Abstract
Non-human primates (NHPs) are used to model human disease owing to their remarkably similar genomes, physiology, and immune systems. Recently, there has been an increased interest in modeling tuberculosis (TB) in NHPs. Macaques are susceptible to infection with different strains of Mycobacterium tuberculosis (Mtb), producing the full spectrum of disease conditions, including latent infection, chronic progressive infection, and acute TB, depending on the route and dose of infection. Clearly, NHPs are an excellent model of human TB. While the initial aim of the NHP model was to allow preclinical testing of candidate vaccines and drugs, it is now also being used to study pathogenesis and immune correlates of protection. Recent advances in this field are discussed in this review. Key questions such as the effect of hypoxia on the biology of Mtb and the basis of reactivation of latent TB can now be investigated through the use of this model.
Keywords: latent, Mycobacterium tuberculosis, non-human primate, reactivation, TB/AIDS coinfection
Global impact of TB
Tuberculosis (TB) is a major infectious disease of mankind, annually causing about 1.5 million deaths [1]. The situation is worsening with an increase in drug-resistant Mtb and the spectacular failure of the global TB vaccination strategy [2, 3]. Rampant coinfection with HIV, the causative agent of AIDS, is also believed to have contributed to a global resurgence in TB cases [4]. Mtb is a highly successful human pathogen and infects over 1/3 of the human population. However, a great majority of humans exposed to Mtb are able to immunologically contain infection in a latent state. With waning immune response, however, latent TB can be reactivated. It is believed that coinfection with HIV contributes to a significant increase in the number of reactivation TB cases [5].
Experimental models of TB
There are no natural hosts of Mtb other than humans. As humans cannot be knowingly exposed to Mtb, numerous experimental models of TB have been employed. Historically, TB was studied in guinea pigs since the times of Koch in the 19th century [6]. Infected guinea pigs produce symptoms and pathology similar to humans and can be used to model chemotherapy and vaccination. However, these animals exhibit exquisite susceptibility to the disease [7]. Rabbits have also been employed as models of TB [8]. Once again, this model faithfully represents the pathophysiological aspects of TB infections, in particular the study of cavitary lesions. However, the limited availability of molecular and immunological research resources for both these models represents a challenge.
The mouse is the most utilized model of TB [9]. It has been extensively utilized to not only study the bacterial factors of virulence, but has also contributed tremendously to our understanding of the immune protection mechanisms that control Mtb infections. Additionally, tools for molecular and immunological research and the availability of defined transgenic and knockout mice render the murine model invaluable. However, the mouse model has some shortcomings. A classical latent infection, represented by immunological response to the infection in the absence of clinical and microbiological evidence of disease, is not attained in the mouse model [10]. Moreover, the gross and microscopic pathology of murine TB is significantly different from TB in humans. This is a major drawback because different host responses reflected in the histopathology of the lesions are predicted to present different challenges and varying degrees of in vivo stress on Mtb, thus resulting in varying response from the pathogen and different outcomes to infection.
Fish infected with Mycobacterium marinum have recently emerged as an excellent surrogate model for studying Mtb infections. Infected zebrafish generate granulomatous lesions [11]. Infected zebrafish embryos are transparent, allowing the emergence of granulomatous pathology to be visually assayed in real time. Coupled with the relative ease of performing host genetics in zebrafish, this renders this fish/M. marinum model attractive for studying factors responsible for both bacterial pathogenesis and host immunity [12].
Non-human primates as surrogate models of human diseases
Non-human primates, because of their genomic, physiological, and immunological similarities to humans, are attractive models of a wide range of infectious diseases. NHP models of AIDS have significantly contributed to our understanding of AIDS pathogenesis including viral latency and development of vaccines, therapeutics, and microbicides to prevent HIV transmission [13–17]. Additionally, NHP models are being utilized to study malaria [18] and a variety of infectious agents such as smallpox [19, 20], ebola [21], Venezuelan equine encephalitis [22], nipah [23], Marburg [24], SARS [25], parainfluenza viruses [26], pneumocystis/AIDS coinfection [27], B. anthracis [28], S. mansoni [29], C. tracomatis [30], L. monocytogenes [31], group-A Streptococcus [32], Leismania [33], Coxiella burnetti [34], and Burkholderia pseudomallei (D. Kaushal, S. Mehra and C. J. Roy, unpublished data). The common element in all these studies has been the similarity between the biology of the species being modeled (humans) and the model itself (NHPs), resulting in the recapitulation of key aspects of the diseases, as they occur in humans.
The non-human primate model of TB
All of the experimental models of TB infection described above have contributed immensely to our understanding of the various phases of Mtb infection and the resulting host response. However, it is widely believed that none of these models reasonably reproduce classical latency and the various types of pathological lesions observed during human infections. Moreover, the lack of a sufficient repertoire of molecular and immunological reagents for some of these models limits their use. It is in this light that the NHP model of TB has gained importance in the last decade. A crucial advantage of the NHP models of TB is the ability to generate clinical correlates of infection, for example, blood CBC counts and chemistries, serum C-reactive protein and differential centrifugation values, thoracic X-rays, tuberculin skin tests, and interferon gamma release assays.
By the 1970s, it was generally agreed that primates can be robustly and reproducibly infected with Mtb and generate several aspects of human-like disease, including protection by vaccination with Bacille Calmette–Guerin (BCG) [35, 36]. Much of this work was performed in the Indian rhesus macaque (Macaca mulatta). The reduced availability of this species in the 1970s because of political reasons and in the 1980s because of the recruitment of this model for AIDS research, coupled with the assumption that infectious disease in general, and TB in particular, was waning, contributed to declining interest in the macaque model of TB. In the modern era, pioneering work by Walsh et al. [37] reinvigorated interest in the NHP model of TB. Most of the recent work on the NHP model of TB has primarily utilized either the cynomolgus macaque (Macaca fascicularis) of Philippine or Mauritian origin [37–50] or captive bread rhesus macaque (Macaca mulatta) of Indian or Chinese origin [51–72]. Significant advances in this field are summarized in Table 1. Important variables such as the inoculum dose, route of infection, and the strain of Mtb used are also listed in Table 1. As part of these studies, macaques have been routinely infected with Mtb strains Erdman, H37Rv, and CDC1551 (Table 1). These inoculations typically used either the intratracheal instillation using a bronchoscope or a true, head-only aerosol delivery method. There are advantages to both methods. While employing the former method, it is relatively easy to consistently quantitate the delivered dose of the inocula, while this is difficult to achieve via the aerosol method. This is due to the fact that NHPs are expensive and unlike mice cannot be euthanized at day 1 with the aim of identifying the initial inocula. Therefore, techniques such as whole-body plethysmography are used in conjugation with aerosol delivery to accurately identify the animals breathing rate and voidal volume. On the other hand, aerosol delivery of Mtb accurately mimics the natural infection of human beings. Intratracheal deposition is localized to one lobe, while with aerosol route, infection can be initiated in all lobes of both lungs.
Table 1.
Key advances in the research on the NHP model of TB are tabulated, along with the description of Mtb strains, route of infection, and the dose inoculated
| Year | Mycobacterial agent | Dose/route of infection | Significant advance | Citation |
|---|---|---|---|---|
| 1996 | Mtb Erdman | 101–102 (low), 103 (moderate), 104–105 (high); intratracheal | Establishment of the cynomolgus macaque as a model of human TB | [37] |
| 2001 | M. bovis BCG (Pasteur) | 103 (low), 106 (moderate), 108 (high); intravenous | Reactivation of BCG infection in rhesus macaques by SIV coinfection | [52] |
| 2004 | Mtb Erdman Mtb H37Rv | 101 (low), 3 × 101 (moderate), 1.5 × 102 (high); intratracheal 3 × 101 (low), 2 × 102 (moderate), 106 (high); intratracheal | Establishment of a rhesus macaque model of asymptomatic infection with Mtb | [55] |
| 2005 | Mtb Erdman | 3 × 103 cfu; 103 cfu; intratracheal | Use of the NHP model to test a candidate vaccine against TB | [39] |
| 2006 | Mtb Erdman | ~2.5 × 101 cfu; intratracheal | Description of the early events upon infection of cynomolgus macaques with Mtb | [40] |
| 2008 | Mtb Erdman | ~2.5 × 101 cfu; intratracheal | Study of hypoxia in caseous lesions of NHPs infected with Mtb | [41] |
| 2010 | Mtb Erdman | ~2.5 × 101 cfu; intratracheal | Reactivation of latent TB in cynomolgus macaques by SIV coinfection | [45] |
| 2010 | Mtb Erdman | ~2.5 × 101 cfu; intratracheal | Reactivation of latent TB in cynomolgus macaques by TNF depletion | [46] |
| 2010 | Mtb CDC1551 | ~5 × 103 cfu; aerosol | Infection phenotype of Mtb mutants in rhesus macaques via the aerosol route | [63] |
| 2011 | Mtb CDC1551 | ~2.5 × 102–5 × 102 cfu; aerosol | Reactivation of latent TB in rhesus macaques by SIV coinfection | [67] |
BCG, Bacille Calmette–Guerin; NPHs, non-human primates; SIV, simian immunodeficiency virus; TB, tuberculosis; TNF, tumor necrosis factor.
The cynomolgus macaque as a model of human TB
Cynomolgus macaques infected with a very low dose [101–102 colony-forming units (cfu)] of virulent Mtb Erdman strain via the intratracheal route generated a spectrum of TB disease conditions based on the choice of dose and the relative infectiousness of the strain. A high dose of the Mtb Erdman strain (104–105 cfu) instilled into the trachea resulted in acute and fatal tuberculous pneumonia in all cynomolgus macaques, while a moderate dose of Mtb Erdman (~103 cfu) delivered via the identical route led to the development of localized, slowly progressing TB in a majority of animals [37] (Table 1). The extremely low dose (101–102 cfu Mtb Erdman) caused the animals to maintain infection in a subclinical, latent state for long periods of time [37]. Similar results with this species were obtained in a later study [38]. More than one-third of the animals infected with a low dose (~25 cfu) of Mtb Erdman strain failed to develop any clinical signs of disease in this study, in spite of signs of infection, thus mirroring the human population latently infected with Mtb. In this model, gross granulomatous lesions can be observed as early as 3 weeks post-infection and were characterized by extensive necrosis. Adaptive immune response, characterized by IFNγ production, could only be discerned after 4 weeks of infection [40]. A subsequent, more detailed description of the lung pathology using identical strain and dose of Mtb points to a spectrum of granulomatous lesions, primary as well as post-primary in the lungs of these animals, akin to those observed in chronically infected humans [42]. This model has since been utilized to study the effectiveness of candidate anti-tubercular vaccines with varying degrees of success [39, 43, 44, 50]. Recently, this model has also been used to explore the central role of tumor necrosis factor (TNF) in controlling latent TB infection [47] and to study whether regulatory T cells play a role in this process [46]. These macaques are also being used to model the reactivation of latent Mtb infection through the use of anti-TNF antibodies to neutralize TNF activity [47] and through coinfection with simian immunodeficiency virus (SIV) to model TB/AIDS coinfections in the human population [45, 48] (Table 1).
The rhesus macaque as a model of human TB
It was initially believed that rhesus macaques do not exhibit latent phase of Mtb infection. This notion stemmed from the fact that a comparable BCG vaccination even appeared to better protect cynomolgus rather than rhesus macaques from challenge with highly virulent Mtb Erdman [72]. However, recent data from the same research institute point out that rhesus macaques can be protected against Mtb Erdman by BCG vaccination [59]. It is now accepted that rhesus macaques, much like the cynomolgus, can develop asymptomatic [56], chronic latent [68], or acute, rapidly fatal pulmonary TB [62, 64, 66] following infection with appropriate doses of Mtb (Table 1).
Much of the earlier work with rhesus macaques utilized the vaccine strain BCG as the mycobacterial infecting agent [52–54, 57, 58]. In these studies, high doses of BCG (103 as a low dose, 106 as a moderate dose, and 109 as a high dose) were employed, and simultaneous coinfection with SIV was performed. As a result of the high dose of BCG and simultaneous coinfection with an immunodeficiency-inducing viral agent, BCG was able to generate a TB-like disease, and it was possible to study the reactivation and progression of the disease [52, 53, 57]. This model laid the foundation for significant studies on the role of CD8+ [60] and Th17 [65, 70] cells in the immune control of Mtb infections (Table 1).
Infection via the aerosol route has been established in the rhesus macaque model, allowing a more natural deposition of infecting bacilli onto the pulmonary surfaces [62–64, 66, 70]. However, the aerosol route is challenging for administration of extremely low cfus to infect macaques. The use of Mtb strains with reduced pathogenicity, for example, CDC1551, has allowed the development of a model of human TB where macaques can be exposed to moderate numbers of viable bacilli via the inhalation route and result in a latent to chronic, rather than an acute, disease outcome [70].
The rhesus macaque model has also been used to test the efficacy or safety of numerous anti-tubercular vaccine candidates [59, 61, 63, 69]. It has been shown beyond doubt that BCG-vaccinated rhesus macaques exhibit protection from disease [59]. These new results clarify the confusion that has existed in the past about the high susceptibility to Mtb infection and low levels of protection following BCG vaccination in rhesus, relative to cynomolgus (Table 1).
Key advantages of the NHP model of TB
By definition, no animal model can completely mimic human disease. This is particularly true for TB, which presents as remarkably different diseases based on strain virulence, host genetics, and a variety of confounding environmental factors. However, Mtb-infected macaques offer a significant advantage while modeling key aspects of human TB.
Macaques develop true, human-like latent infection characterized by the absence of any clinical signs of infection in the presence of antigen-specific immunological response, which can be measured by a tuberculin skin test (TST) or an IFNγ release assay (IGRA) developed specifically for primates (PRIMAGAM) [62]. Clinical and radiological diagnostic means to assess Mtb infection are well developed for NHPs and comparable to humans [38–40, 62, 66, 68].
As discussed earlier, one of the key advantages of the macaque model is its ability to recapitulate the complete spectrum of granulomatous lesions that occur in human disease. As shown in Fig. 1, in rhesus macaques infected with Mtb, classical lesions with central caseous and necrotic cores are commonly found (Fig. 1A). However, other types of lesions such as fibrotic (Fig. 1B) or cavitary (Fig. 1E) may also be present, albeit more rarely. In some cases, mineralization or calcification of caseous lesions is also observed (Fig. 1C). Often, caseous lesions also exhibit the formation of numerous multinucleated giant cells in the peripheral region (Fig. 1D). In rhesus macaques coinfected with SIV, post-primary lesions were readily observed in the vicinity of primary lesions (Fig. 1F). These results strongly reinforce the point that macaque models of TB recapitulate the wide variety of pathologic TB lesions observed in infected humans.
Fig. 1.
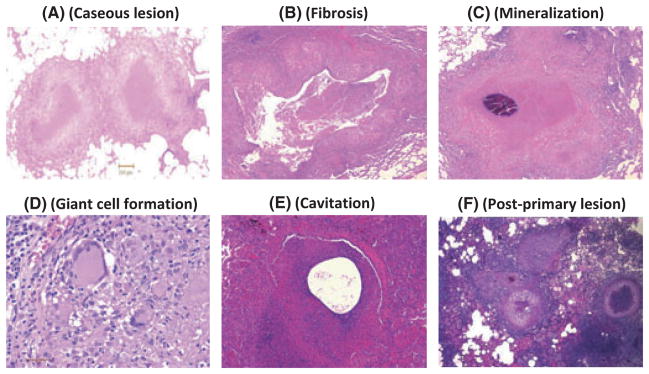
Different types of histopathological lesions observed during various Mtb infections of rhesus macaques. A. Centrally caseous lesion with peripheral rim of immune cells is the most typical type of pathology observed in NHPs infected with Mtb. B. A rare fibrotic lesion in a rhesus macaque infected with Mtb. C. Mineralization of a caseous lesion over time in a rhesus macaque infected with Mtb. D. Formation of highly inflamed multinucleated giant macrophages in a lesion from a rhesus macaque infected with Mtb. E. A rare lesion in a rhesus macaque infected with Mtb with a pathology that could be a precursor for cavitation. F. Post-primary lesions in the vicinity of primary, centrally caseous lesions in a rhesus macaque coinfected with Mtb and simian immunodeficiency virus.
In all of the macaque models described in this review, it has been possible to reactivate latent or chronic TB into an acute form characterized by rapid multiplication of bacilli and pneumonia using either SIV coinfection or blockade of the TNFα pathway [46, 47, 68].
Coinfection with AIDS is a major reason for the global resurgence of TB in the last several decades. It is expected that these robust TB/AIDS coinfection models using Mtb (or BCG) and SIV will not only allow preclinical testing of therapeutics and vaccines but also lend insights into the molecular and cellular mechanisms of reactivation. Typically, coinfection of rhesus or cynomolgus macaques already latently or chronically infected with Mtb via either aerosol or intratracheal route is performed by inoculating with SIVmac239 or SIVmac251, intravenously. For rhesus macaques, a dose of 3 × 102 TCID50 appears to be sufficient for reactivation of chronic TB. The required dosage may be higher for cynomolgus macaques because SIVmac viruses are host adapted for rhesus macaques. It has recently been possible to identify lung as well as lymph node cells from coinfected rhesus macaques as harboring both Mtb and SIV [68]. We have recently identified these coinfected cells as lung macrophages by multilabel confocal microscopy to colocalize cell type–specific and TB-specific antigens in the same cell (S. Mehra et al., unpublished data). Such novel observations on the biology of TB/AIDS coinfection are impossible to generate in any other model besides the natural host itself. The macaque model also provides advantages over studying Mtb/HIV coinfection in humans including but not limited to defined timing of infection, control of the dose, and delivery method of Mtb and SIV including the availability of defined gene deletion mutants of both SIV and Mtb, the ability to collect longitudinal samples, and the control of environmental, dietary, and social factors. In future, it will be important to study whether coinfected macrophages in the lung are deficient in the control of bacterial replication.
Application of novel technologies to the NHP model
In the last few years, several novel applications have been incorporated into the NHP model for TB. The ability to infect NHPs via the inhalation aerosol route is one such example. It is conceivable that the ability to use this natural route with the NHP model would have a similar positive impact on TB research, as did the ability to infect mice via the aerosol route two decades ago.
Use of imaging techniques in the NHP model of TB
Several NHP studies have used thoracic radiography to image and determine the progression and extent of TB in NHPs. However, state-of-the-art imaging tools are now being applied to the NHP model. Once such major advance occurred, Lewinsohn et al. [73] employed X-ray computed tomography (CT) scanning to capture high-resolution real-time images of lung lesions in rhesus macaques infected with high doses of Mtb. These results significantly correlated with pulmonary histopathology, thus providing an accurate, yet noninvasive assessment of lesion development and disease progression. Flynn et al. have used positron emission tomography (PET) coupled with CT (PET/CT) to enable an assessment of both functional (PET) and structural (CT) aspects of the development and progression of pulmonary granulomatous lesions [J.L. Flynn, personal communication]. Using this technology, these researchers can differentiate lesions that have the potential to reactivate Mtb replication from those that are truly latent. Clearly, such advanced imaging technology can be useful while assessing the protective efficacy of candidate vaccines or the therapeutic potential of candidate drugs.
Use of Mtb mutant libraries to study mechanisms of pathogenesis in the NHP model of TB
Mtb encodes over 4000 different open reading frames. A significant number of these genes do not have any defined homologs in other species. Therefore, it is difficult to assign function to many such genes. Researchers have resorted to the use of Mtb mutant libraries to define genes that are absolutely required for pathogenesis in vitro and in vivo, using the murine model [74–76]. Our laboratory recently applied this approach to the rhesus model. Mixed pools of over 300 defined, distinct Mtb mutants were used to infect rhesus macaques via the aerosol route, and their infection phenotype was studied using microarray-based survival readouts [62]. We found that during acute TB, over 33% of all mutants tested were attenuated for survival and multiplication in macaques. This was in stark contrast to when these mutants were tested in mice, where a significantly lower number of mutants (6–10%) were attenuated. It is conceivable that better structural organization of the NHP TB lesions contributes to a more effective and robust immune response, which is able to clear mutants lacking certain proteins required by the pathogen to survive in vivo. This study identified potentially novel bacterial pathways that could be targeted for anti-Mtb drug development, such as DNA repair [77] and molybdenum biosynthesis [78].
Study of hypoxia in NHPs infected with Mtb
It is believed that human TB lesions evolve highly necrotic centers filled with debris and cellular contents from destroyed infected macrophages. As lesions mature over time, this environment becomes hypoxic [79]. It has been speculated that hypoxia must have a significant effect on the biology of the pathogen [80], primarily because Mtb is known to mount a significant transcriptional response to changes in oxygen concentration, governed by the transcription factor DosR [81] via sensor kinases DosT and DosS [82]. This hypothesis was tested by analyzing lesions obtained from macaques infected with Mtb [41]. Pimonidazole hydrochloride, an imaging agent bioreductively activated specifically in hypoxic environment, was injected into macaques prior to necropsy. Discrete regions exhibiting activated pimonidazole adduct in the central caseous regions of tubercular granulomas were evident, clearly confirming that NHP lesions are hypoxic. These experiments begin to provide the tools to examine the role of the local microenvironment on Mtb biology and virulence in vivo.
Use of genome-wide techniques to study TB infections in NHPs
Genome-wide systems biology approaches have been extensively used to study biological problems in the last decade. These include transcriptome- and proteome-wide investigations. Recently, some of these approaches have been applied to the NHP model of TB. In the first such study, we employed rhesus macaque–specific whole-genome microarrays to study host gene expression in tuberculous lesions obtained from the lungs of infected animals, at an acute and a chronic stage of infection. Our results indicate that change in Mtb biology rapidly modulates the host granuloma environment, with the initial Th1 type gene expression signatures giving way to anti-inflammatory gene expression markers [64]. Recently, macaque transcriptomics has also been used to identify pathogen-specific gene expression signatures in peripheral blood [83]. It is expected that several such studies will be reported in the near future.
Another advantage of the NHP models is the rapidly growing availability of the rhesus/cynomolgus macaque genome sequence and their similarity to the human genome. We expect that in the recent future, MHC typing data from the infected macaques will allow us to validate resistance and susceptibility hypotheses derived from the analyses of human populations.
Future directions
The interest in the NHP model of TB stems from its accurate portrayal of key aspects of human syndrome. These models were initially developed with a focus on preclinical testing of diagnostic, therapeutic, and vaccine candidates. While this aim remains important, the NHP model appears to be extremely valuable for the study of pathogenesis including both host and bacterial determinants of disease and immune protection. In particular, we think this model will be crucial to examining TB/AIDS coinfections and reactivation TB in general. The model will also be useful for the design of computational models of TB latency, reactivation, and reinfection based on macaque response to different types of Mtb infections. Key questions related to Mtb virulence can also be addressed in the model including the effect of hypoxia on the pathogen and its long-term survival; the ability of Mtb to reprogram its metabolism to adapt to the lung environment and the nutrients available therein; how a productive adaptive response is unable to provide sterilizing immunity and how Mtb modulates the host immune response to its benefit.
Acknowledgments
The Tulane National Primate Research Center is accredited by the AALAC. All animal-related work at the TNPRC is performed under the guidelines of AALAC and is approved by the Institutional Animal Care and Use Committee. This work was supported by NIH grants RR000164, RR020159, AI089323, HL106790, AI091457, AI058609, RR026006, Howard Hughes Medical Institute, Louisiana Vaccine Center, Tulane Research Enhancement Fund, and a postdoctoral award from Tulane Center for Infectious Diseases (to SM).
References
- 1.Dye C, Scheele C, Dolin P, Pathania V, Raviglione MC. Global burden of tuberculosis: estimated incidence, prevalence, and mortality by country. JAMA. 1999;282:677–86. doi: 10.1001/jama.282.7.677. [DOI] [PubMed] [Google Scholar]
- 2.Shah NS, Wright A, Bai GH, Barrera L, Boulahbal F, Martín-Casabona N, Drobniewski F, Drobniewski F, Gilpin C, Havelková M, Lepe R, Lumb R, Metchock B, Portaels F, Rodrigues MF, Rüsch-Gerdes S, Van Deun A, Vincent V, Laserson K, Wells C, Cegielski JP. Worldwide emergence of extensively drug-resistant tuberculosis. Emerg Infect Dis. 2007;13:380–7. doi: 10.3201/eid1303.061400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fletcher HA. Correlates of immune protection from tuberculosis. Curr Mol Med. 2007;7:319–25. doi: 10.2174/156652407780598520. [DOI] [PubMed] [Google Scholar]
- 4.Harrington M. From HIV to tuberculosis and back again: a tale of activism in 2 pandemics. Clin Infect Dis. 2010;50:S260–6. doi: 10.1086/651500. [DOI] [PubMed] [Google Scholar]
- 5.von Reyn CF, Kimambo S, Mtei L, Arbeit RD, Maro I, Bakari M, Matee M, Lahey T, Adams LV, Black W, Mackenzie T, Lyimo J, Tvaroha S, Waddell R, Kreiswirth B, Horsburgh CR, Pallangyo K. Disseminated tuberculosis in human immunodeficiency virus infection: ineffective immunity, polyclonal disease and high mortality. Int J Tuberc Lung Dis. 2011;15:1087–92. doi: 10.5588/ijtld.10.0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koch R. Classics in infectious diseases. The etiology of tuberculosis: Robert Koch. Berlin, Germany. Rev Infect Dis. 1982;4:1270–4. [PubMed] [Google Scholar]
- 7.Padilla-Carlin DJ, McMurray DN, Hickey AJ. The guinea pig as a model of infectious diseases. Comp Med. 2008;58:324–40. [PMC free article] [PubMed] [Google Scholar]
- 8.Nedeltchev GG, Raghunand TR, Jassal MS, Lun S, Cheng QJ, Bishai WR. Extrapulmonary dissemination of Mycobacterium bovis but not Mycobacterium tuberculosis in a bronchoscopic rabbit model of cavitary tuberculosis. Infect Immun. 2009;77:598–603. doi: 10.1128/IAI.01132-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beamer GL, Turner J. Murine models of susceptibility to tuberculosis. Arch Immunol Ther Exp. 2005;53:469–83. [PubMed] [Google Scholar]
- 10.Flynn JL. Lessons from experimental Mycobacterium tuberculosis infections. Microbes Infect. 2006;8:1179–88. doi: 10.1016/j.micinf.2005.10.033. [DOI] [PubMed] [Google Scholar]
- 11.Volkman HE, Pozos TC, Zheng J, Davis JM, Rawls JF, Ramakrishnan L. Tuberculous granuloma induction via interaction of a bacterial secreted protein with host epithelium. Science. 2010;327:466–9. doi: 10.1126/science.1179663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis JM, Clay H, Lewis JL, Ghori N, Herbomel P, Ramakrishnan L. Real-time visualization of mycobacterium-macrophage interactions leading to initiation of granuloma formation in zebrafish embryos. Immunity. 2002;17:693. doi: 10.1016/s1074-7613(02)00475-2. [DOI] [PubMed] [Google Scholar]
- 13.Hirsch VM, Lifson JD. Simian immunodeficiency virus infection of monkeys as a model system for the study of AIDS pathogenesis, treatment, and prevention. Adv Pharmacol. 2000;49:437–77. doi: 10.1016/s1054-3589(00)49034-4. [DOI] [PubMed] [Google Scholar]
- 14.Lackner AA, Veazey RS. Current concepts in AIDS pathogenesis: insights from the SIV/macaque model. Annu Rev Med. 2007;58:461–76. doi: 10.1146/annurev.med.58.082405.094316. [DOI] [PubMed] [Google Scholar]
- 15.Deere JD, Schinazi RF, North TW. Simian immunodeficiency virus macaque models of HIV latency. Curr Opin HIV AIDS. 2011;6:57–61. doi: 10.1097/COH.0b013e32834086ce. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Staprans SI, Feinberg MB, Shiver JW, Casimiro DR. Role of nonhuman primates in the evaluation of candidate AIDS vaccines: an industry perspective. Curr Opin HIV AIDS. 2010;5:377–85. doi: 10.1097/COH.0b013e32833d2e19. [DOI] [PubMed] [Google Scholar]
- 17.Veazey RS, Ketas TJ, Dufour J, Moroney-Rasmussen T, Green LC, Klasse PJ, Moore JP. Protection of rhesus macaques from vaginal infection by vaginally delivered maraviroc, an inhibitor of HIV-1 entry via the CCR5 co-receptor. J Infect Dis. 2010;202:739–44. doi: 10.1086/655661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pichyangkul S, Kum-Arb U, Yongvanitchit K, Limsalakpetch A, Gettayacamin M, Lanar DE, Ware LA, Stewart VA, Heppner DG, Mettens P, Cohen JD, Ballou WR, Fukuda MM. Preclinical evaluation of the safety and immunogenicity of a vaccine consisting of Plasmodium falciparum liver-stage antigen 1 with adjuvant AS01B administered alone or concurrently with the RTS,S/AS01B vaccine in rhesus primates. Infect Immun. 2008;76:229–38. doi: 10.1128/IAI.00977-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purcell RH, Emerson SU. Animal models of hepatitis A and E. ILAR J. 2001;42:161–77. doi: 10.1093/ilar.42.2.161. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RF, Yellayi S, Cann JA, Johnson A, Smith AL, Paragas J, Jahrling PB, Blaney JE. Cowpox virus infection of cynomolgus macaques as a model of hemorrhagic smallpox. Virology. 2011;418:102–12. doi: 10.1016/j.virol.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reed DS, Lackemeyer MG, Garza NL, Sullivan LJ, Nichols DK. Aerosol exposure to Zaire ebolavirus in three nonhuman primate species: differences in disease course and clinical pathology. Microbes Infect. 2011;13:930–6. doi: 10.1016/j.micinf.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 22.Dupuy LC, Richards MJ, Reed DS, Schmaljohn CS. Immunogenicity and protective efficacy of a DNA vaccine against Venezuelan equine encephalitis virus aerosol challenge in nonhuman primates. Vaccine. 2010;28:7345–50. doi: 10.1016/j.vaccine.2010.09.005. [DOI] [PubMed] [Google Scholar]
- 23.Geisbert TW, Daddario-DiCaprio KM, Hickey AC, Smith MA, Chan YP, Wang LF, Mattapallil JJ, Geisbert JB, Bossart KN, Broder CC. Development of an acute and highly pathogenic nonhuman primate model of Nipah virus infection. PLoS ONE. 2010;5:e10690. doi: 10.1371/journal.pone.0010690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Swenson DL, Warfield KL, Larsen T, Alves DA, Coberley SS, Bavari S. Monovalent virus-like particle vaccine protects guinea pigs and nonhuman primates against infection with multiple Marburg viruses. Expert Rev Vaccines. 2008;7:417–29. doi: 10.1586/14760584.7.4.417. [DOI] [PubMed] [Google Scholar]
- 25.Lawler JV, Endy TP, Hensley LE, Garrison A, Fritz EA, Lesar M, Baric RS, Kulesh DA, Norwood DA, Wasieloski LP, Ulrich MP, Slezak TR, Vitalis E, Huggins JW, Jahrling PB, Paragas J. Cynomolgus macaque as an animal model for severe acute respiratory syndrome. PLoS Med. 2006;3:e149. doi: 10.1371/journal.pmed.0030149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durbin AP, Elkins WR, Murphy BR. African green monkeys provide a useful nonhuman primate model for the study of human parainfluenza virus types-1, -2, and -3 infection. Vaccine. 2000;18:2462–9. doi: 10.1016/s0264-410x(99)00575-7. [DOI] [PubMed] [Google Scholar]
- 27.Shipley TW, Kling HM, Morris A, Patil S, Kristoff J, Guyach SE, Murphy JE, Shao X, Sciurba FC, Rogers RM, Richards T, Thompson P, Montelaro RC, Coxson HO, Hogg JC, Norris KA. Persistent pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis. 2010;202:302–12. doi: 10.1086/653485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Twenhafel NA. Pathology of inhalational anthrax animal models. Vet Pathol. 2010;47:819–30. doi: 10.1177/0300985810378112. [DOI] [PubMed] [Google Scholar]
- 29.Zhang W, Ahmad G, Torben W, Noor Z, Le L, Damian RT, Wolf RF, White GL, Chavez-Suarez M, Podesta RB, Kennedy RC, Siddiqui AA. Sm-p80-based DNA vaccine provides baboons with levels of protection against Schistosoma mansoni infection comparable to those achieved by the irradiated cercarial vaccine. J Infect Dis. 2010;201:1105–12. doi: 10.1086/651147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kari L, Whitmire WM, Crane DD, Reveneau N, Carlson JH, Goheen MM, Peterson EM, Pal S, de la Maza LM, Caldwell HD. Chlamydia trachomatis native major outer membrane protein induces partial protection in nonhuman primates: implication for a trachoma transmission-blocking vaccine. J Immunol. 2009;182:8063–70. doi: 10.4049/jimmunol.0804375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MA, Takeuchi K, Anderson G, Ware GO, McClure HM, Raybourne RB, Mytle N, Doyle MP. Dose-response model for Listeria monocytogenes-induced stillbirths in nonhuman primates. Infect Immun. 2008;76:726–31. doi: 10.1128/IAI.01366-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Virtaneva K, Graham MR, Porcella SF, Hoe NP, Su H, Graviss EA, Gardner TJ, Allison JE, Lemon WJ, Bailey JR, Parnell MJ, Musser JM. Group A Streptococcus gene expression in humans and cynomolgus macaques with acute pharyngitis. Infect Immun. 2003;71:2199–207. doi: 10.1128/IAI.71.4.2199-2207.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Campos-Neto A, Porrozzi R, Greeson K, Coler RN, Webb JR, Seiky YA, Reed SG, Grimaldi G., Jr Protection against cutaneous leishmaniasis induced by recombinant antigens in murine and nonhuman primate models of the human disease. Infect Immun. 2001;69:4103–8. doi: 10.1128/IAI.69.6.4103-4108.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Waag DM, Byrne WR, Estep J, Gibbs P, Pitt ML, Banfield CM. Evaluation of cynomolgus (Macaca fascicularis) and rhesus (Macaca mulatta) monkeys as experimental models of acute Q fever after aerosol exposure to phase-I Coxiella burnetii. Lab Anim Sci. 1999;49:634–8. [PubMed] [Google Scholar]
- 35.Barclay WR, Anacker RL, Brehmer W, Leif W, Ribi E. Aerosol induced tuberculosis in subhuman primates and the course of the disease after intravenous BCG vaccination. Infect Immun. 1970;2:574–82. doi: 10.1128/iai.2.5.574-582.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chaparas SD, Good RC, Janicki BW. Tuberculin-induced lymphocyte transformation and skin reactivity in monkeys vaccinated or not vaccinated with Bacille Calmette-Guerin, then challenged with virulent Mycobacterium tuberculosis. Am Rev Respir Dis. 1975;112:43–7. doi: 10.1164/arrd.1975.112.1.43. [DOI] [PubMed] [Google Scholar]
- 37.Walsh GP, Tan EV, dela Cruz EC, Abalos RM, Villahermosa LG, Young LJ, Cellona RV, Nazareno JB, Horwitz MA. The Philippine cynomolgus monkey (Macaca fasicularis) provides a new nonhuman primate model of tuberculosis that resembles human disease. Nat Med. 1996;2:430–6. doi: 10.1038/nm0496-430. [DOI] [PubMed] [Google Scholar]
- 38.Capuano SV, 3rd, Croix DA, Pawar S, Zinovik A, Myers A, Lin PL, Bissel S, Fuhrman C, Klein E, Flynn JL. Experimental Mycobacterium tuberculosis infection of cynomolgus macaques closely resembles the various manifestations of human M. tuberculosis infection. Infect Immun. 2003;71:5831–44. doi: 10.1128/IAI.71.10.5831-5844.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Langermans JA, Doherty TM, Vervenne RA, van der Laan T, Lyashchenko K, Greenwald R, Agger EM, Aagaard C, Weiler H, van Soolingen D, Dalemans W, Thomas AW, Andersen P. Protection of macaques against Mycobacterium tuberculosis infection by a subunit vaccine based on a fusion protein of antigen 85B and ESAT-6. Vaccine. 2005;23:2740–50. doi: 10.1016/j.vaccine.2004.11.051. [DOI] [PubMed] [Google Scholar]
- 40.Lin PL, Pawar S, Myers A, Pegu A, Fuhrman C, Reinhart TA, Capuano SV, Klein E, Flynn JL. Early events in Mycobacterium tuberculosis infection in cynomolgus macaques. Infect Immun. 2006;74:3790–803. doi: 10.1128/IAI.00064-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Via LE, Lin PL, Ray SM, Carrillo J, Allen SS, Eum SY, Taylor K, Klein E, Manjunatha U, Gonzales J, Lee EG, Park SK, Raleigh JA, Cho SN, McMurray DN, Flynn JL, Barry CE., 3rd Tuberculous granulomas are hypoxic in guinea pigs, rabbits, and nonhuman primates. Infect Immun. 2008;76:2333–40. doi: 10.1128/IAI.01515-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lin PL, Rodgers M, Smith L, Bigbee M, Myers A, Bigbee C, Chiosea I, Capuano SV, Fuhrman C, Klein E, Flynn JL. Quantitative comparison of active and latent tuberculosis in the cynomolgus macaque model. Infect Immun. 2009;77:4631–42. doi: 10.1128/IAI.00592-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reed SG, Coler RN, Dalemans W, Tan EV, DeLa Cruz EC, Basaraba RJ, Orme IM, Skeiky YA, Alderson MR, Cowgill KD, Prieels JP, Abalos RM, Dubois MC, Cohen J, Mettens P, Lobet Y. Defined tuberculosis vaccine, Mtb72F/AS02A, evidence of protection in cynomolgus monkeys. Proc Natl Acad Sci USA. 2009;106:2301–6. doi: 10.1073/pnas.0712077106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Larsen MH, Biermann K, Chen B, Hsu T, Sambandamurthy VK, Lackner AA, Aye PP, Didier P, Huang D, Shao L, Wei H, Letvin NL, Frothingham R, Haynes BF, Chen ZW, Jacobs WR., Jr Efficacy and safety of live attenuated persistent and rapidly cleared Mycobacterium tuberculosis vaccine candidates in non-human primates. Vaccine. 2009;27:4709–17. doi: 10.1016/j.vaccine.2009.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diedrich CR, Mattila JT, Klein E, Janssen C, Phuah J, Sturgeon TJ, Montelaro RC, Lin PL, Flynn JL. Reactivation of latent tuberculosis in cynomolgus macaques infected with SIV is associated with early peripheral T cell depletion and not virus load. PLoS ONE. 2010;5:e9611. doi: 10.1371/journal.pone.0009611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin PL, Myers A, Smith L, Bigbee C, Bigbee M, Fuhrman C, Grieser H, Chiosea I, Voitenek NN, Capuano SV, Klein E, Flynn JL. Tumor necrosis factor neutralization results in disseminated disease in acute and latent Mycobacterium tuberculosis infection with normal granuloma structure in a cynomolgus macaque model. Arthritis Rheum. 2010;62:340–50. doi: 10.1002/art.27271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Green AM, Mattila JT, Bigbee CL, Bongers KS, Lin PL, Flynn JL. CD4(+) regulatory T cells in a cynomolgus macaque model of Mycobacterium tuberculosis infection. J Infect Dis. 2010;202:533–41. doi: 10.1086/654896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mattila JT, Diedrich CR, Lin PL, Phuah J, Flynn JL. Simian immunodeficiency virus-induced changes in T cell cytokine responses in cynomolgus macaques with latent Mycobacterium tuberculosis infection are associated with timing of reactivation. J Immunol. 2011;186:3527–37. doi: 10.4049/jimmunol.1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ford CB, Lin PL, Chase MR, Shah RR, Iartchouk O, Galagan J, Mohaideen N, Ioerger TR, Sacchettini JC, Lipsitch M, Flynn JL, Fortune SM. Use of whole genome sequencing to estimate the mutation rate of Mycobacterium tuberculosis during latent infection. Nat Genet. 2011;43:482–6. doi: 10.1038/ng.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin PL, Dietrich J, Tan E, Abalos RM, Burgos J, Bigbee C, Bigbee M, Milk L, Gideon HP, Rodgers M, Cochran C, Guinn KM, Sherman DR, Klein E, Janssen C, Flynn JL, Andersen P. The multistage vaccine H56 boosts the effects of BCG to protect cynomolgus macaques against active tuberculosis and reactivation of latent Mycobacterium tuberculosis infection. J Clin Invest. 2012;122:303–14. doi: 10.1172/JCI46252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Croix DA, Capuano S, 3rd, Simpson L, Fallert BA, Fuller CL, Klein EC, Reinhart TA, Murphey-Corb M, Flynn JL. Effect of mycobacterial infection on virus loads and disease progression in simian immunodeficiency virus-infected rhesus monkeys. AIDS Res Hum Retroviruses. 2000;16:1895–908. doi: 10.1089/08892220050195856. [DOI] [PubMed] [Google Scholar]
- 52.Shen Y, Shen L, Sehgal P, Zhou D, Simon M, Miller M, Enimi EA, Henckler B, Chalifoux L, Sehgal N, Gastron M, Letvin NL, Chen ZW. Antiretroviral agents restore Mycobacterium-specific T-cell immune responses and facilitate controlling a fatal tuberculosis-like disease in Macaques coinfected with simian immunodeficiency virus and Mycobacterium bovis BCG. J Virol. 2001;75:8690–6. doi: 10.1128/JVI.75.18.8690-8696.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shen Y, Zhou D, Chalifoux L, Shen L, Simon M, Zeng X, Lai X, Li Y, Sehgal P, Letvin NL, Chen ZW. Induction of an AIDS virus-related tuberculosis-like disease in macaques: a model of simian immunodeficiency virus-mycobacterium coinfection. Infect Immun. 2002;70:869–77. doi: 10.1128/IAI.70.2.869-877.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shen Y, Zhou D, Qiu L, Lai X, Simon M, Shen L, Kou Z, Wang Q, Jiang L, Estep J, Hunt R, Clagett M, Sehgal PK, Li Y, Zeng X, Morita CT, Brenner MB, Letvin NL, Chen ZW. Adaptive immune response of Vgamma2Vdelta2+ T cells during mycobacterial infections. Science. 2002;295:2255–8. doi: 10.1126/science.1068819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Safi H, Gormus BJ, Didier PJ, Blanchard JL, Lakey DL, Martin LN, Murphey-Corb M, Vankayalapati R, Barnes PF. Spectrum of manifestations of Mycobacterium tuberculosis infection in primates infected with SIV. AIDS Res Hum Retroviruses. 2003;19:585–95. doi: 10.1089/088922203322230950. [DOI] [PubMed] [Google Scholar]
- 56.Gormus BJ, Blanchard JL, Alvarez XH, Didier PJ. Evidence for a rhesus monkey model of asymptomatic tuberculosis. J Med Primatol. 2004;33:134–45. doi: 10.1111/j.1600-0684.2004.00062.x. [DOI] [PubMed] [Google Scholar]
- 57.Qiu L, Huang D, Chen CY, Wang R, Shen L, Shen Y, Hunt R, Estep J, Haynes BF, Jacobs WR, Jr, Letvin N, Du G, Chen ZW. Severe tuberculosis induces unbalanced up-regulation of gene networks and overexpression of IL-22, MIP-1alpha, CCL27, IP-10, CCR4, CCR5, CXCR3, PD1, PDL2, IL-3, IFN-beta, TIM1, and TLR2 but low antigen-specific cellular responses. J Infect Dis. 2008;198:1514–9. doi: 10.1086/592448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shen L, Shen Y, Huang D, Qiu L, Sehgal P, Du GZ, Miller MD, Letvin NL, Chen ZW. Development of Vgamma2Vdelta2+ T cell responses during active mycobacterial coinfection of simian immunodeficiency virus-infected macaques requires control of viral infection and immune competence of CD4+ T cells. J Infect Dis. 2004;19:1438–47. doi: 10.1086/423939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Verreck FA, Vervenne RA, Kondova I, van Kralingen KW, Remarque EJ, Braskamp G, van der Werff NM, Kersbergen A, Ottenhoff TH, Heidt PJ, Gilbert SC, Gicquel B, Hill AV, Martin C, McShane H, Thomas AW. MVA.85A boosting of BCG and an attenuated, phoP deficient M. tuberculosis vaccine both show protective efficacy against tuberculosis in rhesus macaques. PLoS ONE. 2009;4:e5264. doi: 10.1371/journal.pone.0005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen CY, Huang D, Wang RC, Shen L, Zeng G, Yao S, Shen Y, Halliday L, Fortman J, McAllister M, Estep J, Hunt R, Vasconcelos D, Du G, Porcelli SA, Larsen MH, Jacobs WR, Jr, Haynes BF, Letvin NL, Chen ZW. A critical role for CD8 T cells in a nonhuman primate model of tuberculosis. PLoS Pathog. 2009;5:e1000392. doi: 10.1371/journal.ppat.1000392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sugawara I, Sun L, Mizuno S, Taniyama T. Protective efficacy of recombinant BCG Tokyo (Ag85A) in rhesus monkeys (Macaca mulatta) infected intratracheally with H37Rv Mycobacterium tuberculosis. Tuberculosis. 2009;89:62–7. doi: 10.1016/j.tube.2008.09.008. [DOI] [PubMed] [Google Scholar]
- 62.Dutta NK, Mehra S, Didier PJ, Roy CJ, Doyle LA, Alvarez X, Ratterree M, Be NA, Lamichhane G, Jain SK, Lacey MR, Lackner AA, Kaushal D. Genetic requirements for the survival of tubercle bacilli in primates. J Infect Dis. 2010;201:1743–52. doi: 10.1086/652497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharpe SA, McShane H, Dennis MJ, Basaraba RJ, Gleeson F, Hall G, McIntyre A, Gooch K, Clark S, Beveridge NE, Nuth E, White A, Marriott A, Dowall S, Hill AV, Williams A, Marsh PD. Establishment of an aerosol challenge model of tuberculosis in rhesus macaques and an evaluation of endpoints for vaccine testing. Clin Vaccine Immunol. 2010;17:1170–82. doi: 10.1128/CVI.00079-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mehra S, Pahar B, Dutta NK, Conerly CN, Philippi-Falkenstein K, Alvarez X, Kaushal D. Transcriptional reprogramming in nonhuman primate (rhesus macaque) tuberculosis granulomas. PLoS ONE. 2010;5:e12266. doi: 10.1371/journal.pone.0012266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yao S, Huang D, Chen CY, Halliday L, Zeng G, Wang RC, Chen ZW. Differentiation, distribution and gammadelta T cell-driven regulation of IL-22-producing T cells in tuberculosis. PLoS Pathog. 2010;6:e1000789. doi: 10.1371/journal.ppat.1000789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mehra S, Golden NA, Stuckey KJ, Didier PJ, Doyle LA, Russell-Lodrigue KE, Sugimoto C, Hasegawa A, Sivasubramani SK, Kuroda MJ, Blanchard JL, Lackner AA, Kaushal D. The Mycobacterium tuberculosis SigH is required for bacterial burden as well as immunopathology in primates. J Infect Dis. 2012;205 doi: 10.1093/infdis/jis102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Luciw PA, Oslund KL, Yang XW, Adamson L, Ravindran R, Canfield DR, Tarara R, Hirst L, Christensen M, Lerche NW, Offenstein H, Lewinsohn D, Ventimiglia F, Brignolo L, Wisner ER, Hyde DM. Stereological analysis of bacterial load and lung lesions in nonhuman primates (rhesus macaques) experimentally infected with Mycobacterium tuberculosis. Am J Physiol Lung Cell Mol Physiol. 2011;301:L731–8. doi: 10.1152/ajplung.00120.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Mehra S, Golden NA, Dutta NK, Midkiff CC, Alvarez X, Doyle LA, Asher M, Russell-Lodrigue K, Monjure C, Roy CJ, Blanchard JL, Didier PJ, Veazey RS, Lackner AA, Kaushal D. Reactivation of latent tuberculosis in rhesus macaques by coinfection with simian immunodeficiency virus. J Med Primatol. 2011;40:233–43. doi: 10.1111/j.1600-0684.2011.00485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sampson SL, Mansfield KG, Carville A, Magee DM, Quitugua T, Howerth EW, Bloom BR, Hondalus MK. Extended safety and efficacy studies of a live attenuated double leucine and pantothenate auxotroph of Mycobacterium tuberculosis as a vaccine candidate. Vaccine. 2011;29:4839–47. doi: 10.1016/j.vaccine.2011.04.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zeng G, Chen CY, Huang D, Yao S, Wang RC, Chen ZW. Membrane-bound IL-22 after de novo production in tuberculosis and anti-Mycobacterium tuberculosis effector function of IL-22+ CD4+ T cells. J Immunol. 2011;187:190–9. doi: 10.4049/jimmunol.1004129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sharpe SA, Eschelbach E, Basaraba RJ, Gleeson F, Hall GA, McIntyre A, Williams A, Kraft SL, Clark S, Gooch K, Hatch G, Orme IM, Marsh PD, Dennis MJ. Determination of lesion volume by MRI and stereology in a macaque model of tuberculosis. Tuberculosis. 2009;89:405–16. doi: 10.1016/j.tube.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 72.Langermans JA, Andersen P, van Soolingen D, Vervenne RA, Frost PA, van der Laan T, van Pinxteren LA, van den Hombergh J, Kroon S, Peekel I, Florquin S, Thomas AW. Divergent effect of bacillus Calmette-Guérin (BCG) vaccination on Mycobacterium tuberculosis infection in highly related macaque species: implications for primate models in tuberculosis vaccine research. Proc Natl Acad Sci USA. 2001;98:11497–502. doi: 10.1073/pnas.201404898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lewinsohn DM, Tydeman IS, Frieder M, Grotzke JE, Lines RA, Ahmed S, Prongay KD, Primack SL, Colgin LMA, Lewis AD, Lewinsohn DA. High resolution radiographic and fine immunologic definition of TB disease progression in the rhesus macaque. Microbes Infect. 2006;8:2587–98. doi: 10.1016/j.micinf.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 74.Sassetti CM, Boyd DH, Rubin EJ. Comprehensive identification of conditionally essential genes in mycobacteria. Proc Natl Acad Sci USA. 2001;98:12712–7. doi: 10.1073/pnas.231275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rengarajan J, Bloom BR, Rubin EJ. Genome-wide requirements for Mycobacterium tuberculosis adaptation and survival in macrophages. Proc Natl Acad Sci USA. 2005;102:8327–32. doi: 10.1073/pnas.0503272102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Griffin JE, Gawronski JD, DeJesus MA, Loerger TR, Akerley BA, Sassetti CM. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog. 2011;7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kurthkoti K, Varshney U. Distinct mechanisms of DNA repair in mycobacteria and their implications in attenuation of the pathogen growth. Mech Ageing Dev. 2011 doi: 10.1016/j.mad.2011.09.003. in press. [DOI] [PubMed] [Google Scholar]
- 78.Williams MJ, Kana BD, Mizrahi V. Functional analysis of molybdopterin biosynthesis in mycobacteria identifies a fused molybdopterin synthase in Mycobacterium tuberculosis. J Bacteriol. 2011;193:98–106. doi: 10.1128/JB.00774-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Russell DG. Who puts the tubercle in tuberculosis? Nat Rev Microbiol. 2007;5:39–47. doi: 10.1038/nrmicro1538. [DOI] [PubMed] [Google Scholar]
- 80.Kaufmann SH, Cole ST, Mizrahi V, Rubin E, Nathan C. Mycobacterium tuberculosis and the host response. J Exp Med. 2005;201:1693–7. doi: 10.1084/jem.20050842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Park HD, Guinn KM, Harrell MI, Liao R, Voskuil MI, Tompa M, Schoolnik GK, Sherman DR. Rv3133c/dosR is a transcription factor that mediates the hypoxic response of Mycobacterium tuberculosis. Mol Microbiol. 2003;48:833–43. doi: 10.1046/j.1365-2958.2003.03474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Roberts DM, Liao RP, Wisedchaisri G, Hol WG, Sherman DR. Two sensor kinases contribute to the hypoxic response of Mycobacterium tuberculosis. J Biol Chem. 2004;279:23082–7. doi: 10.1074/jbc.M401230200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Zinman G, Brower-Sinning R, Emeche CH, Ernst J, Huang GT, Mahony S, Myers AJ, O’Dee DM, Flynn JL, Nau GJ, Ross TM, Salter RD, Benos PV, Bar Joseph Z, Morel PA. Large scale comparison of innate responses to viral and bacterial pathogens in mouse and macaque. PLoS ONE. 2011;6:e22401. doi: 10.1371/journal.pone.0022401. [DOI] [PMC free article] [PubMed] [Google Scholar]


