Abstract
Growth factor-binding domains identified in various extracellular matrix (ECM) proteins have been shown to regulate growth factor activity in many ways. Recently we identified a fibronectin peptide (P12) that can bind platelet-derived growth factor BB (PDGF-BB) and promote adult human dermal fibroblast (AHDF) survival under stress. In vivo experiments in a porcine burn injury model showed that P12 limited burn injury progression, suggesting an active role in tissue survival. In this report, we explored the molecular mechanism of this peptide in ADHF under nutrient deprivation. Our results showed that P12 acted like some cell penetrating peptides (CPPs) in that it redirected ligand-bound PDGFR from the clathrin-dependent endocytic pathway to a slower, macropinocytosis-like pathway. P12 slowed internalization and degradation of PDGF-BB, augmented its survival signals, and promoted cell survival after nutrient-removal. Our findings demonstrate a mechanism for a potential therapeutic peptide that increases cell and tissue survival by acting as a cofactor to PDGF-BB.
Keywords: cell survival, cell-penetrating peptide, endocytosis, Akt, JNK, PI3kinase
Introduction
Many growth factor-binding sites have been identified in ECM (Macri et al., 2007). They may act as reservoirs and serve to sequester and protect growth factors from degradation, and/or enhance their activity (Schultz and Wysocki, 2009). Recently, our lab identified a peptide P12 from the first type III repeat of fibronectin with significant binding affinity to PDGF-BB (Lin et al., 2013). Previously we demonstrated that P12 can work as a co-factor of PDGF-BB to promote AHDF survival under reactive oxygen (ROS)- or tunicamycin-induced ER stress. P12 was also shown to limit burn injury progression in a rat hot comb burn model (Lin et al., 2013). These studies has been confirmed in a porcine hot comb burn model (Clark et al., 2011); however, the molecular mechanism of P12 remains unclear.
Much of the tissue damage in burn patients results from injury progression, a dynamic process of tissue death around the burned area occurring in the first 24 to 48h after burn (Lanier et al., 2011). Others in our laboratory have shown significant blood vessel plugging with red blood cells in the first few hours after burn injury (Zhou et al., 2011). This occlusion likely causes hypoxia and nutrient deprivation in peri-burn tissue. In addition, a band of apoptosis in the deep dermis occurred by 24h after burn injury (Lanier et al., 2011). This suggests that progressive tissue death characteristic of burn injury progression is related to nutrient deprivation/hypoxia in peri-burn tissue.
A clue to its mechanism of action is that P12 is a 14-residue peptide with an isoelectic point (pI) of 11.5. Such a structure resembles cell-penetrating peptides (CPPs). CPPs are highly cationic peptides of 10 to 20 amino acids. Many, but not all, CPPS initially bring cargo (proteins, peptides, nucleic acids, etc.) into mammalian cells in an energy- and temperature-dependent manner with characteristics consistent with macropinocytosis (Kaplan et al., 2005; Said Hassane et al., 2010; Schmidt et al., 2010). Subsequently, through some poorly defined process(s), CPPs either penetrate vesicular membranes or are otherwise released from vesicles. In the first step of internalization such CPPs appear to interact electrostatically with cell surface proteoglycans leading to endocytic internalization (Madani et al., 2011). Since P12 fits the structural definition of a typical CPP (4 cationic residues out of a total of 14), it may affect endocytic entry of PDGF-BB and its receptor.
Endocytic entry of growth factor receptors following ligand binding plays an important role in the regulation of growth factor signaling (Sorkin and von Zastrow, 2009). After internalization, epidermal growth factor (EGF) can still generate survival signals from endosomes (Dobrowolski and De Robertis, 2012; Miaczynska and Bar-Sagi, 2010). Moreover, ERK1/2 activation is dependent on endocytosis of the EGF receptor (EGFR) (Galperin and Sorkin, 2008). Importantly, route of entry, downstream adaptor/signal proteins present, and resident-time in endocytic vesicles before degradation or recycling to the plasma membrane dictate the amplitude, specificity and duration of signal (Sorkin and von Zastrow, 2009). Similar to EGF/EGFR, endosomal signaling can occur from ligand-bound PDGFR and is sufficient for cell survival (Wang et al., 2004). Furthermore, different endocytic pathways used to internalize PDGF/PDGFR generate differential signals, leading either to proliferation, or migration (De Donatis et al., 2008).
Interestingly, the macropinosome, as a signaling platform for PDGF-BB, can generate stronger survival signals than other locations (Schmees et al., 2012). In fact, H-Ras-transformed fibroblasts can employ macropinocytosis to generate enhanced PDGF-BB survival signals. The increased localization of PDGFR in macropinosomes led to stronger receptor activation, prolonged Akt phosphorylation and enhanced cell survival (Schmees et al., 2012).
Although PDGF-BB induces macropinocytosis, ligated PDGFR is mainly internalized through clathrin-mediated endocytosis (Kapeller et al., 1993) or at high PDGF-BB dose through, raft/caveolin-mediated endocytosis (De Donatis et al., 2008). During both clathrin- and caveolin-mediated endocytosis, the GTPase dynamin is required for vesicle fission, whereas macropinocytosis occurs independently of dynamin activation (Lim and Gleeson, 2011; Mayor and Pagano, 2007). In this report, we investigated the effect of P12 on PDGF-BB endocytosis and PDGF signaling. Our results suggest that by shifting the balance between clathrin-/dynamin-dependent and independent endocytic pathways, P12 slowed internalization and degradation of PDGF-BB, augmented survival signal, and promoted cell survival under nutrient-removal stress.
Results
P12 enhanced PDGF-BB ability to support AHDF survival under nutrient deprivation
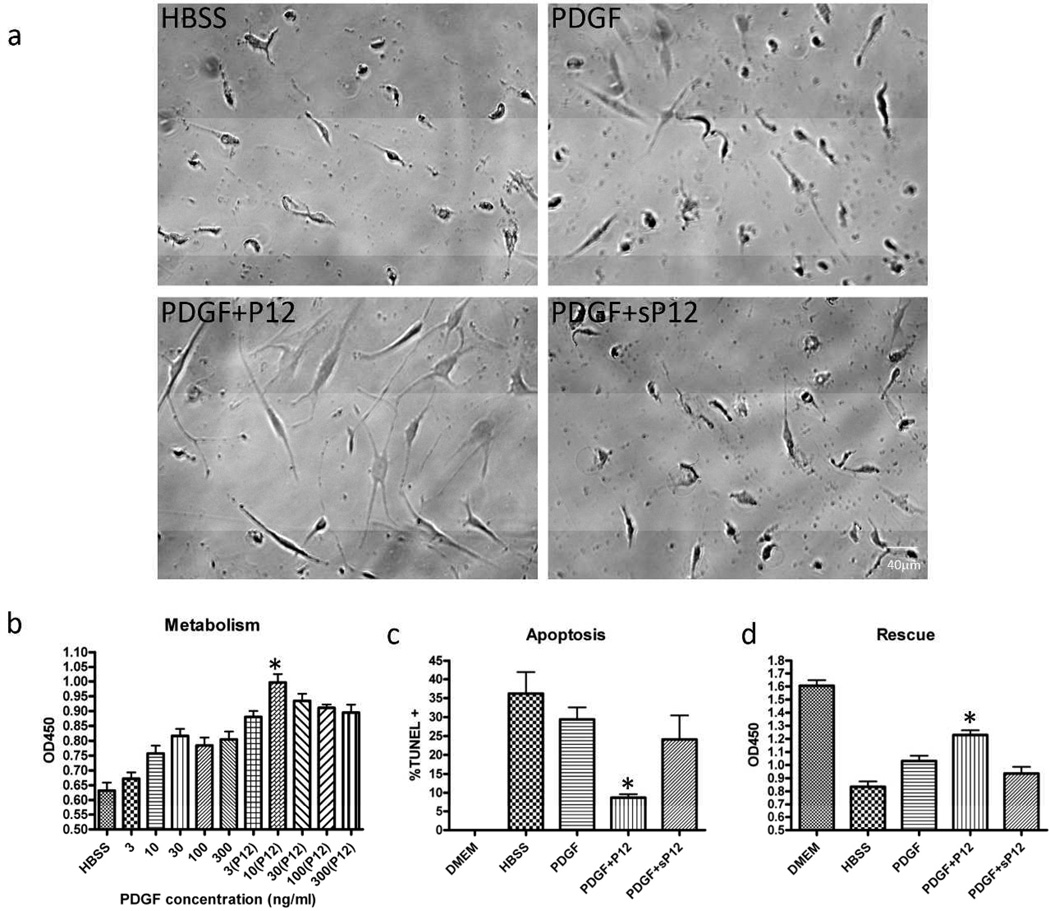
Since P12 worked synergistically with PDGF to promote AHDF survival under reactive oxygen and ER stress (Lin et al., 2013), we investigated whether P12 had a similar effect under acute nutrient deprivation. For this purpose, we employed initially a nutrient deprivation system (Wei et al., 2008) with or without hypoxia (1% oxygen). As an indicator for cell viability we used the XTT assay, which measures mitochondria dehydrogenase activity. While P12 by itself does not stimulate cell metabolism (Figure S1), P12 enhanced the ability of PDGF-BB to support AHDF in Hank’s balanced salt solution (HBSS) devoid of all growth factors and amino acids (Figure 1). AHDFs were rounded or shriveled after 3 days under these starvation conditions, even in the presence of PDGF-BB (Figure 1a). In contrast, AHDFs treated with PDGF-BB+P12, but not PDGF-BB±sP12, were well-spread with a healthy appearing morphology (Figure 1a). Cell metabolism dropped significantly after nutrient deprivation compared to cells in DMEM (OD450 = 1.6). However, P12 increased cell metabolism at all PDGF-BB doses (Figure 1b) and protected cells from nutrient deprivation-induced apoptosis (Figure 1c). Almost 40% of the AHDFs underwent apoptosis after 3 days of starvation. While PDGF-BB reduced apoptosis to 30%, P12/PDGF-BB treatment lowered apoptosis to less than 10% (Figure 1c). To determine whether cells treated with P12/PDGF-BB survived 72h starvation better than cell treated with PDGF-BB alone, cells were switched to full-serum medium and assayed by the XTT 24h later (Figure 1d). Indeed metabolism was significantly higher with P12/PDGF-BB than PDGF-BB alone. Similar results were obtained with cells cultured under nutrient deprivation conditions and hypoxia (1% oxygen) (data not shown).
Figure 1. P12+PDGF promoted AHDF survival under nutrient deprivation.
AHDFs were treated with PDGF ± P12 in HBSS for 72 h. Unless otherwise indicated, all experiments were performed in HBSS with 1nM PDGF and 10 µM P12. a) Cell morphology as judged by phase contrast microscopy. (sP12=scrambled P12). Scale bar: 40 µm. b) Cell metabolism was measured by the XTT assay (asterisk, P<0.05 compared to PDGF alone, n=6), Mean ± SD. c) Apoptosis was measured by the TUNEL assay. %TUNEL+ = TUNEL positive/DAPI positive. >800 cells counted (asterisk, P<0.05 compared to PDGF alone, n=6). Mean ± SD. d) After 72h of nutrient deprivation, cells were rescued with 10% FBS for 24h and metabolism was measured by the XTT assay (asterisk, P<0.05 compared to PDGF alone, n=6). Mean ± SD.
P12 with Alexa488 cargo was internalized into endocytic vesicles
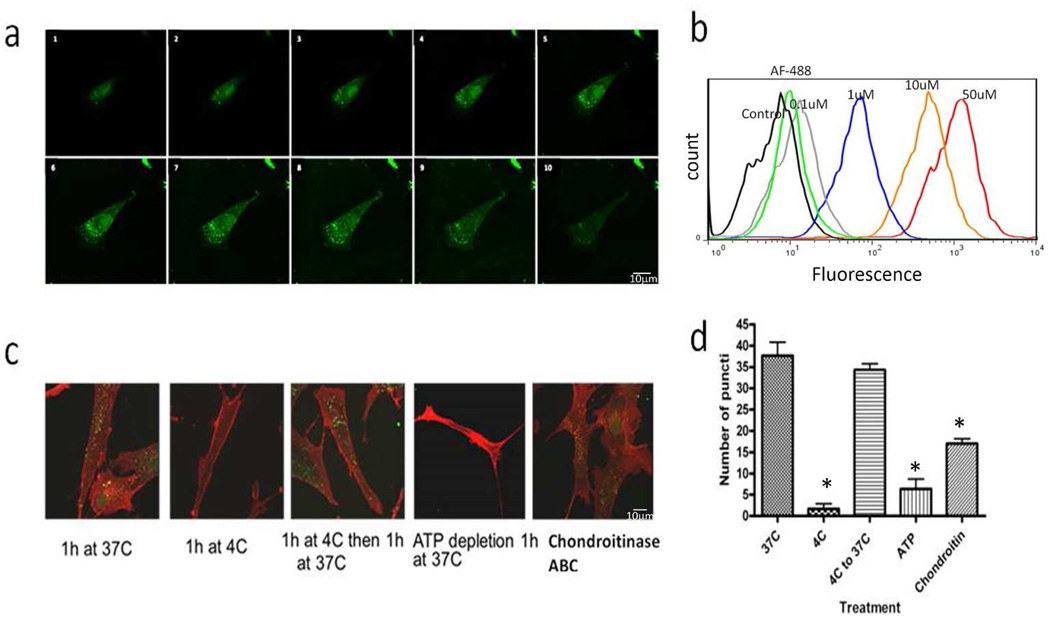
P12 is similar to a cell penetrating peptide in structure (10 to 20 amino acids long; rich in arginine and lysine). Many, but not all, CPP attached to cargo can enter cell through macropinocytosis followed by endosomal escape into cytoplasm (Schmidt et al., 2010). Thus, we studied whether P12 fused with Alexa488 can enter cells through endocytosis. To test this Cys-tagged P12 was labeled with Alexa Fluor 488 (P12-Alexa488), incubated with AHDF, and cells were examined by one micron step-sections with immunofluorescence confocal microscopy (Figure 2a). Importantly, N- or C-terminal blocking with amidation, acetylation or Cys-tagging did not change P12 bioactivity (data not shown). P12-Alexa488 was detectable in endocytic structures within 1h at 37°C. FACS analysis demonstrated that Alexa488 internalization required P12, as Alexa488 itself did not enter AHDF (Figure 2b). To differentiate between cell association and uptake, cells were trypsinized to remove surface P12 before the FACS analysis.
Figure 2. Alexa488-P12 was endocytosed by an energy-dependent pathway.
All experiments were performed with 10% FBS in DMEM. a) AHDF were treated with 1 µM of Alexa488-P12 for 1 h at 37°C then cross-setion imaged at 0.74 µm. Scale bar: 10 µm. b) AHDF were treated with 0.1,1, 10, or 50 µM of Alexa488-P12 or 1 µM Alexa Fluor-488 for 1 h at 37°C, then trypsinized and analyzed by flow cytometry. c) AHDF were treated with 1 µM of Alexa488-P12 after no pretreatment, ATP depletion with 10 mM of sodium azide and 2-deoxyglucose, or 0.1 U/ml chondroitinase ABC digestion. Red signal is Cellmask™ membrane stain. Scale bar: 40 µm. d) Quantification of vesicles/cell. Over 50 cells were counted for each condition (asterisk, P<0.05 compared to 37°C, n=3) Mean ± SD.
To further characterize the P12 internalization pathway, P12-Alexa488 was incubated with cells at low temperature, with ATP depletion or chondroitin sulfate removal since internalization of highly cationic peptides by macropinocytosis requires active metabolism and interaction with cell surface GAGs (Rullo et al., 2011). Alexa488-P12 uptake was almost completely inhibited at 4°C or when cells were pre-incubated with sodium azide and deoxy-glucose to deplete cellular ATP, and greatly inhibited after digestion with chondrointinase ABC (Figure 2c and d). Together these data support the contention that P12 can carry cargo into endocytic vesicles.
P12 slowed PDGF-BB/PDGFR-β internalization/degradation
Although PDGF-BB can induce macropinocytosis (Schmees et al., 2012), activated PDGFR is mainly internalized through clathrin-mediated endocytosis (Kapeller et al., 1993). By contrast, when molecules are internalized through macropinocytosis it is a slower process than clathrin/dynamin endocytosis (Al Soraj et al., 2012; Jones, 2007). Because P12 is a small, highly cationic peptide that can enter cells through an energy- and temperature-dependent pathway and binds strongly to PDGF-BB, it is possible that P12 can change the internalization pathway of PDGF-BB/PDGFR-β and thereby affect the internalization/degradation rate. To test this possibility, we analyzed Alexa Fluor-350 labeled-PDGF-BB uptake in HBSS±P12. Importantly, Alexa Fluor-350 did not affect PDGF-BB bioactivity (Figure S2a). After incubation with AHDF for 1h at 37°C, cellular uptake (indirectly measured by residual PDGF in the medium) was slower in the presence of P12 for both labeled and unlabeled PDGF-BB (Figure S2b and c).
Internalization through clathrin-mediated endocytosis (Muratoglu et al., 2010) and subsequent receptor degradation is a negative regulation that prevents sustained signaling from PDGFR-β on the cell surface (Heldin and Westermark, 1999). However, P12 may have altered the internalization of PDGF-BB pathway in such a way to slow the rate of internalization, thus leaving more PDGF in the medium. In order to determine whether PDGFR-β degradation is also affected by P12, we next used Western blot and immunofluorescence to analyze PDGFR-β levels in cells. After PDGF-BB±P12 treatment in HBSS, we found, in fact, that P12 significantly slowed PDGFR-β degradation (Figure S2d, e, f).
Taken together, the results demonstrated that P12 slowed the internalization/degradation of both PDGF-BB and PDGFR-β. One possible explanation of these findings is that P12-binding to PDGF-BB interfered with receptor recognition. However, Biacore experiments showed that the interaction between PDGF-BB and PDGFR extracellular domain was not affected by the presence of P12 (Figure S3). An alternate possibility is that P12 slowed PDGF-BB/PDGFR-β internalization and degradation by shifting endocytosis from a clathrin-mediated mechanism to a slower route such as macropinocytosis.
P12 shifted endocytosis of PDGF-BB/PDGFR-β to a macropinocytosis-like pathway
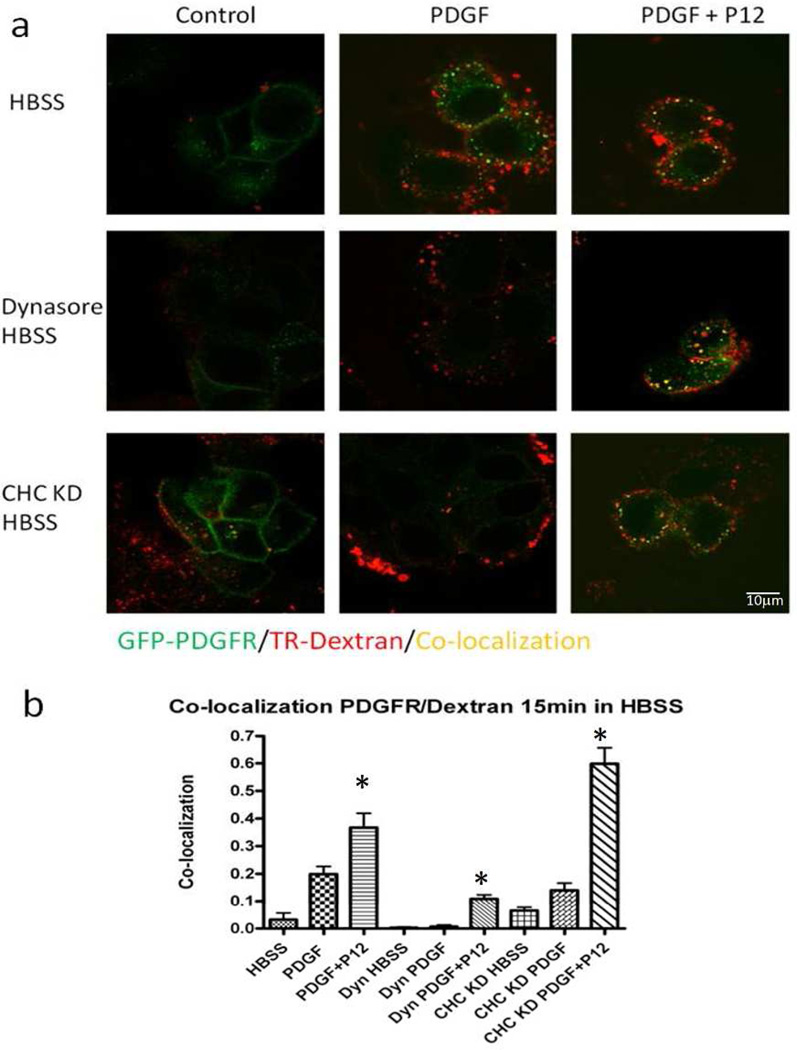
To study how P12 altered PDGF-BB/PDGFR-β entry into the cell, Alexa Fluor 488-label PDGF-BB was attempted. However, the sensitivity was too low at physiological concentrations of PDGF-BB. We next tried Q-dot labeled PDGF-BB (Lidke et al., 2004; Lidke et al., 2007) However, the PDGF-Q-dot complex was internalized by macropinocytosis probably secondary to the large size of Qdots (data not shown). Therefore, a MCF-7 cell line expressing PDGFR-GFP (Reddi et al., 2007) was used to track PDGF-BB/PDGFR-β complexes with 70 kD dextran-Texas red (TR-dextran) as a macropinosome marker (Kerr and Teasdale, 2009). In controls (HBSS without PDGF-BB) most PDGFR-GFP signal was localized uniformly on the cell surface (Figure 3a, HBSS, Control). When PDGF-BB was added PDGFR-GFP signal was observed in green vesicles, despite PDGF-BB-induction of macropinocytosis denoted by red vesicles (Figure 3a: HBSS, PDGF). However, PDGF+P12 promoted co-localization of PDGFR and TR-dextran as noted by yellow vesicles (Figure 3a: HBSS, PDGF+P12), consistent with the hypothesis that P12 shifted PDGF-BB entry to a macropinocytosis-like pathway. In HBSS P12 alone did not induce MCF-7 cell macropinocytosis (Figure S4). Furthermore, scrambled P12 (sP12) had no significant effect on GFP-PDGFR co-localization with TR-Dextran (Figure 3b) To further verify that P12 shifted the endocytic pathway of PDGFR, we used dynasore, a small chemical inhibitor of dynamin that blocks clathrin- and caveola-dependent endocytosis (Macia et al., 2006). In the presence of dynasore, PDGF-BB could only stimulate minimal internalization of PDGFR-GFP. However, P12/PDGF enhanced uptake into vesicles that co-localized PDGFR-GFP with TR-dextran (Figure 3). Complementary to this, we observed in AHDF that PDGFR-β internalization/degradation was slowed significantly by dynasore, and that P12 partially overcame the dynasore block of receptor degradation (Figure S5).
Figure 3. P12 increased PDGF-BB/PDGFR-β internalization into macropinosomes.
a) MCF-7 cells expressing GFP-PDGFR-β were treated with TR-dextran and 1 nM PDGF-BB ± 10 µM P12 for 15 min in HBSS, and then analyzed by confocal microscope. For dynamin inhibition, cells were pre-incubated with 80 µM dynasore for 1 h in serum-free-DMEM (See Figure S6 for CHC KD). Scale bar: 10 µm. b) Co-localization of red and green signal was used to indicate the degree of co-localization between PDGFR-β and TR-dextran (Threshold for both channels were set to 70). >100 cells per condition were counted. The bar graph represents the co-localization index, calculated as the number of yellow pixels divided by the number of yellow + green pixels. Experiments were repeated 3 times (asterisk, P<0.05 compared to PDGF alone, n=3). Mean ± SD.
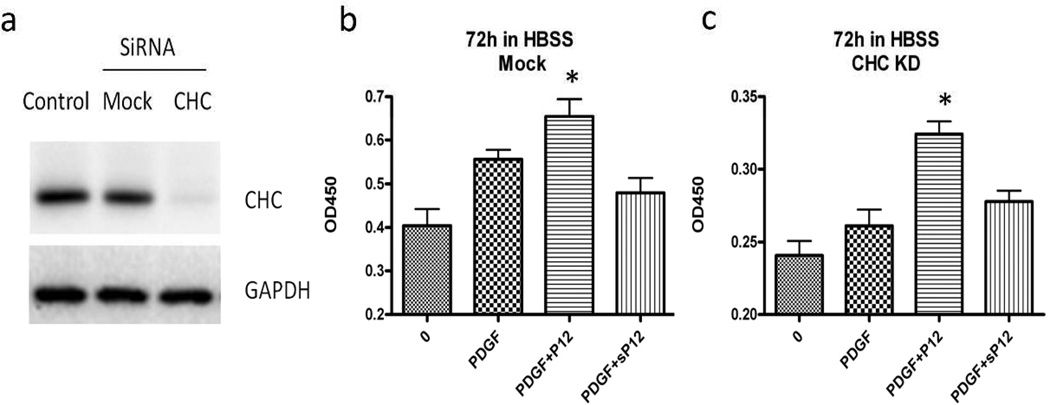
Besides dynasore, clathrin heavy chain knock-down (CHC KD) blocks clathrin-mediated endocytosis (Huang et al., 2004). Therefore, to further define P12/PDGF-BB trafficking into cells we used siRNA to knock down CHC expression in MCF-7 cells (Figure S6). Indeed, PDGF-BB-stimulated PDGFR-β entry into the cell was significantly suppressed by CHC knockdown, but addition of P12 overcame this block (Figure 3). Together these results strongly support the contention that P12 shifted PDGF-BB/PDGFR-β entry into cells from clathrin-mediated endocytosis to a macropinocytosis-like pathway.
P12 enhanced PDGFR-β activation and p85 recruitment
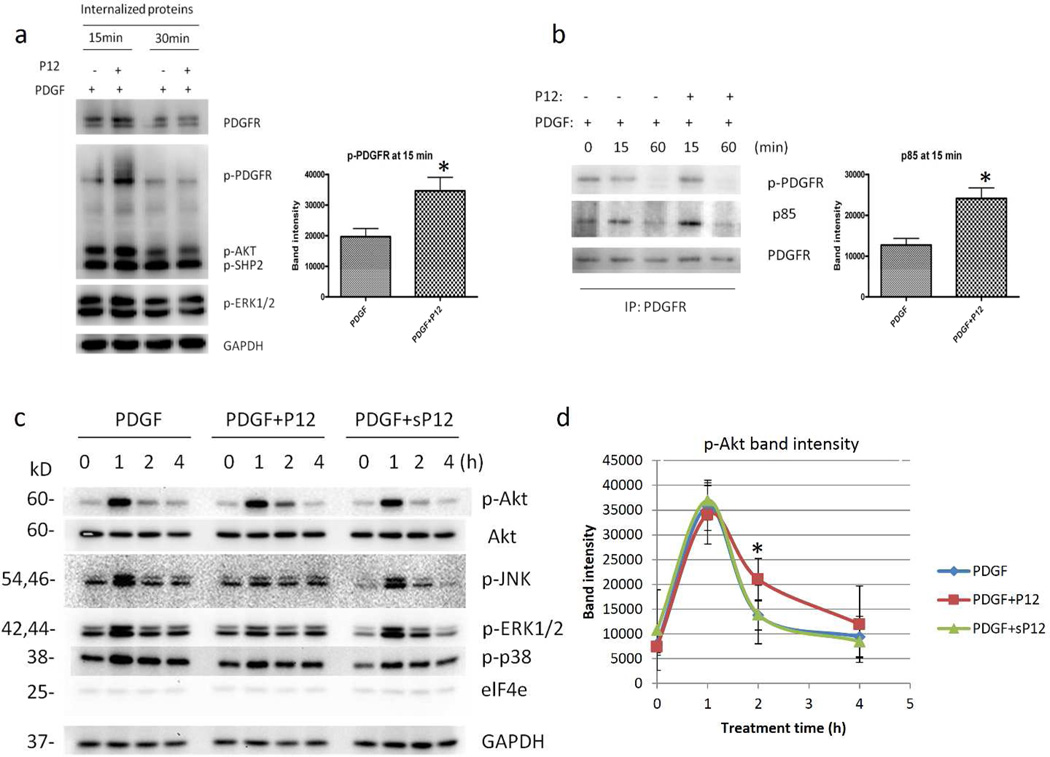
The endocytic route of PDGF-BB/PDGFR-β effects downstream signaling (De Donatis et al., 2008). Furthermore, PDGF-BB can generate a more robust survival signal through macropinocytosis compared to clathrin-mediated endocytosis (Schmees et al., 2012). To determine how P12 affects PDGFR signaling, we first examined the effect of P12 on PDGFR-β activation. AHDF were treated with PDGF±P12 in HBSS followed by isolation of internalized PDGFR-β (see Material & Methods). Enhanced PDGFR-β activation as judged by phosphorylation at Tyr751 was observed in cells treated with P12/PDGF-BB compared to those treated with PDGF-BB alone (Figure 4a). Furthermore, when biotinylated PDGFR-β was pulled-down by streptavidin beads and probed with an antibody to the PI3K p85 subunit, we observed increased recruitment of p85 to PDGFR in P12/PDGF-BB treated cells compared to cells treated with PDGF-BB alone (Figure 4b). These data suggest that P12 enhanced PDGFR-β activation by PDGF-BB and increased PI3K signaling that is required to activate downstream survival pathways (Klinghoffer et al., 1996).
Figure 4. P12 enhanced PDGFR-β activation, p85 recruitment to PDGFR and prolonged Akt phosphorylation.
a) AHDFs were treated with 1 nM PDGF-BB ± 10 µM P12 in HBSS. The PDGFR-β internalized during treatment was separated (See methods) and probed with antibodies against p-PDGFR-β (Tyr751) and p-ERK1/2. GAPDH was used as loading control. b) AHDF cells were treated with 1 nM PDGF-BB ± 10 µM P12 in HBSS. PDGFR-β in total cell lysate was immunoprecipitated. p-PDGFR (Y751) and p85 were probed. c) AHDFs were treated with 1 nM PDGF-BB ± 10µM P12 (sP12) in HBSS. p-Akt, Akt, p-JNK, p-p38 and elF4e were probed. GAPDH was used as a loading control. a, b, d) Digital quantification of p-Akt band intensity using Kodak IM ver4.0.3. Data from 3 independent experiments (asterisk, P<0.05 compared to PDGF alone, n=3). Mean ± SD.
P12 prolonged Akt phosphorylation induced by PDGF-BB
To test whether there is a link between differential PDGF-BB trafficking into the cell and enhanced cell survival signals, we studied whether P12 also affected PDGF-BB survival signals downstream of PI3K. The Akt/PI3K signaling pathway is one of the major signaling pathways activated by PDGF-BB and is important for cell survival under conditions including oxidative stress, ER stress and nutrient deprivation (Franke, 2008). Based on increased PDGFR-β activation and p85 recruitment in P12 treated cells, it is possible that P12 can enhance PDGF-BB signaling downstream in the Akt/PI3K pathway under nutrient deprivation. Indeed, P12/PDGF-BB, compared to PDGF-BB alone, prolonged Akt phosphorylation (Ser473) and inhibited JNK phosphorylation (T183/Y185) (Figure 4c and d) while not effecting p38 phosphorylation and elF4e protein levels 1h after treatment. Similar results were obtained with AHDF (AG09605) from another individual (data not shown). Importantly, P12 alone did not effect PDGFR pathway signaling (Figure S7). Since prolonged Akt and decreased JNK phosphorylation are linked to increased cell survival under starvation (Rovida et al., 2008), these data are consonant with the posit that P12 promotes cell survival by modulating PDGF-BB signaling.
To put this in context of wound healing, we tested biologically relevant doses of PDGF-BB (Figure S8). P12 increased p-Akt at PDGF-BB levels observed in both acute wound fluid and in serum (Baker et al., 2008; Raines and Ross, 1982). As an important control, P12, which does not bind EGF (data not shown), did not effect EGF-stimulated Akt phosphorylation (Figure S8). Previous reports demonstrated that dynasore can block sustained p-Akt and p-ERK1/2 signaling from CSF-1 (Huynh et al., 2012). In our system we observed a similar inhibition of PDGF-BB stimulated p-Akt signal in the presence of dynasore. However, addition of P12 bypassed the dynamin blockade and restored PDGF-BB signaling to control levels (Figure S9). These results supported the contention that P12 can mediate PDGF signaling through a dynamin-independent pathway.
P12 bypassed clathrin heavy chain knockdown to stimulate cell metabolism
Clathrin heavy chain (CHC) knockdown has been shown to block clathrin-mediated endocytosis of growth factor receptors (Huang et al., 2004). Here we used siRNA to knock down the CHC in AHDF cells and measured cell metabolism after 3 days of nutrient deprivation (Figure 5a). Mock transfection had little or no effect on either PDGF- or PDGF/P12-sustained metabolism (Figure 5b, compared to Figure 1a). In contrast, PDGF-stimulated cell metabolism dropped in the KD cells almost to control level, while P12 abrogated this effect (Figure 5c). Taken together our results are consonant with P12 shifting PDGF-BB trafficking from clathrin-mediated endocytosis to a macropinocytosis-like pathway, thereby generating stronger survival signals.
Figure 5. P12 rescued PDGF survival activity in CHC knock down cells.
a) CHC SiRNA was employed to knockdown CHC expression in AHDF cells. The protein level dropped significantly after 2 consecutive transfections (36 hours apart) with Hiperfect transfection reagents. b) Mock cells were treated with 1 nM PDGF-BB ± 10 µM P12 (sP12) in HBSS for 3 days and cell metabolism was measured with XTT assay. Experiment was repeated 3 times (asterisk, P<0.05 compared to PDGF alone, n=6). Error bar indicates SD. c) CHC knockdown cells were treated with 1 nM PDGF-BB ± 10 µM P12 (sP12) in HBSS for 3 days and cell metabolism was measured with XTT assay. Experiment was repeated 3 times (asterisk, P<0.05 compared to PDGF alone, n=6). Mean ± SD.
Discussion
Numerous clinical trials with recombinant growth factors have been largely disappointing. Most growth factors alone have minimal to no activity toward improving wound repair. Interestingly, the trials that demonstrated growth factor benefit all contained a biomaterial carrier, suggesting that spatiotemporal control over growth factor bioactivity is crucial to achieve tangible therapeutic effect (Lee et al., 2011). We believe that the absence of fibronectin (FN) secondary to protease degradation is one of the reasons that chronic wounds and burns fail to heal (Clark et al., 2003; Grinnell and Zhu, 1996; Wysocki et al., 1993). It is possible that the deficiency in growth factor activity in chronic wounds and burns is partially secondary to the absence of FN growth factor-binding sites. If this is so, administered P12 might increase growth factor bioactivity to a level that would promote wound healing.
Multiple layered interactions between ECM and growth factors have created a rapidly developing and complex field of study. For example, ECM proteins in the cell microenvironment can generate signals through integrins that act in synergy with growth factor signaling (Schultz and Wysocki, 2009). ECM can also bind soluble growth factors and regulate their distribution, activation and presentation to cells (Hynes, 2009). Specifically, growth factor binding-domains on FN have been proposed to function as reservoir for growth factors that provide spatial and temporal regulation for growth factor signaling (Kim et al., 2011). Also, proteolytic processing of the ECM proteins generate matrikines that directly activate growth factor receptors (Tran et al., 2004). Our study demonstrates that a growth factor-binding peptide from ECM can regulate growth factor signaling by affecting growth factor endocytosis and thereby enhance survival signals. This finding adds another layer to the complexity between ECM and growth factor signaling.
It has been demonstrated that some 10–20 residue, highly cationic peptides can bring protein cargo into mammalian cells through macropinocytosis (Al Soraj et al., 2012; Kaplan et al., 2005; Wadia et al., 2004). Here, P12, a 14-residue, highly cationic peptide, can bind PDGF-BB/PDGFR-β and shift its entry into cells from dynamin-dependent endocytosis to a macropinocytosis-like pathway. By entering the cell through different endocytic pathways, growth factors can generate differential signals due to the exposure to unique adaptor and signal relay proteins in different cell compartments (Benmerah, 2004; Chen, 2009; De Donatis et al., 2008; Wiley and Burke, 2001). In this study we show that P12 while by itself does not affect PDGFR signaling (Figure S7), can bind to PDGF-BB and shift PDGF-BB/PDGFR-β internalization to a dynamin-independent, macropinocytosis-like pathway. As a consequence, it transiently generates enhanced survival signals to promote AHDF survival under stress. Importantly, P12 by itself does not induce macropinocytosis (Figure S4) which is dependent on PI3K signaling from PDGF-BB (Koivusalo, 2010 #864).
Molecular modeling data has showed a potential binding pocket of P12 is at the interface between PDGF-BB and PDGFR-β (unpublished observations). Thus, as an alternative to the CPP hypothesis, P12 at the interface between PDGF-BB and PDGFR-β could induce conformational change in PDGFR-β and thus affect downstream signaling. To test this possibility, we have initiated a crystallography study to determine whether P12 binding induces conformational change in PDGF-BB/PDGFR-β complexes. Another possibility is that P12 somehow slowed PDGFR degradation from dynamin-dependent endocytosis and the slower degradation of PDGFR-β is responsible for the sustained signaling from PDGFR-β. Because the ubiquitination state of PDGFR-β determines the degradation and signal duration from PDGFR-β (Takayama et al., 2005), one of the future studies will be to investigate if the ubiquitination on PDGFR-β is affected by P12 treatment. Regardless of the exact molecular mechanisms at play, our findings here demonstrate a unique role for ECM proteins in modulating growth factor signaling and cell survival. In addition, these observations have important implications for tissue engineering and for growth factor treatments of wounds.
Material and methods
Materials
P12, sP12 (scrambled P12) and C-terminal Cys-tagged P12 were purchased from American Peptide (Sunnyvale, CA). Scrambled P12 (sP12) is a synthetic peptide sharing same amino acid composition as P12 with scramble sequence. PDGF-BB was purchased from Invitrogen (#PHG0043). Antibody against PDGFR (#4564), p-PDGFR-β (Tyr751) (#3161), p-Akt (Ser473)(#4058), p-JNK (Thr183/Tyr185)(#9251), p-ERK1/2 (Thr202/Tyr204)(#4370), p-P38 (Thr180/Tyr182)(#9211), eIF4E (2067#) and GAPDH(#2118) are purchased from Cell signaling Technology (Dernvers, MA). Antibody for PDGFR (ab32570) was purchased from Abcam (Cambridge, MA). AHDF (CF31) primary cells are isolated from dermis of a 31 year old female. Hank’s Balanced Salt Buffer from Sigma (St. Louis, MO). Cell Proliferation kit II (XTT) and In Situ Cell Death Detection kit (TUNEL reaction mixture) was purchased from Roche Diagnostics (Indianapolis, IN). SiRNA for CHC KD (sc-35067) was purchased from Santa Cruz Biotechnology (Dallas, TX). Allstar negative control siRNA and Hiperfect transfection reagents were purchased from Qiagen (Valencia, CA). EZ-Link Sulfo NHS-SS Biotinylation Kit was purchased from Pierce (Rockford, IL).
Cell culture and Transfections
AHDFs and MCF-7 were maintained in DMEM (Gibco) with 10% FBS at 37°C and 5% CO2 in a humidified atmosphere. AHDFs from passage 8–12 were used. For experimental conditions unless otherwise indicated, P12 (sP12) was used at 10uM and PDGF-BB was used at 1 nM. SiRNA and mock was resuspended to 10 µM prior to transfection. Cells were transfected twice with Hiperfect Transfection Reagent according to manufacturer’s recommendations at 36-h intervals.
Peptide Labeling
Cys-P12 was incubated with equal amount of Alexa Fluor 488 C5 maleimide at 100 µM in 500ul of PBS for 2h at room temperature. Then it was purified by cation exchange chromatography (CM-C50). The column was washed, and labeled peptides were eluted with pH 11.5 Hepes buffer. The pH was adjusted to 7.5 with HCl and concentration of P12-488 is determined by OD 280.
Confocal Microscopy
CF31 cells grown in Lab-Tek Chamers were incubated with 1 µM of P12-Alexa488 for 1h at 37C, and washed. Plasma membranes were stained with 5 ug/ml Cellmask Deep Red plasma membrane stain for 2 min at 37C, fixed with 3.7% formaldehyde, and analyzed by confocal microscopy (Zeiss LSM 510 META NLO Two-Photon Laser Scanning Confocal Microscope System) with excitation light at 488 nm and 633 nm.
Flow cytometry
2 × 105 cells were incubated with 1 µM of P12-488 dissolved in DMEM medium. Subsequently, cells were incubated for 2 min with 0.25% trypsin to detach the cells and remove surface-bound peptide. For immunostaining experiments, cells were treated with 1 nM PDGF ± 10 µM P12 for 1 h then fixed with 3.7% formaldehyde and Permeablized with 0.4% Triton-X100. Blocked with 1% BSA and stained with primary and secondary antibodies each for 1h. Cells were resuspended in PBS and analyzed by flow cytometer.
Separation of Internalized PDGFR-β
Surface proteins were labeled with EZ-Link Sulfo NHS-SS Biotinylation Kit (contained a cleavable S-S to allow removal) following kit instructions. The cells were prewarmed for 5 min at 37°C, followed by stimulation with 10 ng/ml PDGF-BB±P12 for the indicated times in Figure 4. Cells were put on ice and remaining surface-exposed biotin was removed by 20 mM sodium 2-mercaptoethanesulfonate in 50 mM Tris followed by 20 mM iodoacetic acid on ice. After cell lysis, biotinylated proteins were pulled down with streptavidin agarose.
TUNEL staining
AHDF treated according to figure legend was fixed and permeabilized in 0.1% Triton X-100 for 2 min at 4°C, stained according to kit protocol. Cell nuclei were stained with DAPI. Images were captured with a 10×, aperture 0.4 objective on an inverted Diaphot-TMD fluorescent microscope (Nikon) using a CCD camera (Molecular Devices).
Western blot
Cells were frozen and dissolved in lysis buffer from Cell Signaling (#9803) containing 20 mM Tris-HCl (pH 7.5), 150 mM NaCl, 1 mM Na2EDTA, 1 mM EGTA, 1% Triton, 2.5 mM sodium pyrophosphate, 1 mM beta-glycerophosphate, 1 mM Na3VO4, 1 µg/ml leupeptin supplemented with 1 mM PMSF, scraped and sonicated, then spun and boiled in running buffer. Equal amounts of protein were separated by 10% SDS-PAGE and transferred onto nitrocellulose membranes. Membranes were then blocked by 2% BSA in TBST and probed with primary antibodies over night at 4°C. HRP conjugated secondary antibodes were used for detection. Images were taken with a Kodaq image station 440CF (Rochester, NY). Intensity of the chemiluminescent signal was quantified by kodaq molecular imaging software v4.0.3.
Supplementary Material
Acknowledgements
This work was support by the Armed Forces Institute of Regenerative Medicine (RAFC) and NIH/AR063445 (RAFC).
Abbreviations
- AHDF
adult human dermal fibroblast
- CPP
cell penetrating peptide
- PDGF
platelet-derived growth factor
- ECM
extracellular matrix
- PBS
phosphate buffered saline
- HBSS
Hank’s balanced salt buffer
- CHC
Clathrin heavy chain
- Dyn
dynasore
- KD
knockdown
Footnotes
Conflict of Interest:
Richard Clark and Fubao Lin co-discovered P12. Richard Clark is President and Founder of NeoMatrix Formulations, a biotechnology company engaged in preclinical studies for P12 treatment of burns.
References
- Al Soraj M, He L, Peynshaert K, Cousaert J, Vercauteren D, Braeckmans K, De Smedt SC, Jones AT. siRNA and pharmacological inhibition of endocytic pathways to characterize the differential role of macropinocytosis and the actin cytoskeleton on cellular uptake of dextran and cationic cell penetrating peptides octaarginine (R8) and HIV-Tat. J Control Release. 2012;161:132–141. doi: 10.1016/j.jconrel.2012.03.015. [DOI] [PubMed] [Google Scholar]
- Baker EA, Kumar S, Melling AC, Whetter D, Leaper DJ. Temporal and quantitative profiles of growth factors and metalloproteinases in acute wound fluid after mastectomy. Wound Repair Regen. 2008;16:95–101. doi: 10.1111/j.1524-475X.2007.00322.x. [DOI] [PubMed] [Google Scholar]
- Benmerah A. Endocytosis: signaling from endocytic membranes to the nucleus. Curr Biol. 2004;14:R314–R316. doi: 10.1016/j.cub.2004.03.053. [DOI] [PubMed] [Google Scholar]
- Chen YG. Endocytic regulation of TGF-beta signaling. Cell Res. 2009;19:58–70. doi: 10.1038/cr.2008.315. [DOI] [PubMed] [Google Scholar]
- Clark RA, An JQ, Greiling D, Khan A, Schwarzbauer JE. Fibroblast migration on fibronectin requires three distinct functional domains. J Invest Dermatol. 2003;121:695–705. doi: 10.1046/j.1523-1747.2003.12484.x. [DOI] [PubMed] [Google Scholar]
- Clark RAF, Lin F, Tonnesen MG, Singer AJ. P12, a fibronectin-derived peptide, reduces burn injury progression in a porcine burn model. J Invest Dermatol. 2011;131:S140. [Google Scholar]
- De Donatis A, Comito G, Buricchi F, Vinci MC, Parenti A, Caselli A, Camici G, Manao G, Ramponi G, Cirri P. Proliferation versus migration in platelet-derived growth factor signaling: the key role of endocytosis. J Biol Chem. 2008;283:19948–19956. doi: 10.1074/jbc.M709428200. [DOI] [PubMed] [Google Scholar]
- Dobrowolski R, De Robertis EM. Endocytic control of growth factor signalling: multivesicular bodies as signalling organelles. Nat Rev Mol Cell Biol. 2012;13:53–60. doi: 10.1038/nrm3244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franke TF. PI3K/Akt: getting it right matters. Oncogene. 2008;27:6473–6488. doi: 10.1038/onc.2008.313. [DOI] [PubMed] [Google Scholar]
- Galperin E, Sorkin A. Endosomal targeting of MEK2 requires RAF, MEK kinase activity and clathrin-dependent endocytosis. Traffic. 2008;9:1776–1790. doi: 10.1111/j.1600-0854.2008.00788.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grinnell F, Zhu M. Fibronectin degradation in chronic wounds depends on the relative levels of elastase, alpha1-proteinase inhibitor, and alpha2-macroglobulin. J Invest Dermatol. 1996;106:335–341. doi: 10.1111/1523-1747.ep12342990. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Westermark B. Mechanism of action and in vivo role of platelet-derived growth factor. Physiol Rev. 1999;79:1283–1316. doi: 10.1152/physrev.1999.79.4.1283. [DOI] [PubMed] [Google Scholar]
- Huang F, Khvorova A, Marshall W, Sorkin A. Analysis of clathrin-mediated endocytosis of epidermal growth factor receptor by RNA interference. J Biol Chem. 2004;279:16657–16661. doi: 10.1074/jbc.C400046200. [DOI] [PubMed] [Google Scholar]
- Huynh J, Kwa MQ, Cook AD, Hamilton JA, Scholz GM. CSF-1 receptor signalling from endosomes mediates the sustained activation of Erk1/2 and Akt in macrophages. Cell Signal. 2012;24:1753–1761. doi: 10.1016/j.cellsig.2012.04.022. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AT. Macropinocytosis: searching for an endocytic identity and role in the uptake of cell penetrating peptides. J Cell Mol Med. 2007;11:670–684. doi: 10.1111/j.1582-4934.2007.00062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapeller R, Chakrabarti R, Cantley L, Fay F, Corvera S. Internalization of activated platelet-derived growth factor receptor-phosphatidylinositol-3' kinase complexes: potential interactions with the microtubule cytoskeleton. Mol Cell Biol. 1993;13:6052–6063. doi: 10.1128/mcb.13.10.6052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan IM, Wadia JS, Dowdy SF. Cationic TAT peptide transduction domain enters cells by macropinocytosis. J Control Release. 2005;102:247–253. doi: 10.1016/j.jconrel.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Kerr MC, Teasdale RD. Defining macropinocytosis. Traffic. 2009;10:364–371. doi: 10.1111/j.1600-0854.2009.00878.x. [DOI] [PubMed] [Google Scholar]
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- Klinghoffer RA, Duckworth B, Valius M, Cantley L, Kazlauskas A. Platelet-derived growth factor-dependent activation of phosphatidylinositol 3-kinase is regulated by receptor binding of SH2-domain-containing proteins which influence Ras activity. Mol Cell Biol. 1996;16:5905–5914. doi: 10.1128/mcb.16.10.5905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanier ST, McClain SA, Lin F, Singer AJ, Clark RA. Spatiotemporal progression of cell death in the zone of ischemia surrounding burns. Wound repair and regeneration : official publication of the Wound Healing Society [and] the European Tissue Repair Society. 2011;19:622–632. doi: 10.1111/j.1524-475X.2011.00725.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Silva EA, Mooney DJ. Growth factor delivery-based tissue engineering: general approaches and a review of recent developments. J R Soc Interface. 2011;8:153–170. doi: 10.1098/rsif.2010.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidke DS, Nagy P, Heintzmann R, Arndt-Jovin DJ, Post JN, Grecco HE, Jares-Erijman EA, Jovin TM. Quantum dot ligands provide new insights into erbB/HER receptor-mediated signal transduction. Nat Biotechnol. 2004;22:198–203. doi: 10.1038/nbt929. [DOI] [PubMed] [Google Scholar]
- Lidke DS, Nagy P, Jovin TM, Arndt-Jovin DJ. Biotin-ligand complexes with streptavidin quantum dots for in vivo cell labeling of membrane receptors. Methods Mol Biol. 2007;374:69–79. doi: 10.1385/1-59745-369-2:69. [DOI] [PubMed] [Google Scholar]
- Lim JP, Gleeson PA. Macropinocytosis: an endocytic pathway for internalising large gulps. Immunol Cell Biol. 2011;89:836–843. doi: 10.1038/icb.2011.20. [DOI] [PubMed] [Google Scholar]
- Lin F, Zhu J, Tiara B, Tonnesen MG, McClain SA, Singer AJ, Clark RAF. Novel fibronectin peptides bind PDGF-BB and enhance cell and tissue survival under stress. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.420. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macia E, Ehrlich M, Massol R, Boucrot E, Brunner C, Kirchhausen T. Dynasore, a cell-permeable inhibitor of dynamin. Dev Cell. 2006;10:839–850. doi: 10.1016/j.devcel.2006.04.002. [DOI] [PubMed] [Google Scholar]
- Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Madani F, Lindberg S, Langel U, Futaki S, Graslund A. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011;2011:414729. doi: 10.1155/2011/414729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayor S, Pagano RE. Pathways of clathrin-independent endocytosis. Nat Rev Mol Cell Biol. 2007;8:603–612. doi: 10.1038/nrm2216. [DOI] [PubMed] [Google Scholar]
- Miaczynska M, Bar-Sagi D. Signaling endosomes: seeing is believing. Curr Opin Cell Biol. 2010;22:535–540. doi: 10.1016/j.ceb.2010.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muratoglu SC, Mikhailenko I, Newton C, Migliorini M, Strickland DK. Low density lipoprotein receptor-related protein 1 (LRP1) forms a signaling complex with platelet-derived growth factor receptor-beta in endosomes and regulates activation of the MAPK pathway. J Biol Chem. 2010;285:14308–14317. doi: 10.1074/jbc.M109.046672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raines EW, Ross R. Platelet-derived growth factor. I. High yield purification and evidence for multiple forms. J Biol Chem. 1982;257:5154–5160. [PubMed] [Google Scholar]
- Reddi AL, Ying G, Duan L, Chen G, Dimri M, Douillard P, Druker BJ, Naramura M, Band V, Band H. Binding of Cbl to a phospholipase Cgamma1-docking site on platelet-derived growth factor receptor beta provides a dual mechanism of negative regulation. J Biol Chem. 2007;282:29336–29347. doi: 10.1074/jbc.M701797200. [DOI] [PubMed] [Google Scholar]
- Rovida E, Navari N, Caligiuri A, Dello Sbarba P, Marra F. ERK5 differentially regulates PDGF-induced proliferation and migration of hepatic stellate cells. J Hepatol. 2008;48:107–115. doi: 10.1016/j.jhep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- Rullo A, Qian J, Nitz M. Peptide-glycosaminoglycan cluster formation involving cell penetrating peptides. Biopolymers. 2011;95:722–731. doi: 10.1002/bip.21641. [DOI] [PubMed] [Google Scholar]
- Said Hassane F, Saleh AF, Abes R, Gait MJ, Lebleu B. Cell penetrating peptides: overview and applications to the delivery of oligonucleotides. Cell Mol Life Sci. 2010;67:715–726. doi: 10.1007/s00018-009-0186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmees C, Villasenor R, Zheng W, Ma H, Zerial M, Heldin CH, Hellberg C. Macropinocytosis of the PDGF beta-receptor promotes fibroblast transformation by H-RasG12V. Mol Biol Cell. 2012;23:2571–2582. doi: 10.1091/mbc.E11-04-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt N, Mishra A, Lai GH, Wong GC. Arginine-rich cell-penetrating peptides. FEBS Lett. 2010;584:1806–1813. doi: 10.1016/j.febslet.2009.11.046. [DOI] [PubMed] [Google Scholar]
- Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Sorkin A, von Zastrow M. Endocytosis and signalling: intertwining molecular networks. Nat Rev Mol Cell Biol. 2009;10:609–622. doi: 10.1038/nrm2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayama Y, May P, Anderson RG, Herz J. Low density lipoprotein receptor-related protein 1 (LRP1) controls endocytosis and c-CBL-mediated ubiquitination of the platelet-derived growth factor receptor beta (PDGFR beta) J Biol Chem. 2005;280:18504–18510. doi: 10.1074/jbc.M410265200. [DOI] [PubMed] [Google Scholar]
- Tran KT, Griffith L, Wells A. Extracellular matrix signaling through growth factor receptors during wound healing. Wound Repair Regen. 2004;12:262–268. doi: 10.1111/j.1067-1927.2004.012302.x. [DOI] [PubMed] [Google Scholar]
- Wadia JS, Stan RV, Dowdy SF. Transducible TAT-HA fusogenic peptide enhances escape of TAT-fusion proteins after lipid raft macropinocytosis. Nat Med. 2004;10:310–315. doi: 10.1038/nm996. [DOI] [PubMed] [Google Scholar]
- Wang Y, Pennock SD, Chen X, Kazlauskas A, Wang Z. Platelet-derived growth factor receptor-mediated signal transduction from endosomes. J Biol Chem. 2004;279:8038–8046. doi: 10.1074/jbc.M311494200. [DOI] [PubMed] [Google Scholar]
- Wei Y, Pattingre S, Sinha S, Bassik M, Levine B. JNK1-mediated phosphorylation of Bcl-2 regulates starvation-induced autophagy. Mol Cell. 2008;30:678–688. doi: 10.1016/j.molcel.2008.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley HS, Burke PM. Regulation of receptor tyrosine kinase signaling by endocytic trafficking. Traffic. 2001;2:12–18. doi: 10.1034/j.1600-0854.2001.020103.x. [DOI] [PubMed] [Google Scholar]
- Wysocki AB, Staiano-Coico L, Grinnell F. Wound fluid from chronic leg ulcers contains elevated levels of metalloproteinases MMP-2 and MMP-9. J Invest Dermatol. 1993;101:64–68. doi: 10.1111/1523-1747.ep12359590. [DOI] [PubMed] [Google Scholar]
- Zhou W, Hirth DA, Tonnesen MG, McClain SA, Singer AJ, Clark RAF. Burn injury induces early erythrocyte occlusion of surrounding cutaneous microvasculature prior to delayed microthrombus formation. J Invest Dermatol. 2011;131:S137. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.