Abstract
New therapies are needed for metastatic breast cancer patients. Oncolytic herpes simplex virus (oHSV) is an exciting therapy being developed for use against aggressive tumors and established metastases. Although oHSV have been demonstrated safe in clinical trials, a lack of sufficient potency has slowed the clinical application of this approach. We utilized histone deacetylase (HDAC) inhibitors, which have been noted to impair the innate antiviral response and improve gene transcription from viral vectors, to enhance the replication of oHSV in breast cancer cells. A panel of chemically diverse HDAC inhibitors were tested at three different doses (<, = , and >LD50) for their ability to modulate the replication of oHSV in breast cancer cells. Several of the tested HDAC inhibitors enhanced oHSV replication at low multiplicity of infection (MOI) following pre-treatment of the metastatic breast cancer cell line MDA-MB-231 and the oHSV-resistant cell line 4T1, but not in the normal breast epithelial cell line MCF10A. Inhibitors of class I HDACs, including pan-selective compounds, were more effective for increasing oHSV replication compared to inhibitors that selectively target class II HDACs. These studies demonstrate that select HDAC inhibitors increase oHSV replication in breast cancer cells and provides support for pre-clinical evaluation of this combination strategy.
Introduction
The metastasis of breast cancer to distant organs remains the most challenging aspect for the clinical management of this disease. As the five-year survival rate for patients with distant metastases at the time of diagnosis is less than 25% [1], it is clear that new treatments are needed for metastatic breast cancer. Existing therapies are limited in their effectiveness and can cause undesired side effects. As examples, two recent studies have underscored the long term risks of heart disease posed to breast cancer patients treated with either trastuzumab or anthracycline chemotherapeutics [2] as well as radiotherapy [3]. In contrast, oncolytic viruses have been proposed as a therapy that potentially avoids these long term risks due to their ability to selectively replicate in and destroy tumor cells while sparing normal cells [4]. Among the many oncolytic viruses under investigation, oncolytic herpes simplex virus (oHSV) has several advantages and is one of the most well studied [5]. While much of the initial interest in oHSV focused on its use as a therapy for brain tumors, an increasing number of preclinical studies have demonstrated that oHSVs can be effective against a variety of tumor types, including breast cancer. The safety of this approach has been established for several different cancers. However, these clinical trials have also illustrated the need for greater antitumor efficacy. For this reason, there is growing interest in the combination of oHSV with other treatment modalities, such as radiotherapy or chemotherapy, in an effort to enhance viral efficacy [6], [7].
Many cancers, including breast cancer, exhibit aberrant histone deacetylase (HDAC) expression or activity. HDAC inhibitors have been found to exert multiple antitumor effects, paving the way for clinical trials of these agents in several cancers, including breast cancer [8]. Two inhibitors, vorinostat and romidepsin, have recently been approved by the U.S. Food and Drug Administration (FDA) for the treatment of cutaneous T-cell lymphomas. Of particular interest to the field of oncolytic virotherapy, it has been recognized that HDAC inhibitors can also suppress expression of interferon response genes [9]. oHSV is commonly generated through deletion of the diploid γ134.5 gene, which attenuates the virus in non-cycling cells. The γ134.5 gene encodes the primary neurovirulence factor [10], which enables the virus to overcome the host cell's protein kinase R-mediated block of late viral protein translation and also contributes to the ability of HSV to inhibit host cell interferon response. Consequently, it has been shown in a limited number of studies that HDAC inhibitors may have the ability to improve oHSV virotherapy [11]–[13].
In this report, we screened a panel of HDAC inhibitors comprising several different chemical classes for their potential to augment the replication of oHSV in breast cancer cells. Because many of these inhibitors have not been tested in the cell lines used in this study, we first determined LD50 values for each inhibitor. Viral replication was assessed by pre- and co-treatment with the HDAC inhibitors at concentrations greater than, less than, and near their LD50 and at both high and low multiplicity of infection (MOI). Select HDAC inhibitors improved oHSV replication in the cancer cells but not in normal cells. Because many of these HDAC inhibitors and oHSV constructs are being evaluated in clinical trials, this combination may be an effective strategy for the treatment of metastatic breast cancer.
Materials and Methods
Chemicals
HDAC inhibitors ( Table 1 ) were purchased from the following: belinostat (Cat. No. S1085; CAS No. 414864-00-9, Selleck Chemicals, Houston, TX), entinostat (E-3866; CAS No. 209783-80-2) and panobinostat (P-3703; CAS No. 404950-80-7, LC Laboratories, Woburn, MA), and remaining compounds were from Sigma-Aldrich, St. Louis, MO: APHA (3-(4-aroyl-1H-2-pyrrolyl)-N-hydroxypropenamide) compound 8 (A2478; CAS 676599-90-9), MC1568 (M1824; CAS No. 852475-26-4), 1-naphthohydroxamic acid (SML0078; CAS 6953-61-3), SAHA (suberoylanilide hydroxamic acid, Vorinostat; SML0061; CAS 149647-78-9), sodium butyrate (B5887; CAS No. 156-54-7), trichostatin A (T-8552; CAS No. 58880-19-6), tubastatin A hydrochloride (SML0044; CAS 1310693-92-5), valproic acid (PHR1061; CAS 99-66-1). Valproic acid was supplied as a solution whereas all other inhibitors were supplied in powdered form. A concentrated stock solution of sodium butyrate was prepared in sterile water. Concentrated stock solutions of the remaining inhibitors were prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich).
Table 1. HDAC Inhibitors Used in This Study.
| Inhibitor | Chemical type | Selectivity | Potency |
| APHA Compound 8 (APHA 8) | hydroxamic acid | Class I | μM |
| Belinostat (BEL), PDX101 | hydroxamic acid | Pan | μM |
| Entinostat (ENT), MS-275 | benzamide | Class I1 | μM |
| MC1568 | hydroxamic acid | Class II | nM |
| 1-Naphtholhydroxamic Acid (1NHA) | hydroxamic acid | HDAC 8 | μM |
| Panobinostat (PAN), LBH-589 | hydroxamic acid | Pan | nM |
| Sodium Butyrate (NaB) | short chain fatty acid | Class I, IIa | mM |
| Suberoylanilide Hydroxamic Acid (SAHA), Vorinostat | hydroxamic acid | Pan | μM |
| Trichostatin A (TSA) | hydroxamic acid | Pan | nM |
| Tubastatin A (TBSA) | benzamide | HDAC 6 | nM |
| Valproic Acid (VPA) | short chain fatty acid | Class I, IIa | mM |
Also inhibits the Class IIa enzyme HDAC 9.
Cells and Viruses
The human metastatic breast cancer cell line MDA-MB-231 was described previously [14]. MCF10A is an immortalized human mammary epithelial cell line described previously [15]. The 4T1 murine mammary carcinoma cell line and the Vero African Green Monkey kidney cell line were obtained from American Type Culture Collection (Manassas, VA). MDA-MB-231 and 4T1 cells were maintained in a 1∶1 (v/v) mixture of Dulbecco's modified Eagle medium and Ham's F12 (DMEM/F12; Life Technologies, Carlsbad, CA) supplemented with 2 mM L-glutamine (Life Technologies) and 5% v/v FBS (Life Technologies). MCF10A cells were maintained in DMEM/F12 supplemented with 5% FBS, 10 ng/ml human epidermal growth factor, 100 ng/ml cholera toxin, 10 μg/ml insulin, 500 ng/ml hydrocortisone (Sigma-Aldrich), 2 mM L-glutamine (Life Technologies), and 1X non-essential amino acids (Life Technologies). Vero cells were maintained in Eagle's minimum essential medium, alpha modification (α-MEM; Sigma-Aldrich) supplemented with 7% FBS, 2 mM L-glutamine, 100 U/ml penicillin, and 100 μg/ml streptomycin (Mediatech, Manassas, VA).
M002 is a genetically-engineered human herpes simplex virus (HSV) that is derived from the HSV-1 (F) clinical isolate. M002 lacks both copies of the γ134.5 neurovirulence gene and expresses murine interleukin 12 (IL-12) under the early growth response-1 promoter, and has been described previously [16].
Cell viability assays
MDA-MB-231, 4T1 and MCF10A were seeded in 96-well plates at 1000 cells per well. Working dilutions of the HDAC inhibitors were prepared in DMEM/F12, 5% FBS and each dilution was added to the cells in triplicate, with five dilutions tested per inhibitor. The cells were allowed to incubate for 3 days and viability was assessed by a 2 hour incubation with AlamarBlue reagent (Life Technologies), according to the manufacturer's instructions. Fluorescence was determined at 570/580 nm excitation/emission with a Hitachi F-7000 fluorescence spectrophotometer. Dose-response curves and the dose lethal to 50% of the cells (LD50) were calculated using SigmaPlot 10.0 software.
Viral replication assays
MDA-MB-231, 4T1, and MCF10A cells were seeded in 24-well plates at 50,000 cells/well and treated with HDAC inhibitors either 6 hours prior to (pre-treatment) or immediately following (co-treatment) viral infection. Inhibitors were diluted as above and added to the media at low (<LD50), middle (near LD50) and high (>LD50) doses ( Table 2 ). Cells were infected with M002 at a multiplicity of infection (MOI) of 0.1 and 10 plaque-forming units (PFU) per cell as previously described [17]. Briefly, cells were rinsed with PBS, infected with M002 diluted in DMEM/F12 with 1% FBS for 2 hours. At 48 hours post-infection, cells and media were harvested and subjected to three freeze/thaw cycles followed by sonication. This time point was chosen to assay replication at the maximum level. Vero cells were then infected with serial dilutions of each sample. After 48 hours, plaques were stained with 1% w/v crystal violet (Sigma-Aldrich) in 70% ethanol and quantified. The titers from the HDAC inhibitor-treated samples were normalized to infected, untreated cells and expressed as fold change +/- the standard error.
Table 2. Doses of HDAC Inhibitors Used in Viral Replication Experiments.
| LOW | MID | HIGH | |
| APHA 8 | 1 μM | 10 μM | 50 μM |
| BEL | 0.01 μM | 0.25 μM | 1 μM |
| ENT | 0.1 μM | 1 μM | 5 μM |
| MC1568 | 10 μM | 50 μM | 100 μM |
| NaB | 0.1 mM | 1 mM | 10 mM |
| 1-NHA | 10 μM | 50 μM | 100 μM |
| PAN | 5 nm | 10 nm | 100 nm |
| SAHA | 0.1 μM | 1 μM | 10 μM |
| TBSA | 1 μM | 50 μM | 100 μM |
| TSA | 0.1 μM | 0.25 μM | 1 μM |
| VPA | 1 mM | 10 mM | 50 mM |
Results
HDAC inhibitor LD50 values in breast cancer cells
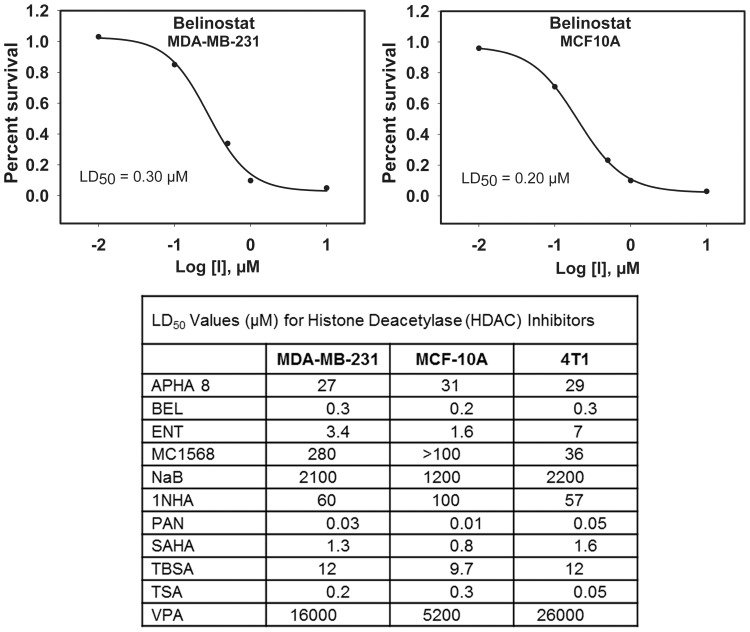
Prior to analyzing the effect of HDAC inhibitors on the replication of oHSV, it was first necessary to determine LD50 values since those have not been reported in the breast cancer cells used in this study for most of the inhibitors. We obtained a panel of inhibitors representing several different chemical classes, HDAC selectivities, and potencies ( Table 1 ). An emphasis was placed on compounds currently under clinical investigation or in clinical use. LD50 values were calculated for each inhibitor in MDA-MB-231, 4T1 and MCF10A cell lines ( Figure 1 ). Generally, the LD50 values were similar in all three cell lines and for most compounds were in the micromolar range. Three inhibitors, BEL, PAN, and TSA (all hydroxamic acids), yielded LD50 values in the submicromolar range. In contrast, the LD50 values for the two short chain fatty acids (NaB and VPA) were in the millimolar range.
Figure 1. Approximate LD50 values determined for a panel of HDAC inhibitors in breast cancer cells.
Proliferating breast cancer (MDA-MB-231), murine mammary carcinoma (4T1) and normal breast epithelial (MCF10A) cells were treated with a panel of histone deacetylase inhibitors at a range of concentrations, and cell viability was assessed after three days. Shown are representative dose-response curves for MDA-MB-231 and MCF10A cells treated with belinostat (upper panels) and a table of approximate LD50 values calculated from dose-response curves for the entire panel of inhibitors.
Pre-treatment of breast cancer cells with HDAC inhibitors enhances the replication of oHSV
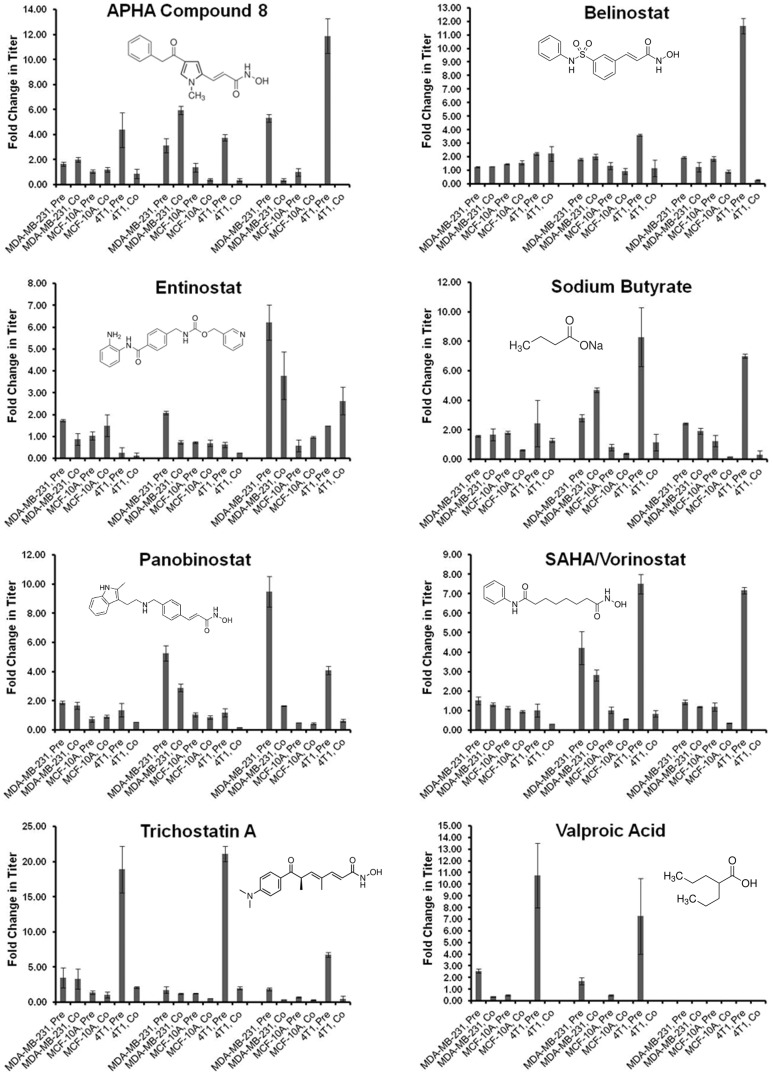
Having determined LD50 values for our panel of HDAC inhibitors, we then sought to examine how treatment of breast cancer cells with these inhibitors modulated the replication of the γ134.5-deleted oHSV M002. The inhibitors were added to the cells at three different doses: a low dose (<LD50), middle dose (near the LD50) and a high dose (>LD50), as listed in Table 2 . The same doses were used for all cell lines since the LD50 values were comparable. Two treatment schemes were utilized: six hours prior to viral infection (designated pre-treatment) and immediately following viral infection (co-treatment). After 48 hours, cells with media were harvested and the titers of plaque-forming units per ml were determined by standard plaque assay. Increases in replication were calculated as the fold change in titer of HDAC inhibitor-treated samples relative to infected, untreated cells (titers averaged 1.5×107 and 1.4×105 PFU/ml for MDA-MB-231 and 4T1, respectively). HDAC inhibitor treatment did not increase replication in cells infected at a high MOI (10 PFU/cell; data not shown). However, in cells infected at low MOI (0.1 PFU/cell), examination of fold changes revealed a number of trends. Most of the tested compounds increased replication at least 2 fold in the MDA-MB-231 cells, whereas replication in the normal breast cell line was not significantly affected (fold changes +/− standard error are given for all inhibitors in Table S1). In the MDA-MB-231 cells, some inhibitors enhanced replication as both a pre-treatment and a co-treatment (low dose: TSA; mid dose: APHA8, NaB, PAN, SAHA; high dose: ENT), but with the exception of APHA8 and NaB (mid dose) the highest magnitude of increase for each of these inhibitors was obtained by pre-treatment. Additionally, for some inhibitors (such as ENT, VPA, TBSA, 1NHA), replication was reduced by co-treatment at certain doses.A few compounds were particularly effective at increasing replication in the MDA-MB-231 cells, with fold changes greater than 5 for APHA8 (mid, high doses), ENT (high dose), and PAN (mid, high doses; Figure 2 ). The greatest increase (approximately 1 log) in viral replication was obtained by pre-treatment with PAN at the highest dose.
Figure 2. HDAC inhibitors enhance oHSV replication in breast cancer cells, but not in normal breast epithelial cells.
MDA-MB-231 human breast cancer, MCF10A normal breast epithelial, and 4T1 murine mammary carcinoma cells were treated with the indicated HDAC inhibitors either 6 hours prior (Pre-) or immediately following (Co-) infection with M002 oHSV at 0.1 PFU/cell. Shown are fold changes in viral titer versus replication in untreated cells, at 48 hours post infection. From left to right, the three sets of bars within each graph indicate inhibitor concentrations below LD50, near LD50 and above LD50.
HDAC inhibitors enhance the replication of oHSV in an HSV-resistant cancer cell line
In a previous study, we have shown that while other mouse carcinoma cell lines are similarly permissive for oHSV as human cancer cells, the aggressive murine mammary carcinoma 4T1 cell line is relatively resistant to γ134.5-deleted oHSVs [18], a result that has also been shown by others [19]. Having observed that HDAC inhibitor treatment enhanced oHSV replication in tumor cells but not normal cells, we postulated that HDAC inhibitor treatment might make an oHSV-resistant line more susceptible to viral replication. We selected 4T1 as a representative oHSV-resistant carcinoma cell line. With the exception of MC1568, all of the inhibitors increased oHSV replication in the 4T1 cells, particularly with pre-treatment at the mid and high doses ( Figure 2 and Table S1). In fact, the increases were more pronounced than those observed in the MDA-MB-231 cells. As with the MDA-MB-231 cells, pre-treatment yielded the highest magnitude in fold increase for most of the effective compounds (with the exception of ENT) whereas co-treatment frequently yielded reduced replication at the mid and high doses in the 4T1 cells. Many of the HDAC inhibitors tested yielded increases in replication greater than 5 fold (low dose: TSA, VPA; mid dose: NaB, SAHA, TSA, VPA; high dose: APHA8, BEL, NaB, SAHA, TSA) and several yielded increases greater than 10 fold (APHA 8, BEL, TSA, VPA), with the highest increase (>20 fold) obtained by TSA at the mid dose. Overall, these data indicate that select HDAC inhibitor treatment can render an oHSV-resistant cell line more susceptible to viral replication.
Discussion
There remains an urgent need for more effective therapies for metastatic breast cancer. Here, we have investigated the potential utility of combining two developing therapeutics: oHSV and HDAC inhibitors. We have determined LD50 values for a panel of inhibitors that includes compounds belonging to several different chemical classes to directly compare cell death in metastatic breast cancer versus normal mammary epithelial cells. In general, the LD50 values were in the same range for all of the cell lines tested. Several of the inhibitors have not been previously tested in breast cancer cells, and a comprehensive comparison of multiple compounds in these cells has not previously been reported.
In the viral replication experiments, we characterized the ability of HDAC inhibitors to enhance the replication of γ134.5-deleted oHSV in breast cancer cells. Although oHSVs with a variety of mutations have been described, those based on mutation of the γ134.5 gene have advanced the furthest in clinical testing. We selected the γ134.5-deleted oHSV M002 due to its enhanced antitumor potency versus non-cytokine oHSVs such as G207 [20], [21], which has been evaluated in Phase I [22] and Phase Ib [23] clinical trials. M002 expresses murine IL-12 to promote an adaptive antitumor immune response and carries a wild type ribonucleotide reductase gene, enabling more efficient replication in cancer cells than G207 [21]. All of the inhibitors in this study mediated at least modest (>2 fold) increases in viral replication in the cancer cell lines, although for some compounds this increase may have been within the range of titration error. The most effective compounds (fold changes >5) were broad-spectrum inhibitors APHA8, BEL, ENT, NaB, PAN, SAHA, TSA, and VPA. In contrast, the least effective inhibitors included the isoform specific compounds MC1568 (Class II HDACs), 1NHA (HDAC 8), and TBSA (HDAC 6).Although not conclusive, these data suggest that broad-spectrum HDAC inhibitors, particularly those that inhibit Class I HDACs, are likely to be more useful than class-specific compounds at enhancing oHSV replication. However, mechanistic studies will be needed to determine which individual HDAC(s) might be the most critical for inhibition.
The most detailed studies of HDAC inhibitors used in combination with oHSV have been in the context of glioma [12], [13], [24], colon cancer [12] and squamous cell carcinoma [11]. The potential for use against breast cancer has not been extensively explored, although Liu et al. reported an additive cytotoxic effect of TSA in combination with oHSV in the MCF-7 cell line that was not due to increased viral replication [12]. In those studies, detailed analyses have only been conducted with TSA [11]–[13] and VPA [13], [24]. The replication of other oncolytic viruses including vesicular stomatitis virus [25], vaccinia virus [25], [26] and others [27] have also been shown to be enhanced by some HDAC inhibitors.
In this study, we provide further evidence that the timing of administration is important, as pre-treatment was found to yield higher increases in viral replication than co-treatment. Similarly, Otsuki et al. report that pre-treatment with VPA enhances viral replication, but co-treatment does not [13]. Katsura et al. demonstrated that co-treatment with TSA enhanced replication at 24 hours post-infection but gave no benefit at 12 or 36 hours, and did not examine pre-treatment [11]. The effectiveness of particular treatment schedules may also be cell line specific. In our study, treatment schedule appeared to have little influence on enhancement of viral replication in the TSA-treated MDA-MB-231 cells, but greatly influenced replication in the oHSV-resistant 4T1 cells.
For some HDAC inhibitors, viral replication was reduced to the extent that no plaques were detected at the dilutions assayed (resulting in “not determined”, Table S1). This may have been the result of cell death limiting viral replication, since this was most often seen with co-treatment at the mid and high doses. Nonetheless, increased replication was observed for some inhibitors even at concentrations above the LD50 (particularly for pre-treatment). This is presumably because a dose sufficient to kill cells over a 72 hour treatment (as in the determination of the LD50 values) is not lethal within the time frame of the viral replication experiments (48 h). Although co-treatment lead to decreased replication in some cases, it is possible that synergistic cell death from combined oHSV/HDAC inhibitor treatment would result in enhanced antitumor effect in vivo. Whether pre-treatment or co-treatment is more advantageous therapeutically will have to be determined in an in vivo system, and may depend on the inhibitor used.
Work by other investigators has identified several mechanisms by which HDAC inhibitors enhance the antitumor efficacy of oHSV, both at the cellular level and at the level of the tumor microenvironment (reviewed by Nguyen et al. [27]). In a glioma model, VPA was shown to enhance oHSV gene expression and inhibit the expression of interferon-responsive genes, enabling increased viral replication [13]. Studies focused on TSA in combination with oHSV have shown that a reduction in cyclin D1 levels [12] and enhancement of nuclear factor kappaB (NFκB) activation via p65 acetylation [11] are also contributing factors. Additional studies are needed to identify other potential mechanisms, such as changes in the efficiency of infection or modification of viral genome-associated histones.
Two HDAC inhibitors (TSA and VPA) have been tested in vivo with oHSV [12], [13]. In murine models of both glioma and colon cancer, the combination of oHSV with TSA and VPA, respectively, has been shown to enhance antitumor effect over oHSV alone. Because HDAC inhibitors exert a variety of antitumor effects independent of their effect on oncolytic viral replication, and because the combination of these two treatment strategies can yield synergistic effects, this could allow for the administration of lower inhibitor doses, thereby avoiding the toxicities of HDAC inhibitors as a monotherapy.
One potential concern is that HDAC inhibitors might mitigate the cancer-selective replication of an oHSV and enable replication in otherwise non-permissive normal cells. However, our results show that HDAC inhibitor treatment did not enhance replication of M002 in the MCF10A normal mammary epithelial cells. A lack of increased oHSV replication has also been noted in primary prostate epithelial cells and quiescent endothelial cells [12] as well as normal human astrocytes [13]. It is unclear why HDAC inhibitors selectively increase oHSV replication in cancer cells but not normal cells, but tumor cell reliance on aberrant HDAC activity or the full complement of antiviral pathways active in normal cells are two possibilities. An additional safety concern is that HDAC inhibitor treatment might enhance the replication of wild-type HSV. Liu et al. showed that the combination of TSA and wild-type HSV yielded no effect on viral replication in quiescent cells or normal primary prostate epithelial cells [12]. Additionally, mice given intracranial wild-type HSV showed no enhancement of encephalitis by VPA administration [24]. In additional experiments, we have similarly observed that wild-type HSV replication was not enhanced by HDAC inhibitors in MCF10A cells, although it was increased in the MDA-MB-231 and 4T1 cell lines (data not shown). Overall, these results indicate that the use of HDAC inhibitors is unlikely to compromise the excellent safety record of oHSV.
In conclusion, we have shown that HDAC inhibitors can be used to increase the replication of an oHSV in breast cancer cells without increasing replication in normal breast epithelial cells, and we have shown that an oHSV-resistant tumor cell line can be made more susceptible to oHSV replication. The magnitude of replication increase is dependent upon cell line, treatment schedule, viral MOI, and inhibitor dose. We did not observe that a particular chemical class of inhibitor was uniformly more effective than others, nor did we observe that a single inhibitor was the most effective in both cancer cell lines. Rather, our data suggest that the selectivity profile of the inhibitor is the most important determinant in how well oHSV replication is enhanced. Our data indicate that broad spectrum inhibitors or those that inhibit Class I HDACs in particular are more effective for increasing viral replication than selective inhibitors targeting class II HDACs ( Table 3 ). Of the inhibitors we tested, 8 increased oHSV replication >5 fold. Of these, 5 are currently being evaluated in clinical trials for breast cancer (BEL, PAN, SAHA, ENT, VPA). This is encouraging for the purposes of clinical relevance, since it provides several potential combinations to pursue.
Table 3. Summary of Increased oHSV Replication in Cancer Cell Lines Pre-treated with HDAC Inhibitors.
| Inhibitor Selectivity | Compound | Clinical Status1 | Replication Increase | ||||
| MDA-MB-231 | 4T1 | ||||||
| MID | HIGH | MID | HIGH | ||||
| Pan | BEL | Phase I | − | − | + | +++ | |
| PAN | Phase II | ++ | ++ | − | + | ||
| SAHA2 | Phase II | + | − | ++ | ++ | ||
| TSA | Preclinical | − | − | +++ | ++ | ||
| Class I | APHA 8 | Preclinical | ++ | ++ | + | +++ | |
| ENT3 | Phase II | + | ++ | − | + | ||
| Class I and IIa | VPA | Phase II | − | − | ++ | − | |
| NaB | Preclinical | + | + | ++ | ++ | ||
| Class II | MC1568 | Preclinical | − | − | − | − | |
| HDAC 6 (Class IIb) | TBSA | Preclinical | + | − | − | + | |
| HDAC 8 (Class I) | 1-NHA | Preclinical | − | − | − | + | |
No increase (-) or increases in replication of >2 fold (+), >5 fold (++) and >10 fold (+++) are shown.
For breast cancer.
Clinically approved for the treatment of cutaneous T cell lymphoma.
Also inhibits the Class IIa enzyme HDAC 9.
Supporting Information
Fold changes in viral titer (+/− standard error) in cell lines pre-treated or co-treated with the indicated HDAC inhibitors, normalized to titer from untreated cells.
(DOCX)
Acknowledgments
The authors would like to thank Jennifer M. Coleman and the members of the Markert lab for providing the viral preparations used in this study. We would also like to thank the members of the Hurst lab for valuable discussions in the preparation of this manuscript.
Funding Statement
This work was supported in part by funding from the American Cancer Society RSG-11-259-01-CSM (DRH), METAvivor Research and Support, Inc. (DRH), and the National Institutes of Health P50 CA097247 and P01 CA71933 (JMM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Siegel R, Naishadham D, Jemal A (2013) Cancer statistics, 2013. CA Cancer J Clin 63: 11–30 10.3322/caac.21166 [doi] [DOI] [PubMed] [Google Scholar]
- 2. Bowles EJ, Wellman R, Feigelson HS, Onitilo AA, Freedman AN, et al. (2012) Risk of heart failure in breast cancer patients after anthracycline and trastuzumab treatment: a retrospective cohort study. J Natl Cancer Inst 104: 1293–1305 djs317 [pii]; 10.1093/jnci/djs317 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Darby SC, Ewertz M, McGale P, Bennet AM, Blom-Goldman U, et al. (2013) Risk of ischemic heart disease in women after radiotherapy for breast cancer. N Engl J Med 368: 987–998 10.1056/NEJMoa1209825 [doi] [DOI] [PubMed] [Google Scholar]
- 4. Russell SJ, Peng KW, Bell JC (2012) Oncolytic virotherapy. Nat Biotechnol 30: 658–670 nbt.2287 [pii]; 10.1038/nbt.2287 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Campadelli-Fiume G, De GC, Gatta V, Nanni P, Lollini PL, et al. (2011) Rethinking herpes simplex virus: the way to oncolytic agents. Rev Med Virol 21: 213–226 10.1002/rmv.691 [doi] [DOI] [PubMed] [Google Scholar]
- 6. Kanai R, Wakimoto H, Cheema T, Rabkin SD (2010) Oncolytic herpes simplex virus vectors and chemotherapy: are combinatorial strategies more effective for cancer? Future Oncol 6: 619–634 10.2217/fon.10.18 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ottolino-Perry K, Diallo JS, Lichty BD, Bell JC, McCart JA (2010) Intelligent design: combination therapy with oncolytic viruses. Mol Ther 18: 251–263 mt2009283 [pii]; 10.1038/mt.2009.283 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Smith KT, Workman JL (2009) Histone deacetylase inhibitors: anticancer compounds. Int J Biochem Cell Biol 41: 21–25 S1357-2725(08)00387-7 [pii]; 10.1016/j.biocel.2008.09.008 [doi] [DOI] [PubMed] [Google Scholar]
- 9. Chang HM, Paulson M, Holko M, Rice CM, Williams BR, et al. (2004) Induction of interferon-stimulated gene expression and antiviral responses require protein deacetylase activity. Proc Natl Acad Sci U S A 101: 9578–9583 10.1073/pnas.0400567101 [doi];0400567101 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chou J, Kern ER, Whitley RJ, Roizman B (1990) Mapping of herpes simplex virus-1 neurovirulence to gamma 134.5, a gene nonessential for growth in culture. Science 250: 1262–1266. [DOI] [PubMed] [Google Scholar]
- 11. Katsura T, Iwai S, Ota Y, Shimizu H, Ikuta K, et al. (2009) The effects of trichostatin A on the oncolytic ability of herpes simplex virus for oral squamous cell carcinoma cells. Cancer Gene Ther 16: 237–245 cgt200881 [pii]; 10.1038/cgt.2008.81 [doi] [DOI] [PubMed] [Google Scholar]
- 12. Liu TC, Castelo-Branco P, Rabkin SD, Martuza RL (2008) Trichostatin A and oncolytic HSV combination therapy shows enhanced antitumoral and antiangiogenic effects. Mol Ther 16: 1041–1047 mt200858 [pii]; 10.1038/mt.2008.58 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Otsuki A, Patel A, Kasai K, Suzuki M, Kurozumi K, et al. (2008) Histone deacetylase inhibitors augment antitumor efficacy of herpes-based oncolytic viruses. Mol Ther 16: 1546–1555 mt2008155 [pii]; 10.1038/mt.2008.155 [doi] [DOI] [PubMed] [Google Scholar]
- 14. Hurst DR, Xie Y, Vaidya KS, Mehta A, Moore BP, et al. (2008) Alterations of BRMS1-ARID4A interaction modify gene expression but still suppress metastasis in human breast cancer cells. J Biol Chem 283: 7438–7444 M709446200 [pii]; 10.1074/jbc.M709446200 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hurst DR, Xie Y, Edmonds MD, Welch DR (2009) Multiple forms of BRMS1 are differentially expressed in the MCF10 isogenic breast cancer progression model. Clin Exp Metastasis 26: 89–96 10.1007/s10585-008-9216-9 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, et al. (2000) Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci U S A 97: 2208–2213 10.1073/pnas.040557897 [doi] 040557897 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Andreansky S, He B, van Cott J, McGhee J, Markert JM, et al. (1998) Treatment of intracranial gliomas in immunocompetent mice using herpes simplex viruses that express murine interleukins. 1998/04/16: 121–130 10.1038/sj.gt.3300550. [DOI] [PubMed] [Google Scholar]
- 18. Cody JJ, Scaturro P, Cantor AB, Yancey GG, Parker JN, et al. (2012) Preclinical evaluation of oncolytic deltagamma(1)34.5 herpes simplex virus expressing interleukin-12 for therapy of breast cancer brain metastases. Int J Breast Cancer 2012: 628697 10.1155/2012/628697 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thomas DL, Fraser NW (2003) HSV-1 therapy of primary tumors reduces the number of metastases in an immune-competent model of metastatic breast cancer. 8: : 543–551. S1525001603002363 [pii]. [DOI] [PubMed] [Google Scholar]
- 20. Hellums EK, Markert JM, Parker JN, He B, Perbal B, et al. (2005) Increased efficacy of an interleukin-12-secreting herpes simplex virus in a syngeneic intracranial murine glioma model. Neuro Oncol 7: 213–224 10.1215/S1152851705000074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Markert JM, Cody JJ, Parker JN, Coleman JM, Price KH, et al. (2012) Preclinical evaluation of a genetically engineered herpes simplex virus expressing interleukin-12. J Virol 86: : 5304–5313. JVI.06998-11 [pii] 10.1128/JVI.06998-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Markert JM, Medlock MD, Rabkin SD, Gillespie GY, Todo T, et al. (2000) Conditionally replicating herpes simplex virus mutant, G207 for the treatment of malignant glioma: results of a phase I trial. Gene Ther 7: 867–874 10.1038/sj.gt.3301205. [DOI] [PubMed] [Google Scholar]
- 23.Markert JM, Liechty PG, Wang W, Gaston S, Braz E, et al. (2009) Phase Ib trial of mutant herpes simplex virus G207 inoculated pre-and post-tumor resection for recurrent GBM. Mol Ther 17: : 199–207. mt2008228 [pii] 10.1038/mt.2008.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Alvarez-Breckenridge CA, Yu J, Price R, Wei M, Wang Y, et al. (2012) The histone deacetylase inhibitor valproic acid lessens NK cell action against oncolytic virus-infected glioblastoma cells by inhibition of STAT5/T-BET signaling and generation of gamma interferon. J Virol 86: 4566–4577 JVI.05545-11 [pii]; 10.1128/JVI.05545-11 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Nguyen TL, Abdelbary H, Arguello M, Breitbach C, Leveille S, et al. (2008) Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc Natl Acad Sci U S A 105: 14981–14986 0803988105 [pii]; 10.1073/pnas.0803988105 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacTavish H, Diallo JS, Huang B, Stanford M, Le BF, et al. (2010) Enhancement of vaccinia virus based oncolysis with histone deacetylase inhibitors. PLoS One 5: e14462 10.1371/journal.pone.0014462 [doi] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Nguyen TL, Wilson MG, Hiscott J (2010) Oncolytic viruses and histone deacetylase inhibitors—a multi-pronged strategy to target tumor cells. Cytokine Growth Factor Rev 21: 153–159 S1359-6101(10)00027-4 [pii]; 10.1016/j.cytogfr.2010.03.002 [doi] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fold changes in viral titer (+/− standard error) in cell lines pre-treated or co-treated with the indicated HDAC inhibitors, normalized to titer from untreated cells.
(DOCX)