Abstract
Plakophilin-1 (PKP-1) is an armadillo family protein critical for desmosomal adhesion and epidermal integrity. In the autoimmune skin blistering disease pemphigus vulgaris (PV), autoantibodies (IgG) target the desmosomal cadherin desmoglein 3 (Dsg3) and compromise keratinocyte cell-cell adhesion. Here, we report that enhanced expression of PKP-1 protects keratinocytes from PV IgG-induced loss of cell-cell adhesion. PKP-1 prevents loss of Dsg3 and other desmosomal proteins from cell-cell borders and prevents alterations in desmosome ultrastructure in keratinocytes treated with PV IgG. Using a series of Dsg3 chimeras and deletion constructs, we find that PKP-1 clusters Dsg3 with the desmosomal plaque protein desmoplakin in a manner dependent upon the plakoglobin binding domain of the Dsg3 tail. Furthermore, PKP-1 expression transforms desmosome adhesion from a calcium-dependent to a calcium-independent and hyper-adhesive state. These results demonstrate that manipulating the expression of a single desmosomal plaque protein can block the pathogenic effects of PV IgG on keratinocyte adhesion.
INTRODUCTION
Desmosomes are intercellular junctions that provide strong adhesion between keratinocytes by anchoring keratin intermediate filaments to cell-cell contact sites. Desmosomal adhesion is critical in tissues that experience mechanical stress, such as in the skin and heart (Getsios et al., 2004). These highly regulated, complex macromolecular junctions are comprised mainly of proteins from three major families: the desmosomal cadherins, desmocollins (Dsc1–3) and desmogleins (Dsg1–4); armadillo proteins, plakoglobin (PG) and the plakophilins (PKP 1–3); and the plakin family protein, desmoplakin (DP) (Green and Simpson, 2007). The transmembrane desmosomal cadherins mediate calcium-sensitive adhesive interactions between adjacent cells (Saito et al., 2012b). The armadillo and plakin family members form the desmosomal plaque that tethers the keratin intermediate filament cytoskeleton to cadherin intracellular domains (Delva et al., 2009). The importance of desmosomes in regulating tissue integrity, differentiation and morphogenesis is evidenced by numerous inherited and autoimmune disorders in which desmosome function is compromised (Brooke et al., 2012; Simpson et al., 2011; Thomason et al., 2010).
Pemphigus vulgaris (PV) is a life-threatening autoimmune disease that presents clinically as either a mucosal dominant form caused by IgG autoantibodies directed against desmoglein-3 (Dsg3), or as a mucocutaneous form characterized by antibodies directed against both Dsg3 and Dsg1 (Amagai et al., 1991; Amagai and Stanley, 2012). PV IgG binding to Dsg3 results in desmosome disruption and the loss of cell-cell adhesion between cells in the lower layers of stratified epithelia where Dsg3 is highly expressed (Sharma et al., 2007). In PV patients, this loss of adhesion, or acantholysis, manifests as severe non-healing erosions of mucous membranes and blisters in the epidermis (Kottke et al., 2006). Although an extensive literature has accumulated on the mechanisms by which PV IgG might disrupt desmosomal adhesion, the precise pathomechanisms of PV are not fully understood. Studies suggest distinct, but possibly synergistic pemphigus pathomechanisms which include interference of extracellular Dsg3 cis or trans interactions (steric hindrance), endocytosis and depletion of cell surface Dsg3, and activation of cellular signaling pathways (Di Zenzo et al., 2012; Getsios et al., 2010; Kitajima and Aoyama, 2007). Furthermore, inhibition of various PV pathomechanisms can reinforce desmosome adhesion and prevent acantholysis in both cell culture and animal model systems (Waschke, 2008). These findings collectively suggest that reinforcing desmosomal adhesion, alone or in addition to immune suppression, may hold promise in the search for better pemphigus therapies. Apart from the potential to discover alternative disease treatments, these studies contribute to our understanding of the complex mechanisms that regulate desmosome adhesion.
A fundamental feature of desmosome adhesion not fully understood is the tissue and differentiation specific expression of desmosomal components (Dusek et al., 2007). Changes in the molecular composition of desmosomes correlate with distinct structural and functional differences in desmosomal adhesion (Holthofer et al., 2007). The armadillo family protein plakophilin-1 (PKP-1) is expressed preferentially in the upper epidermal layers and contributes to keratinocyte adhesion by promoting desmosome formation (Bornslaeger et al., 2001; Hatzfeld, 2007; Hatzfeld et al., 2000; Kowalczyk et al., 1999; Smith and Fuchs, 1998). PKP-1 promotes desmosome formation by recruiting and clustering desmosomal proteins at the plasma membrane and within desmosomes (Bornslaeger et al., 2001; Kowalczyk et al., 1999; Wahl, 2005). PKP-1 binds to DP, Dsg1, Dsc1, actin and keratin (Hatzfeld et al., 2000; Hofmann et al., 2000; Kapprell et al., 1988; Smith and Fuchs, 1998). Mutations in PKP-1 cause the autosomal recessive disorder ectodermal dysplasia-skin fragility syndrome (EDSF) (McGrath et al., 1997). EDSF patient skin biopsies revealed a significant reduction in the number and size of desmosomes in the spinous and granular layers (McMillan et al., 2003). Similar to PV, these patients suffer from mechanical stress-induced skin blistering (McGrath and Mellerio, 2010). Finally, PKP-1 has been associated with the formation of calciumin-dependent desmosomes (South et al., 2003), which exhibit increased intercellular adhesion strength and decreased sensitivity to depletion of extracellular calcium (Garrod and Kimura, 2008).
The observation that PKP-1 stabilizes and enhances desmosome adhesion raised the possibility that keratinocytes expressing PKP-1 might assemble desmosomes that are refractory to PV IgG. To test this idea, exogenous PKP-1 was expressed in primary keratinocyte cultures and responses to PV IgG were tested. Our results demonstrate that increased expression of a desmosomal plaque protein, PKP-1, can modulate keratinocyte sensitivity to the pathogenic effects of PV IgG.
RESULTS
PKP-1 promotes desmosome formation
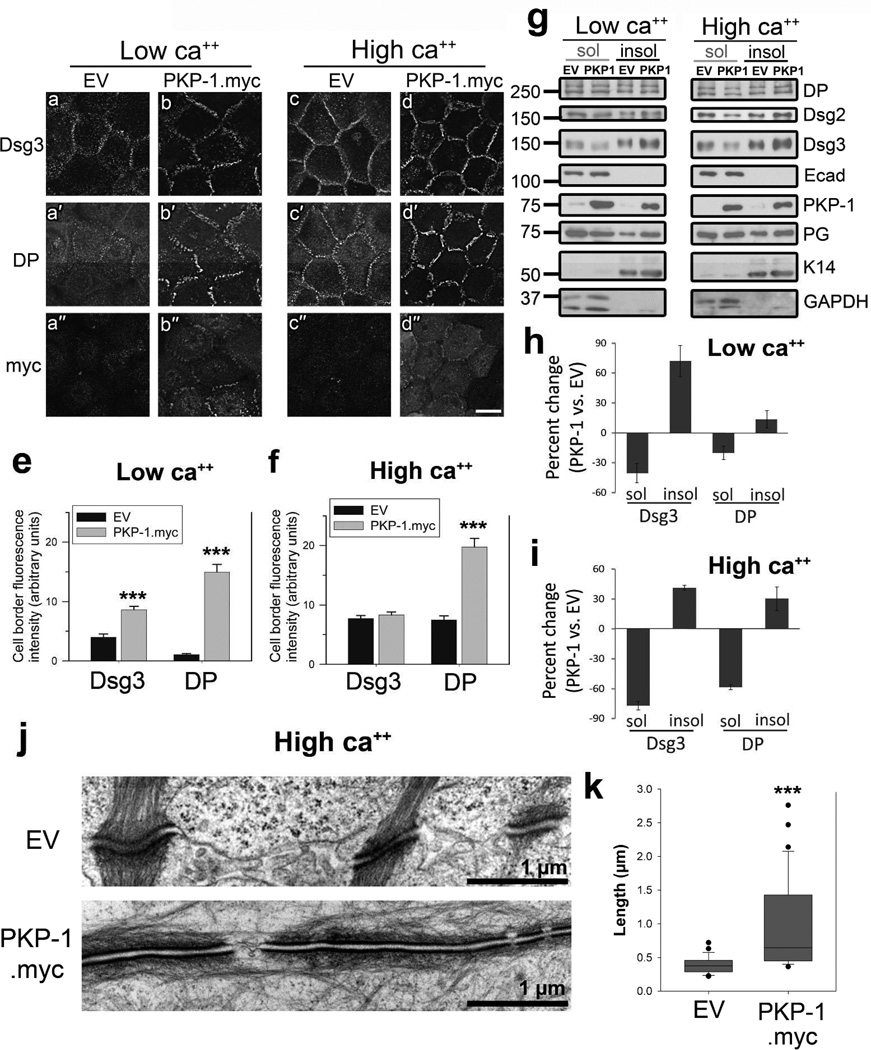
To determine the role of PKP-1 in modulating keratinocyte adhesion and responsiveness to PV IgG, we used an adenoviral delivery system to manipulate PKP-1 expression in primary human epidermal keratinocytes. We first examined the effect of increased PKP-1 expression on Dsg3 and DP localization in cells cultured in low or high calcium media (Figure 1a–d). To detect Dsg3, cells were labeled for 30 minutes prior to fixation with AK15, a non-pathogenic anti-Dsg3 monoclonal antibody (mAb). Total DP and PKP-1.myc were detected after methanol fixation. In empty vector (EV) control keratinocytes, minimal Dsg3 and DP localization was observed at cell-cell contacts in low calcium medium (Figure 1a, a′). Upon the addition of calcium, both proteins localized in punctate patterns at cell-cell contacts (Figure 1c, c′). Interestingly, in low calcium, PKP-1.myc expression substantially increased Dsg3 and DP localization at cell-cell contacts (Figure 1e, compare b, b′ to a, a′). Under high calcium conditions, Dsg3 fluorescence intensity was similar between control and cells with increased PKP-1 expression, whereas DP localization at cell-cell contacts increased significantly with increased PKP-1 expression (Figure 1f, compare c, c′ to d, d′). Sequential detergent extractions from keratinocyte cell lysates followed by western blot analysis was used to compare partitioning of desmosomal proteins between the membrane-associated pool (Triton X-100 soluble) and the desmosomal, cytoskeleton-associated pool (Triton X-100 insoluble). In cells with increased PKP-1 expression, Triton-soluble pools of Dsg3 and DP decreased, while Triton-insoluble pools of Dsg3 and DP increased (Figure 1g–i). Ultrastructural analysis confirmed that PKP-1.myc expression enhanced desmosome formation. Desmosomes in keratinocytes expressing PKP-1.myc more than doubled in length compared to control, increasing from a mean of ~0.4 to ~1.0 Hm (Figure 1i, k). Together, these results demonstrate that PKP-1 expression increases desmosomal protein accumulation at cell-cell contacts and increases desmosome size in primary basal keratinocytes.
Figure 1. PKP-1 promotes desmosome formation.
(a–d) Immunofluorescence images and (e, f) quantification of cell-cell border fluorescence intensity from keratinocytes expressing empty vector (EV) or PKP-1.myc in low (0.1mM) or high calcium media (0.6mM) for 24 hours and immunostained for surface Dsg3, total desmoplakin (DP) and myc (PKP-1.myc). Scale bar: 20Hm. (e, f) Mean ± SEM (n= 50 borders per group); *** p < 0.001 compared with EV (Mann Whitney). (g,h,i) Sequential detergent extraction and western blot analysis of Triton X-100 soluble (sol) and insoluble (insol) proteins. Quantification represents the mean of three independent experiments. (j) Electron micrographs and (k) quantification of desmosome lengths (n= 25 desmosomes per group); *** p < 0.001 compared with EV (Mann Whitney). Scale bars 1Hm.
PKP-1 prevents PV IgG-induced desmosome disruption and loss of cell-cell adhesion
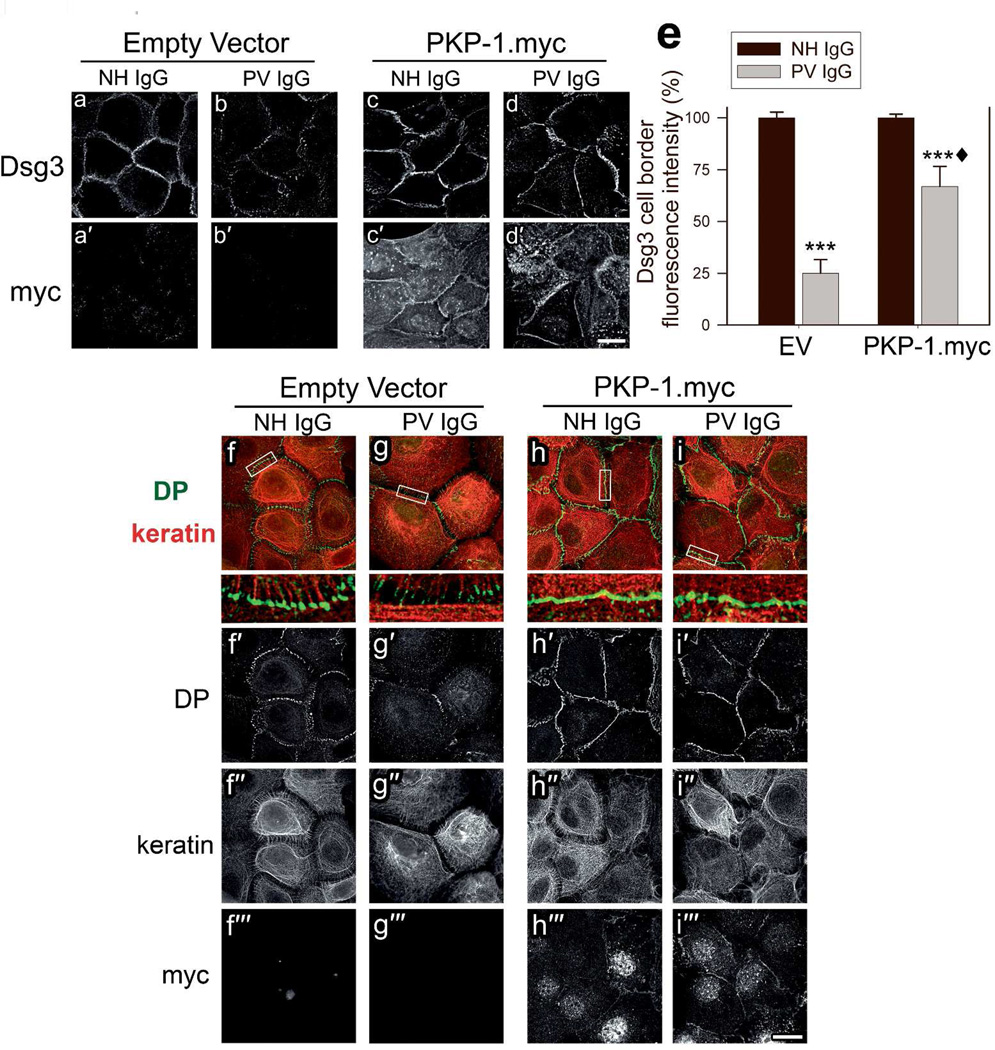
To determine if desmosomes in cells with enhanced PKP-1 expression are responsive to PV IgG-mediated disruption, we examined desmosomal protein distribution and keratinocyte adhesion strength in cells expressing EV or PKP-1.myc, treated with NH IgG or PV IgG. To monitor cell surface Dsg3 after 24 hours of PV IgG treatment, cells were labeled with AK15 just prior to fixation. Similar to previous reports, compared to NH IgG treated cells, PV IgG disrupted Dsg3 localization and significantly reduced the amount of Dsg3 at cell-cell contacts (Figure 2a–b, e) (Calkins et al., 2006; Jennings et al., 2011). Interestingly, expression of PKP-1.myc reduced the loss of Dsg3 border localization in PV IgG treated cells (Figure 2c–e). Furthermore, PV IgG treatment of control cells disrupted DP localization and caused keratin filament retraction from cell-cell borders (Figure 2f–g). High magnification views reveal keratin filaments traversing both in parallel to cell-cell contacts as well as associating with desmosomal plaques, and PV IgG treatment causing dissociation of keratin tonofilament bundles from regions of DP staining (Figure 2f–g enlarged white boxes). PV IgG treated cells expressing PKP-1.myc displayed prominent DP localization at cell-cell contacts with extensive keratin associations at desmosomes (Figure 2i). Control experiments verified that PV IgG, as well as two well characterized Dsg3 mAbs directed against the EC1 (AK23) and EC2–3 (AK15) domains of Dsg3 (Tsunoda et al., 2003), bound to keratinocyte cell surfaces in both control and PKP-1.myc expressing cells (supplementary Figure 1). These findings suggest that PKP-1 expression results in desmosomes that are refractory to disruption when Dsg3 is occupied by pathogenic antibodies.
Figure 2. PKP-1 protects desmosomal components from disruption by PV IgG.
Keratinocytes expressing empty vector (EV) or PKP-1.myc were treated with NH or PV IgG for 24 hours. (a–d) The mAb AK15 was used live to detect cell surface Dsg3 and total myc was detected after methanol fixation (a′–d′). (e) Quantification of Dsg3 fluorescence intensity at cell-cell borders. Data are mean percentages normalized to EV and PKP-1 NH IgG controls. Mean ± SEM (n= 50 borders per group); *** p < 0.001 compared with EV-NH IgG, ♦ compared with EV-PV IgG and PKP-1.myc-NH IgG (Two-way ANOVA, Holm-Sidak method). (f–i) Cells were briefly pre-extracted prior to fixation and immunostained for DP (green, f′–i′), cytokeratin (keratin, red, f″–i″) and myc (f′″–i′″). Data represent four independent experiments. Scale bars, 20Hm.
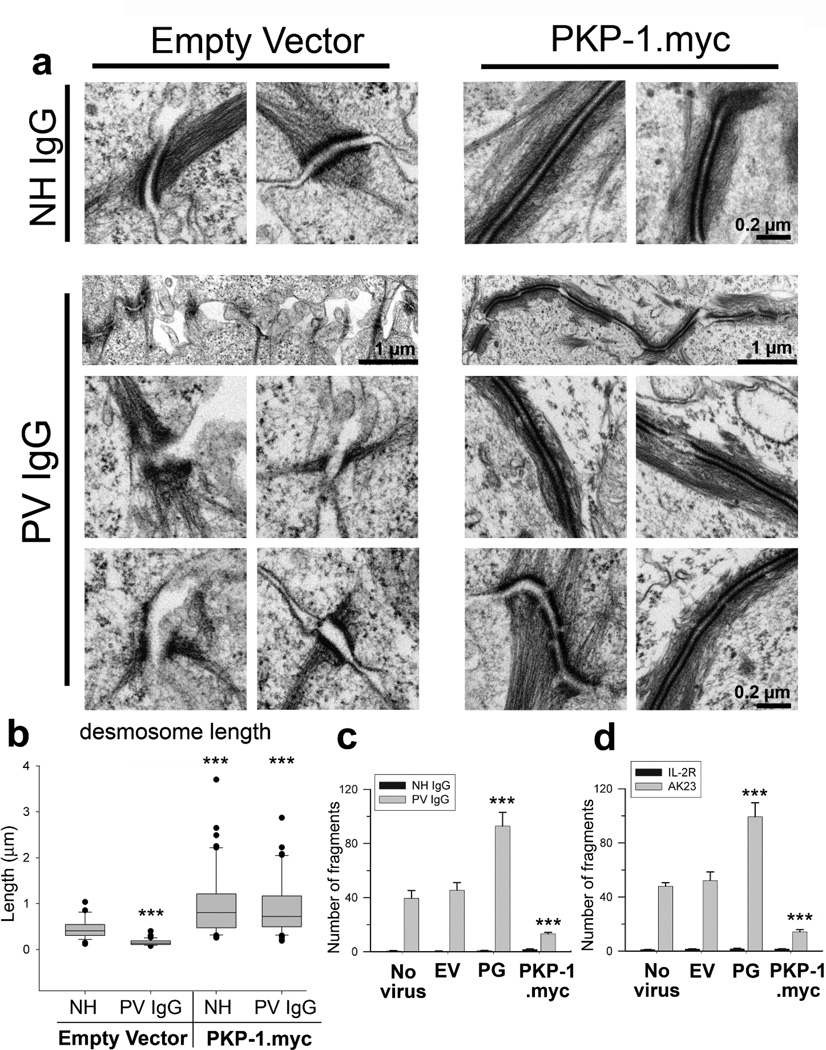
At the ultrastructural level, desmosomes exhibited a variety of severe abnormalities in PV IgG treated keratinocytes, including desmosomes with disordered electron dense plaques and few keratin attachments, as well as desmosomes separated at the midline (Figure 3a). Additionally, PV IgG treatment of control cells caused a substantial decrease in mean desmosome size, from ~0.4 to ~0.1Hm (Figure 3b). In contrast, PV IgG treated keratinocytes expressing PKP-1.myc exhibited elongated desmosomes with well-organized and electron dense plaques with extensive keratin filament attachments (Figure 3a). Furthermore, desmosome lengths in keratinocytes expressing PKP-1.myc did not change with PV IgG treatment (Figure 3b). Lastly, keratinocyte adhesion strength was assessed by performing cell-dissociation assays (Ishii et al., 2005) on keratinocytes treated with PV IgG or the pathogenic mAb AK23. PV IgG and AK23 both caused substantial monolayer fragmentation, indicating the loss of intercellular adhesion strength (Figure 3c, d). Interestingly, increased expression of plakoglobin, which has been implicated in mediating PV IgG effects (Caldelari et al., 2001), exacerbated the effects of PV IgG and AK23 (Figure 3c–d). In contrast, PKP-1 expression maintained strong intercellular adhesion and significantly reduced fragmentation of keratinocytes exposed to pathogenic antibodies (Figure 3c–d). Collectively, these results demonstrate that increasing PKP-1 expression in basal keratinocytes protects desmosomes from PV IgG-induced disruption and loss of keratinocyte adhesion strength.
Figure 3.
PKP-1 protects desmosome ultrastructure and keratinocyte adhesion strength from disruption by PV IgG
(a) Electron micrographs and (b) quantification of desmosome lengths from keratinocytes expressing empty vector (EV) or PKP-1.myc treated with NH or PV IgG for 24 hours (n= 25–50 desmosomes per group); *** p < 0.001 compared with EV-NH IgG (Mann Whitney). (c, d) Quantification of monolayer fragmentation after cell dissociation assays using control keratinocytes (no virus, EV, and plakoglobin (PG)) or keratinocytes expressing PKP-1.myc exposed for 24 hours to (c) NH or PV IgG or (d) mAb IL-2R or AK23, a pathogenic Dsg3 mAb. Mean number of fragments ± SEM;*** p < 0.001 compared with no virus and EV (Two-way ANOVA, Holm-Sidak method). Scale bars, 0.2 or 1 Hm as indicated.
PKP-1 clusters Dsg3 with DP
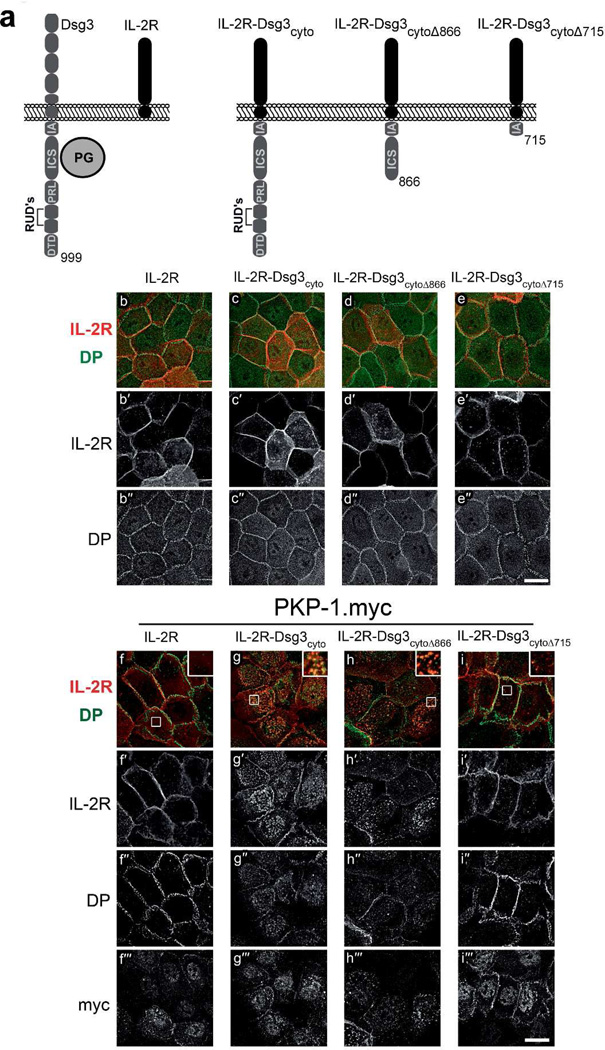
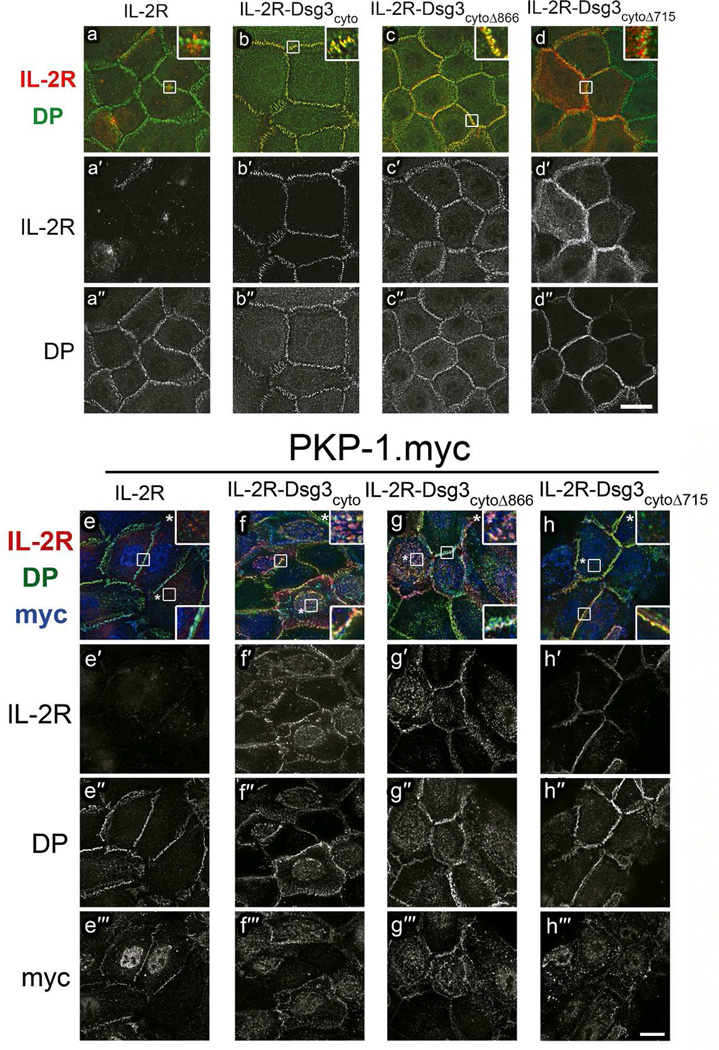
To investigate cytoplasmic interactions between PKP-1 and Dsg3 independently of Dsg3 adhesive interactions, we fused either the full length Dsg3 cytoplasmic domain (Dsg3cyto), or truncated versions of the Dsg3 cytoplasmic tail (Dsg3cytoΔ866, Dsg3cytoΔ715), to the non-adhesive extracellular and transmembrane domains of the interleukin-2 receptor alpha-chain (IL-2R) (Figure 4a). The IL-2R-Dsg3cytoΔ866 chimera lacks the unique desmoglein sequences including the proline-rich linker (PRL), repeating unit domains (RUD) and the desmoglein terminal domain (DTD). The IL-2R-Dsg3cytoΔ715 chimera lacks these domains and the intracellular cadherin-specific domain (ICS), which binds plakoglobin. These proteins were expressed in keratinocytes, with or without PKP-1.myc, and their localization at the cell surface was monitored by live cell IL-2R antibody labeling prior to fixation. Without PKP-1.myc co-expression, all four IL-2R proteins localized to the cell surface and at cell-cell contacts (Figure 4b–e). However, detergent pre-extraction revealed that only the IL-2R-Dsg3cyto and IL-2R-Dsg3cytoΔ866 chimeras co-localized with DP (Figure 5a–d insets). Moreover, the addition of Dsg3 cytoplasmic sequences to the IL-2R conferred resistance to detergent pre-extraction in both control and PKP-1.myc keratinocytes (Figure 5 compare a′ to b′–d′ and e′, to f′–h′). Interestingly, co-expression of PKP-1.myc induced extensive co-clustering of IL-2R-Dsg3cyto and IL-2R-Dsg3cytoΔ866 chimeras with DP, but not the IL-2R or the IL-2R-Dsg3cytoΔ715 chimera (Figure 4f–i, Figure 5e–h). These clusters were present at the cell surface and included the presence of endogenous Dsg3 (Supplemental Figure 2a–d). These results demonstrate that PKP-1 mediates lateral co-clustering of Dsg3 and DP and this activity requires the plakoglobin binding domain on the Dsg3 tail.
Figure 4. PKP-1 clusters the cytoplasmic tail of Dsg3 with DP.
(a) Portions of the Dsg3 cytoplasmic tail (grey) were fused to the extracellular and transmembrane domains of the interleukin-2 receptor alpha chain (IL-2R, black) to create chimeric IL-2R-Dsg3 proteins. IA, intracellular anchor; ICS, intracellular cadherin-specific; IPL, proline-rich linker; RUD, repeating unit domain; DTD; desmoglein terminal domain; cyto, entire Dsg3 cytoplasmic tail; cytoΔ866 and cytoΔ715, Dsg3 cytoplasmic tail truncated at residues 866 and 715. (b–e) IL-2R and IL-2R-Dsg3 chimeras expressed in keratinocytes, methanol fixed and immunostained for cell-surface IL-2R (red, b′–e′) and total DP (green, b″–e″). (f–i) IL-2R and IL-2R-Dsg3 chimeras were co-expressed with PKP-1.myc and immunostained for cell surface IL-2R (red, f′–i′), total desmoplakin (DP) (green, f″–i″) and myc (f′″–i′″). Scale bars 20Hm.
Figure 5. Dsg3 cytoplasmic sequences mediate co-localization with DP and differential sensitivity to detergent pre-extraction.
(a–h) Images of IL-2R and IL-2RDsg3 chimeras expressed in keratinocytes, with and without PKP-1.myc co-expression. Cells were labeled live with a mAb IL-2R (red and a′–h′) for 30 minutes and then immediately subjected to a 45 second detergent pre-extraction with 0.2% Triton-X100. Following fixation, the cells were immunostained for total DP (green and a″–h″) and myc (blue and e′″–h′″). Scale bars 20Hm.
PKP-1 expression induces the formation of calcium-independent and hyper-adhesive desmosomes
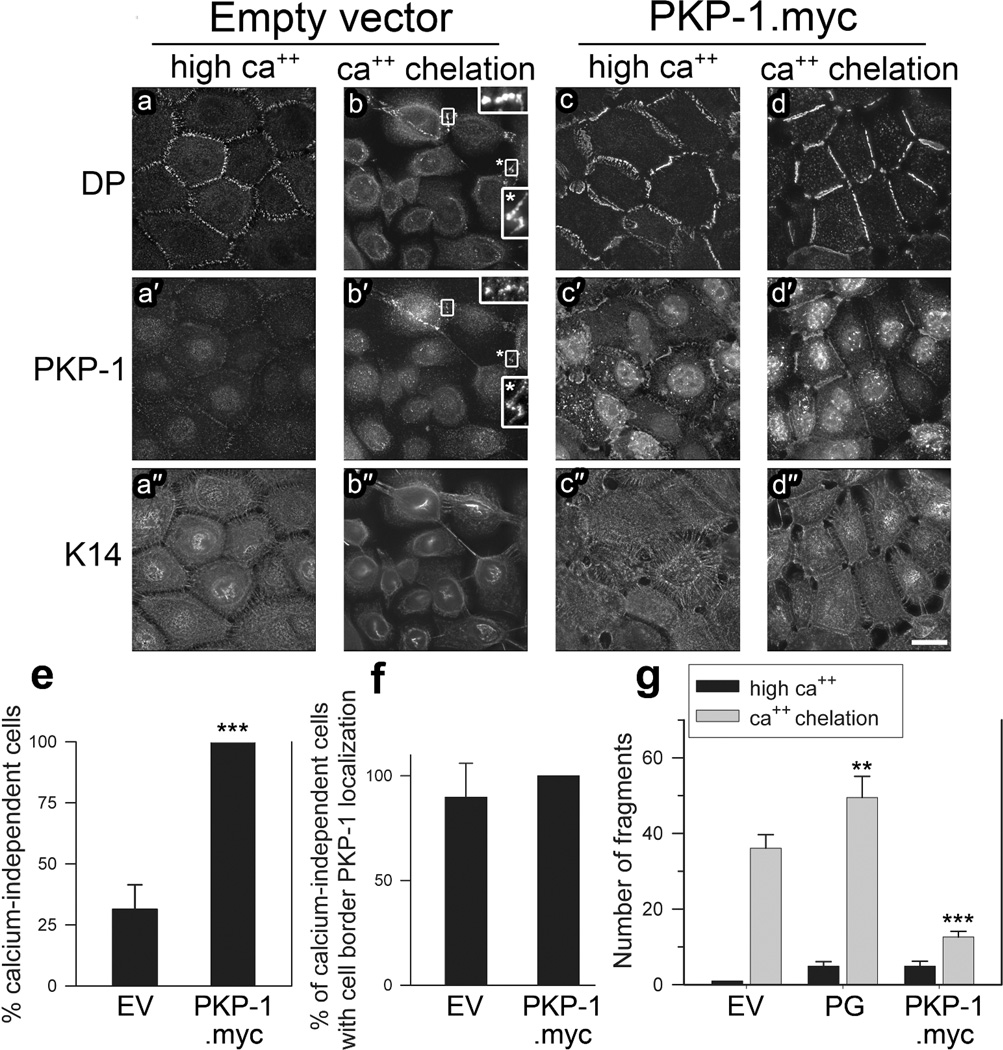
Previous studies indicate that desmosomes can become resistant to calcium depletion (Garrod, 2012) and that calcium-independent desmosomes are resistant to PV IgG (Cirillo et al., 2010). Therefore, we performed ‘calcium chelation assays’ to determine if expression of exogenous PKP-1 increases the formation of calcium-independent desmosomes. Sub-confluent basal keratinocytes pre-incubated in high calcium medium (14–18 hours) were subjected to calcium chelation for 4 hours (calcium-free medium with 3 mM EGTA). After calcium chelation, the localization of PKP-1, DP and keratin-14 was examined. Cells were scored as calcium-independent if cell-cell contacts stained positive for DP. Similar to previous findings (Kimura et al., 2007), the majority of sub-confluent keratinocytes were calcium-dependent, with only ~34% being calcium-independent (Figure 6e). Interestingly, within this subset of calcium-independent keratinocytes, over 90% were positive for endogenous PKP-1 localization at cell-cell contacts (Figure 6f, b, b′ insets,). Although ~34% of control keratinocytes were calcium-independent, the entire population exhibited a rounded morphology, extensive keratin retraction and reduced cell border DP localization after calcium chelation (Figure 6 compare a-a″ to b-b″). Conversely, keratinocytes expressing exogenous PKP-1.myc exhibited a flattened, cobblestone morphology with prominent DP localization and keratin attachments at cell-cell contacts (Figure 6 compare d-d″ to b-b″). Remarkably, we observed that 100% of sub-confluent keratinocytes with increased PKP-1.myc expression possessed calcium-independent desmosomes (Figure 6d, e). To determine if these desmosomes maintain adhesive strength after calcium chelation, we performed cell-dissociation assays. Fragmentation of keratinocyte monolayers expressing PKP-1.myc after calcium chelation was reduced significantly compared to control monolayers (Figure 6g). These data indicate that PKP-1 induces the formation of calcium-independent and hyper-adhesive desmosomes that are resistant to PV IgG.
Figure 6. PKP-1 induces the formation of calcium-independent, hyper-adhesive desmosomes.
(a–d) Keratinocytes expressing empty vector (EV) or PKP-1.myc in high calcium (ca++) medium or after 4 hours calcium chelation, immunostained for desmoplakin (DP), PKP-1 and keratin-14 (K14), bar 20Hm. (e) Percent calcium-independent cells per microscope field. Cells were scored as calcium-independent if cell-cell contact sites were positive for DP. Means ± SEM (n=24 fields);*** p < 0.001 compared with EV (Mann Whitney). (f) Percent cells with PKP-1 localization at cell-cell contacts among calcium-independent cells per microscope field. Means ± SEM (n=24 fields). (g) Quantification of cell dissociation assays using monolayers subjected to 4 hours calcium chelation. Means ± SEM;*** p < 0.001 compared with EV (Two-way ANOVA, Holm-Sidak).
DISCUSSION
We report here that the armadillo family protein PKP-1 prevents PV IgG-induced desmosome disruption in primary human keratinocytes. Keratinocytes with increased PKP-1 expression maintained strong intercellular adhesion in the presence of PV IgG or the pathogenic Dsg3 antibody AK23. Furthermore, PKP-1 co-clusters Dsg3 with DP, resulting in the formation of calcium-independent and hyper-adhesive desmosomes. Our findings raise the interesting possibility that modulating desmosomal plaque protein expression represents a novel approach to reduce blister formation in PV patients.
Pemphigus and related autoimmune disorders are typically treated using immune suppressive therapies (Kasperkiewicz et al., 2012). The use of B-cell depleting agents have proven particularly effective for PV, although these approaches can cause undesirable side effects (Yeh et al., 2005; Zakka et al., 2012). Therefore, recent efforts in the field have sought to find approaches that render the skin resistant to an ongoing autoimmune response. These studies have revealed signaling pathways that regulate desmosome responses to PV IgG, including p38 MAPK, c-myc, EGFR, among others (Sharma et al., 2007). Here, we report that enhanced expression of a single desmosomal plaque protein, PKP-1, renders keratinocytes resistant from PV IgG-induced loss of adhesion by inducing the formation of calcium-independent, hyper-adhesive desmosomes. Our work suggests that manipulating desmosomal plaque protein expression could be considered as an additional approach to facilitate desmosome resistance to autoimmune attack in pemphigus. Similarly, existing treatments such as corticosteroids may work in part by modulating the expression of PKP-1 or other desmosomal genes, and thereby stabilize keratinocyte adhesion in addition to suppressing immune activity (Nguyen et al., 2004).
Previously, we found that desmosome disassembly resulted from disruptive clustering of Dsg3 and other desmosomal proteins in response to polyclonal PV IgG (Saito et al., 2012a), a finding consistent with immunofluorescence results of Dsg3 clustering in the epidermis of PV patients (Oktarina et al., 2011). In this study, we observed that desmosomes exposed to PV IgG exhibited a variety of ultrastructural abnormalities, including disordered electron dense plaques, reduced keratin filament attachment and reduced desmosome size (Figure 3). Importantly, we found that increased expression of PKP-1 reduced PV IgG-induced disruption and loss of Dsg3 at cell-cell borders (Figure 1a–d). Moreover, PKP-1 prevented desmosome structural abnormalities in response to PV IgG (Figure 3). Together, these findings suggest that PKP-1 may prevent the disruptive clustering and reorganization of desmosomal proteins that contribute to desmosome disassembly and loss of adhesion in response to PV IgG. Interestingly, although PKP-1 is expressed in the upper layers of the epidermis, pemphigus foliaceus IgG cause blistering in the granular layer of the skin, (Kneisel and Hertl, 2011) where PKP-1 is expressed at high levels (Hatzfeld, 2007). Thus, the mechanisms of blistering in different forms of pemphigus may vary, or alternatively, endogenous levels of PKP-1 in human epidermis may be insufficient to prevent the loss of desmosomal adhesion caused by pemphigus autoantibodies.
Desmosomes in confluent cell cultures undergo a transition from a calcium-dependent to a calcium-independent state over time (Garrod, 2010). Although the mechanism of desmosome transition to hyper-adhesion is not fully understood, one model proposed to explain hyper-adhesion suggests that calcium-independent desmosomes exhibit a more highly ordered arrangement of cadherin extracellular domains (Garrod et al., 2005). Because PKC signaling has been shown to modulate desmosome hyper-adhesion (Thomason et al., 2010), it has been suggested that an 'inside-out' transmembrane signal is responsible for the transition of desmosomes to a hyper-adhesive, calcium-independent state (Garrod, 2010; Garrod et al., 2005). Importantly, our immunofluorescence analysis revealed that the small percentage of calcium-independent desmosomes that exist in sub-confluent keratinocytes stain positive for PKP-1. Furthermore, in sub-confluent cultures, exogenous PKP-1 expression resulted in a complete transition of desmosomes to a calcium-independent state. These data and others (Hobbs and Green, 2012) suggest that the composition of the desmosomal plaque, along with intracellular signaling pathways (Garrod, 2010), mediate 'inside-out' transitions between various desmosome adhesion states (Garrod, 2010; Garrod et al., 2005).
Previous studies demonstrate that PKP-1 enhances DP recruitment to desmosomes (Hatzfeld, 2007). Another mechanism by which PKP-1 stabilizes and increases desmosome formation is likely to involve increased adhesion mediated by Dsg3 or other desmosomal cadherins (Bornslaeger et al., 2001; South et al., 2003). Our results indicate that while desmosome size increases upon PKP-1 expression (Figure 1j–k), the overall amount of desmosomal proteins expressed does not appear to increase dramatically (Figure 1g). Rather, we observed that upon PKP-1 expression, the non-desmosomal, membrane-associated pool of Dsg3 decreased, while the desmosomal, Triton-insoluble pool increased (Figure 1g–i), suggesting increased Dsg3 incorporation into desmosomes. We also demonstrate that PKP-1 co-clusters Dsg3 with DP through interactions dependent upon the plakoglobin binding domain in the cadherin tail (Figure 4,5). These findings suggest that, along with enhancing DP recruitment, PKP-1 increases desmosome size by clustering and incorporating cell surface desmosomal protein complexes into desmosomes.
Considering that PKP-1 co-clusters Dsg3 with DP, PKP-1 may act to stabilize lateral interactions between desmosomal cadherins and desmosomal plaque proteins. In addition to cytoplasmic associations, lateral cadherin clustering is also mediated by cis-binding interactions in the cadherin extracellular domain (Al-Amoudi et al., 2007; Brasch et al., 2012). A recent study demonstrated that PV IgG bind to putative Dsg3 cis-interacting sequences in the cadherin extracellular domain (Di Zenzo et al., 2012). An intriguing possibility is that PKP-1 clustering of the Dsg3 tail stabilizes cis-interactions between Dsg3 extracellular domains and thereby protects desmosomes from PV IgG mediated disruption. Further studies using structural and biophysical approaches will be needed to test these possibilities, and to fully understand the basis for the transition of desmosomes to a calcium-independent and PV IgG resistant state. Such studies are likely to yield insights into the fundamental mechanisms that govern desmosome assembly as well as new therapeutic approaches to treat PV and related disorders.
MATERIALS AND METHODS
Cell culture
Normal human epidermal keratinocytes (NHEKs) isolated from neonatal foreskin were propagated in low calcium (0.1mM) keratinocyte growth media (KBM-Gold 00192151 withKGM-GoldSingleQuot 00192152, Lonza, Walkersville, MD) as previously described (Calkins et al., 2006). Sub-confluent (50–80%) NHEKs, passages 2–4, were switched into KGM containing 0.6mM CaCl 14–18 hours prior to experimentation.
Antibodies
PV patient sera were kind gifts from M. Amagai (Keio University, Tokyo), J. Stanley (University of Pennsylvania, Philadelphia, PA), and R. Swerlick (Emory University, Atlanta, GA). PV sera recognized Dsg3, but not Dsg1, determined by ELISA. PV IgG was purified using a Melon Gel IgG Purification Kit (45212, ThermoFisher Scientific, Rockford, IL). Mouse mAb AK15 and AK23 were generated as previously described (Tsunoda et al., 2003). NH and PV IgG were used at 100–150Hg/ml, while AK23, AK15 and IL-2R were added at 10–15Hg/ml. Other antibodies: NH IgG (Invitrogen), rabbit anti-DP (NW6; gift KJ Green, Northwestern University), rabbit anti-gamma catenin (plakoglobin, H-80) and rabbit anti-GAPDH (25778) (Santa Cruz Biotechnology, Santa Cruz, CA), chicken (5560) and rabbit anti-keratin 14 (gift J. Segre, NIH, Bethesda, MD), polyclonal chicken anti-c-myc (A190-103A; Bethyl Laboratories, Montgomery, TX), mouse anti-Dsg2 (AH12.2; gift A. Nusrat, Emory University (Nava et al., 2007)), mouse anti-IL-2R IgG2a alpha (7G7B6; American Type Culture Collection, Manasssas, VA), mouse anti-E-cadherin (61081; BD Transduction Laboratories), mouse anti-PKP-1 (14B11; gift J. Wahl III, University of Nebraska (Sobolik-Delmaire et al., 2006)), mouse anti-cytokeratin (Immunotech, Marseille, France). Secondary antibodies: AlexaFluor 647, 555, 488 and HRP-conjugated goat IgGs (Invitrogen).
Adenoviruses
Myc-tagged full length PKP-1 was subcloned into pAd-Track and adenoviruses made from pAdEeasy adenovirus-packaging system that co-expresses GFP with the cDNA of interest (Delva et al., 2008). The full-length “a” form of PKP-1 originally cloned into pCMV-Script was described previously (Hatzfeld et al., 1994; Kowalczyk et al., 1999). The IL-2R and IL-2R-Dsg3 chimeras were generated as described previously (Saito et al., 2012a). NHEKs were infected with adenoviruses 24–36 hours prior to experimentation for adenoviral protein expression. All constructs and their expression were verified by DNA sequencing, western blotting, and immunohistochemistry. High titers were generated as described previously (Setzer et al., 2004; Xiao et al., 2003). Infection rates (70–90%) were determined visually by GFP and immunofluorescence of the protein of interest.
Immunofluorescence and cell-cell border quantification
NHEKs on glass coverslips were washed 3x with phosphate-buffered saline (PBS+), fixed on ice using either −20°C methanol (2 minutes) or were pre-extracted with 0.2% TritonX-100 + 3mM sucrose (45 seconds) and fixed with 4% paraformaldehyde (10 minutes) followed by extraction with 0.2% TritonX-100 (5 minutes). To detect only surface proteins, cells were fixed with 4% paraformaldehyde (10 minutes) without permeabilization. Live cell labeling with mAb AK15, AK23 or IL-2R was performed for 30 minutes prior to fixation at 37°C. To remove surface bound antibody (supplemental Figure 1), cells were incubated on ice with a low pH wash (PBS+ with 100 mM glycine, 20 mM magnesium acetate, and 50 mM potassium chloride, pH 2.2) for 30 minutes prior to fixation. Image acquisition, deconvolution and quantification was conducted with Simple PCI software (version 6.6, Hamamatsu Corporation, Sewickley, PA) on a wide-field fluorescence microscope (DMRXA2; Leica) equipped with 63×/1.32 NA, and 100×/1.40 NA oil immersion objectives, and a CCD camera (ORCA; Hamamatsu Photonics). Images shown are deconvolved z-stack projections. For quantification, exposure times and fluorescence intensity thresholds were consistent between experimental data sets. Images were thresholded, and the average intensity above threshold was calculated within 4.5Hm×4.5Hm region of interests (ROIs) selecting cell borders from individual images.
Calcium chelation assays
NHEKs on glass coverslips, infected with the appropriate adenoviruses in 0.1mM calcium KGM, were switched to high calcium media (0.6mM) for 14–18 hours prior to three, one-minute washes in calcium and magnesium-free PBS supplemented with 3mM Methylene glycol-bis(2-aminoethylether)-N,N,N′,N′-tetraacetic acid (EGTA). Cells were incubated in calcium free KBM with 3mM EGTA for 4 hours and processed for immunofluorescence. Images were captured at random (63x oil objective), the total number of cells counted and scored as calcium-independent if cell-cell contact sites were positive for DP staining (8 images per group, per experiment (~ 40 cells per image)). This number, a proportion of the total cell count, is displayed as the percentage of cells that are calcium-independent.
Cell-Dissociation assays
Dispase based cell-dissociation assays were performed as described previously (Saito et al., 2012a). Briefly, 100% confluent NHEKs in 1.9cm2 culture dishes were switched to high calcium medium ~16 hours before addition of the appropriate antibodies. Recombinant exfoliative toxin A (ETA, 0.25Hg/ml) cleaves Dsg1 and was added 2 hours prior to the assay. After overnight incubation with antibodies, monolayers were released (1U/ml dispase, Roche) and subjected to mechanical stress (pipetting 25× with a 1ml micropipette tip). Monolayers were fixed, stained and fragments counted using a dissecting microscope. In assays with calcium chelation, monolayers were released from the culture dish prior to calcium chelation.
Electron microscopy
NHEKs on coverslips, infected with the appropriate adenovirus, were treated with NH or PV IgG as described above. Cells were fixed (2.5% glutaraldehyde in 0.1M cacodylate) and processed for conventional electron microscopy (Robert P. Apkarian Integrated Electron Microscopy Core, Emory University).
Sequential detergent extraction and western blot analysis
NHEKs were extracted sequentially in Triton buffer (1% TritonX-100, 10mM Tris-pH 7.5, 140mM NaCl, 5mM EDTA, 2mM EGTA, (cOmplete, Mini, EDTA-free Protease Inhibitor Tablets)) followed by extraction with urea–SDS buffer (1% SDS, 8M urea, 10mM Tris-HCL-pH 7.5, 5mM EDTA, 2mM EGTA, 70 mM NaCl) as described previously (Calkins et al., 2006). The triton-soluble samples were 2x the insoluble samples for visualization.
Supplementary Material
ACKNOWLEDGMENTS
We acknowledge advice and comments from Kowalczyk laboratory members and Drs. Kathleen Green, Molly Ogle and Chris Capaldo. We recognize Benjamin Nanes for help with illustrations and data analysis, and Susan Summers for adenovirus production and keratinocyte isolations. We thank Hong Yi for technical assistance at the Emory University Robert P. Apkarian Integrated Electron Microscopy Core. This work was supported by the NIH/NIAMS (R01 AR048266). DKT was supported in part by T32GM008367.
Abbreviations
- Dsg3
desmoglein 3
- DP
desmoplakin
- IL-2R
interleukin-2 receptor alpha-chain
- mAb
monoclonal antibody
- NH IgG
normal human IgG
- PV IgG
pemphigus vulgaris IgG
- PKP-1
Plakophilin-1
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
References
- Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–837. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- Amagai M, Klaus-Kovtun V, Stanley JR. Autoantibodies against a novel epithelial cadherin in pemphigus vulgaris, a disease of cell adhesion. Cell. 1991;67:869–877. doi: 10.1016/0092-8674(91)90360-b. [DOI] [PubMed] [Google Scholar]
- Amagai M, Stanley JR. Desmoglein as a Target in Skin Disease and Beyond. J Invest Dermatol. 2012;132:776–784. doi: 10.1038/jid.2011.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bornslaeger EA, Godsel LM, Corcoran CM, Park JK, Hatzfeld M, Kowalczyk AP, et al. Plakophilin 1 interferes with plakoglobin binding to desmoplakin, yet together with plakoglobin promotes clustering of desmosomal plaque complexes at cell-cell borders. J Cell Sci. 2001;114:727–738. doi: 10.1242/jcs.114.4.727. [DOI] [PubMed] [Google Scholar]
- Brasch J, Harrison OJ, Honig B, Shapiro L. Thinking outside the cell: how cadherins drive adhesion. Trends in Cell Biology. 2012;22:299–310. doi: 10.1016/j.tcb.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooke MA, Nitoiu D, Kelsell DP. Cell–cell connectivity: desmosomes and disease. The Journal of Pathology. 2012;226:158–171. doi: 10.1002/path.3027. [DOI] [PubMed] [Google Scholar]
- Caldelari R, de Bruin A, Baumann D, Suter MM, Bierkamp C, Balmer V, et al. A central role for the armadillo protein plakoglobin in the autoimmune disease pemphigus vulgaris. J Cell Biol. 2001;153:823–834. doi: 10.1083/jcb.153.4.823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkins CC, Setzer SV, Jennings JM, Summers S, Tsunoda K, Amagai M, et al. Desmoglein Endocytosis and Desmosome Disassembly Are Coordinated Responses to Pemphigus Autoantibodies. J Biol Chem. 2006;281:7623–7634. doi: 10.1074/jbc.M512447200. [DOI] [PubMed] [Google Scholar]
- Cirillo N, Lanza A, Prime SS. Induction of hyper-adhesion attenuates autoimmune-induced keratinocyte cell-cell detachment and processing of adhesion molecules via mechanisms that involve PKC. Exp Cell Res 2010. 2010 Feb 15;316(4):580–592. doi: 10.1016/j.yexcr.2009.10.005. Epub 2009 Oct 8. [DOI] [PubMed] [Google Scholar]
- Delva E, Jennings JM, Calkins CC, Kottke MD, Faundez V, Kowalczyk AP. Pemphigus vulgaris IgG-induced desmoglein-3 endocytosis and desmosomal disassembly are mediated by a clathrin and dynamin-independent mechanism. J Biol Chem. 2008 doi: 10.1074/jbc.M710046200. M710046200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delva E, Tucker DK, Kowalczyk AP. The desmosome. Cold Spring Harb Perspect Biol. 2009;1:a002543. doi: 10.1101/cshperspect.a002543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Zenzo G, Di Lullo G, Corti D, Calabresi V, Sinistro A, Vanzetta F, et al. Pemphigus autoantibodies generated through somatic mutations target the desmoglein-3 cis-interface. The Journal of Clinical Investigation. 2012;122:3781–3790. doi: 10.1172/JCI64413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusek RL, Godsel LM, Green KJ. Discriminating roles of desmosomal cadherins: Beyond desmosomal adhesion. Journal of Dermatological Science. 2007;45:7–21. doi: 10.1016/j.jdermsci.2006.10.006. [DOI] [PubMed] [Google Scholar]
- Garrod D. Desmosomes In Vivo. Dermatology Research and Practice. 2010;2010 doi: 10.1155/2010/212439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod D, Kimura TE. Hyper-adhesion: a new concept in cell-cell adhesion. Biochem Soc Trans 2008. 2008 Apr;36(Pt 2):195–201. doi: 10.1042/BST0360195. [DOI] [PubMed] [Google Scholar]
- Garrod DR. The Assay that Defines Desmosome Hyper-Adhesion. J Invest Dermatol. 2012 doi: 10.1038/jid.2012.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrod DR, Berika MY, Bardsley WF, Holmes D, Tabernero L. Hyperadhesion in desmosomes: its regulation in wound healing and possible relationship to cadherin crystal structure. Journal of Cell Science. 2005;118:5743–5754. doi: 10.1242/jcs.02700. [DOI] [PubMed] [Google Scholar]
- Getsios S, Huen AC, Green KJ. Working out the strength and flexibility of desmosomes. Nat Rev Mol Cell Biol. 2004;5:271–281. doi: 10.1038/nrm1356. [DOI] [PubMed] [Google Scholar]
- Getsios S, Waschke J, Borradori L, Hertl M, Muller EJ. From Cell Signaling to Novel Therapeutic Concepts: International Pemphigus Meeting on Advances in Pemphigus Research and Therapy. J Invest Dermatol. 2010;130:1764–1768. doi: 10.1038/jid.2010.111. [DOI] [PubMed] [Google Scholar]
- Green KJ, Simpson CL. Desmosomes: new perspectives on a classic. J Invest Dermatol. 2007;127:2499–2515. doi: 10.1038/sj.jid.5701015. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M. Plakophilins: Multifunctional proteins or just regulators of desmosomal adhesion? Biochimica et Biophysica Acta (BBA) - Molecular Cell Research. 2007;1773:69–77. doi: 10.1016/j.bbamcr.2006.04.009. [DOI] [PubMed] [Google Scholar]
- Hatzfeld M, Haffner C, Schulze K, Vinzens U. The Function of Plakophilin 1 in Desmosome Assembly and Actin Filament Organization. J Cell Biol. 2000;149:209–222. doi: 10.1083/jcb.149.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatzfeld M, Kristjansson GI, Plessmann U, Weber K. Band 6 protein, a major constituent of desmosomes from stratified epithelia, is a novel member of the armadillo multigene family. Journal of Cell Science. 1994;107:2259–2270. doi: 10.1242/jcs.107.8.2259. [DOI] [PubMed] [Google Scholar]
- Hobbs RP, Green KJ. Desmoplakin regulates desmosome hyperadhesion. J Invest Dermatol. 2012;132:482–485. doi: 10.1038/jid.2011.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann I, Mertens C, Brettel M, Nimmrich V, Schnolzer M, Herrmann H. Interaction of plakophilins with desmoplakin and intermediate filament proteins: an in vitro analysis. J Cell Sci. 2000;113:2471–2483. doi: 10.1242/jcs.113.13.2471. [DOI] [PubMed] [Google Scholar]
- Holthöfer B, Windoffer R, Troyanovsky S, Leube RE. International Review of Cytology. Vol. Volume 264. Academic Press; 2007. Structure and Function of Desmosomes. In: pp. 65–163. [DOI] [PubMed] [Google Scholar]
- Ishii K, Harada R, Matsuo I, Shirakata Y, Hashimoto K, Amagai M. In Vitro Keratinocyte Dissociation Assay for Evaluation of the Pathogenicity of Anti-Desmoglein 3 IgG Autoantibodies in Pemphigus Vulgaris. J Investig Dermatol. 2005;124:939–946. doi: 10.1111/j.0022-202X.2005.23714.x. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Tucker DK, Kottke MD, Saito M, Delva E, Hanakawa Y, et al. Desmosome disassembly in response to pemphigus vulgaris IgG occurs in distinct phases and can be reversed by expression of exogenous dsg3. J Invest Dermatol. 2011;131:706–718. doi: 10.1038/jid.2010.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapprell HP, Owaribe K, Franke WW. Identification of a basic protein of Mr 75,000 as an accessory desmosomal plaque protein in stratified and complex epithelia. The Journal of Cell Biology. 1988;106:1679–1691. doi: 10.1083/jcb.106.5.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasperkiewicz M, Schmidt E, Zillikens D. Current therapy of the pemphigus group. Clinics in Dermatology. 2012;30:84–94. doi: 10.1016/j.clindermatol.2011.03.014. [DOI] [PubMed] [Google Scholar]
- Kimura TE, Merritt AJ, Garrod DR. Calcium-Independent Desmosomes of Keratinocytes are Hyper-Adhesive. J Invest Dermatol. 2007;127:775–781. doi: 10.1038/sj.jid.5700643. [DOI] [PubMed] [Google Scholar]
- Kitajima Y, Aoyama Y. A Perspective of Pemphigus from Bedside and Laboratory-Bench. Clinical Reviews in Allergy & Immunology. 2007;33:57–66. doi: 10.1007/s12016-007-0036-5. [DOI] [PubMed] [Google Scholar]
- Kneisel A, Hertl M. Autoimmune bullous skin diseases. Part 1: Clinical manifestations. JDDG: Journal der Deutschen Dermatologischen Gesellschaft. 2011;9:844–857. doi: 10.1111/j.1610-0387.2011.07793.x. [DOI] [PubMed] [Google Scholar]
- Kottke MD, Delva E, Kowalczyk AP. The desmosome: cell science lessons from human diseases. J Cell Sci. 2006;119:797–806. doi: 10.1242/jcs.02888. [DOI] [PubMed] [Google Scholar]
- Kowalczyk AP, Hatzfeld M, Bornslaeger EA, Kopp DS, Borgwardt JE, Corcoran CM, et al. The head domain of plakophilin-1 binds to desmoplakin and enhances its recruitment to desmosomes. Implications for cutaneous disease. J Biol Chem. 1999;274:18145–18148. doi: 10.1074/jbc.274.26.18145. [DOI] [PubMed] [Google Scholar]
- McGrath JA, McMillan JR, Shemanko CS, Runswick SK, Leigh IM, Lane EB, et al. Mutations in the plakophilin 1 gene result in ectodermal dysplasia/skin fragility syndrome. Nat Genet. 1997 Oct 17;1997(2):240–244. doi: 10.1038/ng1097-240. [DOI] [PubMed] [Google Scholar]
- McGrath JA, Mellerio JE. Ectodermal Dysplasia-Skin Fragility Syndrome. Dermatologic clinics. 2010;28:125–129. doi: 10.1016/j.det.2009.10.014. [DOI] [PubMed] [Google Scholar]
- McMillan JR, Haftek M, Akiyama M, South AP, Perrot H, McGrath JA, et al. Alterations in Desmosome Size and Number Coincide with the Loss of Keratinocyte Cohesion in Skin with Homozygous and Heterozygous Defects in the Desmosomal Protein Plakophilin 1. J Investig Dermatol. 2003;121:96–103. doi: 10.1046/j.1523-1747.2003.12324.x. [DOI] [PubMed] [Google Scholar]
- Nava P, Laukoetter MG, Hopkins AM, Laur O, Gerner-Smidt K, Green KJ, et al. Desmoglein-2: A Novel Regulator of Apoptosis in the Intestinal Epithelium. Mol Biol Cell. 2007;18:4565–4578. doi: 10.1091/mbc.E07-05-0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Arredondo J, Chernyavsky AI, Kitajima Y, Pittelkow M, Grando SA. Pemphigus vulgaris IgG and methylprednisolone exhibit reciprocal effects on keratinocytes. J Biol Chem. 2004;279:2135–2146. doi: 10.1074/jbc.M309000200. [DOI] [PubMed] [Google Scholar]
- Oktarina DA, van der Wier G, Diercks GF, Jonkman MF, Pas HH. IgGinduced clustering of desmogleins 1 and 3 in skin of patients with pemphigus fits with the desmoglein nonassembly depletion hypothesis. The British journal of dermatology. 2011;165:552–562. doi: 10.1111/j.1365-2133.2011.10463.x. [DOI] [PubMed] [Google Scholar]
- Saito M, Stahley SN, Caughman CY, Mao X, Tucker DK, Payne AS, et al. Signaling Dependent and Independent Mechanisms in Pemphigus Vulgaris Blister Formation. PLoS One. 2012a;7:e50696. doi: 10.1371/journal.pone.0050696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito M, Tucker DK, Kohlhorst D, Niessen CM, Kowalczyk AP. Classical and desmosomal cadherins at a glance. Journal of Cell Science. 2012b;125:2547–2552. doi: 10.1242/jcs.066654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setzer SV, Calkins CC, Garner J, Summers S, Green KJ, Kowalczyk AP. Comparative analysis of armadillo family proteins in the regulation of a431 epithelial cell junction assembly, adhesion and migration. J Invest Dermatol. 2004;123:426–433. doi: 10.1111/j.0022-202X.2004.23319.x. [DOI] [PubMed] [Google Scholar]
- Sharma P, Mao X, Payne AS. Beyond steric hindrance: the role of adhesion signaling pathways in the pathogenesis of pemphigus. J Dermatol Sci. 2007;48:1–14. doi: 10.1016/j.jdermsci.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Simpson CL, Patel DM, Green KJ. Deconstructing the skin: cytoarchitectural determinants of epidermal morphogenesis. Nat Rev Mol Cell Biol. 2011;12:565–580. doi: 10.1038/nrm3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith EA, Fuchs E. Defining the Interactions Between Intermediate Filaments and Desmosomes. The Journal of Cell Biology. 1998;141:1229–1241. doi: 10.1083/jcb.141.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobolik-Delmaire T, Katafiasz D, Wahl JK., III Carboxyl Terminus of Plakophilin-1 Recruits It to Plasma Membrane, whereas Amino Terminus Recruits Desmoplakin and Promotes Desmosome Assembly. J Biol Chem. 2006;281:16962–16970. doi: 10.1074/jbc.M600570200. [DOI] [PubMed] [Google Scholar]
- South AP, Wan H, Stone MG, Dopping-Hepenstal PJC, Purkis PE, Marshall JF, et al. Lack of plakophilin 1 increases keratinocyte migration and reduces desmosome stability. J Cell Sci. 2003;116:3303–3314. doi: 10.1242/jcs.00636. [DOI] [PubMed] [Google Scholar]
- Thomason HA, Scothern A, McHarg S, Garrod DR. Desmosomes: adhesive strength and signalling in health and disease. Biochemical Journal. 2010;429:419–433. doi: 10.1042/BJ20100567. [DOI] [PubMed] [Google Scholar]
- Tsunoda K, Ota T, Aoki M, Yamada T, Nagai T, Nakagawa T, et al. Induction of Pemphigus Phenotype by a Mouse Monoclonal Antibody Against the Amino-Terminal Adhesive Interface of Desmoglein 3. The Journal of Immunology. 2003;170:2170–2178. doi: 10.4049/jimmunol.170.4.2170. [DOI] [PubMed] [Google Scholar]
- Wahl JK. A role for plakophilin-1 in the initiation of desmosome assembly. Journal of Cellular Biochemistry. 2005;96:390–403. doi: 10.1002/jcb.20514. [DOI] [PubMed] [Google Scholar]
- Waschke J. The desmosome and pemphigus. Histochemistry and Cell Biology. 2008 doi: 10.1007/s00418-008-0420-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao K, Allison DF, Kottke MD, Summers S, Sorescu GP, Faundez V, et al. Mechanisms of VE-cadherin processing and degradation in microvascular endothelial cells. J Biol Chem. 2003;278:19199–19208. doi: 10.1074/jbc.M211746200. [DOI] [PubMed] [Google Scholar]
- Yeh SW, Sami N, Ahmed RA. Treatment of Pemphigus Vulgaris: Current and Emerging Options. American Journal of Clinical Dermatology. 2005;6:327–342. doi: 10.2165/00128071-200506050-00006. [DOI] [PubMed] [Google Scholar]
- Zakka LR, Shetty SS, Ahmed AR. Rituximab in the Treatment of Pemphigus Vulgaris. Dermatology and therapy. 2012;2:17. doi: 10.1007/s13555-012-0017-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.