Abstract
Despite decades of study, subarachnoid hemorrhage (SAH) continues to be a serious and significant health problem in the United States and worldwide. The mechanisms contributing to brain injury after SAH remain unclear. Traditionally, most in vivo research has heavily emphasized the basic mechanisms of SAH over the pathophysiological or morphological changes of delayed cerebral vasospasm after SAH. Unfortunately, the results of clinical trials based on this premise have mostly been disappointing, implicating some other pathophysiological factors, independent of vasospasm, as contributors to poor clinical outcomes. Delayed cerebral vasospasm is no longer the only culprit. In this review, we summarize recent data from both experimental and clinical studies of SAH and discuss the vast array of physiological dysfunctions following SAH that ultimately lead to cell death. Based on the progress in neurobiological understanding of SAH, the terms “early brain injury” and “delayed brain injury” are used according to the temporal progression of SAH-induced brain injury. Additionally, a new concept of the vasculo-neuronal-glia triad model for SAH study is highlighted and presents the challenges and opportunities of this model for future SAH applications.
Keywords: Subarachnoid Hemorrhage, Vasospasm, Early Brain Injury, Delayed Brain Injury, Vasculo-neuronal-glia Triad Model
1.Introduction: It is Time to Reawaken Interest in the Mechanisms of Subarachnoid Hemorrhage Pathophysiology
Subarachnoid hemorrhage (SAH) is a devastating cerebrovascular disease with complex mechanisms that threaten brain perfusion and function. The definition of a SAH has recently been updated to mean bleeding into the subarachnoid space, i.e., the area between the arachnoid membrane and the pia mater of the brain or spinal cord (Sacco et al., 2013). Despite recent improvements our knowledge of SAH pathophysiology and the management of ruptured aneurysms, which can include surgical clipping or endovascular treatment, SAH remains a serious and significant health problem in the United States and throughout the world (Sehba et al., 2012). Although it accounts for only 5% of all strokes, its burden to society is significant, given the young age at which it occurs, its high rates of mortality and disability, and poor clinical outcomes (Venti, 2012). Approximately one in six patients die during the sudden onset of bleeding. The mean age at which SAH occurs is 50 years old, and the onset of SAH in this younger population renders members of an otherwise productive age group unable to return to work. Additionally, it necessitates long-term care. Those who survive initially may succumb to early rebleeding or a delayed ischemic neurological deficit (DIND) that occurs with or without cerebral vasospasm.
Hippocrates demonstrated the presentation of spontaneous SAH followed by subsequent delayed neurological deficient nearly 2,400 years ago. Delayed cerebral vasospasm, a syndrome first reported in 1951 (Ecker and Riemenschneider, 1951) was regarded as the single most crucial and treatable cause of mortality and morbidity after SAH in subsequent decades. The Fisher Grade was applied in predicting the onset of vasospasms (Fisher et al., 1980). Arterial vasospasm after SAH can be visualized and evaluated by digital subtraction angiography or magnetic resonance angiography in clinical research and by India ink angiography, synchrotron radiation angiography or H&E staining in basic research (Bederson et al., 1998; Cai et al., 2012; Suzuki et al., 2010a).
Historically, considerable efforts have been made to investigate vasospasm as the primary mechanism underlying SAH injury that leads to tissue ischemia, and ultimately to infarctions and poor neurological outcome. However, in recent years, that theory is being questioned increasingly. First, the peak incidence of angiographic vasospasm during the second week post-SAH is approximately 70% (Dorsch and King, 1994), but the incidence of clinically delayed cerebral ischemia (DCI) is only around 30% (Dorsch, 2011). Secondly, the relationship between vessel constriction and cerebral infarction is somewhat poor (Minhas et al., 2003). It is unlikely that all cases of cerebral infarction are due to a SAH-induced vasospasm, because in some instances infarction can occur immediately after a SAH, and a vasospasm in the territorial artery is not always detected by angiography (Naidech et al., 2006). Furthermore, the presence of angiographic or Transcranial Doppler (TCD) vasospasm had only 67% positive predictive value, but 72% negative predictive value for the occurrence of cerebral infarction, respectively (Rabinstein et al., 2005). Cerebral infarction can develop even in an unaffected vascular distribution without vasoconstriction after SAH (Brown et al., 2013; Naidech et al., 2006). Cerebral infarction contributed to poor outcome by both vasospasm-dependent and -independent effects in the majority of 194 patients with moderate to severe vasospasm (Naidech et al., 2006). Therefore, poor outcome seems to be directly dependent on infarction, but independent of vasospasm (Vergouwen et al., 2011). Thirdly, a wide range of cerebral perfusion disturbances has been observed among patients who developed delayed neurological deficits after SAH (Minhas et al., 2003). Cerebral blood flow (CBF) measured via CT perfusion in areas supplied by vessels with vasospasm ranged from 26.4 to 41.5 ml/100 g/min (Dankbaar et al., 2009; Sviri et al., 2006; Wintermark et al., 2006), which is higher than the assumed threshold for ischemic injury (25 ml/100 g/min) (Murphy et al., 2006). This raises a critical question for future studies: Are the effects of vasospasm on CBF sufficient to cause cerebral ischemia and brain infarct?
The treatment of SAH has not improved despite nearly four decades experimental studies targeting vasospasms. Additionally, the calcium channel antagonist nimodipine, which is the only proven drug treatment to improve outcomes after SAH, seems to provide beneficial effects without angiographic evidence of cerebral vasodilation (Petruk et al., 1988). Finally, treating vasospasm does not always lead to improvement in functional outcomes (Macdonald et al., 2008; Polin et al., 2000). Disappointing results were observed in the two randomized, double-blind, placebo-controlled, phase III trials using endothelin A receptor antagonist, clazosentan (CONSCIOUS-2 and CONSCIOUS-3) (Macdonald et al., 2011; Macdonald et al., 2012). Clazosentan reduced vasospasm in patients after SAH, but failed to reduce mortality and to ameliorate neurological deficits. In recent years, increasing evidence has revealed that some new mechanisms, such as early brain injury (EBI), cortical spreading depolarization (CSD) and impaired microcirculatory function, may closely dictate patients’ prognosis following SAH. Hence, a new review is necessary to appraise those research advances of SAH.
SAH, as an untreatable CNS disease, promotes collaborative efforts from neurosciences, neurosurgery, neurology, neuro-ICU and other interrelated fields. Recent advances in SAH research suggest that we need to look beyond vasospasm after SAH and target other coexisting factors that might be involved in the pathogenesis of delayed cerebral ischemia, which should improve outcomes in patients after SAH (Vergouwen et al., 2011). This review aims to summarize the evolving new pathophysiological mechanisms that are implicated in brain injury after SAH and to improve our understanding of these mechanisms in order to explore potential novel therapeutic options for patients with SAH.
2. SAH Background
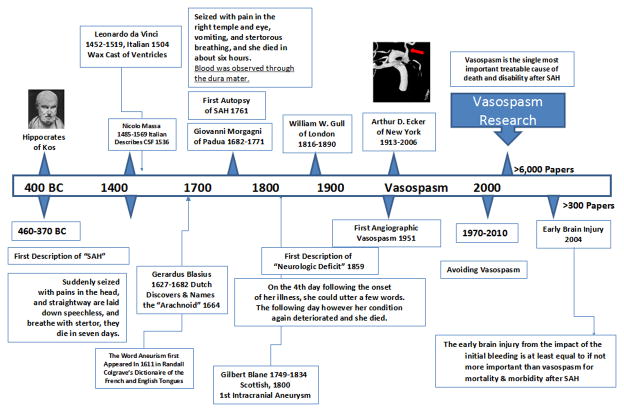
“When persons in good health are suddenly seized with pains in the head, and straightway are laid down speechless, and breathe with stertor, they die in seven days,”(Clarke, 1963). His description is similar to the classic and dramatic presentation of patients with SAH, such as a middle-aged person who collapses at restroom, reports a sudden onset of the “worst headache of my life,” subsequently projectile vomits, briefly loses consciousness, and presents with subhyaloid ocular hemorrhages and a stiff-neck. A brief history of SAH is summarized in Figure 1.
Figure 1. Schematic of a brief SAH history.
2.1 Etiology of spontaneous SAH
A SAH can be caused by trauma; however, only spontaneous, nontraumatic SAH is included under the definition of stroke (Sacco et al., 2013). Approximately 85% of cases of spontaneous SAH are due to the rupture of an intracranial aneurysm and bleeds into the subarachnoid space. Cerebral aneurysms may be present in 2–3% of the population with the annual risk of rupture being relatively low, about 0.7–4% (Rinkel et al., 1998). Spontaneous SAH also can occur in the absence of an aneurysmal rupture. Indeed, perimesencephalic non-aneurysmal conditions account for 10% of SAH (Inagawa, 2010). The normal angiographic findings in these instances are consistent with a venous origin of the bleeding, which can occur due to the rupture of a prepontine or interpeduncular vein (Hashimoto et al., 2000). Approximately 5% of SAH are associated with other medical conditions such as arteriovenous malformation, intracranial artery dissections, mycotic aneurysms, bleeding disorders, reversible cerebral vasoconstriction syndrome, vasculitis, moyamoya disease, cerebral amyloid angiopathy, or drug abuse (Cabral et al., 2013; Cuvinciuc et al., 2010; Rinkel et al., 1993; Santos Carvalho et al., 2013; Venti, 2012; Viswanathan et al., 2012). This review focuses only on current neurobiological knowledge of aneurysmal SAH.
2.2 Epidemiology: incidence, mortality and racial differences
A large multinational World Health Organization epidemiological investigation reported that the annual incidence of aneurysmal SAH ranges from 2.0 cases in China to 22.5 cases in Finland per 100,000 in age-adjusted adults (Ingall et al., 2000). In the United States, the annual prevalence of aneurysmal SAH is approximately 30,000 persons (Bederson et al., 2009; Loftspring, 2010). The incidence of SAH has been reported to be age-related with a higher incidence among individuals aged 40 to 60 years, peaking at age 55. It also appears to be gender-dependent, with women having an incidence rate that is approximately 1.6 times higher than that of men (Rinkel et al., 1998). Previous studies suggest the risk of SAH further varies based on a female’s hormonal status. Premenopausal women (Longstreth et al., 1994), women of older age at the birth of their first child, and those of older age at the onset of menarche have lower risk for SAH (Okamoto et al., 2001).
Most studies report mortality rates ranging from 25% to 35% in high-income countries and up to 48% in low-income countries (Feigin et al., 2009). The median mortality rate of SAH in the United States alone is as high as 32% (Connolly et al., 2012). However, because these data do not include pre-hospital deaths, the actual rate of mortality for SAH is likely to be much higher than that that reported (Inagawa, 1997). Indeed, approximately 12% of patients die before receiving medical attention or are misdiagnosed (Fridriksson et al., 2001; Huang and van Gelder, 2002). Nevertheless, the rates of morbidity and mortality due to SAH have slowly decreased, with nearly a 1% reduction in the mortality rate per year since 1974 (Johnston et al., 1998).
There also appear to be racial differences in the risk of SAH (Brinjikji et al., 2012). Black Americans are at higher risk than are white Americans (Broderick et al., 1992). Mortality rates for SAH also seem to vary by race. White Americans have a lower mortality rate than do African Americans, Hispanics, American Indians/Alaskan Natives, and Asian/Pacific Islanders residing in the United States (Connolly et al., 2012).
2.3 Perspectives on the Current Status of SAH Animal Models
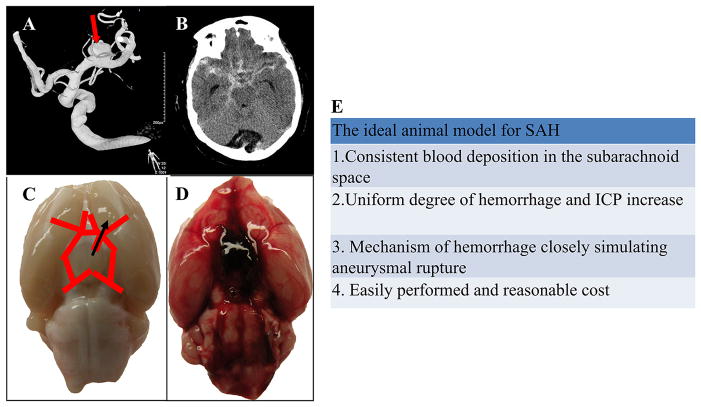
There is a clear need for rigorous translational research using animal models so that we can make further progress in the management of patients with SAH (Figure 2). Indeed, although the human cerebral artery may be considered an ideal tool for studying the pathogenesis and treatment options of SAH, using live human brain vessels in vivo is not a viable option. Moreover, postmortem evaluation of arteries taken from humans who died from SAH provide only minimal information (Crompton, 1964).
Figure 2. Comparison of subarachnoid hemorrhage (SAH) in human subjects and experimental endovascular perforation rat model.
A, a ruptured aneurysm (red arrow) in a middle cerebral artery causes SAH in the human brain. B, a brain computed tomography (CT) scan showed a high density area in the cistern. C, SAH was produced by the endovascular filament (slim black arrow) of the internal carotid artery in a rat. D, an image of rat brain post-SAH showed a thick blood clot around the circle of Willis. E, a table summarizes the conditions of the ideal SAH model.
Various animal species, including mice, rats, rabbits, dogs, primates, cats, pigs, and goats have been used to produce SAH models to closely mimic the physiological situation in humans (Marbacher et al., 2010a). Non-human primates are very similar in their genome, anatomy, and physiology to humans, and thus the models of non-human primates’ SAH are believed to be the best candidates for replicating clinical SAH. The puncture monkey model through a small anterior craniotomy was first reported in 1968 (Simeone et al., 1968). Non-human primates are usually time-consuming and demand complex surgical manipulation, such as anesthesia, angiography, craniotomy, etc, and are also not cost effective. Therefore, the puncture technique was also adapted to small animals with the advancement of microsurgical technology. For example, the rat is an excellent species, because it is relatively lower cost and that easy to manipulate in a laboratory setting. The endovascular puncture SAH model has also been produced in the mouse, though it is technically more challenging (Kamii et al., 1999). Recently, the therapeutic effect of potential drug candidates against EBI has been examined on the mouse endovascular puncture model (Altay et al., 2012a). The advantage of a mouse SAH model would be the option to use transgenic mice, which is becoming the preferred research tool for gene-specific silencing in vivo. Using transgenic mice with spontaneous aneurysmal SAH would better mimic the natural history seen in patients with SAH.
Because there are no spontaneously occurring animal models of SAH, a number of animal models of SAH have been developed in various species to simulate SAH. SAH researchers can choose from a number of SAH animal models to suit their research objectives. The SAH models that mainly utilize two techniques: 1) endovascular punctures, in which puncture of an intracranial artery allows blood to quickly spread in the subarachnoid space, and 2) blood injection into the subarachnoid space after blood is obtained by the surgical exposure of a distant artery or vein.
Each animal model has its own advantages and disadvantages. It is generally accepted that the endovascular perforation model of SAH better mimics EBI, whereas the blood injection model better mimics the vasospasm that can occur after SAH (Lee et al., 2009b). In recent years, as the concept of EBI gains more popularity over delayed vasospasm, the blood injection model has been less favored as compared to the monofilament puncture model, since researchers are beginning to explore changes not only in the large cerebral arteries but also in the brain parenchyma (Bederson et al., 1995; Titova et al., 2009).
The endovascular puncture model was independently described in 1995 by Bederson et al. and Veelken et al. (Bederson et al., 1995; Veelken et al., 1995). The surgical procedure involves perforation of the internal carotid bifurcation by a sharpened suture, which is inserted through the external carotid artery without craniotomy. Because this method does not entail craniotomy, it best represents a clinical scenario, as it mimics the acute pathophysiological changes of an aneurysmal rupture in humans (Schwartz et al., 2000a; Schwartz et al., 2000b). It results in with considerably high rate of mortality, which ranges from 30% to 50% within 24 hr after SAH (Prunell et al., 2004). However, the brief period of ischemia caused by temporal clip, the lack of control over the volume of the hemorrhage, and the high rate of morbidity and mortality significantly impacts its use for studies exploring possible therapeutic options. Furthermore, the CBF on the side with the perforation tends to be lower than that of the non-perforated side, thus suggesting that this model results in some degree of laterality to the hemorrhagic event.
The blood injection model, in which blood is directly injected into the subarachnoid space either once or twice, is another widely used technique for inducing experimental SAH. This technique elicits early and delayed vasospasm in a variety of species, although its presence depends on the site of injection. More recently, Marbacher et al. reported vasospasm in a number of SAH models: The most frequently injected volume of blood amount (ml), the peak onset of the vasospasm (day), and amount by which the blood vessel narrowed (%): mice endovascular puncture (a range, day 3, 20–62%); rat single injection (0.3 ml, day 2, 19–29%); rat double injection (0.3 ml, two times, day 7, 28–47%); rabbit single injection (3 ml, day 3, 19–55%); rabbit double injection (not established, day 5, not established); dog double injection (4–5 ml, two times, day 7, 45–66%); primate clot placement (5 ml, day 7, 32–52%) (Marbacher et al., 2010a). Recently, a novel technique of inducing SAH by extra-intracranial blood shunt has been established to trigger delayed cerebral vasospasm under a controlled intracranial pressure (ICP) (Marbacher et al., 2010b). In the future, experimental models of cerebral vasospasm should be improved so as to better mimic human SAH, in terms of having a direct hemorrhagic brain lesion under systolic pressure (Pluta et al., 2009).
Given the availability of various types of SAH models, it is imperative that the model chosen is appropriate for the objective of the study and that the model is replicable. In addition, genome-wide association studies are helpful to identify novel genetic factors that contribute to intracranial aneurysm susceptibility. Consequently, it is possible to produce an aneurysm on cerebral vessels experimentally using genetic technology (Suda et al., 2013). Furthermore, ensuring reproducibility of results, the efficacy of a treatment option should be confirmed in multiple species and in multiple laboratories before beginning translation to a clinical study (Tajiri et al., 2013).
2.4 Failure in Current SAH Studies
Despite the promising results seen in animal models, clinical studies have failed to translate the outcomes seen in animal models to human subjects (Feuerstein et al., 2008; Savitz, 2007). The failure to translate animal studies could be due to methodological flaws during animal experiments including the following: First, studies do not always indicate if the animals used in the experiments were randomized or how the randomization was performed. Yet, studies may not be translatable the animals used in the experiments were not randomly allocated. Furthermore, control groups are often inadequate, and some studies do not even include a control group. In addition, evaluations performed during experiments are not always blinded, which is essential for a non-biased meaningful study. Second, most studies utilize young, non-diseased male animals, which, therefore, does not simulate the age, physical condition, or gender-specific risks seen in patients with SAH, who are more likely to be (45–55 years old, have hypertension, and be female) (Kassell et al., 1996; Kongable et al., 1996; Lanzino and Kassell, 1999). Furthermore, young rats are more resistant to the effect of the products of bilirubin oxidation compared to aged rats post-SAH (Clark et al., 2011). Third, sample size is not always adequate for statistical analysis, which can lead to incorrect conclusions about the results of an experiment. Accurate power and statistical analyses are crucial for drawing valid conclusions from a study.
Further, in depth evaluation of outcomes in preclinical models is crucial to measure an agent’s efficacy for the successful clinical translation of novel therapies (Knight et al., 2013). Given the high mortality rates in patients with SAH, mortality should be examined in experimental studies. Neurological function also should be thoroughly evaluated, because this information is crucial for translation of animal studies. Clinical evaluations of patients after SAH commonly reveal cognitive deficits, and this information is critical to providing for proper care (Wong et al., 2013). However, cognitive function, fatigue, and emotional disturbances are seldom evaluated in preclinical experiments (Boyko et al., 2013; Tso and Loch Macdonald, 2013). Additionally, for many patients with SAH and their relatives, the activities of daily living is as important as, or even more important than, the life prolonged. Therefore, efficacy of therapies should be evaluated using tests to examine not only sensory motor function but also cognitive function, speech, and memory. Experimental studies need to incorporate an assessment of neurobehavioral outcomes in the acute phase as well as a more detailed evaluation of long-term neurological function.
It also is important to examine potential side effects of compounds being tested in animal studies in order to eliminate drugs that may be too toxic for clinical use. Indeed, recent failures in the CONSCIOUS trials may have occurred due to adverse effects, such as hypotension and pulmonary complications, that are common after taking endothelin receptor antagonists (Macdonald, 2012). For example, complications such as hypotension, pulmonary complications, and anemia were encountered more often in patients who were treated with clazosentan than in those who received placebos (Macdonald et al., 2012). Similarly, use of several of the synthetic N-methyl D-aspartate (NMDA) antagonists have been abandoned because of concerns regarding drug toxicity, particularly in strokes (Muir, 2006). Using two or three gradual dosages is helpful to show dose dependent and potential toxic effects in experimental studies. The successful clinical translation of future studies requires the careful design of experiments, adequate controls, and rigorous testing in experimental SAH models. It may be prudent to develop a set of guidelines for SAH translational studies (Lapchak et al., 2013).
2.5 Major Complications after SAH
2.5.1 Acute and Chronic Hydrocephalus
SAH often can lead to hydrocephalus, an outcome with a frequency that varies from 9% to 67% in some patients (Milhorat et al., 1970; Woernle et al., 2013; Yasargil et al., 1973). Traditionally, it has been believed that patients with SAH suffer from hydrocephalus that is due to two major problems: 1) blockage of cerebrospinal fluid (CSF) pathways in the ventricular system, and 2) compromised reuptake of CSF within the subarachnoid granulations. The obstruction of CSF pathways in the acute setting, largely due to the mass effect of blood clots, has been established as the cause of acute hydrocephalus. Several studies have shown correlations between the amount of cisternal and ventricular blood and the likelihood of developing acute hydrocephalus after SAH (Graff-Radford et al., 1989). Recently, CSF overproduction by stimulation of the irritant receptor glossopharyngeal and vagal nerve endings has been suggested to the etiology of early hydrocephalus after SAH (Kanat et al., 2012), which, although plausible, has yet to be confirmed with substantial scientific evidence (Shah and Komotar, 2013). In contrast, the latter usually occurs in the chronic setting secondary to fibrosis of the arachnoid villi as a result of inflammatory reaction or blood clotting products, which prevents the reabsorption of CSF (Massicotte and Del Bigio, 1999; Suzuki et al., 2008).
Previous analyses also indicate multifactorial causes in the development of chronic hydrocephalus after SAH. These include poor neurological condition upon the admission of a patient, the presence of intraventricular hemorrhage, ruptured vertebral artery aneurysm, surgical clipping and endovascular coiling, meningitis, hyperglycemia, gender, increased sympathetic activity, and a prolonged period of external ventricular drainage (de Oliveira et al., 2007; Dorai et al., 2003; Graff-Radford et al., 1989; Lai and Morgan, 2013; Lambert et al., 2002; Yang et al., 2013). In experimental SAH, rats seem to develop hydrocephalus as measured by magnetic resonance imaging if the SAH grade is high or if there is intracerebroventricular bleeding, and if the cerebroventricular wall is damaged (Okubo et al., 2013). The hepatocyte growth factor and vascular endothelial growth factor may participate in the periventricular white matter injury in rats with chronic hydrocephalus after SAH (Chu et al., 2011). The cascade of transforming growth factor β1-Smad3 induced by thrombin might be a mechanism of communicating hydrocephalus after SAH in rats (Li et al., 2013). To clarify and strengthen these observations, future studies should differentiate in detail suitable SAH models that target this complication. More studies of the neurobiological mechanisms of hydrocephalus, including prospective, double-blinded, randomized trials are needed.
2.5.2 Seizures
Seizures are a well-recognized complication of SAH, although the administration of prophylactic antiepileptic medication following an aneurysmal SAH is still controversial (Ibrahim et al., 2013). Seizures occur in 5–35% of survivors of SAH, most commonly onset time in the first 24 hr (Baker et al., 1995; Connolly et al., 2012; Ohman, 1990). They are associated with higher Hunt-Hess grade and Fisher scores, lower admission Glasgow Coma Scale scores, hypertension, infarction, and rebleeding (Cabral et al., 2009; Hasan et al., 1993). However, reports about the association between seizures and functional outcomes are heterogeneous (Butzkueven et al., 2000; Choi et al., 2009; Rhoney et al., 2000). Therefore, further neurobiological investigation is warranted to understand the mechanism of epilepsy and seizure disorders after SAH. To clarify and strengthen these observations, a suitable model of SAH should be developed to target this complication. Then, the efficacy and timing of prophylactic antiepileptic medication should be tested carefully in that model before a multiple-center, double-blinded, randomized prospective evaluation.
3. 3. Neurobiological Response after SAH
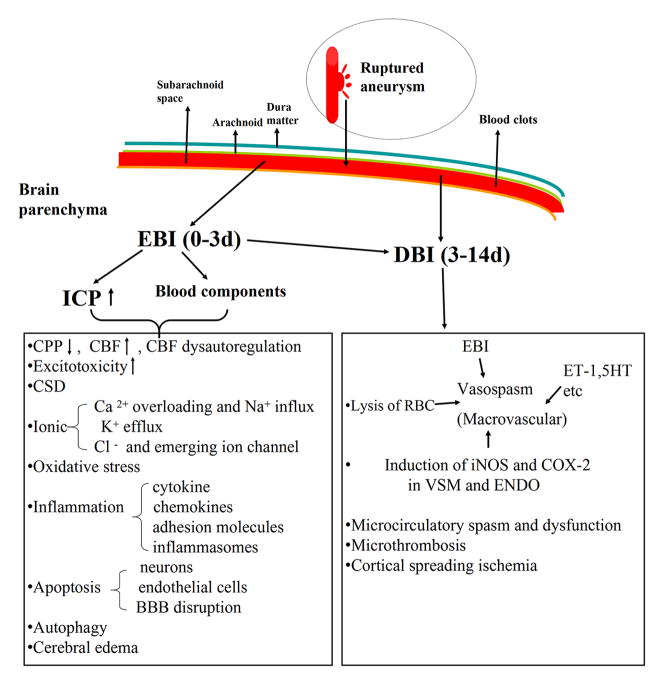
In cases when SAH is not immediately fatal, secondary complications can occur after the initial bleeding (Figure 3). Recently, we have begun to see that SAH clearly has a complex, multisystem, and multifaceted pathogenesis that involves several ongoing processes other than vasospasm of the major cerebral vessels (Cahill et al., 2006; Hansen-Schwartz et al., 2007; Macdonald et al., 2007). In this review, the term “EBI” and “delayed brain injury” (DBI) are used according to the temporal progression of SAH-induced brain injury. In fact, the term “EBI” was first coined in 2004 to explain the acute pathophysiological event that occurs within the first 72 hr of the SAH (Kusaka et al., 2004). With advances in understanding the pathophysiology of SAH, in the present review the term “DBI” is used to demonstrate a host of critical, interrelated pathological pathways that arise in the late phase of SAH as a consequence of EBI. Many of the pathogenic triggers of DBI are interrelated, and furthermore, the mechanisms leading to delayed vasospasm and DBI are not mutually exclusive. We believe that delayed vasospasm is a clinical manifestation and not a separate entity of the many mechanisms of DBI after SAH.
Figure 3. Mechanisms of early brain injury (EBI) and delayed brain injury (DBI) after SAH.
The neurobiological responses contributing to all outcomes are listed. RBC, red blood cell; CPP, cerebral perfusion pressure; CBF, cerebral blood flow; CSD, cortical spreading depolarization; BBB, blood-brain barrier; ET-1, endothelin-1; 5-HT, 5-hydroxytryptamine; COX-2, cyclooxygenase-2; VSM, vascular smooth muscle; ENDO, endothelium.
3.1 Early Brain Injury
More than 300 articles in a PubMed/MEDLINE search provide an extensive coverage of advances in the research on EBI over the last ten years; however, most of that information comes from animal studies. The term “EBI” refers to global brain injury that starts immediately after aneurysms rupture (Sabri et al., 2013b). A number of animal studies and some data from human autopsies provide evidence that EBI begins within minutes after the initial bleeding (Bederson et al., 1998; Nau et al., 2002). This correlates well with the clinical scenario in which clinical deterioration is commonly observed within the first 2 hr (Miyazaki et al., 2006; Ohkuma et al., 2001), and SAH fatalities occur within 48 hr of the ictus (Ingall et al., 2000).
The major causes of death within 72 hr after the initial bleed are the effects of the initial hemorrhage and aneurysms’ rebleeding (Broderick et al., 1994). Indeed, survivors with SAH can succumb to injury at a later period or present with severe deficits, due to secondary insult. Brain injury following SAH is not only limited to the distribution of the ruptured vessel, but also extends to brain regions distant to the site of hemorrhage. In recent years, increasing study efforts have been directed at elucidating the mechanisms of EBI after SAH, which challenges the already tenuous link between vasospasm and DIND. The cascade of events that occur with EBI is responsible for the initial signs and symptoms of patients with SAH, which is believed to be a precursor for both delayed vasospasm and DIND. In addition, a range of physiological derangements that occurs early on can trigger a number of devastating cascades that lead to blood brain barrier (BBB) dysfunction, inflammation, necrosis, apoptosis and oxidative stress (Ayer and Zhang, 2010). The physiological changes that occur with EBI and the mechanisms responsible for EBI are discussed below.
3.1.1 Initial Physiological Changes: A Culprit of EBI
3.1.1.1 Intracranial Pressure (ICP)
Once an aneurysm ruptures, blood extravasates from a ruptured defect and spreads into the subarachnoid space. The size of the ruptured defect in vessel correlates with the amount of the blood clot. At the time of the SAH occurrence, ICP sharply elevates, and the rate of increase is indicative of the severity of the initial bleed. The rapid increase in ICP accounts for the “thunderclap” headache, which is the classical presentation seen in patients at the clinics. ICP is normally less than 13mmHg. There are two types of ICP increase. First, ICP rapidly increases to a value approximating the arterial pressure (~120 mmHg), then decreases to a level slightly above baseline (Bederson et al., 1995). Within 1–2 min after SAH induction, ICP rapidly rises to peak values in a new blood shunt model, and it decreases to a plateau that is higher than the baseline values within 5–10 min (Marbacher et al., 2012). This initial increase in the ICP is thought to be a protective mechanism, which arrests the initial aneurysmal bleed and preventing rebleeding, the so-called “brain tamponade” (Nornes, 1973). This pattern is usually accompanied by a small volume hematoma.
The other pattern of ICP increase is characterized by a sustained increase in ICP, which may be due to an enlarging hematoma volume, vasoparalysis, distal cerebral arteriolar vasodilation or the development of acute hydrocephalus (Asano and Sano, 1977; Brinker et al., 1990; Grote and Hassler, 1988; Nornes and Magnaes, 1972). This pattern is less common. The peak ICP elevation is a response to both the amount of blood released into the subarachnoid space and the volume of hemorrhage (Bederson et al., 1998; Schwartz et al., 2000a). High ICP predicts poor outcome after SAH (Czosnyka et al., 2005; Soehle et al., 2007), as it disrupts cerebral perfusion pressure (CPP), the pressure driving cerebral blood flow, and may lead to a critical loss of cerebrovascular autoregulation (Czosnyka et al., 2005). The time course assessment of ICP suggests that it may contribute to a high rate of early clinical deterioration in patients (Ohkuma et al., 2001). The results of the study conducted by Westermaier et al. describe that a sharp increase of ICP does not correlate with the development of edema (Westermaier et al., 2012). Unfortunately, early ICP monitoring of patients is generally not feasible, so that the accurate impact of early ICP remains unclear.
3.1.1.2 Cerebral Perfusion Pressure (CPP)
CPP is the net pressure gradient of blood flow to the brain. Normal CPP, which is equal to mean arterial blood pressure minus ICP, approximates 80 mm Hg (Huang et al., 2006). The relationship between ICP and CPP is imprecisely understood, although it is predicted to be related to the “Monroe-Kelly” doctrine (Ayer and Zhang, 2010; Schoffer et al., 2002). A profound fall in CPP to almost zero has been observed immediately after SAH in animals and humans (Marbacher et al., 2012; Nornes, 1973; Voldby and Enevoldsen, 1982). After a few minutes, CPP gradually recovers with the decline of ICP. This pattern looks like global cerebral ischemia with gradual reperfusion. Interestingly, some experimental studies suggest that decrease in CPP was negatively associated with ICP (Kuyama et al., 1984). While CPP recovers, the reduction of CBF persists, which indicates acute vasoconstriction within 6 hr (Westermaier et al., 2009). However, reduction in CPP does not always correlate with a poor clinical outcome (Heuer et al., 2004).
3.1.1.3 Cerebral Blood Flow (CBF)
The rise in ICP and subsequent fall in the CPP results in a significant decrease in the CBF, which can drop to zero after the initial impact of SAH in experimental studies (Bederson et al., 1995). Once there is a transitory fall of CBF, the consequences are significant, both short and long term. In some instances, severe global hypoperfusion can exhibit as syncope or unconsciousness in patients with SAH (Jakobsen, 1992). Also, mortality rate increases with severe reduction in the CBF. In an experimental study, Bederson et al. observed that CBF reduction to less than 40% of baseline in the first hour after SAH predicted 100% mortality and was defined as “lethal” SAH, while a lesser degree of CBF reduction resulted in 19% mortality (Bederson et al., 1998). Furthermore, the study showed that the mortality rate was independent of ICP and CPP in the first hour after SAH. It appears that marked acute cerebral vasoconstriction can occur at this critical time independent of changes in CPP and ICP, which likely contribute to the cerebral ischemia (Bederson et al., 1998; Friedrich et al., 2012a).
Cerebral perfusion is essential for life, since the brain has high metabolic demands and is very sensitive to hypoxia-ischemia. Early CBF reduction was accompanied by a lessened cerebral metabolic rate of oxygen investigated by positron emission tomography analysis (Frykholm et al., 2004). Positron emission tomography has the potential to increase our knowledge of the role of cellular hypoxia in SAH, which described that hypoxia (increased (18) F-FMISO uptake) was present in symptomatic patients with SAH (Sarrafzadeh et al., 2010).
3.1.2 Disturbance in Cerebral Autoregulation
Cerebral autoregulation is a process in mammals that plays an important role in maintaining adequate and stable cerebral blood flow (Paulson et al., 1990). Cerebral autoregulation is able to deliver sufficient blood containing oxygen and nutrients to the brain tissue for metabolic needs, and removes CO2 and other waste products (Muller et al., 2002). Under normal circumstances, CBF is regulated through adjustment in the arteriolar size, which, in turn, drives the changes in vascular resistance according to Hagen–Poiseuille’s Law (Kontos et al., 1978). Three different mechanisms contribute to the process of cerebral autoregulation, including metabolic, myogenic and neurogenic (Aries et al., 2010; Paulson et al., 1990).
Haemodynamic communication between neurons and the vasculature is necessary to efficiently regulate CBF by neuronal activation (Attwell et al., 2010). In humans, CBF autoregulation typically operates between mean arterial pressures of the order of 60 and 150 mmHg. The initial aneurysmal rupture seems to lead to acute impairment of cerebral autoregulation (Lang et al., 2001; Ratsep and Asser, 2001). CBF, therefore, is dependent on changes in CPP, blood viscosity, and systemic arterial pressure. Additionally, a degree of cerebral dysautoregulation is also observed to occur throughout the subacute stage following SAH as well during the delayed stage at the time of delayed vasospasm (Budohoski et al., 2013).
Currently, TCD remains the most commonly used noninvasive tool to assess early alterations in vascular diameter. Clinical studies have described primary autoregulation failure measured by TCD as a direct result of SAH and, therefore, it is proportional to severity of the ictus (Budohoski et al., 2012). In addition, experimental analysis has revealed numerous activated molecular pathways that could lead to the observed dysfunction of cerebral autoregulation in the acute phase after SAH (Cho et al., 2003; Shin et al., 2002). In consideration of the existing reports on cerebral autoregulation following SAH, it is imperative to determine the exact time course of autoregulatory impairment following SAH and to determine the most appropriate methodology to detect the impairment, which might be beneficial in patients especially who present with mild neurological deterioration.
3.1.3 Excitotoxicity, Spreading Depolization and Ionic Imbalance: A Path to Neuroprotection
After the onset of SAH, there is a derangement in neurotransmitter release and inhibition of the reuptake (Jung et al., 2012; Kahn et al., 2012) occurrence of CSD (Hartings et al., 2013; Winkler et al., 2012) and loss of energy stores, which causes ionic imbalance (Aihara et al., 2004; Hubschmann and Nathanson, 1985). Excitotoxicity, CSD, and ionic imbalance are inextricably linked and contribute to SAH-induced cell death (Ohta et al., 2001; Petzold et al., 2005b; Sakowitz et al., 2013), and have been implicated in poor outcomes in comatose patients after SAH.
3.1.3.1 Excitotoxicity
Excitotoxicity is defined as toxicity resulting from the excessive activation of ionotropic and metabotropic glutamate receptors (Puyal et al., 2013). Excessive glutamate causes neurotoxicity effects. Mounting evidence describes that glutamate, a major excitatory transmitter, is elevated in the CSF after SAH, suggesting that it may play a significant role in the pathophysiology of the ictus (Jung et al., 2013a). Glutamate is not synthesized in neurons nor acquired from circulation (Hertz et al., 2007), but rather synthesized and released by activated astrocytes and microglia through the hemichannels of gap junctions (Takeuchi et al., 2006). Maintaining a minimal level of glutamate is critical for normal neuronal function after brain injury (Zlotnik et al., 2012).
Glutamate concentration was significantly high in double-hemorrhage SAH rats from day 1 to day 7 after SAH, especially at day 1, which has an important role in neuronal death (Wu et al., 2011). In the double-injection SAH rat model, an excessive and prolonged increase level of glutamate in the CSF and a reduced level of glutamate transporters GTs, including glutamate/aspartate transporter, glutamate transporter-1, and excitatory amino acid carrier 1 were observed on day 7, which was accompanied by wall thickness of the basilar artery and neuronal degeneration in hippocampus (Wu et al., 2011). Moreover, the toxicity effect mediated by glutamate includes excessive activation of the NMDA receptor leading to massive Ca2+ influx and subsequent apoptotic cell death and necrosis (Owens et al., 1997). Ionotropic glutamate receptors promoted an excessive influx of sodium with concomitant cell swelling and edema. It has been reported that the NMDA receptor antagonist, felbamate, improved neurological performance and limited the BBB disruption in a single-injection rat model of SAH (Germano et al., 2007).
Currently, various strategies have been explored to target glutamate that include inhibiting its synthesis, blocking its release from the presynaptic terminals, antagonizing its receptors on the postsynaptic terminal, and accelerating its reuptake from the synaptic cleft. Blood glutamate scavengers, oxaloacetate and pyruvate, have been shown to effectively reduce blood glutamate concentrations, ameliorate BBB disruption, and improve neurological outcome after SAH in rats (Boyko et al., 2012). Therefore, preventing glutamate-induced neurotoxicity can potentially improve neurological outcomes after SAH. However, it should be noted that clinical trials using NMDA receptor antagonists after ischemic stroke have not lived up to the expectations from the experimental data in animals, because the blockade of NMDA receptor-mediated synaptic transmission hinders neuronal survival (Ikonomidou and Turski, 2002).
3.1.3.2 Cortical Spreading Depolarization (CSD)
The term “cortical spreading depolarization” has been characterized by Aristides Leão as a wave in the cortex of the CNS (Leao, 1947). It is a self-propagating wave of neuronal and glial depolarization, which travels at a characteristic 2 to 5 mm/min and can be induced by a variety of noxious stimuli (Somjen, 2001). However, it does not spontaneously originate in normal brain tissue. CSD near-completely results in a breakdown of ion gradients and a sustained depolarization in individual metabolic impact observed as a result of cytotoxic edema after SAH (Canals et al., 2005; Dijkhuizen et al., 1999; Dreier et al., 1998; Takano et al., 2007). The magnitude of CSD-induced the disturbances of electrical, ionic, and metabolic is even larger than those of epileptic seizure activity (Dreier, 2011). CSD results in massive ion translocation between the intra- and extracellular space, redistribution of neurotransmitters, neurons swelling, distortion of dendritic spines, slowing of electrical potential, and silencing of electrical activity (Dreier, 2011; Somjen, 2001). Furthermore, CSD is associated with severe vasoconstriction (Dreier et al., 1998). Under conditions similar to those of SAH, in the presence of extravascular hemoglobin and elevated extra-cellular K+ or low glucose, CSD causes a vascular response ranging from hyperemia to hypoperfusion. The combination of decreased CBF and increased energy requirements imposed by CSD may further worsen neuronal injury (Strong et al., 2007). Thus, CSD represents an electrical circle between intra- and extra-cellular space, and represents a biochemical and morphological alteration after SAH.
Unequivocal electrophysiological evidence indicates that CSD frequently occurs in a clinical setting after SAH (Sanchez-Porras et al., 2013): During the past few years, several studies using subdural electrode strips have confirmed the presence of CSD in the cortex of patients with SAH (Dreier et al., 2006; Schlenk et al., 2008). Neocortical application of a solution containing hemoglobin with high K+ or a low level of glucose triggered CSD (Dreier et al., 2000). Thus, CSD can be ignited by intense neural activity resulting in increases of extracellular K+ and excitatory neurotransmitters.
In vivo, the noncompetitive NMDA receptor antagonist MK-801 inhibited CSD propagation under physiological conditions, but an elevated baseline [K+]o reduced the efficacy of MK-801 on depolarization (Petzold et al., 2005b). Furthermore, antagonists of NMDA channels or voltage-gated Na+ channels or certain types of Ca2+ channels can postpone or mitigate CSD (Addae et al., 2011). The recent demonstration that SAH is associated with waves of CSD has revealed yet another potential mechanism for DCI after SAH (Sanchez-Porras et al., 2013).
In view of experimental and their clinical evidence, Dreier et al. proposed that clustered CSD with prolonged periods of depression is an early indicator of the start of delayed neurological deterioration after SAH (Dreier et al., 1998; Dreier et al., 2006). However, at the present an important issue is whether CSD is a possible new culprit for DCI, which would require a more comprehensive understanding of CSD after SAH. If CSD is an early manifestation of reversible neuronal dysfunction after SAH, then therapies that suppress CSD may lead to decreased DCI and eventually improved clinical outcomes. However, if CSD is manifested only as a terminal metabolic failure, then treatments targeting CSD may not revive brain tissue already fated to die.
3.1.3.3 Ion and Ion Channels Change
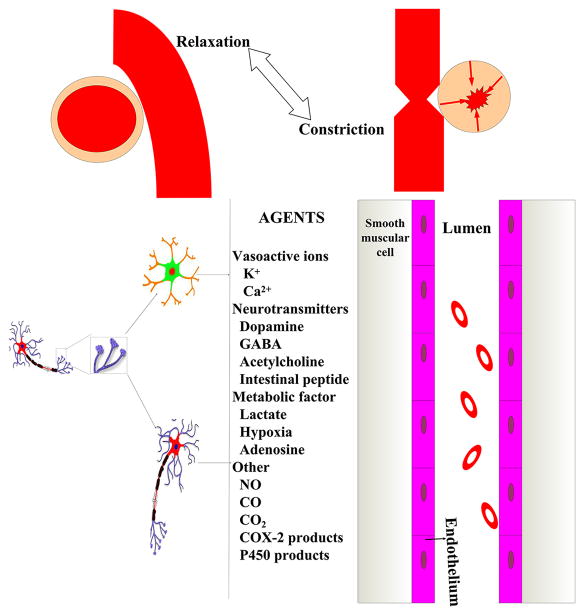
Approximately 20% of the body’s resting metabolism is consumed by the brain to establish ionic gradients (Mies and Paschen, 1984). Several different ion channels are expressed in cerebrovascular myocytes, including those for potassium, sodium, calcium, and often ignored anions, specifically, chloride. The myocytes control cerebral autoregulation. More specifically, they regulate resting membrane potential, vascular diameter, and vascular tone. Ionic distribution in the intra- and extra-cellular space and ion channel expression in the brain is rapidly and severely impaired after SAH, which thereby promotes a disturbance in electrical activity (Kamp et al., 2012).
3.1.3.3.1 Potassium (K+) and Its Channels
A high level of serum K+ has been detected after SAH and this varies by gender (Fukui et al., 2004). K+ can be released into the CSF from the blood clot in the subarachnoid space due to a decrease in sodium pump activity. Microthrombosis, endogenous digitalis-like compounds, as well as activation of neuronal K+ channels play a key role in the rise of basal K+ after SAH (Dreier et al., 2000). Subarachnoid hemoglobin combined with a high concentration of K+ causes widespread constriction of cerebral arteries and a decrease in CBF, eventually leading to cell necrosis in the cortex. Several authors have emphasized the role of loss of functional voltage-gated K+ channel (Kv) in arterial constriction in dog double-injection SAH models (Jahromi et al., 2008; Weyer et al., 2006). For instance, Kv1.5, Kv2.1 and Kv2.2 transcripts and protein levels were reduced in basilar arteries of dogs in response to SAH, suggesting that Kv dysfunction contributes to the pathogenesis of delayed cerebral vasospasm after SAH (Aihara et al., 2004; Jahromi et al., 2008). Furthermore, Ishiguro et al. demonstrated downregulation of Kv1.5 protein in rabbit cerebral arteries after oxyhemoglobin exposure (Ishiguro et al., 2006). The study also showed that oxyhemoglobin-induced suppression of Kv1.5 channels was mediated by increased tyrosine phosphorylation-dependent trafficking of the channels from the cell surface (Ishiguro et al., 2006). Therapeutic manipulation of the K+ channels has been attempted to reduce vasospasm after SAH. The KATP channel activator, levcromakalim, or endogenous activator, calcitonin gene-related peptide, displayed vasorelaxation after SAH in dogs, rabbits and monkeys (Ahmad et al., 1996; Sugai et al., 1999; Zuccarello et al., 1996). However, it failed to significantly attenuate vasospasm to a greater degree than that provided by standard care in a randomized multicenter single-blind clinical trial.
3.1.3.3.2 Sodium (Na+) and Related Syndromes
Hyponatraemia, which can occur in 10–30% of patients with SAH, is strongly linked to adverse outcomes and should be avoided in all victims of SAH (Naval et al., 2006). It is an early biochemical change seen in patients with SAH that is difficult to remedy (Berendes et al., 1997). Patients with hyponatraemia are at higher risk of developing cerebral ischemia and infarction, and mortality (Hasan et al., 1990; Wijdicks et al., 1985). The cerebral salt-wasting syndrome (CSWS) and inappropriate secretion of anti-diuretic hormones (SIADH) are suggested as the mechanisms underlying SAH-induced hyponatremia (Bruder et al., 2009), though still debated (Benvenga, 2006). CSWS is probably related to the outcome of the patients with severe SAH, and can occur due to high sympathetic tone, hyperreninemic hypoaldosteronism syndrome, and enhanced natriuretic peptides release (Audibert et al., 2009). Atrial natriuretic peptide (ANP) has been suggested as a causal natriuretic factor in CSWS. By the time a patient is admitted, both plasma ANP and brain natriuretic peptide (BNP) have been found to be increased, whereas levels in CSF were unaffected (Espiner et al., 2002).
Kleindienst et al. concluded that ICP generates the delayed CSWS, and ADH release does not mediate natriuresis in a rat model of SAH (Kleindienst et al., 2012). SIADH, the most common etiology of hyponatremia (Sherlock et al., 2006), is a condition in which the body produces excessive antidiuretic hormones and clinically manifests with hypertonic urine, hypo-osmolar serum, and apparent euvolemia without renal, adrenal, or thyroid disorders (Kao et al., 2009). In a retrospective case-note study consisting of 316 patients with SAH, Sherlock et al. found that 56.6% of patients developed hyponatremia, which was related to SIADH (Sherlock et al., 2006).
For clinicians it is important to distinguish between the two conditions that may account for hyponatremia after SAH, because the treatment strategy is opposite for the two conditions: They should restrict fluids and sodium in cases of CSWS but order increase sodium intake for SIADH. Many biochemical parameters are similar between CSWS and SIADH, and a compensatory hypersecretion of ADH to correct fluid depletion can occur with CSWS. The status of blood volume is helpful to differentiate between these two situations; patients with CSWS are hypovolemic, but SIADH patients are either normovolemic or hypervolemic (Audibert et al., 2009).
3.1.3.3.3 Calcium (Ca2+) Channels and Magnesium (Mg2+)
Ca2+ is a cofactor in multiple intracellular processes. Disruption of intracellular Ca2+ regulation and Ca2+ overloading have been hypothesized to play an important role in the process of cell injury, which results in vasoconstriction and sometimes cell death following exposure to oxyhemoglobin. In cultured cerebrovascular smooth muscle cells from primates, a significant increase in free intracellular Ca2+ was observed as early as 2 min after application of oxyhemoglobin and remained continuously elevated for 7 d (Takanashi et al., 1992). In vivo studies suggest that intracellular Ca2+ starts to increase at a very early stage – as soon as 15 min after SAH (Ishiguro et al., 2008; Kohno et al., 1991; Meguro et al., 2001). Endothelin and oxyhemoglobin, but not bilirubin, induced acute dose-dependent increases in intracellular Ca2+ concentration in cultured vascular smooth muscle cells (Takenaka et al., 1991a; Takenaka et al., 1991b). It has been shown that hemolysate released Ca2+ from endoplasmic reticulum and promoted Ca2+ influx from voltage-independent Ca2+ channels in endothelial cells of cerebral arteries (Zhang et al., 1996). Moreover, non-L-type Ca2+ channels play an important role in the oxyhemoglobin-induced rise in intracellular Ca2+ to produce acute vasoconstriction after exposure of the vessel to blood (Takenaka et al., 1991a). Long-term oxyhemoglobin exposure enhanced the expression of voltage-gated calcium channels (VGCC), pointing toward important roles for VGCC in delayed vasoconstriction after SAH (Ishiguro et al., 2008).
In addition to R-type VGCCs, the components of low-voltage-activated (T-type) channels Cav3.1 and Cav3.3 have been shown to be significantly increased in the dog basilar artery after SAH (Nikitina et al., 2010); however the functional significance of this kind of channel in SAH is a matter of debate (Cook et al., 2012). Intracellular Ca2+ can activate endonucleases responsible for the cleavage of double stranded DNA (Moss et al., 1997). Furthermore, increased intracellular Ca2+ can trigger apoptosis of endothelial and smooth muscle cells, which is one of the factors attributed to BBB disruption and vasospasm (Macdonald et al., 1995; Zhang et al., 2013b; Zhang et al., 2001). In addition, a defect in the ionic mechanisms regulating Ca2+ permeability in the membrane of smooth muscle after SAH impairs smooth muscle relaxation (Aihara et al., 2004).
Treatments that target Ca2+ dysregulation have been explored in clinical and preclinical studies. In a model of SAH, glibenclamide, a selective antagonist of the Sur1-NCCa-ATP channel, attenuated several pathologic effects associated with the inflammatory response in response to extravasated blood (Simard et al., 2009; Simard et al., 2012b). Nimodipine, an L-type Ca2+ channel antagonist, is currently the only pharmacologic agent that has been shown to consistently improve neurological outcomes. However, it has not been found to be effective in clinical trials of cerebral vasospasm in patients with SAH (Dorhout Mees et al., 2007).
Mg2+ is a physiological antagonist of Ca2+, which plays a crucial role in maintaining the intracellular concentration of Ca2+. Moreover, it is neuroprotective and has a well documented clinical profile (McLean, 1994). The total level of Mg2+ in serum normally remained unchanged, but the level of biologically active free ionized Mg2+ decreased after traumatic brain injury (Memon et al., 1995). The various mechanisms of action of Mg2+ include reduction of excitatory amino acid release, blockage of the NMDA-glutamate receptor and voltage-dependent calcium channels, inhibition of platelet aggregation, inhibition of endothelin-1 synthesis, vasodilation through the release of endothelial nitric oxide (NO), and increased synthesis of prostacyclins (Berthon et al., 2003; Nadler et al., 1987; van den Bergh et al., 2004; Yang et al., 2000). Both animal studies and pilot clinical trials using magnesium sulfate have reported trends toward improved outcomes (Mori et al., 2011, 2012). In the Field Administration of Stroke Therapy–Magnesium (FAST-MAG) pilot trial, prehospital administration of magnesium is feasible and safe in acute ischemia stroke patients (Saver et al., 2004). Therefore, the results from FAST-MAG phase III are worthy of exception. However, intravenous magnesium sulfate administered after SAH did not improve the clinical outcome for patients in a phase 3 randomized, double-blind, placebo controlled, multicenter study (MASH-2 and IMASH) (Dorhout Mees et al., 2012; Wong et al., 2010). Likewise, intravenous magnesium sulfate did not benefit patients with ischemic stroke and intracerebral hemorrhage in the Intravenous MAGnesium Efficacy in acute Strokes (IMAGES) trial (Muir et al., 2004). There are some possible reasons for the unsatisfactory results from the IMAGES trial. First, magnesium solutions were administered at the median time of 7 hr after stroke, which might be somewhat longer than the short-time window. In fact, only 3% of participants received the treatment within 3 hr of stroke onset. Second, unfortunately, the IMAGES trial did not measure the initial stroke severity, which is the most important predictor for outcomes. Finally, the detrimental effects from magnesium treatment in some patients may obscure the beneficial effects in others.
3.1.3.3.4 Chloride (Cl−), a Neglected, but Important, Ion
Injury to the CNS by SAH results in a loss of ionic homeostasis that can lead to neuronal death. An increase in intracellular cations has been well established, but there are no studies of changes in intracellular anions after SAH (Galeffi et al., 2004). A rise in intracellular Cl− has been observed in other CNS diseases except SAH, such as ischemia, seizure, neurodevelopmental disorders, and pain (Ben-Ari et al., 2012; Galeffi et al., 2004; Yeo et al., 2013; Zhang et al., 2013c). One of the major consequences of a rise in intracellular Cl− is that GABAA becomes depolarizing, whereas it is normally hyperpolarizing. A loss of GABAA-mediated inhibition may contribute to neuronal hyperexcitability observed after stroke, leading to neuronal damage (Gao et al., 1999; Urban et al., 1989; Zhang et al., 2013c). The Na-K-Cl cotransporter and the K-Cl cotransporter play a particularly important role in controlling the intracellular concentration of Cl− (Ben-Ari et al., 2012). Thus, these two ion channels are considered novel targets for brain edema (Walcott et al., 2012).
Cl− is a major anion in the brain (Terry, 1994), but to the best of our knowledge, there are no studies addressing changes in chloride and the expression of its cotransporter in pathophysiological events of SAH. As previously mentioned, a balance in intracellular Cl− is necessary for the hyperpolarizing effects of GABAA in the adult brain. It is possible that attenuation of neuronal concentration of Cl− post-SAH prevents the depolarizing effects of GABAA, thereby influencing secondary brain injury. Future studies are needed to explore changes in intracellular Cl− and its functional roles in SAH-induced neuronal death, which may provide a novel therapeutic target for SAH treatment.
3.1.3.3.5 Promising Ion Channels
The hyperpolarization-activated/cyclic nucleotide (HCN) channels are important regulators of neuronal excitability and network activity in a variety of nervous system diseases, such as neuropathic pain, epilepsy, and SAH (Jung et al., 2007; Tibbs et al., 2013). Li et al. found that oxyhemoglobin-induced neuronal hyperexcitability was mediated by HCN channels (Li et al., 2012). An important observation made in the study was that whole-cell recordings in rat brain slices indicated that perfusion of bloody CSF promoted neuronal hyperexcitability and blocked HCN currents in CA1 pyramidal neurons by scavenging NO (Li et al., 2012). Given the evidence of a potential role in SAH, these channels may emerge as an attractive therapeutic target to attenuate neurovascular dysfunction after SAH; however, this necessitates more research.
Another promising ion channel that warrants further investigation in regard to SAH is the purinergic receptor. Cytotoxic events following SAH, such as extracellular accumulation of ATP, may activate the purinergic receptor to stimulate the innate immune response and apoptotic/pyroptotic cell death as seen in ischemic stroke and CNS trauma (Burnstock et al., 2011; Dahl and Keane, 2012). Bloody CSF elicits a steep, transient rise in Ca2+ by activating ATP-sensitive P2 receptors in human astrocytes culture (Kasseckert et al., 2013). Depending on the activation scheme, in vivo purinergic receptors are non-selective cation channels (Locovei et al., 2006; Sperlagh et al., 2006). These receptors result exclusively in the formation of non-selective large ion pores, termed pannexin (Locovei et al., 2007). Based on the properties of these receptors, purinergic signaling may be a potential therapeutic target for limiting ion distribution after SAH.
3.1.4 Oxidative Stress: An Opportunity for Intervention
Reactive oxygen radicals are a key mediator of SAH pathology. Mounting data supports the early generation of reactive oxygen species (ROS) and oxidative stress after a SAH. The ROS produced include superoxide anion (O2•), hydroxyl radical (OH•), hydrogen peroxide (H2O2), NO, and peroxynitrate(ONOO•) (Asano and Matsui, 1999; Ayer and Zhang, 2008; Gaetani et al., 1994; Lin et al., 2006; Marzatico et al., 1993; Petzold et al., 2005a; Schulz et al., 2000). The major origin of ROS following SAH is the leakage of O2• from disrupted mitochondria due to a disturbance in the electron transport chain, and from the auto-oxidation of hemoglobin upon the lysis of erythrocytes into the subarachnoid space (Asano, 1999; Marzatico et al., 1993; Piantadosi and Zhang, 1996; Sercombe et al., 2002). Other sources of ROS include increased nitric oxide synthase (NOS) activity (Ayer and Zhang, 2008; Sehba et al., 2004a), hypoxic conversion of endothelial xanthine dehydrogenase to xanthine oxidase (Sermet et al., 2000), lipid peroxidation (Schulz et al., 2000), and an upregulation of NADPH oxidase post-SAH (Liu et al., 2007). Additionally, high levels of Ca2+, Na+, and ADP in the damaged cells stimulate excessive production of ROS by mitochondria (Martin et al., 2011; Viola and Hool, 2013).
There are several enzymatic antioxidant systems that are activated to combat free radical production during the typical cellular damage that occurs after SAH. Superoxide dismutase (SOD), glutathione peroxidases, and catalase are the major enzymatic scavengers in brain tissue (Lewen et al., 2000). However, levels of those endogenous antioxidant enzymes normally are not adequate to eradicate excess free radical formation. In both experimental models and clinical studies, there is an imbalance between the intrinsic antioxidant system and the production of ROS in the brain after SAH (Gaetani et al., 1998a; Kaynar et al., 2005; Marzatico et al., 1993; Marzatico et al., 1998). Thus, oxidative stress has been speculated to be one of the factors involved in the short- and long-term pathogenesis of SAH (Asaeda et al., 2005; Liu et al., 2007; Pyne-Geithman et al., 2009).
A large number of studies have provided evidence that oxidative stress plays a significant role in EBI (Zhuang et al., 2012). Experimental studies in rats indicate that the activities of enzymatic and non-enzymatic antioxidant systems (Cu-Zn and Mn SOD) decreased 1 hr after SAH and remained so until 48 hr later (Marzatico et al., 1993). The metabolic products of lipid peroxidation increased from 1 to 6 hr after SAH (Gaetani et al., 1990). Similarly, in humans, a decrease in antioxidant capacity and an increase in lipid peroxidation products were found within 72 hr after ictus and correlated well with poor clinical status and eventual outcome (Gaetani et al., 1997; Gaetani et al., 1998a; Hsieh et al., 2009; Kamezaki et al., 2002). The nuclear factor E2-related factor 2/antioxidant-response element (Nrf2/ARE) pathway, an important antioxidant defense (Zhang et al., 2013a), was activated in the brain after SAH, playing a beneficial role in EBI (Chen et al., 2011b; Zhao and Aronowski, 2013).
ROS can damage elements of the neurovascular unit by promoting lipid peroxidation, protein breakdown, and DNA damage (Ostrowski et al., 2006a). Some of the consequences of oxidative stress after SAH include neuroinflammation, disruption of the BBB, and production of spasmogens (Gaetani et al., 1990; Yun et al., 2013). Intracellular ROS activate NF-κB to upregulate NOS 2 (Chen et al., 2012). In addition, ROS can activate apoptotic signals, including p53, caspase-3 and caspase-9 to promote apoptotic cell death (Lu et al., 2013). Overexpressing CuZn-SOD in transgenic mice prevented apoptotic cell death (Matz et al., 2000), and reduced mortality via activation of Akt/GSK3 beta survival signaling after SAH (Endo et al., 2007).
Therapeutic avenues to reduce EBI and vasospasm after SAH by targeting oxidative stress have been explored. Treatment with hydrogen, a medical gas used in novel experimental studies of SAH, alleviated EBI and vasospasm by decreasing the oxidative stress-induced injury (Hong et al., 2012; Zhan et al., 2012). Hyperbaric oxygen suppressed NADPH oxidase and the level of oxidative stress in cerebral tissues at 24 hr after SAH (Ostrowski et al., 2006b). Although systemic antioxidants showed substantial success in preventing oxidative stress and decreasing vasospasm in experimental SAH, there has been minimal success in translating this approach into clinical trials (Germano et al., 1998; Zhang et al., 2010). Also, oxidative stress seems to occur soon after SAH; thus, an effective therapeutic window is the biggest challenge for clinical translation. It is possible that the injury caused by free radicals may occur even before a patient can receive effective treatment (Haley et al., 1997; Kassell et al., 1996; Lanzino and Kassell, 1999; Lanzino et al., 1999), which is a possible explanation as to why clinical trials of free radical scavengers have failed.
3.1.5 Inflammation: A Promising Area of Research for New Treatments
A burgeoning body of researches suggests that many components of the inflammatory response contribute to the progression of an injury after an aneurysm ruptures. Microglias are the resident immunocompetent and phagocytic cells in the CNS, which play a crucial role in neuroinflammation (Kim et al., 2013; Xiao et al., 2013). Similar to microglias, astrocytes are capable of synthetizing and secreting inflammatory factors, such as cytokines and chemokines (Hutchison et al., 2013). The existing literature provides evidence that a variety of factors that are linked to the development of inflammatory lesions. It emphasizes brain injury mediated by inflammatory cells, which proliferate in response to the secretion of cytokines from leukocytes and glia cells, which itself is in response to the free radical-inducing properties of extravascular hemoglobin in the subarachnoid space.
3.1.5.1 Cytokines
A variety of inflammatory cytokines, including interleukin (IL)-1α, IL-1β, IL-6, IL-8, and tumor necrosis factor (TNF)-α, have been shown to be strongly associated with brain injury in both patients and animals after SAH (Gaetani et al., 1998b; Greenhalgh et al., 2012; Larysz-Brysz et al., 2012). High-mobility group box 1, a potent proinflammatory mediator, was increased after SAH and has been proposed to be a useful, complementary tool for predicting functional outcome and mortality after SAH (Zhu et al., 2012). In addition, convincing data implicate cytokines in the development and maintenance of neurovascular injury. IL-1β, IL-6, and matrix metalloproteinase (MMP)-9 expressions were elevated over time. They showed an early increase at around 6 hr, and a late peak between 48–72 hr, post-SAH in the cerebral arteries. This occurred via early activation of the mitogen activated protein kinase kinase (MEK)- extracellular signal-regulated kinase 1/2 (ERK1/2) pathway (Maddahi et al., 2012). A significant difference was observed in the mRNA expression of IL-1α, IL-6, and IL-8 in the canine basilar artery over the course of days after SAH, with maximal expression during the peak of vasospasm (Aihara et al., 2001).
Additionally, the role of NF-κB, a key transcriptional regulator of inflammatory genes, has been elaborately studied after SAH. You et al. recently demonstrated that the activated NF-κB in neurons plays an important role in regulating the expression of inflammatory genes in the brain and ultimately contributes to delayed brain injury after SAH (You et al., 2013).
A number of anti-inflammatory strategies have been utilized to reduce inflammation after SAH. Pyrrolidine dithiocarbamate, an NF-κB inhibitor, reduced the levels of TNF-α and IL-1β mRNA 5 d after SAH in a rabbit model and thereby suppressed the post-SAH inflammatory response (You et al., 2013). Neutralization of IL-1β by an anti-rat antibody resulted in a significant decrease of both endothelin-1 and TNF-α, but not of IL-6, in the peripheral blood (Larysz-Brysz et al., 2012). Furthermore, inhibition of IL-1β by its pharmacological antagonist attenuated EBI via the inhibition of c-Jun N-terminal kinase (JNK)-mediated induction of MMP-9 and consequent preservation of tight junction protein zonula occludens-1 after SAH (Sozen et al., 2009). Thus, a novel, safe, and effective anti-inflammatory drug might be a promising strategy to improve the outcome of patients with SAH (Sercombe et al., 2002).
3.1.5.2 Chemokines
Chemokines are a class of small cytokines or signaling proteins that induce the migration of nearby blood borne inflammatory cells toward the source (Lakhan et al., 2009; Li and Ransohoff, 2008; Reaux-Le Goazigo et al., 2013). Consequently, these molecules play an important role in cell recruitment in the damaging inflammatory processes after stroke. They also are involved in cell to cell communication (Hughes et al., 2002; Kim et al., 1995).
Expression of chemokines, such as monocyte chemotactic protein-1 (MCP-1) (Wang et al., 2011), chemokine (C-C motif) ligand 5 (CCL5) (Smithason et al., 2012), and chemokine (C-X-C motif) ligand 1 (CXCL1), are strongly upregulated in the cortex following SAH. The elevation of chemokines CCL5 and CXCL1 were suppressed in mice with myeloid cell depletion after SAH (Smithason et al., 2012). The level of MCP-1 was increased in a time course parallel to the development of cerebral vasospasm in a prospective clinical study (Kim et al., 2008). These findings suggest that the administration of specific MCP-1 antagonists might prevent cerebral vasospasm and improve the poor outcome caused by SAH (Lu et al., 2009). It also implies that serum MCP-1 could be a possible biomarker for SAH.
Another report that evaluated the human cerebral response of the neurotrophins fibroblast growth factor-2 (FGF2) following SAH shows that FGF2 peaked 2 d after SAH. This study also identified the potential threshold values for the chemokine to serve as a monitoring indicator in the neurosurgical intensive care unit (Mellergard et al., 2010). In conclusion, chemokines are a fascinating family of peptide mediators that contribute to neuron-neuron, glia-glia, or neuron-glia communications relevant to SAH and are possible targets for developing new therapeutic approaches for SAH.
3.1.5.3 Cellular Adhesion Molecules
Mounting evidence shows that the leukocyte-endothelial interaction exerts a crucial effect in the pathogenesis of SAH. Elevated leukocyte count has been reported to increase the risk of experiencing symptoms of vasospasm, and it was closely associated with a 90% rate of poor outcomes. Adhesion molecules are important mediators of inflammation after stroke, and essentially help cells stick to each other or to their surroundings. They are proteins located on the surface of cells including the immunoglobulin superfamily, integrins, cadherins, and selectins, all of which have been detected in patients with SAH. These adhesion molecules are well known to mediate endothelial capture, adhesion, extravasation of leukocytes, and recruitment to the site of injury (Chaichana et al., 2010; Polin et al., 1998; Yang et al., 2012).
Studies have demonstrated that the levels of soluble forms of E-selectin, intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) were significantly elevated in the CSF and serum of patients after SAH as compared to normal controls (Kubo et al., 2008). ICAM-1 is heavily glycosylated and possesses binding sites for a number of immune-associated ligands. ICAM-1 binds to LFA-1 (Lymphocyte Function-Associated Antigen-1), a glycoprotein receptor found on the surface of leukocytes (Marlin and Springer, 1987). When activated, leukocytes bind to endothelial cells via ICAM-1/LFA-1 and then transmigrate into brain tissue through the vascular endothelium (Yang et al., 2006).
In a rat model of SAH, the expression of ICAM-1 was enhanced in the endothelial layer of the basilar artery (Handa et al., 1995). The level of ICAM-1 mRNA increased early soon after SAH, and peaked around day 7 in parallel with the persistent contraction of the basilar artery (Aihara et al., 2001). The anti-ICAM-1 antibody reduced vasospasm by 22% following SAH (Bavbek et al., 1998). 6-mercaptopurine was effective in preventing and reversing arterial narrowing by inhibiting ICAM-1 and E-selectin in a rodent model of SAH (Chang et al., 2010).
E-selectin and P-selectin may also play an important role in mediating SAH-induced inflammation. A marked increase in the concentration of E-selectin was observed in both the CSF and serum from patients with SAH as compared to control (Tanriverdi et al., 2005). It was especially high in patients who later developed moderate to severe vasospasm (Polin et al., 1998). Furthermore, it has been reported that the monoclonal antibody against E-selectin has a therapeutic effect similar to that of the anti-ICAM-1 antibody in terms of attenuating vascular injury after SAH (Lin et al., 2005). A higher serum level of neutrophil P-selectin glycoprotein ligand-1 at time of admission suggests an impending DCI in patients with aneurysmal SAH (Yang et al., 2012).
Additionally, a new target for SAH is the adhesion molecule vascular adhesion protein-1 (VAP-1), a novel type of adhesion molecule with semicarbazide-sensitive monoamine oxidases activity. Mounting evidence suggests that VAP-1 is an inflammation-inducible endothelial glycoprotein. The expression and role of VAP-1 has been explored in both patients and animal models of stroke (Airas et al., 2008; Ma et al., 2011). However, there are no studies exploring the role of VAP-1 in SAH, which has the potential to be a target of pharmaceutical interest.
3.1.5.4 Inflammasomes: An Emerging Inflammatory Mediator
The nucleotide-binding domain, leucine-rich repeat containing (NLR) recently received attention because of its role in innate immune regulation and genetic linkage to human inherited autoinflammatory syndromes (Jha and Ting, 2009). Inflammasome complexes that are assembled in response to danger signals lead to the autoactivation of pro-caspase-1, which in turn activates pro-IL-1β/IL-18 (Deroide et al., 2013; Lippai et al., 2013). Since there are more than 20 NLR members, the next step would be to identify which of the inflammasomes are involved in the SAH pathology. Understanding inflammasome pathways may provide insight into the development of neuroinflammation after SAH.
3.1.6 Apoptosis: A Target for Future Therapeutic Intervention
Apoptosis is a potentially reversible process that is characterized by energy-dependent programmed cell death to dispose of redundant cells (Taylor et al., 2008). Apoptosis after SAH may be caused by elevated ICP, the neurotoxicity of blood breakdown components, ischemia, and reperfusion, as well as by acute vasospasm (Bederson et al., 1998; Matz et al., 2001). Even a brief brain insult is sufficient to trigger complex cellular events that subsequently can lead to progressive apoptotic cell death.
Apoptosis was first identified in a patient who died of SAH-induced cerebral vasospasm (Zubkov et al., 2000). A number of studies have since revealed apoptotic pathways and cascades within the cortical, subcortical or hippocampal neurons, endothelium, and vascular cells following the onset of SAH (Ostrowski et al., 2006a). A number of intrinsic and extrinsic apoptotic pathways are activated after SAH, including the death receptor pathway, the caspase-dependent and -independent pathways, as well as the mitochondrial pathway (Cahill et al., 2006; Cheng et al., 2009; Endo et al., 2007; Hasegawa et al., 2011b).
3.1.6.1 Apoptosis in Neuronal Cells
If the initial bleed following SAH is severe enough to block blood flow into the brain, as in the case of a global stroke, it is unlikely that the cerebral tissue will survive. Apoptosis might play an important role in SAH pathology, and neuronal apoptosis can occur after SAH (Cahill et al., 2006). Matz et al. injected hemolysate into the subarachnoid space and then observed apoptotic cells in the neocortex closest to the injection site (Matz et al., 2001). In an endovascular perforation rat model of SAH, pathological changes of apoptosis were noted in most brain regions, especially in the basal cerebral cortex and hippocampus (Park et al., 2004). Caspase-3 and apoptotic cells were present not only in the basal cerebral cortex, which was exposed to bloody CSF, but were also evident in the hippocampal dentate gyrus and CA1 region, which is the region most vulnerable to damage. Further, apoptosis related proteins, including apoptosis-inducing factor, cytochrome C, P53, and caspases, have been shown to be activated after SAH (Simard et al., 2012a; Yuksel et al., 2012). Endoplasmic reticulum stress has also been implicated in orchestrating neuronal apoptosis via C/EBP homologous protein (CHOP) in response to SAH (He et al., 2012a).
The role of mitogen-activated protein kinases (MAPKs), including ERK1/2, JNK, and p38, in EBI induced apoptosis has been studied. JNK and p38 were activated in response to apoptotic cascades (Hasegawa et al., 2011b), while ERK1/2 was significantly decreased in the dentate gyrus but, not in the cortex or hippocampus (Lin et al., 2009). Yatsushige et al. demonstrated the neuronal programmed death was mediated through the activation of JNK/c-Jun pathway (Yatsushige et al., 2007). MAPKs alter both a variety of proapoptotic proteins, including c-Jun, p53, bim, and bax, and anti-apoptotic proteins, such as Bcl-2 and Bcl-xl following SAH. Furthermore, the “tropomyosin-related kinase” receptor family is usually associated with cell survival, differentiation and apoptosis (Boulle et al., 2012). Preservation of tropomyosin-related kinase B signaling by sodium orthovanadate attenuates neuronal apoptosis after SAH (Hasegawa et al., 2011a).
Akt, a serine/threonine kinase, is one of the key anti-apoptotic signaling molecules downstream of phosphoinositide 3-kinase, which mediates a protective effect against neuronal apoptosis and vasospasm after SAH (Duris et al., 2011; Endo et al., 2006; Sugawara et al., 2008; Zhuang et al., 2011). An elevated expression of phospho-Akt and phospho-GSK3βwas described in the cerebral tissue after SAH, but it was not sufficient to protect the brain. Administration of pharmacological agents, such as the α7 nicotinic acetylcholine receptor agonist and simvastatin, enhanced p-Akt and p-GSK3β and attenuated neuronal apoptosis (Cheng et al., 2010; Duris et al., 2011). Recently studies have shown that anti-apoptotic drugs ameliorate negative outcomes after SAH in animal models (Zhou et al., 2004; Zubkov et al., 2002). Current anti-apoptotic therapies for SAH focus on the MAPK pathway (Cahill et al., 2007; Suzuki et al., 2010b), the tumor suppressor p53 (Chen et al., 2011a; Li et al., 2010), and hypoxia inducible factor-1 (HIF-1) target genes. Additionally, intravenous mesenchymal stem cell administration in vivo provided neuroprotective effects by ameliorating neural cell apoptosis in SAH animal models (Khalili et al., 2012).
3.1.6.2 Apoptosis in Endothelial Cells and Blood Brain Barrier (BBB) Disruption
Under normal physiological conditions, the BBB permits only water, ions, small lipophilic molecules, and a limited number of nutrients transported via receptor-mediated transcytosis (i.e., glucose, some amino acids, heparin, and transferrin) to enter the brain (Broadwell et al., 1996). Plasma-borne macromolecules and most cellular elements are prevented from crossing the BBB because of continuous-type endothelial cells and the junction complexes that maintain the integrity of the BBB. A remarkable increase in capillary permeability occurs at a very acute stage after experimental SAH (Peterson et al., 1990). Increased extravasation of Evans blue dye in the ipsilateral hemisphere occurred as early as 3 hr after SAH in rats, with a maximal extravasation at 48 hr (Doczi et al., 1986a; Doczi et al., 1986b). A difference in barrier disruption of the intraparenchymal vessels located from proximal to distal to cisternal clots was detected following SAH in rabbits (Johshita et al., 1990). Early impairment of the BBB contributes to vasogenic edema, which results in brain volume expansion and prolongs ICP elevation after SAH.
Endothelial cells are an important component of the BBB and essential for maintaining the integrity of the BBB. Apoptotic death of endothelial cells can lead to destruction of the BBB and directly exposes smooth muscle cells to harmful blood components that can ultimately worsen the consequences of SAH. Zubkov et al. introduced the concept of apoptosis of cerebral endothelial cells in relation to a delayed cerebral vasospasm in a patient with SAH (Zubkov et al., 2000). Early apoptosis of endothelial cells may trigger, aggravate, and maintain delayed cerebral vasospasm (Chen et al., 2008; Zubkov et al., 2001). The mechanism of apoptosis in endothelial cells involves the TNF-α receptor-1 and the caspase-8 and caspase-3 pathways. Caspase-3 activation and DNA fragmentation in the endothelial cells starts within 10 min of SAH (Friedrich et al., 2012b). Additionally, upregulated p53 modulation of apoptosis induced apoptosis of microvascular endothelial cells in the hippocampus may play a significant role in the disruption of the BBB that occurs after SAH (Yan et al., 2011).
A variety of anti-apoptotic strategies have shown favorable results in treating endothelial apoptosis after SAH. Beneficial effects have been reported upon inhibition of caspase activity in endothelial cells after SAH (Gules et al., 2003), and caspase inhibitors may also have a potential role in the treatment of cerebral vasospasm (Zhou et al., 2004). For example, CHOP siRNA reduced apoptosis signaling effectors, bim and caspase-3, to ameliorate EBI. Furthermore, inhibition of CHOP effectively combats apoptotic mechanisms of cerebral vasospasm set in the basilar artery endothelia (He et al., 2012b). The beneficial effect of recombinant human erythropoietin on vasospasm after SAH may be related to its anti-apoptotic effects of the endothelial cells in the basilar arteries, mediated in part by the JAK2/signal transducer and activator of the transcription signaling pathway (Chen et al., 2009). Treatment with antioxidants such as alpha lipoic acid attenuated the severity of endothelial apoptosis (Erdi et al., 2011).
3.1.7 Autophagy: A New Player in EBI
Autophagy is a cellular process that causes degradation of long-lived proteins and organelles and recycling of cellular constituent to ensure cell survival. It plays a role in regulating the turnover of cellular constituents, which plays a role in mediating in cell homeostasis. Autophagy has recently been recognized as a form of cell death that occurs after SAH, as do apoptosis and necrosis. Indeed, Lee et al. identified the autophagic death of neurons as the third mode of cell death after SAH (Lee et al., 2009a).
To date, autophagy has been studied extensively in stroke, but only to a limited degree in SAH. In experimental SAH, autophagy was found to be activated in neurons as early as 6 hr after experimental SAH (Zhao et al., 2013). Moreover, the conversion of light chain-3 I to light chain-3 II and expression of beclin-1 increased significantly, thus suggesting that autophagy is activated during EBI (Lee et al., 2009a; Zhao et al., 2013). However, it remains an open issue as to whether activation of autophagy is an inducer of death as a part of harmful response or a stopper of death as a part of an endogenous neuroprotective response (Carloni et al., 2008; Wen et al., 2008).
After an interaction between apoptosis and autophagy was demonstrated, rapamycin and simvastatin were tested and shown to inhibit apoptosis by activating post-SAH autophagy (Zhao et al., 2013). Conversely, ischemic insult triggered autophagy, and thus an autophagic mechanism may contribute to neuronal injury after cerebral ischemia (Wen et al., 2008). When autophagy was inhibited by 3-methyladenine and wortmann in SAH rats, neuronal apoptosis were increased and neurological function deteriorated. Brain edema and disruption of the BBB were further aggravated, suggesting that autophagy may have a beneficial effect (Jing et al., 2012; Wang et al., 2012). However, the precise role of autophagy in the pathogenesis following SAH is requires further investigation, as in other CNS diseases. Next, the cross-talk between autophagy (self-eating) and apoptosis (self-killing) merit the imagination of stroke scientists.
3.1.8 Necrosis: An Unregulated Cell Death
SAH simultaneously activates both apoptotic and necrotic pathways. Apoptosis and necrosis can be differentiated according to the morphological and biochemical differences, especially in early membrane disruption. Conventional knowledge categorizes necrosis to be an accidental and uncontrolled mode of cell demise, characterized by swollen organelles, increased cell volume, early membrane integrity loss, and cellular collapse. Currently, necrosis is observed to contribute to a wide range of pathological forms of cell death following SAH. However, little research effort has been made to eliminate necrosis after SAH, possibly due to the widely held belief in its unregulated manner. A study using cultured cells showed a high oxyhemoglobin concentration induced necrosis in aortic smooth muscle cells, suggesting necrosis contributes to vascular wall changes after SAH (Ogihara et al., 2001). Oxyhemoglobin also exerts a necrotic effect on cultured astrocytes (Rollins et al., 2002). Considering the discrepancy between in vitro study and in vivo studies, animal SAH experiments must be performed. After continuous superfusion with artificial CSF containing hemoglobin, necrosis was induced in focal areas of the cortex (Dreier et al., 2000). Additionally, tumor necrosis factor levels are elevated after SAH, which can lead to necrosis (Lin et al., 2004). Neuronal necrosis began very early in SAH rat models (Friedrich et al., 2012b). Some mechanisms are responsible for SAH-induced necrosis. ATP depletion is an initial trigger in SAH-induced necrotic cell death. The other potential candidates are calcium and ROS. ROS stimulates oxidative stress that damages lipids, proteins, and DNA, subsequently leading to mitochondrial dysfunction, ion balance deregulation, rapid loss of membrane integrity, and finally necrosis. Elevated cytosolic calcium levels result in mitochondrial calcium overload and activation of calcium-dependent proteases and phospholipases, which trigger necrosis. Bloody CSF from hemorrhagic patients causes necrosis in cultured human astrocytes via increased calcium levels. An ATP-sensitive P2 receptor antagonist inhibited the bloody CSF-induced calcium peak and consequent necrosis (Kasseckert et al., 2013).
Recently, accumulating studies revealed necrosis could be a programmed process like apoptosis, which challenged the traditional view of necrosis. A new form of non-apoptotic caspase-independent cell demise, termed as necroptosis, has attracted attention in the CNS (Fricker et al., 2013). Our next step is to evaluate whether necroptosis is a novel death pathway after SAH.
3.1.9 Cerebral Edema: A Major Contributor to Poor Outcomes
Global cerebral edema is a common and important feature of both experimental and clinical SAH (Altay et al., 2012b; Westermaier et al., 2012). It is evident in 6–8% of patients at admission and develops in an additional 12% over the first 6 d, as assessed by CT scans (Claassen et al., 2002; Kassell et al., 1990). Four types of cerebral edema have been characterized: vasogenic, cytotoxic, osmotic, and interstitial. In SAH, cerebral edema that develops soon after the initial bleeding could be classified mainly as both a primary vasogenic and secondary cytotoxic component (Barry et al., 2012). Global cerebral edema after SAH reflects disruption of the BBB due to the apoptosis of endothelial cells, degradation of the basal-lamina by proteases, diffuse inflammatory reaction or neurotoxic effects of blood and its degradation products, ischemic injury due to transient ictal cerebral circulatory arrest, abnormal autoregulation due to microvascular damage, or dysfunction of vasomotor centers located in the brainstem (Claassen et al., 2002; Keep et al., 2005; Sehba et al., 2007).
In experimental studies, cerebral edema has historically been quantified as a change in the percentage of water content in brain (i.e., water content divided by wet weight) (Adachi and Feigin, 1966). However, that measurement can be influenced by the technical abilities of the investigator and by other conditions, such as room temperature, humidity, and air flow. Therefore, if not measured carefully, the number can, be misleading as “small” changes in the percentage water content actually mirror much larger changes in brain swelling. Thus, constant temperature and humidity are needed when measuring brain edema using the dry/wet method. Furthermore, some investigators have even suggested that it would be valuable to find a new equation that might better reflect the impact of edema after SAH (Keep et al., 2012). Until then, it is recommended that researchers measure brain swelling by imaging techniques, such as computed tomography and magnetic resonance imaging, or by thermal conductivity (Keric et al., 2012; Ko et al., 2012).
3.2 Delayed Brain Injury (DBI)
DIND is the leading cause of morbidity and mortality in patients who survive the initial impact of SAH and have had their aneurysm effectively treated (Crowley et al., 2008; Fergusen and Macdonald, 2007). Recent studies have demonstrated a host of critical, interrelated pathologies arising in the subacute phase of SAH as a result of EBI, and this phenomenon is designated as DBI. With advances in understanding the pathophysiology of SAH, it has become clear that the mechanisms leading to EBI and DBI are not mutually exclusive. In fact, many of the pathogenic triggers are interrelated.
3.2.1 Delayed Cerebral Vasospasm: From Extravascular Blood to Vessel Lumen
Historically, cerebral vasospasm after SAH was considered to be a prolonged contraction of the smooth muscle cells and abnormal endothelial hypertrophy, inflammatory changes, and gene expression in the cerebral arteries, finally leading to tissue ischemia (Rothoerl and Ringel, 2007). The central event in the contraction of vascular smooth muscles is an increase in the concentration of intracellular Ca2+. Indeed, in the last century, there was a wide assumption that vasospasm was the main cause of poor outcomes for patients with SAH (Kassell et al., 1990). Several studies found a strong link between radiologically confirmed vasospasm and clinical symptoms of DCI (Fergusen and Macdonald, 2007; Rabinstein et al., 2004). As mentioned above, SAH-induced DCI is a clinical syndrome of focal neurological and cognitive disorder. Unpredictably, it occurs in 30% patients from 3–14 d after the initial bleeding (Dorsch and King, 1994).
In vasospasm, overflowed blood from a ruptured aneurysm triggers a chain reaction of cerebral artery vasoconstriction, brain tissue infarction, and clinical condition deterioration. The most powerful predictors of vasospasm is including the volume, density, and prolonged presence of subarachnoid blood surrounding the arteries (Fisher et al., 1980; Loch Macdonald, 2006; Reilly et al., 2004; Suzuki et al., 1980). Conversely, vasospasm was invariably absent in those patients with only a minimal subarachnoid blood load (Fisher et al., 1980). In the filament model of SAH, contraction of the large arteries in the Willis circle in rats after SAH is ascribed to the persisting subarachnoid blood clot. Vasospasm began on the third day after the onset of SAH, and was maximal at 6–8 d, eventually lasting 2–3 wk (Wilkins, 1990). In sum, it is reasonable to suppose that cerebral vasospasm commonly takes place during DBI. However, there is not adequate evidence to conclude that vasospasm, in and of itself, could be used as a surrogate marker to monitor SAH progression and the efficacy of interventions (Nolan and Macdonald, 2006).
The effects of vasoactive agents have long been studied in regard to the pathogenesis of vasospasm (Roman et al., 2006). These include neurogenic factors, biogenic amines (such as histamine and norepinephrine), 20-hydroxyeicosatetraenoic acid (Mulligan and MacVicar, 2004), eicosanoids (such as prostaglandins, thromboxans and leukotrienes), ion (Girouard et al., 2010), and free radicals (Nishizawa and Laher, 2005). Astrocytes and leukocytes release endothelin-1, a potent vasoconstrictor in response to SAH. In addition, endothelin receptors are upregulated (Beg et al., 2006; Fassbender et al., 2000; Hansen-Schwartz et al., 2003b; Pluta et al., 1997).
SAH increased the expression of G-protein-coupled vasoconstrictor receptors in cerebral arteries, including ETB, 5-HT1B, AT1, and TXA2 receptors (Hansen-Schwartz et al., 2003a; Povlsen et al., 2012). The Rho/Rho-kinase pathway is the main regulator of these actin-dependent cell functions in the pathogenesis of sustained smooth muscle cell contraction and vasospasm (Satoh et al., 2012; Takai et al., 1995). Therefore, the combination of treatment with an inhibitor of RhoA, pitavastatin, and an inhibitor of Rho-kinase, fasudil, could extensively prevent cerebral vasospasm after SAH (Naraoka et al., 2013). Furthermore, phosphorylations trigger SAH-induced vasculopathy in cerebral arteries, as determined by quantitative mass spectrometry, including focal adhesion complexes, ERK1/2, calcium calmodulin-dependent kinase II, STAT3, and c-Jun (Parker et al., 2013). Tenascin-C, a matricellular protein, induces cerebral vasospasm via TLR4 and activation of JNK and p38 (Fujimoto et al., 2013; Suzuki et al., 2013). Animal experiments have shown a benefit from inhibiting the PKC and MAPK pathways on cerebral vasoconstriction (Beg et al., 2007; Hansen-Schwartz et al., 2008) thus engendering enthusiasm that preconditioning stimuli may protect against SAH, as vasospasm-induced brain damage is anticipated after SAH (Wang et al., 2013b).
3.2.2 Microcirculatory Spasm and Dysfunction
Study of the changes in the microcirculation due to SAH started about 30 years ago (Herz et al., 1975; Wiernsperger et al., 1981). The concept of microvascular spasm is evolving, and recently, more attention has been paid to the functional and structural changes in microcirculation (Kozniewska et al., 2006). Under normal conditions, the cerebral microvasculature can accommodate, to a certain extent, decreased perfusion pressure through vasodilation, a process underlying pressure autoregulation (Paulson et al., 1990). SAH resulted in failure of the microcirculation, including a significant decrease in the mass density of capillaries, vasocontraction in small arteries, and changes of the vessel wall that might lead to laminar, triangular, or round cortical infarcts (Ohkuma et al., 2000; Weidauer et al., 2008). Herz et al., demonstrated that topical applications of homologous blood alone could result in a 33% vasoconstriction in guinea pigs, which was consistent with a subsequent cortical infarction (Herz et al., 1975). Progressive disturbances of the microcirculation result in, and/or contribute, to formation of microthrombi (Sabri et al., 2012). Disturbances in the microcirculation were found to be accompanied by disruption of the barriers of the intraparenchymal microvessels located distal or proximal to experimental clots in rats (Johshita et al., 1990). The thrombin receptor (protease-activated receptor-1) has been observed as playing a role in disruption of the endothelial barrier of hippocampus tissue (Yan et al., 2013). In addition, a spasm may occur in microcirculation in dissociation with the state of extraparenchymal vessels (Ohkuma and Suzuki, 1999).
Aside from measuring CBF, which is the more common method, a study utilizing orthogonal polarizing spectral imaging showed that mono- and multi-segmental cortical arterioles constrict after SAH with a reduction in diameter of up to 75% (Uhl et al., 2003). Ohkuma et al. noted that CBF was clearly associated with a prolonged circulation time in the cerebrum, even in patients without demonstrable spasms of large vessel, thus suggesting the impact of microcirculatory changes on constriction (Ohkuma et al., 2000). Although these data suggest that there are likely some microvascular perturbations after SAH, how these factors independently contribute to poor outcomes after SAH remains unknown.
3.2.3 Microthrombosis: An Additional Explanation for DCI
Microthrombosis has been attributed to the aggregation of platelets subsequent to the activation and amplification of the coagulation cascade after SAH. The presence of multiple microthrombi were, for the first time, confirmed in the autopsy of an SAH patient in 1983 (Suzuki et al., 1983), and have since then been confirmed in larger studies. A number of markers of microthormbosis are significantly upregulated after SAH, including fibrinopeptide A, tissue factor, and thrombin-antithrombin complexes (Stein et al., 2006; Vergouwen et al., 2008). Endothelial NOS knockout and decreased P-selectin reduce the formation of microthrombi after SAH (Sabri et al., 2012; Sabri et al., 2013a). Furthermore, the concentrations of these thrombotic markers were significantly elevated within days of SAH in patients who eventually developed DCI (Dorsch, 2011).
A very recent study showed that approximately 30% of constricted arterioles were occluded by the microthrombi, suggesting that microthrombosis may be an etiology of the perfusion deficits and CPP-independent decrease in CBF after SAH (Friedrich et al., 2012a). Another line of investigation has suggested that microthrombi not only mechanically occlude the vessel, but also mediate vascular damage and constriction as the platelets aggregate and neutrophil infiltrates (Friedrich et al., 2011; Sehba et al., 2005). Intraluminal platelet aggregates can occur as soon as 10 min after SAH and are associated with injury to the vessel wall, including damage to the endothelium and the microvascular basal lamina (Friedrich et al., 2010). Further, platelet aggregates can be amplified and stabilized through thrombin activation as well. The intracellular contents of platelets include serotonin, thrombin and adenosine diphosphate and MMP-9, which are known to potentially mediate vasoconstriction, degrade collagen IV in the microcirculation, and thus lead to disruption of the BBB (Scholler et al., 2007; Sehba et al., 2004b).
3.2.4 Cortical Spreading Ischemia
Cortical spreading ischemia is a newly described phenomenon in which ischemia is the consequence of neuronal/glial depolarization in experimental SAH and in patients with SAH (Dreier et al., 1998; Dreier et al., 2009). Spreading ischemia in the cortex occurred when CBF reached 40%–70% compared to baseline, which was deemed to be 100%. Cortical spreading ischemia produced widespread cortical necrosis. In animal models, a number of pathologic conditions have been demonstrated to trigger CSD with spreading ischemia, such as hypoxia, hypotension, transient ischemia from microemboli, low glucose level, NO depletion, and free hemoglobin and high extracellular K+ from erythrocytes (Dreier et al., 2000; Dreier et al., 1998; Nozari et al., 2010).
3.3 Are We Close to Solving the Mystery of SAH?
Recent data challenge the traditional concept of delayed vasospasm as the sole cause of DCI after SAH (Kusaka et al., 2004; Macdonald et al., 2007). Beyond vasospasm, an emerging body of evidence at present suggests that DIND is likely to have a multifactorial etiology. Recently, in the randomized, double-blind, placebo-controlled, phase III trial, clazosentan (15mg/h), an endothelin receptor antagonist, significantly decreased vasospasm-related morbidity, but did not significantly improve functional outcome in patients with aneurysmal SAH (Macdonald et al., 2012; Sabri et al., 2011). The mismatch between treatment of vasospasm and poor clinical outcome after SAH could have resulted from other underlying mechanisms of injury as opposed to vasospasm. To date, clinical trials have concentrated on vasospasm, which may be a wrong focus for SAH studies, since our increasing understanding of the SAH pathophysiology reveals EBI to be a major player in SAH-induced injury (Gomis et al., 2010).
A severe increase of ICP during the initial bleeding event has been identified as triggering subsequent pathophysiological changes after SAH. In recent years, EBI has evolved as a potential target to implement treatment modalities in patients with SAH, which could ameliorate some of the devastating injuries in the long term (Cahill et al., 2006). It can be suggested that vasospasm might be only a late manifestation of EBI (Povlsen et al., 2013), because they share many of the same pathogenic factors. Further, EBI may also contribute to the pathogenesis of delayed neurological deficits. Nevertheless, research on EBI after SAH has been relatively limited; further studies are needed to clarify the exact mechanisms involved in EBI, which may reveal new therapeutic avenues that can be exploited in combination with anti-vasospasm medications. A comprehensive assessment of patterns of biomarkers during the past three years for predicting the SAH outcome is provided in Table 1.
Table 1.
Agents listed were new biomarkers for predicting the outcome of SAH in the past three years
| Agents | Sample | Main finding |
|---|---|---|
| Bradykinin (BK) | CSF | Elevated BK is correlation with brain edema (Kunz et al., 2013) |
| TNF-α | serum CSF | High level is associated with DCI, poor outcome and hydrocephalus, but not vasospasm (Beeftink et al., 2011; Chou et al., 2012) |
| Tau protein | CSF | Tau level is proportional to SAH severity (Zanier et al., 2008; Zanier et al., 2013) |
| TGF-β1 | CSF | TGF-β1 was upregulated in SAH-induced hydrocephalus (Lee et al., 2012) |
| Mitochondrial DNA | CSF | Higher CSF DNA levels on presentation are associated with worse outcomes (Wang et al., 2013a) |
| Adrenomedullin (AM) | CSF | AM concentration 8 days after SAH is related to appetite loss and DIND (Kubo et al., 2013) |
| Ceramide | CSF | Ceramide is associated with the occurrence of symptomatic vasospasm and poor neurological outcome (Testai et al., 2012) |
| Catecholamine | CSF | Epinephrine serves as an useful index of outcome (Moussouttas et al., 2012) |
| 20-hydroxyeicosat etraenoic acid (20-HETE) | CSF | 20-HETE concentrations are associated with DCI and poor outcomes (Crago et al., 2011) |
| Heart-type fatty acid binding protein (H-FABP) | serum CSF | The Hunt and Hess and Fisher grading scales are correlated with an increase in H-FABP level on administration (Yilman et al., 2012; Zanier et al., 2008; Zanier et al., 2013) |
| Monomethylated L-arginine (L-NMMA) | CSF serum | L-NMMA is associated with the occurrence of cerebral ischemic events (Jung et al., 2013b) |
| Neuropeptide Y (NPY) | CSF serum | Higher levels of NPY were in patients with cerebral infarction caused by vasospasm (Schebesch et al., 2011) |
| Haptoglobin (Hp) phenotype | serum | The Hp phenotype is associated with angiographical vasospams and clinical deterioration by DCI but does not affect the cerebral infarction(Ohnishi et al., 2013) |
| Interleukin-6 (IL-6) | serum | Higher IL-6 levels are associated with worse clinical outcome and the occurrence of DIND (Muroi et al., 2013) |
| S100B | serum | S100B is a suitable marker for ischemia after SAH (Hassan et al., 2012; Jung et al., 2013a) |
| Kallikrein 6(KLK6) | serum | Decreased KLK6 is correlated with poor outcome after SAH (Martinez-Morillo et al., 2012) |
| High-mobility group box 1(HMGB1) | serum | HMGB1 on admission predicts poor outcome and mortality and vasospasm (Zhu et al., 2012) |
| Myeloperoxidase (MPO) | serum | Elevated MPO correlates with clinically vasospasm (Lim et al., 2012) |
| Free fatty acid (FFA) | serum | n-6:n-3 FFA ratio is associated with DCI (Badjatia et al., 2012) |
| Glucose | blood | Glucose levels at admission are predictive of an elevated 1-year mortality rate (Bian et al., 2012) |
| Copeptin | plasma | Copeptin indicates clinical severity of the initial bleeding and has prognostic value for outcome of patients with SAH (Fung et al., 2013; Zhu et al., 2011) |
| Angiopoietin-1 (Ang-1) | serum | Ang-1 is significantly altered in patients suffering from cerebral ischemia (Fischer et al., 2011) |
| C-reactive protein(CRP) | plasma | CRP levels correlate with outcome but do not seem to predict DCI or infarction (Juvela et al., 2012; Romero et al., 2012) |
| Asymmetric dimethyl arginine (ADMA) | plasma | ADMA ratios predict mortality after SAH (Staalso et al., 2013) |
| Taurine | plasma | Taurine concentrations on admission predict a poor outcome (Barges-Coll et al., 2013) |
| B-type natriuretic peptide (BNP) | plasma | BNP is useful in detecting patients at risk for adverse outcomes without large vessel vasospasm (Taub et al., 2011) |
| Matrix metalloproteinase-9 (MMP-9) | Micro-dialysis sample, CSF Blood | MMP-9 was related to World Federation of Neurological Surgeons (WFNS) grade severity and SAH outcome (Sarrafzadeh et al., 2012) |
| Metabolic ratio | Metabolic ratio is a reliable marker for predicting the outcome of poor-grade patients with SAH (Barcelos et al., 2013) | |
| Prolonged QT interval and tachycardia | Prolonged QT interval and tachycardia are independently associated with Angiographic vasospasm (Ibrahim and Macdonald, 2012) |
Spontaneous recovery can occur in other types of stroke. For instance, in rats, a small fraction of dead striatal neurons is replaced by new neurons after ischemic stroke (Arvidsson et al., 2002). Neurogenesis in the hippocampus may affect functional outcome 30 d after SAH (Mino et al., 2003). However, the involvement of neurogenesis in SAH has not yet been sufficiently examined. It will be important to clarify whether and how SAH induces the differentiation of new neurons. Furthermore, signaling molecules that attract neuronal migration to the damaged area should be identified. A novel therapeutic strategy could be explored if neuronal regeneration can be stimulated, and the developing neurons can sustain and remain functional at the injury site after SAH.
4. The Vasculo-Neuronal-Glia Triad Model in SAH—A Paradigm
In recent years, strategies targeting neurons have been uniformly unhelpful in clinical trials. Mechanistic investigations into brain function have shifted from a purely “neurocentric” focus into emphasizing a more integrative perspective that involves all cell types in the CNS diseases, including stroke and neurodegenerative diseases (Gorelick et al., 2011; Guo and Lo, 2009; Lecrux and Hamel, 2011; Zlokovic, 2011).
Traditionally, the neurovascular unit was though of as a structural arrangement, with microvessel components and neurons connect via common astrocytes. It is a conceptual framework that links microvesicular and neuronal functions and their responses to injury without including the large vessels (del Zoppo, 2012). Here, we propose an emerging concept of the vasculo-neuronal-glia triad model in SAH pathology, in order to emphasize the interactions among different types of cells, including neurons, astrocytes, capillary and noncapillary endothelial cells, pericytes, smooth muscle cells, perivascular nerves, fibroblasts, smooth muscle progenitor cells, and veins (Figure 4).
Figure 4. The components of the Vasculo-Neuronal-Glia Triad Model.
A. Schematic representation showing the traditional neurovascular unit as a component of the Vasculo-Neuronal-Glia triad model, which includes neurons, astrocytes, capillary endothelial cells, pericytes, smooth muscle cells, noncapillary endothelial cells, perivascular nerves, smooth muscle progenitor cells, and veins. The Vasculo-Neuronal-Glia triad model is larger than the neurovascular unit, therefore comprising all cells and structures required to maintain cerebral blood flow under physiological and pathological conditions.
4.1 The Rationale of Vasculo-Neuronal-Glia Triad Model in SAH
The brain receives up to 20% of its blood from cardiac output. It does not possess any reserve of energy or oxygen. Thus, it relies on a constant perfusion to fulfill its energetic demands, particularly when neuronal activity is increased (Siesjo and Plum, 1971). The blood can adequately deliver supplies of oxygen, glucose, and other nutrients, while taking away metabolic products, such as lactic acid and carbon dioxide. When blood flow to the brain stops or is greatly reduced, the functions of the brain stop within seconds and damage to neurons may occur within minutes (Girouard and Iadecola, 2006). Thus, a normal neuronal-vascular link is critical for maintaining normal brain function, and it is estimated that nearly every neuron in the human brain is coupled with its own capillary (Zlokovic, 2005). Conversely, neuronal activity plays a major role in regulating vascular tone and controlling blood flow (Figure 5).
Figure 5. Agents implicated in the connection of neurons and vessels.
The concept of a “neurovascular unit” was first described by Cohen et al. in 1996 to describe the intimate interaction of a triad consisting of neurons, astrocytes, and blood vessels (Cohen et al., 1996). Nowadays, the neurovascular unit is an emerging issue in several CNS diseases, including ischemic stroke, SAH, and Alzheimer’s disease (Koide et al., 2013b; Urra and Chamorro, 2013). The large arteries are critical in determining the delivery of blood to the parenchymal circulation in SAH. The large-vessels occurs contract after SAH (Rajajee et al., 2012). This raises an interesting question as to whether information from the traditional concept of neurovascular unit might be conveyed “upstream” to the arterioles of the pia-arachnoid.
In this review, we endorse the concept of “the vasculo-neuronal-glia triad model” in SAH over that of “the neurovascular unit”, which includes neurons, glial and vascular cells, especially large arteries. In sum, the rationale for this model in SAH involves the observations that (i) SAH is a vascular disorder with pathophysiological consequences affecting the large arteries to the brain parenchyma, (ii) neurological consequences are associated with all cell types in the CNS and large vessels, (iii) neuron–vascular communication is interdependent and its interaction, acute cell–cell anatomic and physiological relationships, and neurotransmission are fixed in the adult brain, (iv) SAH causes inversion of neuron-vascular communication on both the local and global levels.
4.2 Neurons in the Model
Neurons are the core components of the nervous system. According to a traditional neuron-centric view of SAH, neurons are the primary targets affected by the disease process. Neuronal death results in edema. Activity within a localized brain region elicits increased blood flow to that region, supplying oxygen and nutrients to the active neurons, termed as functional hyperemia (Koide et al., 2013b), thereby releasing vasodilatory substances (Attwell et al., 2010; Filosa et al., 2006; Gordon et al., 2007). However, under certain conditions, neuronal activation can also lead to parenchymal arteriolar constriction by triggering sufficient K+ efflux from large-conductance Ca2+-activated K+ (BK) channels (Gordon et al., 2008; Metea and Newman, 2006). Localized neuronal activation can elevate astrocytic endfeet Ca2+ and produce vasoconstricting agents such as 20-hydroxyeicosatetraenoic acid (Newman, 2005; Schipke and Kettenmann, 2004), resulting in decreased CBF (Alkayed et al., 1996).
Neuron-stimulating vasoconstriction may represent a pathological status promoting a decrease. Although the mechanisms remain elusive, evidence suggests that elevated perivascular K+ may underlie this inversion of neurovascular coupling with vasodilative effects at concentrations of extracellular K+ below 20 mM, and constrictive effects when the threshold of 20 mM is exceeded. This inversion of neurovascular coupling contributes to decreased CBF and development of neurological deficits following SAH (Koide et al., 2013a). Neuronal activity by electrical stimulation elevated astrocytic endfeet Ca2+ that caused vasodilation in brain slices from normal animals. However, in brain slices from SAH animals, neuronal activity by electrical stimulation induced a similar increase in astrocytic endfeet Ca2+ that caused arteriolar constriction rather than vasodilation (Koide et al., 2013a). Inversion of neurovascular coupling by SAH depends on large-conductance BK channels (Koide et al., 2012). Moreover, neuron-to-glia signaling-evoked vasoactivity was regulated by neuronal release of ATP and was interrupted by a purinergic antagonist (Metea and Newman, 2006; Newman, 2005). Conversion of the neurovascular response from vasodilation to vasoconstriction after SAH may act in concert with other reactions, such as direct constrictor effects on parenchymal arterioles, microthrombi formation, and cortical spreading depolarization, which compromises cortical blood flow.
4.3 Astrocytes in the Model
In neurovascular coupling, astrocytes are in close contacts with both neurons and capillary endothelial cells through their microdomains or “foot processes” (Anderson and Nedergaard, 2003). Additionally, their endfeet are completely encased parenchymal arterioles (Gordon et al., 2007). Astrocytes tightly maintain local homeostasis, deliver glucose and provide metabolic substrates. This unique position of the astrocytes might contribute to their role in neurovascular coupling, which synchronizes neuronal activity and metabolic demands with local regulation of CBF (Zonta et al., 2003). If the availability of oxygen is lowered, the concentration of astrocytic Ca2+ is elevated, which maximizes astrocytic glycolysis and the release of lactate. Astrocytes also clear neuronal waste, including not only metabolic byproducts but also neurotransmitters released during synaptic transmission, which are sequestered through active uptake. In short, astrocytes are multifunctional cells that, by their housekeeping functions, allow neurons to progressively become the perivascular nerves and vascular endothelial cells that regulate blood flow. Several mediators of astrocyte-neuron signaling have been identified, including glutamate, ATP, adenosine, and gap-junction signaling. Astrocytes contribute to brain communication pathways by regulating synaptic transmission (Newman, 2003), neuronal firing thresholds, and plasticity (Nedergaard et al., 2003).
A vasodilating effect of astrocytes in the control of cerebral microcirculation mediated by P450 2C11-catalyzed conversion of arachidonic acid to epoxyeicosatrienoic acids was observed, which amplified K+-evoked current in the vascular smooth muscle cells of large cerebral arteries (Alkayed et al., 1996). Neuronal stimulation up to ~300 nM by Ca2+ elevated astrocytic endfeet Ca2+ to ~400 nM and subsequently caused vasoconstriction in post-SAH brain slices; however it dilated parenchymal arterioles in control brain slices (Koide et al., 2012). It is possible that components of the blood such as oxyhemoglobin or their breakdown products act directly to alter Ca2+ signaling in astrocytic endfeet (Asano, 1999; Nishizawa and Laher, 2005). The cytosolic concentration of Ca2+ in human astrocytes was elevated by blood CSF via activation of ATP-sensitive P2 receptors and subsequent inositol 1,4,5-trisphosphate-dependent Ca2+ release from endoplasmic reticulum (Kasseckert et al., 2013). In cortical brain slices from rats, the responses of parenchymal arterioles to increases of astrocytic endfeet Ca2+ evoked by electrical field stimulation were of three distinct types: (I) 40% exhibited a sustained vasoconstriction, (II) 30% exhibited a transient vasoconstriction (diameter restored within 1 min after stimulation), and (III) 20% responded with a biphasic response (brief vasodilation followed by vasoconstriction) (Koide et al., 2013a). BK channels on astrocytic endfeet play an important role in transducing altered astrocytic Ca2+ signaling pathway into alternations in the diameter of arteries and blood flow within the brain, perhaps leading to the development of neurological deficits following SAH (Koide et al., 2012). The high-amplitude spontaneous events after SAH promote BK channel activity to elevate basal [K+]o in the perivascular space between astrocyte endfeet and arteriolar muscle cells. This inversion of neurovascular coupling may play a particularly important role in the development of DBI following SAH.
4.3 Vascular Cells in the Model
The brain cortex is at risk if blood flow through parenchymal arterioles is restricted. Matching the blood flow to regional brain function and metabolism involves the coordinated activity of neurons, astrocytes, and parenchymal arterioles (Filosa et al., 2004). A persistent interruption of blood flow results in subsequent swelling of perivascular astrocytes, neuronal cells, and capillary endothelium. However, perhaps owing to the contemporary idea that arterial cell types are only minor players in the pathophysiology of stroke, the physiological importance of vascular smooth muscle cells in the traditional neurovascular unit has not been emphasized (Zhang et al., 2012). Vascular smooth muscle cells were included in the original model of the neuron–astrocyte–vasculature triad in 1996, but were replaced by capillary endothelial cells in the revised definition published in 2002. Pathological circumstances after SAH modulate the magnitude and timing of the vasomotor components. Under such conditions the vascular response becomes predominantly vasoconstrictive, that is, inverted (Sonn and Mayevsky, 2000; Sukhotinsky et al., 2010). The smooth muscle cells in blood vessels are highly dynamic, and their phenotype is influenced by multiple factors in the perivascular environment. Acute applications of the blood component oxyhemoglobin, suppress voltage-gated K+ channels in parenchymal arteriolar myocytes through heparin-binding epidermal growth factor (EGF)-like growth factor-mediated activation of EGF receptors. Activation of the EGF/EGF receptor pathway contributes to the suppression of K(V) current and enhanced constriction of the parenchymal arterioles after SAH (Koide and Wellman, 2013). This acute oxyhemoglobin-induced K(V) channel suppression is mediated via a cell-signaling pathway involving activation of MMP-2 in SAH animals. SAH-induced depolarization of membrane potentials, which involves altered K+ homeostasis, leads to enhanced activity of voltage-dependent Ca2+ channels, increased cytosolic Ca2+ in smooth muscles, and the constriction of parenchymal arterioles (Wellman and Koide, 2013). Perivascular nerve fibers can promote smooth muscle growth and differentiation (Hamel, 2006). SAH significantly reduced the nerve fibers, which resulted in deleterious changes in the function and phenotype of cerebrovascular smooth muscle cells (Alabadi et al., 1994). More importantly, in all arteries, vascular smooth muscle cells exhibit multiple coexisting functions and phenotypic characteristics, including migration, proliferation, secretion of extracellular matrix proteins, and contraction (Alexander and Owens, 2012).
In addition, endothelial cells release numerous factors that can influence the proliferation and differentiation of adjacent vascular smooth muscle cells. Endothelial cells can mediate both vasodilatation and vasoconstriction by releasing vasoactive substances including both vasodilator (acetylcholine, nitric oxide) and vasoconstrictor (endothelin) (Burnstock and Ralevic, 1994). Additionally, vascular function during cerebral injury can be modulated through active substances, including platelet-derived growth factors, vascular endothelial growth factors, brain-derived neurotrophic factors, nerve growth factors, angiopoietins, adenosine, and glutamate (Alexander and Owens, 2012).
5. Conclusions
Despite extensive research and vast efforts, patient outcomes following SAH remain poor. More recently, EBI has emerged as a new frontier and requires further investigation and consideration in devising therapeutic strategies for improving SAH outcomes. In addition, a better understanding of animal models, evaluation methods, and the guidelines for translational studies are essential for the successful translation of basic science research into human trials of SAH. The vasculo-neuronal-glia triad model should provide a critical platform in identifying potential therapies to ameliorate SAH injury. Success may come with the development of a monotherapy targeting the contents of the model.
Highlights.
A better understanding of the role of vasospasm after SAH.
Summary of recent data on the neurobiological role of EBI and DBI after SAH.
Description of the possible role of the vasculo-neuronal-glia triad model in SAH.
Acknowledgments
Our research and the writing of this article were supported by grants from by NIH NS053407 to JH Zhang and by National Natural Science Foundation of China (No.81171096 and No. 81371433) to JM Zhang.
Abbreviations
- SAH
subarachnoid hemorrhage
- DIND
delayed ischemic neurological deficit
- DCI
delayed cerebral ischemia
- TCD
transcranial Doppler
- EBI
early brain injury
- DBI
delayed brain injury
- ICP
intracranial pressure
- CPP
cerebral perfusion pressure
- CBF
cerebral blood flow
- CSD
cortical spreading depolarization
- BBB
blood-brain barrier
- NMDA
N-methyl D-aspartate
- ANP
atrial natriuretic peptide
- BNP
brain natriuretic peptide
- CSWS
cerebral salt-wasting syndrome
- SIADH
syndrome of inappropriate secretion of anti-diuretic hormone
- VGCC
voltage-gated calcium channel
- VAP-1
vascular adhesion protein-1
- MAPKs
Mitogen-activated protein kinases
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun Nterminal kinase
- STAT3
signal transducer and activator of transcription
- Kv
voltage-gated K+ channel
- NO
nitric oxide
- NOS
nitric oxide synthase
- MEK
mitogen activated protein kinase kinase
- SOD
Superoxide dismutase
- IL-1α
interleukin-1α
- TNF-α
tumor necrosis factor-α
- MCP-1
chemotactic protein-1
- CCL5
chemokine (C-C motif) ligand 5
- CXCL1
chemokine (C-X-C motif) ligand 1
- CAMs
Cell adhesion molecules
- ICAM-1
intercellular adhesion molecule-1
- VCAM-1
vascular cell adhesion molecule-1
- CHOP
C/EBP homologous protein
- EGF
epidermal growth factor
Footnotes
Conflicts of Interest: None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adachi M, Feigin I. Cerebral oedema and the water content of normal white matter. J Neurol Neurosurg Psychiatry. 1966;29:446–450. doi: 10.1136/jnnp.29.5.446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addae JI, Ali N, Stone TW. Effects of AMPA and clomethiazole on spreading depression cycles in the rat neocortex in vivo. Eur J Pharmacol. 2011;653:41–46. doi: 10.1016/j.ejphar.2010.11.021. [DOI] [PubMed] [Google Scholar]
- Ahmad I, Imaizumi S, Shimizu H, Kaminuma T, Ochiai N, Tajima M, Yoshimoto T. Development of calcitonin gene-related peptide slow-release tablet implanted in CSF space for prevention of cerebral vasospasm after experimental subarachnoid haemorrhage. Acta Neurochir (Wien) 1996;138:1230–1240. doi: 10.1007/BF01809753. [DOI] [PubMed] [Google Scholar]
- Aihara Y, Jahromi BS, Yassari R, Nikitina E, Agbaje-Williams M, Macdonald RL. Molecular profile of vascular ion channels after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:75–83. doi: 10.1097/01.WCB.0000095803.98378.D8. [DOI] [PubMed] [Google Scholar]
- Aihara Y, Kasuya H, Onda H, Hori T, Takeda J. Quantitative analysis of gene expressions related to inflammation in canine spastic artery after subarachnoid hemorrhage. Stroke. 2001;32:212–217. doi: 10.1161/01.str.32.1.212. [DOI] [PubMed] [Google Scholar]
- Airas L, Lindsberg PJ, Karjalainen-Lindsberg ML, Mononen I, Kotisaari K, Smith DJ, Jalkanen S. Vascular adhesion protein-1 in human ischaemic stroke. Neuropathol Appl Neurobiol. 2008;34:394–402. doi: 10.1111/j.1365-2990.2007.00911.x. [DOI] [PubMed] [Google Scholar]
- Alabadi JA, Torregrosa G, Salom JB, Miranda FJ, Barbera MD, Mayordomo F, Alborch E. Changes in the adrenergic mechanisms of cerebral arteries after subarachnoid hemorrhage in goats. Neurosurgery. 1994;34:1027–1033. doi: 10.1227/00006123-199406000-00011. discussion 1033–1024. [DOI] [PubMed] [Google Scholar]
- Alexander MR, Owens GK. Epigenetic control of smooth muscle cell differentiation and phenotypic switching in vascular development and disease. Annu Rev Physiol. 2012;74:13–40. doi: 10.1146/annurev-physiol-012110-142315. [DOI] [PubMed] [Google Scholar]
- Alkayed NJ, Narayanan J, Gebremedhin D, Medhora M, Roman RJ, Harder DR. Molecular characterization of an arachidonic acid epoxygenase in rat brain astrocytes. Stroke. 1996;27:971–979. doi: 10.1161/01.str.27.5.971. [DOI] [PubMed] [Google Scholar]
- Altay O, Hasegawa Y, Sherchan P, Suzuki H, Khatibi NH, Tang J, Zhang JH. Isoflurane delays the development of early brain injury after subarachnoid hemorrhage through sphingosine-related pathway activation in mice. Crit Care Med. 2012a;40:1908–1913. doi: 10.1097/CCM.0b013e3182474bc1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altay O, Suzuki H, Hasegawa Y, Caner B, Krafft PR, Fujii M, Tang J, Zhang JH. Isoflurane attenuates blood-brain barrier disruption in ipsilateral hemisphere after subarachnoid hemorrhage in mice. Stroke. 2012b;43:2513–2516. doi: 10.1161/STROKEAHA.112.661728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson CM, Nedergaard M. Astrocyte-mediated control of cerebral microcirculation. Trends Neurosci. 2003;26:340–344. doi: 10.1016/S0166-2236(03)00141-3. author reply 344–345. [DOI] [PubMed] [Google Scholar]
- Aries MJ, Elting JW, De Keyser J, Kremer BP, Vroomen PC. Cerebral autoregulation in stroke: a review of transcranial Doppler studies. Stroke. 2010;41:2697–2704. doi: 10.1161/STROKEAHA.110.594168. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Asaeda M, Sakamoto M, Kurosaki M, Tabuchi S, Kamitani H, Yokota M, Watanabe T. A non-enzymatic derived arachidonyl peroxide, 8-iso-prostaglandin F2 alpha, in cerebrospinal fluid of patients with aneurysmal subarachnoid hemorrhage participates in the pathogenesis of delayed cerebral vasospasm. Neurosci Lett. 2005;373:222–225. doi: 10.1016/j.neulet.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Asano T. Oxyhemoglobin as the principal cause of cerebral vasospasm: a holistic view of its actions. Crit Rev Neurosurg. 1999;9:303–318. doi: 10.1007/s003290050147. [DOI] [PubMed] [Google Scholar]
- Asano T, Matsui T. Antioxidant therapy against cerebral vasospasm following aneurysmal subarachnoid hemorrhage. Cell Mol Neurobiol. 1999;19:31–44. doi: 10.1023/A:1006908422937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asano T, Sano K. Pathogenetic role of no-reflow phenomenon in experimental subarachnoid hemorrhage in dogs. J Neurosurg. 1977;46:454–466. doi: 10.3171/jns.1977.46.4.0454. [DOI] [PubMed] [Google Scholar]
- Attwell D, Buchan AM, Charpak S, Lauritzen M, Macvicar BA, Newman EA. Glial and neuronal control of brain blood flow. Nature. 2010;468:232–243. doi: 10.1038/nature09613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Audibert G, Steinmann G, de Talance N, Laurens MH, Dao P, Baumann A, Longrois D, Mertes PM. Endocrine response after severe subarachnoid hemorrhage related to sodium and blood volume regulation. Anesth Analg. 2009;108:1922–1928. doi: 10.1213/ane.0b013e31819a85ae. [DOI] [PubMed] [Google Scholar]
- Ayer R, Zhang J. Connecting the early brain injury of aneurysmal subarachnoid hemorrhage to clinical practice. Turk Neurosurg. 2010;20:159–166. doi: 10.5137/1019-5149.JTN.2714-09.0. [DOI] [PubMed] [Google Scholar]
- Ayer RE, Zhang JH. Oxidative stress in subarachnoid haemorrhage: significance in acute brain injury and vasospasm. Acta Neurochir Suppl. 2008;104:33–41. doi: 10.1007/978-3-211-75718-5_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badjatia N, Seres D, Carpenter A, Schmidt JM, Lee K, Mayer SA, Claassen J, Connolly ES, Elkind MS. Free Fatty acids and delayed cerebral ischemia after subarachnoid hemorrhage. Stroke. 2012;43:691–696. doi: 10.1161/STROKEAHA.111.636035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker CJ, Prestigiacomo CJ, Solomon RA. Short-term perioperative anticonvulsant prophylaxis for the surgical treatment of low-risk patients with intracranial aneurysms. Neurosurgery. 1995;37:863–870. doi: 10.1227/00006123-199511000-00003. discussion 870–861. [DOI] [PubMed] [Google Scholar]
- Barcelos GK, Tholance Y, Grousson S, Renaud B, Perret-Liaudet A, Dailler F, Zimmer L. Outcome of poor-grade subarachnoid hemorrhage as determined by biomarkers of glucose cerebral metabolism. Neurocrit Care. 2013;18:234–244. doi: 10.1007/s12028-012-9810-1. [DOI] [PubMed] [Google Scholar]
- Barges-Coll J, Perez-Neri I, Avendano J, Mendez-Rosito D, Gomez-Amador JL, Rios C. Plasma taurine as a predictor of poor outcome in patients with mild neurological deficits after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2013 doi: 10.3171/2013.4.JNS121558. [DOI] [PubMed] [Google Scholar]
- Barry C, Turner RJ, Corrigan F, Vink R. New therapeutic approaches to subarachnoid hemorrhage. Expert Opin Investig Drugs. 2012;21:845–859. doi: 10.1517/13543784.2012.683113. [DOI] [PubMed] [Google Scholar]
- Bavbek M, Polin R, Kwan AL, Arthur AS, Kassell NF, Lee KS. Monoclonal antibodies against ICAM-1 and CD18 attenuate cerebral vasospasm after experimental subarachnoid hemorrhage in rabbits. Stroke. 1998;29:1930–1935. doi: 10.1161/01.str.29.9.1930. discussion 1935–1936. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Connolly ES, Jr, Batjer HH, Dacey RG, Dion JE, Diringer MN, Duldner JE, Jr, Harbaugh RE, Patel AB, Rosenwasser RH. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a statement for healthcare professionals from a special writing group of the Stroke Council, American Heart Association. Stroke. 2009;40:994–1025. doi: 10.1161/STROKEAHA.108.191395. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Germano IM, Guarino L. Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke. 1995;26:1086–1091. doi: 10.1161/01.str.26.6.1086. discussion 1091–1082. [DOI] [PubMed] [Google Scholar]
- Bederson JB, Levy AL, Ding WH, Kahn R, DiPerna CA, Jenkins AL, 3rd, Vallabhajosyula P. Acute vasoconstriction after subarachnoid hemorrhage. Neurosurgery. 1998;42:352–360. doi: 10.1097/00006123-199802000-00091. discussion 360–352. [DOI] [PubMed] [Google Scholar]
- Beeftink MM, Ruigrok YM, Rinkel GJ, van den Bergh WM. Relation of serum TNF-alpha and TNF-alpha genotype with delayed cerebral ischemia and outcome in subarachnoid hemorrhage. Neurocrit Care. 2011;15:405–409. doi: 10.1007/s12028-011-9556-1. [DOI] [PubMed] [Google Scholar]
- Beg SA, Hansen-Schwartz JA, Vikman PJ, Xu CB, Edvinsson LI. ERK1/2 inhibition attenuates cerebral blood flow reduction and abolishes ET(B) and 5-HT(1B) receptor upregulation after subarachnoid hemorrhage in rat. J Cereb Blood Flow Metab. 2006;26:846–856. doi: 10.1038/sj.jcbfm.9600236. [DOI] [PubMed] [Google Scholar]
- Beg SS, Hansen-Schwartz JA, Vikman PJ, Xu CB, Edvinsson LI. Protein kinase C inhibition prevents upregulation of vascular ET(B) and 5-HT(1B) receptors and reverses cerebral blood flow reduction after subarachnoid haemorrhage in rats. J Cereb Blood Flow Metab. 2007;27:21–32. doi: 10.1038/sj.jcbfm.9600313. [DOI] [PubMed] [Google Scholar]
- Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18:467–486. doi: 10.1177/1073858412438697. [DOI] [PubMed] [Google Scholar]
- Benvenga S. What is the pathogenesis of hyponatremia after subarachnoid hemorrhage? Nat Clin Pract Endocrinol Metab. 2006;2:608–609. doi: 10.1038/ncpendmet0302. [DOI] [PubMed] [Google Scholar]
- Berendes E, Walter M, Cullen P, Prien T, Van Aken H, Horsthemke J, Schulte M, von Wild K, Scherer R. Secretion of brain natriuretic peptide in patients with aneurysmal subarachnoid haemorrhage. Lancet. 1997;349:245–249. doi: 10.1016/s0140-6736(96)08093-2. [DOI] [PubMed] [Google Scholar]
- Berthon N, Laurant P, Fellmann D, Berthelot A. Effect of magnesium on mRNA expression and production of endothelin-1 in DOCA-salt hypertensive rats. J Cardiovasc Pharmacol. 2003;42:24–31. doi: 10.1097/00005344-200307000-00004. [DOI] [PubMed] [Google Scholar]
- Bian L, Liu L, Wang C, Hussain M, Yuan Y, Liu G, Wang W, Zhao X. Hyperglycemia within day 14 of aneurysmal subarachnoid hemorrhage predicts 1-year mortality. Clin Neurol Neurosurg. 2012 doi: 10.1016/j.clineuro.2012.09.026. [DOI] [PubMed] [Google Scholar]
- Boulle F, Kenis G, Cazorla M, Hamon M, Steinbusch HW, Lanfumey L, van den Hove DL. TrkB inhibition as a therapeutic target for CNS-related disorders. Prog Neurobiol. 2012;98:197–206. doi: 10.1016/j.pneurobio.2012.06.002. [DOI] [PubMed] [Google Scholar]
- Boyko M, Azab AN, Kuts R, Gruenbaum BF, Gruenbaum SE, Melamed I, Brotfain E, Shapira Y, Cesnulis E, Zlotnik A. The neuro-behavioral profile in rats after subarachnoid hemorrhage. Brain Res. 2013;1491:109–116. doi: 10.1016/j.brainres.2012.10.061. [DOI] [PubMed] [Google Scholar]
- Boyko M, Melamed I, Gruenbaum BF, Gruenbaum SE, Ohayon S, Leibowitz A, Brotfain E, Shapira Y, Zlotnik A. The effect of blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome in a rat model of subarachnoid hemorrhage. Neurotherapeutics. 2012;9:649–657. doi: 10.1007/s13311-012-0129-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinjikji W, Rabinstein AA, Lanzino G, Cloft HJ. Racial and ethnic disparities in the treatment of unruptured intracranial aneurysms: a study of the Nationwide Inpatient Sample 2001–2009. Stroke. 2012;43:3200–3206. doi: 10.1161/STROKEAHA.112.671214. [DOI] [PubMed] [Google Scholar]
- Brinker T, Seifert V, Stolke D. Acute changes in the dynamics of the cerebrospinal fluid system during experimental subarachnoid hemorrhage. Neurosurgery. 1990;27:369–372. doi: 10.1097/00006123-199009000-00005. [DOI] [PubMed] [Google Scholar]
- Broadwell RD, Baker-Cairns BJ, Friden PM, Oliver C, Villegas JC. Transcytosis of protein through the mammalian cerebral epithelium and endothelium. III. Receptor-mediated transcytosis through the blood-brain barrier of blood-borne transferrin and antibody against the transferrin receptor. Exp Neurol. 1996;142:47–65. doi: 10.1006/exnr.1996.0178. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott T, Tomsick T, Huster G, Miller R. The risk of subarachnoid and intracerebral hemorrhages in blacks as compared with whites. N Engl J Med. 1992;326:733–736. doi: 10.1056/NEJM199203123261103. [DOI] [PubMed] [Google Scholar]
- Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A. Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke. 1994;25:1342–1347. doi: 10.1161/01.str.25.7.1342. [DOI] [PubMed] [Google Scholar]
- Brown RJ, Kumar A, Dhar R, Sampson TR, Diringer MN. The relationship between delayed infarcts and angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2013;72:702–708. doi: 10.1227/NEU.0b013e318285c3db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruder N, Ichai C, Gelb AW. Hyponatremia and subarachnoid hemorrhage: will that be one pinch or two of salt? Anesth Analg. 2009;108:1734–1735. doi: 10.1213/ane.0b013e3181a32872. [DOI] [PubMed] [Google Scholar]
- Budohoski KP, Czosnyka M, Kirkpatrick PJ, Smielewski P, Steiner LA, Pickard JD. Clinical relevance of cerebral autoregulation following subarachnoid haemorrhage. Nat Rev Neurol. 2013;9:152–163. doi: 10.1038/nrneurol.2013.11. [DOI] [PubMed] [Google Scholar]
- Budohoski KP, Czosnyka M, Smielewski P, Kasprowicz M, Helmy A, Bulters D, Pickard JD, Kirkpatrick PJ. Impairment of cerebral autoregulation predicts delayed cerebral ischemia after subarachnoid hemorrhage: a prospective observational study. Stroke. 2012;43:3230–3237. doi: 10.1161/STROKEAHA.112.669788. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Krugel U, Abbracchio MP, Illes P. Purinergic signalling: from normal behaviour to pathological brain function. Prog Neurobiol. 2011;95:229–274. doi: 10.1016/j.pneurobio.2011.08.006. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ralevic V. New insights into the local regulation of blood flow by perivascular nerves and endothelium. Br J Plast Surg. 1994;47:527–543. doi: 10.1016/0007-1226(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Butzkueven H, Evans AH, Pitman A, Leopold C, Jolley DJ, Kaye AH, Kilpatrick CJ, Davis SM. Onset seizures independently predict poor outcome after subarachnoid hemorrhage. Neurology. 2000;55:1315–1320. doi: 10.1212/wnl.55.9.1315. [DOI] [PubMed] [Google Scholar]
- Cabral KP, Fraser GL, Duprey J, Gibbons BA, Hayes T, Florman JE, Seder DB. Prothrombin complex concentrates to reverse warfarin-induced coagulopathy in patients with intracranial bleeding. Clin Neurol Neurosurg. 2013;115:770–774. doi: 10.1016/j.clineuro.2012.07.006. [DOI] [PubMed] [Google Scholar]
- Cabral NL, Goncalves AR, Longo AL, Moro CH, Costa G, Amaral CH, Fonseca LA, Eluf-Neto J. Incidence of stroke subtypes, prognosis and prevalence of risk factors in Joinville, Brazil: a 2 year community based study. J Neurol Neurosurg Psychiatry. 2009;80:755–761. doi: 10.1136/jnnp.2009.172098. [DOI] [PubMed] [Google Scholar]
- Cahill J, Calvert JW, Marcantonio S, Zhang JH. p53 may play an orchestrating role in apoptotic cell death after experimental subarachnoid hemorrhage. Neurosurgery. 2007;60:531–545. doi: 10.1227/01.NEU.0000249287.99878.9B. discussion 545. [DOI] [PubMed] [Google Scholar]
- Cahill J, Calvert JW, Zhang JH. Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:1341–1353. doi: 10.1038/sj.jcbfm.9600283. [DOI] [PubMed] [Google Scholar]
- Cai J, Sun Y, Yuan F, Chen L, He C, Bao Y, Chen Z, Lou M, Xia W, Yang GY, Ling F. A novel intravital method to evaluate cerebral vasospasm in rat models of subarachnoid hemorrhage: a study with synchrotron radiation angiography. PLoS One. 2012;7:e33366. doi: 10.1371/journal.pone.0033366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals S, Makarova I, Lopez-Aguado L, Largo C, Ibarz JM, Herreras O. Longitudinal depolarization gradients along the somatodendritic axis of CA1 pyramidal cells: a novel feature of spreading depression. J Neurophysiol. 2005;94:943–951. doi: 10.1152/jn.01145.2004. [DOI] [PubMed] [Google Scholar]
- Carloni S, Buonocore G, Balduini W. Protective role of autophagy in neonatal hypoxia-ischemia induced brain injury. Neurobiol Dis. 2008;32:329–339. doi: 10.1016/j.nbd.2008.07.022. [DOI] [PubMed] [Google Scholar]
- Chaichana KL, Pradilla G, Huang J, Tamargo RJ. Role of inflammation (leukocyte-endothelial cell interactions) in vasospasm after subarachnoid hemorrhage. World Neurosurg. 2010;73:22–41. doi: 10.1016/j.surneu.2009.05.027. [DOI] [PubMed] [Google Scholar]
- Chang CZ, Lin CL, Kassel NF, Kwan AL, Howng SL. 6-Mercaptopurine attenuates adhesive molecules in experimental vasospasm. Acta Neurochir (Wien) 2010;152:861–867. doi: 10.1007/s00701-010-0602-0. [DOI] [PubMed] [Google Scholar]
- Chen D, Tang J, Khatibi NH, Zhu M, Li Y, Wang C, Jiang R, Tu L, Wang S. Treatment with Z-ligustilide, a component of Angelica sinensis, reduces brain injury after a subarachnoid hemorrhage in rats. J Pharmacol Exp Ther. 2011a;337:663–672. doi: 10.1124/jpet.110.177055. [DOI] [PubMed] [Google Scholar]
- Chen G, Fang Q, Zhang J, Zhou D, Wang Z. Role of the Nrf2-ARE pathway in early brain injury after experimental subarachnoid hemorrhage. J Neurosci Res. 2011b;89:515–523. doi: 10.1002/jnr.22577. [DOI] [PubMed] [Google Scholar]
- Chen G, Wu J, Sun C, Qi M, Hang C, Gong Y, Han X, Shi J. Potential role of JAK2 in cerebral vasospasm after experimental subarachnoid hemorrhage. Brain Res. 2008;1214:136–144. doi: 10.1016/j.brainres.2008.03.085. [DOI] [PubMed] [Google Scholar]
- Chen G, Zhang S, Shi J, Ai J, Hang C. Effects of recombinant human erythropoietin (rhEPO) on JAK2/STAT3 pathway and endothelial apoptosis in the rabbit basilar artery after subarachnoid hemorrhage. Cytokine. 2009;45:162–168. doi: 10.1016/j.cyto.2008.11.015. [DOI] [PubMed] [Google Scholar]
- Chen LC, Hsu C, Chiueh CC, Lee WS. Ferrous citrate up-regulates the NOS2 through nuclear translocation of NFkappaB induced by free radicals generation in mouse cerebral endothelial cells. PLoS One. 2012;7:e46239. doi: 10.1371/journal.pone.0046239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng G, Chunlei W, Pei W, Zhen L, Xiangzhen L. Simvastatin activates Akt/glycogen synthase kinase-3beta signal and inhibits caspase-3 activation after experimental subarachnoid hemorrhage. Vascul Pharmacol. 2010;52:77–83. doi: 10.1016/j.vph.2009.12.001. [DOI] [PubMed] [Google Scholar]
- Cheng G, Wei L, Zhi-Dan S, Shi-Guang Z, Xiang-Zhen L. Atorvastatin ameliorates cerebral vasospasm and early brain injury after subarachnoid hemorrhage and inhibits caspase-dependent apoptosis pathway. BMC Neurosci. 2009;10:7. doi: 10.1186/1471-2202-10-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HG, Shin HK, Shin YW, Lee JH, Hong KW. Role of nitric oxide in the CBF autoregulation during acute stage after subarachnoid haemorrhage in rat pial artery. Fundam Clin Pharmacol. 2003;17:563–573. doi: 10.1046/j.1472-8206.2003.00185.x. [DOI] [PubMed] [Google Scholar]
- Choi KS, Chun HJ, Yi HJ, Ko Y, Kim YS, Kim JM. Seizures and Epilepsy following Aneurysmal Subarachnoid Hemorrhage : Incidence and Risk Factors. J Korean Neurosurg Soc. 2009;46:93–98. doi: 10.3340/jkns.2009.46.2.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou SH, Feske SK, Atherton J, Konigsberg RG, De Jager PL, Du R, Ogilvy CS, Lo EH, Ning M. Early elevation of serum tumor necrosis factor-alpha is associated with poor outcome in subarachnoid hemorrhage. J Investig Med. 2012;60:1054–1058. doi: 10.231/JIM.0b013e3182686932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu SH, Feng DF, Ma YB, Zhang H, Zhu ZA, Li ZQ, Zhang ZH. Expression of HGF and VEGF in the cerebral tissue of adult rats with chronic hydrocephalus after subarachnoid hemorrhage. Mol Med Rep. 2011;4:785–791. doi: 10.3892/mmr.2011.500. [DOI] [PubMed] [Google Scholar]
- Claassen J, Carhuapoma JR, Kreiter KT, Du EY, Connolly ES, Mayer SA. Global cerebral edema after subarachnoid hemorrhage: frequency, predictors, and impact on outcome. Stroke. 2002;33:1225–1232. doi: 10.1161/01.str.0000015624.29071.1f. [DOI] [PubMed] [Google Scholar]
- Clark JF, Harm A, Saffire A, Biehle SJ, Lu A, Pyne-Geithman GJ. Bilirubin oxidation products seen post subarachnoid hemorrhage have greater effects on aged rat brain compared to young. Acta Neurochir Suppl. 2011;110:157–162. doi: 10.1007/978-3-7091-0353-1_27. [DOI] [PubMed] [Google Scholar]
- Clarke E. Apoplexy in the Hippocratic Writings. Bull Hist Med. 1963;37:301–314. [PubMed] [Google Scholar]
- Cohen Z, Bonvento G, Lacombe P, Hamel E. Serotonin in the regulation of brain microcirculation. Prog Neurobiol. 1996;50:335–362. doi: 10.1016/s0301-0082(96)00033-0. [DOI] [PubMed] [Google Scholar]
- Connolly ES, Jr, Rabinstein AA, Carhuapoma JR, Derdeyn CP, Dion J, Higashida RT, Hoh BL, Kirkness CJ, Naidech AM, Ogilvy CS, Patel AB, Thompson BG, Vespa P. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/american Stroke Association. Stroke. 2012;43:1711–1737. doi: 10.1161/STR.0b013e3182587839. [DOI] [PubMed] [Google Scholar]
- Cook DJ, Kan S, Ai J, Kasuya H, Macdonald RL. Cisternal sustained release dihydropyridines for subarachnoid hemorrhage. Curr Neurovasc Res. 2012;9:139–148. doi: 10.2174/156720212800410894. [DOI] [PubMed] [Google Scholar]
- Crago EA, Thampatty BP, Sherwood PR, Kuo CW, Bender C, Balzer J, Horowitz M, Poloyac SM. Cerebrospinal fluid 20-HETE is associated with delayed cerebral ischemia and poor outcomes after aneurysmal subarachnoid hemorrhage. Stroke. 2011;42:1872–1877. doi: 10.1161/STROKEAHA.110.605816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crompton MR. The Pathogenesis of Cerebral Infarction Following the Rupture of Cerebral Berry Aneurysms. Brain. 1964;87:491–510. doi: 10.1093/brain/87.3.491. [DOI] [PubMed] [Google Scholar]
- Crowley RW, Medel R, Kassell NF, Dumont AS. New insights into the causes and therapy of cerebral vasospasm following subarachnoid hemorrhage. Drug Discov Today. 2008;13:254–260. doi: 10.1016/j.drudis.2007.11.010. [DOI] [PubMed] [Google Scholar]
- Cuvinciuc V, Viguier A, Calviere L, Raposo N, Larrue V, Cognard C, Bonneville F. Isolated acute nontraumatic cortical subarachnoid hemorrhage. AJNR Am J Neuroradiol. 2010;31:1355–1362. doi: 10.3174/ajnr.A1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czosnyka M, Steiner L, Balestreri M, Schmidt E, Smielewski P, Hutchinson PJ, Pickard JD. Concept of “true ICP” in monitoring and prognostication in head trauma. Acta Neurochir Suppl. 2005;95:341–344. doi: 10.1007/3-211-32318-x_70. [DOI] [PubMed] [Google Scholar]
- Dahl G, Keane RW. Pannexin: from discovery to bedside in 11+/−4 years? Brain Res. 2012;1487:150–159. doi: 10.1016/j.brainres.2012.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dankbaar JW, Rijsdijk M, van der Schaaf IC, Velthuis BK, Wermer MJ, Rinkel GJ. Relationship between vasospasm, cerebral perfusion, and delayed cerebral ischemia after aneurysmal subarachnoid hemorrhage. Neuroradiology. 2009;51:813–819. doi: 10.1007/s00234-009-0575-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira JG, Beck J, Setzer M, Gerlach R, Vatter H, Seifert V, Raabe A. Risk of shunt-dependent hydrocephalus after occlusion of ruptured intracranial aneurysms by surgical clipping or endovascular coiling: a single-institution series and meta-analysis. Neurosurgery. 2007;61:924–933. doi: 10.1227/01.neu.0000303188.72425.24. discussion 933–924. [DOI] [PubMed] [Google Scholar]
- del Zoppo GJ. Aging and the neurovascular unit. Ann N Y Acad Sci. 2012;1268:127–133. doi: 10.1111/j.1749-6632.2012.06686.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deroide N, Li X, Lerouet D, Van Vre E, Baker L, Harrison J, Poittevin M, Masters L, Nih L, Margaill I, Iwakura Y, Ryffel B, Pocard M, Tedgui A, Kubis N, Mallat Z. MFGE8 inhibits inflammasome-induced IL-1beta production and limits postischemic cerebral injury. J Clin Invest. 2013;123:1176–1181. doi: 10.1172/JCI65167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dijkhuizen RM, Beekwilder JP, van der Worp HB, Berkelbach van der Sprenkel JW, Tulleken KA, Nicolay K. Correlation between tissue depolarizations and damage in focal ischemic rat brain. Brain Res. 1999;840:194–205. doi: 10.1016/s0006-8993(99)01769-2. [DOI] [PubMed] [Google Scholar]
- Doczi T, Joo F, Adam G, Bozoky B, Szerdahelyi P. Blood-brain barrier damage during the acute stage of subarachnoid hemorrhage, as exemplified by a new animal model. Neurosurgery. 1986a;18:733–739. doi: 10.1227/00006123-198606000-00010. [DOI] [PubMed] [Google Scholar]
- Doczi T, Joo F, Sonkodi S, Adam G. Increased vulnerability of the blood-brain barrier to experimental subarachnoid hemorrhage in spontaneously hypertensive rats. Stroke. 1986b;17:498–501. doi: 10.1161/01.str.17.3.498. [DOI] [PubMed] [Google Scholar]
- Dorai Z, Hynan LS, Kopitnik TA, Samson D. Factors related to hydrocephalus after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2003;52:763–769. doi: 10.1227/01.neu.0000053222.74852.2d. discussion 769–771. [DOI] [PubMed] [Google Scholar]
- Dorhout Mees SM, Algra A, Vandertop WP, van Kooten F, Kuijsten HA, Boiten J, van Oostenbrugge RJ, Al-Shahi Salman R, Lavados PM, Rinkel GJ, van den Bergh WM. Magnesium for aneurysmal subarachnoid haemorrhage (MASH-2): a randomised placebo-controlled trial. Lancet. 2012;380:44–49. doi: 10.1016/S0140-6736(12)60724-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorhout Mees SM, Rinkel GJ, Feigin VL, Algra A, van den Bergh WM, Vermeulen M, van Gijn J. Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2007:CD000277. doi: 10.1002/14651858.CD000277.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch N. A clinical review of cerebral vasospasm and delayed ischaemia following aneurysm rupture. Acta Neurochir Suppl. 2011;110:5–6. doi: 10.1007/978-3-7091-0353-1_1. [DOI] [PubMed] [Google Scholar]
- Dorsch NW, King MT. A review of cerebral vasospasm in aneurysmal subarachnoid haemorrhage Part I: Incidence and effects. J Clin Neurosci. 1994;1:19–26. doi: 10.1016/0967-5868(94)90005-1. [DOI] [PubMed] [Google Scholar]
- Dreier JP. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17:439–447. doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Ebert N, Priller J, Megow D, Lindauer U, Klee R, Reuter U, Imai Y, Einhaupl KM, Victorov I, Dirnagl U. Products of hemolysis in the subarachnoid space inducing spreading ischemia in the cortex and focal necrosis in rats: a model for delayed ischemic neurological deficits after subarachnoid hemorrhage? J Neurosurg. 2000;93:658–666. doi: 10.3171/jns.2000.93.4.0658. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Korner K, Ebert N, Gorner A, Rubin I, Back T, Lindauer U, Wolf T, Villringer A, Einhaupl KM, Lauritzen M, Dirnagl U. Nitric oxide scavenging by hemoglobin or nitric oxide synthase inhibition by N-nitro-L-arginine induces cortical spreading ischemia when K+ is increased in the subarachnoid space. J Cereb Blood Flow Metab. 1998;18:978–990. doi: 10.1097/00004647-199809000-00007. [DOI] [PubMed] [Google Scholar]
- Dreier JP, Major S, Manning A, Woitzik J, Drenckhahn C, Steinbrink J, Tolias C, Oliveira-Ferreira AI, Fabricius M, Hartings JA, Vajkoczy P, Lauritzen M, Dirnagl U, Bohner G, Strong AJ. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132:1866–1881. doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreier JP, Woitzik J, Fabricius M, Bhatia R, Major S, Drenckhahn C, Lehmann TN, Sarrafzadeh A, Willumsen L, Hartings JA, Sakowitz OW, Seemann JH, Thieme A, Lauritzen M, Strong AJ. Delayed ischaemic neurological deficits after subarachnoid haemorrhage are associated with clusters of spreading depolarizations. Brain. 2006;129:3224–3237. doi: 10.1093/brain/awl297. [DOI] [PubMed] [Google Scholar]
- Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH. alpha7 nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke. 2011;42:3530–3536. doi: 10.1161/STROKEAHA.111.619965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker A, Riemenschneider PA. Arteriographic demonstration of spasm of the intracranial arteries, with special reference to saccular arterial aneurysms. J Neurosurg. 1951;8:660–667. doi: 10.3171/jns.1951.8.6.0660. [DOI] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Yu F, Chan PH. Akt/GSK3beta survival signaling is involved in acute brain injury after subarachnoid hemorrhage in rats. Stroke. 2006;37:2140–2146. doi: 10.1161/01.STR.0000229888.55078.72. [DOI] [PubMed] [Google Scholar]
- Endo H, Nito C, Kamada H, Yu F, Chan PH. Reduction in oxidative stress by superoxide dismutase overexpression attenuates acute brain injury after subarachnoid hemorrhage via activation of Akt/glycogen synthase kinase-3beta survival signaling. J Cereb Blood Flow Metab. 2007;27:975–982. doi: 10.1038/sj.jcbfm.9600399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erdi MF, Guney O, Kiyici A, Esen H. The effects of alpha lipoic acid on cerebral vasospasm following experimental subarachnoid hemorrhage in the rabbit. Turk Neurosurg. 2011;21:527–533. [PubMed] [Google Scholar]
- Espiner EA, Leikis R, Ferch RD, MacFarlane MR, Bonkowski JA, Frampton CM, Richards AM. The neuro-cardio-endocrine response to acute subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2002;56:629–635. doi: 10.1046/j.1365-2265.2002.01285.x. [DOI] [PubMed] [Google Scholar]
- Fassbender K, Hodapp B, Rossol S, Bertsch T, Schmeck J, Schutt S, Fritzinger M, Horn P, Vajkoczy P, Wendel-Wellner M, Ragoschke A, Kuehl S, Brunner J, Schurer L, Schmiedeck P, Hennerici M. Endothelin-1 in subarachnoid hemorrhage: An acute-phase reactant produced by cerebrospinal fluid leukocytes. Stroke. 2000;31:2971–2975. doi: 10.1161/01.str.31.12.2971. [DOI] [PubMed] [Google Scholar]
- Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009;8:355–369. doi: 10.1016/S1474-4422(09)70025-0. [DOI] [PubMed] [Google Scholar]
- Fergusen S, Macdonald RL. Predictors of cerebral infarction in patients with aneurysmal subarachnoid hemorrhage. Neurosurgery. 2007;60:658–667. doi: 10.1227/01.NEU.0000255396.23280.31. discussion 667. [DOI] [PubMed] [Google Scholar]
- Feuerstein GZ, Zaleska MM, Krams M, Wang X, Day M, Rutkowski JL, Finklestein SP, Pangalos MN, Poole M, Stiles GL, Ruffolo RR, Walsh FL. Missing steps in the STAIR case: a Translational Medicine perspective on the development of NXY-059 for treatment of acute ischemic stroke. J Cereb Blood Flow Metab. 2008;28:217–219. doi: 10.1038/sj.jcbfm.9600516. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Nelson MT. Calcium dynamics in cortical astrocytes and arterioles during neurovascular coupling. Circ Res. 2004;95:e73–81. doi: 10.1161/01.RES.0000148636.60732.2e. [DOI] [PubMed] [Google Scholar]
- Filosa JA, Bonev AD, Straub SV, Meredith AL, Wilkerson MK, Aldrich RW, Nelson MT. Local potassium signaling couples neuronal activity to vasodilation in the brain. Nat Neurosci. 2006;9:1397–1403. doi: 10.1038/nn1779. [DOI] [PubMed] [Google Scholar]
- Fischer M, Broessner G, Dietmann A, Beer R, Helbok R, Pfausler B, Chemelli A, Schmutzhard E, Lackner P. Angiopoietin-1 is associated with cerebral vasospasm and delayed cerebral ischemia in subarachnoid hemorrhage. BMC Neurol. 2011;11:59. doi: 10.1186/1471-2377-11-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher CM, Kistler JP, Davis JM. Relation of cerebral vasospasm to subarachnoid hemorrhage visualized by computerized tomographic scanning. Neurosurgery. 1980;6:1–9. doi: 10.1227/00006123-198001000-00001. [DOI] [PubMed] [Google Scholar]
- Fricker M, Vilalta A, Tolkovsky AM, Brown GC. Caspase inhibitors protect neurons by enabling selective necroptosis of inflamed microglia. J Biol Chem. 2013;288:9145–9152. doi: 10.1074/jbc.M112.427880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridriksson S, Hillman J, Landtblom AM, Boive J. Education of referring doctors about sudden onset headache in subarachnoid hemorrhage. A prospective study. Acta Neurol Scand. 2001;103:238–242. [PubMed] [Google Scholar]
- Friedrich B, Muller F, Feiler S, Scholler K, Plesnila N. Experimental subarachnoid hemorrhage causes early and long-lasting microarterial constriction and microthrombosis: an in-vivo microscopy study. J Cereb Blood Flow Metab. 2012a;32:447–455. doi: 10.1038/jcbfm.2011.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich V, Flores R, Muller A, Bi W, Peerschke EI, Sehba FA. Reduction of neutrophil activity decreases early microvascular injury after subarachnoid haemorrhage. J Neuroinflammation. 2011;8:103. doi: 10.1186/1742-2094-8-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich V, Flores R, Muller A, Sehba FA. Escape of intraluminal platelets into brain parenchyma after subarachnoid hemorrhage. Neuroscience. 2010;165:968–975. doi: 10.1016/j.neuroscience.2009.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedrich V, Flores R, Sehba FA. Cell death starts early after subarachnoid hemorrhage. Neurosci Lett. 2012b;512:6–11. doi: 10.1016/j.neulet.2012.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frykholm P, Andersson JL, Langstrom B, Persson L, Enblad P. Haemodynamic and metabolic disturbances in the acute stage of subarachnoid haemorrhage demonstrated by PET. Acta Neurol Scand. 2004;109:25–32. doi: 10.1034/j.1600-0404.2003.00174.x. [DOI] [PubMed] [Google Scholar]
- Fujimoto M, Suzuki H, Shiba M, Shimojo N, Imanaka-Yoshida K, Yoshida T, Kanamaru K, Matsushima S, Taki W. Tenascin-C induces prolonged constriction of cerebral arteries in rats. Neurobiol Dis. 2013;55:104–109. doi: 10.1016/j.nbd.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Fukui S, Katoh H, Tsuzuki N, Ishihara S, Otani N, Uozumi Y, Ooigawa H, Toyooka T, Ohnuki A, Miyazawa T, Nawashiro H, Shima K. Gender disparities in serum electrolytes levels after subarachnoid hemorrhage. J Clin Neurosci. 2004;11:606–609. doi: 10.1016/j.jocn.2003.02.016. [DOI] [PubMed] [Google Scholar]
- Fung C, De Marchis GM, Katan M, Seiler M, Arnold M, Gralla J, Raabe A, Beck J. Copeptin as a marker for severity and prognosis of aneurysmal subarachnoid hemorrhage. PLoS One. 2013;8:e53191. doi: 10.1371/journal.pone.0053191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaetani P, Cafe C, Rodriguez y Baena R, Tancioni F, Torri C, Tartara F, Marzatico F. Superoxide dismutase activity in cisternal cerebrospinal fluid after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 1997;139:1033–1037. doi: 10.1007/BF01411556. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Marzatico F, Rodriguez y Baena R, Pacchiarini L, Vigano T, Grignani G, Crivellari MT, Benzi G. Arachidonic acid metabolism and pathophysiologic aspects of subarachnoid hemorrhage in rats. Stroke. 1990;21:328–332. doi: 10.1161/01.str.21.2.328. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Pasqualin A, Rodriguez y Baena R, Borasio E, Marzatico F. Oxidative stress in the human brain after subarachnoid hemorrhage. J Neurosurg. 1998a;89:748–754. doi: 10.3171/jns.1998.89.5.0748. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Rodriguez y Baena R, Quaglini S, Bellazzi R, Cafe C, Torri C, Marzatico F. Experimental subarachnoid hemorrhage: events related to anti-oxidant enzymatic systems and eicosanoid peroxide enhancement. Neurochem Res. 1994;19:839–844. doi: 10.1007/BF00967453. [DOI] [PubMed] [Google Scholar]
- Gaetani P, Tartara F, Pignatti P, Tancioni F, Rodriguez y Baena R, De Benedetti F. Cisternal CSF levels of cytokines after subarachnoid hemorrhage. Neurol Res. 1998b;20:337–342. doi: 10.1080/01616412.1998.11740528. [DOI] [PubMed] [Google Scholar]
- Galeffi F, Sah R, Pond BB, George A, Schwartz-Bloom RD. Changes in intracellular chloride after oxygen-glucose deprivation of the adult hippocampal slice: effect of diazepam. J Neurosci. 2004;24:4478–4488. doi: 10.1523/JNEUROSCI.0755-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao TM, Pulsinelli WA, Xu ZC. Changes in membrane properties of CA1 pyramidal neurons after transient forebrain ischemia in vivo. Neuroscience. 1999;90:771–780. doi: 10.1016/s0306-4522(98)00493-x. [DOI] [PubMed] [Google Scholar]
- Germano A, Caffo M, Angileri FF, Arcadi F, Newcomb-Fernandez J, Caruso G, Meli F, Pineda JA, Lewis SB, Wang KK, Bramanti P, Costa C, Hayes RL. NMDA receptor antagonist felbamate reduces behavioral deficits and blood-brain barrier permeability changes after experimental subarachnoid hemorrhage in the rat. J Neurotrauma. 2007;24:732–744. doi: 10.1089/neu.2006.0181. [DOI] [PubMed] [Google Scholar]
- Germano A, Imperatore C, d’Avella D, Costa G, Tomasello F. Antivasospastic and brain-protective effects of a hydroxyl radical scavenger (AVS) after experimental subarachnoid hemorrhage. J Neurosurg. 1998;88:1075–1081. doi: 10.3171/jns.1998.88.6.1075. [DOI] [PubMed] [Google Scholar]
- Girouard H, Bonev AD, Hannah RM, Meredith A, Aldrich RW, Nelson MT. Astrocytic endfoot Ca2+ and BK channels determine both arteriolar dilation and constriction. Proc Natl Acad Sci U S A. 2010;107:3811–3816. doi: 10.1073/pnas.0914722107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girouard H, Iadecola C. Neurovascular coupling in the normal brain and in hypertension, stroke, and Alzheimer disease. J Appl Physiol. 2006;100:328–335. doi: 10.1152/japplphysiol.00966.2005. [DOI] [PubMed] [Google Scholar]
- Gomis P, Graftieaux JP, Sercombe R, Hettler D, Scherpereel B, Rousseaux P. Randomized, double-blind, placebo-controlled, pilot trial of high-dose methylprednisolone in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2010;112:681–688. doi: 10.3171/2009.4.JNS081377. [DOI] [PubMed] [Google Scholar]
- Gordon GR, Choi HB, Rungta RL, Ellis-Davies GC, MacVicar BA. Brain metabolism dictates the polarity of astrocyte control over arterioles. Nature. 2008;456:745–749. doi: 10.1038/nature07525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon GR, Mulligan SJ, MacVicar BA. Astrocyte control of the cerebrovasculature. Glia. 2007;55:1214–1221. doi: 10.1002/glia.20543. [DOI] [PubMed] [Google Scholar]
- Gorelick PB, Scuteri A, Black SE, Decarli C, Greenberg SM, Iadecola C, Launer LJ, Laurent S, Lopez OL, Nyenhuis D, Petersen RC, Schneider JA, Tzourio C, Arnett DK, Bennett DA, Chui HC, Higashida RT, Lindquist R, Nilsson PM, Roman GC, Sellke FW, Seshadri S. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke. 2011;42:2672–2713. doi: 10.1161/STR.0b013e3182299496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford NR, Torner J, Adams HP, Jr, Kassell NF. Factors associated with hydrocephalus after subarachnoid hemorrhage. A report of the Cooperative Aneurysm Study. Arch Neurol. 1989;46:744–752. doi: 10.1001/archneur.1989.00520430038014. [DOI] [PubMed] [Google Scholar]
- Greenhalgh AD, Brough D, Robinson EM, Girard S, Rothwell NJ, Allan SM. Interleukin-1 receptor antagonist is beneficial after subarachnoid haemorrhage in rat by blocking haem-driven inflammatory pathology. Dis Model Mech. 2012;5:823–833. doi: 10.1242/dmm.008557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grote E, Hassler W. The critical first minutes after subarachnoid hemorrhage. Neurosurgery. 1988;22:654–661. doi: 10.1227/00006123-198804000-00006. [DOI] [PubMed] [Google Scholar]
- Gules I, Satoh M, Nanda A, Zhang JH. Apoptosis, blood-brain barrier, and subarachnoid hemorrhage. Acta Neurochir Suppl. 2003;86:483–487. doi: 10.1007/978-3-7091-0651-8_99. [DOI] [PubMed] [Google Scholar]
- Guo S, Lo EH. Dysfunctional cell-cell signaling in the neurovascular unit as a paradigm for central nervous system disease. Stroke. 2009;40:S4–7. doi: 10.1161/STROKEAHA.108.534388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haley EC, Jr, Kassell NF, Apperson-Hansen C, Maile MH, Alves WM. A randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in North America. J Neurosurg. 1997;86:467–474. doi: 10.3171/jns.1997.86.3.0467. [DOI] [PubMed] [Google Scholar]
- Hamel E. Perivascular nerves and the regulation of cerebrovascular tone. J Appl Physiol. 2006;100:1059–1064. doi: 10.1152/japplphysiol.00954.2005. [DOI] [PubMed] [Google Scholar]
- Handa Y, Kubota T, Kaneko M, Tsuchida A, Kobayashi H, Kawano H. Expression of intercellular adhesion molecule 1 (ICAM-1) on the cerebral artery following subarachnoid haemorrhage in rats. Acta Neurochir (Wien) 1995;132:92–97. doi: 10.1007/BF01404854. [DOI] [PubMed] [Google Scholar]
- Hansen-Schwartz J, Ansar S, Edvinsson L. Cerebral vasoconstriction after subarachnoid hemorrhage--role of changes in vascular receptor phenotype. Front Biosci. 2008;13:2160–2164. doi: 10.2741/2831. [DOI] [PubMed] [Google Scholar]
- Hansen-Schwartz J, Hoel NL, Xu CB, Svendgaard NA, Edvinsson L. Subarachnoid hemorrhage-induced upregulation of the 5-HT1B receptor in cerebral arteries in rats. J Neurosurg. 2003a;99:115–120. doi: 10.3171/jns.2003.99.1.0115. [DOI] [PubMed] [Google Scholar]
- Hansen-Schwartz J, Hoel NL, Zhou M, Xu CB, Svendgaard NA, Edvinsson L. Subarachnoid hemorrhage enhances endothelin receptor expression and function in rat cerebral arteries. Neurosurgery. 2003b;52:1188–1194. 1194–1185. [PubMed] [Google Scholar]
- Hansen-Schwartz J, Vajkoczy P, Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm: looking beyond vasoconstriction. Trends Pharmacol Sci. 2007;28:252–256. doi: 10.1016/j.tips.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Hartings JA, Wilson JA, Look AC, Vagal A, Shutter LA, Dreier JP, Ringer A, Zuccarello M. Full-band electrocorticography of spreading depolarizations in patients with aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:131–141. doi: 10.1007/978-3-7091-1192-5_27. [DOI] [PubMed] [Google Scholar]
- Hasan D, Schonck RS, Avezaat CJ, Tanghe HL, van Gijn J, van der Lugt PJ. Epileptic seizures after subarachnoid hemorrhage. Ann Neurol. 1993;33:286–291. doi: 10.1002/ana.410330310. [DOI] [PubMed] [Google Scholar]
- Hasan D, Wijdicks EF, Vermeulen M. Hyponatremia is associated with cerebral ischemia in patients with aneurysmal subarachnoid hemorrhage. Ann Neurol. 1990;27:106–108. doi: 10.1002/ana.410270118. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Altay O, Zhang JH. Preservation of tropomyosin-related kinase B (TrkB) signaling by sodium orthovanadate attenuates early brain injury after subarachnoid hemorrhage in rats. Stroke. 2011a;42:477–483. doi: 10.1161/STROKEAHA.110.597344. [DOI] [PubMed] [Google Scholar]
- Hasegawa Y, Suzuki H, Sozen T, Altay O, Zhang JH. Apoptotic mechanisms for neuronal cells in early brain injury after subarachnoid hemorrhage. Acta Neurochir Suppl. 2011b;110:43–48. doi: 10.1007/978-3-7091-0353-1_8. [DOI] [PubMed] [Google Scholar]
- Hashimoto H, Iida J, Shin Y, Hironaka Y, Sakaki T. Spinal dural arteriovenous fistula with perimesencephalic subarachnoid haemorrhage. J Clin Neurosci. 2000;7:64–66. doi: 10.1054/jocn.1998.0145. [DOI] [PubMed] [Google Scholar]
- Hassan T, Nassar M, Elhadi SM, Radi WK. Effect of magnesium sulfate therapy on patients with aneurysmal subarachnoid hemorrhage using serum S100B protein as a prognostic marker. Neurosurg Rev. 2012;35:421–427. doi: 10.1007/s10143-011-0368-8. discussion 427. [DOI] [PubMed] [Google Scholar]
- He Z, Ostrowski RP, Sun X, Ma Q, Huang B, Zhan Y, Zhang JH. CHOP silencing reduces acute brain injury in the rat model of subarachnoid hemorrhage. Stroke. 2012a;43:484–490. doi: 10.1161/STROKEAHA.111.626432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Ostrowski RP, Sun X, Ma Q, Tang J, Zhang JH. Targeting C/EBP homologous protein with siRNA attenuates cerebral vasospasm after experimental subarachnoid hemorrhage. Exp Neurol. 2012b;238:218–224. doi: 10.1016/j.expneurol.2012.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz L, Peng L, Dienel GA. Energy metabolism in astrocytes: high rate of oxidative metabolism and spatiotemporal dependence on glycolysis/glycogenolysis. J Cereb Blood Flow Metab. 2007;27:219–249. doi: 10.1038/sj.jcbfm.9600343. [DOI] [PubMed] [Google Scholar]
- Herz DA, Baez S, Shulman K. Pial microcirculation in subarachnoid hemorrhage. Stroke. 1975;6:417–424. doi: 10.1161/01.str.6.4.417. [DOI] [PubMed] [Google Scholar]
- Heuer GG, Smith MJ, Elliott JP, Winn HR, LeRoux PD. Relationship between intracranial pressure and other clinical variables in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:408–416. doi: 10.3171/jns.2004.101.3.0408. [DOI] [PubMed] [Google Scholar]
- Hong Y, Guo S, Chen S, Sun C, Zhang J, Sun X. Beneficial effect of hydrogen-rich saline on cerebral vasospasm after experimental subarachnoid hemorrhage in rats. J Neurosci Res. 2012;90:1670–1680. doi: 10.1002/jnr.22739. [DOI] [PubMed] [Google Scholar]
- Hsieh YP, Lin CL, Shiue AL, Yin H, Morrow JD, Hsu JC, Hsieh TC, Wei HJ, Yen HC. Correlation of F4-neuroprostanes levels in cerebrospinal fluid with outcome of aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2009;47:814–824. doi: 10.1016/j.freeradbiomed.2009.06.026. [DOI] [PubMed] [Google Scholar]
- Huang J, van Gelder JM. The probability of sudden death from rupture of intracranial aneurysms: a meta-analysis. Neurosurgery. 2002;51:1101–1105. doi: 10.1097/00006123-200211000-00001. discussion 1105–1107. [DOI] [PubMed] [Google Scholar]
- Huang SJ, Chang L, Han YY, Lee YC, Tu YK. Efficacy and safety of hypertonic saline solutions in the treatment of severe head injury. Surg Neurol. 2006;65:539–546. doi: 10.1016/j.surneu.2005.11.019. discussion 546. [DOI] [PubMed] [Google Scholar]
- Hubschmann OR, Nathanson DC. The role of calcium and cellular membrane dysfunction in experimental trauma and subarachnoid hemorrhage. J Neurosurg. 1985;62:698–703. doi: 10.3171/jns.1985.62.5.0698. [DOI] [PubMed] [Google Scholar]
- Hughes PM, Allegrini PR, Rudin M, Perry VH, Mir AK, Wiessner C. Monocyte chemoattractant protein-1 deficiency is protective in a murine stroke model. J Cereb Blood Flow Metab. 2002;22:308–317. doi: 10.1097/00004647-200203000-00008. [DOI] [PubMed] [Google Scholar]
- Hutchison ER, Kawamoto EM, Taub DD, Lal A, Abdelmohsen K, Zhang Y, Wood WH, 3rd, Lehrmann E, Camandola S, Becker KG, Gorospe M, Mattson MP. Evidence for miR-181 involvement in neuroinflammatory responses of astrocytes. Glia. 2013 doi: 10.1002/glia.22483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim GM, Fallah A, Macdonald RL. Clinical, laboratory, and radiographic predictors of the occurrence of seizures following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2013 doi: 10.3171/2013.3.JNS122097. [DOI] [PubMed] [Google Scholar]
- Ibrahim GM, Macdonald RL. Electrocardiographic changes predict angiographic vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2012;43:2102–2107. doi: 10.1161/STROKEAHA.112.658153. [DOI] [PubMed] [Google Scholar]
- Ikonomidou C, Turski L. Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury? Lancet Neurol. 2002;1:383–386. doi: 10.1016/s1474-4422(02)00164-3. [DOI] [PubMed] [Google Scholar]
- Inagawa T. What are the actual incidence and mortality rates of subarachnoid hemorrhage? Surg Neurol. 1997;47:47–52. doi: 10.1016/s0090-3019(96)00370-9. discussion 52–43. [DOI] [PubMed] [Google Scholar]
- Inagawa T. Risk factors for the formation and rupture of intracranial saccular aneurysms in Shimane, Japan. World Neurosurg. 2010;73:155–164. doi: 10.1016/j.surneu.2009.03.007. discussion e123. [DOI] [PubMed] [Google Scholar]
- Ingall T, Asplund K, Mahonen M, Bonita R. A multinational comparison of subarachnoid hemorrhage epidemiology in the WHO MONICA stroke study. Stroke. 2000;31:1054–1061. doi: 10.1161/01.str.31.5.1054. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Morielli AD, Zvarova K, Tranmer BI, Penar PL, Wellman GC. Oxyhemoglobin-induced suppression of voltage-dependent K+ channels in cerebral arteries by enhanced tyrosine kinase activity. Circ Res. 2006;99:1252–1260. doi: 10.1161/01.RES.0000250821.32324.e1. [DOI] [PubMed] [Google Scholar]
- Ishiguro M, Murakami K, Link T, Zvarova K, Tranmer BI, Morielli AD, Wellman GC. Acute and chronic effects of oxyhemoglobin on voltage-dependent ion channels in cerebral arteries. Acta Neurochir Suppl. 2008;104:99–102. doi: 10.1007/978-3-211-75718-5_19. [DOI] [PubMed] [Google Scholar]
- Jahromi BS, Aihara Y, Ai J, Zhang ZD, Nikitina E, Macdonald RL. Voltage-gated K+ channel dysfunction in myocytes from a dog model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2008;28:797–811. doi: 10.1038/sj.jcbfm.9600577. [DOI] [PubMed] [Google Scholar]
- Jakobsen M. Role of initial brain ischemia in subarachnoid hemorrhage following aneurysm rupture. A pathophysiological survey. Acta Neurol Scand Suppl. 1992;141:1–33. [PubMed] [Google Scholar]
- Jha S, Ting JP. Inflammasome-associated nucleotide-binding domain, leucine-rich repeat proteins and inflammatory diseases. J Immunol. 2009;183:7623–7629. doi: 10.4049/jimmunol.0902425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing CH, Wang L, Liu PP, Wu C, Ruan D, Chen G. Autophagy activation is associated with neuroprotection against apoptosis via a mitochondrial pathway in a rat model of subarachnoid hemorrhage. Neuroscience. 2012;213:144–153. doi: 10.1016/j.neuroscience.2012.03.055. [DOI] [PubMed] [Google Scholar]
- Johnston SC, Selvin S, Gress DR. The burden, trends, and demographics of mortality from subarachnoid hemorrhage. Neurology. 1998;50:1413–1418. doi: 10.1212/wnl.50.5.1413. [DOI] [PubMed] [Google Scholar]
- Johshita H, Kassell NF, Sasaki T. Blood-brain barrier disturbance following subarachnoid hemorrhage in rabbits. Stroke. 1990;21:1051–1058. doi: 10.1161/01.str.21.7.1051. [DOI] [PubMed] [Google Scholar]
- Jung CS, Lange B, Zimmermann M, Seifert V. The CSF concentration of ADMA, but not of ET-1, is correlated with the occurrence and severity of cerebral vasospasm after subarachnoid hemorrhage. Neurosci Lett. 2012;524:20–24. doi: 10.1016/j.neulet.2012.06.076. [DOI] [PubMed] [Google Scholar]
- Jung CS, Lange B, Zimmermann M, Seifert V. CSF and Serum Biomarkers Focusing on Cerebral Vasospasm and Ischemia after Subarachnoid Hemorrhage. Stroke Res Treat. 2013a;2013:560305. doi: 10.1155/2013/560305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung CS, Lange B, Zimmermann M, Seifert V. Role of endogenous monomethylated L-arginine (L-NMMA) after subarachnoid Hemorrhage. Neurol Res. 2013b doi: 10.1179/1743132813Y.0000000194. [DOI] [PubMed] [Google Scholar]
- Jung S, Jones TD, Lugo JN, Jr, Sheerin AH, Miller JW, D’Ambrosio R, Anderson AE, Poolos NP. Progressive dendritic HCN channelopathy during epileptogenesis in the rat pilocarpine model of epilepsy. J Neurosci. 2007;27:13012–13021. doi: 10.1523/JNEUROSCI.3605-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juvela S, Kuhmonen J, Siironen J. C-reactive protein as predictor for poor outcome after aneurysmal subarachnoid haemorrhage. Acta Neurochir (Wien) 2012;154:397–404. doi: 10.1007/s00701-011-1243-7. [DOI] [PubMed] [Google Scholar]
- Kahn DE, Ferraro N, Benveniste RJ. 3 cases of primary intracranial hemorrhage associated with “Molly”, a purified form of 3,4-methylenedioxymethamphetamine (MDMA) J Neurol Sci. 2012;323:257–260. doi: 10.1016/j.jns.2012.08.031. [DOI] [PubMed] [Google Scholar]
- Kamezaki T, Yanaka K, Nagase S, Fujita K, Kato N, Nose T. Increased levels of lipid peroxides as predictive of symptomatic vasospasm and poor outcome after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2002;97:1302–1305. doi: 10.3171/jns.2002.97.6.1302. [DOI] [PubMed] [Google Scholar]
- Kamii H, Kato I, Kinouchi H, Chan PH, Epstein CJ, Akabane A, Okamoto H, Yoshimoto T. Amelioration of vasospasm after subarachnoid hemorrhage in transgenic mice overexpressing CuZn-superoxide dismutase. Stroke. 1999;30:867–871. doi: 10.1161/01.str.30.4.867. discussion 872. [DOI] [PubMed] [Google Scholar]
- Kamp MA, Dibue M, Schneider T, Steiger HJ, Hanggi D. Calcium and potassium channels in experimental subarachnoid hemorrhage and transient global ischemia. Stroke Res Treat. 2012;2012:382146. doi: 10.1155/2012/382146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanat A, Turkmenoglu O, Aydin MD, Yolas C, Aydin N, Gursan N, Tumkaya L, Demir R. Toward Changing of the Pathophysiologic Basis of Acute Hydrocephalus After Subarachnoid Hemorrhage: A Preliminary Experimental Study. World Neurosurg. 2012 doi: 10.1016/j.wneu.2012.12.020. [DOI] [PubMed] [Google Scholar]
- Kao L, Al-Lawati Z, Vavao J, Steinberg GK, Katznelson L. Prevalence and clinical demographics of cerebral salt wasting in patients with aneurysmal subarachnoid hemorrhage. Pituitary. 2009;12:347–351. doi: 10.1007/s11102-009-0188-9. [DOI] [PubMed] [Google Scholar]
- Kasseckert SA, Shahzad T, Miqdad M, Stein M, Abdallah Y, Scharbrodt W, Oertel M. The mechanisms of energy crisis in human astrocytes after subarachnoid hemorrhage. Neurosurgery. 2013;72:468–474. doi: 10.1227/NEU.0b013e31827d0de7. discussion 474. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Haley EC, Jr, Apperson-Hansen C, Alves WM. Randomized, double-blind, vehicle-controlled trial of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage: a cooperative study in Europe, Australia, and New Zealand. J Neurosurg. 1996;84:221–228. doi: 10.3171/jns.1996.84.2.0221. [DOI] [PubMed] [Google Scholar]
- Kassell NF, Torner JC, Haley EC, Jr, Jane JA, Adams HP, Kongable GL. The International Cooperative Study on the Timing of Aneurysm Surgery. Part 1: Overall management results. J Neurosurg. 1990;73:18–36. doi: 10.3171/jns.1990.73.1.0018. [DOI] [PubMed] [Google Scholar]
- Kaynar MY, Tanriverdi T, Kemerdere R, Atukeren P, Gumustas K. Cerebrospinal fluid superoxide dismutase and serum malondialdehyde levels in patients with aneurysmal subarachnoid hemorrhage: preliminary results. Neurol Res. 2005;27:562–567. doi: 10.1179/016164105X17288. [DOI] [PubMed] [Google Scholar]
- Keep RF, Andjelkovic AV, Stamatovic SM, Shakui P, Ennis SR. Ischemia-induced endothelial cell dysfunction. Acta Neurochir Suppl. 2005;95:399–402. doi: 10.1007/3-211-32318-x_81. [DOI] [PubMed] [Google Scholar]
- Keep RF, Hua Y, Xi G. Brain water content. A misunderstood measurement? Transl Stroke Res. 2012;3:263–265. doi: 10.1007/s12975-012-0152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keric N, Maier GS, Samadani U, Kallenberg K, Dechent P, Brueck W, Heuer J, Rohde V. Tissue Plasminogen Activator Induced Delayed Edema in Experimental Porcine Intracranial Hemorrhage: Reduction with Plasminogen Activator Inhibitor-1 Administration. Transl Stroke Res. 2012;3:88–93. doi: 10.1007/s12975-012-0188-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalili MA, Anvari M, Hekmati-Moghadam SH, Sadeghian-Nodoushan F, Fesahat F, Miresmaeili SM. Therapeutic benefit of intravenous transplantation of mesenchymal stem cells after experimental subarachnoid hemorrhage in rats. J Stroke Cerebrovasc Dis. 2012;21:445–451. doi: 10.1016/j.jstrokecerebrovasdis.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Kim GH, Kellner CP, Hahn DK, Desantis BM, Musabbir M, Starke RM, Rynkowski M, Komotar RJ, Otten ML, Sciacca R, Schmidt JM, Mayer SA, Connolly ES., Jr Monocyte chemoattractant protein-1 predicts outcome and vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2008;109:38–43. doi: 10.3171/JNS/2008/109/7/0038. [DOI] [PubMed] [Google Scholar]
- Kim JS, Gautam SC, Chopp M, Zaloga C, Jones ML, Ward PA, Welch KM. Expression of monocyte chemoattractant protein-1 and macrophage inflammatory protein-1 after focal cerebral ischemia in the rat. J Neuroimmunol. 1995;56:127–134. doi: 10.1016/0165-5728(94)00138-e. [DOI] [PubMed] [Google Scholar]
- Kim SU, Park YH, Min JS, Sun HN, Han YH, Hua JM, Lee TH, Lee SR, Chang KT, Kang SW, Kim JM, Yu DY, Lee SH, Lee DS. Peroxiredoxin I is a ROS/p38 MAPK-dependent inducible antioxidant that regulates NF-kappaB-mediated iNOS induction and microglial activation. J Neuroimmunol. 2013;259:26–36. doi: 10.1016/j.jneuroim.2013.03.006. [DOI] [PubMed] [Google Scholar]
- Kleindienst A, Schlaffer SM, Sharma N, Linde L, Buchfelder M, Verbalis JG. Development of an experimental model to study the pathophysiology of cerebral salt wasting following subarachnoid hemorrhage. Acta Neurochir Suppl. 2012;114:399–403. doi: 10.1007/978-3-7091-0956-4_77. [DOI] [PubMed] [Google Scholar]
- Knight DR, Jr, Smith AH, Schroeder RL, Huang C, Beebe DA, Sokolowski SA, Wang M. Effects of age on noninvasive assessments of vascular function in nonhuman primates: implications for translational drug discovery. J Transl Med. 2013;11:101. doi: 10.1186/1479-5876-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko SB, Choi HA, Parikh G, Schmidt JM, Lee K, Badjatia N, Claassen J, Connolly ES, Mayer SA. Real time estimation of brain water content in comatose patients. Ann Neurol. 2012;72:344–350. doi: 10.1002/ana.23619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K, Sakaki S, Ohue S, Kumon Y, Matsuoka K. Intracellular calcium levels in canine basilar artery smooth muscle following experimental subarachnoid hemorrhage: an electron microscopic cytochemical study. Acta Neuropathol. 1991;81:664–669. doi: 10.1007/BF00296377. [DOI] [PubMed] [Google Scholar]
- Koide M, Bonev AD, Nelson MT, Wellman GC. Inversion of neurovascular coupling by subarachnoid blood depends on large-conductance Ca2+-activated K+ (BK) channels. Proc Natl Acad Sci U S A. 2012;109:E1387–1395. doi: 10.1073/pnas.1121359109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Bonev AD, Nelson MT, Wellman GC. Subarachnoid blood converts neurally evoked vasodilation to vasoconstriction in rat brain cortex. Acta Neurochir Suppl. 2013a;115:167–171. doi: 10.1007/978-3-7091-1192-5_32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Sukhotinsky I, Ayata C, Wellman GC. Subarachnoid hemorrhage, spreading depolarizations and impaired neurovascular coupling. Stroke Res Treat. 2013b;2013:819340. doi: 10.1155/2013/819340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koide M, Wellman GC. SAH-induced suppression of voltage-gated K(+) (K (V)) channel currents in parenchymal arteriolar myocytes involves activation of the HB-EGF/EGFR pathway. Acta Neurochir Suppl. 2013;115:179–184. doi: 10.1007/978-3-7091-1192-5_34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kongable GL, Lanzino G, Germanson TP, Truskowski LL, Alves WM, Torner JC, Kassell NF. Gender-related differences in aneurysmal subarachnoid hemorrhage. J Neurosurg. 1996;84:43–48. doi: 10.3171/jns.1996.84.1.0043. [DOI] [PubMed] [Google Scholar]
- Kontos HA, Wei EP, Navari RM, Levasseur JE, Rosenblum WI, Patterson JL., Jr Responses of cerebral arteries and arterioles to acute hypotension and hypertension. Am J Physiol. 1978;234:H371–383. doi: 10.1152/ajpheart.1978.234.4.H371. [DOI] [PubMed] [Google Scholar]
- Kozniewska E, Michalik R, Rafalowska J, Gadamski R, Walski M, Frontczak-Baniewicz M, Piotrowski P, Czernicki Z. Mechanisms of vascular dysfunction after subarachnoid hemorrhage. J Physiol Pharmacol. 2006;57(Suppl 11):145–160. [PubMed] [Google Scholar]
- Kubo Y, Koji T, Kashimura H, Otawara Y, Ogawa A, Ogasawara K. Adrenomedullin concentration in the cerebrospinal fluid is related to appetite loss and delayed ischemic neurological deficits after subarachnoid hemorrhage. Neurol Res. 2013 doi: 10.1179/1743132813Y.0000000222. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Ogasawara K, Kakino S, Kashimura H, Tomitsuka N, Sugawara A, Ogawa A. Serum inflammatory adhesion molecules and high-sensitivity C-reactive protein correlates with delayed ischemic neurologic deficits after subarachnoid hemorrhage. Surg Neurol. 2008;69:592–596. doi: 10.1016/j.surneu.2008.02.014. discussion 596. [DOI] [PubMed] [Google Scholar]
- Kunz M, Nussberger J, Holtmannspoetter M, Bitterling H, Plesnila N, Zausinger S. Bradykinin in Blood and CSF after Acute Cerebral Lesions -Correlations with Cerebral Edema and Intracranial Pressure. J Neurotrauma. 2013 doi: 10.1089/neu.2012.2774. [DOI] [PubMed] [Google Scholar]
- Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH. Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:916–925. doi: 10.1097/01.WCB.0000125886.48838.7E. [DOI] [PubMed] [Google Scholar]
- Kuyama H, Ladds A, Branston NM, Nitta M, Symon L. An experimental study of acute subarachnoid haemorrhage in baboons: changes in cerebral blood volume, blood flow, electrical activity and water content. J Neurol Neurosurg Psychiatry. 1984;47:354–364. doi: 10.1136/jnnp.47.4.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai L, Morgan MK. Predictors of in-hospital shunt-dependent hydrocephalus following rupture of cerebral aneurysms. J Clin Neurosci. 2013 doi: 10.1016/j.jocn.2012.09.033. [DOI] [PubMed] [Google Scholar]
- Lakhan SE, Kirchgessner A, Hofer M. Inflammatory mechanisms in ischemic stroke: therapeutic approaches. J Transl Med. 2009;7:97. doi: 10.1186/1479-5876-7-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambert G, Naredi S, Eden E, Rydenhag B, Friberg P. Monoamine metabolism and sympathetic nervous activation following subarachnoid haemorrhage: influence of gender and hydrocephalus. Brain Res Bull. 2002;58:77–82. doi: 10.1016/s0361-9230(02)00762-1. [DOI] [PubMed] [Google Scholar]
- Lang EW, Diehl RR, Mehdorn HM. Cerebral autoregulation testing after aneurysmal subarachnoid hemorrhage: the phase relationship between arterial blood pressure and cerebral blood flow velocity. Crit Care Med. 2001;29:158–163. doi: 10.1097/00003246-200101000-00031. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part II. A cooperative study in North America. J Neurosurg. 1999;90:1018–1024. doi: 10.3171/jns.1999.90.6.1018. [DOI] [PubMed] [Google Scholar]
- Lanzino G, Kassell NF, Dorsch NW, Pasqualin A, Brandt L, Schmiedek P, Truskowski LL, Alves WM. Double-blind, randomized, vehicle-controlled study of high-dose tirilazad mesylate in women with aneurysmal subarachnoid hemorrhage. Part I. A cooperative study in Europe, Australia, New Zealand, and South Africa. J Neurosurg. 1999;90:1011–1017. doi: 10.3171/jns.1999.90.6.1011. [DOI] [PubMed] [Google Scholar]
- Lapchak PA, Zhang JH, Noble-Haeusslein LJ. RIGOR Guidelines: Escalating STAIR and STEPS for Effective Translational Research. Transl Stroke Res. 2013;4:279–285. doi: 10.1007/s12975-012-0209-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larysz-Brysz M, Lewin-Kowalik J, Czuba Z, Kotulska K, Olakowska E, Marcol W, Liskiewicz A, Jedrzejowska-Szypulka H. Interleukin-1beta increases release of endothelin-1 and tumor necrosis factor as well as reactive oxygen species by peripheral leukocytes during experimental subarachnoid hemorrhage. Curr Neurovasc Res. 2012;9:159–166. doi: 10.2174/156720212801619045. [DOI] [PubMed] [Google Scholar]
- Leao AA. Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol. 1947;10:409–414. doi: 10.1152/jn.1947.10.6.409. [DOI] [PubMed] [Google Scholar]
- Lecrux C, Hamel E. The neurovascular unit in brain function and disease. Acta Physiol (Oxf) 2011;203:47–59. doi: 10.1111/j.1748-1716.2011.02256.x. [DOI] [PubMed] [Google Scholar]
- Lee JH, Park DH, Back DB, Lee JY, Lee CI, Park KJ, Kang SH, Cho TH, Chung YG. Comparison of cerebrospinal fluid biomarkers between idiopathic normal pressure hydrocephalus and subarachnoid hemorrhage-induced chronic hydrocephalus: a pilot study. Med Sci Monit. 2012;18:PR19–25. doi: 10.12659/MSM.883586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JY, He Y, Sagher O, Keep R, Hua Y, Xi G. Activated autophagy pathway in experimental subarachnoid hemorrhage. Brain Res. 2009a;1287:126–135. doi: 10.1016/j.brainres.2009.06.028. [DOI] [PubMed] [Google Scholar]
- Lee JY, Sagher O, Keep R, Hua Y, Xi G. Comparison of experimental rat models of early brain injury after subarachnoid hemorrhage. Neurosurgery. 2009b;65:331–343. doi: 10.1227/01.NEU.0000345649.78556.26. discussion 343. [DOI] [PubMed] [Google Scholar]
- Lewen A, Matz P, Chan PH. Free radical pathways in CNS injury. J Neurotrauma. 2000;17:871–890. doi: 10.1089/neu.2000.17.871. [DOI] [PubMed] [Google Scholar]
- Li B, Luo C, Tang W, Chen Z, Li Q, Hu B, Lin J, Zhu G, Zhang JH, Feng H. Role of HCN channels in neuronal hyperexcitability after subarachnoid hemorrhage in rats. J Neurosci. 2012;32:3164–3175. doi: 10.1523/JNEUROSCI.5143-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ransohoff RM. Multiple roles of chemokine CXCL12 in the central nervous system: a migration from immunology to neurobiology. Prog Neurobiol. 2008;84:116–131. doi: 10.1016/j.pneurobio.2007.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Zhang P, Yuan B, Zhao D, Chen Y, Zhang X. Thrombin-induced TGF-beta1 pathway: a cause of communicating hydrocephalus post subarachnoid hemorrhage. Int J Mol Med. 2013;31:660–666. doi: 10.3892/ijmm.2013.1253. [DOI] [PubMed] [Google Scholar]
- Li Y, Tang J, Khatibi NH, Zhu M, Chen D, Zheng W, Wang S. Ginsenoside Rbeta1 reduces neurologic damage, is anti-apoptotic, and down-regulates p53 and BAX in subarachnoid hemorrhage. Curr Neurovasc Res. 2010;7:85–94. doi: 10.2174/156720210791184952. [DOI] [PubMed] [Google Scholar]
- Lim M, Bower RS, Wang Y, Sims L, Bower MR, Camara-Quintana J, Li G, Cheshier S, Harsh GRt, Steinberg GK, Guccione S. The predictive value of serum myeloperoxidase for vasospasm in patients with aneurysmal subarachnoid hemorrhage. Neurosurg Rev. 2012;35:413–419. doi: 10.1007/s10143-012-0375-4. discussion 419. [DOI] [PubMed] [Google Scholar]
- Lin CL, Dumont AS, Calisaneller T, Kwan AL, Hwong SL, Lee KS. Monoclonal antibody against E selectin attenuates subarachnoid hemorrhage-induced cerebral vasospasm. Surg Neurol. 2005;64:201–205. doi: 10.1016/j.surneu.2005.04.038. discussion 205–206. [DOI] [PubMed] [Google Scholar]
- Lin CL, Dumont AS, Tsai YJ, Huang JH, Chang KP, Kwan AL, Hong YR, Howng SL. 17Beta-estradiol activates adenosine A(2a) receptor after subarachnoid hemorrhage. J Surg Res. 2009;157:208–215. doi: 10.1016/j.jss.2008.08.021. [DOI] [PubMed] [Google Scholar]
- Lin CL, Hsu YT, Lin TK, Morrow JD, Hsu JC, Hsu YH, Hsieh TC, Tsay PK, Yen HC. Increased levels of F2-isoprostanes following aneurysmal subarachnoid hemorrhage in humans. Free Radic Biol Med. 2006;40:1466–1473. doi: 10.1016/j.freeradbiomed.2005.12.019. [DOI] [PubMed] [Google Scholar]
- Lin Y, Choksi S, Shen HM, Yang QF, Hur GM, Kim YS, Tran JH, Nedospasov SA, Liu ZG. Tumor necrosis factor-induced nonapoptotic cell death requires receptor-interacting protein-mediated cellular reactive oxygen species accumulation. J Biol Chem. 2004;279:10822–10828. doi: 10.1074/jbc.M313141200. [DOI] [PubMed] [Google Scholar]
- Lippai D, Bala S, Petrasek J, Csak T, Levin I, Kurt-Jones EA, Szabo G. Alcohol-induced IL-1beta in the brain is mediated by NLRP3/ASC inflammasome activation that amplifies neuroinflammation. J Leukoc Biol. 2013 doi: 10.1189/jlb.1212659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Tang J, Ostrowski RP, Titova E, Monroe C, Chen W, Lo W, Martin R, Zhang JH. Oxidative stress after subarachnoid hemorrhage in gp91phox knockout mice. Can J Neurol Sci. 2007;34:356–361. doi: 10.1017/s031716710000682x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loch Macdonald R. Management of cerebral vasospasm. Neurosurg Rev. 2006;29:179–193. doi: 10.1007/s10143-005-0013-5. [DOI] [PubMed] [Google Scholar]
- Locovei S, Scemes E, Qiu F, Spray DC, Dahl G. Pannexin1 is part of the pore forming unit of the P2X(7) receptor death complex. FEBS Lett. 2007;581:483–488. doi: 10.1016/j.febslet.2006.12.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Locovei S, Wang J, Dahl G. Activation of pannexin 1 channels by ATP through P2Y receptors and by cytoplasmic calcium. FEBS Lett. 2006;580:239–244. doi: 10.1016/j.febslet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Loftspring MC. Iron and early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2010;30:1791–1792. doi: 10.1038/jcbfm.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longstreth WT, Nelson LM, Koepsell TD, van Belle G. Subarachnoid hemorrhage and hormonal factors in women. A population-based case-control study. Ann Intern Med. 1994;121:168–173. doi: 10.7326/0003-4819-121-3-199408010-00002. [DOI] [PubMed] [Google Scholar]
- Lu H, Shi JX, Chen HL, Hang CH, Wang HD, Yin HX. Expression of monocyte chemoattractant protein-1 in the cerebral artery after experimental subarachnoid hemorrhage. Brain Res. 2009;1262:73–80. doi: 10.1016/j.brainres.2009.01.017. [DOI] [PubMed] [Google Scholar]
- Lu X, Xu H, Sun B, Zhu Z, Zheng D, Li X. Enhanced neuroprotective effects of resveratrol delivered by nanoparticles on hydrogen peroxide-induced oxidative stress in rat cortical cell culture. Mol Pharm. 2013;10:2045–2053. doi: 10.1021/mp400056c. [DOI] [PubMed] [Google Scholar]
- Ma Q, Manaenko A, Khatibi NH, Chen W, Zhang JH, Tang J. Vascular adhesion protein-1 inhibition provides antiinflammatory protection after an intracerebral hemorrhagic stroke in mice. J Cereb Blood Flow Metab. 2011;31:881–893. doi: 10.1038/jcbfm.2010.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL. Endothelin antagonists in subarachnoid hemorrhage: what next? Crit Care. 2012;16:171. doi: 10.1186/cc11822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Marr A, Roux S, Kassell N. Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomised, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2) Lancet Neurol. 2011;10:618–625. doi: 10.1016/S1474-4422(11)70108-9. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Higashida RT, Keller E, Mayer SA, Molyneux A, Raabe A, Vajkoczy P, Wanke I, Bach D, Frey A, Nowbakht P, Roux S, Kassell N. Randomized trial of clazosentan in patients with aneurysmal subarachnoid hemorrhage undergoing endovascular coiling. Stroke. 2012;43:1463–1469. doi: 10.1161/STROKEAHA.111.648980. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Kassell NF, Mayer S, Ruefenacht D, Schmiedek P, Weidauer S, Frey A, Roux S, Pasqualin A. Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke. 2008;39:3015–3021. doi: 10.1161/STROKEAHA.108.519942. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Pluta RM, Zhang JH. Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol. 2007;3:256–263. doi: 10.1038/ncpneuro0490. [DOI] [PubMed] [Google Scholar]
- Macdonald RL, Zhang J, Sima B, Johns L. Papaverine-sensitive vasospasm and arterial contractility and compliance after subarachnoid hemorrhage in dogs. Neurosurgery. 1995;37:962–967. doi: 10.1227/00006123-199511000-00016. discussion 967–968. [DOI] [PubMed] [Google Scholar]
- Maddahi A, Povlsen GK, Edvinsson L. Regulation of enhanced cerebrovascular expression of proinflammatory mediators in experimental subarachnoid hemorrhage via the mitogen-activated protein kinase kinase/extracellular signal-regulated kinase pathway. J Neuroinflammation. 2012;9:274. doi: 10.1186/1742-2094-9-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marbacher S, Andereggen L, Neuschmelting V, Widmer HR, von Gunten M, Takala J, Jakob SM, Fandino J. A new rabbit model for the study of early brain injury after subarachnoid hemorrhage. J Neurosci Methods. 2012;208:138–145. doi: 10.1016/j.jneumeth.2012.05.010. [DOI] [PubMed] [Google Scholar]
- Marbacher S, Fandino J, Kitchen ND. Standard intracranial in vivo animal models of delayed cerebral vasospasm. Br J Neurosurg. 2010a;24:415–434. doi: 10.3109/02688691003746274. [DOI] [PubMed] [Google Scholar]
- Marbacher S, Sherif C, Neuschmelting V, Schlappi JA, Takala J, Jakob SM, Fandino J. Extra-intracranial blood shunt mimicking aneurysm rupture: intracranial-pressure-controlled rabbit subarachnoid hemorrhage model. J Neurosci Methods. 2010b;191:227–233. doi: 10.1016/j.jneumeth.2010.07.004. [DOI] [PubMed] [Google Scholar]
- Marlin SD, Springer TA. Purified intercellular adhesion molecule-1 (ICAM-1) is a ligand for lymphocyte function-associated antigen 1 (LFA-1) Cell. 1987;51:813–819. doi: 10.1016/0092-8674(87)90104-8. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Adams NA, Pan Y, Price A, Wong M. The mitochondrial permeability transition pore regulates nitric oxide-mediated apoptosis of neurons induced by target deprivation. J Neurosci. 2011;31:359–370. doi: 10.1523/JNEUROSCI.2225-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Morillo E, Diamandis A, Romaschin AD, Diamandis EP. Kallikrein 6 as a serum prognostic marker in patients with aneurysmal subarachnoid hemorrhage. PLoS One. 2012;7:e45676. doi: 10.1371/journal.pone.0045676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzatico F, Gaetani P, Cafe C, Spanu G, Rodriguez y Baena R. Antioxidant enzymatic activities after experimental subarachnoid hemorrhage in rats. Acta Neurol Scand. 1993;87:62–66. doi: 10.1111/j.1600-0404.1993.tb04077.x. [DOI] [PubMed] [Google Scholar]
- Marzatico F, Gaetani P, Tartara F, Bertorelli L, Feletti F, Adinolfi D, Tancioni F, Rodriguez y Baena R. Antioxidant status and alpha1-antiproteinase activity in subarachnoid hemorrhage patients. Life Sci. 1998;63:821–826. doi: 10.1016/s0024-3205(98)00338-5. [DOI] [PubMed] [Google Scholar]
- Massicotte EM, Del Bigio MR. Human arachnoid villi response to subarachnoid hemorrhage: possible relationship to chronic hydrocephalus. J Neurosurg. 1999;91:80–84. doi: 10.3171/jns.1999.91.1.0080. [DOI] [PubMed] [Google Scholar]
- Matz PG, Copin JC, Chan PH. Cell death after exposure to subarachnoid hemolysate correlates inversely with expression of CuZn-superoxide dismutase. Stroke. 2000;31:2450–2459. doi: 10.1161/01.str.31.10.2450. [DOI] [PubMed] [Google Scholar]
- Matz PG, Fujimura M, Lewen A, Morita-Fujimura Y, Chan PH. Increased cytochrome c-mediated DNA fragmentation and cell death in manganese-superoxide dismutase-deficient mice after exposure to subarachnoid hemolysate. Stroke. 2001;32:506–515. doi: 10.1161/01.str.32.2.506. [DOI] [PubMed] [Google Scholar]
- McLean RM. Magnesium and its therapeutic uses: a review. Am J Med. 1994;96:63–76. doi: 10.1016/0002-9343(94)90117-1. [DOI] [PubMed] [Google Scholar]
- Meguro T, Chen B, Parent AD, Zhang JH. Caspase inhibitors attenuate oxyhemoglobin-induced apoptosis in endothelial cells. Stroke. 2001;32:561–566. doi: 10.1161/01.str.32.2.561. [DOI] [PubMed] [Google Scholar]
- Mellergard P, Sjogren F, Hillman J. Release of VEGF and FGF in the extracellular space following severe subarachnoidal haemorrhage or traumatic head injury in humans. Br J Neurosurg. 2010;24:261–267. doi: 10.3109/02688690903521605. [DOI] [PubMed] [Google Scholar]
- Memon ZI, Altura BT, Benjamin JL, Cracco RQ, Altura BM. Predictive value of serum ionized but not total magnesium levels in head injuries. Scand J Clin Lab Invest. 1995;55:671–677. doi: 10.3109/00365519509075397. [DOI] [PubMed] [Google Scholar]
- Metea MR, Newman EA. Glial cells dilate and constrict blood vessels: a mechanism of neurovascular coupling. J Neurosci. 2006;26:2862–2870. doi: 10.1523/JNEUROSCI.4048-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mies G, Paschen W. Regional changes of blood flow, glucose, and ATP content determined on brain sections during a single passage of spreading depression in rat brain cortex. Exp Neurol. 1984;84:249–258. doi: 10.1016/0014-4886(84)90222-x. [DOI] [PubMed] [Google Scholar]
- Milhorat TH, Clark RG, Hammock MK. Experimental hydrocephalus. 2. Gross pathological findings in acute and subacute obstructive hydrocephalus in the dog and monkey. J Neurosurg. 1970;32:390–399. doi: 10.3171/jns.1970.32.4.0390. [DOI] [PubMed] [Google Scholar]
- Minhas PS, Menon DK, Smielewski P, Czosnyka M, Kirkpatrick PJ, Clark JC, Pickard JD. Positron emission tomographic cerebral perfusion disturbances and transcranial Doppler findings among patients with neurological deterioration after subarachnoid hemorrhage. Neurosurgery. 2003;52:1017–1022. discussion 1022–1014. [PubMed] [Google Scholar]
- Mino M, Kamii H, Fujimura M, Kondo T, Takasawa S, Okamoto H, Yoshimoto T. Temporal changes of neurogenesis in the mouse hippocampus after experimental subarachnoid hemorrhage. Neurol Res. 2003;25:839–845. doi: 10.1179/016164103771953934. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Ohta F, Moritake K, Nagase A, Kagawa T. The key to improving prognosis for aneurysmal subarachnoid hemorrhage remains in the pre-hospitalization period. Surg Neurol. 2006;65:360–365. doi: 10.1016/j.surneu.2005.10.025. discussion 365–366. [DOI] [PubMed] [Google Scholar]
- Mori K, Yamamoto T, Miyazaki M, Hara Y, Aiko Y, Koike N, Sakamoto S, Nakao Y, Esaki T. Optimal cerebrospinal fluid magnesium ion concentration for vasodilatory effect and duration after intracisternal injection of magnesium sulfate solution in a canine subarachnoid hemorrhage model. J Neurosurg. 2011;114:1168–1175. doi: 10.3171/2010.10.JNS10866. [DOI] [PubMed] [Google Scholar]
- Mori K, Yamamoto T, Miyazaki M, Hara Y, Aiko Y, Koike N, Sakamoto S, Nakao Y, Esaki T. Effect of intrathecal magnesium sulfate solution injection via a microcatheter in the cisterna magna on cerebral vasospasm in the canine subarachnoid haemorrhage model. Br J Neurosurg. 2012;26:64–68. doi: 10.3109/02688697.2011.591948. [DOI] [PubMed] [Google Scholar]
- Moss J, Zolkiewska A, Okazaki I. ADP-ribosylarginine hydrolases and ADP-ribosyltransferases. Partners in ADP-ribosylation cycles. Adv Exp Med Biol. 1997;419:25–33. doi: 10.1007/978-1-4419-8632-0_3. [DOI] [PubMed] [Google Scholar]
- Moussouttas M, Huynh TT, Khoury J, Lai EW, Dombrowski K, Pello S, Pacak K. Cerebrospinal fluid catecholamine levels as predictors of outcome in subarachnoid hemorrhage. Cerebrovasc Dis. 2012;33:173–181. doi: 10.1159/000334660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Muir KW, Lees KR, Ford I, Davis S. Magnesium for acute stroke (Intravenous Magnesium Efficacy in Stroke trial): randomised controlled trial. Lancet. 2004;363:439–445. doi: 10.1016/S0140-6736(04)15490-1. [DOI] [PubMed] [Google Scholar]
- Muller T, Lohle M, Schubert H, Bauer R, Wicher C, Antonow-Schlorke I, Sliwka U, Nathanielsz PW, Schwab M. Developmental changes in cerebral autoregulatory capacity in the fetal sheep parietal cortex. J Physiol. 2002;539:957–967. doi: 10.1113/jphysiol.2001.012590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan SJ, MacVicar BA. Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature. 2004;431:195–199. doi: 10.1038/nature02827. [DOI] [PubMed] [Google Scholar]
- Muroi C, Hugelshofer M, Seule M, Tastan I, Fujioka M, Mishima K, Keller E. Correlation among systemic inflammatory parameter, occurrence of delayed neurological deficits, and outcome after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2013;72:367–375. doi: 10.1227/NEU.0b013e31828048ce. discussion 375. [DOI] [PubMed] [Google Scholar]
- Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Hachinski V, Chan R, Lee TY. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- Nadler JL, Goodson S, Rude RK. Evidence that prostacyclin mediates the vascular action of magnesium in humans. Hypertension. 1987;9:379–383. doi: 10.1161/01.hyp.9.4.379. [DOI] [PubMed] [Google Scholar]
- Naidech AM, Drescher J, Tamul P, Shaibani A, Batjer HH, Alberts MJ. Acute physiological derangement is associated with early radiographic cerebral infarction after subarachnoid haemorrhage. J Neurol Neurosurg Psychiatry. 2006;77:1340–1344. doi: 10.1136/jnnp.2006.089748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naraoka M, Munakata A, Matsuda N, Shimamura N, Ohkuma H. Suppression of the Rho/Rho-Kinase Pathway and Prevention of Cerebral Vasospasm by Combination Treatment with Statin and Fasudil After Subarachnoid Hemorrhage in Rabbit. Transl Stroke Res. 2013;4:368–374. doi: 10.1007/s12975-012-0247-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nau R, Haase S, Bunkowski S, Bruck W. Neuronal apoptosis in the dentate gyrus in humans with subarachnoid hemorrhage and cerebral hypoxia. Brain Pathol. 2002;12:329–336. doi: 10.1111/j.1750-3639.2002.tb00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naval NS, Stevens RD, Mirski MA, Bhardwaj A. Controversies in the management of aneurysmal subarachnoid hemorrhage. Crit Care Med. 2006;34:511–524. doi: 10.1097/01.ccm.0000198331.45998.85. [DOI] [PubMed] [Google Scholar]
- Nedergaard M, Ransom B, Goldman SA. New roles for astrocytes: redefining the functional architecture of the brain. Trends Neurosci. 2003;26:523–530. doi: 10.1016/j.tins.2003.08.008. [DOI] [PubMed] [Google Scholar]
- Newman EA. New roles for astrocytes: regulation of synaptic transmission. Trends Neurosci. 2003;26:536–542. doi: 10.1016/S0166-2236(03)00237-6. [DOI] [PubMed] [Google Scholar]
- Newman EA. Calcium increases in retinal glial cells evoked by light-induced neuronal activity. J Neurosci. 2005;25:5502–5510. doi: 10.1523/JNEUROSCI.1354-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikitina E, Kawashima A, Takahashi M, Zhang ZD, Shang X, Ai J, Macdonald RL. Alteration in voltage-dependent calcium channels in dog basilar artery after subarachnoid hemorrhage. Laboratory investigation. J Neurosurg. 2010;113:870–880. doi: 10.3171/2010.2.JNS091038. [DOI] [PubMed] [Google Scholar]
- Nishizawa S, Laher I. Signaling mechanisms in cerebral vasospasm. Trends Cardiovasc Med. 2005;15:24–34. doi: 10.1016/j.tcm.2004.12.002. [DOI] [PubMed] [Google Scholar]
- Nolan CP, Macdonald RL. Can angiographic vasospasm be used as a surrogate marker in evaluating therapeutic interventions for cerebral vasospasm? Neurosurg Focus. 2006;21:E1. doi: 10.3171/foc.2006.21.3.1. [DOI] [PubMed] [Google Scholar]
- Nornes H. The role of intracranial pressure in the arrest of hemorrhage in patients with ruptured intracranial aneurysm. J Neurosurg. 1973;39:226–234. doi: 10.3171/jns.1973.39.2.0226. [DOI] [PubMed] [Google Scholar]
- Nornes H, Magnaes B. Intracranial pressure in patients with ruptured saccular aneurysm. J Neurosurg. 1972;36:537–547. doi: 10.3171/jns.1972.36.5.0537. [DOI] [PubMed] [Google Scholar]
- Nozari A, Dilekoz E, Sukhotinsky I, Stein T, Eikermann-Haerter K, Liu C, Wang Y, Frosch MP, Waeber C, Ayata C, Moskowitz MA. Microemboli may link spreading depression, migraine aura, and patent foramen ovale. Ann Neurol. 2010;67:221–229. doi: 10.1002/ana.21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara K, Aoki K, Zubkov AY, Zhang JH. Oxyhemoglobin produces apoptosis and necrosis in cultured smooth muscle cells. Brain Res. 2001;889:89–97. doi: 10.1016/s0006-8993(00)03120-6. [DOI] [PubMed] [Google Scholar]
- Ohkuma H, Manabe H, Tanaka M, Suzuki S. Impact of cerebral microcirculatory changes on cerebral blood flow during cerebral vasospasm after aneurysmal subarachnoid hemorrhage. Stroke. 2000;31:1621–1627. doi: 10.1161/01.str.31.7.1621. [DOI] [PubMed] [Google Scholar]
- Ohkuma H, Suzuki S. Histological dissociation between intra- and extraparenchymal portion of perforating small arteries after experimental subarachnoid hemorrhage in dogs. Acta Neuropathol. 1999;98:374–382. doi: 10.1007/s004010051097. [DOI] [PubMed] [Google Scholar]
- Ohkuma H, Tsurutani H, Suzuki S. Incidence and significance of early aneurysmal rebleeding before neurosurgical or neurological management. Stroke. 2001;32:1176–1180. doi: 10.1161/01.str.32.5.1176. [DOI] [PubMed] [Google Scholar]
- Ohman J. Hypertension as a risk factor for epilepsy after aneurysmal subarachnoid hemorrhage and surgery. Neurosurgery. 1990;27:578–581. doi: 10.1097/00006123-199010000-00012. [DOI] [PubMed] [Google Scholar]
- Ohnishi H, Iihara K, Kaku Y, Yamauchi K, Fukuda K, Nishimura K, Nakai M, Satow T, Nakajima N, Ikegawa M. Haptoglobin phenotype predicts cerebral vasospasm and clinical deterioration after aneurysmal subarachnoid hemorrhage. J Stroke Cerebrovasc Dis. 2013;22:520–526. doi: 10.1016/j.jstrokecerebrovasdis.2013.02.005. [DOI] [PubMed] [Google Scholar]
- Ohta K, Graf R, Rosner G, Heiss WD. Calcium ion transients in peri-infarct depolarizations may deteriorate ion homeostasis and expand infarction in focal cerebral ischemia in cats. Stroke. 2001;32:535–543. doi: 10.1161/01.str.32.2.535. [DOI] [PubMed] [Google Scholar]
- Okamoto K, Horisawa R, Kawamura T, Asai A, Ogino M, Takagi T, Ohno Y. Menstrual and reproductive factors for subarachnoid hemorrhage risk in women: a case-control study in nagoya, Japan. Stroke. 2001;32:2841–2844. doi: 10.1161/hs1201.099383. [DOI] [PubMed] [Google Scholar]
- Okubo S, Strahle J, Keep RF, Hua Y, Xi G. Subarachnoid hemorrhage-induced hydrocephalus in rats. Stroke. 2013;44:547–550. doi: 10.1161/STROKEAHA.112.662312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res. 2006a;28:399–414. doi: 10.1179/016164106X115008. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Tang J, Zhang JH. Hyperbaric oxygen suppresses NADPH oxidase in a rat subarachnoid hemorrhage model. Stroke. 2006b;37:1314–1318. doi: 10.1161/01.STR.0000217310.88450.c3. [DOI] [PubMed] [Google Scholar]
- Owens J, Wyper DJ, Patterson J, Brown DR, Elliott AT, Teasdale GM, McCulloch J. First SPET images of glutamate (NMDA) receptor activation in vivo in cerebral ischaemia. Nucl Med Commun. 1997;18:149–158. doi: 10.1097/00006231-199702000-00010. [DOI] [PubMed] [Google Scholar]
- Park S, Yamaguchi M, Zhou C, Calvert JW, Tang J, Zhang JH. Neurovascular protection reduces early brain injury after subarachnoid hemorrhage. Stroke. 2004;35:2412–2417. doi: 10.1161/01.STR.0000141162.29864.e9. [DOI] [PubMed] [Google Scholar]
- Parker BL, Larsen MR, Edvinsson LI, Povlsen GK. Signal transduction in cerebral arteries after subarachnoid hemorrhage-a phosphoproteomic approach. J Cereb Blood Flow Metab. 2013 doi: 10.1038/jcbfm.2013.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson OB, Strandgaard S, Edvinsson L. Cerebral autoregulation. Cerebrovasc Brain Metab Rev. 1990;2:161–192. [PubMed] [Google Scholar]
- Peterson JW, Roussos L, Kwun BD, Hackett JD, Owen CJ, Zervas NT. Evidence of the role of hemolysis in experimental cerebral vasospasm. J Neurosurg. 1990;72:775–781. doi: 10.3171/jns.1990.72.5.0775. [DOI] [PubMed] [Google Scholar]
- Petruk KC, West M, Mohr G, Weir BK, Benoit BG, Gentili F, Disney LB, Khan MI, Grace M, Holness RO, et al. Nimodipine treatment in poor-grade aneurysm patients. Results of a multicenter double-blind placebo-controlled trial. J Neurosurg. 1988;68:505–517. doi: 10.3171/jns.1988.68.4.0505. [DOI] [PubMed] [Google Scholar]
- Petzold A, Rejdak K, Belli A, Sen J, Keir G, Kitchen N, Smith M, Thompson EJ. Axonal pathology in subarachnoid and intracerebral hemorrhage. J Neurotrauma. 2005a;22:407–414. doi: 10.1089/neu.2005.22.407. [DOI] [PubMed] [Google Scholar]
- Petzold GC, Windmuller O, Haack S, Major S, Buchheim K, Megow D, Gabriel S, Lehmann TN, Drenckhahn C, Peters O, Meierkord H, Heinemann U, Dirnagl U, Dreier JP. Increased extracellular K+ concentration reduces the efficacy of N-methyl-D-aspartate receptor antagonists to block spreading depression-like depolarizations and spreading ischemia. Stroke. 2005b;36:1270–1277. doi: 10.1161/01.STR.0000166023.51307.e0. [DOI] [PubMed] [Google Scholar]
- Piantadosi CA, Zhang J. Mitochondrial generation of reactive oxygen species after brain ischemia in the rat. Stroke. 1996;27:327–331. doi: 10.1161/01.str.27.2.327. discussion 332. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Boock RJ, Afshar JK, Clouse K, Bacic M, Ehrenreich H, Oldfield EH. Source and cause of endothelin-1 release into cerebrospinal fluid after subarachnoid hemorrhage. J Neurosurg. 1997;87:287–293. doi: 10.3171/jns.1997.87.2.0287. [DOI] [PubMed] [Google Scholar]
- Pluta RM, Hansen-Schwartz J, Dreier J, Vajkoczy P, Macdonald RL, Nishizawa S, Kasuya H, Wellman G, Keller E, Zauner A, Dorsch N, Clark J, Ono S, Kiris T, Leroux P, Zhang JH. Cerebral vasospasm following subarachnoid hemorrhage: time for a new world of thought. Neurol Res. 2009;31:151–158. doi: 10.1179/174313209X393564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polin RS, Bavbek M, Shaffrey ME, Billups K, Bogaev CA, Kassell NF, Lee KS. Detection of soluble E-selectin, ICAM-1, VCAM-1, and L-selectin in the cerebrospinal fluid of patients after subarachnoid hemorrhage. J Neurosurg. 1998;89:559–567. doi: 10.3171/jns.1998.89.4.0559. [DOI] [PubMed] [Google Scholar]
- Polin RS, Coenen VA, Hansen CA, Shin P, Baskaya MK, Nanda A, Kassell NF. Efficacy of transluminal angioplasty for the management of symptomatic cerebral vasospasm following aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000;92:284–290. doi: 10.3171/jns.2000.92.2.0284. [DOI] [PubMed] [Google Scholar]
- Povlsen GK, Johansson SE, Larsen CC, Samraj AK, Edvinsson L. Early events triggering delayed vasoconstrictor receptor upregulation and cerebral ischemia after subarachnoid hemorrhage. BMC Neurosci. 2013;14:34. doi: 10.1186/1471-2202-14-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Povlsen GK, Waldsee R, Ahnstedt H, Kristiansen KA, Johansen FF, Edvinsson L. In vivo experimental stroke and in vitro organ culture induce similar changes in vasoconstrictor receptors and intracellular calcium handling in rat cerebral arteries. Exp Brain Res. 2012;219:507–520. doi: 10.1007/s00221-012-3108-6. [DOI] [PubMed] [Google Scholar]
- Prunell GF, Mathiesen T, Svendgaard NA. Experimental subarachnoid hemorrhage: cerebral blood flow and brain metabolism during the acute phase in three different models in the rat. Neurosurgery. 2004;54:426–436. doi: 10.1227/01.neu.0000103670.09687.7a. discussion 436–427. [DOI] [PubMed] [Google Scholar]
- Puyal J, Ginet V, Clarke PG. Multiple interacting cell death mechanisms in the mediation of excitotoxicity and ischemic brain damage: A challenge for neuroprotection. Prog Neurobiol. 2013;105:24–48. doi: 10.1016/j.pneurobio.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Pyne-Geithman GJ, Caudell DN, Prakash P, Clark JF. Glutathione peroxidase and subarachnoid hemorrhage: implications for the role of oxidative stress in cerebral vasospasm. Neurol Res. 2009;31:195–199. doi: 10.1179/174313209X393906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabinstein AA, Friedman JA, Weigand SD, McClelland RL, Fulgham JR, Manno EM, Atkinson JL, Wijdicks EF. Predictors of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2004;35:1862–1866. doi: 10.1161/01.STR.0000133132.76983.8e. [DOI] [PubMed] [Google Scholar]
- Rabinstein AA, Weigand S, Atkinson JL, Wijdicks EF. Patterns of cerebral infarction in aneurysmal subarachnoid hemorrhage. Stroke. 2005;36:992–997. doi: 10.1161/01.STR.0000163090.59350.5a. [DOI] [PubMed] [Google Scholar]
- Rajajee V, Fletcher JJ, Pandey AS, Gemmete JJ, Chaudhary N, Jacobs TL, Thompson BG. Low pulsatility index on transcranial Doppler predicts symptomatic large-vessel vasospasm after aneurysmal subarachnoid hemorrhage. Neurosurgery. 2012;70:1195–1206. doi: 10.1227/NEU.0b013e3182417dca. discussion 1206. [DOI] [PubMed] [Google Scholar]
- Ratsep T, Asser T. Cerebral hemodynamic impairment after aneurysmal subarachnoid hemorrhage as evaluated using transcranial doppler ultrasonography: relationship to delayed cerebral ischemia and clinical outcome. J Neurosurg. 2001;95:393–401. doi: 10.3171/jns.2001.95.3.0393. [DOI] [PubMed] [Google Scholar]
- Reaux-Le Goazigo A, Van Steenwinckel J, Rostene W, Melik Parsadaniantz S. Current status of chemokines in the adult CNS. Prog Neurobiol. 2013;104:67–92. doi: 10.1016/j.pneurobio.2013.02.001. [DOI] [PubMed] [Google Scholar]
- Reilly C, Amidei C, Tolentino J, Jahromi BS, Macdonald RL. Clot volume and clearance rate as independent predictors of vasospasm after aneurysmal subarachnoid hemorrhage. J Neurosurg. 2004;101:255–261. doi: 10.3171/jns.2004.101.2.0255. [DOI] [PubMed] [Google Scholar]
- Rhoney DH, Tipps LB, Murry KR, Basham MC, Michael DB, Coplin WM. Anticonvulsant prophylaxis and timing of seizures after aneurysmal subarachnoid hemorrhage. Neurology. 2000;55:258–265. doi: 10.1212/wnl.55.2.258. [DOI] [PubMed] [Google Scholar]
- Rinkel GJ, Djibuti M, Algra A, van Gijn J. Prevalence and risk of rupture of intracranial aneurysms: a systematic review. Stroke. 1998;29:251–256. doi: 10.1161/01.str.29.1.251. [DOI] [PubMed] [Google Scholar]
- Rinkel GJ, van Gijn J, Wijdicks EF. Subarachnoid hemorrhage without detectable aneurysm. A review of the causes. Stroke. 1993;24:1403–1409. doi: 10.1161/01.str.24.9.1403. [DOI] [PubMed] [Google Scholar]
- Rollins S, Perkins E, Mandybur G, Zhang JH. Oxyhemoglobin produces necrosis, not apoptosis, in astrocytes. Brain Res. 2002;945:41–49. doi: 10.1016/s0006-8993(02)02562-3. [DOI] [PubMed] [Google Scholar]
- Roman RJ, Renic M, Dunn KM, Takeuchi K, Hacein-Bey L. Evidence that 20-HETE contributes to the development of acute and delayed cerebral vasospasm. Neurol Res. 2006;28:738–749. doi: 10.1179/016164106X152016. [DOI] [PubMed] [Google Scholar]
- Romero FR, de Bertolini EF, Figueiredo EG, Teixeira MJ. Serum C-reactive protein levels predict neurological outcome after aneurysmal subarachnoid hemorrhage. Arq Neuropsiquiatr. 2012;70:202–205. doi: 10.1590/s0004-282x2012000300009. [DOI] [PubMed] [Google Scholar]
- Rothoerl RD, Ringel F. Molecular mechanisms of cerebral vasospasm following aneurysmal SAH. Neurol Res. 2007;29:636–642. doi: 10.1179/016164107X240224. [DOI] [PubMed] [Google Scholar]
- Sabri M, Ai J, Lakovic K, D’Abbondanza J, Ilodigwe D, Macdonald RL. Mechanisms of microthrombi formation after experimental subarachnoid hemorrhage. Neuroscience. 2012;224:26–37. doi: 10.1016/j.neuroscience.2012.08.002. [DOI] [PubMed] [Google Scholar]
- Sabri M, Ai J, Lass E, D’Abbondanza J, Macdonald RL. Genetic elimination of eNOS reduces secondary complications of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2013a doi: 10.1038/jcbfm.2013.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabri M, Ai J, Macdonald RL. Dissociation of vasospasm and secondary effects of experimental subarachnoid hemorrhage by clazosentan. Stroke. 2011;42:1454–1460. doi: 10.1161/STROKEAHA.110.604728. [DOI] [PubMed] [Google Scholar]
- Sabri M, Lass E, Macdonald RL. Early brain injury: a common mechanism in subarachnoid hemorrhage and global cerebral ischemia. Stroke Res Treat. 2013b;2013:394036. doi: 10.1155/2013/394036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, Elkind MS, George MG, Hamdan AD, Higashida RT, Hoh BL, Janis LS, Kase CS, Kleindorfer DO, Lee JM, Moseley ME, Peterson ED, Turan TN, Valderrama AL, Vinters HV. An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2013 doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakowitz OW, Santos E, Nagel A, Krajewski KL, Hertle DN, Vajkoczy P, Dreier JP, Unterberg AW, Sarrafzadeh AS. Clusters of spreading depolarizations are associated with disturbed cerebral metabolism in patients with aneurysmal subarachnoid hemorrhage. Stroke. 2013;44:220–223. doi: 10.1161/STROKEAHA.112.672352. [DOI] [PubMed] [Google Scholar]
- Sanchez-Porras R, Zheng Z, Santos E, Scholl M, Unterberg AW, Sakowitz OW. The role of spreading depolarization in subarachnoid hemorrhage. Eur J Neurol. 2013 doi: 10.1111/ene.12139. [DOI] [PubMed] [Google Scholar]
- Santos Carvalho C, Resende F, Joao Centeno M, Ribeiro I, Moreira J. Anesthetic Approach of Pregnant Woman with Cerebral Arteriovenous Malformation and Subarachnoid Hemorrhage during Pregnancy: Case Report. Rev Bras Anestesiol. 2013;63:223–226. doi: 10.1016/j.bjane.2012.05.004. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh A, Copin JC, Bengualid DJ, Turck N, Vajkoczy P, Bijlenga P, Schaller K, Gasche Y. Matrix metalloproteinase-9 concentration in the cerebral extracellular fluid of patients during the acute phase of aneurysmal subarachnoid hemorrhage. Neurol Res. 2012;34:455–461. doi: 10.1179/1743132812Y.0000000018. [DOI] [PubMed] [Google Scholar]
- Sarrafzadeh AS, Nagel A, Czabanka M, Denecke T, Vajkoczy P, Plotkin M. Imaging of hypoxic-ischemic penumbra with (18)F-fluoromisonidazole PET/CT and measurement of related cerebral metabolism in aneurysmal subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2010;30:36–45. doi: 10.1038/jcbfm.2009.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh S, Takayasu M, Kawasaki K, Ikegaki I, Hitomi A, Yano K, Shibuya M, Asano T. Antivasospastic effects of hydroxyfasudil, a Rho-kinase inhibitor, after subarachnoid hemorrhage. J Pharmacol Sci. 2012;118:92–98. doi: 10.1254/jphs.11075fp. [DOI] [PubMed] [Google Scholar]
- Saver JL, Kidwell C, Eckstein M, Starkman S. Prehospital neuroprotective therapy for acute stroke: results of the Field Administration of Stroke Therapy-Magnesium (FAST-MAG) pilot trial. Stroke. 2004;35:e106–108. doi: 10.1161/01.STR.0000124458.98123.52. [DOI] [PubMed] [Google Scholar]
- Savitz SI. A critical appraisal of the NXY-059 neuroprotection studies for acute stroke: a need for more rigorous testing of neuroprotective agents in animal models of stroke. Exp Neurol. 2007;205:20–25. doi: 10.1016/j.expneurol.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Schebesch KM, Brawanski A, Kagerbauer SM, Martin J, Bele S, Herbst A, Feigl G, Stoerr EM, Lohmeier A, Proescholdt M. The possible role of neuropeptide Y after spontaneous subarachnoid hemorrhage. Acta Neurochir (Wien) 2011;153:1663–1668. doi: 10.1007/s00701-011-1056-8. discussion 1668. [DOI] [PubMed] [Google Scholar]
- Schipke CG, Kettenmann H. Astrocyte responses to neuronal activity. Glia. 2004;47:226–232. doi: 10.1002/glia.20029. [DOI] [PubMed] [Google Scholar]
- Schlenk F, Nagel A, Graetz D, Sarrafzadeh AS. Hyperglycemia and cerebral glucose in aneurysmal subarachnoid hemorrhage. Intensive Care Med. 2008;34:1200–1207. doi: 10.1007/s00134-008-1044-5. [DOI] [PubMed] [Google Scholar]
- Schoffer KL, Benstead TJ, Grant I. Spontaneous intracranial hypotension in the absence of magnetic resonance imaging abnormalities. Can J Neurol Sci. 2002;29:253–257. doi: 10.1017/s0317167100002031. [DOI] [PubMed] [Google Scholar]
- Scholler K, Trinkl A, Klopotowski M, Thal SC, Plesnila N, Trabold R, Hamann GF, Schmid-Elsaesser R, Zausinger S. Characterization of microvascular basal lamina damage and blood-brain barrier dysfunction following subarachnoid hemorrhage in rats. Brain Res. 2007;1142:237–246. doi: 10.1016/j.brainres.2007.01.034. [DOI] [PubMed] [Google Scholar]
- Schulz MK, Wang LP, Tange M, Bjerre P. Cerebral microdialysis monitoring: determination of normal and ischemic cerebral metabolisms in patients with aneurysmal subarachnoid hemorrhage. J Neurosurg. 2000;93:808–814. doi: 10.3171/jns.2000.93.5.0808. [DOI] [PubMed] [Google Scholar]
- Schwartz AY, Masago A, Sehba FA, Bederson JB. Experimental models of subarachnoid hemorrhage in the rat: a refinement of the endovascular filament model. J Neurosci Methods. 2000a;96:161–167. doi: 10.1016/s0165-0270(00)00156-4. [DOI] [PubMed] [Google Scholar]
- Schwartz AY, Sehba FA, Bederson JB. Decreased nitric oxide availability contributes to acute cerebral ischemia after subarachnoid hemorrhage. Neurosurgery. 2000b;47:208–214. doi: 10.1097/00006123-200007000-00042. discussion 214–205. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Chereshnev I, Maayani S, Friedrich V, Jr, Bederson JB. Nitric oxide synthase in acute alteration of nitric oxide levels after subarachnoid hemorrhage. Neurosurgery. 2004a;55:671–677. doi: 10.1227/01.neu.0000134557.82423.b2. discussion 677–678. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Friedrich V, Jr, Makonnen G, Bederson JB. Acute cerebral vascular injury after subarachnoid hemorrhage and its prevention by administration of a nitric oxide donor. J Neurosurg. 2007;106:321–329. doi: 10.3171/jns.2007.106.2.321. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Hou J, Pluta RM, Zhang JH. The importance of early brain injury after subarachnoid hemorrhage. Prog Neurobiol. 2012;97:14–37. doi: 10.1016/j.pneurobio.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehba FA, Mostafa G, Friedrich V, Jr, Bederson JB. Acute microvascular platelet aggregation after subarachnoid hemorrhage. J Neurosurg. 2005;102:1094–1100. doi: 10.3171/jns.2005.102.6.1094. [DOI] [PubMed] [Google Scholar]
- Sehba FA, Mostafa G, Knopman J, Friedrich V, Jr, Bederson JB. Acute alterations in microvascular basal lamina after subarachnoid hemorrhage. J Neurosurg. 2004b;101:633–640. doi: 10.3171/jns.2004.101.4.0633. [DOI] [PubMed] [Google Scholar]
- Sercombe R, Dinh YR, Gomis P. Cerebrovascular inflammation following subarachnoid hemorrhage. Jpn J Pharmacol. 2002;88:227–249. doi: 10.1254/jjp.88.227. [DOI] [PubMed] [Google Scholar]
- Sermet A, Tasdemir N, Deniz B, Atmaca M. Time-dependent changes in superoxide dismutase, catalase, xanthine dehydrogenase and oxidase activities in focal cerebral ischaemia. Cytobios. 2000;102:157–172. [PubMed] [Google Scholar]
- Shah AH, Komotar RJ. Pathophysiology of Acute Hydrocephalus After Subarachnoid Hemorrhage. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.01.110. [DOI] [PubMed] [Google Scholar]
- Sherlock M, O’Sullivan E, Agha A, Behan LA, Rawluk D, Brennan P, Tormey W, Thompson CJ. The incidence and pathophysiology of hyponatraemia after subarachnoid haemorrhage. Clin Endocrinol (Oxf) 2006;64:250–254. doi: 10.1111/j.1365-2265.2006.02432.x. [DOI] [PubMed] [Google Scholar]
- Shin HK, Lee JH, Kim KY, Kim CD, Lee WS, Rhim BY, Hong KW. Impairment of autoregulatory vasodilation by NAD(P)H oxidase-dependent superoxide generation during acute stage of subarachnoid hemorrhage in rat pial artery. J Cereb Blood Flow Metab. 2002;22:869–877. doi: 10.1097/00004647-200207000-00012. [DOI] [PubMed] [Google Scholar]
- Siesjo BK, Plum F. Cerebral energy metabolism in normoxia and in hypoxia. Acta Anaesthesiol Scand Suppl. 1971;45:81–101. doi: 10.1111/j.1399-6576.1971.tb00662.x. [DOI] [PubMed] [Google Scholar]
- Simard JM, Geng Z, Woo SK, Ivanova S, Tosun C, Melnichenko L, Gerzanich V. Glibenclamide reduces inflammation, vasogenic edema, and caspase-3 activation after subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2009;29:317–330. doi: 10.1038/jcbfm.2008.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Tosun C, Ivanova S, Kurland DB, Hong C, Radecki L, Gisriel C, Mehta R, Schreibman D, Gerzanich V. Heparin Reduces Neuroinflammation and Transsynaptic Neuronal Apoptosis in a Model of Subarachnoid Hemorrhage. Transl Stroke Res. 2012a;3:155–165. doi: 10.1007/s12975-012-0166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simard JM, Woo SK, Schwartzbauer GT, Gerzanich V. Sulfonylurea receptor 1 in central nervous system injury: a focused review. J Cereb Blood Flow Metab. 2012b;32:1699–1717. doi: 10.1038/jcbfm.2012.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simeone FA, Ryan KG, Cotter JR. Prolonged experimental cerebral vasospasm. J Neurosurg. 1968;29:357–366. doi: 10.3171/jns.1968.29.4.0357. [DOI] [PubMed] [Google Scholar]
- Smithason S, Moore SK, Provencio JJ. Systemic administration of LPS worsens delayed deterioration associated with vasospasm after subarachnoid hemorrhage through a myeloid cell-dependent mechanism. Neurocrit Care. 2012;16:327–334. doi: 10.1007/s12028-011-9651-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehle M, Chatfield DA, Czosnyka M, Kirkpatrick PJ. Predictive value of initial clinical status, intracranial pressure and transcranial Doppler pulsatility after subarachnoid haemorrhage. Acta Neurochir (Wien) 2007;149:575–583. doi: 10.1007/s00701-007-1149-6. [DOI] [PubMed] [Google Scholar]
- Somjen GG. Mechanisms of spreading depression and hypoxic spreading depression-like depolarization. Physiol Rev. 2001;81:1065–1096. doi: 10.1152/physrev.2001.81.3.1065. [DOI] [PubMed] [Google Scholar]
- Sonn J, Mayevsky A. Effects of brain oxygenation on metabolic, hemodynamic, ionic and electrical responses to spreading depression in the rat. Brain Res. 2000;882:212–216. doi: 10.1016/s0006-8993(00)02827-4. [DOI] [PubMed] [Google Scholar]
- Sozen T, Tsuchiyama R, Hasegawa Y, Suzuki H, Jadhav V, Nishizawa S, Zhang JH. Role of interleukin-1beta in early brain injury after subarachnoid hemorrhage in mice. Stroke. 2009;40:2519–2525. doi: 10.1161/STROKEAHA.109.549592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperlagh B, Vizi ES, Wirkner K, Illes P. P2X7 receptors in the nervous system. Prog Neurobiol. 2006;78:327–346. doi: 10.1016/j.pneurobio.2006.03.007. [DOI] [PubMed] [Google Scholar]
- Staalso JM, Bergstrom A, Edsen T, Weikop P, Romner B, Olsen NV. Low plasma arginine:asymmetric dimethyl arginine ratios predict mortality after intracranial aneurysm rupture. Stroke. 2013;44:1273–1281. doi: 10.1161/STROKEAHA.111.000605. [DOI] [PubMed] [Google Scholar]
- Stein SC, Browne KD, Chen XH, Smith DH, Graham DI. Thromboembolism and delayed cerebral ischemia after subarachnoid hemorrhage: an autopsy study. Neurosurgery. 2006;59:781–787. doi: 10.1227/01.NEU.0000227519.27569.45. discussion 787–788. [DOI] [PubMed] [Google Scholar]
- Strong AJ, Anderson PJ, Watts HR, Virley DJ, Lloyd A, Irving EA, Nagafuji T, Ninomiya M, Nakamura H, Dunn AK, Graf R. Peri-infarct depolarizations lead to loss of perfusion in ischaemic gyrencephalic cerebral cortex. Brain. 2007;130:995–1008. doi: 10.1093/brain/awl392. [DOI] [PubMed] [Google Scholar]
- Suda N, Moriyama K, Ganburged G. Effect of angiotensin II receptor blocker on experimental periodontitis in a mouse model of Marfan syndrome. Infect Immun. 2013;81:182–188. doi: 10.1128/IAI.00886-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugai K, Yaganisawa T, Motohashi O, Suzuki M, Yoshimoto T. Levcromakalim decreases cytoplasmic Ca2+ and vascular tone in basilar artery of SAH model dogs. J Cardiovasc Pharmacol. 1999;33:868–875. doi: 10.1097/00005344-199906000-00006. [DOI] [PubMed] [Google Scholar]
- Sugawara T, Ayer R, Jadhav V, Chen W, Tsubokawa T, Zhang JH. Simvastatin attenuation of cerebral vasospasm after subarachnoid hemorrhage in rats via increased phosphorylation of Akt and endothelial nitric oxide synthase. J Neurosci Res. 2008;86:3635–3643. doi: 10.1002/jnr.21807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukhotinsky I, Yaseen MA, Sakadzic S, Ruvinskaya S, Sims JR, Boas DA, Moskowitz MA, Ayata C. Perfusion pressure-dependent recovery of cortical spreading depression is independent of tissue oxygenation over a wide physiologic range. J Cereb Blood Flow Metab. 2010;30:1168–1177. doi: 10.1038/jcbfm.2009.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Hasegawa Y, Chen W, Kanamaru K, Zhang JH. Recombinant osteopontin in cerebral vasospasm after subarachnoid hemorrhage. Ann Neurol. 2010a;68:650–660. doi: 10.1002/ana.22102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH. Mechanisms of osteopontin-induced stabilization of blood-brain barrier disruption after subarachnoid hemorrhage in rats. Stroke. 2010b;41:1783–1790. doi: 10.1161/STROKEAHA.110.586537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H, Kinoshita N, Imanaka-Yoshida K, Yoshida T, Taki W. Cerebrospinal fluid tenascin-C increases preceding the development of chronic shunt-dependent hydrocephalus after subarachnoid hemorrhage. Stroke. 2008;39:1610–1612. doi: 10.1161/STROKEAHA.107.505735. [DOI] [PubMed] [Google Scholar]
- Suzuki H, Shiba M, Fujimoto M, Kawamura K, Nanpei M, Tekeuchi E, Matsushima S, Kanamaru K, Imanaka-Yoshida K, Yoshida T, Taki W. Matricellular protein: a new player in cerebral vasospasm following subarachnoid hemorrhage. Acta Neurochir Suppl. 2013;115:213–218. doi: 10.1007/978-3-7091-1192-5_39. [DOI] [PubMed] [Google Scholar]
- Suzuki J, Komatsu S, Sato T, Sakurai Y. Correlation between CT findings and subsequent development of cerebral infarction due to vasospasm in subarachnoid haemorrhage. Acta Neurochir (Wien) 1980;55:63–70. doi: 10.1007/BF01808921. [DOI] [PubMed] [Google Scholar]
- Suzuki S, Suzuki M, Iwabuchi T, Kamata Y. Role of multiple cerebral microthrombosis in symptomatic cerebral vasospasm: with a case report. Neurosurgery. 1983;13:199–203. doi: 10.1227/00006123-198308000-00018. [DOI] [PubMed] [Google Scholar]
- Sviri GE, Britz GW, Lewis DH, Newell DW, Zaaroor M, Cohen W. Dynamic perfusion computed tomography in the diagnosis of cerebral vasospasm. Neurosurgery. 2006;59:319–325. doi: 10.1227/01.NEU.0000222819.18834.33. discussion 319–325. [DOI] [PubMed] [Google Scholar]
- Tajiri N, Dailey T, Metcalf C, Mosley YI, Lau T, Staples M, van Loveren H, Kim SU, Yamashima T, Yasuhara T, Date I, Kaneko Y, Borlongan CV. In vivo animal stroke models: a rationale for rodent and non-human primate models. Transl Stroke Res. 2013;4:308–321. doi: 10.1007/s12975-012-0241-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takai Y, Sasaki T, Tanaka K, Nakanishi H. Rho as a regulator of the cytoskeleton. Trends Biochem Sci. 1995;20:227–231. doi: 10.1016/s0968-0004(00)89022-2. [DOI] [PubMed] [Google Scholar]
- Takanashi Y, Weir BK, Vollrath B, Kasuya H, Macdonald RL, Cook D. Time course of changes in concentration of intracellular free calcium in cultured cerebrovascular smooth muscle cells exposed to oxyhemoglobin. Neurosurgery. 1992;30:346–350. doi: 10.1227/00006123-199203000-00006. [DOI] [PubMed] [Google Scholar]
- Takano T, Tian GF, Peng W, Lou N, Lovatt D, Hansen AJ, Kasischke KA, Nedergaard M. Cortical spreading depression causes and coincides with tissue hypoxia. Nat Neurosci. 2007;10:754–762. doi: 10.1038/nn1902. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Yamada H, Sakai N, Ando T, Nakashima T, Nishimura Y, Okano Y, Nozawa Y. Cytosolic calcium changes in cultured rat aortic smooth-muscle cells induced by oxyhemoglobin. J Neurosurg. 1991a;74:620–624. doi: 10.3171/jns.1991.74.4.0620. [DOI] [PubMed] [Google Scholar]
- Takenaka K, Yamada H, Sakai N, Ando T, Okano Y, Nozawa Y. Intracellular Ca2+ changes in cultured vascular smooth muscle cells by treatment with various spasmogens. Neurol Res. 1991b;13:168–172. doi: 10.1080/01616412.1991.11739985. [DOI] [PubMed] [Google Scholar]
- Takeuchi H, Jin S, Wang J, Zhang G, Kawanokuchi J, Kuno R, Sonobe Y, Mizuno T, Suzumura A. Tumor necrosis factor-alpha induces neurotoxicity via glutamate release from hemichannels of activated microglia in an autocrine manner. J Biol Chem. 2006;281:21362–21368. doi: 10.1074/jbc.M600504200. [DOI] [PubMed] [Google Scholar]
- Tanriverdi T, Sanus GZ, Ulu MO, Tureci E, Uzun H, Aydin S, Kaynar MY. Serum and cerebrospinal fluid concentrations of E-selectin in patients with aneurysmal subarachnoid hemorrhage. Braz J Med Biol Res. 2005;38:1703–1710. doi: 10.1590/s0100-879x2005001100020. [DOI] [PubMed] [Google Scholar]
- Taub PR, Fields JD, Wu AH, Miss JC, Lawton MT, Smith WS, Young WL, Zaroff JG, Ko NU. Elevated BNP is associated with vasospasm-independent cerebral infarction following aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2011;15:13–18. doi: 10.1007/s12028-011-9535-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Terry J. The major electrolytes: sodium, potassium, and chloride. J Intraven Nurs. 1994;17:240–247. [PubMed] [Google Scholar]
- Testai FD, Hillmann M, Amin-Hanjani S, Gorshkova I, Berdyshev E, Gorelick PB, Dawson G. Changes in the cerebrospinal fluid ceramide profile after subarachnoid hemorrhage. Stroke. 2012;43:2066–2070. doi: 10.1161/STROKEAHA.112.650390. [DOI] [PubMed] [Google Scholar]
- Tibbs GR, Rowley TJ, Sanford RL, Herold KF, Proekt A, Hemmings HC, Jr, Andersen OS, Goldstein PA, Flood PD. HCN1 Channels as Targets for Anesthetic and Nonanesthetic Propofol Analogs in the Amelioration of Mechanical and Thermal Hyperalgesia in a Mouse Model of Neuropathic Pain. J Pharmacol Exp Ther. 2013;345:363–373. doi: 10.1124/jpet.113.203620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Titova E, Ostrowski RP, Zhang JH, Tang J. Experimental models of subarachnoid hemorrhage for studies of cerebral vasospasm. Neurol Res. 2009;31:568–581. doi: 10.1179/174313209X382412. [DOI] [PubMed] [Google Scholar]
- Tso M, Loch Macdonald R. A Need for a Standardized Cognitive Outcome Measure in Subarachnoid Hemorrhage Clinical Studies. World Neurosurg. 2013 doi: 10.1016/j.wneu.2013.03.020. [DOI] [PubMed] [Google Scholar]
- Uhl E, Lehmberg J, Steiger HJ, Messmer K. Intraoperative detection of early microvasospasm in patients with subarachnoid hemorrhage by using orthogonal polarization spectral imaging. Neurosurgery. 2003;52:1307–1315. doi: 10.1227/01.neu.0000065154.04824.9e. disacussion 1315–1307. [DOI] [PubMed] [Google Scholar]
- Urban L, Neill KH, Crain BJ, Nadler JV, Somjen GG. Postischemic synaptic physiology in area CA1 of the gerbil hippocampus studied in vitro. J Neurosci. 1989;9:3966–3975. doi: 10.1523/JNEUROSCI.09-11-03966.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urra X, Chamorro A. Emerging issues in acute ischemic stroke. J Neurol. 2013 doi: 10.1007/s00415-013-6919-x. [DOI] [PubMed] [Google Scholar]
- van den Bergh WM, Dijkhuizen RM, Rinkel GJ. Potentials of magnesium treatment in subarachnoid haemorrhage. Magnes Res. 2004;17:301–313. [PubMed] [Google Scholar]
- Veelken JA, Laing RJ, Jakubowski J. The Sheffield model of subarachnoid hemorrhage in rats. Stroke. 1995;26:1279–1283. doi: 10.1161/01.str.26.7.1279. discussion 1284. [DOI] [PubMed] [Google Scholar]
- Venti M. Subarachnoid and intraventricular hemorrhage. Front Neurol Neurosci. 2012;30:149–153. doi: 10.1159/000333625. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Ilodigwe D, Macdonald RL. Cerebral infarction after subarachnoid hemorrhage contributes to poor outcome by vasospasm-dependent and -independent effects. Stroke. 2011;42:924–929. doi: 10.1161/STROKEAHA.110.597914. [DOI] [PubMed] [Google Scholar]
- Vergouwen MD, Vermeulen M, Coert BA, Stroes ES, Roos YB. Microthrombosis after aneurysmal subarachnoid hemorrhage: an additional explanation for delayed cerebral ischemia. J Cereb Blood Flow Metab. 2008;28:1761–1770. doi: 10.1038/jcbfm.2008.74. [DOI] [PubMed] [Google Scholar]
- Viola HM, Hool LC. Role of the cytoskeleton in communication between L-type Ca(2+) channels and mitochondria. Clin Exp Pharmacol Physiol. 2013;40:295–304. doi: 10.1111/1440-1681.12072. [DOI] [PubMed] [Google Scholar]
- Viswanathan G, Nair S, Chandrasekhar K, Vishnupuri R. Cerebellar low-grade oligoastrocytoma presenting with subarachnoid haemorrhage. Turk Neurosurg. 2012;22:382–385. doi: 10.5137/1019-5149.JTN.3644-10.1. [DOI] [PubMed] [Google Scholar]
- Voldby B, Enevoldsen EM. Intracranial pressure changes following aneurysm rupture. Part 1: clinical and angiographic correlations. J Neurosurg. 1982;56:186–196. doi: 10.3171/jns.1982.56.2.0186. [DOI] [PubMed] [Google Scholar]
- Walcott BP, Kahle KT, Simard JM. Novel treatment targets for cerebral edema. Neurotherapeutics. 2012;9:65–72. doi: 10.1007/s13311-011-0087-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, Yang TM, Lin WC, Lin YJ, Tsai NW, Liou CW, Kwan AL, Lu CH. The value of serial plasma and cerebrospinal fluid nuclear and mitochondrial deoxyribonucleic acid levels in aneurysmal subarachnoid hemorrhage. J Neurosurg. 2013a;118:13–19. doi: 10.3171/2012.8.JNS112093. [DOI] [PubMed] [Google Scholar]
- Wang MM, Xi G, Keep RF. Should the STAIR criteria be modified for preconditioning studies? Transl Stroke Res. 2013b;4:3–14. doi: 10.1007/s12975-012-0219-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Shi XY, Yin J, Zuo G, Zhang J, Chen G. Role of autophagy in early brain injury after experimental subarachnoid hemorrhage. J Mol Neurosci. 2012;46:192–202. doi: 10.1007/s12031-011-9575-6. [DOI] [PubMed] [Google Scholar]
- Wang Z, Zuo G, Shi XY, Zhang J, Fang Q, Chen G. Progesterone administration modulates cortical TLR4/NF-kappaB signaling pathway after subarachnoid hemorrhage in male rats. Mediators Inflamm. 2011;2011:848309. doi: 10.1155/2011/848309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidauer S, Vatter H, Beck J, Raabe A, Lanfermann H, Seifert V, Zanella F. Focal laminar cortical infarcts following aneurysmal subarachnoid haemorrhage. Neuroradiology. 2008;50:1–8. doi: 10.1007/s00234-007-0294-1. [DOI] [PubMed] [Google Scholar]
- Wellman GC, Koide M. Impact of subarachnoid hemorrhage on parenchymal arteriolar function. Acta Neurochir Suppl. 2013;115:173–177. doi: 10.1007/978-3-7091-1192-5_33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen YD, Sheng R, Zhang LS, Han R, Zhang X, Zhang XD, Han F, Fukunaga K, Qin ZH. Neuronal injury in rat model of permanent focal cerebral ischemia is associated with activation of autophagic and lysosomal pathways. Autophagy. 2008;4:762–769. doi: 10.4161/auto.6412. [DOI] [PubMed] [Google Scholar]
- Westermaier T, Jauss A, Eriskat J, Kunze E, Roosen K. Time-course of cerebral perfusion and tissue oxygenation in the first 6 h after experimental subarachnoid hemorrhage in rats. J Cereb Blood Flow Metab. 2009;29:771–779. doi: 10.1038/jcbfm.2008.169. [DOI] [PubMed] [Google Scholar]
- Westermaier T, Stetter C, Raslan F, Vince GH, Ernestus RI. Brain edema formation correlates with perfusion deficit during the first six hours after experimental subarachnoid hemorrhage in rats. Exp Transl Stroke Med. 2012;4:8. doi: 10.1186/2040-7378-4-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyer GW, Jahromi BS, Aihara Y, Agbaje-Williams M, Nikitina E, Zhang ZD, Macdonald RL. Expression and function of inwardly rectifying potassium channels after experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2006;26:382–391. doi: 10.1038/sj.jcbfm.9600193. [DOI] [PubMed] [Google Scholar]
- Wiernsperger N, Schulz U, Gygax P. Physiological and morphometric analysis of the microcirculation of the cerebral cortex under acute vasospasm. Stroke. 1981;12:624–627. doi: 10.1161/01.str.12.5.624. [DOI] [PubMed] [Google Scholar]
- Wijdicks EF, Vermeulen M, ten Haaf JA, Hijdra A, Bakker WH, van Gijn J. Volume depletion and natriuresis in patients with a ruptured intracranial aneurysm. Ann Neurol. 1985;18:211–216. doi: 10.1002/ana.410180208. [DOI] [PubMed] [Google Scholar]
- Wilkins RH. Cerebral vasospasm. Crit Rev Neurobiol. 1990;6:51–77. [PubMed] [Google Scholar]
- Winkler MK, Chassidim Y, Lublinsky S, Revankar GS, Major S, Kang EJ, Oliveira-Ferreira AI, Woitzik J, Sandow N, Scheel M, Friedman A, Dreier JP. Impaired neurovascular coupling to ictal epileptic activity and spreading depolarization in a patient with subarachnoid hemorrhage: possible link to blood-brain barrier dysfunction. Epilepsia. 2012;53(Suppl 6):22–30. doi: 10.1111/j.1528-1167.2012.03699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wintermark M, Ko NU, Smith WS, Liu S, Higashida RT, Dillon WP. Vasospasm after subarachnoid hemorrhage: utility of perfusion CT and CT angiography on diagnosis and management. AJNR Am J Neuroradiol. 2006;27:26–34. [PMC free article] [PubMed] [Google Scholar]
- Woernle CM, Winkler KM, Burkhardt JK, Haile SR, Bellut D, Neidert MC, Bozinov O, Krayenbuhl N, Bernays RL. Hydrocephalus in 389 patients with aneurysm-associated subarachnoid hemorrhage. J Clin Neurosci. 2013 doi: 10.1016/j.jocn.2012.07.015. [DOI] [PubMed] [Google Scholar]
- Wong GK, Lam SW, Wong A, Ngai K, Poon WS, Mok V. Comparison of montreal cognitive assessment and mini-mental state examination in evaluating cognitive domain deficit following aneurysmal subarachnoid haemorrhage. PLoS One. 2013;8:e59946. doi: 10.1371/journal.pone.0059946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong GK, Poon WS, Chan MT, Boet R, Gin T, Ng SC, Zee BC. Intravenous magnesium sulphate for aneurysmal subarachnoid hemorrhage (IMASH): a randomized, double-blinded, placebo-controlled, multicenter phase III trial. Stroke. 2010;41:921–926. doi: 10.1161/STROKEAHA.109.571125. [DOI] [PubMed] [Google Scholar]
- Wu CT, Wen LL, Wong CS, Tsai SY, Chan SM, Yeh CC, Borel CO, Cherng CH. Temporal changes in glutamate, glutamate transporters, basilar arteries wall thickness, and neuronal variability in an experimental rat model of subarachnoid hemorrhage. Anesth Analg. 2011;112:666–673. doi: 10.1213/ANE.0b013e318207c51f. [DOI] [PubMed] [Google Scholar]
- Xiao Y, Jin J, Chang M, Chang JH, Hu H, Zhou X, Brittain GC, Stansberg C, Torkildsen O, Wang X, Brink R, Cheng X, Sun SC. Peli1 promotes microglia-mediated CNS inflammation by regulating Traf3 degradation. Nat Med. 2013;19:595–602. doi: 10.1038/nm.3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Li L, Khatibi NH, Yang L, Wang K, Zhang W, Martin RD, Han J, Zhang J, Zhou C. Blood-brain barrier disruption following subarchnoid hemorrhage may be faciliated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp Neurol. 2011;230:240–247. doi: 10.1016/j.expneurol.2011.04.022. [DOI] [PubMed] [Google Scholar]
- Yan J, Manaenko A, Chen S, Klebe D, Ma Q, Caner B, Fujii M, Zhou C, Zhang JH. Role of SCH79797 in Maintaining Vascular Integrity in Rat Model of Subarachnoid Hemorrhage. Stroke. 2013;44:1410–1417. doi: 10.1161/STROKEAHA.113.678474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L, Kowalski JR, Zhan X, Thomas SM, Luscinskas FW. Endothelial cell cortactin phosphorylation by Src contributes to polymorphonuclear leukocyte transmigration in vitro. Circ Res. 2006;98:394–402. doi: 10.1161/01.RES.0000201958.59020.1a. [DOI] [PubMed] [Google Scholar]
- Yang TC, Chang CH, Liu YT, Chen YL, Tu PH, Chen HC. Predictors of Shunt-Dependent Chronic Hydrocephalus after Aneurysmal Subarachnoid Haemorrhage. Eur Neurol. 2013;69:296–303. doi: 10.1159/000346119. [DOI] [PubMed] [Google Scholar]
- Yang TM, Lin YJ, Tsai NW, Lin WC, Ho JT, Chang WN, Cheng BC, Kung CT, Lee TH, Huang CC, Wang HC, Lu CH. The prognostic value of serial leukocyte adhesion molecules in post-aneurysmal subarachnoid hemorrhage. Clin Chim Acta. 2012;413:411–416. doi: 10.1016/j.cca.2011.10.007. [DOI] [PubMed] [Google Scholar]
- Yang ZW, Gebrewold A, Nowakowski M, Altura BT, Altura BM. Mg(2+)-induced endothelium-dependent relaxation of blood vessels and blood pressure lowering: role of NO. Am J Physiol Regul Integr Comp Physiol. 2000;278:R628–639. doi: 10.1152/ajpregu.2000.278.3.R628. [DOI] [PubMed] [Google Scholar]
- Yasargil MG, Yonekawa Y, Zumstein B, Stahl HJ. Hydrocephalus following spontaneous subarachnoid hemorrhage. Clinical features and treatment. J Neurosurg. 1973;39:474–479. doi: 10.3171/jns.1973.39.4.0474. [DOI] [PubMed] [Google Scholar]
- Yatsushige H, Ostrowski RP, Tsubokawa T, Colohan A, Zhang JH. Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J Neurosci Res. 2007;85:1436–1448. doi: 10.1002/jnr.21281. [DOI] [PubMed] [Google Scholar]
- Yeo M, Berglund K, Hanna M, Guo JU, Kittur J, Torres MD, Abramowitz J, Busciglio J, Gao Y, Birnbaumer L, Liedtke WB. Bisphenol A delays the perinatal chloride shift in cortical neurons by epigenetic effects on the Kcc2 promoter. Proc Natl Acad Sci U S A. 2013;110:4315–4320. doi: 10.1073/pnas.1300959110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilman M, Cokluk C, Baydin A, Yardan T, Kati C, Gunay M, Meric M. Is there a relationship between serum heart- type fatty acid binding protein level and clinical severity in patients with subarachnoid hemorrhage? Turk Neurosurg. 2012;22:695–700. doi: 10.5137/1019-5149.JTN.5847-12.2. [DOI] [PubMed] [Google Scholar]
- You WC, Wang CX, Pan YX, Zhang X, Zhou XM, Zhang XS, Shi JX, Zhou ML. Activation of nuclear factor-kappaB in the brain after experimental subarachnoid hemorrhage and its potential role in delayed brain injury. PLoS One. 2013;8:e60290. doi: 10.1371/journal.pone.0060290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuksel S, Tosun YB, Cahill J, Solaroglu I. Early brain injury following aneurysmal subarachnoid hemorrhage: emphasis on cellular apoptosis. Turk Neurosurg. 2012;22:529–533. doi: 10.5137/1019-5149.JTN.5731-12.1. [DOI] [PubMed] [Google Scholar]
- Yun X, Maximov VD, Yu J, Zhu H, Vertegel AA, Kindy MS. Nanoparticles for targeted delivery of antioxidant enzymes to the brain after cerebral ischemia and reperfusion injury. J Cereb Blood Flow Metab. 2013;33:583–592. doi: 10.1038/jcbfm.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanier ER, Longhi L, Fiorini M, Cracco L, Bersano A, Zoerle T, Branca V, Monaco S, Stocchetti N. Increased levels of CSF heart-type fatty acid-binding protein and tau protein after aneurysmal subarachnoid hemorrhage. Acta Neurochir Suppl. 2008;102:339–343. doi: 10.1007/978-3-211-85578-2_65. [DOI] [PubMed] [Google Scholar]
- Zanier ER, Zoerle T, Fiorini M, Longhi L, Cracco L, Bersano A, Branca V, Benedetti MD, De Simoni MG, Monaco S, Stocchetti N. Heart-fatty acid-binding and tau proteins relate to brain injury severity and long-term outcome in subarachnoid haemorrhage patients. Br J Anaesth. 2013 doi: 10.1093/bja/aet149. [DOI] [PubMed] [Google Scholar]
- Zhan Y, Chen C, Suzuki H, Hu Q, Zhi X, Zhang JH. Hydrogen gas ameliorates oxidative stress in early brain injury after subarachnoid hemorrhage in rats. Crit Care Med. 2012;40:1291–1296. doi: 10.1097/CCM.0b013e31823da96d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Weir BK, Macdonald RL, Marton LS, Solenski NJ, Kwan AL, Lee KS. Mechanisms of [Ca++]i elevation induced by erythrocyte components in endothelial cells. J Pharmacol Exp Ther. 1996;277:1501–1509. [PubMed] [Google Scholar]
- Zhang JH, Badaut J, Tang J, Obenaus A, Hartman R, Pearce WJ. The vascular neural network--a new paradigm in stroke pathophysiology. Nat Rev Neurol. 2012;8:711–716. doi: 10.1038/nrneurol.2012.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M, An C, Gao Y, Leak RK, Chen J, Zhang F. Emerging roles of Nrf2 and phase II antioxidant enzymes in neuroprotection. Prog Neurobiol. 2013a;100:30–47. doi: 10.1016/j.pneurobio.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Wang L, Liu M, Wu B. Tirilazad for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2010:CD006778. doi: 10.1002/14651858.CD006778.pub2. [DOI] [PubMed] [Google Scholar]
- Zhang X, Yeung PK, McAlonan GM, Chung SS, Chung SK. Transgenic mice over-expressing endothelial endothelin-1 show cognitive deficit with blood-brain barrier breakdown after transient ischemia with long-term reperfusion. Neurobiol Learn Mem. 2013b;101:46–54. doi: 10.1016/j.nlm.2013.01.002. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Zhang H, Duan DD. Chloride channels in stroke. Acta Pharmacol Sin. 2013c;34:17–23. doi: 10.1038/aps.2012.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZD, Yamini B, Komuro T, Ono S, Johns L, Marton LS, Weir B, Macdonald RL. Vasospasm in monkeys resolves because of loss of and encasement of subarachnoid blood clot. Stroke. 2001;32:1868–1874. doi: 10.1161/01.str.32.8.1868. [DOI] [PubMed] [Google Scholar]
- Zhao H, Ji Z, Tang D, Yan C, Zhao W, Gao C. Role of autophagy in early brain injury after subarachnoid hemorrhage in rats. Mol Biol Rep. 2013;40:819–827. doi: 10.1007/s11033-012-2120-z. [DOI] [PubMed] [Google Scholar]
- Zhao X, Aronowski J. Nrf2 to pre-condition the brain against injury caused by products of hemolysis after ICH. Transl Stroke Res. 2013;4:71–75. doi: 10.1007/s12975-012-0245-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou C, Yamaguchi M, Kusaka G, Schonholz C, Nanda A, Zhang JH. Caspase inhibitors prevent endothelial apoptosis and cerebral vasospasm in dog model of experimental subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2004;24:419–431. doi: 10.1097/00004647-200404000-00007. [DOI] [PubMed] [Google Scholar]
- Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM. Detection of copeptin in peripheral blood of patients with aneurysmal subarachnoid hemorrhage. Crit Care. 2011;15:R288. doi: 10.1186/cc10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu XD, Chen JS, Zhou F, Liu QC, Chen G, Zhang JM. Relationship between plasma high mobility group box-1 protein levels and clinical outcomes of aneurysmal subarachnoid hemorrhage. J Neuroinflammation. 2012;9:194. doi: 10.1186/1742-2094-9-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang Z, Zhao X, Wu Y, Huang R, Zhu L, Zhang Y, Shi J. The anti-apoptotic effect of PI3K-Akt signaling pathway after subarachnoid hemorrhage in rats. Ann Clin Lab Sci. 2011;41:364–372. [PubMed] [Google Scholar]
- Zhuang Z, Zhou ML, You WC, Zhu L, Ma CY, Sun XJ, Shi JX. Hydrogen-rich saline alleviates early brain injury via reducing oxidative stress and brain edema following experimental subarachnoid hemorrhage in rabbits. BMC Neurosci. 2012;13:47. doi: 10.1186/1471-2202-13-47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular mechanisms of Alzheimer’s neurodegeneration. Trends Neurosci. 2005;28:202–208. doi: 10.1016/j.tins.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Sinelnikov I, Gruenbaum BF, Gruenbaum SE, Dubilet M, Dubilet E, Leibowitz A, Ohayon S, Regev A, Boyko M, Shapira Y, Teichberg VI. Effect of glutamate and blood glutamate scavengers oxaloacetate and pyruvate on neurological outcome and pathohistology of the hippocampus after traumatic brain injury in rats. Anesthesiology. 2012;116:73–83. doi: 10.1097/ALN.0b013e31823d7731. [DOI] [PubMed] [Google Scholar]
- Zonta M, Angulo MC, Gobbo S, Rosengarten B, Hossmann KA, Pozzan T, Carmignoto G. Neuron-to-astrocyte signaling is central to the dynamic control of brain microcirculation. Nat Neurosci. 2003;6:43–50. doi: 10.1038/nn980. [DOI] [PubMed] [Google Scholar]
- Zubkov AY, Aoki K, Parent AD, Zhang JH. Preliminary study of the effects of caspase inhibitors on vasospasm in dog penetrating arteries. Life Sci. 2002;70:3007–3018. doi: 10.1016/s0024-3205(02)01550-3. [DOI] [PubMed] [Google Scholar]
- Zubkov AY, Ogihara K, Bernanke DH, Parent AD, Zhang J. Apoptosis of endothelial cells in vessels affected by cerebral vasospasm. Surg Neurol. 2000;53:260–266. doi: 10.1016/s0090-3019(99)00187-1. [DOI] [PubMed] [Google Scholar]
- Zubkov AY, Tibbs RE, Clower B, Ogihara K, Aoki K, Zhang JH. Apoptosis in basilar endothelial cells in a canine double hemorrhage model. Acta Neurochir Suppl. 2001;77:29–31. doi: 10.1007/978-3-7091-6232-3_7. [DOI] [PubMed] [Google Scholar]
- Zuccarello M, Boccaletti R, Tosun M, Rapoport RM. Role of extracellular Ca2+ in subarachnoid hemorrhage-induced spasm of the rabbit basilar artery. Stroke. 1996;27:1896–1902. doi: 10.1161/01.str.27.10.1896. [DOI] [PubMed] [Google Scholar]