Abstract
Individual animals differ in their propensity to engage in dangerous situations, or in their risk-taking behavior. There is a heritable basis to some of this variation, but the environment plays an important role in shaping individuals’ risk-taking propensity as well. This chapter describes some of the challenges in studying the genetic basis of individual differences in risk-taking behavior, arguing new insights will emerge from studies which take a whole-genome approach and which simultaneously consider both genetic and environmental influences on the behavior. The availability of genomic tools for three-spined stickleback, a small fish renowned for its variable behavior, opens up new possibilities for studying the genetic basis of natural, adaptive variation in risk-taking behavior. After introducing the general biology of sticklebacks, the chapter summarizes the existing literature on the genetic and environmental influences on risk-taking behavior, and describes the overall strategy that our group is taking to identify inherited and environmentally responsive genes related to risk-taking behavior in this species. Insights gleaned from such studies will be relevant to our understanding of similar behaviors in other organisms, including ourselves.
I. INTRODUCTION
In a wide variety of organisms, individuals differ in their propensity to take risks in different situations. For example, mice show interindividual differences in behavior in an open field (Defries et al., 1966), monkeys vary in aggressiveness (Suomi, 1987), and individual fish differ in their reaction to a predator (Huntingford, 1976). For the purposes of this review, I adopt a very inclusive definition of risk-taking behavior that can encompass this diversity. What is common to all of the examples above is that there is an element of danger involved in the behavior—from the chance of encountering a threat in a new environment, to the probability of injury in fights, to the possibility of death during interactions with a predator. Broadly construed, risk-taking behaviors are expressed in dangerous situations and increase the chance that an individual is injured or even dies.
Individual differences in the propensity to engage in risk-taking behavior have consequences for social groups for several reasons. First, risk-taking behaviors can influence other members of the social group. For example, high levels of aggression can influence other members of a social group by excluding nonaggressive individuals from access to resources, or via the effects of winning or losing fights on subsequent levels of aggression of others (the winner–loser effect). Diverse behaviors such as predator inspection behavior in fishes, mobbing behavior in birds, or alarm calling in rodents could reflect individual differences in the propensity to engage in dangerous situations around predators, and have consequences for the safety of other members of the group. Second, it is likely that the fitness of any given behavioral type (e.g., risk averse versus risk prone) depends on the frequency of other behavioral types within the social group, that is, fitness is frequency dependent. Therefore, understanding the causes and consequences of individual differences in risk-taking behavior would need to take into consideration the social context in which risk-taking behavior is expressed. Third, social dynamics could be influenced by the frequency of risk-taking phenotypes within the social group. Imagine a group composed entirely of risk-taking behavioral types, versus a group composed of risk-averse behavioral types. It is likely that such groups have different population dynamics and even fitness. Indeed, an intriguing question is whether there might be an optimal combination of behavioral types within the social group (Sih and Watters, 2005). Finally, individual differences in exploratory behavior, or response to novelty, have consequences for dispersal and the colonization of new environments by a founding social group of individuals (Duckworth and Badyaev, 2007).
Individual differences in the propensity to engage in dangerous situations are often influenced by both genetic and environmental factors, and have relevance for human health and disease. Indeed, problems such as self-harm, addiction, sexual risk-taking, and violence have serious adverse consequences in humans. Despite the obvious costs to individuals and society and importance for health, we know relatively little about the etiology of risk-taking behaviors associated with these afflictions. While there are several candidate genes for related behaviors, candidate genes explain only a small fraction of the total genetic variation, suggesting that a whole-genome approach is likely to identify novel genes and pathways that are currently unknown. After describing some of the challenges that confront us as we try to understand the genetic basis of risk-taking behaviors in humans and other organisms, I describe the approach that we are taking to identify inherited and environmentally responsive genes related to risk-taking behaviors in a fresh new model system, three-spined stickleback fish.
II. CHALLENGES AND APPROACHES FOR STUDYING THE GENETICS OF RISK-TAKING BEHAVIOR IN HUMANS AND OTHER ANIMALS
There is a large literature investigating the genetic and neurochemical causes of variation in risk-taking behavior in humans and nonhuman animals. Some of the most recent findings have confirmed that human risk-taking behaviors related to psychopathology are influenced by inherited genetic factors (Kendler et al., 2003). Animal models for related behaviors such as fearlessness and impulsiveness (Strandberg et al., 2005), fearfulness (Boissy, 1995), aggression (Miczek et al., 2001), anxiety and depression (Adamec et al., 2006; Flint et al., 1995), and substance dependence (Crabbe, 2002) have extended these findings by elucidating the mechanisms underlying the specific behaviors. Progress in this field has been facilitated by the use of powerful tools such as microarrays (Kroes et al., 2006) and knockouts (Adamec et al., 2006) to find genes related to behavior.
However, many challenges remain as we try to study the genetic basis of risk-taking behavior, especially in identifying particular genes that might be related to variation in risk-taking behaviors. An immediate challenge is that like all complex traits, it is likely that a large number of genes, each of small effect, are involved in regulating risk-taking behavior (Kendler and Greenspan, 2006; Mackay, 2009). The sample sizes needed to identify quantitative trait loci (QTL) of small effect are prohibitively large (n >1000), especially when we wish to understand how genes are responsive to the environment (Mackay, 2004; Plomin, 2005), and require huge numbers of markers (>1000) in order to find QTL, making this an approach available to traditional model organisms, such as mouse (reviewed in Hovatta and Barlow, 2008; Willis-Owen and Flint, 2007) and some domesticated animals, for example, chickens (Wiren et al., 2009), quail (Beaumont et al., 2005), and cattle (Gutierrez-Gil et al., 2008).
Indeed, even when candidate genes have been proposed based on neurochemical hypotheses or QTL studies, specific candidate genes (e.g., DRD4, SERT, MAOA) for related behaviors only explain a small fraction of the total genetic variation (Reif and Lesch, 2003), indicating that we have yet to learn the identity of most of the important genes. Another recurring problem that has plagued candidate gene studies is the failure to replicate. While some studies, for example, find an association between the serotonin transporter polymorphism and behavior, other studies do not (reviewed in Reif and Lesch, 2003). At this point it is unclear how much of the inconsistency among studies is due to methodological problems, that is, population stratification, or due to real genetic differences between populations or species in the genetic mechanisms underlying similar behaviors.
Another challenge is that although there often appears to be some genetic component to risk-taking behavior, it cannot be denied that early experience affects related behaviors in both humans (Farrington, 2005) and nonhuman animals (Caldji et al., 2000; Meaney, 2001), and there is mounting evidence that the environment can influence behavior in a genotype-specific way (G×E interaction) (Caspi and Moffitt, 2006; Eaves et al., 2003). Arguably, the ubiquity (and effect size, >75% of the phenotypic variation in some cases) of genotype by environment interactions (Caspi and Moffitt, 2006; Kaufman et al., 2006) is an indication that studies will have the biggest impact if they simultaneously consider both genetic and environmental factors.
There has been considerable interest in recent years for using gene expression microarrays to identify genes related to behavior. Some of the advantages of measuring whole genome expression using microarrays are that the approach is unbiased and open-ended, and that it considers the coordinated action of the entire genome rather than focusing on one gene at a time. Moreover, microarrays are efficient, in that each microarray is its own self-contained experiment, which facilitates our ability to compare relative expression across genes. Also, microarrays are good for nonmodel systems in which it is not feasible to use traditional forward genetic approaches.
Behavioral experiments using microarrays have compared gene expression following different kinds of experiences, across different behavioral types, or among individuals from different populations or strains. Some studies have compared whole genome expression between groups that have or have not experienced some treatment or challenge that elicits a behavioral response, assuming that whatever genes are differentially expressed are related to the differing behavioral reactions. For example, studies have examined the genomic response to song in birds (London et al., 2009), female response to courtship or mating in Drosophila (Carney, 2007; Kapelnikov et al., 2008; Lawniczak and Begun, 2004; Mack et al., 2006; McGraw et al., 2008), swordtail fish (Cummings et al., 2008), and honeybees (Kocher et al., 2008), and male aggression in Drosophila (Wang et al., 2008a,b). Related to risk-taking behavior, a number of studies have taken this strategy to identify gene expression correlates of anxiety in mice (Joo et al., 2009; Sherrin et al., 2009; Wang et al., 2008a, b), Drosophila (Ibi et al., 2008), monkeys (Sabatini et al., 2007), and rats (Kabbaj et al., 2004; Kroes et al., 2006). However, many questions remain about how to interpret these experiments, how to compare across studies, and what treatment differences imply about the genetics of behavior (see Gibson, 2008). For example, it is difficult to know whether the expression differences are due to the application of the treatment rather than to the execution of the behavior itself. Moreover, the timing of sampling is critical; the genes involved in the immediate genomic response to the treatment could be different from those involved in the execution of the actual behavior. It is also unclear whether the same genetic mechanisms underlie treatment level differences and behavioral differences between individuals.
Another approach is to compare baseline gene expression differences between different genotypes or individuals that differ in behavior, assuming that whatever expression differences observed are related to behavioral differences (Aubin-Horth et al., 2005; Ben-Shahar et al., 2002; Dierick and Greenspan, 2006; Edwards et al., 2006; Gammie et al., 2007; Kim et al., 2007, 2009; Quilter et al., 2008; Renn et al., 2008; Wang et al., 2008a, b; Whitfield et al., 2003). However, differences in transcript abundance could be a consequence, rather than a cause of differences in behavior. Despite this caveat, showing differential expression can be an important first step toward identifying the causative genetic polymorphisms underlying the difference in gene expression.
III. APPROACHING THE GENOMICS OF RISK-TAKING BEHAVIORS IN STICKLEBACK FISH
Obviously, we still have a lot to learn about the etiology of risk-taking behaviors in humans and other organisms. While traditional animal models have been useful in identifying mechanisms underlying some behaviors, such studies have typically been carried out on genetically homogeneous and lab-adapted strains. New insights might emerge from studying natural variation in behavior that resembles human behavioral variation.
Three-spined sticklebacks (Gasterosteus aculeatus) are renowned for their natural variation in behavior, morphology, and physiology (Bell and Foster, 1994). Therefore, in our work, we are using three-spined sticklebacks to identify candidate genes for natural variation in risk-taking behaviors. Sticklebacks are small (4–5 cm standard length at maturity) teleost fish that occur in the northern hemisphere. Sticklebacks commence breeding in the spring and typically live for 1 year. We are taking a whole-genome approach to understand genetic and environmental effects on suites of covarying risk-taking behaviors.
Our hypothesis is that there are inherited and environmentally responsive genes that affect risk-taking behaviors in sticklebacks, and those genes are shared with other animals, including humans. Later, we describe how risk-taking behaviors in this species show adaptive, natural variation within and between populations. Behaviors such as aggression and predator inspection are influenced by both inherited and experiential factors (Tulley and Huntingford, 1987), and they covary (Bell, 2005; Bell and Stamps, 2004; Huntingford, 1976). Moreover, the species is experimentally tractable—a favorite subject of the classical ethologists (Tinbergen, 1972; Wootton, 1984), the behaviors of sticklebacks are well characterized and are amenable to laboratory investigation. In addition, there are genomic resources for sticklebacks, including a whole-genome sequence (11× coverage by the Broad Institute), BAC and cDNA libraries, an EST project and a whole genome-linkage map (Peichel et al., 2001).
Sticklebacks have another key attribute that makes them an especially good model: they have an unusual evolutionary history that has produced a replicated natural experiment. Freshwater populations of sticklebacks are the descendants of marine ancestors which independently colonized freshwater environments following glacial retreat ~12,000 years ago. Isolated postglacial freshwater populations rapidly adapted to local environmental conditions, resulting in incredible phenotypic diversity among close relatives (Bell and Foster, 1994). Independent populations rapidly evolved convergent phenotypes in response to similar selective pressures, such that the same traits arose repeatedly and independently from the same ancestor. This system has already had great success in identifying the genetic basis of morphological traits (Colosimo et al., 2005; Cresko et al., 2004; Peichel et al., 2001; Shapiro et al., 2004).
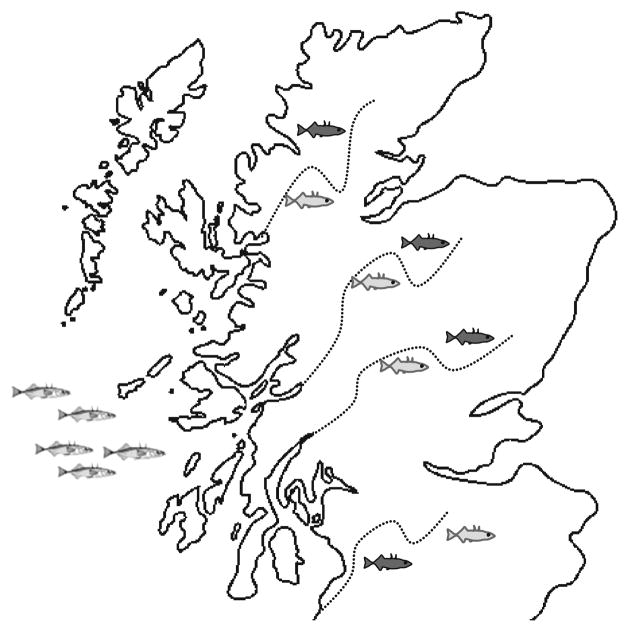
This important feature of the stickleback system means that we have a built-in solution to a problem that has plagued candidate gene studies: the failure to replicate. After we identify a set of genes associated with risk-taking behaviors, we can replicate the experiment by comparing the genes in additional sets of populations. If the same genes are differentially expressed between an independent pair of risk-averse and risk-prone populations, we can conclude that the differential expression is related to the behavior and is not simply a consequence of other factors such as genetic drift (Fig. 4.1).
Figure 4.1.
The replicated evolution of risk-taking behaviors in sticklebacks provides a natural experiment for testing candidate genes. This idealized map of Scotland shows the movement of sticklebacks from the ocean (on left) into freshwater rivers following glacial retreat. Sticklebacks inhabiting water bodies with abundant predators independently evolved increased levels of risk-taking behavior (dark fish) compared to sticklebacks inhabiting water bodies without predators (light fish).
A. Risk-taking behavior in sticklebacks
In the following sections, I describe how sticklebacks show natural variation in risk-taking behaviors such as predator inspection and aggression which are amenable to manipulative experimentation, and which resemble familiar human tendencies such as sensation-seeking, fearlessness, and disinhibition. Like the spectrum of externalizing behaviors (conduct disorder, antisocial personality, and substance abuse) in humans (Krueger et al., 2002), risk-taking behaviors in sticklebacks also covary and are influenced by both heredity and early life stress. Another key similarity between the stickleback model of risk-taking and human externalizing behaviors is that they share the same neuroendocrine substrates, as described below. The recent availability of new genomic tools for sticklebacks means that these ecologically relevant behaviors are genetically tractable.
1. Individuals differ in their propensity to take risks in different situations
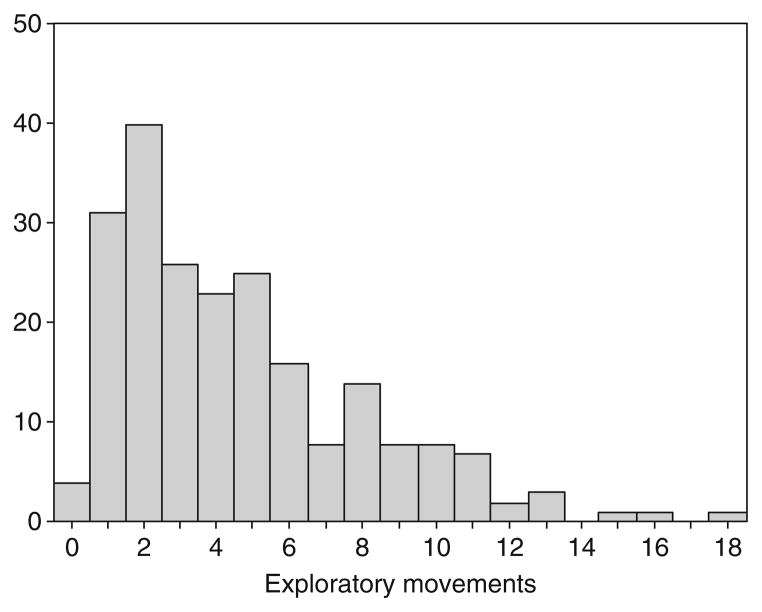
A consistent result of all of our studies is that there is substantial variation among both wild-caught and lab-reared individuals in how they behave in dangerous situations. For example, in an assay similar to the open field test (Yalcin et al., 2004), some individual sticklebacks actively move around an unfamiliar and dangerous environment while others scarcely leave the safety of a refuge (Fig. 4.2). Therefore, we can use genetic correlates of this individual variation as a tool for finding candidate genes related to risk-taking behavior.
Figure 4.2.
Individuals vary in their propensity to take risks. This histogram shows the distribution of exploratory movements of individuals in the presence of a predator. Methods: Juvenile sticklebacks from several populations were brought into the lab and the number of times an individual moved during 15 min in the presence of a predator was recorded. Mean=4.6, S.D.=3.39, n =218.
Another dangerous situation in which we observe individual differences in behavior is during confrontation by a potential predator. While some individuals hide in the presence of a predator, others swim up to the predator’s mouth and face the predator head-on. The same individuals that engage in this dangerous behavior, known as predator inspection, are also relatively aggressive toward other sticklebacks and are more willing to take risks in order to gain food than their risk-averse conspecifics (Bell, 2005; Bell and Stamps, 2004; Huntingford, 1976).
Individual sticklebacks can be classified as either risk-prone or risk-averse in several other contexts. Predator inspection is one of the most obvious forms of risk-taking behaviors and has been widely studied in sticklebacks and other small fishes (e.g., Pitcher et al., 1986). Despite the obvious danger involved in performing this behavior (Dugatkin and Godin, 1992; Milinski et al., 1997), it is thought that predator inspection can provide reliable information about predation risk via both olfactory and visual cues.
Individual differences in risk-taking behavior are also observed when individuals balance the benefits of feeding against the costs of potential predation (Bell, 2005). Some risk-prone individuals are more willing to assume the risk of predation in order to get food than others, even when differences in size, sex, or hunger level are controlled for (e.g., Krause et al., 1998). Therefore, differences in the willingness to forage in the presence of a predator can reflect intrinsic differences in the propensity to engage in risk-taking behaviors.
Fighting with conspecifics is another dangerous situation in which individual differences in risk-taking behavior are observed (Bell, 2005; Bell and Stamps, 2004; Huntingford, 1976). Intraspecific aggression in sticklebacks occurs during competition over access to resources, including food and territories and is manifested as biting, chasing, and attacking conspecifics. These behaviors are dangerous because in addition to energetic costs (Thorpe et al., 1995), aggression can result in injury (Neat et al., 1998) and exposure to predators while fighting (Diaz-Uriarte, 1999). The territorial aggression of male sticklebacks is especially well characterized (Bakker, 1994), but juveniles and females can also be aggressive (Bakker, 1986).
Interestingly, in sticklebacks, individuals that engage in higher levels of predator inspection are also more aggressive toward conspecifics (Bell, 2005; Bell and Stamps, 2004; Huntingford, 1976). Such covariation has parallels with the externalizing spectrum in humans, and with the observation that diverse psychiatric disorders in humans co-occur; antisocial behaviors, substance dependence, impulsivity, and behavioral disinhibition are comorbid (Kendler and Greenspan, 2006). Covariation among behavioral responses to dangerous situations in sticklebacks suggests that there is variation in the tendency to engage in risk-taking behaviors, and this tendency is manifested in multiple contexts (Sih et al., 2004).
In humans, there is evidence that the broad, underlying tendency is more heritable than the particular manifestations (Krueger et al., 2002). For example, whereas a significant portion of the variance in the externalizing latent trait can be traced to common genetic factors (>80% of the variance; Krueger et al., 2002), heritabilities for single behaviors (e.g., conduct disorder, alcohol dependence) are generally much lower. If the same is true for risk-taking behaviors in sticklebacks, we might be more likely to find genes related to risk-taking behaviors if we look for genetic correlates of the propensity to be generally risk-averse or risk-prone.
2. There is a genetic basis to risk-taking behaviors in sticklebacks
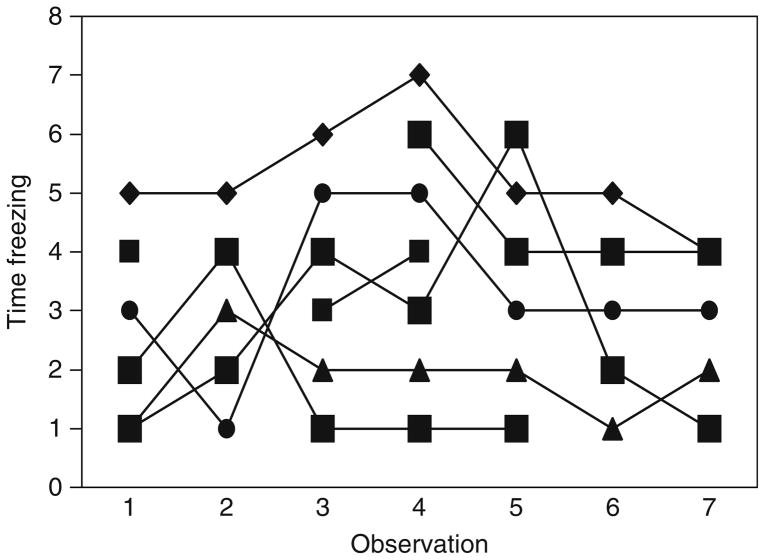
Several lines of evidence suggest that there is a genetic component to risk-taking behaviors in sticklebacks. First, risk-taking behavior is repeatable. Figure 4.3 shows the results of an experiment in which different individual sticklebacks were measured for their behavioral reaction to a live fish predator (pike) on several occasions (Bell et al., 2009). Individuals differed in the total amount of time they spent freezing (remaining motionless) in the presence of a predator. Freezing is negatively related to risk-taking behaviors and we interpret it as reflecting fearfulness. Individual differences in freezing were consistent through time. Specifically, individuals that froze in response to the predator the first time they were measured also froze when measured on subsequent occasions. These data are important because they show that an individual’s willingness to take risks is a stable attribute, supporting the hypothesis that there is a genetic basis to risk-taking behaviors in sticklebacks (Boake, 1994).
Figure 4.3.
Risk-taking behavior in sticklebacks is repeatable. The Y-axis shows the rank order time spent freezing in the presence of a live predator. Time (the observation number) is on the X-axis. Each line represents a different individual; each point represents the measure of that individual on each observation day. Methods: On seven different occasions over the course of 2 months, individual sticklebacks were presented with a live pike and their behavioral response recorded. Repeatability was calculated as in Lessels and Boag (1987), R =0.68, F6,42 =13, p <0.001. From Bell et al. (2009).
Another line of evidence for a genetic component to risk-taking behavior is that there is significant genetic variation for risk-taking behavior among families (Bell, 2005; Bell and Stamps, 2004). When confronted with an unfamiliar environment, members of certain families actively explored the environment while members of other families did not. As can be seen from the data in Fig. 4.4, there is substantial variation among families within populations in their willingness to explore an unfamiliar environment. These data are important because they show that there is heritable genetic variation within stickleback populations for risk-taking behaviors.
Figure 4.4.
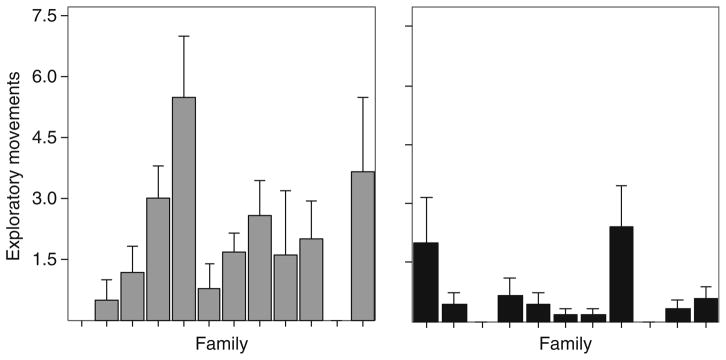
There is genetic variation for risk-taking behaviors among families. The data show the mean (±S.E.) number of exploratory movements in an unfamiliar environment of different families from two different populations, Putah Creek (gray) and the Navarro River (black). Methods: The exploratory behavior of lab-reared sticklebacks from 22 different families (2–5 full sibs/family) were measured at 7 months of age. Data were analyzed with a nested ANOVA with family and population as fixed factors (Population: F21,102 =21, p <0.0001; Family(Population): F20,102 =2.1, p =0.003).
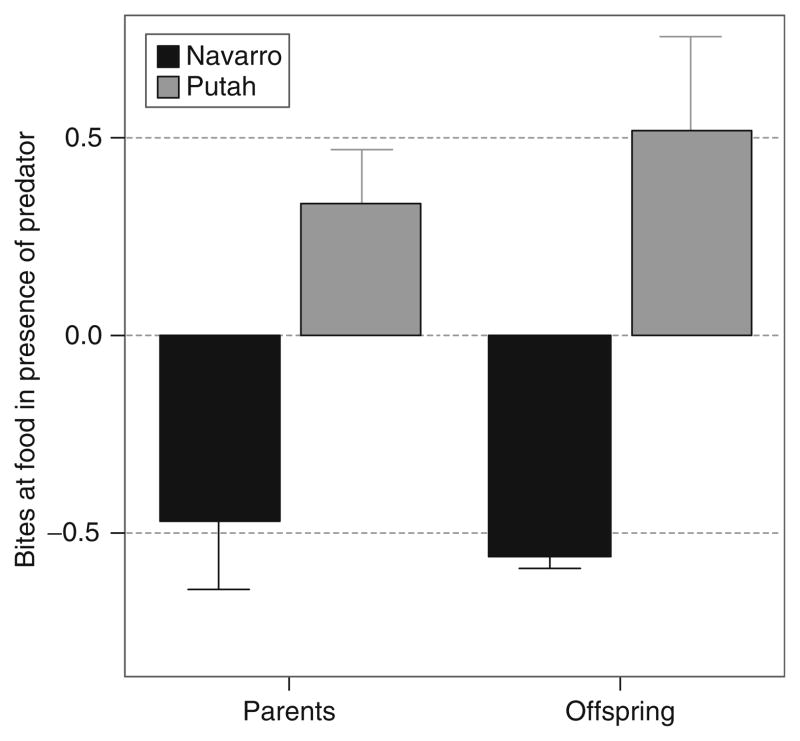
Common garden experiments also show that there is a genetic basis to risk-taking behaviors (Bell, 2005; Bell and Stamps, 2004). As can be seen from the data in Fig. 4.5, sticklebacks from one population were more willing to eat in the presence of a predator compared to sticklebacks from another population. This difference was apparent both for wild-caught parents and their lab-reared offspring that were reared under standardized laboratory conditions. These data are important because they show that the behavioral difference between the two populations has a genetic component. Moreover, it illustrates another important point about sticklebacks: the two populations differ in predation pressure which is a major source of variation for many stickleback phenotypes, and is discussed further later (Huntingford et al., 1994; Reimchen, 1994).
Figure 4.5.
There is a genetic basis to behavioral differences between populations. These data show the standardized mean (±S.E.) number of bites at food while in the presence of a predator for wild-caught parents and their lab-reared offspring from two different populations, Navarro and Putah (Bell, 2005). Methods: The willingness to forage in the presence of a predator was measured on wild-caught adults from the two populations. Full-sib offspring from the two populations were reared under standardized laboratory conditions and measured for willingness to forage in the presence of a predator at adulthood. Both the parents and offspring of fish from Putah Creek took more bites in the presence of a predator (Parents: F1,75 =14.3, p <0.0001; Offspring: F1,44 =18.4, p <0.0001).
A heritable basis to behaviors that deter predation (antipredator behaviors) is further substantiated by other studies on fishes, which have shown that differences between populations in antipredator behavior are inherited and arise without any experience of predators or without exposure to threatening situations (Magurran, 1990). There is also a heritable basis to aggression in sticklebacks (Bakker, 1986).
3. Risk-taking behavior is also influenced by the environment
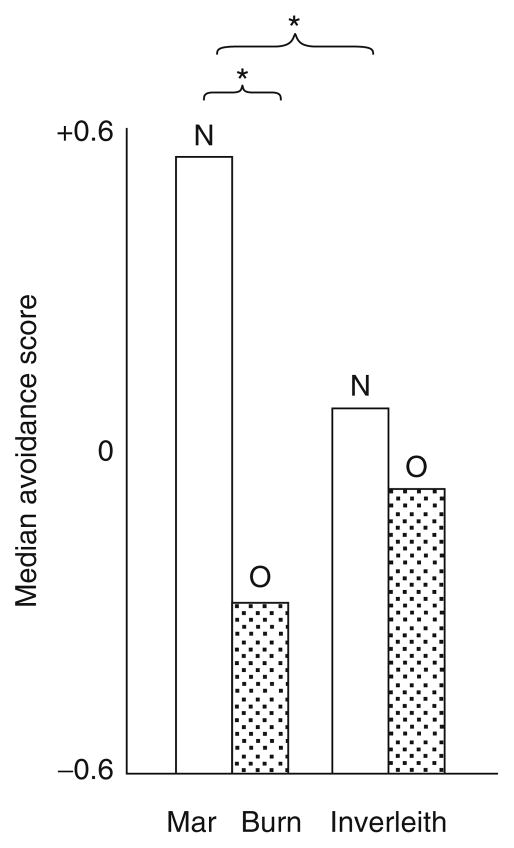
However, we also know that risk-taking behaviors in sticklebacks are influenced by the environment, including early interactions with parents. During the reproductive season, which is triggered by lengthening day lengths and increasing temperatures, male sticklebacks defend nesting territories. Males attract females to spawn in their nests and defend the breeding territory from intruders and predators. After spawning, the female leaves the male’s territory and the male is solely responsible for the care of the eggs. During the ~9-day incubation period, the male “fans” (oxygenates) the eggs, removes rotten eggs and debris, and defends the territory. A breeding male stickleback tends his newly hatched offspring for ~10 days. The fathers chase and catch fry that stray from the nest and spit them back into the nest. The fry are not injured during these interactions, but Tulley and Huntingford (1987), following a suggestion by Benzie (1965), showed that these interactions help to prepare the stickleback fry for later encounters with predators. Figure 4.6, reproduced from Tulley and Huntingford (1987), shows that sticklebacks reared by their father (“N”) avoided a model pike predator more than sticklebacks reared without paternal care (orphans, “O”). Interestingly, the behavioral difference between father-reared and orphan sticklebacks was only apparent among sticklebacks from a population where predators are abundant (the Mar Burn population on the left).
Figure 4.6.
Paternal care improves antipredator behavior. This is Fig. 1 from Tulley and Huntingford (1987). The strength of avoidance shown to a model pike by sticklebacks from the Mar Burn, where predators are abundant, and from Inverleith Pond, where predators are absent with (“N”) and without (“O”) paternal care.
In addition to the influence of their father, young fishes also learn from their own direct experience with predators, and these early interactions affect subsequent risk-taking behaviors later in life (e.g., Vilhunen, 2006). Interestingly, the effect of both paternal care and direct experience appears to be especially strong for fish from high predation localities. The fact that fish from high predation localities learn faster about predators suggests an inherited predisposition to respond to experience (Huntingford and Wright, 1992). Similar G×E interactions, in which some genotypes (or populations) are more responsive to the environment, have been found for risk-taking behaviors in other fish species (e.g., Gerlai and Csanyi, 1990).
4. Sticklebacks from populations experiencing higher levels of predation engage in more risk-taking behaviors than their counterparts from safer environments
As mentioned above, sticklebacks are widely distributed throughout the northern hemisphere and have a penchant for rapidly adapting to their local environments. Populations that occur in similar environments have evolved similar behaviors. One of the most important selective forces shaping stickleback populations is predation pressure. That is, some lakes contain many predators which prey on sticklebacks (“high predation”) while other lakes are relatively predator-free (“low predation”). We have been comparing the risk-taking behaviors of sticklebacks in a set of populations in Scotland that vary in predation pressure (Bell et al., 2009). Scotland is especially well suited for studying variation in risk-taking behavior in response to predation pressure because the country is teeming with postglacial waterbodies. Many lochs and ponds have been isolated for long enough (up to 15,000 generations) to independently evolve adaptations to high or low predation pressure and to become genetically differentiated from each other, but still capable of interbreeding (Malhi et al., 2006).
Interestingly, sticklebacks from areas where there are high levels of predation tend to be more risk-prone (i.e., they show higher levels of risk-taking behaviors) than their counterparts in safer environments (Huntingford and Coulter, 1989; Huntingford et al., 1994; Walling et al., 2003, 2004). This pattern has been documented in other small fish species as well, such as guppies (Magurran, 1986). While risky behavior in a dangerous environment might seem nonintuitive, this result is predicted by life history theory. The reason is that small individuals are especially vulnerable to predation, so when predation pressure is high, individuals that grow quickly will be favored because they are not small and vulnerable for long. Therefore, risk-taking behaviors that improve growth rate such as active foraging and aggression that results in access to resources should be favored when predation pressure is high (Mangel and Stamps, 2001). It is also worth noting that increased levels of risk-taking behaviors in humans have been documented in harsh or impoverished environments (Farrington, 2005; Kendler et al., 1995).
5. Risk-taking behaviors in sticklebacks resemble risk-taking behaviors in other organisms
Our contention that the genetic mechanisms underlying risk-taking behaviors in sticklebacks are shared with humans and other animals is supported by evidence that the neuroendocrine mechanisms underlying risk-taking behaviors are the same. If we can show that the neuroendocrine substrate underlying risk-taking behaviors in sticklebacks are conserved, then it is likely that the candidate genes that are identified in the stickleback system are likely to be good candidates for other animals, including humans.
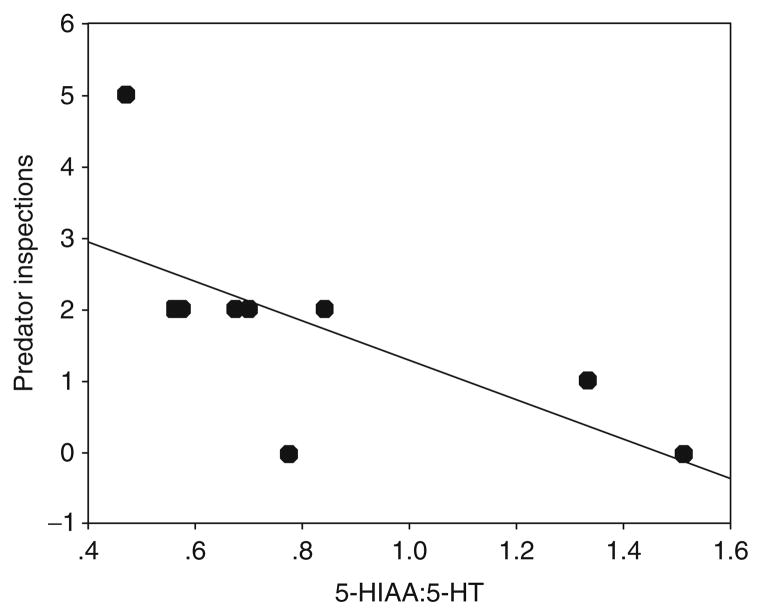
For example, risk-taking behavior in sticklebacks is associated with serotonin turnover (Bell et al., 2007). As can be seen in Fig. 4.7, individuals that engaged in more frequent predator inspection behavior had lower serotonergic activity. Low serotonergic activity is associated with sensation seeking in humans (Netter et al., 1996) and aggression in several other animals (Higley et al., 1996).
Figure 4.7.
Risk-takers have lower serotoninergic activity. Predator inspection was negatively correlated with serotonergic activity, as measured by the turnover of serotonin (5-HT) to its metabolite 5-hydroxyphenylacetic acid (5-HIAA), 15 min after presentation of the predator. From Bell et al. (2007). Methods: Juvenile sticklebacks were measured for their behavioral reaction to a live pike predator and then sacrificed after 15 min, their brains removed and quickly deep frozen. Tissue was homogenized in 4% perchloric acid with an internal standard and monoamines were measured using HPLC with electrochemical detection as in Øverli et al. (1999). The correlation between individual levels of predator inspection and serotonergic activity was statistically significant (r =−0.669, n =9, p =0.049).
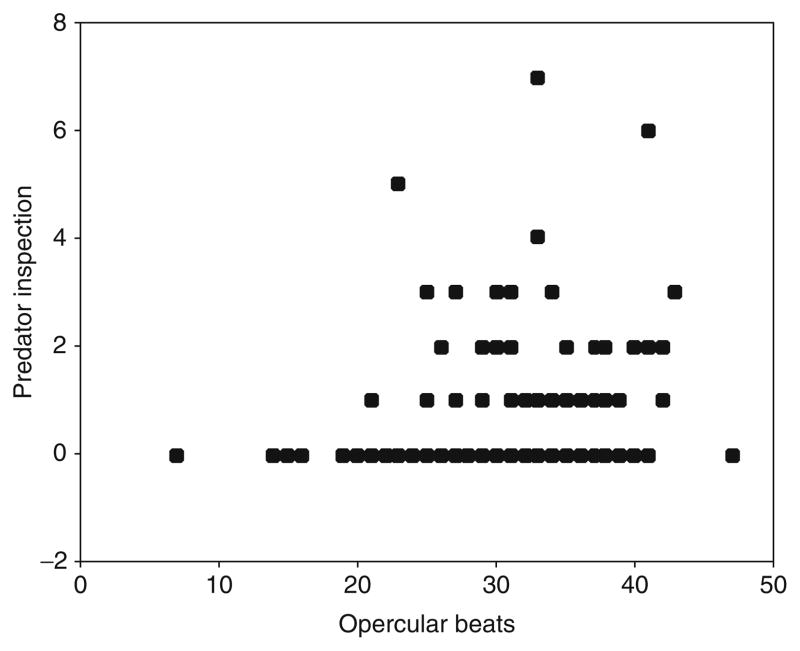
Second, breathing rate (opercular beats) is predictive of an individual’s risk-taking tendency in sticklebacks. We measured opercular beats in response to handling stress and then the same individuals’ behavioral reaction to a predator 24 h later. As can be seen from the data in Fig. 4.8, fish that breathed faster in response to handling stress were more likely to engage in risk-taking behavior (Bell et al., 2009). Unlike some other physiological measures, opercular beats can be measured noninvasively and repeatedly on the same individuals. Therefore, opercular beat rate is an appealing possible endophenotype linking genes and behavior. Studies on other organisms have also found associations between metabolic rate and risk-taking behaviors (Carere and Van Oers, 2004; Heim and Nemeroff, 2001).
Figure 4.8.
Breathing rate is an endophenotype for risk-taking behavior. The number of opercular beats in 15 s after handling is positively correlated with predator inspection (Bell et al., 2009). Methods: Individuals were subjected to brief handling stress and the number of opercular beats was counted. At least 24 h after, the fish was presented with a live pike and the number of predator inspections in 15 min was recorded. The partial correlation coefficient (controlling for population) is statistically significant (r =0.211, n =168, p =0.006) and was not related to body size.
6. Genomic resources for sticklebacks
Genomic resources for stickleback research are rapidly expanding. The first release of the stickleback genome, sequenced and assembled at the Broad Institute, comprises 460 Mb. Gene models include approximately 21,000 known, novel and pseudogenes with an estimated 44,000 Genscan predictions. Furthermore, close to 250,000, 3′ and 5′ expressed sequence tags (ESTs) from various tissues and developmental stages have been submitted to GenBank and comprise 15,087 Unigene clusters (Kingsley et al., 2004).
IV. FUTURE DIRECTIONS AND CONCLUSIONS
Based on the work described earlier, we are taking a multipronged strategy for investigating the genetic correlates and causes of risk-taking behaviors in sticklebacks. We hypothesize that inherited and environmentally responsive genes that affect risk-taking behaviors in sticklebacks have orthologs with common function in other animal genomes, including human. Therefore, using whole genome microarrays, we are searching for candidate genes related to the propensity to engage in risk-taking behaviors that are both inherited and responsive to the environment. First, we are comparing baseline brain gene expression differences between risk-prone and risk-averse individuals and populations. Second, we are also identifying genes underlying risk-taking behaviors that are responsive to adverse environmental conditions by comparing the brain gene expression profiles of individuals exposed to different early life stressors. Third, we are testing candidate genes related to risk-taking behaviors in replicated populations of sticklebacks.
Future studies on the genetics of risk-taking behaviors in sticklebacks and other animals should focus on understanding the mechanisms by which candidate genes influence the behavior. Manipulations such as morpholinos, RNAi, and transgenics (Hosemann et al., 2004; Kingsley et al., 2004) will help to make the causal link between candidate genes and risk-taking behaviors; ultimately, making a risk-averse individual risk-prone. The future is also bright for whole genome association studies to study the genetic basis underlying phenotypes in natural populations, such as being done already in humans (Butcher et al., 2008; Dina et al., 2005; Donner et al., 2008; Doyle et al., 2008; Stallings et al., 2005) and dogs (Jones et al., 2008). In the not-too-distant future, it is likely that the main barrier to progress will not be genotyping our subjects, but in phenotyping the thousands of animals needed in order to obtain sufficient power to detect relationships between minor loci and behavior.
Acknowledgments
The author thanks Edelyn Verona for insights about anxiety, fear, and risk-taking behavior in humans and other organisms. This work is funded by NIH R01 GM082937 to A. M. B.
References
- Adamec RE, Burton P, et al. Vulnerability to mild predator stress in serotonin transporter knockout mice. Behav Brain Res. 2006;170:126–140. doi: 10.1016/j.bbr.2006.02.012. [DOI] [PubMed] [Google Scholar]
- Aubin-Horth N, Landry CR, et al. Alternative life histories shape brain gene expression profiles in males of the same population. Proc R Soc B-Biol Sci. 2005;272:1655–1662. doi: 10.1098/rspb.2005.3125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker TCM. Aggressiveness in sticklebacks (Gasterosteus aculeatus) a behavior-genetic study. Behaviour. 1986;98:1–144. [Google Scholar]
- Bakker TCM. Evolution of aggressive behaviour in the threespine stickleback. In: Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford: 1994. pp. 345–379. [Google Scholar]
- Beaumont C, Roussot O, et al. A genome scan with AFLP((TM)) markers to detect fearfulness-related QTLs in Japanese quail. Anim Genet. 2005;36:401–407. doi: 10.1111/j.1365-2052.2005.01336.x. [DOI] [PubMed] [Google Scholar]
- Bell AM. Differences between individuals and populations of threespined stickleback. J Evol Biol. 2005;18:464–473. doi: 10.1111/j.1420-9101.2004.00817.x. [DOI] [PubMed] [Google Scholar]
- Bell MA, Foster SA. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford: 1994. [Google Scholar]
- Bell AM, Stamps JA. The development of behavioural differences between individuals and populations of stickleback. Anim Behav. 2004;68:1339–1348. [Google Scholar]
- Bell AM, Backstrom T, et al. Variable behavioral and neuroendocrine responses to ecologically-relevant challenges in sticklebacks. Physiol Behav. 2007;91:15–25. doi: 10.1016/j.physbeh.2007.01.012. [DOI] [PubMed] [Google Scholar]
- Bell AM, Henderson L, et al. Behavioral and respiratory responses to stressors in multiple populations of three-spined sticklebacks that differ in predation pressure. J Comp Physiol. 2009 doi: 10.1007/s00360-009-0395-8. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Shahar Y, Robichon A, et al. Influence of gene action across different time scales on behavior. Science. 2002;296:741–744. doi: 10.1126/science.1069911. [DOI] [PubMed] [Google Scholar]
- Benzie V. PhD thesis Zoology. Oxford University; 1965. Some aspects of the anti-predator responses of two species of stickleback. [Google Scholar]
- Boake CRB. Quantitative Genetic Studies of Behavioral Evolution. University of Chicago Press; Chicago: 1994. [Google Scholar]
- Boissy A. Fear and fearfulness in animals. Quarterly Review of Biology. 1995;70:165–191. doi: 10.1086/418981. [DOI] [PubMed] [Google Scholar]
- Butcher LM, Davis OSP, et al. Genome-wide quantitative trait locus association scan of general cognitive ability using pooled DNA and 500 K single nucleotide polymorphism microarrays. Genes Brain Behav. 2008;7:435–446. doi: 10.1111/j.1601-183X.2007.00368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldji C, Diorio J, et al. Variations in maternal care in infancy regulate the development of stress reactivity. Biol Psychiatry. 2000;48:1164–1174. doi: 10.1016/s0006-3223(00)01084-2. [DOI] [PubMed] [Google Scholar]
- Carere C, Van Oers K. Shy and bold great tits (Parus major): Body temperature and breath rate in response to handling stress. Physiol Behav. 2004;82:905–912. doi: 10.1016/j.physbeh.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Carney GE. A rapid genome-wide response to Drosophila melanogaster social interactions. BMC Genomics. 2007:8. doi: 10.1186/1471-2164-8-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE. Opinion—Gene–environment interactions in psychiatry: Joining forces with neuroscience. Nat Rev Neurosci. 2006;7:583–590. doi: 10.1038/nrn1925. [DOI] [PubMed] [Google Scholar]
- Colosimo PF, Hosemann KE, et al. Widespread parallel evolution in sticklebacks by repeated fixation of ectodysplasin alleles. Science. 2005;307:1928–1933. doi: 10.1126/science.1107239. [DOI] [PubMed] [Google Scholar]
- Crabbe JC. Genetic contributions to addiction. Annu Rev Psychol. 2002;53:435–462. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- Cresko WA, Amores A, et al. Parallel genetic basis for repeated evolution of armor loss in Alaskan threespine stickleback populations. Proc Natl Acad Sci USA. 2004;101:6050–6055. doi: 10.1073/pnas.0308479101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings ME, Larkins-Ford J, et al. Sexual and social stimuli elicit rapid and contrasting genomic responses. Proc R Soc B-Biol Sci. 2008;275:393–402. doi: 10.1098/rspb.2007.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defries JC, Hegmann JP, et al. Open-field behavior in mice—Evidence for a major gene effect mediated by visual system. Science. 1966;154:1577. doi: 10.1126/science.154.3756.1577. [DOI] [PubMed] [Google Scholar]
- Diaz-Uriarte R. Anti-predator behaviour changes following an aggressive encounter in the lizard Tropidurus hispidus. Proc R Soc Lond B Biol Sci. 1999;266:2457–2464. doi: 10.1098/rspb.1999.0946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dierick HA, Greenspan RJ. Molecular analysis of flies selected for aggressive behavior. Nat Genet. 2006;38:1023–1031. doi: 10.1038/ng1864. [DOI] [PubMed] [Google Scholar]
- Dina C, Nemanov L, et al. Fine mapping of a region on chromosome 8p gives evidence for a QTL contributing to individual differences in an anxiety-related personality trait: TPQ harm avoidance. Am J Med Genet Part B-Neuropsychiatr Genet. 2005;132B:104–108. doi: 10.1002/ajmg.b.30099. [DOI] [PubMed] [Google Scholar]
- Donner J, Pirkola S, et al. An association analysis of murine anxiety genes in humans implicates novel candidate genes for anxiety disorders. Biol Psychiatry. 2008;64:672–680. doi: 10.1016/j.biopsych.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle AE, Ferreira MAR, et al. Multivariate genomewide linkage scan of neurocognitive traits and ADHD symptoms: Suggestive linkage to 3q13. Am J Med Genet Part B-Neuropsychiatr Genet. 2008;147B:1399–1411. doi: 10.1002/ajmg.b.30868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth RA, Badyaev AV. Coupling of dispersal and aggression facilitates the rapid range expansion of a passerine bird. Proc Natl Acad Sci USA. 2007;104:15017–15022. doi: 10.1073/pnas.0706174104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugatkin LA, Godin JJ. Predator inspection shoaling and foraging under predation hazard in the Trinidadian guppy, Poecilia reticulata. Environ Biol Fishes. 1992;34:265–276. [Google Scholar]
- Eaves LJ, Silberg J, et al. Resolving multiple epigenetic pathways to adolescent depression. J Child Psychol Psychiatry. 2003;44:1006–1014. doi: 10.1111/1469-7610.00185. [DOI] [PubMed] [Google Scholar]
- Edwards AC, Rollmann SM, et al. Quantitative genomics of aggressive behavior in Drosophila melanogaster. PLoS Genet. 2006;2:1386–1395. doi: 10.1371/journal.pgen.0020154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrington DP. Childhood origins of antisocial behavior. Clin Psychol Psychother. 2005;12:177–190. [Google Scholar]
- Flint J, Corley R, et al. A simple genetic basis for a complex psychological trait in laboratory mice. Science. 1995;269:1432–1435. doi: 10.1126/science.7660127. [DOI] [PubMed] [Google Scholar]
- Gammie SC, Auger AP, et al. Altered gene expression in mice selected for high maternal aggression. Genes Brain Behav. 2007;6:432–443. doi: 10.1111/j.1601-183X.2006.00271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerlai R, Csanyi V. Genotype–environment interaction and the correlation structure of behavioral elements in paradise fish. Physiol Behav. 1990;47:343–356. doi: 10.1016/0031-9384(90)90153-u. [DOI] [PubMed] [Google Scholar]
- Gibson G. The environmental contribution to gene expression profiles. Nat Rev Genet. 2008;9:575–581. doi: 10.1038/nrg2383. [DOI] [PubMed] [Google Scholar]
- Gutierrez-Gil B, Ball N, et al. Identification of quantitative trait loci affecting cattle temperament. J Hered. 2008;99:629–638. doi: 10.1093/jhered/esn060. [DOI] [PubMed] [Google Scholar]
- Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: Preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–1039. doi: 10.1016/s0006-3223(01)01157-x. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, et al. CSF testosterone and 5-HIAA correlate with different types of aggressive behaviors. Biol Psychiatry. 1996;40:1067–1082. doi: 10.1016/S0006-3223(95)00675-3. [DOI] [PubMed] [Google Scholar]
- Hosemann KE, Colosimo PE, et al. A simple and efficient microinjection protocol for making transgenic sticklebacks. Behaviour. 2004;141:1345–1355. [Google Scholar]
- Hovatta I, Barlow C. Molecular genetics of anxiety in mice and men. Ann Med. 2008;40:92–109. doi: 10.1080/07853890701747096. [DOI] [PubMed] [Google Scholar]
- Huntingford FA. The relationship between anti-predator behaviour and aggression among conspecifics in the three-spined stickleback. Anim Behav. 1976;24:245–260. [Google Scholar]
- Huntingford FA, Coulter RM. Habituation of predator inspection in the three-spined stickleback Gasterosteus aculeatus. J Fish Biol. 1989;35:153–154. [Google Scholar]
- Huntingford FA, Wright PJ. Inherited population differences in avoidance conditioning in threespined sticklebacks, Gasterosteus aculeatus. Behaviour. 1992;122:264–273. [Google Scholar]
- Huntingford FA, Wright PJ, et al. Adaptive variation and antipredator behaviour in threespine stickleback. In: Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford: 1994. pp. 277–295. [Google Scholar]
- Ibi D, Takuma K, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- Jones P, Chase K, et al. Single-nucleotide-polymorphism-based association mapping of dog stereotypes. Genetics. 2008;179:1033–1044. doi: 10.1534/genetics.108.087866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo Y, Choi KM, et al. Chronic immobilization stress induces anxiety- and depression-like behaviors and decreases transthyretin in the mouse cortex. Neurosci Lett. 2009;461:121–125. doi: 10.1016/j.neulet.2009.06.025. [DOI] [PubMed] [Google Scholar]
- Kabbaj M, Evans S, et al. The search for the neurobiological basis of vulnerability to drug abuse: Using microarrays to investigate the role of stress and individual differences. Neuropharmacology. 2004;47:111–122. doi: 10.1016/j.neuropharm.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Kapelnikov A, Zelinger E, et al. Mating induces an immune response and developmental switch in the Drosophila oviduct. Proc Natl Acad Sci USA. 2008;105:13912–13917. doi: 10.1073/pnas.0710997105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman J, Yang BZ, et al. Brain-derived neurotrophic factor-5-HTTLPR gene interactions and environmental modifiers of depression in children. Biol Psychiatry. 2006;59:673–680. doi: 10.1016/j.biopsych.2005.10.026. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Greenspan RJ. The nature of genetic influences on behavior: Lessons from ‘simpler’ organisms. Am J Psychiatry. 2006;163:1683–1694. doi: 10.1176/ajp.2006.163.10.1683. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Kessler RC, et al. Stressful life events, genetic liability and onset of an episode of major depression in women. Am J Psychiatry. 1995;152:833–842. doi: 10.1176/ajp.152.6.833. [DOI] [PubMed] [Google Scholar]
- Kendler KS, Jacobsen KC, et al. Specificity of genetic and environmental risk factors for use and abuse/dependence of cannabis, cocaine, hallucinogens, sedatives, stimulates and opiates in male twins. Am J Psychiatry. 2003;160:687–695. doi: 10.1176/appi.ajp.160.4.687. [DOI] [PubMed] [Google Scholar]
- Kim S, Choi KH, et al. Suicide candidate genes associated with bipolar disorder and schizophrenia: An exploratory gene expression profiling analysis of post-mortem prefrontal cortex. BMC Genomics. 2007;8:413. doi: 10.1186/1471-2164-8-413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Zhang SM, et al. An E3 ubiquitin ligase, really interesting new gene (RING) finger 41, is a candidate gene for anxiety-like behavior and beta-carboline-induced seizures. Biol Psychiatry. 2009;65:425–431. doi: 10.1016/j.biopsych.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingsley DM, Zhu BL, et al. New genomic tools for molecular studies of evolutionary change in threespine sticklebacks. Behaviour. 2004;141:1331–1344. [Google Scholar]
- Kocher SD, Richard FJ, et al. Genomic analysis of post-mating changes in the honey bee queen (Apis mellifera) BMC Genomics. 2008;9:232. doi: 10.1186/1471-2164-9-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause J, Loader SP, et al. Refuge use by fish as a function of body length-related metabolic expenditure and predation risks. Proc R Soc Lond Ser B. 1998;265:2373–2379. [Google Scholar]
- Kroes RA, Panksepp J, et al. Modeling depression: Social dominance–submission gene expression patterns in rat neocortex. Neuroscience. 2006;137:37–49. doi: 10.1016/j.neuroscience.2005.08.076. [DOI] [PubMed] [Google Scholar]
- Krueger RF, Hicks BM, et al. Etiologic connections among substance dependence, antisocial behavior, and personality: Modeling the externalizing spectrum. J Abnorm Psychol. 2002;111:411–424. [PubMed] [Google Scholar]
- Lawniczak MKN, Begun DJ. A genome-wide analysis of courting and mating responses in Drosophila melanogaster females. Genome. 2004;47:900–910. doi: 10.1139/g04-050. [DOI] [PubMed] [Google Scholar]
- Lessels CM, Boag PT. Unrepeatable repeatabilities: A common mistake. The Auk. 1987;104:116–121. [Google Scholar]
- London SE, Dong S, et al. Developmental shifts in gene expression in the auditory forebrain during the sensitive period for song learning. Dev Neurobiol. 2009;69:437–450. doi: 10.1002/dneu.20719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mack PD, Kapelnikov A, et al. Mating-responsive genes in reproductive tissues of female Drosophila melanogaster. Proc Natl Acad Sci USA. 2006;103:10358–10363. doi: 10.1073/pnas.0604046103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackay TFC. The genetic architecture of quantitative traits: Lessons from Drosophila. Curr Opin Genet Dev. 2004;14:253–257. doi: 10.1016/j.gde.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Mackay TFC. The genetic architecture of complex behaviors: Lessons from Drosophila. Genetica. 2009;136:295–302. doi: 10.1007/s10709-008-9310-6. [DOI] [PubMed] [Google Scholar]
- Magurran AE. Predator inspection behaviour in minnow shoals: Differences between populations and individuals. Behav Ecol Sociobiol. 1986;19:267–273. [Google Scholar]
- Magurran AE. The inheritance and development of minnow anti-predator behaviour. Anim Behav. 1990;39:834–842. [Google Scholar]
- Malhi RS, Rhett G, et al. Mitochondrial DNA evidence of an early holocene population expansion of threespine sticklebacks from Scotland. Mol Phylogenet Evol. 2006;40:148–154. doi: 10.1016/j.ympev.2006.02.019. [DOI] [PubMed] [Google Scholar]
- Mangel M, Stamps J. Trade-offs between growth and mortality and the maintenance of individual variation in growth. Evol Ecol Res. 2001;3:583–593. [Google Scholar]
- McGraw LA, Clark AG, et al. Post-mating gene expression profiles of female Drosophila melanogaster in response to time and to four male accessory gland proteins. Genetics. 2008;179:1395–1408. doi: 10.1534/genetics.108.086934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ. Maternal care, gene expression, and the transmission of individual differences in stress reactivity across generations. Annu Rev Neurosci. 2001;24:1161–1192. doi: 10.1146/annurev.neuro.24.1.1161. [DOI] [PubMed] [Google Scholar]
- Miczek KA, Maxson SC, et al. Aggressive behavioral phenotypes in mice. Behav Brain Res. 2001;125:167–181. doi: 10.1016/s0166-4328(01)00298-4. [DOI] [PubMed] [Google Scholar]
- Milinski M, Luthi JH, et al. Cooperation under predation risk: Experiments on costs and benefits. Proc R Soc Lond B Biol Sci. 1997;264:831–837. [Google Scholar]
- Neat FC, Taylor AC, et al. Proximate costs of fighting in male cichlid fish: The role of injuries and energy metabolism. Anim Behav. 1998;55:875–882. doi: 10.1006/anbe.1997.0668. [DOI] [PubMed] [Google Scholar]
- Netter P, Henning J, et al. Serotonin and dopamine as mediators of sensation seeking behavior. Neuropsychobiology. 1996;34:155–165. doi: 10.1159/000119318. [DOI] [PubMed] [Google Scholar]
- Øverli O, Harris CA, et al. Short-term effects of fights for social dominance and the establishment of dominant–subordinate relationships on brain monoamines and cortisol in rainbow trout. Brain Behav Evol. 1999;54:263–275. doi: 10.1159/000006627. [DOI] [PubMed] [Google Scholar]
- Peichel CL, Nereng KS, et al. The genetic architecture of divergence between threespine stickleback species. Nature. 2001;414:901–905. doi: 10.1038/414901a. [DOI] [PubMed] [Google Scholar]
- Pitcher TJ, Green DA, et al. Dicing with death: Predator inspection behavior in minnow (Phoxinus phoxinus) shoals. J Fish Biol. 1986;28:439–448. [Google Scholar]
- Plomin R. Finding genes in child psychology and psychiatry: When are we going to be there? J Child Psychol Psychiatry. 2005;46:1030–1038. doi: 10.1111/j.1469-7610.2005.01524.x. [DOI] [PubMed] [Google Scholar]
- Quilter CR, Gilbert CL, et al. Gene expression profiling in porcine maternal infanticide: A model for puerperal psychosis. Am J Med Genet Part B-Neuropsychiatr Genet. 2008;147B:1126–1137. doi: 10.1002/ajmg.b.30734. [DOI] [PubMed] [Google Scholar]
- Reif A, Lesch KP. Toward a molecular architecture of personality. Behav Brain Res. 2003;139:1–20. doi: 10.1016/s0166-4328(02)00267-x. [DOI] [PubMed] [Google Scholar]
- Reimchen TE. Predators and morphological evolution in threespine stickleback. In: Bell MA, Foster SA, editors. The Evolutionary Biology of the Threespine Stickleback. Oxford University Press; Oxford: 1994. pp. 240–273. [Google Scholar]
- Renn SCP, Aubin-Horth N, et al. Fish and chips: Functional genomics of social plasticity in an African cichlid fish. J Exp Biol. 2008;211:3041–3056. doi: 10.1242/jeb.018242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabatini MJ, Ebert P, et al. Amygdala gene expression correlates of social behavior in monkeys experiencing maternal separation. J Neurosci. 2007;27:3295–3304. doi: 10.1523/JNEUROSCI.4765-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro MD, Marks, Peichel CL, et al. Genetic and developmental basis of evolutionary pelvic reduction in threespine sticklebacks. Nature. 2004;428:717–723. doi: 10.1038/nature02415. [DOI] [PubMed] [Google Scholar]
- Sherrin T, Blank T, et al. Region specific gene expression profile in mouse brain after chronic corticotropin releasing factor receptor 1 activation: The novel role for diazepam binding inhibitor in contextual fear conditioning. Neuroscience. 2009;162:14–22. doi: 10.1016/j.neuroscience.2009.04.012. [DOI] [PubMed] [Google Scholar]
- Sih A, Watters JV. The mix matters: Behavioural types and group dynamics in water striders. Behaviour. 2005;142:1417–1431. [Google Scholar]
- Sih A, Bell AM, et al. Behavioral syndromes: An integrative overview. Q Rev Biol. 2004;79:241–277. doi: 10.1086/422893. [DOI] [PubMed] [Google Scholar]
- Stallings MC, Corley RP, et al. A genome-wide search for quantitative trait loci that influence antisocial drug dependence in adolescence. Arch Gen Psychiatry. 2005;62:1042–1051. doi: 10.1001/archpsyc.62.9.1042. [DOI] [PubMed] [Google Scholar]
- Strandberg E, Jacobsson J, et al. Direct genetic, maternal and litter effects on behaviour in German shepherd dogs in Sweden. Livestock Prod Sci. 2005;93:33–42. [Google Scholar]
- Suomi JS. Genetic and maternal contributions to individual differences in Rhesus monkey biobehavioral development. In: Krasnagor N, editor. Psychobiological Aspects of Behavioral Development. Academic Press; New York: 1987. pp. 397–419. [Google Scholar]
- Thorpe KE, Taylor AC, et al. How costly is fighting? Physiological effects of sustained exercise and fighting in swimming crabs. Necora puber Anim Behav. 1995;50:1657–1666. [Google Scholar]
- Tinbergen N. The Animal in its World; Explorations of an Ethologist. Harvard University Press; Cambridge: 1972. [Google Scholar]
- Tulley JJ, Huntingford FA. Paternal care and the development of adaptive variation in anti-predator responses in sticklebacks. Anim Behav. 1987;35:1570–1572. [Google Scholar]
- Vilhunen S. Repeated antipredator conditioning: A pathway to habituation or to better avoidance? J Fish Biol. 2006;68:25–43. [Google Scholar]
- Walling CA, Dawnay N, et al. Do competing males cooperate? Familiarity and its effect on cooperation during predator inspection in male three-spined sticklebacks (Gasterosteus aculeatus) J Fish Biol. 2003;63:243–244. [Google Scholar]
- Walling CA, Dawnay N, et al. Predator inspection behaviour in three-spined sticklebacks (Gasterosteus aculeatus): Body size, local predation pressure and cooperation. Behav Ecol Sociobiol. 2004;56:164–170. [Google Scholar]
- Wang J, Ross KG, et al. Genome-wide expression patterns and the genetic architecture of a fundamental social trait. PLoS Genet. 2008a;4:e1000127. doi: 10.1371/journal.pgen.1000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang LM, Dankert H, et al. A common genetic target for environmental and heritable influences on aggressiveness in Drosophila. Proc Natl Acad Sci USA. 2008b;105:5657–5663. doi: 10.1073/pnas.0801327105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield CW, Cziko AM, et al. Gene expression profiles in the brain predict behavior in individual honey bees. Science. 2003;302:296–299. doi: 10.1126/science.1086807. [DOI] [PubMed] [Google Scholar]
- Willis-Owen SAG, Flint J. Identifying the genetic determinants of emotionality in humans; insights from rodents. Neurosci Biobehav Rev. 2007;31:115–124. doi: 10.1016/j.neubiorev.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Wiren A, Gunnarsson U, et al. Domestication-related genetic effects on social behavior in chickens—Effects of genotype at a major growth quantitative trait locus. Poult Sci. 2009;88:1162–1166. doi: 10.3382/ps.2008-00492. [DOI] [PubMed] [Google Scholar]
- Wootton RJ. A Functional Biology of Sticklebacks. University of California Press; Berkeley: 1984. [Google Scholar]
- Yalcin B, Willis-Owen SAG, et al. Genetic dissection of a behavioral quantitative trait locus shows that Rgs2 modulates anxiety in mice. Nat Genet. 2004;36:1197–1202. doi: 10.1038/ng1450. [DOI] [PubMed] [Google Scholar]