Abstract
Magnetic resonance spectroscopy (MRS) characteristics of dementia with Lewy bodies (DLB) Alzheimer’s disease (AD) and cognitively normal controls (CN) were compared. DLB (n=34), AD (n=35) and CN (n=148) participated in a MRS study from frontal, posterior cingulate and occipital voxels. We investigated DLB patients with preserved hippocampal volumes to determine the MRS changes in DLB with low probability of overlapping AD pathology. DLB patients were characterized by decreased NAA/Cr in the occipital voxel. AD patients were characterized by lower NAA/Cr in the frontal and posterior cingulate voxels. Normal NAA/Cr levels in the frontal voxel differentiated DLB patients with preserved hippocampal volumes from AD patients. DLB and AD patients had elevated Cho/Cr and mI/Cr in the posterior cingulate. MRS abnormalities associated with loss of neuronal integrity localized to the occipital lobes in DLB, and the posterior cingulate gyri and frontal lobes in AD. This pattern of MRS abnormalities may have a role in differential diagnosis of DLB and in distinguishing DLB patients with overlapping AD pathology.
Keywords: Dementia with Lewy Bodies, Magnetic resonance spectroscopy, Alzheimer’s disease
1. Introduction
Dementia with Lewy bodies (DLB) is the second most common cause of neurodegenerative dementia after Alzheimer’s disease (AD).(Zaccai, et al., 2005) Patients with DLB often have coexisting AD pathology (Galasko, et al., 1994, Gomez-Isla, et al., 1999, Schneider, et al., 2007). The pathological overlap makes it difficult to identify the specific molecular pathology in order to collect relatively homogenous groups for clinical trials. Hippocampal volume on antemortem structural MRI is associated with the presence and severity of neurofibrillary tangle pathology of AD.(Kantarci, et al., 2012a) in the hippocampus of patients with DLB(Murray, et al., 2012). However, hippocampal volumes alone are not adequate as 25% of AD cases have atypical pathologic involvement with 11% being hippocampal sparing AD.(Murray, et al., 2011) Scintigraphy with [(123)I] metaiodobenzyl guanidine quantifies postganglionic sympathetic cardiac innervation and has been used to distinguish DLB from AD.(Yoshita, et al., 2001) Proton magnetic resonance spectroscopy (MRS) may provide novel information about cerebral physiology that cannot be obtained by structural MR.
MRS is unique among imaging modalities as it provides quantitative in vivo assessment of several metabolites during a single scan that are associated with different pathophysiological processes. MRS has been well studied in AD dementia but fewer studies have investigated the MRS findings in DLB. The typical MRS pattern in AD dementia is decreased neuronal integrity marker N-acetylaspartate (NAA) and elevated possible glial marker myo-Inositol (mI).(Huang, et al., 2001, Kantarci, et al., 2000, Miller, et al., 1993, Pfefferbaum, et al., 1999, Schuff, et al., 2002) The MRS in DLB studies are difficult to interpret because many patients with DLB have both AD and Lewy-related pathology in their brains. AD dementia, frontotemporal dementia (FTD), and vascular dementia (VaD) have similarly reduced NAA/Cr, but patients with clinically probable DLB have normal NAA/Cr levels in the posterior cingulate gyri.(Jones and Waldman, 2004, Kantarci, et al., 2004, Kattapong, et al., 1996) Reduction in NAA/Cr in the hippocampi and white matter of DLB subjects has been described (Molina, et al., 2002, Xuan, et al., 2008), but the relative contribution of AD pathology to these findings in these studies is unclear.
Our primary objective in this study was to determine the MRS findings in the posterior cingulate, medial frontal, and medial occipital lobes in DLB. In an attempt to identify DLB patients without overlapping AD pathology, we used preserved hippocampal volumes to characterize DLB patients with a low probability of overlapping AD pathology and autopsy confirmation when available. Thus our secondary objective was to determine the regional MRS findings in patients with probable isolated Lewy body disease.
2. Methods
2.1. Participants
Consecutive patients with DLB (n=34) and AD dementia (n=35) who consented to the MRS study were recruited from the Mayo Clinic Alzheimer Disease Research Center (ADRC) from 8/26/2005 to 7/23/2010. DLB patients met the third Report of the DLB Consortium criteria for probable DLB,(McKeith, et al., 2005) and AD dementia patients met the NINCDS-ADRDA criteria for probable AD. (McKhann, et al., 1984) Cognitively normal (CN) older subjects (n=148) were recruited from both the Mayo Clinic ADRC and Mayo Clinic Study of Aging, which is a longitudinal population-based study (Roberts, et al., 2008) during the same time. Exclusion criteria were: 1) presence of structural abnormalities that could cause cognitive impairment or dementia such as brain tumors 2) concurrent illnesses or treatments interfering with cognitive function other than AD or DLB. Autopsy confirmation was available in 20 subjects through the ADRC Neuropathology Core.
Presence of clinical features of DLB was recorded using the following criteria: 1) Severity of parkinsonism was rated with the Unified Parkinson’s Disease Rating Scale (UPDRS); 2) Visual hallucinations are present if they are fully formed, occurring on more than one occasion and not attributable to medical factors (e.g. infection, postoperative confusion), medications or advanced dementia; 3) Fluctuations are considered present if the patient scored 3 or 4 on the Mayo Fluctuations Questionnaire (Ferman, et al., 2004); 4) Patients with probable REM sleep behavior disorder (pRBD) met the International Classification of Sleep Disorders-II diagnostic criteria B for pRBD (AASM, 2005). All subjects underwent clinical evaluation, MRI/MRS studies within a five month period.
2.2 MRI
MRI examinations were performed at 1.5 Tesla (GE Healthcare). A 3-D high resolution magnetization prepared rapid gradient echo (MPRAGE) acquisition with TR/TE/TI = 7/3/900 ms; flip angle, 8 degrees; in-plane resolution of 1.0 mm, and a slice thickness of 1.2 mm was performed for the automated segmentation of hippocampal volumes and for MRS voxel placements.
Volumes of the hippocampi were computed by combining the right and left hippocampal volumes. Each subject’s high resolution T1-weighted MRI scan was spatially normalized to a custom template with the in-house modified anatomical labeling atlas labels and segmented into grey matter, white matter, and CSF using the unified segmentation model of SPM5. Then for each subject, inverse of the normalization transformation was applied in order to warp the atlas hippocampal ROIs to the subject’s native anatomical space to determine the gray matter volume in the segmented T1-weighted MRIs.
2.3 MRS
MRS studies were performed with the automated single-voxel MRS package (PROBE/SV; GE Healthcare).(Webb, et al., 1994) An 8cm3 (2×2×2 cm) voxel was placed by a trained MRI technologist (MM) on a midsagittal T1-weighted image covering: 1) Right and left posterior cingulate gyri and inferior precunei (posterior cingulate voxel); 2) Medial portion of the right and left superior frontal gyrus (frontal voxel); 3) Right and left medial occipital lobes (occipital voxel) (Figure 1). Point-resolved spectroscopy (PRESS) pulse sequence with repetition time of 2,000 milliseconds, echo time of 30 milliseconds, 2,048 data points, and 128 excitations was used for the examinations. The prescan algorithm of PROBE automatically adjusts the transmitter and receiver gains and center frequency. The local magnetic field homogeneity is optimized with the three-plane auto-shim procedure, and the flip angle of the third water suppression pulse is adjusted for chemical shift water suppression (CHESS) prior to PRESS acquisition. Metabolite intensity ratios are automatically calculated at the end of each PROBE/SV acquisition with the PROBE/QUANT software (Webb, et al., 1994). MRS variables that were analyzed for this study were NAA/Cr, Cho/Cr, and mI/Cr ratios. A number of subjects were missing MRS data because the scan was not completed or had poor quality (full with at half Maximum <4Hz., lipid signal contamination artifacts and poor water suppression) that failed quantitative analysis in the frontal (one CN, one DLB, one AD), posterior cingulate (three CN, one DLB), and occipital (seven CN, two DLB) voxels.
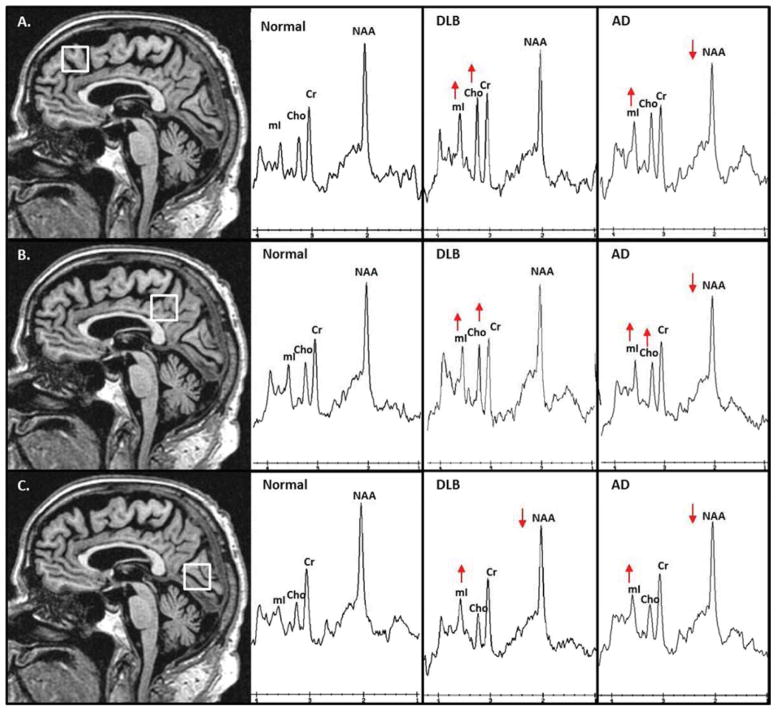
Figure 1. Voxel Locations and Examples of Proton MR Spectra from autopsy confirmed cases.
Frontal Lobe voxel (A) (2×2×2cm) is placed on a mid-sagittal 3D T1-weighted image. Posterior inferior corner of the voxel is the cingulate sulcus at the level of the anterior margin of the lateral ventricles. Spectra from the frontal lobe voxel demonstrate decreased N-acetylaspartate (NAA) and elevated myo-inositol (mI) in the patient with AD; elevated Choline (Cho) and mI in the patient with DLB. Posterior cingulate voxel (B) (2×2×2cm) is placed on a mid-sagittal 3D T1-weighted image. Anterior inferior corner of the voxel is the anterior border of the splenium of the corpus callosum. Spectra from the posterior cingulate voxel demonstrate decreased NAA and elevated mI and Cho in the patient with AD; elevated Cho and mI in the patient with DLB. Occipital voxel (C) (2×2×2cm) is placed on a mid-sagittal 3D T1-weighted image. Anterior superior corner of the voxel is the parieto-occipital sulcus. Spectra from the occipital voxel demonstrate decreased NAA and elevated mI in patients with AD and DLB. All MR spectra are scaled to the creatine (Cr) peak.
2.4 Statistical Analysis
Characteristics of subjects in the CN, DLB, and AD clinical groups were described with means, standard deviations, counts and proportions, and compared using analysis of variance and chi-square tests. Analyses of variance adjusting for age and gender to assess differences in metabolites between clinical groups were performed. For continuous variables, Tukey’s honest significance tests were used to assess pair-wise differences between the means of metabolites for the clinical groups, and for categorical variables, chi-square tests adjusted for false discovery rates were used to compare the proportions. These tests account for multiple comparisons. We first used all DLB patients as a single clinical group, and then we separated the subjects into DLB with low probability AD based on hippocampal volumes. We performed a logistic regression analysis based on data from a prior study in an independent autopsy-confirmed AD and DLB sample(Kantarci, et al., 2012a) to get the cutoff hippocampal volume as a percentage of the total intracranial volume (TIV) of 0.4488585. DLB patients with hippocampal volumes below the cutoff volume (%TIV) were considered to have a high probability, and DLB patients with hippocampal volumes (%TIV) above the cutoff were considered to have low probability of co-existing AD related pathology. Correlations between UPDRS ratings and MRS metabolites in DLB patients were described and tested with Pearson correlations. Differences between MRS metabolites in patients with and without a specific DLB clinical feature were tested with t-tests. Data from autopsy confirmed cases were displayed graphically but statistical analysis was not performed on this data due to small sample sizes.
3. Results
3.1 Patient Characteristics
Table 1 describes the characteristics of subjects in each group. Patients with DLB were younger (p=0.003) and a greater proportion of DLB patients were male (p=0.002) compared to AD patients. For this reason, all analyses were adjusted for age and sex. Hippocampal volumes were significantly different between the three clinical groups with the AD patients having the smallest volumes. We further separated DLB subjects with preserved hippocampal volumes (n=20) (i.e. hippocampal volume (%TIV) > 0.4488585) as DLB with low probability of AD. Mean UPDRS in patients with DLB was 9.9 with a standard deviation of 6.9. Of the 34 DLB patients 22 (65%) had visual hallucinations, 12 (36%) had fluctuations, and 29 (85%) had pRBD.
Table 1.
Patient Characteristics by clinical diagnosis
| CN (n = 148) | DLB (n = 34) | DLB - Low Probability AD (n = 20) | AD (n = 35) | |
|---|---|---|---|---|
| No. female, n (%). | 68 (46) | 5 (15) | 1 (5) | 18 (51) |
| Age at scan, yr. | 77±6 | 73±7 | 72±5 | 79±11 |
| Education, yr. | 14±3 | 14±3 | 14±3 | 14±4 |
| No. APOE4 carrier, n (%) | 39 (26) | 15 (47) | 11 (55) | 17 (49) |
| Short Test of Mental Status | 35.1±2.4 | 24.6±6.4 | 25.0±5.8 | 23.7±6.7 |
| CDR Sum of Boxes | 0.03±0.16 | 5.60±4.23 | 4.75±3.13 | 4.75±3.05 |
| Atlas HP (TIV adjusted) | 0.50±0.05 | 0.46±0.07 | 0.50±0.04 | 0.41±0.07 |
Mean±SD is reported for continuous variables.
APOE: Apolipoprotein E; CDR: Clinical Dementia Rating; TIV: Total intracranial volume
Significant differences from cognitively normal subjects.
3.2 NAA/Cr
Patients with DLB had lower NAA/Cr only in the occipital voxel compared to CN subjects (Table 2) (p=0.006). In contrast, patients with AD had a trend to lower occipital NAA/Cr (p=0.13) and had lower NAA/Cr in the posterior cingulate (p=0.02) and frontal (p=0.02) voxels compared to CN subjects. Whereas no NAA/Cr differences were identified when comparing patients with DLB to those with AD, DLB patients with low probability of AD (i.e. preserved hippocampal volumes) had higher NAA/Cr in the frontal lobes compared to patients with AD (p=0.02).
Table 2.
MRS findings (mean ± standard deviation) by clinical diagnosis.
| Voxel and Metabolite ratio | CN (n = 148) | DLB (n = 34) | DLB - Low Probability AD (n = 20) | AD (n = 35) | DLB vs. CN p-value | DLB – Low Prob. AD vs. CN p-value |
|---|---|---|---|---|---|---|
| NAA/Cr | ||||||
| Frontal | 1.41±0.12 | 1.37±0.17 | 1.43±0.13 | 1.34±0.10 | 0.25 | 0.75 |
| Occipital | 1.59±0.12 | 1.52±0.15 | 1.56±0.07 | 1.55±0.12 | 0.006 | 0.52 |
| Posterior cingulate | 1.50±0.10 | 1.45±0.12 | 1.50±0.10 | 1.44±0.13 | 0.06 | 0.99 |
| Cho/Cr | ||||||
| Frontal | 0.82±0.11 | 0.85±0.13 | 0.88±0.13 | 0.85±0.11 | 0.39 | 0.049 |
| Occipital | 0.47±0.06 | 0.47±0.06 | 0.46±0.06 | 0.49±0.05 | 0.98 | 0.99 |
| Posterior cingulate | 0.66±0.07 | 0.70±0.09 | 0.70±0.09 | 0.72±0.10 | 0.04 | 0.13 |
| MI/Cr | ||||||
| Frontal | 0.69±0.11 | 0.73±0.08 | 0.74±0.07 | 0.74±0.12 | 0.12 | 0.13 |
| Occipital | 0.61±0.09 | 0.65±0.08 | 0.66±0.08 | 0.64±0.09 | 0.06 | 0.03 |
| Posterior cingulate | 0.67±0.08 | 0.71±0.10 | 0.73±0.09 | 0.75±0.13 | 0.04 | 0.03 |
Tukey’s honest significance tests were used to assess pair-wise differences.
NAA: N-acetylaspartate; Cr: Creatine; mI: myo-inositol; Cho: Choline
3.3 Cho/Cr
Both patients with DLB and AD (p=0.04 and p<0.001 respectively) had elevated Cho/Cr in the posterior cingulate voxel compared to CN subjects. Whereas frontal lobe Cho/Cr levels were elevated in DLB patients with low probability of AD (i.e. preserved hippocampal volumes) (p=0.049), frontal lobe Cho/Cr of AD patients was not different from the CN subjects. No differences in occipital Cho/Cr levels were identified among the clinical groups.
3.4 mI/Cr
Both patients with DLB and AD had elevated mI/Cr in the posterior cingulate voxel compared to CN subjects (p=0.04 and p<0.001 respectively). Whereas patients with AD had elevated mI/Cr in the frontal voxel (p=0.03), DLB patients with low probability of AD (i.e. preserved hippocampal volumes) had elevated mI/Cr in the occipital voxel compared to CN subjects (p=0.03).
3.5 Clinical features and MRS Metabolites
We found no relationship between UPDRS ratings and MRS metabolite ratios in the frontal, posterior cingulate and occipital voxels (p=0.15), neither did we find differences in metabolite ratios in DLB patients with and without fluctuations (p≥0.20). However, we found some evidence that occipital voxel Cho/Cr levels were lower in DLB patients with visual hallucinations compared to those without visual hallucinations (p=0.04).
3.6 Autopsy confirmation
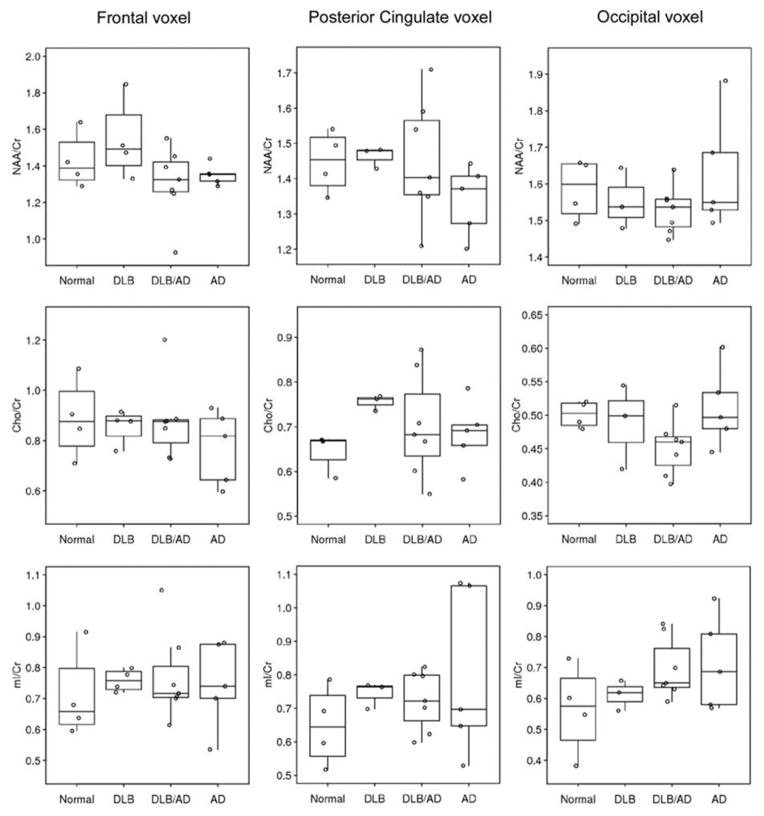
We performed an exploratory analysis on cases with autopsy confirmation of the underlying pathology. There were 4 cases with a low likelihood AD and no LB pathology (i.e. normal), 4 cases with high likelihood DLB (i.e. DLB), 5 with high likelihood AD (i.e. AD), and 7 with low to intermediate likelihood DLB (i.e. AD/DLB: presence of both AD and Lewy body related pathology). The metabolite ratios in four pathologic groups are displayed in Figure 2. The autopsy confirmed disease groups, in general, showed similar findings compared to the clinically diagnosed patients. The only apparent MRS metabolite differences observed among patients with autopsy confirmed AD and DLB was decreased NAA/Cr levels in the posterior cingulate and frontal voxels in patients with AD and preserved NAA/Cr levels in patients with DLB compared to normal. Occipital NAA/Cr levels were similarly decreased in both autopsy confirmed AD and DLB/AD patients, but autopsy confirmed pure DLB patients had relatively preserved NAA/Cr in the occipital lobe. Patients with mixed AD/DLB pathology tended to have similarly decreased NAA/Cr levels as the autopsy confirmed AD patients. Examples of MR spectra from the autopsy confirmed normal, AD and DLB patients are displayed in Figure 1.
Figure 2.
MRS Metabolite ratios in frontal, posterior cingulate and occipital voxels in autopsy confirmed disease groups.
4. Discussion
In this study, we report the MRS abnormalities in patients with DLB and AD from the frontal, posterior cingulate, and occipital voxels which are differentially affected with the pathophysiological processes of AD and DLB. We found distinct differences among the MRS metabolite abnormalities in AD and DLB patients compared to controls as patients with DLB were characterized by MRS metabolite abnormalities of decreased NAA/Cr and increased mI/Cr ratios in the occipital voxel. On the other hand patients with AD dementia were characterized by similar metabolite abnormalities in the posterior cingulate and the frontal voxels. Our study extends prior findings because we demonstrate MRS results from multiple voxels and in DLB patients with a presumed low probability of AD pathology.
DLB is frequently accompanied by AD pathology in patients with dementia.(Hamilton, 2000) In fact, in some cohorts, it is more common for DLB to occur in conjunction with AD than for DLB to occur in isolation.(Gomez-Isla, et al., 1999) We used hippocampal atrophy as a biomarker for AD pathology. In this study, we demonstrate that DLB patients without hippocampal atrophy, have normal NAA/Cr levels in the posterior cingulate gyri consistent with the finding of preserved neuronal numbers in DLB patients at autopsy.(Gomez-Isla, et al., 1999) In fact, decreased posterior cingulate and frontal NAA/Cr levels in autopsy confirmed DLB patients with additional AD pathology suggest that coexisting AD pathology is responsible for NAA/Cr decreases in the frontal and posterior cingulate voxels in patients with DLB. Preservation of NAA/Cr levels in patients with DLB may be analogous to the characteristic fluorodeoxyglucose positron emission tomography signature of DLB of relative preservation of posterior cingulate cortex glucose metabolism (i.e. cingulate island sign), which differentiates patients with DLB from AD dementia.(Lim, et al., 2009) The cingulate island sign has been reported to have a specificity of near 100% and sensitivity of 62–82%, although this was based primarily on clinically not autopsy diagnosed DLB.(Lim, et al., 2009) Taken together with our findings of decreased posterior cingulate NAA/Cr in cases with mixed AD and Lewy body pathology, the lower sensitivity of the cingulate island sign may be driven by the cases of DLB with coexisting AD pathology.
While DLB patients were found to have low NAA/Cr in the occipital voxel, DLB patients without coexisting AD pathology did not differ from controls in the occipital voxel. Similarly, the autopsy confirmed pure DLB patients did not differ from controls in the occipital voxel. On the contrary, AD patients had a trend towards lower NAA/Cr in the occipital voxel. Perhaps, the patients with a combination of DLB/AD pathology drove the occipital NAA/Cr finding in the clinical DLB group resulting in a loss of this finding when they were excluded by hippocampal atrophy.
In agreement with a previous study from our group in a separate cohort, patients with DLB had elevated Cho/Cr in the posterior cingulate voxel compared to controls.(Kantarci, et al., 2004) While Cho/Cr ratio is elevated in AD dementia and DLB in the posterior cingulate voxel, it is normal in VaD.(Kantarci, et al., 2004, Kattapong, et al., 1996, MacKay, et al., 1996) The Cho peak in MRS is thought to be composed of phospholipid synthesis and breakdown products.(Klein, 2000) The Cho/Cr elevation in dementia is thought to be related to dying back of the neuropil or, alternatively, a compensation of decreased acetylcholine by breakdown of membrane phosphatidylcholine to provide substrate for more acetylcholine.(MacKay, et al., 1996, Wurtman RJ, 1985) While the posterior cingulate voxel Cho/Cr is significantly elevated in the DLB patients compared to controls, the difference was only a trend in DLB patients with preserved hippocampal volumes despite the mean ratio being the same likely due to smaller sample size. Furthermore, in autopsy confirmed DLB patients with no additional AD pathology, we also found high Cho/Cr levels. In addition, the elevation of Cho/Cr in the posterior cingulate in the pathologically confirmed DLB subjects demonstrates that the elevation of Cho/Cr is a characteristic feature of DLB and may be independent of AD pathology. Although DLB patients had normal Cho/Cr in the occipital lobe, lower occipital lobe Cho/Cr ratio was associated with visual hallucinations. The discrepancy of findings on Cho/Cr levels in the posterior cingulate and occipital voxels in DLB may be associated with the differential metabolic involvement of the posterior cingulate gyrus and occipital lobes in DLB. Preserved posterior cingulate gyrus metabolism with respect to the decreased occipital metabolism characterizes FDG PET findings in DLB(Lim, et al., 2009), which may explain the discrepant Cho/Cr findings in these voxels.
MI/Cr levels were elevated in patients with DLB and AD compared to controls in the posterior cingulate voxel. Interestingly, occipital voxel mI/Cr levels were elevated only in DLB patients with preserved hippocampal volumes relative to controls. Although the significance of mI elevation in neurodegenerative diseases is unclear, mI is present in glial cells and elevated mI is thought to be associated with glial proliferation.(Bitsch, et al., 1999, Glanville, et al., 1989, Kantarci, et al., 2010, Oz, et al., 2010, Ross, et al., 1998) The anatomical localization of elevated mI/Cr in the occipital voxel in DLB patients with preserved hippocampal volumes who presumably do not have significant AD pathology is consistent with the FDG PET findings in DLB, which show greater occipital lobe hypometabolism in DLB compared to mild AD.(Kantarci, et al., 2012b) This distinction is lost as disease progresses and AD pathology involves the occipital lobes. Occipital involvement may be the reason for the elevated occipital mI/Cr levels observed in autopsy confirmed AD subjects.
Investigation of autopsy confirmed subgroups in the current study further confirmed the preservation of neuronal integrity marker NAA/Cr in the posterior cingulate and frontal lobe voxels in DLB patients relative to patients with DLB/AD pathology, highlighting that AD pathology may be driving the decrease in NAA/Cr in these regions
Although we reported the regional MRS metabolite changes in autopsy confirmed DLB and mixed DLB and AD pathology, a limitation of our study is the availability of autopsy confirmation in a relatively small subset of the patients (n=20). By classifying DLB patients with preserved hippocampal volumes as DLB with low probability of AD, we were able to exclude DLB patients who likely have additional hippocampal AD pathology. However it is possible that we could not distinguish patients with DLB with the hippocampal sparing subtype of AD pathology, if there are any in our sample. Nonetheless, localization of MRS abnormalities to the occipital lobe in DLB with low probability of AD, is in agreement with the findings in autopsy confirmed DLB patients. We did not attempt to quantify metabolite concentrations. We quantified metabolite ratios to Cr for applicability to the clinical practice using tools available in clinical scanners, because many ex-vivo and in-vivo studies in mouse models and humans consistently found stable Cr levels in degenerative dementias(Chao, et al., 2005, Dedeoglu, et al., 2004, Valenzuela and Sachdev, 2001), particularly in AD. Therefore it is unlikely that our findings are influenced by altered Cr levels associated with AD. In contrast, others have shown that in non-demented elderly decreased NAA/Cr correlated with cognitive performance but higher Cr explained the reduction in NAA/Cr better than reduction in NAA (Ferguson, et al., 2002) and, Cr levels may increase with age in cognitively normal individuals.(Pfefferbaum, et al., 1999, Saunders, et al., 1999, Schuff, et al., 2001) Lastly, Cr levels have also been reported to be higher in gray matter compared to white matter.(Pfefferbaum, et al., 1999, Schuff, et al., 2001) It is also possible that Cr levels change in DLB, which needs to be investigated. Furthermore, this study was conducted at 1.5 Tesla. MRS studies at higher magnetic fields such as 3 Tesla may reveal further abnormalities in glutamine and glutamate peaks that may be useful in distinguishing AD and DLB.(Antuono, et al., 2001, Bartha, et al., 2008, Hattori, et al., 2002, Kantarci, et al., 2003, Lin, et al., 2003, Rupsingh, et al., 2011)
MRS abnormalities are associated with early β-amyloid pathology in cognitively normal older adults and may provide valuable information on AD-related pathology in the preclinical stages.(Kantarci, et al., 2011, Kantarci, et al., 2013) Clinical trials for the prevention of AD are already planned in older adults with preclinical AD. DLB patients, many of whom have additional AD pathology may also benefit from agents targeting AD pathology. Thus, imaging markers that help determine the presence of AD pathology in DLB will be critical when enrolling patients with mixed pathologies into clinical trials on targeted agents. For example, presence of imaging evidence of AD pathology in patients with clinically probable DLB predicts treatment response to acetylcholinesterase inhibitors.(Graff-Radford, et al., 2012) MRS may have a complimentary role in antemortem pathological diagnoses helping determine whether clinically probable DLB patients have DLB versus DLB with AD pathology as NAA/Cr reduction in the posterior cingulate and frontal voxels appear to be a marker of coexisting AD pathology in patients with DLB. The pathologic basis of elevated Cho/Cr in DLB even in the absence of AD pathology requires further investigation.
Acknowledgments
Study Funding
NIH [K23 AG030935, R01 AG040042, R01 AG11378, P50 AG44170, P50 AG16574, U01 AG06786, C06 RR018898], Mangurian Foundation, and the Robert H. and Clarice Smith and Abigail Van Buren Alzheimer s Disease Research Program
Footnotes
Disclosure Statement
Dr. Graff-Radford, Dr. Murray, Dr. Lesnick, Ms. Tosakulwong and Ms. Maroney-Smith report no disclosures.
Dr. Boeve has served as an investigator for a clinical trial sponsored by Cephalon, Inc. He has received honoraria from the American Academy of Neurology. He receives research support from the National Institute on Aging (P50-AG16574 [Co-I], U01 AG06786 [Co-I], R01-AG15866 [Co-I], and U24-AG26395 [Co-I]), and the Alzheimer’s Association (IIRG-05–14560 [PI]).
Dr. Ferman is funded by the NIH [Mayo Clinic Alzheimer’s Disease Research Center/Project 1-P50-AG16574/P1 [Co-I]).
Dr. Smith is funded by the NIH (P50-AG16574).
Dr. Knopman serves as Deputy Editor for Neurology®; served on a Data Safety Monitoring Board for Lilly Pharmaceuticals; served as a consultant to TauRx, was an investigator in clinical trials sponsored by Baxter, Elan Pharmaceuticals, and Forest Pharmaceuticals in the past 2 years; and receives research support from the NIH.
Dr. Jack serves as a consultant for Janssen, Bristol-Meyer-Squibb, General Electric, Siemens, and Johnson and Johnson and is involved in clinical trials sponsored by Allon and Baxter, Inc. He receives research funding from the National Institutes of Health (R01-AG011378, RO1-AG037551, U01-HL096917, U01-AG032438, U01-AG024904), and the Alexander Family Alzheimer’s Disease Research Professorship of the Mayo Foundation. Family.
Dr. Petersen serves on scientific advisory boards for Elan Pharmaceuticals, Wyeth Pharmaceuticals, and GE Healthcare and receives research support from the NIH (P50-AG16574 [PI] and U01-AG06786 [PI], R01-AG11378 [Co-I], and U01–24904 [Co-I]).
Dr. Dickson is funded by the NIH (P50-AG16574/Neuropathology Core [PI], P01AG017216 [PI], P50-NS072187 [PI], and R01-AG040042 [Co-I])
Dr. Kantarci serves on the data safety monitoring board for Pfizer Inc. and Takeda Global Research & Development Center, Inc.; and she is funded by the NIH [R01AG040042 (PI), R21 NS066147 (PI), P50 AG44170/Project 2 (PI), P50 AG16574/Project 1 (PI), and R01 AG11378 (Co-I)]
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- AASM. International Classification of Sleep Disorders—2: Diagnostic and Coding Manual. American Academy of Sleep Medicine; Chicago: 2005. [Google Scholar]
- Antuono PG, Jones JL, Wang Y, Li SJ. Decreased glutamate + glutamine in Alzheimer’s disease detected in vivo with (1)H-MRS at 0.5 T. Neurology. 2001;56(6):737–42. doi: 10.1212/wnl.56.6.737. [DOI] [PubMed] [Google Scholar]
- Bartha R, Smith M, Rupsingh R, Rylett J, Wells JL, Borrie MJ. High field (1)H MRS of the hippocampus after donepezil treatment in Alzheimer disease. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32(3):786–93. doi: 10.1016/j.pnpbp.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Bitsch A, Bruhn H, Vougioukas V, Stringaris A, Lassmann H, Frahm J, Bruck W. Inflammatory CNS demyelination: histopathologic correlation with in vivo quantitative proton MR spectroscopy. American Journal of Neuroradiology. 1999;20(9):1619–27. [PMC free article] [PubMed] [Google Scholar]
- Chao LL, Schuff N, Kramer JH, Du AT, Capizzano AA, O’Neill J, Wolkowitz OM, Jagust WJ, Chui HC, Miller BL, Yaffe K, Weiner MW. Reduced medial temporal lobe N-acetylaspartate in cognitively impaired but nondemented patients. Neurology. 2005;64(2):282–9. doi: 10.1212/01.WNL.0000149638.45635.FF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dedeoglu A, Choi JK, Cormier K, Kowall NW, Jenkins BG. Magnetic resonance spectroscopic analysis of Alzheimer’s disease mouse brain that express mutant human APP shows altered neurochemical profile. Brain research. 2004;1012(1–2):60–5. doi: 10.1016/j.brainres.2004.02.079. [DOI] [PubMed] [Google Scholar]
- Ferguson KJ, MacLullich AMJ, Marshall I, Deary IJ, Starr JM, Seckl JR, Wardlaw JM. Magnetic resonance spectroscopy and cognitive function in healthy elderly men. Brain. 2002;125:2743–9. doi: 10.1093/Brain/Awf278. [DOI] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Boeve BF, Ivnik RJ, Petersen RC, Knopman D, Graff-Radford N, Parisi J, Dickson DW. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology. 2004;62(2):181–7. doi: 10.1212/wnl.62.2.181. [DOI] [PubMed] [Google Scholar]
- Galasko D, Hansen LA, Katzman R, Wiederholt W, Masliah E, Terry R, Hill LR, Lessin P, Thal LJ. Clinical-neuropathological correlations in Alzheimer’s disease and related dementias. Arch Neurol. 1994;51(9):888–95. doi: 10.1001/archneur.1994.00540210060013. [DOI] [PubMed] [Google Scholar]
- Glanville NT, Byers DM, Cook HW, Spence MW, Palmer FB. Differences in the metabolism of inositol and phosphoinositides by cultured cells of neuronal and glial origin. Biochimica et Biophysica Acta. 1989;1004(2):169–79. doi: 10.1016/0005-2760(89)90265-8. [DOI] [PubMed] [Google Scholar]
- Gomez-Isla T, Growdon WB, McNamara M, Newell K, Gomez-Tortosa E, Hedley-Whyte ET, Hyman BT. Clinicopathologic correlates in temporal cortex in dementia with Lewy bodies. Neurology. 1999;53(9):2003–9. doi: 10.1212/wnl.53.9.2003. [DOI] [PubMed] [Google Scholar]
- Graff-Radford J, Boeve BF, Pedraza O, Ferman TJ, Przybelski S, Lesnick TG, Vemuri P, Senjem ML, Smith GE, Knopman DS, Lowe V, Jack CR, Jr, Petersen RC, Kantarci K. Imaging and acetylcholinesterase inhibitor response in dementia with Lewy bodies. Brain. 2012;135(Pt 8):2470–7. doi: 10.1093/brain/aws173aws173. [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RL. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 2000;10(3):378–84. doi: 10.1111/j.1750-3639.2000.tb00269.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori N, Abe K, Sakoda S, Sawada T. Proton MR spectroscopic study at 3 Tesla on glutamate/glutamine in Alzheimer’s disease. Neuroreport. 2002;13(1):183–6. doi: 10.1097/00001756-200201210-00041. [DOI] [PubMed] [Google Scholar]
- Huang W, Alexander GE, Chang L, Shetty HU, Krasuski JS, Rapoport SI, Schapiro MB. Brain metabolite concentration and dementia severity in Alzheimer’s disease: a (1)H MRS study. Neurology. 2001;57(4):626–32. doi: 10.1212/wnl.57.4.626. [DOI] [PubMed] [Google Scholar]
- Jones RS, Waldman AD. 1H-MRS evaluation of metabolism in Alzheimer’s disease and vascular dementia. Neurological Research. 2004;26(5):488–95. doi: 10.1179/016164104225017640. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Boeve BF, Wszolek ZK, Rademakers R, Whitwell JL, Baker MC, Senjem ML, Samikoglu AR, Knopman DS, Petersen RC, Jack CR., Jr MRS in presymptomatic MAPT mutation carriers: a potential biomarker for tau-mediated pathology. Neurology. 2010;75(9):771–8. doi: 10.1212/WNL.0b013e3181f073c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Ferman TJ, Boeve BF, Weigand SD, Przybelski S, Vemuri P, Murray MM, Senjem ML, Smith GE, Knopman DS, Petersen RC, Jack CR, Jr, Parisi JE, Dickson DW. Focal atrophy on MRI and neuropathologic classification of dementia with Lewy bodies. Neurology. 2012a;79(6):553–60. doi: 10.1212/WNL.0b013e31826357a5. WNL.0b013e31826357a5 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Jack CR, Jr, Xu YC, Campeau NG, O’Brien PC, Smith GE, Ivnik RJ, Boeve BF, Kokmen E, Tangalos EG, Petersen RC. Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease: A 1H MRS study. Neurology. 2000;55(2):210–7. doi: 10.1212/wnl.55.2.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe V, Przybelski SA, Senjem ML, Weigand SD, Ivnik RJ, Roberts R, Geda YE, Boeve BF, Knopman DS, Petersen RC, Jack CR., Jr Magnetic resonance spectroscopy, beta-amyloid load, and cognition in a population-based sample of cognitively normal older adults. Neurology. 2011;77(10):951–8. doi: 10.1212/WNL.0b013e31822dc7e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe VJ, Boeve BF, Weigand SD, Senjem ML, Przybelski SA, Dickson DW, Parisi JE, Knopman DS, Smith GE, Ferman TJ, Petersen RC, Jack CR., Jr Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol Aging. 2012b;33(9):2091–105. doi: 10.1016/j.neurobiolaging.2011.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Petersen RC, Boeve BF, Knopman DS, Tang-Wai DF, O’Brien PC, Weigand SD, Edland SD, Smith GE, Ivnik RJ, Ferman TJ, Tangalos EG, Jack CR., Jr 1H MR spectroscopy in common dementias. Neurology. 2004;63(8):1393–8. doi: 10.1212/01.wnl.0000141849.21256.ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Reynolds G, Petersen RC, Boeve BF, Knopman DS, Edland SD, Smith GE, Ivnik RJ, Tangalos EG, Jack CR., Jr Proton MR spectroscopy in mild cognitive impairment and Alzheimer disease: comparison of 1.5 and 3 T. AJNR Am J Neuroradiol. 2003;24(5):843–9. [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Weigand SD, Przybelski SA, Preboske GM, Pankratz VS, Vemuri P, Senjem ML, Murphy MC, Gunter JL, Machulda MM, Ivnik RJ, Roberts RO, Boeve BF, Rocca WA, Knopman DS, Petersen RC, Jack CR., Jr MRI and MRS predictors of mild cognitive impairment in a population-based sample. Neurology. 2013;81(2):126–33. doi: 10.1212/WNL.0b013e31829a3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kattapong VJ, Brooks WM, Wesley MH, Kodituwakku PW, Rosenberg GA. Proton magnetic resonance spectroscopy of vascular- and Alzheimer-type dementia. Arch Neurol. 1996;53(7):678–80. doi: 10.1001/archneur.1996.00550070116019. [DOI] [PubMed] [Google Scholar]
- Klein J. Membrane breakdown in acute and chronic neurodegeneration: focus on choline-containing phospholipids. Journal of Neural Transmission. 2000;107(8–9):1027–63. doi: 10.1007/s007020070051. [DOI] [PubMed] [Google Scholar]
- Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, Bradshaw J, Merory J, Woodward M, Hopwood M, Rowe CC. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J Nucl Med. 2009;50(10):1638–45. doi: 10.2967/jnumed.109.065870. jnumed.109.065870 [pii] [DOI] [PubMed] [Google Scholar]
- Lin AP, Shic F, Enriquez C, Ross BD. Reduced glutamate neurotransmission in patients with Alzheimer’s disease -- an in vivo (13)C magnetic resonance spectroscopy study. Magma. 2003;16(1):29–42. doi: 10.1007/s10334-003-0004-x. [DOI] [PubMed] [Google Scholar]
- MacKay S, Ezekiel F, Di Sclafani V, Meyerhoff DJ, Gerson J, Norman D, Fein G, Weiner MW. Alzheimer disease and subcortical ischemic vascular dementia: evaluation by combining MR imaging segmentation and H-1 MR spectroscopic imaging. Radiology. 1996;198(2):537–45. doi: 10.1148/radiology.198.2.8596863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology. 2005;65(12):1863–72. doi: 10.1212/01.wnl.0000187889.17253.b1. [DOI] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–44. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Miller BL, Moats RA, Shonk T, Ernst T, Woolley S, Ross BD. Alzheimer disease: depiction of increased cerebral myo-inositol with proton MR spectroscopy. Radiology. 1993;187(2):433–7. doi: 10.1148/radiology.187.2.8475286. [DOI] [PubMed] [Google Scholar]
- Molina JA, Garcia-Segura JM, Benito-Leon J, Gomez-Escalonilla C, del Ser T, Martinez V, Viano J. Proton magnetic resonance spectroscopy in dementia with Lewy bodies. European Neurology. 2002;48(3):158–63. doi: 10.1159/000065520. [DOI] [PubMed] [Google Scholar]
- Murray M, Vemuri P, Ferman T, Dugger B, Jack C, Boeve B, Knopman D, Petersen R, Parisi J, Dickson D, Kantarci K. In dementia with Lewy bodies, rapid eye movement sleep behavior disorder is associated with reduced Alzheimer’s disease pathology and hippocampal atrophy. Alzheimers Dement. 2012;8(4):13–4. [Google Scholar]
- Murray ME, Graff-Radford NR, Ross OA, Petersen RC, Duara R, Dickson DW. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: a retrospective study. Lancet neurology. 2011;10(9):785–96. doi: 10.1016/S1474-4422(11)70156-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oz G, Nelson CD, Koski DM, Henry PG, Marjanska M, Deelchand DK, Shanley R, Eberly LE, Orr HT, Clark HB. Noninvasive detection of presymptomatic and progressive neurodegeneration in a mouse model of spinocerebellar ataxia type 1. J Neurosci. 2010;30(10):3831–8. doi: 10.1523/JNEUROSCI.5612-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfefferbaum A, Adalsteinsson E, Spielman D, Sullivan EV, Lim KO. In vivo spectroscopic quantification of the N-acetyl moiety, creatine, and choline from large volumes of brain gray and white matter: effects of normal aging. Magnetic Resonance in Medicine. 1999;41(2):276–84. doi: 10.1002/(sici)1522-2594(199902)41:2<276::aid-mrm10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Roberts RO, Geda YE, Knopman DS, Cha RH, Pankratz VS, Boeve BF, Ivnik RJ, Tangalos EG, Petersen RC, Rocca WA. The Mayo Clinic Study of Aging: design and sampling, participation, baseline measures and sample characteristics. Neuroepidemiology. 2008;30(1):58–69. doi: 10.1159/000115751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross BD, Bluml S, Cowan R, Danielsen E, Farrow N, Tan J. In vivo MR spectroscopy of human dementia. Neuroimaging Clinics of North America. 1998;8(4):809–22. [PubMed] [Google Scholar]
- Rupsingh R, Borrie M, Smith M, Wells JL, Bartha R. Reduced hippocampal glutamate in Alzheimer disease. Neurobiol Aging. 2011;32(5):802–10. doi: 10.1016/j.neurobiolaging.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Saunders DE, Howe FA, van den Boogaart A, Griffiths JR, Brown MM. Aging of the adult human brain: in vivo quantitation of metabolite content with proton magnetic resonance spectroscopy. J Magn Reson Imaging. 1999;9(5):711–6. doi: 10.1002/(sici)1522-2586(199905)9:5<711::aid-jmri14>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69(24):2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schuff N, Capizzano AA, Du AT, Amend DL, O’Neill J, Norman D, Kramer J, Jagust W, Miller B, Wolkowitz OM, Yaffe K, Weiner MW. Selective reduction of N-acetylaspartate in medial temporal and parietal lobes in AD. Neurology. 2002;58(6):928–35. doi: 10.1212/wnl.58.6.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuff N, Ezekiel F, Gamst AC, Amend DL, Capizzano AA, Maudsley AA, Weiner MW. Region and tissue differences of metabolites in normally aged brain using multislice 1H magnetic resonance spectroscopic imaging. Magn Reson Med. 2001;45(5):899–907. doi: 10.1002/mrm.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela MJ, Sachdev P. Magnetic resonance spectroscopy in AD. Neurology. 2001;56(5):592–8. doi: 10.1212/wnl.56.5.592. [DOI] [PubMed] [Google Scholar]
- Webb PG, Sailasuta N, Kohler SJ, Raidy T, Moats RA, Hurd RE. Automated single-voxel proton MRS: technical development and multisite verification. Magnetic Resonance in Medicine. 1994;31(4):365–73. doi: 10.1002/mrm.1910310404. [DOI] [PubMed] [Google Scholar]
- Wurtman RJ, BJ, Marie JC. Autocannibalism of choline-containing membrane phospholipids in the pathogenesis of Alzheimer’s disease. Neurochemistry International. 1985;7:369–72. doi: 10.1016/0197-0186(85)90127-5. [DOI] [PubMed] [Google Scholar]
- Xuan X, Ding M, Gong X. Proton magnetic resonance spectroscopy detects a relative decrease of N-acetylaspartate in the hippocampus of patients with dementia with Lewy bodies. Journal of neuroimaging : official journal of the American Society of Neuroimaging. 2008;18(2):137–41. doi: 10.1111/j.1552-6569.2007.00203.x. [DOI] [PubMed] [Google Scholar]
- Yoshita M, Taki J, Yamada M. A clinical role for [(123)I]MIBG myocardial scintigraphy in the distinction between dementia of the Alzheimer’s-type and dementia with Lewy bodies. J Neurol Neurosurg Psychiatry. 2001;71(5):583–8. doi: 10.1136/jnnp.71.5.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccai J, McCracken C, Brayne C. A systematic review of prevalence and incidence studies of dementia with Lewy bodies. Age Ageing. 2005;34(6):561–6. doi: 10.1093/ageing/afi190. [DOI] [PubMed] [Google Scholar]