Abstract
The logopenic variant of primary progressive aphasia (lvPPA) strongly associates with Alzheimer’s disease, but can also associate with frontotemporal lobar degeneration. We aimed to assess the frequency of lvPPA in patients with speech and language disorders without β-amyloid deposition, and to perform detailed neuroimaging and genetic testing in such lvPPA patients. Seventy-six patients with a neurodegenerative speech and language disorder and Pittsburgh compound B (PiB) PET imaging demonstrating no β-amyloid deposition were analyzed. Six lvPPA patients (8 %) were identified. All six underwent progranulin (GRN) gene testing. Structural abnormality index maps and Cortex ID analysis were utilized to assess individual patterns of grey matter atrophy on MRI and hypometabolism on 18-F fluorodeoxyglucose (FDG) PET. Statistical parametric mapping was used to perform MRI and FDG-PET group comparisons between those with (GRN-positive) and without (GRN-negative) progranulin mutations. All six lvPPA patients showed left temporoparietal atrophy and hypometabolism. Three patients (50 %) were GRN-positive. Speech, language, and neurological and neuropsychological profiles did not differ between GRN-positive and negative patients, although GRN-positive patients had family histories, were on average 8 years younger, and had lower PiB-PET ratios. All six patients showed similar patterns of atrophy and hypometabolism, although, as a group, GRN-positive patients had more severe abnormalities, particularly in anteromedial temporal lobes. Logopenic PPA accounts for a small minority of neurodegenerative speech and language disorders not associated with β-amyloid deposition. Identification of such patients, however, should prompt testing for GRN mutations, since GRN-positive patients do not have distinctive features, yet account for 50 % of this patient population.
Keywords: Progranulin, Logopenic, Primary progressive aphasia, β-amyloid, MRI, FDG-PET
Introduction
Three variants of primary progressive aphasia (PPA) are currently recognized, including agrammatic, semantic, and logopenic PPA (lvPPA) [1]. Agrammatic PPA is characterized by agrammatic and/or telegraphic speech output, while the semantic variant is characterized by grammatically adequate but tangential speech, with associated loss of word meaning [2]. The logopenic variant is characterized by word-finding difficulties, which are observed during conversations as the patient searches to retrieve words, particularly words that are less frequent in his or her vocabulary [3]. This typically results in a speech pattern that is characterized by frequent pauses or hesitation between words or sentences. Formal speech and language evaluation identifies word retrieval deficits and anomia, as well as difficulty with sentence repetition, and the production of sound errors known as phonological or phonemic errors. Recently, consensus criteria were published to guide diagnosis of all three variants of PPA, including lvPPA [1]. Importantly, in addition to the features described above, the consensus criteria include the presence of motor speech disorders and a loss of meaning for words as non-supportive features for an lvPPA diagnosis.
Clinicopathologic and amyloid imaging studies have demonstrated that lvPPA is strongly associated with β-amyloid deposition and Alzheimer’s disease [4–6], while the agrammatic and semantic variants of PPA are strongly associated with frontotemporal lobar degeneration pathology (FTLD) [7–9]. Little is known, however, about lvPPA patients who do not show an association with β-amyloid deposition, and hence likely have an underlying FTLD pathology [5, 10]. For example, it is unknown how frequently lvPPA accounts for the diagnosis in patients presenting with aphasia or apraxia of speech who do not show β-amyloid deposition and, hence, do not have Alzheimer’s disease. It is also unknown whether patients with lvPPA who have a genetic mutation in progranulin (GRN) that causes FTLD [11] would have a unique clinical or imaging signature when compared to lvPPA patients who do not show β-amyloid deposition and do not have GRN gene mutations.
Over the past 4 years. we have been prospectively recruiting patients with progressive speech and language disorders who undergo Pittsburgh compound B (PiB) positron emission tomography (PiB-PET) scanning to document the presence of β-amyloid deposition in the brain. We therefore aimed to determine the frequency of lvPPA in a large cohort of patients with any type of a neurodegenerative progressive apraxia of speech or aphasia, including PPA, who are β-amyloid-negative and hence, most likely have an underlying FTLD pathology. We also aimed to determine whether clinical and imaging features of such lvPPA patients would differ between those with and without progranulin gene mutations.
Methods
Patients
Between July 2010 and November 2013, patients with a neurodegenerative progressive speech and language disorder (apraxia of speech and/or aphasia) who presented to the Department of Neurology at the Mayo Clinic in Rochester, MN, were prospectively recruited and underwent detailed speech, language, and neurological and neuropsychological testing, as previously described [12, 13].
For speech and language evaluation, all patients completed the Western Aphasia Battery-revised test (WAB) [14], a 22-item version of Part V of DeRenzi and Vignolo’s Token Test [15], the 15-item Boston Naming Test (BNT) [16], Action (verb) Fluency [17] and Letter (FAS) Fluency [18] tasks, and the Pyramids and Palm Trees Test (PPTT) [19]. Motor speech was assessed for apraxia of speech (AOS) using an AOS rating scale (ASRS) [12]. The presence of phonological errors was also assessed and rated on a five-point scale (absent, mild, moderate, marked, and severe). The speech and language assessments for all patients were video recorded and reviewed by two study authors (JRD and EAS) in order to render a consensus diagnosis based on modification to our previously published criteria [2]. The following criteria were used to diagnose lvPPA: the presence of any combination of two or more of anomia without loss of word meaning, impaired sentence repetition, phonemic paraphasias, and no features that are more suggestive of another speech and language disorder.
All patients underwent neurological evaluation by a behavioral neurologist (KAJ) and completed the Montreal Cognitive Assessment battery (MoCA) [20], Frontal Behavioral Inventory (FBI) [21], brief questionnaire form of the Neuropsychiatric Inventory (NPI) [22], the limb apraxia subscale of the WAB [14], and the Movement Disorders Society-sponsored version of the Unified Parkinson’s Disease Rating Scale part III (MDS-UPDRS) [23]. Detailed neuropsychological testing was performed by a psychometrist with oversight by a clinical neuropsychologist (MMM). The battery included tests of motor speed with the Trail Making Test (TMT) A [24], executive function with the TMT B [24], and Delis–Kaplan Executive Function System Card Sort (DKEFS) [25], learning and memory with the Auditory Verbal Learning Test (AVLT) [26], and visuospatial and visuoperceptual functions with the Cube Analysis and Incomplete Letters from the Visual Object and Space Perception (VOSP) battery [27] and the Rey-Osterreith Complex Figure Test (Rey-O) [26, 28]. Mayo Older American Normative Studies (MOANS) age and education-adjusted scaled scores [29] were used for all neuropsychological variables, except for the DKEFS Card Sort, VOSP Cube Analysis, and Incomplete Letters. The MOANS and DKEFS Card Sort are constructed to have a mean of 10 and standard deviation of 3 in cognitively healthy participants.
The study was approved by the Mayo Clinic institutional review and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All patients consented for enrolment into the study.
Genetic testing
All six lvPPA patients without β-amyloid deposition underwent apolipoprotein E (APOE) genotype testing, as previously described [30], and were tested for the presence of GRN gene mutations. Exons 0–12 and the 3′ untranslated region of the GRN gene were amplified by polymerase chain reaction (PCR) assay using our previously published primers and protocol [31, 32]. The PCR amplicons were purified using the Multiscreen system (Millipore, Billerica, MA) and then sequenced in both directions using Big Dye chemistry following the manufacturer’s protocol (Applied Biosystems, Foster City, CA). Sequence products were purified using the Montage system (Millipore) prior to being run on an ABI 3730 DNA Analyzer. Sequence data was analyzed using either SeqScape or Sequencer software.
Image acquisition
All patients underwent a standardized magnetic resonance imaging (MRI) protocol at 3.0 T that included a 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence (TR/TE/T1, 2300/3/900 ms; flip angle 8°, 26 cm FOV; 256 × 256 in-plane matrix with a phase FOV of 0.94, slice thickness of 1.2 mm, in-plane resolution of 1 mm). In addition, all patients underwent both an 18-F fluorodeoxyglucose (FDG) and PiB-PET scan, acquired using a PET/CT scanner (GE Healthcare, Milwaukee, Wisconsin). For FDG-PET, patients were injected with FDG (average, 459 MBq; range, 367–576 MBq) in a dimly lit room with minimal auditory stimulation. After a 30-min uptake period, an 8-min FDG scan was performed consisting of four 2-min dynamic frames following a low-dose CT transmission scan. For PiB-PET, patients were injected with PiB (average, 614 MBq; range, 414–695 MBq), and after a 40–60 min uptake period, a 20 min PiB scan was obtained consisting of four 5-min dynamic frames following a low-dose CT transmission scan. Standard corrections were applied. Individual frames of the FDG and PiB dynamic series were realigned if motion was detected, and then a mean image was created. Emission data were reconstructed into a 256 × 256 matrix with a 30 cm FOV. The image thickness was 3.75 mm. Patients were classified as PiB-negative using a cortical-to-cerebellar (SUVR) ratio cut-point of less than 1.5, as previously defined [33].
Image analysis
To visualize the patterns of grey matter atrophy in individual patients, we created age and education-adjusted Z-score maps or STAND-Maps (Structural Abnormality due to NeuroDegeneration-Maps), which indicate the atrophy in terms of Z-scores relative to a group of cognitively normal controls in 120 brain regions [34]. In order to visualize patterns of FDG-PET hypometabolism in individual patients, we employed the clinical three-dimensional, stereotactic surface projections tool using Cortex ID (GE Healthcare, Waukesha, Wisconsin) [35]. Briefly, the activity in each FDG-PET image is normalized to the pons and compared with an age-segmented normative database, yielding a metabolic map of Z scores for each surface pixel.
Group-level analyses of the MPRAGE and FDG-PET scans were also performed using SPM5. All MPRAGE scans were normalized and segmented using customized priors and unified segmentation [36, 37], followed by the hidden Markov random-field clean-up step. Grey matter images were modulated and smoothed at 8-mm full-width-at-half-maximum. The FDG-PET images were then co-registered to the patients’ MPRAGE and normalized to the customized template using the normalization parameters from the MPRAGE normalization. All voxels in the FDG-PET image were normalized to the pons to form FDG uptake-ratio images. Two-sided t tests were performed to compare the GRN-negative and GRN-positive patients to a control cohort of 26 age- and gender-matched subjects that had undergone identical MRI and FDG-PET protocols. Direct comparisons between the GRN-positive and GRN-negative groups were also performed. Results were assessed uncorrected for multiple comparisons at p <0.001 with an extent threshold of 50 voxels. Age and gender were included in all comparisons as covariates.
Results
Patients
Seventy-six patients with an aphasia and/or apraxia of speech had a PiB-PET cut-off score of less than 1.5, and hence were β-amyloid-negative. Of these 76 patients, six patients (8 %) were diagnosed with lvPPA. All six patients had evidence of word retrieval deficits in spontaneous speech and in a picture description task. Demographic and clinical data for these six patients are shown in Table 1. Disease duration in all six patients was ≤3 years. All six patients had aphasia with a WAB aphasia quotient of <94.1 and difficulty comprehending complex sentences as measured by the Token Test. Five had sentence repetition difficulties, five had phonological errors that were mild to moderate in severity, and four had evidence of anomia with a score of <11 on the BNT. No patient had unequivocal evidence of apraxia of speech as measured by the ASRS, and none had a score of 5 or less on the fluency score of the WAB, which would have been evidence for agrammatism. In addition, a review of the spoken and written WAB narrative picture description responses of each patient (Table 2), independent of the WAB fluency score, yielded consensus that agrammatism was not evidence. Patient 3 performed within the abnormal range on the PPT and the Auditory Word Recognition subtest of the WAB, suggesting deficits in object and word knowledge, respectively. This patient, however, was severely impaired across all language, neurological and neuropsychological measures, except for recognizing famous faces. Importantly, the most severe features in this patient were still those typical of lvPPA. No patients had more than mild parkinsonism on the UPDRS, while almost all patients, except patient 1, had executive dysfunction as evident on the neurological and neuropsychological testing. Memory performance on the AVLT was impaired for all patients.
Table 1.
Demographic and clinical characteristics for each patient
|
GRN-positive patients
|
GRN-negative patients
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | ALLb | 4 | 5 | 6 | ALLb | |
| Demographics | ||||||||
| Gender | M | M | F | 67 % male | M | F | M | 67 % male |
| Handedness | R | R | R | 100 % R | R | R | R | 100 % R |
| Education (years) | 14 | 18 | 12 | 14.7 ± 3.1 | 18 | 18 | 17 | 17.7 ± 0.6 |
| Illness duration (years) | 2 | 3 | 2 | 2.3 ± 0.6 | 2 | 3 | 2 | 2.3 ± 0.6 |
| Age at onset (years) | 56 | 61 | 56 | 57.7 ± 2.9* | 65 | 66 | 66 | 65.7 ± 0.6 |
| Family history | Y | Y | Y | 100 % yesc | N | N | N | 0 % yes |
| PiB ratio | 1.1 | 1.2 | 1.1 | 1.1 ± 0.1* | 1.4 | 1.3 | 1.4 | 1.3 ± 0.1 |
| Apolipoprotein E genotype | 3,4 | 3,3 | 3,3 | 25 % e4 | 3,3 | 3,3 | 3,4 | 25 % e4 |
| Neurological | ||||||||
| MoCA (/30) | 22 | 20 | 2 | 14.7 ± 11.0 | 13 | 17 | 18 | 16.0 ± 2.7 |
| FTLD-modified Clinical Dementia Rating Scale Sum of Boxes (/24) | 3 | 1 | 16 | 6.7 ± 8.1 | 2 | 4 | 8 | 4.7 ± 3.1 |
| Frontal Assessment Battery (/18) | 18 | 14 | 4 | 12.0 ± 7.2 | 14 | 6 | 12 | 10.7 ± 4.2 |
| FBI (/72) | 13 | 17 | 20 | 16.7 ± 3.5 | 1 | 3 | 32 | 12.0 ± 17.4 |
| Faces (/10) | 9 | 10 | 10 | 9.7 ± 0.6 | 10 | 10 | 10 | 10.0 ± 0.0 |
| Ideomotor apraxia (/60) | 59 | 57 | 43 | 53.0 ± 8.7 | 59 | 54 | 60 | 57.7 ± 3.2 |
| UPDRS III (/132) | 0 | 8 | 0 | 2.7 ± 4.6 | 4 | 4 | 13 | 7.0 ± 5.2 |
| NPI-Q total score (/36) | 3 | 3 | 2 | 2.7 ± 0.6 | 1 | 1 | 7 | 3.0 ± 3.5 |
| Speech and language | ||||||||
| WAB Aphasia Quotient (/100) | 90.2 | 85.3 | 39.3 | 71.6 ± 28.1 | 74.4 | 79.3 | 89.8 | 81.2 ± 7.9 |
| WAB Repetition (/10) | 7.4 | 8.2 | 3 | 6.2 ± 2.8 | 7.6 | 6.6 | 9.2 | 7.8 ± 1.3 |
| WAB Information content (/10) | 6 | 8 | 8 | 7.3 ± 3.8 | 8 | 8 | 9 | 8.3 ± 0.6 |
| WAB Fluency (/10) | 9 | 8 | 7 | 8.0 ± 1.0 | 6 | 8 | 8 | 7.3 ± 1.2 |
| WAB Auditory recognition (/60) | 60 | 59 | 39 | 52.7 ± 11.9 | 57 | 60 | 60 | 59.0 ± 1.7 |
| Boston naming test (/15) | 14 | 11 | 0 | 8.3 ± 7.4 | 2 | 12 | 11 | 8.3 ± 5.5 |
| Phonological error severity (/5) | 2 | 1 | 2 | 1.7 ± 0.6 | 1 | 0 | 1 | 0.7 ± 0.6 |
| Pyramids and palm trees (/52) | 52 | 51 | 40 | 47.7 ± 6.7 | 46 | 48 | 45 | 46.3 ± 1.5 |
| Token test (/22) | 14 | 7 | 0 | 7.0 ± 7.0 | 7 | 9 | 13 | 9.7 ± 3.1 |
| Reading irregular words (/10) | 10 | 10 | 0 | 6.7 ± 5.8 | 3 | 9 | 9 | 7.0 ± 3.5 |
| Reading non-words (/10) | 10 | 9 | 0 | 6.3 ± 5.5 | 0 | 5 | 3 | 2.7 ± 2.5 |
| ASRS (/60) | 0 | 0 | 2 | 0.7 ± 1.2 | 2 | 0 | 1 | 1.0 ± 1.0 |
| Action fluency | 12 | 7 | 0 | 6.3 ± 6.0 | 9 | 5 | 4 | 6.0 ± 2.7 |
| Letter fluency | 32 | 18 | 0 | 16.7 ± 16.0 | 8 | 11 | 13 | 10.7 ± 2.5 |
| Animal fluency | 20 | 16 | 0 | 12.0 ± 10.6 | 9 | 7 | 11 | 9.0 ± 2.0 |
| Neuropsychological | ||||||||
| TMT A MOANSa | 10 | 12 | 2 | 8.0 ± 5.3 | 9 | 6 | 9 | 8.0 ± 1.7 |
| TMT B MOANSa | 13 | 8 | 0 | 7.0 ± 6.6 | 6 | 2 | 6 | 4.7 ± 2.3 |
| DKEFS card sorta | 8 | 6 | 0 | 4.7 ± 4.2 | 6 | 1 | 4 | 3.7 ± 2.5 |
| AVLT delayed recall MOANSa | 5 | 6 | 2 | 4.3 ± 2.1 | 5 | 3 | 6 | 4.7 ± 1.5 |
| AVLT % retention MOANSa | 6 | 8 | 2 | 5.3 ± 3.1 | 6 | 3 | 14 | 7.7 ± 5.7 |
| Rey-O MOANSa | 11 | 10 | 2 | 7.7 ± 4.9 | 9 | 6 | 10 | 8.3 ± 2.1 |
| VOSP incomplete letters (/20) | 19 | 19 | 9 | 15.7 ± 5.8 | 19 | 20 | 20 | 19.7 ± 0.6 |
| VOSP cube analysis (/10) | 10 | 10 | 4 | 8.0 ± 3.5 | 9 | 10 | 10 | 9.7 ± 0.6 |
Significant difference observed between GRN-positive and GRN-negative patients
The MOANS and DKEFS Card Sort are constructed to have a mean of 10 and standard deviation of 3 in cognitively healthy participants
Data shown as mean ± standard deviation
Patient 1 had a mother with dementia (Alzheimer’s disease) and a maternal uncle who had difficulty speaking and diagnosed with Alzheimer’s disease with age of onset in his 50s. Patient 2 had a brother with primary progressive aphasia. Patient 3 had a maternal aunt and uncle with aphasia
Table 2.
Excerpts from spoken and written WAB picture descriptions for each patient, illustrating the absence of agrammatism, and language output characteristics not incompatible with lvPPA
| GRN-positive patient 1 |
| Verbal picture description: “OK, well there’s, there’s kids, it’s in the summer and they’re havin’ a, by the lake and doin’, flyin’ a kite, and they’re, they’re out there fishing, and sand, she’s makin’ a sand picture.” |
| Written picture description: “This picture is a beautiful summer setting. The kids are having fun.” |
| GRN-positive patient 2 |
| Verbal picture description: “There’s the house and the tree and the car and the flag, and then there’s the woman and the man are having the, uh, picnic basket, and here’s a kid having, um, kite, and the dog is following him…” |
| Written picture description: “My mother and father are eating a picnic on a basket and blanket. My child has been running with a kite and a dog was following my child.” |
| GRN-positive patient 3 |
| Verbal picture description: “Well, it’s the time getting all this crap. And here, there. Yea, I don’t know. Well, the dog, or the thing.” |
| Written picture description: Patient initially attempted to copy the picture. Then, with reinstruction, wrote only “Thanks.” |
| GRN-negative patient 4 |
| Verbal picture description: “It’s a family. And they’re…kids are playing with the dogs…Don’t know what that is there, but looks like some kind of, er, fish.” |
| Written picture description: “Home with family and with kits. Father and wife with f” |
| GRN-negative patient 5 |
| Verbal picture description: “We’re going to go look at some pretty pictures. We’re going to see a sail…dot, and we’re liking to read… playing with the dogs, we like that…we like to have all the sand, and things like that they do for making castles…” |
| Written picture description:” We are going to a lovely home and family…We fish for good times and the sand for the small kids and a great salad and for the family.” |
| GRN-negative patient 6 |
| Verbal picture description: “Well, the picture is of a vacation area, uh, with a young boy with a dog following behind him is leading a cat…kite, up over the house where there is a car.” |
| Written picture description: “The Family of John and Mary went caning on the beach of Mayo.” |
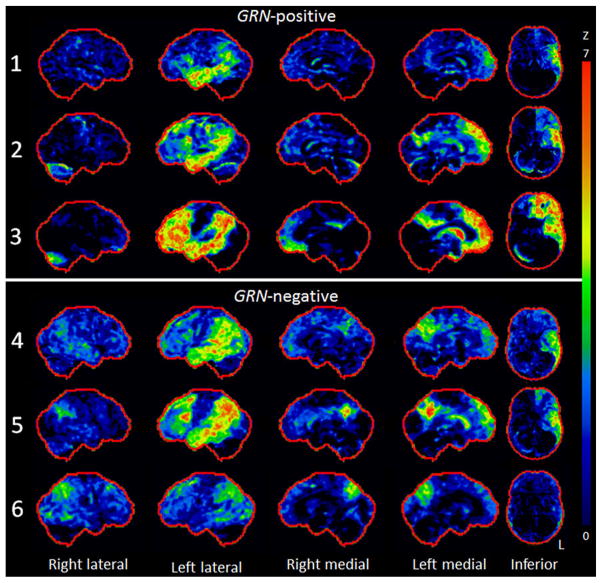
Patients 1–5 all showed asymmetric FDG hypometab-olism throughout the left lateral temporoparietal cortex, with hypometabolism spreading into the lateral anterior temporal lobe in all cases (Fig. 1). Patient 6 showed milder involvement of the left lateral temporal lobe, with more striking and bilateral involvement of the parietal lobe. The left precuneus and posterior cingulate was involved in patients 2–6, with the right precuneus or posterior cingulate involved in cases 3–6. The left lateral and medial frontal lobe also showed hypometabolism in all patients, with very little involvement in the right hemisphere. The left caudate nucleus was involved in all cases. MRI revealed asymmetric left lateral temporal atrophy in all patients, with extension into the parietal cortex in the majority (Fig. 2). Most patients showed some left medial temporal atrophy, although it was severe in patient 3.
Fig. 1.
Stereotactic surface projection maps showing regions of hypometabolism in each GRN-positive and GRN-negative patient. All patients showed left parietal and temporal hypometabolism, which was particularly severe in patient 3, who also showed striking left frontal lobe hypometabolism. Less temporal lobe hypometabolism was observed in patient 6, who also showed a more bilateral pattern
Fig. 2.

STAND maps showing regional grey matter atrophy in each GRN-positive and GRN-negative patient. All patients showed left temporal lobe atrophy, although the GRN-positive patients appeared to have more severe atrophy with extension into the left parietal lobe
GRN-positive versus GRN-negative
Three of the six lvPPA patients were found to have a pathogenic mutation in the GRN gene (patients 1–3, Table 1). Those with a GRN gene mutation (GRN-positive) were younger at onset and had lower PiB ratios compared to those without a GRN gene mutation (GRN-negative). All of the GRN-positive patients, but none of the GRN-negative patients, had a family history of dementia (Table 1). There was no striking difference in speech and language, neurological or neuropsychological profiles, or apolipoprotein genotype, between the GRN-positive and negative patients.
There were no obvious differences on FDG-PET between the GRN-positive and GRN-negative patients (Fig. 1). Similarly, on MRI, the patterns of abnormalities observed in both groups were similar, although the GRN-positive patients by visual inspection appeared to have more atrophy in the affected regions (Fig. 2).
In the group level maps (Fig. 3), the GRN-negative group showed grey matter volume loss in the left inferior temporal gyrus compared to controls. The GRN-positive group showed more widespread involvement of the left lateral and medial temporal lobe. Both groups showed FDG hypometabolism in the left temporoparietal cortex, although the GRN-positive group showed more striking involvement of the medial temporal and frontal lobe, and the GRN-negative group showed greater bilateral involvement of the precuneus.
Fig. 3.
Voxel-level group maps showing grey matter atrophy and hypometabolism in the GRN-positive and GRN-negative groups compared to controls and to each other. Grey matter results are shown in blue and hypometabolism results are shown in green. The GRN-positive and GRN-negative groups showed grey matter loss in the left temporal lobe, with greater loss observed in the anterior temporal lobe in the GRN-positive group. Both groups showed hypometabolism in the left temporoparietal cortex, with greater hypometabolism observed in the medial temporal lobe in the GRN-positive group
On direct comparison between the groups (Fig. 3), the GRN-positive group showed greater grey matter loss and hypometabolism in the left parahippocampal gyrus, and greater grey matter loss in the left anterior temporal lobe and anterior frontal lobe, compared to the GRN-negative group. No regions showed greater involvement in the GRN-negative group compared to the GRN-positive group.
Discussion
In this study, we report six patients with lvPPA who were PiB-negative and hence were not associated with AD pathology, and demonstrate that lvPPA in fact accounts for a small proportion of PiB-negative neurodegenerative apraxia of speech and aphasias.
All six patients in the study presented with features typical for lvPPA, including poor word retrieval in general conversation, anomia, phonological errors, and difficulties with sentence repetition. Consistent with the clinical findings, all six patients displayed asymmetric patterns of atrophy and hypometabolism typical for lvPPA [3, 10, 38], predominantly affecting the left lateral temporal and parietal lobe, with additional involvement of the precuneus and posterior frontal lobe in some patients. Interestingly, the anterior temporal pole was affected in five of the six patients, which is somewhat unusual for lvPPA, and would be more typical of the semantic variant of PPA [39]. Only patient 3 displayed features suggestive of semantic aphasia, with poor performance on the PPT and the Auditory Word Recognition subtest of the WAB. Patient 3, however, was very severely impaired across all clinical and imaging testing, and lvPPA features were still the most prominent. This patient appears to be atypical, having a rapidly progressive phenotype, yet an illness duration of only 2 years [40]. No patients had impairment in recognizing faces and none had surface dyslexia with poor reading of irregular words and preserved reading of non-words. The preserved ability to recognize faces would be consistent with sparing of the right anterior temporal lobe, specifically the fusiform gyrus [41]. Although memory impairment and mild medial temporal atrophy was evident in most patients, none presented with complaints of memory loss, and in all patients it was the language impairment that was affecting activities in daily living, consistent with a diagnosis of PPA. Similarly, although executive dysfunction was identified in all but one patient, it was never a presenting feature. Most likely, executive dysfunction was due to involvement of the left frontal lobe. However, none of the patients presented with behavioral dyscontrol and in only one patient did the spouse endorse significant behavioral dyscontrol. In general, the FDG-PET data showed greater involvement of the parietal lobes than the MRI data, which concurs with our previous findings in a larger cohort of lvPPA patients [38]. It is possible that metabolic changes may be occurring in these regions before the onset of atrophy, or, alternatively, FDG-PET may be more sensitive to abnormalities in these regions in lvPPA patients.
In our cohort of six lvPPA PiB-negative patients, 50 % were found to have mutations in the GRN gene. All of these patients also had a family history of dementia, while the GRN-negative patients did not. The presence of a family history with a negative PiB-PET scan is therefore a strong indicator of the presence of a GRN mutation in lvPPA. In addition, the patients with GRN mutations were the youngest of the six patients, with two of the three patients showing disease onset in their 50s. Young onset in the 5th and 6th decade is typical for patients with GRN mutations [42–44], but is also common for patients with lvPPA due to AD [6, 38], and therefore age by itself may not be predictive; young age in the context of family history may, however, be more compelling. The young age of these patients may also explain why the PiB-PET ratios were slightly lower than those in the patients without GRN mutations, although it could reflect the fact that GRN-positive patients simply have an absent to low β-amyloid burden.
Despite differences in family history and age, there were no other clinical or neuropsychological differences observed between the patients with and without GRN mutations. The groups performed similarly on speech and language testing, neurological testing, and on neuropsychological testing of memory, executive and visuospatial performance. Therefore, while we show that GRN-positive patients can present with lvPPA, we could not confirm that such patients have a unique clinical signature [11]. A larger sample size would be needed to better address this issue. Patterns of atrophy and hypometabolism were also similar across the GRN-positive and GRN-negative groups, although there was a trend for the GRN-positive patients to show more severe patterns of left anterior temporal lobe involvement. Two lvPPA patients with GRN mutations have similarly been reported to show anterior temporal atrophy [45]. One patient in particular (patient 3) that had a mutation in GRN showed a very severe and asymmetric pattern of atrophy and hypometabolism and a very severe clinical profile, with poor performance observed across the majority of tests, despite a disease duration of only 2 years. Rapid disease progression and fast rates of atrophy [46, 47] have indeed been associated with GRN mutations, and likely explain the rapid course in this patient. Highly asymmetric atrophy has also been observed in GRN mutation carriers [48, 49]. The other two patients with GRN mutations were, however, more similar in severity to the GRN-negative patients, and so would likely be difficult to differentiate based on imaging or clinical features alone.
The clinical syndrome of lvPPA accounts for a small minority of β-amyloid-negative speech and language disorders. A high proportion of these patients will likely harbor underlying FTLD pathology. In fact, given the finding of GRN mutations in our cohort, it appears that at least 50 % will have FTLD pathology with deposition of the transactive response (TAR) DNA-binding protein of 43 kDa (FTLD-TDP); specifically FTLD-TDP type A [50], which is always observed in patients with GRN mutations [51]. Therefore, lvPPA is associated with FTLD-TDP type A pathology in PiB-negative patients. Future studies are now needed to compare PiB-negative and PiB-positive lvPPA.
Acknowledgments
This study was funded by a National Institutes of Health (NIH) grant, R01 DC010367 (PI Josephs), and an Alzheimer’s Association grant, NIRG-12-242215 (PI Whitwell).
Footnotes
Conflicts of interest The authors declare that they have no conflicts of interest.
Contributor Information
Keith A. Josephs, Email: josephs.keith@mayo.edu, Department of Neurology (Division of Behavioral Neurology), Mayo Clinic, Rochester, MN, USA
Joseph R. Duffy, Department of Neurology (Division of Speech Pathology), Mayo Clinic, Rochester, MN, USA
Edythe A. Strand, Department of Neurology (Division of Speech Pathology), Mayo Clinic, Rochester, MN, USA
Mary M. Machulda, Department of Psychiatry and Psychology (Division of Neuropsychology), Mayo Clinic, Rochester, MN, USA
Prashanthi Vemuri, Department of Radiology, Mayo Clinic, Rochester, MN, USA.
Matthew L. Senjem, Department of Information Technology, Mayo Clinic, Rochester, MN, USA
Ralph B. Perkerson, Department of Neuroscience, Mayo Clinic, Jacksonville, FL, USA
Matthew C. Baker, Department of Neuroscience, Mayo Clinic, Jacksonville, FL, USA
Val Lowe, Department of Radiology, Mayo Clinic, Rochester, MN, USA.
Clifford R. Jack, Jr., Department of Radiology, Mayo Clinic, Rochester, MN, USA
Rosa Rademakers, Department of Neuroscience, Mayo Clinic, Jacksonville, FL, USA.
Jennifer L. Whitwell, Department of Radiology, Mayo Clinic, Rochester, MN, USA
References
- 1.Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–1014. doi: 10.1212/WNL.0b013e31821103e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Josephs KA, Duffy JR, Fossett TR, Strand EA, Claassen DO, Whitwell JL, et al. Fluorodeoxyglucose F18 positron emission tomography in progressive apraxia of speech and primary progressive aphasia variants. Arch Neurol. 2010;67:596–605. doi: 10.1001/archneurol.2010.78. [DOI] [PubMed] [Google Scholar]
- 3.Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–346. doi: 10.1002/ana.10825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, et al. Subtypes of progressive aphasia: application of the international consensus criteria and validation using beta-amyloid imaging. Brain J Neurol. 2011;134:3030–3043. doi: 10.1093/brain/awr216. [DOI] [PubMed] [Google Scholar]
- 5.Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–719. doi: 10.1002/ana.21388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Abeta amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401. doi: 10.1002/ana.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–49. doi: 10.1212/WNL.0b013e3181c7198e. [DOI] [PubMed] [Google Scholar]
- 8.Josephs KA, Duffy JR, Strand EA, Whitwell JL, Layton KF, Parisi JE, et al. Clinicopathological and imaging correlates of progressive aphasia and apraxia of speech. Brain. 2006;129:1385–1398. doi: 10.1093/brain/awl078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Josephs KA, Hodges JR, Snowden JS, Mackenzie IR, Neumann M, Mann DM, et al. Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol. 2011;122:137–153. doi: 10.1007/s00401-011-0839-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Teichmann M, Kas A, Boutet C, Ferrieux S, Nogues M, Samri D, et al. Deciphering logopenic primary progressive aphasia: a clinical, imaging and biomarker investigation. Brain. 2013;136:3474–3488. doi: 10.1093/brain/awt266. [DOI] [PubMed] [Google Scholar]
- 11.Rohrer JD, Crutch SJ, Warrington EK, Warren JD. Progranulin-associated primary progressive aphasia: a distinct phenotype? Neuropsychologia. 2010;48:288–297. doi: 10.1016/j.neuropsychologia.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Master AV, et al. Characterizing a neurodegenerative syndrome: primary progressive apraxia of speech. Brain J Neurol. 2012;135:1522–1536. doi: 10.1093/brain/aws032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Josephs KA, Duffy JR, Strand EA, Machulda MM, Senjem ML, Lowe VJ, et al. Syndromes dominated by apraxia of speech show distinct characteristics from agrammatic PPA. Neurology. 2013;81:337–345. doi: 10.1212/WNL.0b013e31829c5ed5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kertesz A. Western Aphasia Battery (Revised) Psych-Corp; San Antonio: 2007. [Google Scholar]
- 15.De Renzi E, Vignolo LA. The token test: a sensitive test to detect receptive disturbances in aphasics. Brain. 1962;85:665–678. doi: 10.1093/brain/85.4.665. [DOI] [PubMed] [Google Scholar]
- 16.Lansing AE, Ivnik RJ, Cullum CM, Randolph C. An empirically derived short form of the Boston naming test. Arch Clin Neuropsychol. 1999;14:481–487. [PubMed] [Google Scholar]
- 17.Woods SP, Scott JC, Sires DA, Grant I, Heaton RK, Troster AI. Action (verb) fluency: test-retest reliability, normative standards, and construct validity. J Int Neuropsychol Soc. 2005;11:408–415. [PubMed] [Google Scholar]
- 18.Loonstra AS, Tarlow AR, Sellers AH. COWAT meta-norms across age, education, and gender. Appl Neuropsychol. 2001;8:161–166. doi: 10.1207/S15324826AN0803_5. [DOI] [PubMed] [Google Scholar]
- 19.Howard D, Patterson K. The pyramids and palm trees test: a test of semantic access from words and picture. Thames Valley Test Company; Bury St Edmunds, UK: 1992. [Google Scholar]
- 20.Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 21.Kertesz A, Davidson W, Fox H. Frontal behavioral inventory: diagnostic criteria for frontal lobe dementia. Can J Neurol Sci. 1997;24:29–36. doi: 10.1017/s0317167100021053. [DOI] [PubMed] [Google Scholar]
- 22.Kaufer DI, Cummings JL, Ketchel P, Smith V, MacMillan A, Shelley T, et al. Validation of the NPI-Q, a brief clinical form of the neuropsychiatric inventory. J Neuropsychiatry Clin Neurosci. 2000;12:233–239. doi: 10.1176/jnp.12.2.233. [DOI] [PubMed] [Google Scholar]
- 23.Goetz CG, Tilley BC, Shaftman SR, Stebbins GT, Fahn S, Martinez-Martin P, et al. Movement disorder society-sponsored revision of the unified Parkinson’s disease rating scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord Off J Mov Disord Soc. 2008;23:2129–2170. doi: 10.1002/mds.22340. [DOI] [PubMed] [Google Scholar]
- 24.Spreen O, Strauss E. A compendium of neuropsychological tests: administration, norms, and commentary. Oxford University Press; New York: 1991. [Google Scholar]
- 25.Delis D, Kaplan E, Kramer J. Delis-Kaplan executive function system (DKEFS): examiner’s manual. The Psychological Corporation; San Antonio: 2001. [Google Scholar]
- 26.Rey A. L’examen clinique en psychologie. Presses Universitaires de France; Paris: 1964. [Google Scholar]
- 27.Warrington E, James M. The visual object and space perception battery. Thames Valley Test Company; Bury St Edmonds: 1991. [Google Scholar]
- 28.Osterrieth PA. Le test de copie d’une figure complexe. Archives de Psychologie. 1944;30:206–356. [Google Scholar]
- 29.Ivnik RJ, Malec J, Smith GE, Tangalos EG, Petersen RC, Kokmen E. Mayo’s older American normative studies: WAIS-R, WMS-R and AVLT norms for ages 56–97. Clin Neuropsychol. 1992;6(supplement):1–104. [Google Scholar]
- 30.Josephs KA, Tsuboi Y, Cookson N, Watt H, Dickson DW. Apolipoprotein E epsilon 4 is a determinant for Alzheimer-type pathologic features in tauopathies, synucleinopathies, and frontotemporal degeneration. Arch Neurol. 2004;61:1579–1584. doi: 10.1001/archneur.61.10.1579. [DOI] [PubMed] [Google Scholar]
- 31.Baker M, Mackenzie IR, Pickering-Brown SM, Gass J, Rademakers R, Lindholm C, et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature. 2006;442:916–919. doi: 10.1038/nature05016. [DOI] [PubMed] [Google Scholar]
- 32.Gass J, Cannon A, Mackenzie IR, Boeve B, Baker M, Adamson J, et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum Mol Genet. 2006;15:2988–3001. doi: 10.1093/hmg/ddl241. [DOI] [PubMed] [Google Scholar]
- 33.Jack CR, Jr, Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, et al. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vemuri P, Simon G, Kantarci K, Whitwell JL, Senjem ML, Przybelski SA, et al. Antemortem differential diagnosis of dementia pathology using structural MRI: differential-STAND. Neuroimage. 2011;55:522–531. doi: 10.1016/j.neuroimage.2010.12.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Minoshima S, Frey KA, Koeppe RA, Foster NL, Kuhl DE. A diagnostic approach in Alzheimer’s disease using three-dimensional stereotactic surface projections of fluorine-18-FDG PET. J Nucl Med. 1995;36:1238–1248. [PubMed] [Google Scholar]
- 36.Ashburner J, Friston KJ. Voxel-based morphometry—the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 37.Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 38.Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer’s type. PLoS One. 2013;8:62471. doi: 10.1371/journal.pone.0062471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chan D, Fox NC, Scahill RI, Crum WR, Whitwell JL, Leschziner G, et al. Patterns of temporal lobe atrophy in semantic dementia and Alzheimer’s disease. Ann Neurol. 2001;49:433–442. [PubMed] [Google Scholar]
- 40.Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, et al. Identification of an atypical variant of logopenic progressive aphasia. Brain Lang. 2013;127(2):139–144. doi: 10.1016/j.bandl.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Josephs KA, Whitwell JL, Vemuri P, Senjem ML, Boeve BF, Knopman DS, et al. The anatomic correlate of prosopagnosia in semantic dementia. Neurology. 2008;71:1628–1633. doi: 10.1212/01.wnl.0000334756.18558.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitwell JL, Jack CR, Jr, Baker M, Rademakers R, Adamson J, Boeve BF, et al. Voxel-based morphometry in frontotemporal lobar degeneration with ubiquitin-positive inclusions with and without progranulin mutations. Arch Neurol. 2007;64:371–376. doi: 10.1001/archneur.64.3.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Skoglund L, Brundin R, Olofsson T, Kalimo H, Ingvast S, Blom ES, et al. Frontotemporal dementia in a large Swedish family is caused by a progranulin null mutation. Neurogenetics. 2009;10:27–34. doi: 10.1007/s10048-008-0155-z. [DOI] [PubMed] [Google Scholar]
- 44.Pickering-Brown SM, Rollinson S, Du Plessis D, Morrison KE, Varma A, Richardson AM, et al. Frequency and clinical characteristics of progranulin mutation carriers in the Manchester frontotemporal lobar degeneration cohort: comparison with patients with MAPT and no known mutations. Brain. 2008;131:721–731. doi: 10.1093/brain/awm331. [DOI] [PubMed] [Google Scholar]
- 45.Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2010;49:984–993. doi: 10.1016/j.neuroimage.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mahoney CJ, Beck J, Rohrer JD, Lashley T, Mok K, Shakespeare T, et al. Frontotemporal dementia with the C9ORF72 hexanucleotide repeat expansion: clinical, neuroanatomical and neuropathological features. Brain. 2012;135:736–750. doi: 10.1093/brain/awr361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Whitwell JL, Weigand SD, Gunter JL, Boeve BF, Rademakers R, Baker M, et al. Trajectories of brain and hippocampal atrophy in FTD with mutations in MAPT or GRN. Neurology. 2011;77:393–398. doi: 10.1212/WNL.0b013e318227047f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Whitwell JL, Weigand SD, Boeve BF, Senjem ML, Gunter JL, DeJesus-Hernandez M, et al. Neuroimaging signatures of frontotemporal dementia genetics: C9ORF72, tau, progranulin and sporadics. Brain. 2012;135:794–806. doi: 10.1093/brain/aws001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Beck J, Rohrer JD, Campbell T, Isaacs A, Morrison KE, Goodall EF, et al. A distinct clinical, neuropsychological and radiological phenotype is associated with progranulin gene mutations in a large UK series. Brain. 2008;131:706–720. doi: 10.1093/brain/awm320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mackenzie IR, Neumann M, Baborie A, Sampathu DM, Du Plessis D, Jaros E, et al. A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol. 2011;122:111–113. doi: 10.1007/s00401-011-0845-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bigio EH. Update on recent molecular and genetic advances in frontotemporal lobar degeneration. J Neuropathol Exp Neurol. 2008;67:635–648. doi: 10.1097/NEN.0b013e31817d751c. [DOI] [PMC free article] [PubMed] [Google Scholar]