Abstract
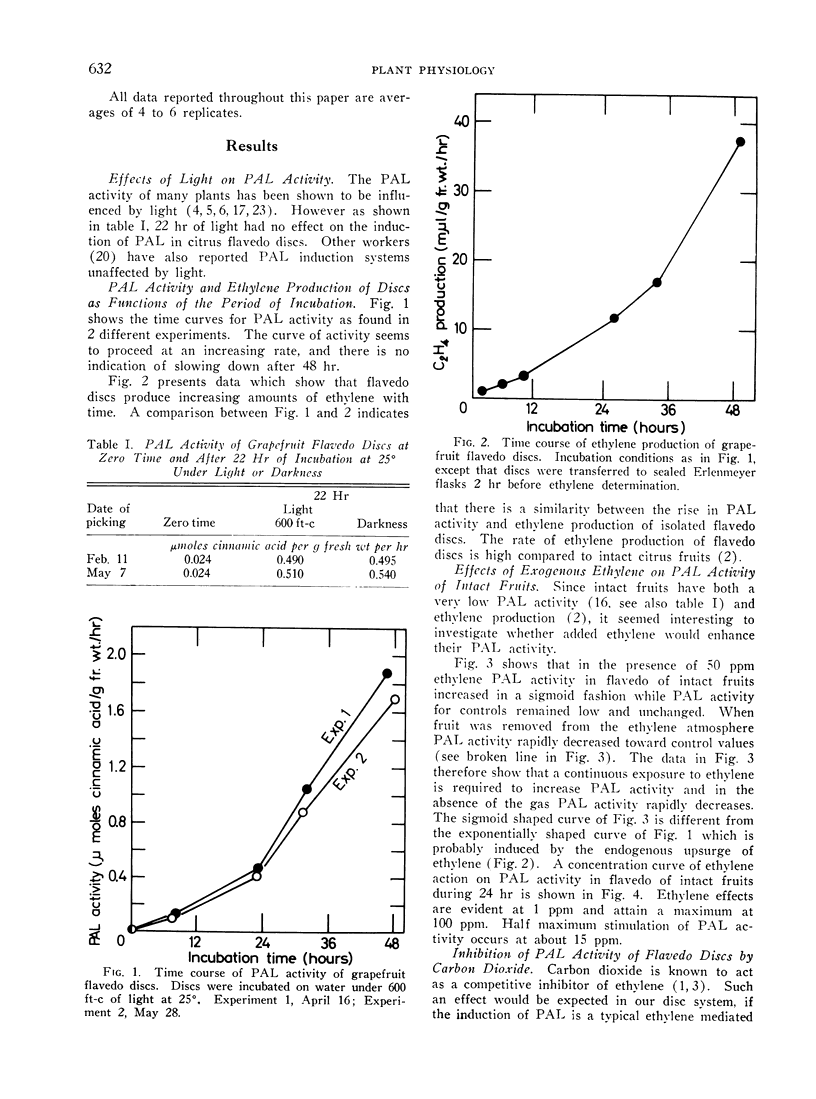
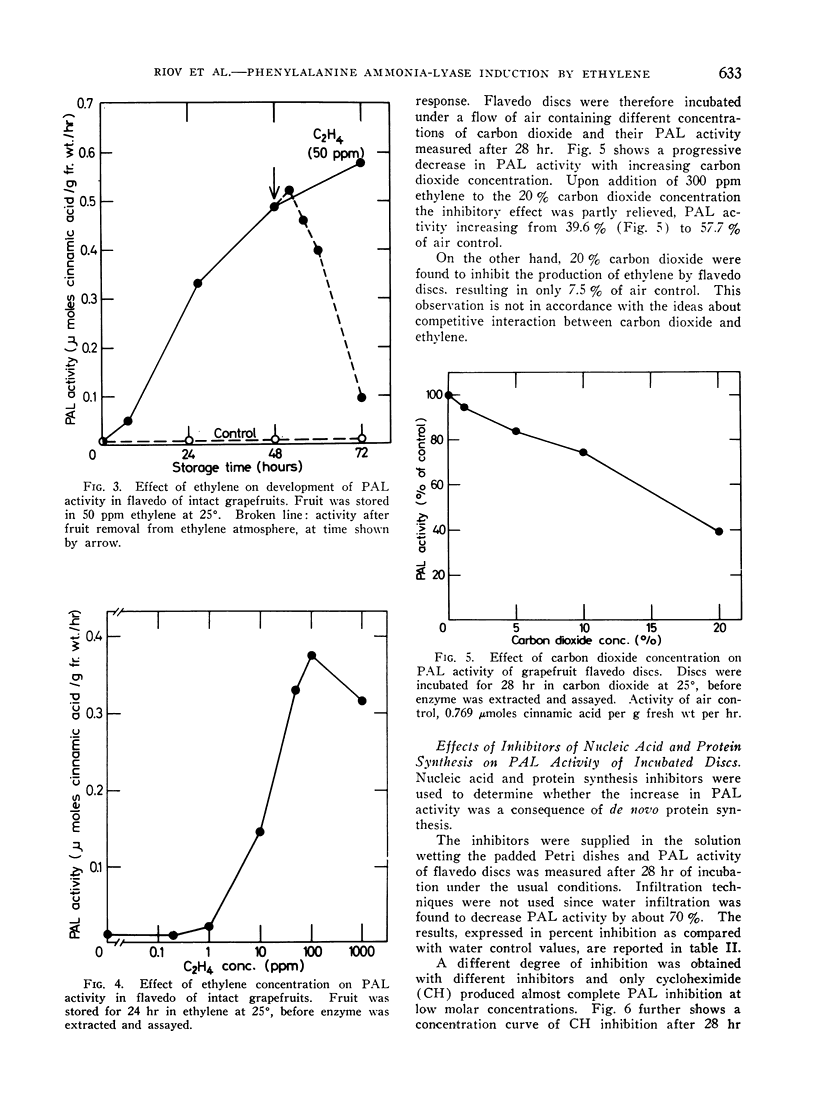
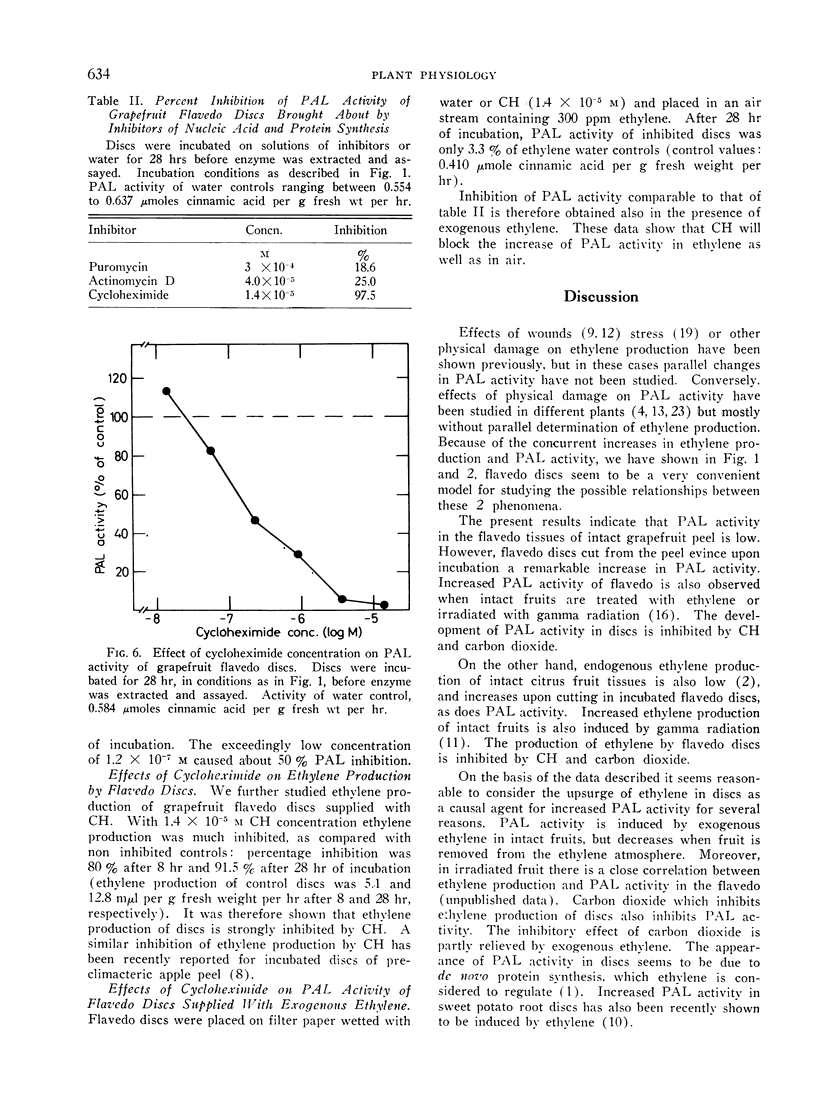
l-Phenylalanine ammonia-lyase (PAL) activity is low in the external layers (flavedo) of intact mature grapefruit peel. Flavedo discs evince upon incubation increasing PAL activity and ethylene production. Light has no effect in enhancing PAL activity in discs. Exogenous ethylene stimulates PAL activity in the flavedo of intact mature grapefruits (half maximum stimulation at 15 ppm); such activity rapidly decreases when fruit is removed from the ethylene containing atmosphere. Carbon dioxide inhibits both ethylene production and PAL activity of discs; exogenous ethylene only partly relieves PAL inhibition. Cycloheximide inhibits both PAL activity and ethylene production by flavedo discs. The same concentration of cycloheximide also inhibits PAL activity of discs in the presence of exogenous ethylene. Protein synthesis seems therefore to be needed at both levels of ethylene evolution and enhancement of PAL activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURG S. P., BURG E. A. ETHYLENE ACTION AND THE RIPENING OF FRUITS. Science. 1965 May 28;148(3674):1190–1196. doi: 10.1126/science.148.3674.1190. [DOI] [PubMed] [Google Scholar]
- Burg S. P., Burg E. A. Role of Ethylene in Fruit Ripening. Plant Physiol. 1962 Mar;37(2):179–189. doi: 10.1104/pp.37.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F., Mohr H. Half-life of phytochrome-induced phenylalanine deaminase in mustard seedlings (Sinapis alba L.). Naturwissenschaften. 1966 Dec;53(24):707–707. doi: 10.1007/BF00602735. [DOI] [PubMed] [Google Scholar]
- Engelsma G. Effect of cycloheximide on the inactivation of phenylalanine deaminase in gherkin seedlings. Naturwissenschaften. 1967 Jun;54(12):319–320. doi: 10.1007/BF00640619. [DOI] [PubMed] [Google Scholar]
- Maxie E. C., Eaks I. L., Sommer N. F., Rae H. L., El-Batal S. Effect of Gamma Radiation on Rate of Ethylene and Carbon Dioxide Evolution by Lemon Fruit. Plant Physiol. 1965 May;40(3):407–409. doi: 10.1104/pp.40.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minamikawa T., Uritani I. Phenylalanine ammonia-lyase in sliced sweet potato roots. J Biochem. 1965 May;57(5):678–688. [PubMed] [Google Scholar]
- Stahmann M. A., Clare B. G., Woodbury W. Increased disease resistance and enzyme activity induced by ethylene and ethylene production of black rot infected sweet potato tissue. Plant Physiol. 1966 Nov;41(9):1505–1512. doi: 10.1104/pp.41.9.1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walton D. C., Sondheimer E. Effects of Abscisin II on Phenylalanine Ammonia-Lyase Activity in Excised Bean Axes. Plant Physiol. 1968 Mar;43(3):467–469. doi: 10.1104/pp.43.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S. F. Biosynthesis of ethylene. Ethylene formation from methional by horseradish peroxidase. Arch Biochem Biophys. 1967 Nov;122(2):481–487. doi: 10.1016/0003-9861(67)90222-6. [DOI] [PubMed] [Google Scholar]
- Zucker M. Induction of Phenylalanine Deaminase by Light and its Relation to Chlorogenic Acid Synthesis in Potato Tuber Tissue. Plant Physiol. 1965 Sep;40(5):779–784. doi: 10.1104/pp.40.5.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker M. Sequential Induction of Phenylalanine Ammonia-lyase and a Lyase-inactivating System in Potato Tuber Disks. Plant Physiol. 1968 Mar;43(3):365–374. doi: 10.1104/pp.43.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]



