Abstract
Introduction
Differences in quality of care (QOC) may contribute to racial variation in outcomes of bladder cancer (BCa). Quality indicators in patients undergoing surgery for BCa include use of high-volume surgeons (HVSs) and hospitals (HVHs), and, when clinically indicated, receipt of pelvic lymphadenectomy (PLND), use of continent urinary diversion and undergoing radical cystectomy (RC) instead of partial cystectomy (PC). We compared these quality indicators, as well as adverse perioperative outcomes in Black and White patients with BCa.
Methods
We used the Healthcare Cost and Utilization Project’s State Inpatient Databases, selecting NY, FL, and MD (1996–2009), because they consistently included race, surgeon and hospital identifiers. Quality indicators were compared across racial groups using regression models adjusting for age, sex, Elixhauser comorbidity sum, insurance, state, and year of surgery, accounting for clustering within hospital.
Results
Black patients were more often treated by lower volume surgeons and hospitals; had significantly lower utilization of PLND and continent diversion; and experienced higher rates of adverse outcomes compared to White patients. These associations remained significant for Black patients receiving treatment by surgeons and hospitals in the top volume decile.
Conclusion
Black BCa patients had lower utilization of experienced providers and institutions for BCa surgery. In addition, QOC for Black patients was lower than for Whites even if treated in a high-volume setting. This gap in QOC requires further investigation.
Keywords: Health disparities, quality of care, bladder cancer, surgery
INTRODUCTION
While bladder cancer (BCa) incidence is twice as high among Whites compared with Blacks, Blacks suffer a disproportionally high rate of BCa mortality, even when accounting for race differences in stage at presentation.1–3 Variability in quality of care (QOC) may contribute to differences in BCa mortality, particularly in the definitive surgical management of aggressive disease (i.e., cystectomy or bladder removal). Such procedures are technically demanding, and a successful outcome depends on specific processes of care and provider competence.4–6
There are several indicators suitable to evaluate quality of BCa care.7, 8 First, partial cystectomy (PC) provides inferior cancer control compared to radical cystectomy (RC), unless strict selection criteria are met.9, 10 Therefore, higher utilization of PC may indicate inferior QOC.9 Second, use of high-volume surgeons (HVS) and hospitals (HVH) is associated with lower complication rates, perioperative mortality, length of stay (LOS) and re-admission rates.11–13 Third, receipt of pelvic lymphadenectomy (PLND) in conjunction with RC for muscle-invasive BCa is considered standard-of-care,14, 15 because of its prognostic and therapeutic value. Finally, the proportion of RC patients receiving continent diversion may also reflect QOC, since some observers consider continent diversion more desirable for urinary function and cosmesis, and the operation is more technically demanding than non-continent diversion.8, 16 These structural (utilization of HVH and HVS) and process (receipt of RC vs. PC, PLND and continent diversion) measures affect treatment outcomes and provide a means to measure differences in QOC.
Thus, the purpose of this study was to determine whether a QOC gap exists between Black and White patients undergoing cystectomy for BCa. We hypothesized that Black patients would have lower utilization of high-quality structural resources and processes of care than Whites, associated with more adverse events (prolonged LOS, blood transfusions, surgical complications, and in-hospital mortality.) Furthermore, we hypothesized that Black and White patients treated at HVH and by HVS would have similar processes of care utilization and perioperative outcomes.
METHODS
Dataset
The State Inpatient Databases (SIDs) are encounter-level administrative data compiled through the Agency for Healthcare Research and Quality (AHRQ)’s Healthcare Cost and Utilization Project (HCUP).17 The SIDs contain over 100 uniformly recorded data elements on virtually all discharges from non-Federal hospitals in HCUP-participating states. Data elements include principal and secondary discharge diagnoses and procedures, patient demographics, expected payment source, and LOS.
We restricted our analysis to states meeting all of the following criteria: 1) race/ethnicity coding was sufficiently complete for the state to be included in HCUP’s disparities analytic file;18 2) surgeon identifier was uniform across hospitals in the state so that inter-hospital procedure volumes could be calculated; 3) data were available through HCUP’s Central Distributor. Three states (Florida, New York, and Maryland) met these criteria. We excluded data from hospitals with suspect coding of race, based on the following criteria, which reflect methods used to assemble AHRQ’s disparities analytic file:18 1) >30% of discharges had race reported as “other”; 2) race was missing in >50% of discharges; 3) all discharges had race coded as White, other, or missing; or 4) 100% of discharges had race coded as White. Data were available from 1996 to 2009.
Cohort Definition
We identified all cases with PC or RC listed as the principal procedure by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) procedure codes (57.6, 57.7, 57.71 and 57.79). We restricted the cohort to patients with BCa (ICD-9-CM principal diagnosis codes 188, 1880, 1889, 2337, 2376, 2394) and included all patients 19 to 94 years of age. We excluded cases performed during admissions coded as ‘urgent’ or ‘emergency’.
Independent Variables
We used the uniformly coded ‘race’ variable to classify patients as ‘White,’ ‘Black,’ or ‘All Others.’ The number of patients identified as ‘Hispanic,’ ‘Asian/Pacific Islander,’ or ‘Native American’ and ‘Other’ was too small to permit meaningful separate analyses, so we grouped them together and focused our analysis on White and Black patients. Our use of the terms ‘White’ and ‘Black’ throughout this manuscript reflects the terminology used in the SIDs.
Other independent variables included patient age; sex; payer (private insurance, Medicare, Medicaid, or Other; number of comorbidities;19 state in which the procedure was performed; and year of surgery.
Outcome Definition
The outcomes of interest were use of RC vs. PC; use of PLND; hospital volume; surgeon volume; in-hospital mortality [yes/no]; blood transfusion [yes/no]; any complications [yes/no]; diversion type [continent/incontinent]; and LOS. On univariate and multivariable analyses, use of RC vs. PC was assessed in the entire analytic cohort. For analyses of the remaining measures, we included only patients who underwent RC, because these measures have been validated to an extent in RC, but not in PC. Use of PLND and continent diversion was determined from ICD-9 procedure codes (403 and 578.7, respectively.) Surgeon volume (SV) was calculated as the number of RC and PC cases performed by a particular surgeon in a particular state, in each calendar year, based on the surgeon identifier. Hospital volume (HV) was similarly calculated. Both SV and HV were evaluated as outcome variables, as well as predictors of the other outcomes.
In-hospital mortality and LOS are reported for all discharges in the SIDs and blood transfusion was identified by ICD-9-CM procedure code (99.04), and/or Uniform Billing (UB-92) codes. Based on previous studies, we composed a binary variable indicating whether the patient had any complications according to the presence of specific secondary discharge diagnoses.20
Statistical Analysis
We used Pearson chi squared and Kruskal-Wallis tests to compare outcomes across racial groups and across surgeon and hospital volume strata. To simplify univariate comparisons, annual RC volumes were classified as low, medium and high, with high volume (≥5 cases per surgeon and ≥ 12 cases per hospital) representing the top volume decile, computed as a weighted average of the top volume decile for all states and all years. However, surgeon and hospital volume were entered into multivariable models as continuous variables.
We examined the relationship between race and each quality indicator using different types of regression models, adjusting for correlation among observations within the same hospital. To assess the associations between race and surgeon and hospital volume, we fit Poisson regression models with the standard errors estimated through cluster bootstrap resampling to account for clustering within hospital.21 We fit logistic regression models predicting PLND, death in hospital, any complications, homologous blood transfusion, type of urinary diversion, and use of RC vs. PC.
For the LOS analysis, we excluded inpatient deaths (n = 345), since the interpretation of LOS is different in these patients, and patients with LOS < 4 days (n = 49), since these probably represent coding errors. We fit a generalized least squares (GLS) regression model, assuming an exchangeable correlation structure, using the log length of stay to correct for non-normality in the residuals.
In each model, we included interaction terms to allow the effect of race on outcome to vary by stratum of other independent variables. Because of the flexibility allowed by the interaction terms, there is no one effect of race to estimate; rather, the effect of race can be estimated for any combination of values of the independent variables. Therefore, we presented estimated effects of race for specific scenarios of patient characteristics. In order to determine if there is an overall effect of race on each outcome, we performed F tests of the null hypothesis that parameters for all model terms involving race are equal to zero. Restricted cubic splines were used with age and year of surgery to allow for nonlinear relationships with the outcome variable. Analyses were performed using R version 2.14.2 (R Core Team, Vienna, Austria).
We obtained an Institutional Review Board review exemption at Vanderbilt University and signed a data-use agreement with AHRQ for use of the public-access versions of the SID.
RESULTS
After excluding 506 patients from hospitals with suspect coding of race and 31 with missing race, we identified 16,864 cases for analysis, 14,834 (88%) White, 795 (5%) Black, and 1235 (7%) Other (Table 1).
Table 1.
Patient Characteristics
| Characteristics | % White | % Black |
|---|---|---|
| N = 14,834 | N = 795 | |
| Median (IQR) age (years) | 71 (63, 77) | 67 (57, 74) |
| Male | 81.7 | 69.1 |
| Hospital state | ||
| FL | 42.9 | 32.9 |
| MD | 11.1 | 25.2 |
| NY | 46.0 | 41.9 |
| Payer | ||
| Medicare | 64.6 | 52.0 |
| Medicaid | 02.2 | 10.1 |
| Private insurance | 30.2 | 32.7 |
| Other | 03.0 | 05.2 |
| Comorbidity count | ||
| 0 | 20.6 | 19.0 |
| 1 | 30.3 | 28.1 |
| 2 | 25.0 | 24.8 |
| 3 | 14.5 | 17.7 |
| 4+ | 09.5 | 10.5 |
Percentages may not sum to 100% due to rounding.
Three to sixteen percent of surgeons performed RC in >1 hospital in each state/year combination, demonstrating that the identifier does indeed cross hospitals. Most surgeons performed 1–2 cases per year (73.7%, 341/463 surgeons on average each year) and 48.4% of patients had their RC performed by surgeons who performed <5 cases in that year. Median annual hospital volume ranged from 2–4, depending on the state and year, and 42.9% of patients had their surgery at hospitals that performed <12 RCs that year.
Black patients consistently used lower-volume hospitals and surgeons than White patients (Table 2), despite an increase in surgeon and hospital volume over time for both groups. Unadjusted analyses showed that Black patients also experienced lower utilization of evidence-based processes of care and had more adverse outcomes compared to Whites (Table 2).
Table 2.
Outcome Measures by Racial Group
| Outcome | % White | % Black | p-value |
|---|---|---|---|
| All patients | N = 14,834 | N = 795 | |
| Cystectomy type | 0.021 | ||
| Partial | 14.8 | 16.2 | |
| Radical cystectomy | N = 12,638 | N = 666 | |
| Median (IQR) surgeon volume | 5 (2, 19) | 3 (1, 9) | < 0.001 |
| Median (IQR) hospital volume | 15 (5, 45) | 9 (4, 28) | < 0.001 |
| Transfusion | 34 | 44 | < 0.001 |
| Any complication | 44 | 52 | < 0.001 |
| In-hospital morality | 2.4 | 3.2 | 0.41 |
| PLND | 59 | 49 | < 0.001 |
| Median (IQR) LOS (days) | 9 (7, 12) | 9 (8, 14) | < 0.001 |
| Diversion type | N = 10816 | N = 555 | |
| Continent | 8.6 | 6.5 | < 0.001 |
Shaded rows show the number of patients available for analysis in the rows below; quality measures besides cystectomy type were studied only in RC patients; 1,933 White and Black patients were missing data for diversion type.
We also examined the associations between surgeon and hospital volume and each process and outcome measure (Table 3). Surgeon and hospital volume were directly associated with use of PLND and continent diversion, and inversely associated with LOS, complications, blood transfusions, and perioperative mortality.
Table 3.
Outcomes by Surgeon and Hospital Volume
| Outcome | % Analytic set | % SV 1–2 | % SV 3–4 | % SV 5 or more | p-value |
|---|---|---|---|---|---|
| N = 16,747 | N = 5,925 | N = 2,847 | N = 7,975 | ||
| Cystectomy type | < 0.001 | ||||
| Partial | 15.0 | 24.7 | 15.0 | 7.9 | |
| Radical cystectomy | N = 14,226 | N = 4,462 | N = 2,419 | N = 7,345 | |
| Transfusion | 35 | 37 | 36 | 33 | < 0.001 |
| Any complication | 44 | 49 | 46 | 41 | < 0.001 |
| In-hospital morality | 2.4 | 3.8 | 2.9 | 1.4 | < 0.001 |
| PLND | 58 | 46 | 49 | 69 | < 0.001 |
| Median (IQR) LOS (days) | 9 (7, 12) | 9 (7,13) | 9 (7, 12) | 8 (7, 11) | < 0.001 |
| Diversion type | N = 12,132 | N = 3,856 | N = 2,114 | N = 6,162 | |
| Continent | 8.8 | 3.1 | 4.7 | 13.8 | < 0.001 |
| Outcome | % Analytic set | % HV 1–5 | % HV 6–11 | % HV 12 or more | p-value |
|---|---|---|---|---|---|
| N = 16,862 | N = 4,867 | N = 3,023 | N = 8,972 | ||
| Cystectomy type | |||||
| Partial | 15.1 | 24.8 | 17.6 | 8.9 | < 0.001 |
| Radical cystectomy | N = 14,322 | N = 3,657 | N = 2,490 | N = 8,175 | |
| Transfusion | 35 | 37 | 35 | 34 | < 0.001 |
| Any complication | 44 | 50 | 46 | 42 | < 0.001 |
| In-hospital morality | 2.4 | 3.6 | 2.7 | 1.8 | < 0.001 |
| PLND | 58 | 45 | 50 | 67 | < 0.001 |
| Median (IQR) LOS (days) | 9 (7, 12) | 9 (7,13) | 9 (7, 12) | 9 (7, 11) | < 0.001 |
| Diversion type | N = 12,210 | N = 3,178 | N = 2,162 | N = 6,870 | |
| Continent | 8.8 | 2.5 | 3.6 | 13.3 | < 0.001 |
Shaded rows show the number of patients available for analysis in the rows below; all quality measures besides cystectomy type were studied only in RC patients; 2,094 and 2,112 patients were missing data for diversion type in the SV and HV analyses, respectively.
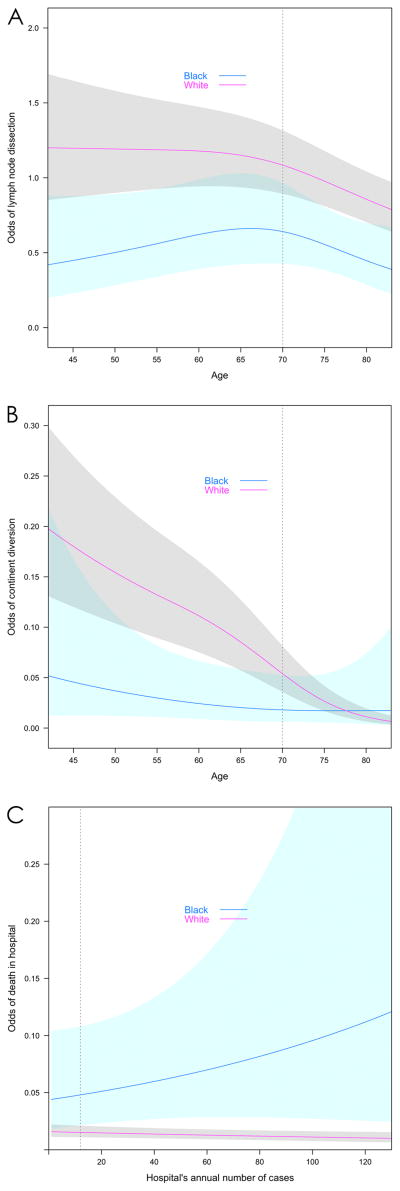
In the multivariable models, Black race was a significant predictor of poorer performance on each quality indicator except use of RC vs. PC and use of blood transfusion (Table 4, Figure 1). However, interaction terms for Black race X SV and Black race X HV were non-significant in each model, suggesting that the QOC deficit was independent of volume. Representative figures demonstrate differences in performance on quality measures in Black vs. White patients, across strata of other independent variables, among patients treated by surgeons and hospitals at the 90th percentile for volume (Figure 1A–C). All remaining independent variables are set to the median for all patients (age = 70; sex = male; comorbidity = 1; state = NY; Year = 2003; Payer = Medicare) to exemplify a “typical” patient. For example, “typical” Black patients treated at HVHs had lower odds of receiving PLND than typical White patients, over a broad range of patient ages (Figure 1A). Black patients treated at HVHs by HVSs had significantly lower odds of continent diversion, but the association became non-significant over the age of 68, beyond which few patients in either group underwent continent diversion (Figure 1B). Black patients treated by HVSs had markedly higher odds of in-hospital mortality compared to White patients, regardless of hospital volume (Figure 1C).
Table 4.
Overall Effect of Black Race on Quality Measures
| Quality Indicator | p value Overall |
|---|---|
| Use of RC | 0.411 |
| Use of PLND | 0.025 |
| Continent Diversion | <0.001 |
| Transfusion | 0.053 |
| Any complications | 0.013 |
| In-hospital mortality | <0.001 |
| Length of stay | <0.001 |
| Surgeon volume** | 0.005 |
| Hospital volume** | <0.001 |
RC = Radical cystectomy; PLND = Pelvic lymph node dissection
p-value for overall effect of Black race on outcome, calculated by an F test of the null hypothesis that coefficients for all terms involving race are zero, including main effect and all interaction terms. Note that the overall effect of race may be significant even when it is not significant in a specific scenario.
Models predicting surgeon and hospital volume do not include surgeon or hospital volume as independent variables, whereas other models do include such terms.
Figure 1. Model predictions based on race and interaction terms.
A. Odds of undergoing Pelvic Lymph Node Dissection by Race over Surgeon Volume
B. Odds of undergoing Continent Diversion by Race over Age
C. Odds of In-Hospital Mortality by Race over Hospital Volume
Independent variables are adjusted to median values for all patients (age = 70; sex = male; comorbidity = 1; state = NY; Year = 2003; Payer = Medicare), except where they are present in the x-axis. Surgeon and hospital volume, are set to the 90th percentile (>5 cases per year for surgeon and>12 cases per year for hospital) when not present in the x-axis.
Vertical bar represents specific scenario in Table 5. Shading indicates 95% confidence interval.
Odds ratios for the specific scenario in which all independent variables were set to the median, except for SV and HV, which were set to the 90th percentile are shown in Table 5. Note that in the models for continent diversion and complications, the Black race was not a significant predictor in this particular scenario, although the overall effect of Black race was significant (Table 4). This is typical of models that include multiple interaction terms, because the magnitude and significance of the effect may vary over strata of another independent variable, as in the interaction between Black race X age on use of continent diversion (Figure 1B.)
Table 5.
Specific Scenario for a “Typical” Patient Treated in a High-Volume Hospital by a High-Volume Surgeon
| Specific Scenario | ||
|---|---|---|
| Quality indicator | OR (Black vs. White) | 95% CI |
| Use of RC | 0.97 | 0.53, 1.75 |
| Use of PLND | 0.59 | 0.38, 0.91 |
| Continent Diversion | 0.35 | 0.10, 1.19 |
| Transfusion | 0.94 | 0.56, 1.59 |
| Any complications | 1.40 | 0.90, 2.20 |
| In-hospital mortality | 3.16 | 1.35, 7.40 |
| R | 95% CI | |
| Length of stay* | 1.24 | 1.13, 1.36 |
RC = Radical cystectomy; PLND = Pelvic lymph node dissection
Independent variables are adjusted to median values for all patients (age = 70; sex = male; comorbidity = 1; state = NY; Year = 2003; Payer = Medicare), except for surgeon and hospital volume, which are set to the 90th percentile (5 cases per year for surgeon and 12 cases per year for hospital).
Estimate for the length of stay model represents the multiplicative effect of Black race compared to White; R = Ratio.
DISCUSSION
In this study, we found that Black patients undergoing cystectomy experienced lower QOC than White patients, as evidenced by use of lower-volume surgeons and hospitals, lower use of evidence-based processes of care, and higher incidence of adverse outcomes. Past studies have reported similar racial variation in QOC and outcomes for BCa.3, 22–27 However, beyond confirming these previous studies, we found Black race to be significantly associated with lower QOC even among patients treated by providers and at hospitals at the 90th percentile for volume.
Limitations in healthcare access may explain why Black patients were treated by lower volume surgeons and at lower volume hospitals. This finding pervades studies of healthcare disparities, and may be influenced by factors including socio-economic status, insurance, transportation, employment and social support.4, 28 Decreased access to care can lead to inferior BCa outcomes via delay in diagnosis, resulting more advanced disease at presentation;22 or via use of less experienced providers and hospitals, resulting in inferior outcomes due to lack of specialty care, lower use of clinical care pathways and deviation from evidence-based practices; or by use of comparable volume, but inferior quality surgeons and facilities, such as those that ‘fail to rescue’ deteriorating patients. 29
Interestingly, we observed that Black patients experienced lower use of PLND and continent diversion, and had more adverse outcomes even when treated in a HVH and by a HVS. It is possible that patients presenting to HVS and HVH differed with respect to the severity of their comorbidities, disease characteristics or surgical indications leading to different treatment recommendations. However, we could not address this due to the limited clinical information in our dataset. Black patients may not advocate for their healthcare as effectively as White patients, which may result in less aggressive (no PLND) or complex (incontinent) therapy. It is also possible that providers tend to recommend less aggressive or less complex therapy to Black patients, either because the provider is appropriately weighing factors such as comorbidity and the lower life expectancy of Black persons compared to Whites. Alternatively, providers may inappropriately allow personal bias to guide their treatment recommendations. Although differential healthcare recommendations or preferences cannot be ruled out by our findings, the increased incidence of adverse outcomes among Black patients suggests that other factors are at play. Despite getting less aggressive and less complex care, Black patients suffered more adverse outcomes, even in the hands of HVS and at HVH, suggesting that unmeasured confounders, such as differences in disease characteristics and patient comorbidity, contribute to both treatment decisions and adverse outcomes.
Whereas Finks et al demonstrated a 14% decline in perioperative mortality in the Medicare RC population between 1999 and 2008, associated with an increase in hospital RC volume during this period30, we found that Blacks had consistently higher inpatient mortality compared to Whites regardless of surgeon and hospital volume. Therefore, it may be important to examine policies that are intended to ensure that all patients benefit from volume-associated improvements in QOC, as market forces, payment reform and other factors lead toward further concentration of care.
These findings must be interpreted in the context of study design. Our dataset lacks important clinical information such as disease characteristics, treatments, and cancer outcomes; patient-level socioeconomic data; and patient and surgeon preferences. While there are known race differences in stage at presentation, it is unlikely that these explain the totality of the observed effects of race on LOS, transfusion, mortality and complications. Additionally, higher stage at presentation should be associated with higher use of RC vs. PC and higher use of PLND, which we did not observe. Importantly, we cannot ascertain the severity of comorbidities, and it is possible that persons with decreased access to care have a greater number of untreated chronic conditions or fewer comorbidities coded. In addition, diversion type was missing in nearly 20% of patients. While we did categorize surgeon and hospital volume to facilitate presentation of the univariate statistics, our multivariable models did not depend upon arbitrary volume cut-offs. Instead, we presented the case in which a patient is treated at a hospital and by a surgeon in the 90th of cystectomy volume, to illustrate the scenario of a patient treated in a high-volume setting. Our analysis of 3 states may limit its generalizability; however, we were guided by previously established criteria for selecting states and hospitals with the most complete reporting of race. The race variable in the SID has two major disadvantages: 1) it captures Hispanic ethnicity in the same variable as race, making it impossible to separate the effects of racial group from those of Hispanic ethnicity; and 2) since race is based on hospital coding, it is subject to variable procedures for collecting this information at different hospitals. Unfortunately, there were too few patients in each of the other racial and ethnic groups to permit meaningful individual analyses of those groups. Many barriers to access reflect underlying socioeconomic disparities between Blacks and Whites, which we cannot completely control for. Finally, our findings are based on patients who underwent cystectomy, and do not necessarily reflect the magnitude of racial variation in QOC for all BCa patients.
These limitations notwithstanding, this dataset is extremely robust because it includes all payers and age ranges, without which it would be difficult to accumulate enough cases to compare meaningful outcomes such as in-hospital mortality. Furthermore, the dataset and modeling techniques enabled us to demonstrate the effect of race on each quality metric at different strata of SV and HV. It also may allow for subsequent analyses of gender disparities in care and the interplay between race and gender, as Black women are known to have poor oncologic outcomes.
In summary, we demonstrated that Black BCa patients are less likely to undergo cystectomy with a HVS or HVH, and have lower use of PLND and continent diversion and more adverse perioperative events than their White counterparts, even when treated by HVS and HVH. Further research efforts should focus on improving access to high-quality care for all patients with BCa, and identifying factors responsible for the apparent QOC gap seen in high-volume settings.
Acknowledgments
Sources of Financial Support: American Cancer Society Internal Review Grant 58-0009-51, administered by the Vanderbilt Ingram Cancer Center; the National Center for Research Resources/National Institutes of Health via the Vanderbilt CTSA grant UL1TR000445.
Footnotes
Relevant Financial Disclosures: None
Conflict of Interest: None
Supplemental Material: None
Note: the opinions expressed in this paper are those of the authors and do not necessarily reflect the position of the US Agency for Healthcare Research and Quality, the National Institutes of Health, or the US Department of Health and Human Services.
References
- 1.Riesl . Cancer of the Urinary Bladder - SEER Survival Monograph. 2007. pp. 1–12. [Google Scholar]
- 2.Yee DS, Ishill NM, Lowrance WT, Herr HW, Elkin EB. Ethnic Differences in Bladder Cancer Survival. Urology. 2011;78:544–549. doi: 10.1016/j.urology.2011.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mallin K, David KA, Carroll PR, Milowsky MI, Nanus DM. Transitional cell carcinoma of the bladder: racial and gender disparities in survival (1993 to 2002), stage and grade (1993 to 2007) J Urol. 2011;185:1631–1636. doi: 10.1016/j.juro.2010.12.049. [DOI] [PubMed] [Google Scholar]
- 4.Jacobs BL, Montgomery JS, Zhang Y, Skolarus TA, Weizer AZ, Hollenbeck BK. Disparities in bladder cancer. Urol Oncol. 2012;30:81–88. doi: 10.1016/j.urolonc.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 5.Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol. 2004;22:2781–2789. doi: 10.1200/JCO.2004.11.024. [DOI] [PubMed] [Google Scholar]
- 6.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 7.Goossens-Laan CA, Kil PJM, Roukema JA, Bosch JLHR, De Vries J. Quality of care indicators for muscle-invasive bladder cancer. Urologia Internationalis. 2011;86:11–18. doi: 10.1159/000319369. [DOI] [PubMed] [Google Scholar]
- 8.Cooperberg MR, Porter MP, Konety B. Candidate quality of care indicators for localized bladder cancer. Urol Oncol. 2009;27:435–442. doi: 10.1016/j.urolonc.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Hollenbeck BK, Taub DA, Dunn RL, Wei JT. Quality of care: partial cystectomy for bladder cancer--a case of inappropriate use? J Urol. 2005;174:1050–1054. doi: 10.1097/01.ju.0000169477.30477.3d. discussion 1054. [DOI] [PubMed] [Google Scholar]
- 10.Holzbeierlein JM, Lopez-Corona E, Bochner BH, et al. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol. 2004;172:878–881. doi: 10.1097/01.ju.0000135530.59860.7d. [DOI] [PubMed] [Google Scholar]
- 11.Begg CB, Cramer LD, Hoskins WJ, Brennan MF. Impact of hospital volume on operative mortality for major cancer surgery. JAMA. 1998;280:1747–1751. doi: 10.1001/jama.280.20.1747. [DOI] [PubMed] [Google Scholar]
- 12.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL. Surgeon volume and operative mortality in the United States. NEJM. 2003;349:2117–2127. doi: 10.1056/NEJMsa035205. [DOI] [PubMed] [Google Scholar]
- 13.Konety BR, Dhawan V, Allareddy V, Joslyn SA. Impact of hospital and surgeon volume on in-hospital mortality from radical cystectomy: data from the health care utilization project. J Urol. 2005;173:1695–1700. doi: 10.1097/01.ju.0000154638.61621.03. [DOI] [PubMed] [Google Scholar]
- 14.Skinner E, Stein J, Skinner D. Surgical benchmarks for the treatment of invasive bladder cancer. Urol Oncol. 2007;25:66–71. doi: 10.1016/j.urolonc.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Herr HW, Bochner BH, Dalbagni G, Donat SM, Reuter VE, Bajorin DF. Impact of the number of lymph nodes retrieved on outcome in patients with muscle invasive bladder cancer. J Urol. 2002;167:1295–1298. [PubMed] [Google Scholar]
- 16.Hautmann RE, Abol-Enein H, Davidsson T, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: urinary diversion. Eur Urol. 2013;63:67–80. doi: 10.1016/j.eururo.2012.08.050. [DOI] [PubMed] [Google Scholar]
- 17.Healthcare Cost and Utilization Project (HCUP) Agency for Healthcare Research and Quality; Rockville, MD: Jun, 2011. [accessed August 5, 2011]. HCUP Databases. www.hcup-us.ahrq.gov/sidoverview.jsp. [Google Scholar]
- 18.Coffey R, Barrett M, Houchens R, et al. Methods Applying AHRQ Quality Indicators to Healthcare Cost and Utilization Project (HCUP) Data for the Eighth (2010) [Accessed 9/1/11];National Healthcare Quality Report (NHQR) and National Healthcare Disparities Report (NHDR). 2010. HCUP Methods Series Report # 2010-06. 2010 at http://www.hcup-us.ahrq.gov/reports/methods.jsp.
- 19.Elixhauser A, Steiner C, Harris DR, Coffey RM. Comorbidity measures for use with administrative data. Med Care. 1998;36:8–27. doi: 10.1097/00005650-199801000-00004. [DOI] [PubMed] [Google Scholar]
- 20.Konety BR, Allareddy V, Herr H. Complications after radical cystectomy: analysis of population-based data. Urology. 2006;68:58–64. doi: 10.1016/j.urology.2006.01.051. [DOI] [PubMed] [Google Scholar]
- 21.Feng Z, McLerran D, Grizzle J. A comparison of statistical methods for clustered data analysis with Gaussian error. Stat Med. 1996;15:1793–1806. doi: 10.1002/(SICI)1097-0258(19960830)15:16<1793::AID-SIM332>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Du XL, Lin CC, Johnson NJ, Altekruse S. Effects of individual-level socioeconomic factors on racial disparities in cancer treatment and survival: findings from the National Longitudinal Mortality Study, 1979–2003. Cancer. 2011;117:3242–3251. doi: 10.1002/cncr.25854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gore JL, Saigal CS, Hanley JM, Schonlau M, Litwin MS Urologic Diseases in America P. Variations in reconstruction after radical cystectomy. Cancer. 2006;107:729–737. doi: 10.1002/cncr.22058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee CT, Dunn RL, Williams C, Underwood W. Racial disparity in bladder cancer: trends in tumor presentation at diagnosis. J Urol. 2006;176:927–933. doi: 10.1016/j.juro.2006.04.074. discussion 933-924. [DOI] [PubMed] [Google Scholar]
- 25.Scosyrev E, Noyes K, Feng C, Messing E. Sex and racial differences in bladder cancer presentation and mortality in the US. Cancer. 2009;115:68–74. doi: 10.1002/cncr.23986. [DOI] [PubMed] [Google Scholar]
- 26.Hollenbeck BK, Dunn RL, Ye Z, Hollingsworth JM, Lee CT, Birkmeyer JD. Racial differences in treatment and outcomes among patients with early stage bladder cancer. Cancer. 2010;116:50–56. doi: 10.1002/cncr.24701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Konety BR, Allareddy V, Carroll PR. Factors affecting outcomes after radical cystectomy in African Americans. Cancer. 2007;109:542–548. doi: 10.1002/cncr.22449. [DOI] [PubMed] [Google Scholar]
- 28.Halpern MT, Ward EM, Pavluck AL, Schrag NM, Bian J, Chen AY. Association of insurance status and ethnicity with cancer stage at diagnosis for 12 cancer sites: a retrospective analysis. Lancet Oncol. 2008;9:222–231. doi: 10.1016/S1470-2045(08)70032-9. [DOI] [PubMed] [Google Scholar]
- 29.Ghaferi AA, Birkmeyer JD, Dimick JB. Variation in hospital mortality associated with inpatient surgery. N Engl J Med. 2009;361:1368–1375. doi: 10.1056/NEJMsa0903048. [DOI] [PubMed] [Google Scholar]
- 30.Finks JF, Osborne NH, Birkmeyer JD. Trends in hospital volume and operative mortality for high-risk surgery. N Engl J Med. 2011;364:2128–2137. doi: 10.1056/NEJMsa1010705. [DOI] [PMC free article] [PubMed] [Google Scholar]