Summary
Study objective
Non-convulsive seizures/status epilepticus occur in approximately 20% of comatose, non-cardiac arrest intensive care unit (ICU) patients, and are associated with increased mortality. The prevalence and clinical significance of seizures in comatose survivors of cardiac arrest undergoing therapeutic hypothermia is not well described.
Methods
At this urban level I trauma center, every patient undergoing therapeutic hypothermia is monitored with continuous video encephalography (cvEEG). We abstracted medical records for all cardiac arrest patients treated with therapeutic hypothermia during 2010. Clinical data were extracted in duplicate. cvEEGs were independently reviewed for seizures by two board-certified epileptologists.
Results
There were 33 patients treated with therapeutic hypothermia after cardiac arrest in 2010 who met inclusion criteria for this study. Median age was 58 (range 28–86 years), 63% were white, 55% were male, and 9% had a history of seizures or epilepsy. During cooling, seizures occurred in 5/33 patients (15%, 95%CI 6%–33%). 11/33 patients (33%, 95% CI 19%–52%) had seizures at some time during hospitalization. 13/33 (39%) survived to discharge and of these, 7/13 (54%) survived to 30 days. 9/11 patients with seizures died during hospitalization, compared with 11/22 patients without seizures (82% vs. 50%; difference 32%, CI951%–63%). No patient with seizures was alive at 30 days.
Conclusions
Seizures are common in comatose patients treated with therapeutic hypothermia after cardiac arrest. All patients with seizures were deceased within 30 days of discharge. Routine use of EEG monitoring could assist in early detection of seizures in this patient population, providing an opportunity for intervention to potentially improve outcomes.
Keywords: Non-convulsive seizures, Seizures, Therapeutic hypothermia, Continuous EEG, Cardiac arrest
Introduction
The chance of neurologically intact survival from cardiac arrest remains low despite significant advances in clinical care, including improved CPR and treatment with therapeutic hypothermia. Less than half of those with return of spontaneous circulation survive to hospital discharge, and 20–30% of survivors are neurologically devastated (Neumar et al., 2008). With up to 300,000 cardiac arrests per year in the United States (Neumar et al., 2008), even small improvements in post-resuscitation care would translate into thousands of lives improved or saved each year. Patients who are successfully resuscitated from cardiac arrest are often comatose and critically ill. A variable number of critically ill patients with altered mental status or coma experience non-convulsive seizures or status epilepticus (NCS), depending on the etiology. The reported incidence of NCS in critically ill patients ranges from 8 to 60% (Towne et al., 2000; Claassen et al., 2004), and the incidence in post-cardiac arrest patients is suggested to be between 15 and 44% (Neumar et al., 2008; Rittenberger et al., 2012; Mani et al., 2012; Rossetti et al., 2010).
The diagnosis of NCS is challenging. It is both under-recognized and undertreated. Many cardiac arrest patients undergoing therapeutic hypothermia are heavily sedated and paralyzed throughout the duration of the cooling and rewarming periods. In comatose patients, over 48 h of continuous electroencephalography (cEEG) monitoring is necessary to achieve 90% sensitivity for detecting electrographic seizures; the sensitivity is less than 50% for a routine 20 min EEG in the comatose ICU patient (Claassen et al., 2004). However, widespread utilization of cEEG is limited by cost and resource availability, the need for specialized equipment, technical support, and trained physicians to interpret the data (Kull and Emerson, 2005).
We contend that a better characterization of the incidence of seizures and associated outcomes in cardiac arrest patients treated with therapeutic hypothermia will lead to increased use of cEEG monitoring, enabling early detection and treatment of seizures or status epilepticus. Our objective was to estimate the incidence of seizures in cardiac arrest patients undergoing therapeutic hypothermia, and to explore the association between seizures and death.
Methodology
Study design, setting and selection of participants
This study was conducted at an urban, academic Level 1 trauma center with greater than 90,000 annual adult visits to the emergency department. The emergency department is the initial point of contact for the majority of patients admitted for resuscitation after cardiac arrest. Patients were identified by primary teams in the ED or ICU, and application of therapeutic hypothermia was performed at the discretion of the Neurocritical Care (NCC) team. NCC specialists consulted on every patient treated with therapeutic hypothermia after cardiac arrest, and these patients were monitored with continuous video electroencephalography (cvEEG) from the time of initiation of therapeutic hypothermia until the patient achieved euthermia after rewarming as a standard component of clinical care. cvEEG monitoring could continue beyond the therapeutic hypothermia period at the discretion of the treating team. After approval from the Institutional Review Board, we conducted a retrospective chart review of every cardiac arrest patient 18 years old or greater who was treated with therapeutic hypothermia between January 1, 2010 and December 31, 2010. Patients for the study were identified from the consultation logs maintained by the Neurocritical care service.
Data collection
Participants’ charts were initially screened to ensure therapeutic hypothermia was conducted and the patient was greater than 18 years of age. Subsequently, each chart underwent dual, independent data review and abstraction. Chart abstractors were clinical study assistants (CSAs) experienced in data collection and chart abstraction. The CSAs were trained by study personnel on the format and terminology of the ED and ICU hospital records and the abstraction methods to be used for this study. They were blinded to the purpose of this study, and they were not involved in the care of cardiac arrest patients treated with therapeutic hypothermia.
Each CSA initially abstracted three test charts for clinical data. The primary investigator reviewed each abstraction and provided feedback. The CSAs then completed two further charts and agreement was checked. Throughout the data abstraction process, the abstractors met routinely with the investigators to review the collected data. Questions regarding variable definitions and charting shortfalls were addressed throughout the data collection period. After the completion of abstraction, data were compared and the principal investigator adjudicated any discrepancies.
Abstracted data included demographic information, laboratory information, written cvEEG reports and final patient disposition, including in-hospital mortality. Thirty-day mortality was assessed via review of the Social Security Death Index, which occurred at least seven months after patient discharge. Standardized abstraction forms were used with pre-specified definitions for all fields and explicitly stated abstraction and coding rules. Missing or unknown data points were recorded as such, and every data form had all entries completed.
The cvEEGs were independently reviewed by two epileptologists board certified in Clinical Neurophysiology for the period of cooling, from the first recorded 34 °C temperature to the initiation of rewarming. Epileptologists were blinded to both the initial cvEEG interpretation and all clinical data. They were instructed to determine whether or not each participant experienced seizures. There was good inter-rater reliability between the two blinded epileptologists (kappa = 0.784). Two cases were discrepant for the diagnosis of seizures and required adjudication by a third blinded epileptologist. An additional five charts were discrepant on other EEG characteristics, and were adjudicated by the same blinded epileptologist. Electrographic seizures were defined as any pattern lasting at least 10 s with one of the following criteria: (Jirsch and Hirsch, 2007).
Repetitive generalized or focal spikes, sharp-waves, spike and wave complexes at ≥3 per second.
Repetitive generalized or focal spikes, sharp-waves, spike and wave complexes at ≤3 per second AND significant improvement in clinical state or appearance of previously absent normal EEG patterns temporally coupled to acute administration of a rapidly acting anti-epileptic drug.
Sequential, rhythmic, periodic, or quasi-periodic waves at ≥1 per second and unequivocal evolution in frequency (increase by at least 1 per second), morphology or location.
The primary aim of this study was to estimate the proportion of patients suffering seizures during the cooling period, and therefore the epileptologist review of the cvEEG recordings was limited to the cooling period. Clinical study assistants reviewed the medical record for the entire hospital stay and documented any additional cvEEG interpretations noted in the record. This allowed the study team to capture all documented seizures occurring during hospitalization, and to have maximal sensitivity to seizures that occurred during the hypothermia period. The hospital’s treatment protocol calls for all patients to be monitored with cvEEG from initiation of therapeutic hypothermia until return to euthermia after rewarming. Monitoring past rewarming was determined by the treating team based on clinical indications.
Primary outcomes
The primary outcome was the presence or absence of seizures in patients undergoing therapeutic hypothermia after cardiac arrest. Secondary outcomes included inhospital and 30-day mortality.
Data management and analysis
Study data were collected on paper case report forms and subsequently managed using REDCap (Research Electronic Data Capture). All analyses were conducted using SPSS 21.0 for Windows (IBM Corporation, Armonk, NY). Continuous data are described using medians and ranges. Categorical data are described using frequencies and percentages. Fisher’s Exact Test was used to compare categorical variables. Differences in proportions were calculated and 95% confidence intervals are used to indicate precision of the estimates.
Results
In 2010, there were 34 patients eligible for therapeutic hypothermia for whom a neurocritical care physician completed a post-cardiac arrest consultation. One patient began to follow verbal commands and subsequently was ineligible for the protocol. The remaining 33 patients were monitored with cvEEG and included in the study.
Median age was 58 years (range 28–86 years, interquartile range 24), 55% were male, and 64% were Caucasian (Table 1). A past history of seizure disorder or epilepsy was reported in 3/33 (9%).
Table 1.
Patient demographics by seizure status. Data are presented in frequency and percent or median and IQR.
| Age | Total (n = 33)
|
No seizure (n = 22)
|
Seizure (n = 11)
|
|||
|---|---|---|---|---|---|---|
| 58 | 24 | 59 | 21 | 54 | 29 | |
| Caucasian | 21 | 63.6% | 12 | 54.5% | 9 | 81.8% |
| Male | 18 | 54.5% | 10 | 45.5% | 8 | 72.7% |
| History of seizure disorder or seizures | 3 | 9.1% | 2 | 9.1% | 1 | 9.1% |
| Bystander CPR | 10 | 30.3% | 7 | 31.8% | 3 | 27.3% |
| Inpatient arrest | 11 | 33.3% | 15 | 68.2% | 8 | 72.7% |
| Documented down time (minutes)a | 18 | 18 | 16 | 13 | 30 | 14 |
| Initial rhythm of arrest | ||||||
| Ventricular fibrillation (V-Fib) | 15 | 45.5% | 12 | 54.5% | 3 | 27.3% |
| Pulseless electrical activity (PEA) | 10 | 30.3% | 4 | 18.2% | 6 | 54.5% |
| Asystole | 7 | 21.2% | 5 | 22.7% | 2 | 18.2% |
| Ventricular tachycardia (V-Tach) | 1 | 3.0% | 1 | 4.5% | 0 | 0.0% |
Documented for 30 patients.
Seizures were confirmed by the blinded epileptologists in 5/33 patients (15%, CI95 6–33%). Myoclonic status epilepticus was diagnosed in 4/5 (80%) patients with electrographic seizures during the cooling period. cEEG monitoring was continued past the initiation of rewarming in 21/33 patients (64%), and 13/33 (39%) had cEEG monitoring past the return to normothermia. During the entire hospital stay, seizures were reported in the cvEEG medical records for an additional six patients for a total of 11/33 patients (33%, CI95 19–52%). Seizures were treated according to local protocol.
Cooling methods to achieve therapeutic hypothermia and the sedative and paralytic medications administered during hypothermia and rewarming are shown in Table 2. Thirty of 33 participants (90.9%) received sedation with midazolam, lorazepam or propofol. One patient did not have any medications documented. The median interval from cardiac arrest to initiation of cvEEG monitoring was 7 h (range 3–26 h IQR 3).
Table 2.
Hypothermia time intervals and cooling methods. Data are presented in frequency and percent or median and IQR.
| Total (n = 33) | No seizure (n = 22) | Seizure (n = 11) | ||||
|---|---|---|---|---|---|---|
| Interval from arrest to target temperature (h) | 7 | 5 | 7 | 5 | 8 | 7 |
| Time at target temperature (h) | 24 | 5 | 24 | 5 | 23 | 6 |
| Duration of rewarming (h) | 8 | 3 | 8 | 4 | 8 | 4 |
| Interval from arrest to cvEEG monitoring initiation (h) | 7 | 3 | 8 | 4 | 7 | 2 |
| Glasgow coma score | ||||||
| Eyes | 1 | 0 | 1 | 0 | 1 | 0 |
| Verbal | 1 | 0 | 1 | 0 | 1 | 0 |
| Motor | 1 | 2 | 1 | 2 | 1 | 0 |
| Methods of cooling | ||||||
| Endovascular coolinga | 22 | 66.7% | 13 | 59.1% | 9 | 81.8% |
| Surface coolinga | 27 | 81.8% | 18 | 81.8% | 9 | 81.8% |
| Surface and endovascular coolinga | 19 | 57.6% | 12 | 54.5% | 7 | 63.6% |
| Any sedation | 30 | 90.9% | 19 | 86.4% | 11 | 100.0% |
| Midazolam | 14 | 42.4% | 7 | 31.8% | 7 | 63.6% |
| Lorazepam | 5 | 15.2% | 1 | 4.5% | 4 | 36.4% |
| Propofol | 24 | 72.7% | 15 | 68.2% | 9 | 81.8% |
Patients could have multiple methods of cooling.
Outcomes are shown in Table 3. Thirteen of 33 participants (40%) survived to discharge. Of these, 7/13 (54%) survived to 30 days. Patients with a seizure during the cooling period were not more likely to die than those without a seizure during hospitalization (60% vs. 61%; difference – 1%, CI9547% – 46%). Similarly, patients with any seizure were not more likely to die than those without a seizure during hospitalization (82% vs. 50%; difference 32%, CI951% – 63%). However, no patient with seizures was alive at 30 days, and patients with any seizure were more likely to die within 30 days than those without a seizure (100% vs. 68%; difference 32%, CI9512% – 51%).
Table 3.
Outcome and discharge disposition by seizure status. Difference in proportions and 95% confidence intervals are provided.
| Total | No seizure (n = 22)
|
Seizure (n = 11)
|
Difference | 95% CI
|
|||||
|---|---|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||||
| Deceased at discharge | 20 | 60.6% | 11 | 50.0% | 9 | 81.8% | 31.8% | 0.9% | 62.7% |
| Deceased at 30 days | 26 | 78.8% | 15 | 68.2% | 11 | 100.0% | 31.8% | 12.4% | 51.3% |
| Discharge disposition | |||||||||
| Death | 20 | 60.6% | 11 | 50.0% | 9 | 81.8% | |||
| Transfer to skilled nursing | 4 | 12.1% | 4 | 18.2% | 0 | 0.0% | |||
| Other facility | 3 | 9.1% | 1 | 4.5% | 2 | 18.2% | |||
| Discharged home | 3 | 9.1% | 3 | 13.6% | 0 | 0.0% | |||
| Rehabilitation facility | 2 | 6.1% | 2 | 9.1% | 0 | 0.0% | |||
| Transfer to long term care | 1 | 3.0% | 1 | 4.5% | 0 | 0.0% | |||
Discussion
These data suggest that seizures occur in up to 1 in 3 patients during the cooling period and the subsequent hospitalization after treatment with therapeutic hypothermia. This study was conducted in a single, urban, academic hospital. Whether the results are generalizable beyond this setting is unknown, although the incidence of seizures observed here is similar to that previously reported in the literature (8–60%) (Rittenberger et al., 2012; Towne et al., 2000; Claassen et al., 2004; Mani et al., 2012; Rossetti et al., 2010; Crepeau et al., 2013).
The incidence of seizures we detected was despite the antiepileptic effect of intravenous benzodiazepine or propofol, which were used for sedation in 91% of the patients; the incidence of seizures might be higher in an unmedicated population. There are several hypotheses regarding the mechanisms of seizures despite continuous intravenous sedation, ranging from failure of the blood–brain barrier to medication resistance of the seizures (Marchi et al., 2011). We have additionally shown the propensity for seizures during the cooling period itself, which refutes prior suggestions that seizures are limited to the rewarming period after therapeutic hypothermia. We do note that while cvEEG was initiated very quickly, with a median time from return of spontaneous circulation to cvEEG initiation of 7 h, it is possible that earlier seizures might have occurred. Further work should consider the importance of earlier initiation of hypothermia (and overall cooling duration) and time to cvEEG monitoring to improve outcomes.
Increased duration of seizures is associated with increased mortality (Towne et al., 1994). Amongst all types of seizures, if a patient seizes for less than 10 h, mortality is reported to be less than 10%; if seizures continue for greater than 20 h, the mortality rises to over 85% (Towne et al., 1994). Earlier diagnosis also portends improved outcomes. If the diagnosis is made in less than ½ an-hour from ictus, mortality is as low as 36%. For a diagnosis made over 24 h from ictus, mortality can be over 75% (Young et al., 1996). Our results suggest early cvEEG monitoring, including during the cooling period, might be beneficial.
In the patients with seizures, the documented down time was longer, and the rate of pulseless electrical activity (PEA) was higher. This could additionally explain differences in mortality, suggesting seizures were a result of a higher rate of brain injury in this group of patients, and not the cause.
As more studies demonstrate the frequent occurrence and poor outcome of seizures in post-cardiac arrest patients (Rittenberger et al., 2012; Mani et al., 2012; Crepeau et al., 2013), developing effective treatments becomes critical. cvEEG allows for the detection of electrographic seizures, and thus prompting treatment of what would be otherwise go unrecognized. In one study, cEEG monitoring led to a change in antiepileptic use in 52% of patients, with initiation of therapy in 14% (Kilbride et al., 2009). As yet, there is little evidence to suggest preferred treatment options. In this study, 30-day mortality for patients who experienced seizures was 100%. Management of seizures and implementation of end-of-life care was not controlled and it is certainly the case that seizures post cardiac arrest, particularly myoclonic status epilepticus, has traditionally been viewed as a herald of poor outcome. Rather than incorporating such findings as a self-fulfilling prophecy, we recommend early diagnosis of seizures post cardiac arrest as the first step toward treatment to ameliorate the devastating consequences. It might even be conjectured that making a diagnosis of seizures could inform end of life decision-making. Families faced with the decision to withdraw care might be inclined to opt for ongoing life-support and treatment if the patient’s neurologic devastation is a result of potentially treatable seizures rather than a cardiac arrest related anoxic brain injury.
The results should be tempered by the limitations inherent in the study design. Although rigorous chart review methods were used, the study is nonetheless limited by the nature of clinical documentation, including missing data, error and inconsistency. The absence of a standardized EEG protocol after the rewarming period is an additional limitation, particularly given the number of seizures noted after the hypothermia period. The small sample size also suggests the need to replicate these findings, particularly in order to support the remarkably high 30-day mortality in patients who experienced seizures. This is higher than previously published reports (Rittenberger et al., 2012; Mani et al., 2012; Rossetti et al., 2010), and supported in a recent study (Crepeau et al., 2013), which suggests that identification and treatment of seizures may be more important than realized.
Conclusions
In this study, seizures occurred in one of every three cardiac arrest patients treated with hypothermia and were associated with increased mortality. Seizures were observed during cooling as well as after rewarming. Applying cvEEG early in the course of care for these patients is recommended to enable earlier diagnosis. Optimized treatment options for seizures might then decrease the high morbidity and mortality associated with cardiac arrest.
Acknowledgments
Funding
This project was supported in part by an award from the Emergency Medicine Foundation and by an Institutional Clinical and Translational Science Award, NIH/NCRR Grant Number 5UL1RR026314-03.
Appendix A
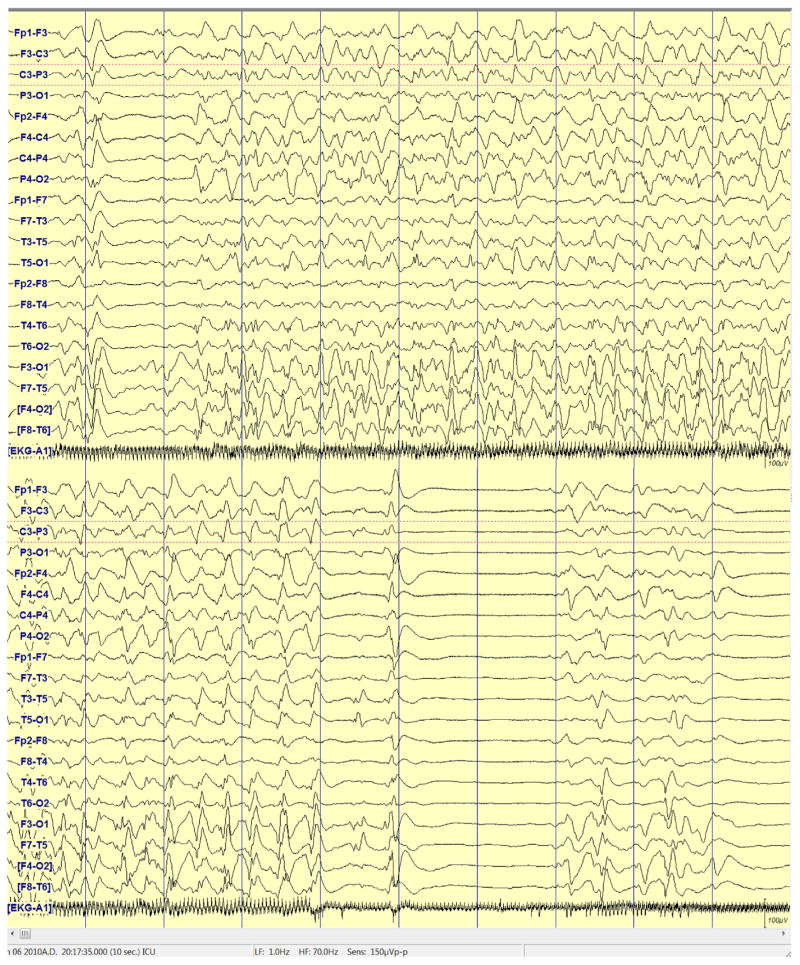
Figure A1.
EEG example showing 2–4 Hz continuous generalized spike and wave.
Figure A2.
EEG showing 6 Hz bifrontal maximal electrographic seizure lasting for 12 s.
Footnotes
Selected results were presented at the Scientific Assembly, American College of Emergency Physicians, October 2011, San Francisco, CA, and at the Neurocritical Care Society annual meeting, October 2011, Montreal, Ontario, Canada.
Conflicts of interest
There are no conflicts of interest from any author as it pertains to this manuscript.
Authors contributions
W.A.K., with guidance from C.J.L., conceived the study, designed the trial and obtained research funding. W.A.K., K.W.H. and C.J.L. supervised the conduct of the trial and data collection. O.M.A., J.B.B., D.M.F., S.P.K., M.D.P. and J.P.S. provided input to the design of the study. D.F., J.S., and M.P. performed independent review of all cEEGs. K.W.H. managed and analyzed the data, including quality control; C.J.L. provided statistical oversight. W.A.K. drafted the manuscript, and all authors contributed substantially to its revision. W.A.K. takes responsibility for the paper as a whole.
Contributor Information
William A. Knight, Email: William.Knight@uc.edu, knightwa@ucmail.uc.edu.
Kimberly W. Hart, Email: Kimberly.Hart@uc.edu.
Opeolu M. Adeoye, Email: Opeolu.Adeoye@uc.edu.
Jordan B. Bonomo, Email: Jordan.Bonomo@uc.edu.
Shaun P. Keegan, Email: Shaun.Keegan@UCHealth.com.
David M. Ficker, Email: David.Ficker@uc.edu.
Jerzy P. Szaflarski, Email: Szaflaj@uab.edu.
Michael D. Privitera, Email: Michael.Privitera@uc.edu.
Christopher J. Lindsell, Email: Christopher.Lindsell@uc.edu.
References
- Neumar RW, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118(December 23):2452–2483. doi: 10.1161/CIRCULATIONAHA.108.190652. [DOI] [PubMed] [Google Scholar]
- Rittenberger JC, Popescu A, Brenner RP, Guyette FX, Callaway CW. Frequency and timing of nonconvulsive status epilepticus in comatose post-cardiac arrest subjects treated with hypothermia. Neurocrit Care. 2012;16:114–122. doi: 10.1007/s12028-011-9565-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–345. doi: 10.1212/wnl.54.2.340. [DOI] [PubMed] [Google Scholar]
- Claassen J, Mayer SA, Kowalski RG, Emerson RG, Hirsch LJ. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. doi: 10.1212/01.wnl.0000125184.88797.62. [DOI] [PubMed] [Google Scholar]
- Mani R, Schmitt SE, Mazer M, Putt ME, Gaieski DF. The frequency and timing of epileptiform activity on continuous electroencephalogram in comatose post-cardiac arrest syndrome patients treated with therapeutic hypothermia. Resuscitation. 2012;83:840–847. doi: 10.1016/j.resuscitation.2012.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kull LL, Emerson RG. Continuous EEG monitoring in the intensive care unit: technical and staffing considerations. J Clin Neurophysiol. 2005;22:107–118. doi: 10.1097/01.wnp.0000158361.24544.2d. [DOI] [PubMed] [Google Scholar]
- Jirsch J, Hirsch LJ. Nonconvulsive seizures: developing a rational approach to the diagnosis and management in the critically ill population. Clin Neurophysiol. 2007;118:1660–1670. doi: 10.1016/j.clinph.2006.11.312. [DOI] [PubMed] [Google Scholar]
- Rossetti AO, Urbano LA, Delodder F, Kaplan PW, Oddo M. Prognostic value of continuous EEG monitoring during therapeutic hypothermia after cardiac arrest. Crit Care. 2010;14:R173. doi: 10.1186/cc9276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towne AR, Pellock JM, Ko D, DeLorenzo RJ. Determinants of mortality in status epilepticus. Epilepsia. 1994;35 (1):27–34. doi: 10.1111/j.1528-1157.1994.tb02908.x. [DOI] [PubMed] [Google Scholar]
- Young GB, Jordan KG, Doig GS. An assessment of non-convulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–89. doi: 10.1212/wnl.47.1.83. [DOI] [PubMed] [Google Scholar]
- Crepeau AZ, Rabinstein AA, Fugate JE, et al. Continuous EEG in therapeutic hypothermia after cardiac arrest: prognostic and clinical value. Neurology. 2013;80:339–344. doi: 10.1212/WNL.0b013e31827f089d. [DOI] [PubMed] [Google Scholar]
- Marchi N, Tierney W, Alexopoulos AV, et al. The etiological role of blood–brain barrier dysfunction in seizure disorders. Cardiovasc Psychiatr Neurol. 2011:Article ID 482415. doi: 10.1155/2011/482415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbride RD, Costello DJ, et al. How seizure detection by continuous electroencephalographic monitoring affects the prescribing of antiepileptic medications. Arch Neurol. 2009;66 (6):723–728. doi: 10.1001/archneurol.2009.100. [DOI] [PubMed] [Google Scholar]