321519a0. [DOI] [PubMed] [Google Scholar]
- Malenka R. C. Postsynaptic factors control the duration of synaptic enhancement in area CA1 of the hippocampus. Neuron. 1991 Jan;6(1):53–60. doi: 10.1016/0896-6273(91)90121-f. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K., Masu M., Ishii T., Shigemoto R., Mizuno N., Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991 Nov 7;354(6348):31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Niidome T., Kim M. S., Friedrich T., Mori Y. Molecular cloning and characterization of a novel calcium channel from rabbit brain. FEBS Lett. 1992 Aug 10;308(1):7–13. doi: 10.1016/0014-5793(92)81038-n. [DOI] [PubMed] [Google Scholar]
- Perlmutter L. S., Siman R., Gall C., Seubert P., Baudry M., Lynch G. The ultrastructural localization of calcium-activated protease "calpain" in rat brain. Synapse. 1988;2(1):79–88. doi: 10.1002/syn.890020111. [DOI] [PubMed] [Google Scholar]
- Petralia R. S., Yokotani N., Wenthold R. J. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994 Feb;14(2):667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randall A., Tsien R. W. Pharmacological dissection of multiple types of Ca2+ channel currents in rat cerebellar granule neurons. J Neurosci. 1995 Apr;15(4):2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman E. I., De Jongh K. S., Florio V., Lai Y., Catterall W. A. Specific phosphorylation of a COOH-terminal site on the full-length form of the alpha 1 subunit of the skeletal muscle calcium channel by cAMP-dependent protein kinase. J Biol Chem. 1992 Aug 15;267(23):16100–16105. [PubMed] [Google Scholar]
- Rotman E. I., Murphy B. J., Catterall W. A. Sites of selective cAMP-dependent phosphorylation of the L-type calcium channel alpha 1 subunit from intact rabbit skeletal muscle myotubes. J Biol Chem. 1995 Jul 7;270(27):16371–16377. doi: 10.1074/jbc.270.27.16371. [DOI] [PubMed] [Google Scholar]
- Sculptoreanu A., Rotman E., Takahashi M., Scheuer T., Catterall W. A. Voltage-dependent potentiation of the activity of cardiac L-type calcium channel alpha 1 subunits due to phosphorylation by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 1993 Nov 1;90(21):10135–10139. doi: 10.1073/pnas.90.21.10135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Imaging of calcium variations in living dendritic spines of cultured rat hippocampal neurons. J Physiol. 1995 Jul 15;486(Pt 2):283–295. doi: 10.1113/jphysiol.1995.sp020811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R., Noszek J. C. Excitatory amino acids activate calpain I and induce structural protein breakdown in vivo. Neuron. 1988 Jun;1(4):279–287. doi: 10.1016/0896-6273(88)90076-1. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Reiner P. B. Ca2+ channels: diversity of form and function. Curr Opin Neurobiol. 1992 Jun;2(3):247–253. doi: 10.1016/0959-4388(92)90111-w. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Tomlinson W. J., Leonard J. P., Gilbert M. M. Distinct calcium channels are generated by alternative splicing and are differentially expressed in the mammalian CNS. Neuron. 1991 Jul;7(1):45–57. doi: 10.1016/0896-6273(91)90073-9. [DOI] [PubMed] [Google Scholar]
- Wagner J. J., Terman G. W., Chavkin C. Endogenous dynorphins inhibit excitatory neurotransmission and block LTP induction in the hippocampus. Nature. 1993 Jun 3;363(6428):451–454. doi: 10.1038/363451a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walaas S. I., Greengard P. Protein phosphorylation and neuronal function. Pharmacol Rev. 1991 Sep;43(3):299–349. [PubMed] [Google Scholar]
- Wang K. K., Roufogalis B. D., Villalobo A. Further characterization of calpain-mediated proteolysis of the human erythrocyte plasma membrane Ca2+-ATPase. Arch Biochem Biophys. 1988 Nov 15;267(1):317–327. doi: 10.1016/0003-9861(88)90037-9. [DOI] [PubMed] [Google Scholar]
- Wang K. K., Yuen P. W. Calpain inhibition: an overview of its therapeutic potential. Trends Pharmacol Sci. 1994 Nov;15(11):412–419. doi: 10.1016/0165-6147(94)90090-6. [DOI] [PubMed] [Google Scholar]
- Wei X., Neely A., Lacerda A. E., Olcese R., Stefani E., Perez-Reyes E., Birnbaumer L. Modification of Ca2+ channel activity by deletions at the carboxyl terminus of the cardiac alpha 1 subunit. J Biol Chem. 1994 Jan 21;269(3):1635–1640. [PubMed] [Google Scholar]
- Westenbroek R. E., Ahlijanian M. K., Catterall W. A. Clustering of L-type Ca2+ channels at the base of major dendrites in hippocampal pyramidal neurons. Nature. 1990 Sep 20;347(6290):281–284. doi: 10.1038/347281a0. [DOI] [PubMed] [Google Scholar]
- Westenbroek R. E., Hell J. W., Warner C., Dubel S. J., Snutch T. P., Catterall W. A. Biochemical properties and subcellular distribution of an N-type calcium channel alpha 1 subunit. Neuron. 1992 Dec;9(6):1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- Westenbroek R. E., Westrum L. E., Hendrickson A. E., Wu J. Y. Ultrastructure of synaptic remodeling in piriform cortex of adult rats after neonatal olfactory bulb removal: an immunocytochemical study. J Comp Neurol. 1988 Aug 15;274(3):334–346. doi: 10.1002/cne.902740304. [DOI] [PubMed] [Google Scholar]
- Williams M. E., Feldman D. H., McCue A. F., Brenner R., Velicelebi G., Ellis S. B., Harpold M. M. Structure and functional expression of alpha 1, alpha 2, and beta subunits of a novel human neuronal calcium channel subtype. Neuron. 1992 Jan;8(1):71–84. doi: 10.1016/0896-6273(92)90109-q. [DOI] [PubMed] [Google Scholar]
- Yokoyama C. T., Westenbroek R. E., Hell J. W., Soong T. W., Snutch T. P., Catterall W. A. Biochemical properties and subcellular distribution of the neuronal class E calcium channel alpha 1 subunit. J Neurosci. 1995 Oct;15(10):6419–6432. doi: 10.1523/JNEUROSCI.15-10-06419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R., Denk W. Dendritic spines as basic functional units of neuronal integration. Nature. 1995 Jun 22;375(6533):682–684. doi: 10.1038/375682a0. [DOI] [PubMed] [Google Scholar]