Abstract
Retinitis pigmentosa (RP) is a hereditary disease that leads to the progressive degeneration of retinal photoreceptor cells and to blindness. It is caused by mutations in several distinct genes, including the ciliary gene FAM161A, which is associated with a recessive form of this disorder. Recent investigations have revealed that defects in FAM161A represent a rather prevalent cause of hereditary blindness in Israel and the Palestinian territories, whereas they seem to be rarely present within patients from Germany. Genetic or clinical data are currently not available for other countries. In this work, we screened a cohort of patients with recessive RP from North America to determine the frequency of FAM161A mutations in this ethnically-mixed population and to assess the phenotype of positive cases. Out of 273 unrelated patients, only 3 subjects had defects in FAM161A. A fourth positive patient, the sister of one of these index cases, was also identified following pedigree analysis. They were all homozygous for the p.T452Sfx3 mutation, which was previously reported as a founder DNA variant in the Israeli and Palestinian populations. Analysis of cultured lymphoblasts from patients revealed that mutant FAM161A transcripts were actively degraded by nonsense-mediated mRNA decay. Electroretinographic testing showed 30 Hz cone flicker responses in the range of 0.10 to 0.60 microvolts in all cases at their first visit (age 12 to 23) (lower norm = 50 μV) and of 0.06 to 0.32 microvolts at their most recent examination (age 27 to 43), revealing an early-onset of this progressive disease. Our data indicate that mutations in FAM161A are responsible for 1% of recessive RP cases in North America, similar to the prevalence detected in Germany and unlike the data from Israel and the Palestinian territories. We also show that, at the molecular level, the disease is likely caused by FAM161A protein deficiency.
Introduction
Retinitis pigmentosa (RP) is a group of hereditary degenerative diseases of the retina displaying a significant phenotypic and genotypic variability and characterized by the progressive decrease of rod and cone photoreceptor function. Patients typically report loss of night vision during adolescence, loss of mid and far peripheral field in young adulthood, and loss of central vision in later life. Characteristic clinical features are elevated final dark adaptation thresholds and reduced and delayed full-field electroretinogram (ERG) signals, measuring the electrical response of the retina to flashes of light [1]. At present, according to the RetNet database (https://sph.uth.edu/retnet/), over 60 genes have been associated with nonsyndromic RP, which account for 50-60% of patients with this condition [2], [3].
Mutations in FAM161A have been found to represent the cause of RP28-associated autosomal recessive RP (arRP) in the initial Indian family in which the RP28 locus was mapped [4], as well as in a cohort of German patients [5]. Frequent nonsense mutations in FAM161A were also identified in patients from Israel and the Palestinian territories [6]. FAM161A is evolutionarily conserved in vertebrates [5], [6] and, despite having multiple splicing variants, only two of them produce stable mRNA transcripts. The main splicing variant contains 6 exons and encodes a protein of 76 kDa, while the second variant contains a supplementary 168 bp in-frame exon between exons 3 and 4 [5], [6]. The protein is mainly expressed in the retina and in the testis and localizes in photoreceptor cells during mouse retinal development [5], [6]. More specifically, FAM161A localizes at the base of the photoreceptor connecting cilium and at the ciliary basal body [7], [8]. Patients with FAM161A mutations have been reported to have symptoms ranging from an atypically late-onset form of RP, detected in the German cohort mentioned above, to an early-onset manifestation of the disease, as observed in Israeli and Palestinian individuals [5], [6]. Unexpectedly, despite these clinical differences, all patients who have been ascertained so far carry the same class of mutations, leading to premature stops of the open reading frame.
Here we report results of our mutational screening of FAM161A in a cohort of patients with autosomal recessive RP from North America, with the aim of assessing the mutation spectrum in this population and investigating any possible genotype/phenotype correlation.
Methods
Patients and Controls
This research was carried out in accordance with the tenets of the Declaration of Helsinki and was approved by the Institutional Review Boards of the University of Lausanne and of Harvard Medical School and the Massachusetts Eye and Ear, where the blood was collected and the patients were followed. Written informed consent was obtained from patients who participated in this study or from their legal guardians before they donated 10-30 ml of their blood for research.
All patients were evaluated with respect to best-corrected Snellen acuities, kinetic visual fields on the Goldmann perimeter with a V-4e white test light, final dark adaptation thresholds with an 11° white test light in a Goldmann-Weekers dark adaptometer, and full-field cone ERGs in response to 30 Hz white flicker obtained with narrow bandpass filtering and computer averaging, as described previously [9]. After dilation, all had a slit-lamp examination, fundus evaluation with direct and indirect ophthalmoscopy, and fundus photography.
DNA from peripheral blood leukocytes was extracted from 273 unrelated patients with arRP from North America, representing an ethnically-mixed population. Controls included 95 individuals with no history of retinal degeneration and 80 subjects with normal ERGs. In addition, DNA samples from 95 ethnically-matched healthy individuals were purchased from the Coriell Institute Repository. Initial screening of FAM161A sequence was performed on whole-genome amplified DNA (REPLI-g Mini Kit, Qiagen). All samples that were found to be positive for DNA changes were then PCR-amplified and sequenced a second time, starting from original DNA.
Mutation Screening
Primer pairs for individual exons and relevant intron boundaries were designed using the CLCbio Genomics Workbench (Table S1). Amplifications by PCR were performed in 25 μl reactions containing 20 ng genomic DNA, 1× GoTaq buffer, 1.2 mM MgCl2, 0.1 mM dNTPs, 0.4 μM of each primer, and 0.01 U/μl of GoTaq polymerase (Promega). Amplification conditions were: an initial step at 95°C for 2 minutes, 35 cycles of denaturation at 94°C for 30 seconds, annealing according to primers' melting temperature for 30 seconds, and extension at 72°C for 1 minute. Before the end of the reaction, a final extension step at 72°C for 5 minutes was performed.
Sequencing reactions were carried out by Sanger sequencing, after purification of PCR products (ExoSAP-IT, USB), by using 1 μl of 3.3 μM sequencing primer (Table S2) and 0.5 μl of BigDye Terminator v1.1 (Applied Biosystems). The products of these sequencing reactions were run on an ABI-3130XLS sequencer (Applied Biosystems).
Cell Culture and Drug Treatment
The Epstein-Barr virus immortalized lymphoblastoid cell lines that were used in this study were derived from two affected patients (003-161 and her sibling 012-001), and were cultured as previously described [10]. Normal control cell lines were purchased from the Coriell Cell Repository. RNA extraction, cDNA synthesis, and treatment of cells with cycloheximide was performed as previously reported [11].
FAM161A primers for cDNA amplification were 5′-ggaagaaacgaaaagaatgg-3′ and 5′–ttctcgttggtattctctcatcc-3′, yielding a 1,115 bp product. 18S ribosomal RNA was used as a housekeeping gene for this analysis, and primers for its cDNA amplification were 5′-cggctaccacatccaaggaa-3′ and 5′–gctggaattaccgcggct-3′ (resulting in a 187 bp product).
RNA Ligase Mediated Rapid Amplification of cDNA Ends (RLM-RACE)
To perform RLM-RACE we used the FirstChoice RLM-RACE kit (Invitrogen), following the manufacturer's instruction. One microgram of total RNA from human retina (Clontech) was used to perform 3′UTR RLM-RACE, and ten micrograms of total RNA from human retina (Clontech) and from retinoblastoma cell line Y79 were used to perform 5′UTR RLM-RACE.
Results
Molecular Genetic Analysis
We screened FAM161A in 273 unrelated patients with arRP from North America, and in 270 ethnically matched healthy individuals. In three of these patients we identified the null mutation p.Thr452SerfsX3, which was previously reported as a founder mutation in an Israeli Jewish population [6]. Following the pedigree analysis of these three index patients, we identified an additional affected family member (012-001, sister of 003-161), who was also found to be positive for the same mutation. All four patients were homozygous for p.Thr452SerfsX3 (Table 1). All controls were negative for this DNA change.
Table 1. Variants identified in this study.
| Patient ID | Nucleotide change | Predicted effect | Polyphena | SIFTb | MutPredc | PMutd | Controls frequency (alleles) | Reference of the variation |
| 003–327 | c.1113 C>G/+ | p.Asp371Glu/+ | benign | tolerated | benign | neutral | 0/540 | This study |
| 003–154, 003–217, 121–847, 121–050, 121–543 | c.1133 T>G/+ | p.Leu378Arg/+ | probably damaging | tolerated | disrupted | pathological | 2/540 | Langmann et al. [5] |
| 003–218 | c.1153 C>G/+ | p.Gln385Glu/+ | probably damaging | tolerated | benign | neutral | 2/540 | Langmann et al. [5] |
| 003–189 | c.1391 A>G/+ | p.His464Arg/+ | benign | tolerated | benign | pathological | 0/540 | This study |
| 003–161, 003–257, 121–385, 012–001 | c.1355_6delCA/c.1355_6delCA | p.Thr452SerfsX3/p.Thr452SerfsX3 | confirmed mutation | 0/540 | Bandah-Rozenfeld et al. [6] | |||
+, wild-type allele.
http://genetics.bwh.harvard.edu/pph2/.
http://sift.jcvi.org/.
http://mutpred.mutdb.org/
http://mmb2.pcb.ub.es:8080/PMut/.
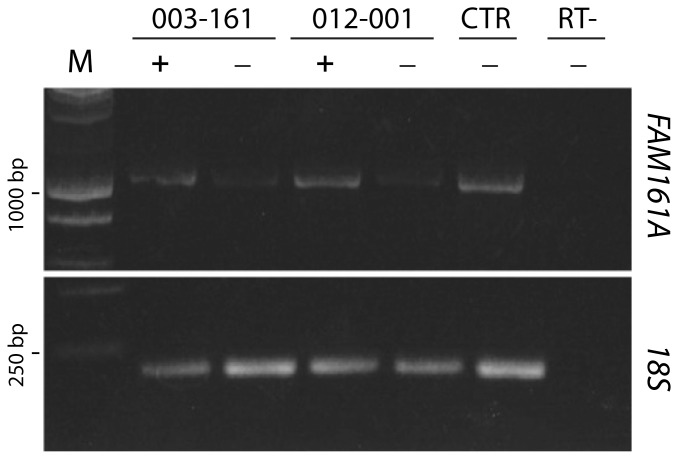
p.Thr452SerfsX3 is predicted to produce a transcript that could be a target for nonsense mediated mRNA decay (NMD) and therefore result in no protein product [12]. In order to test this hypothesis, we analyzed lymphoblastoid cell lines derived from the two siblings carrying this change (003-161 and 012-001). RT-PCRs of FAM161A mRNA from both cell lines, cultured in standard conditions, yielded a rather poor amplification product, indicative of mRNA degradation. Conversely, supplementation with the chemical NMD inhibitor cycloheximide reversed this phenomenon and allowed good amplification, confirming that p.Thr452SerfsX3 results in a null allele (Figure 1).
Figure 1. Mutation c.1355_6delCA (p.T452SfsX3) produces transcripts with a premature stop codon that are targets for NMD degradation.
The image shows an agarose gel on which six RT-PCR products were run: two from patients 003-161 and 102-001, respectively, one from a control cell line (CTR), and one from the same control cell line, following a cDNA synthesis reaction performed without the addition of the reverse transcriptase enzyme (RT-). Presence of cycloheximide in cell cultures is indicated with "+", its absence with "−". M, DNA molecular size marker; 18S, RT-PCR loading control (18S rRNA); 1000 bp and 250 bp, bands of the marker corresponding to these specific sizes, respectively.
In our cohort we also found a few other rare variants, likely having no pathological relevance (Table 1). Variant p.Q385E (rs139266382; Minor Allele Frequency, or MAF<0.01) was previously identified as a heterozygous change in arRP patients and in healthy controls with a frequency of 2/400 alleles [5]. In our screening, we identified it in one patient and in 2 individuals from our control cohort. Variant p.H464R (rs201315315, MAF<0.01) was found as a heterozygous change in one arRP patient and in no healthy controls. Variant p.D371E was neither annotated in the dbSNP database [13] nor was found in the control cohort, but was found in a heterozygous state in one patient. However, it involved an amino acid residue that is not conserved across species and was predicted to result in a benign change by in silico analyses.
Variant p.L378R was previously identified in the heterozygous state in a cohort of German patients with recessive or isolated forms of RP and classified as a rare variant with uncertain pathogenicity [5]. It involves a highly conserved residue and is predicted to be deleterious by 3 out of 4 prediction programs (Polyphen, SIFT, MutPred, and PMut) [14]–[17]. Recently, it has been annotated in the dbSNP database (build 135/138, rs187695569), with a MAF of 0.003. We identified it in a heterozygous state in 5 out of 273 arRP patients and in 2 healthy individuals from the Coriell control cohort.
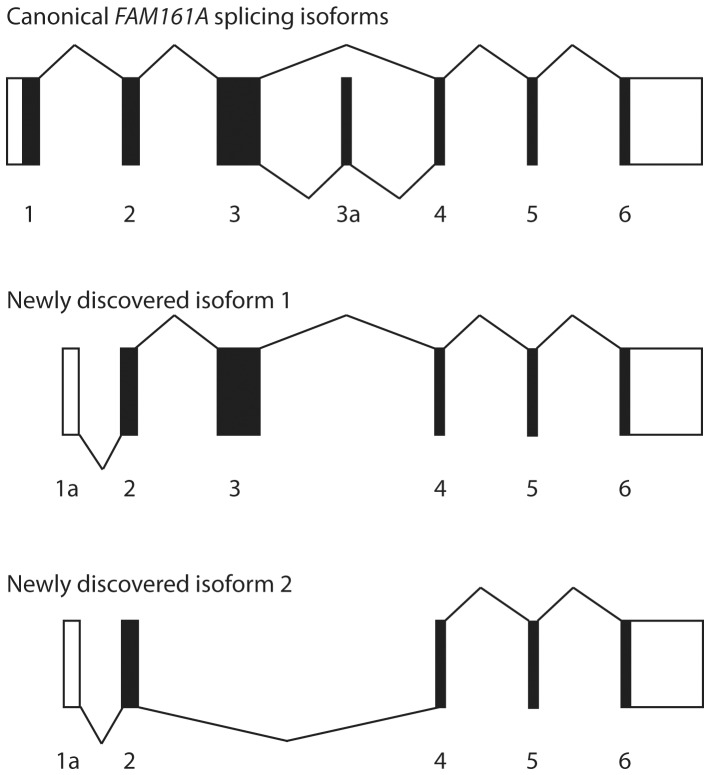
To test the hypothesis that heterozygous changes detected in our cohort of patients could in fact represent unrecognized mutations, we ascertained whether there were other non-annotated FAM161A exons in the human retina. We therefore performed 5′ and 3′ RLM-RACE PCR using pooled retinal RNA from different donors, and identified two additional transcripts with an alternative 5′UTR in intron 1, whose sequence corresponds to the annotated exon 2 of FAM161A-003 isoform. In these transcripts, which were expressed in the retina at lower levels compared to the major isoforms, the translation-initiation codon may lie in exon 2 (Figure 2) [6]. Interestingly, one of them completely skipped the highly-conserved exon 3, where most FAM161A mutations have been so far reported. Furthermore, despite the absence of exon 1 and the skipping of exon 3, in both newly-discovered isoforms the original FAM161A open reading frame appeared to be preserved, possibly leading to translation of these transcripts. We therefore screened this alternative 5′UTR sequence in the patients, but we did not find any likely pathological variant.
Figure 2. Schematic representation of alternative splicing events of FAM161A transcripts.
Boxes represent exons, while lines represent splicing events. Coding regions are in black, noncoding regions are white. The canonical forms of FAM161A mRNA are presented in the top panel, as reference. The bottom two panels show newly-discovered splicing isoforms with an alternative 5′UTR in intron 1 (1a).
Clinical Evaluation of the Patients
Clinical evaluation at initial visits showed preserved visual acuity, constricted visual fields, and reduced but detectable 30 Hz cone ERGs in all 4 patients with known pathogenic mutations (Table 2). All had bone spicule pigment in the periphery (Figure 3). At follow up, 10 to 20 years later, each patient showed preserved acuity in at least one eye and cone ERGs comparable in amplitude to those at their initial visits. Because of a possible floor effect [9], further degeneration was difficult to quantify in these patients due to their small initial ERGs amplitudes.
Table 2. Clinical summary of patients with FAM161A mutations associated with retinitis pigmentosa.
| Time of examination | Patient ID | Age | Sex | EO | VAa OD | VAa OS | ERGb OD | ERGb OS | VFc OD | VFc OS | DAd | Lense OD | Lense OS | Maculaf OD | Maculaf OS | Peripheryg OD | Peripheryg OS |
| First visit | 003–161 | 12 | F | Jewish (European) | 20/40 | 20/30 | 0.10 | 0.10 | 1174 | 2016 | 3.0 | – | – | – | – | + | + |
| First visit | 003–257 | 21 | F | Jewish (Moroccan) | 20/50 | 20/40 | 0.10 | 0.10 | 108 | 76 | 3.0 | – | + | – | – | + | + |
| First visit | 121–385 | 23 | M | Caucasian | 20/20 | 20/20 | 0.24 | 0.60 | 2060 | 1795 | 3.0 | + | + | – | – | + | + |
| First visit | 012–001 | 12 | F | Jewish (European) | 20/40 | 20/40 | 0.10 | 0.10 | 78 | 78 | 3.0 | + | + | – | – | + | + |
| Last visit | 003–161 | 27 | F | Jewish (European) | 20/30 | 20/25 | 0.17 | 0.24 | 123 | 118 | NA | – | – | – | – | + | + |
| Last visit | 003–257 | 38 | F | Jewish (Moroccan) | HM | 20/60 | 0.06 | 0.10 | NA | NA | 4.0 | + | + | AS | + | + | + |
| Last visit | 121–385 | 43 | M | Caucasian | 20/20 | 20/30 | 0.22 | 0.32 | 69 | 65 | 4.0 | PP | PP | + | + | + | + |
| Last visit | 012–001 | 30 | F | Jewish (European) | 20/30 | 20/30 | NA | NA | 43 | 73 | NA | + | + | – | – | + | + |
Visual Acuity: best corrected Snellen visual acuity.
Electroretinograms: full field cone ERG amplitude in microvolts to 30HZ white light (lower norm = 50 microvolts).
Visual Field: Goldmann total field area to V-4e white test light (lower norm = 11,399 degrees squared).
Dark adaptation: final threshold in log units above normal after 45 minutes of dark adaptation.
Lens: −, clear lens; +, central posterior subcapsular cataract.
Macula: −, within normal limits; +, granular.
Periphery: bone spicule or clumped pigment in one or more quadrants: +, present; −, absent.
Abbreviations: F, female; M, male; EO, ethnic origin; OD, right eye; OS, left eye; HM, hand motions; PP, pseudophakia; AS, atrophic scar; NA, not available.
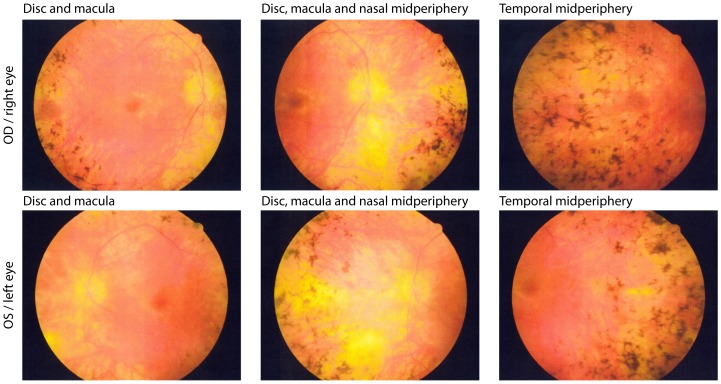
Figure 3. Fundus photographs from patient 121–385 (male, 43 years old).
The patient shows the representative phenotype of this mutation with waxy pallor of the optic disc, retinal arteriolar attenuation, granularity of the macula and intra retinal pigment around the midperiphery in a bone spicule or clumped configuration.
Discussion
Mutations in FAM161A were recently reported to cause autosomal recessive retinitis pigmentosa by two independent studies [5], [6]. However, despite mutations corresponded in all cases to DNA changes that lead to the premature termination of the reading frame, both prevalence and clinical severity of FAM161A-linked disease seem to be population-specific and/or dependent on the presence of other modifier genes. In Germany, this gene is mutated with a prevalence that is comparable to that of most arRP genes (1–2%) and is found in patients with a remarkably late-onset of the disease [5]. In Israel and in the Palestinian territories, FAM161A mutations account for about 12% of recessive RP cases, who have symptoms and signs of disease much earlier in their life [6].
Among 273 unrelated recessive patients screened in our study, only 3 were positive for FAM161A mutations, and in particular for p.T452Sfx3, indicating that this gene is rarely associated with RP in North America. Interestingly, p.T452Sfx3 was previously reported as a founder mutation in the Israeli and Palestinian populations [6], and all but one of our patients reported Jewish ancestry. Furthermore, from our study it emerged that a few patients carried rare heterozygous variants that were absent or had an allele frequency of less than 1% in the general population, and that had an uncertain functional significance.
Patients with FAM161A mutations clinically showed early-onset RP with relatively good acuity and very reduced cone ERGs. Follow-up examinations 10–20 years after the initial visit showed that all retained good acuity in at least one eye, 3 out of 4 showed further loss of visual field, and 1 showed further decline in the ERG. A floor effect may have occurred in those with less than or equal to 0.34 μV at their initial visit that could simulate stabilization or even improvement. Visual field areas to the V-4e white test light were all below normal. The phenotype was that of typical retinitis pigmentosa in all 4 cases. The long-term course of arRP associated with FAM161A mutations remains to be defined.
Finally, our analysis of FAM161A transcripts revealed that p.T452Sfx3 probably results in a functionally null allele, in view of the premature termination codon introduced by the mutation. Since all other pathogenic changes detected so far belong to this same class of mutations, it is likely that the disease is caused by deficiency of FAM161A protein, making the FAM161A gene a good candidate for gene replacement studies.
Supporting Information
Primers for polymerase chain reaction amplification of FAM161A exons.
(PDF)
Primers for Sanger sequencing of FAM161A exons.
(PDF)
Acknowledgments
We would like to thank Alexandra Title, BSc, for technical assistance.
Funding Statement
This work was supported by the Swiss National Science Foundation (Grant 310030_138346), the Gebert Rüf Foundation, Switzerland (Rare Diseases - New Technologies grant), and a Center Grant to the Berman-Gund Laboratory for the Study of Retinal Degenerations, Harvard Medical School, to Dr. Berson from The Foundation Fighting Blindness, Columbia, Maryland. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Berson EL (1993) Retinitis pigmentosa. The Friedenwald Lecture. Invest Ophthalmol Vis Sci 34: 1659–1676. [PubMed] [Google Scholar]
- 2. Hartong DT, Berson EL, Dryja TP (2006) Retinitis pigmentosa. Lancet 368: 1795–1809. [DOI] [PubMed] [Google Scholar]
- 3. Daiger SP, Bowne SJ, Sullivan LS (2007) Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol 125: 151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gu S, Kumaramanickavel G, Srikumari CR, Denton MJ, Gal A (1999) Autosomal recessive retinitis pigmentosa locus RP28 maps between D2S1337 and D2S286 on chromosome 2p11–p15 in an Indian family. J Med Genet 36: 705–707. [PMC free article] [PubMed] [Google Scholar]
- 5. Langmann T, Di Gioia SA, Rau I, Stohr H, Maksimovic NS, et al. (2010) Nonsense Mutations in FAM161A Cause RP28-Associated Recessive Retinitis Pigmentosa. Am J Hum Genet 87: 376–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bandah-Rozenfeld D, Mizrahi-Meissonnier L, Farhy C, Obolensky A, Chowers I, et al. (2010) Homozygosity mapping reveals null mutations in FAM161A as a cause of autosomal-recessive retinitis pigmentosa. Am J Hum Genet 87: 382–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Di Gioia SA, Letteboer SJ, Kostic C, Bandah-Rozenfeld D, Hetterschijt L, et al. (2012) FAM161A, associated with retinitis pigmentosa, is a component of the cilia-basal body complex and interacts with proteins involved in ciliopathies. Hum Mol Genet 21: 5174–5184. [DOI] [PubMed] [Google Scholar]
- 8. Zach F, Grassmann F, Langmann T, Sorusch N, Wolfrum U, et al. (2012) The retinitis pigmentosa 28 protein FAM161A is a novel ciliary protein involved in intermolecular protein interaction and microtubule association. Hum Mol Genet 21: 4573–4586. [DOI] [PubMed] [Google Scholar]
- 9. Berson EL, Rosner B, Sandberg MA, Hayes KC, Nicholson BW, et al. (1993) A randomized trial of vitamin A and vitamin E supplementation for retinitis pigmentosa. Arch Ophthalmol 111: 761–772. [DOI] [PubMed] [Google Scholar]
- 10. Rivolta C, McGee TL, Rio Frio T, Jensen RV, Berson EL, et al. (2006) Variation in retinitis pigmentosa-11 (PRPF31 or RP11) gene expression between symptomatic and asymptomatic patients with dominant RP11 mutations. Hum Mutat 27: 644–653. [DOI] [PubMed] [Google Scholar]
- 11. Rio Frio T, Wade NM, Ransijn A, Berson EL, Beckmann JS, et al. (2008) Premature termination codons in PRPF31 cause retinitis pigmentosa via haploinsufficiency due to nonsense-mediated mRNA decay. J Clin Invest 118: 1519–1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hentze MW, Kulozik AE (1999) A perfect message: RNA surveillance and nonsense-mediated decay. Cell 96: 307–310. [DOI] [PubMed] [Google Scholar]
- 13. Smigielski EM, Sirotkin K, Ward M, Sherry ST (2000) dbSNP: a database of single nucleotide polymorphisms. Nucleic Acids Res 28: 352–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Adzhubei IA, Schmidt S, Peshkin L, Ramensky VE, Gerasimova A, et al. (2010) A method and server for predicting damaging missense mutations. Nat Methods 7: 248–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ng PC, Henikoff S (2003) SIFT: Predicting amino acid changes that affect protein function. Nucleic Acids Res 31: 3812–3814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mort M, Sterne-Weiler T, Li B, Ball EV, Cooper DN, et al. (2014) MutPred Splice: machine learning-based prediction of exonic variants that disrupt splicing. Genome Biol 15: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ferrer-Costa C, Gelpi JL, Zamakola L, Parraga I, de la Cruz X, et al. (2005) PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics 21: 3176–3178. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Primers for polymerase chain reaction amplification of FAM161A exons.
(PDF)
Primers for Sanger sequencing of FAM161A exons.
(PDF)