Abstract
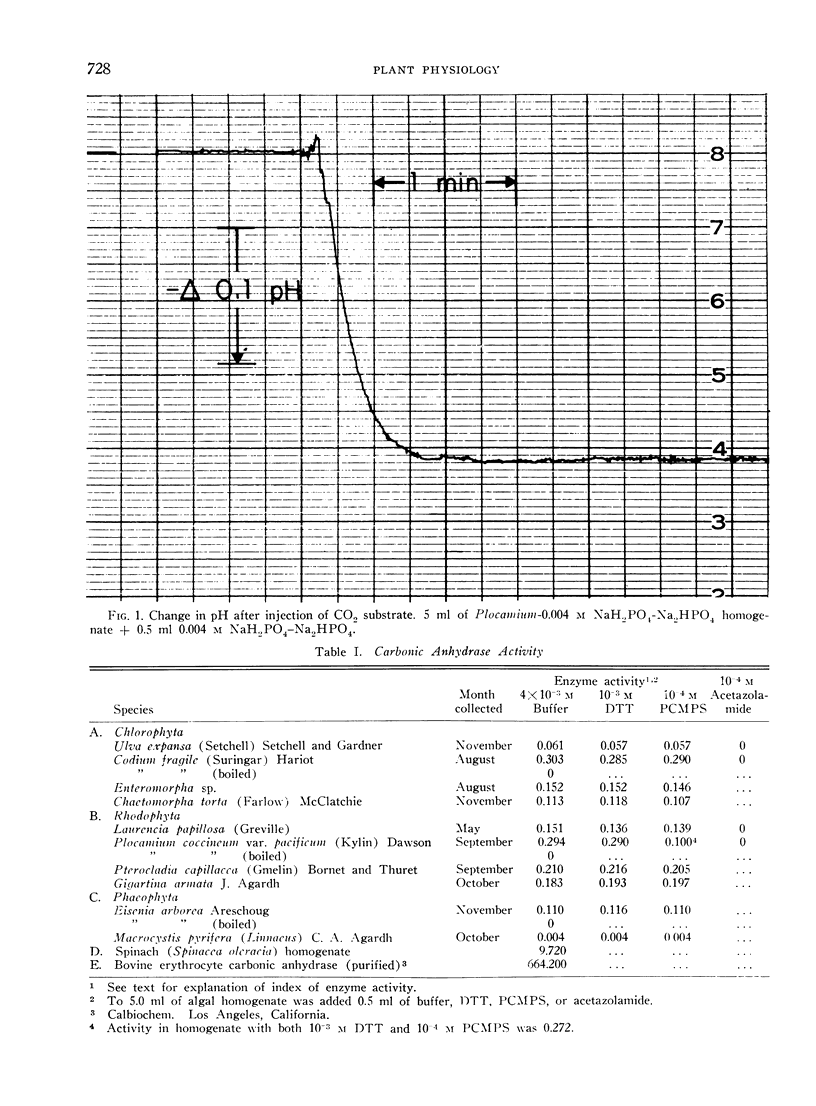
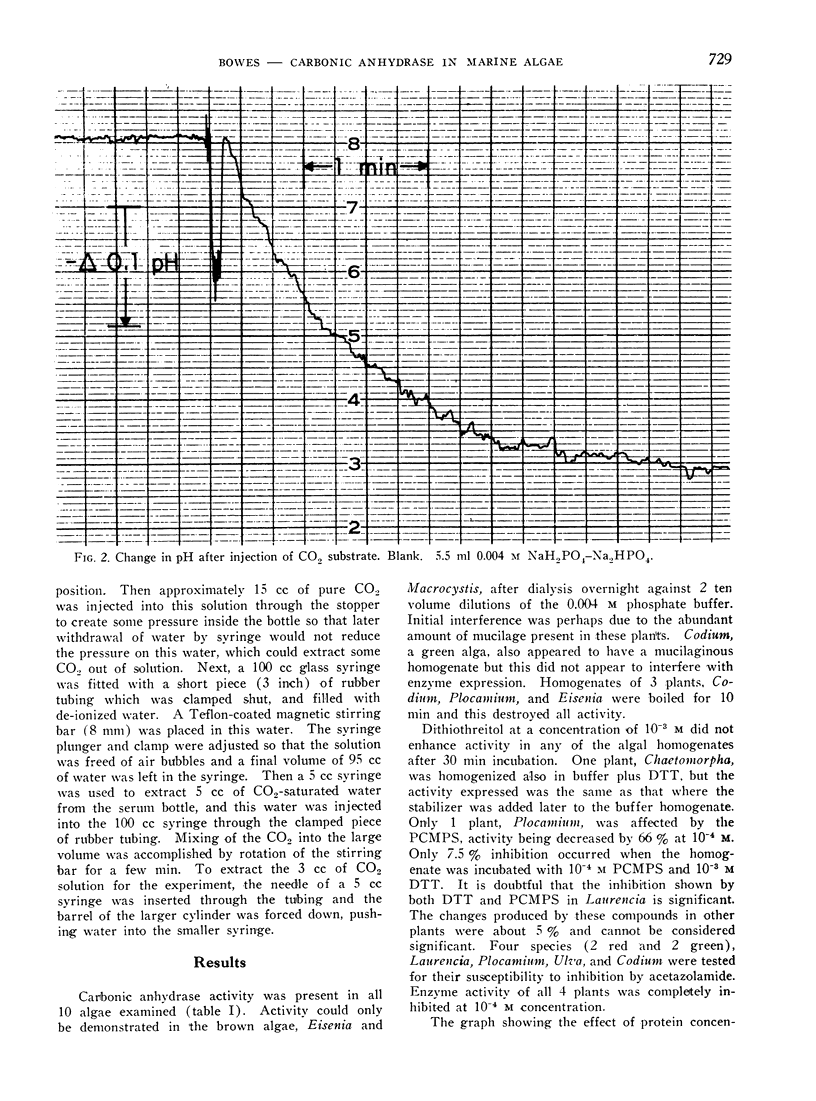
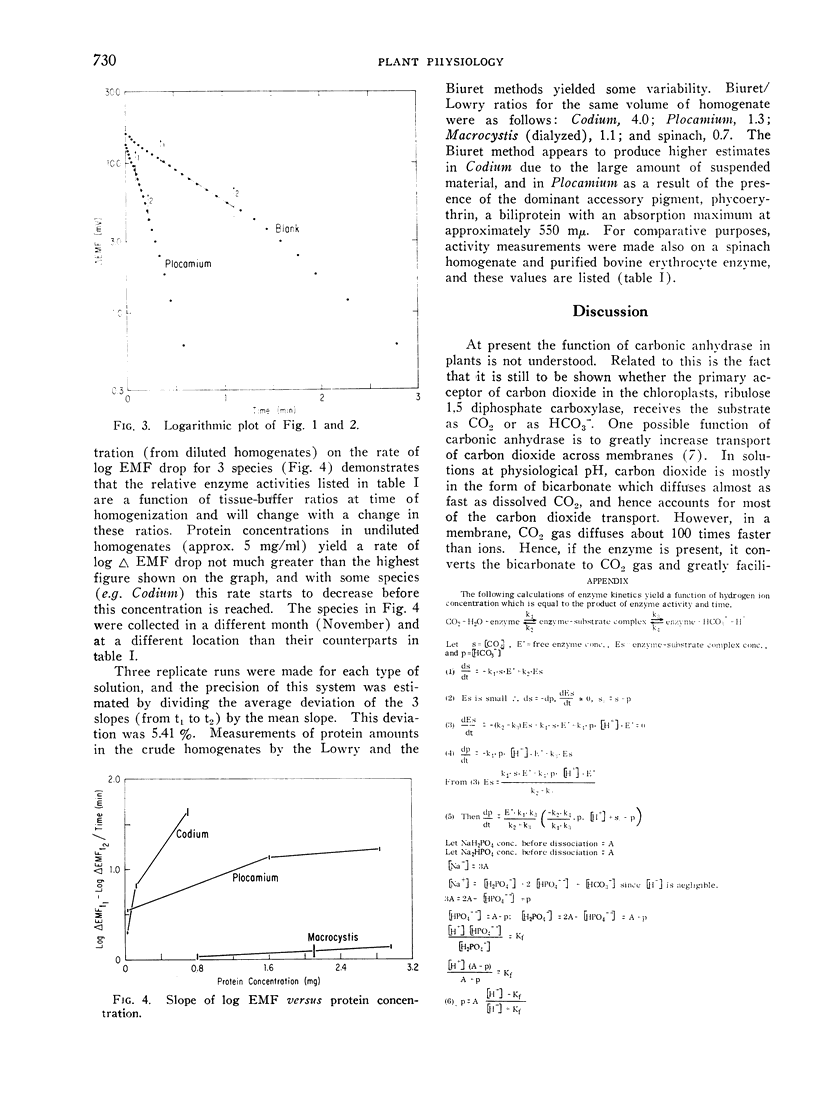
An electrometric system for determination of carbonic anhydrase activity was constructed. Enzyme activity was assayed in homogenates of marine macroscopic Chlorophyta, Rhodophyta, and Phaeophyta. Plants surveyed included Ulva expansa (Setchell) Setchell and Gardner, Codium fragile (Suringar) Hariot, Enteromorpha sp., Chaetomorpha torta (Farlow) McClatchie (Chlorophyta); Laurencia papillosa (Greville). Plocamium coccineum var. pacificum (Kylin) Dawson, Pterocladia capillacea (Gmelin) Bornet and Thuret. Gigartina armata J. Agardh (Rhodophyta); Eisenia arborea Areschoug and Macrocystis pyrifera (Linnaeus) C. A. Agardh (Phaeophyta). Activity was present in all algae; in the Phaeophyta this could be demonstrated only after dialysis. p-Chloromercuriphenylsulfonic acid (10−4m) decreased activity in 1 species, Plocamium; this inhibition could be almost completely overcome with the addition of 10−3m dithiothreitol. In 2 green and 2 red algae assayed for sensitivity to acetazolamide (Diamox), inhibition was complete at 10−4m concentration of inhibitor. Dithiothreitol at a concentration of 10−3m did not enhance activity in any of the homogenates, and was not necessary for enzyme expression.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkman R. The occurrence of carbonic anhydrase in lower marine animals. J Physiol. 1933 Dec 5;80(2):171–173. doi: 10.1113/jphysiol.1933.sp003079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLELAND W. W. DITHIOTHREITOL, A NEW PROTECTIVE REAGENT FOR SH GROUPS. Biochemistry. 1964 Apr;3:480–482. doi: 10.1021/bi00892a002. [DOI] [PubMed] [Google Scholar]
- DAVIS R. P. THE MEASUREMENT OF CARBONIC ANHYDRASE ACTIVITY. Methods Biochem Anal. 1963;11:307–327. doi: 10.1002/9780470110294.ch7. [DOI] [PubMed] [Google Scholar]
- Enns T. Facilitation by carbonic anhydrase of carbon dioxide transport. Science. 1967 Jan 6;155(3758):44–47. doi: 10.1126/science.155.3758.44. [DOI] [PubMed] [Google Scholar]
- FELLNER S. K. ZINC-FREE PLANT CARBONIC ANHYDRASE; LACK OF INHIBITION BY SULFONAMIDES. Biochim Biophys Acta. 1963 Sep 3;77:155–156. doi: 10.1016/0006-3002(63)90483-9. [DOI] [PubMed] [Google Scholar]
- GIBBONS B. H., EDSALL J. T. KINETIC STUDIES OF HUMAN CARBONIC ANHYDRASES B AND C. J Biol Chem. 1964 Aug;239:2539–2544. [PubMed] [Google Scholar]
- KARLER R., WOODBURY D. M. MODIFIED MANOMETRIC METHOD FOR DETERMINATION OF CARBONIC ANHYDRASE IN TISSUES. Anal Biochem. 1963 Nov;6:381–392. doi: 10.1016/0003-2697(63)90091-5. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Polya J. B., Wirtz A. J. Studies on carbonic anhydrase. II. Occurrence of the enzyme in some invertebrates. Enzymologia. 1965 Jul 30;29(1):27–37. [PubMed] [Google Scholar]
- SIBLY P. M., WOOD J. G. The nature of carbonic anhydrase from plant sources. Aust J Sci Res B. 1951 Nov;4(4):500–510. doi: 10.1071/bi9510500. [DOI] [PubMed] [Google Scholar]
- VEITCH F. P., BLANKENSHIP L. C. Carbonic anhydrase in bacteria. Nature. 1963 Jan 5;197:76–77. doi: 10.1038/197076a0. [DOI] [PubMed] [Google Scholar]



