Abstract
Background
Short-term high-frequency nasal ventilation (HFNV) of preterm neonates provides acceptable gas exchange compared to endotracheal intubation and intermittent mandatory ventilation (IMV). Whether long-term HFNV will provide acceptable gas exchange is unknown. We hypothesized that HFNV for up to 21d would lead to acceptable gas exchange at lower inspired oxygen (O2) levels and airway pressures compared to intubation and IMV.
Methods
Preterm lambs were exposed to antenatal steroids, and treated with perinatal surfactant and postnatal caffeine. Lambs were intubated and resuscitated by IMV. At ~3h of age, half of the lambs were switched to non-invasive HFNV. Support was for 3d or 21d. By design, PaO2 and PaCO2 were not different between groups.
Results
At 3d (n=5) and 21d (n=4) of HFNV, fractional inspired O2 (FiO2), peak inspiratory pressure, mean airway, intra-tracheal, and positive end-expiratory pressures, oxygenation index, and Alveolar-arterial gradient were significantly lower than matched periods of intubation and IMV. PaO2/FiO2 ratio was significantly higher at 3d and 21d of HFNV compared to matched intubation and IMV. HFNV led to better alveolarization at 3d and 21d.
Conclusion
Long-term HFNV provides acceptable gas exchange at lower inspired O2 levels and respiratory pressures compared to intubation and IMV.
Introduction
Endotracheal intubation and intermittent mandatory ventilation (IMV) are important risk factors for neonatal chronic lung disease (CLD; bronchopulmonary dysplasia, BPD) (1–5). Experimental animal studies indicate that contributors to lung injury are volutrauma, atelectotrauma, and hyperoxia (6–9). Volutrauma results from cyclic over-distension of the inhomogeneously inflated parenchyma of the immature lung. Atelectotrauma occurs during reopening of collapsed lung parenchyma. Both volutrauma and atelectotrauma expose the parenchymal cells and extracellular matrix to distortion that lead to altered expression of genes involved with lung inflammation and development (10–13). Hyperoxia sets the stage for cytotoxic reactive oxygen species that contribute to cellular injury and reprogramming of developmental processes (14). Disruptions of lung developmental processes are structurally manifest as alveolar simplification.
Early use of non-invasive respiratory support, such as nasal continuous positive airway pressure (CPAP), is associated with better outcomes. For example, nasal CPAP is associated with less use and fewer days of intubation and IMV, lower levels of inspired oxygen (O2) (15), and lower rates of BPD or BPD/death (1, 16, 17). Insights from studies using preterm experimental animal models of evolving neonatal CLD reveal possible mechanisms for better outcomes of non-invasive respiratory support. Functional studies using preterm lambs reveal lower expression of genes involved in acute-phase responses and markers of inflammation at 2h bubble nasal CPAP compared to intubation and IMV (18, 19). Structural studies using preterm baboons demonstrate that early use of non-invasive respiratory support promotes alveolarization compared to invasive respiratory support (9, 20, 21). Our previous study shows that an approach to non-invasive respiratory support for 3d leads to alveolarization compared to intubation and IMV of preterm.
Whether long-term non-invasive support will provide prolonged physiological gas exchange across the lung accompanied by alveolar formation is unknown. Therefore, we used our preterm lamb model of evolving neonatal CLD to prospectively determine the impact of prolonged high-frequency nasal ventilation (HFNV) on physiological outcomes for respiratory gas exchange and respiratory pressures, and morphological outcomes for alveolar formation. We hypothesized that HFNV for up to 21d would lead to acceptable respiratory gas exchange at lower inspired O2 levels and airway pressures, as well as alveolar formation, compared to intubation and IMV. The principal results of our study show that HFNV out to 21d provides acceptable respiratory gas exchange that is accompanied by alveolar formation.
Materials and Methods
Protocols adhered to APS/NIH guidelines for humane use of animals for research, and were prospectively approved by the IACUC at the University of Utah Health Sciences Center.
Surgical Preparation
The methods for chronically ventilating preterm lambs are reported (9, 22, 23). Briefly, time-pregnant ewes that carried one fetus or twin fetuses at 132±2d of gestation (term ~150d gestation) were used. The pregnant ewes were given an intramuscular injection of dexamethasone phosphate (6 mg; Vedco, Inc., St. Joseph, MO), ~36h before operative delivery. At delivery, we intubated all fetal lambs with a cuffed endotracheal tube (3.5 to 4.0 French), through which 10 mL of lung liquid was aspirated and replaced with Survanta (2.5 mL; NDC 0074-1040-08, Ross Products Division, Abbott Laboratories, Columbus, OH).
Additional Surgical Step for HFNV
We prospectively and randomly assigned a subset of all of the intubated preterm lambs to be weaned to high-frequency nasal ventilation (HFNV) within ~3h of life (see the Randomization subsection). This subset had an uncuffed oral/nasal true Murphy tube (3.0 French; 13 cm length) inserted ~5 cm through a nostril. We used a Murphy tube because preliminary studies revealed that preterm lambs did not tolerate nasal prongs or a nasal mask.
Initial Resuscitation of all Preterm Lambs
All lambs were intubated and managed by IMV, with warmed and humidified O2 (Bird VIP ventilator; model 15215, Palm Springs, CA). Initial settings were: respiratory rate 60 breaths/min, inspiratory time 0.3 sec, positive end-expiratory pressure (PEEP) 7 cmH2O, flow 8 L/min of 50% O2, and expiratory tidal volume 5–7 mL/Kg. Within 30 min of delivery, the lambs were given an intravenous loading dose of caffeine citrate (15 mg/Kg, given over 2h, Mead Johnson & Company, Evansville, IN) to stimulate ventilatory drive. Caffeine citrate was given every 24h (5 mg/Kg). Arterial blood gases, pH, electrolytes, and glucose were measured hourly, as was O2 saturation by pulse oximetry. Vascular pressures and heart rate were recorded continuously (model V6400, SurgVet, Inc.; Waukesha, WI). Dextrose was infused intravenously to maintain plasma glucose level between 60 and 90 mg/dL. Plasma concentrations of total protein and hematocrit were measured at 6h intervals.
Arterial partial pressure of oxygen (PaO2) was targeted between 70 and 90 mmHg by adjusting fractional inspired O2 (FiO2). Arterial partial pressure of carbon dioxide (PaCO2) was targeted between 40 and 60 mmHg, and pH between 7.25–7.45, by adjusting peak inspiratory pressure (PIP). Mean pressure in the airway (Paw) also was measured. We calculated oxygenation index (OI) [(Paw × FiO2)/PaO2], Alveolar-arterial (A-a) gradient [((FiO2/100) × (640−47)) − (PaCO2/0.8) − PaO2], and PaO2/FiO2 (P/F) ratio.
Randomization
Randomization to invasive versus non-invasive of respiratory support for 3d or 21d was done using a block randomization approach. We studied two preterm lambs concurrently.
Two Modes of Respiratory Support
Invasive respiratory support was endotracheal intubation and IMV. Non-invasive respiratory support was a unique approach of nasal ventilation with a high-frequency component created by a high-frequency, flow-interruption ventilator (model VDR4, Percussionaire Inc., Sand Point, ID). We call this mode high-frequency nasal ventilation (HFNV). The Percussionaire is a flow-regulated, time-cycled ventilator that provides controlled-pressure delivery of low-frequency breathing cycles with a series of high-frequency, sub-physiologic tidal volumes (24).
Weaning from IMV, begun at 2h of life, was successful when the lambs consistently breathed spontaneously while the ventilator circuit to the Bird VIP ventilator was disconnected from the endotracheal tube (9). At that time, the endotracheal tube was withdrawn and the ventilator circuit from the Percussionaire ventilator was attached to the tube in the nose. Initial ventilator settings were background convective breaths at 5–10 breaths per min PIP of 20–25 cmH2O, PEEP of 5–7 cmH2O, and high-frequency rate at 10 Hz. The ventilator circuit included a gas humidifier. Adjustments were made to the ventilator settings to reach the target ranges for PaO2 and PaCO2. Because a leak-free seal was not possible during HFNV, lung volumes could not be directly measured.
Measurement of Airway Pressures in situ
Phasic and mean pressures from the ventilator to the trachea were measured in situ, using a pressure-tipped wire (Samba Sensor model Preclin 420 LP, Samba Sensors SE, Västra Frölunda, Sweden). The wire was inserted through a rubber-covered side port of the connector piece to the endotracheal tube or nasal tube.
Management of Preterm Lambs
Lambs were kept prone in a veterinary sling mounted on a radiantly heated bed. Antibiotics were given daily (potassium penicillin, 100,000 U/Kg, Pfizer; and gentamicin, 2.5 mg/Kg/d, Hospira, Inc.). Gentamicin was discontinued at 10d. Sedation was provided, using pentobarbital sodium and buprenorphine hydrochloride. Lambs that were randomized to intubation and IMV received 3–5 mg/Kg of pentobarbital sodium (Abbott Laboratories, North Chicago, IL) as needed to minimize discomfort and agitation associated with endotracheal intubation. Lambs that were randomized to HFNV received only 1–2 mg/Kg of pentobarbital as needed to permit spontaneous breathing. All lambs were initially given buprenorphine hydrochloride (5 mcg/Kg every 3h, Reckitt & Colman Pharmaceuticals, Richmond, VA) for the 3d study. For the 21d study, buprenorphine dosage was reduced at 1 wk of life and thereafter for the HFNV group to minimize respiratory depression.
An orogastric feeding tube was used for enteral feedings, using ewe’s colostrum, beginning at 4h of life (3 mL/Kg every 2h) and continued for 3d. The volume was increased gradually by 3–5 mL increments, as tolerated, to attain a goal of 60–80 Kcal/Kg/d of total energy substrate, based on daily weight. After 3d, ewe’s milk was given. Volume was increased as tolerated. We monitored total fluid intake (saline, dextrose, and milk) and output (withdrawn blood, urine, and stool), and made adjustments to maintain fluid homeostasis, as indicated by urine output (>1–2 mL/Kg/h) and mean arterial blood pressure (>45 mmHg). None of the lambs required pressors. Chest radiographs were taken on days of life 1, 2, and 3 of all of the 3d (n=5/group) and 21d lambs (n=4/group), and weekly thereafter for the 21d lambs, to assess lung inflation volume and to identify atelectasis. Indices of infection were monitored by daily leukocyte total and differential cell counts, and rectal temperature.
Terminal Collection of Tissue
Blood samples were collected before the lambs were given heparin (1000 U, intravenously) followed by 5 mg/Kg of pentobarbital (9, 23). Both HFNV groups were intubated and reconnected to the Percussionaire ventilator to maintain lung inflation when the chest was opened to remove the lungs. All lambs were given 60 mg/Kg pentobarbital sodium solution intravenously (Beuthanasia solution, Ovation Pharmaceuticals, Inc., Deerfield, IL). The chest was opened, the trachea was ligated at end-inspiration (to minimize atelectasis), and the lungs and heart were removed. The whole left lung was insufflated with 10% buffered neutral formalin (static pressure of 25 cmH2O). Fixed-lung displacement volume was measured by suspension in formalin before the lung was stored in fixative (4°C, 24h). Paraffin-embedded tissue blocks were prepared for histology.
Quantification of Alveolar Secondary Septation and Distal Airspace Wall Thickness
We used quantitative histology (morphometry and stereology) to statistically compare alveolar secondary septation (radial alveolar count and secondary septal volume density) and distal airspace wall thickness, using methods previously reported by our group (9).
Data Analysis
Continuous variables that were approximately normally distributed are summarized by mean ± standard deviation (SD). These results were compared by unpaired t-test. Continuous variables that were not approximately normally distributed are summarized by the median and interquartile range (IQR). These results were analyzed by Mann-Whitney U-test. Linear mixed effects models were used to test whether FiO2, PIP, and Paw changed differently over time (time effects) during intubation and IMV versus HFNV, and whether the two respiratory support groups changed differently over time (group-time interaction). We used a simple random intercept model, with a variance-covariance structure that assumed compound symmetry across time points. Statistical analyses were done using StatView5 (SAS Institute, Inc., Cary, NC) or R v2.15.0. Statistical significance was assessed at p<0.05.
Results
We defined successful experiments as reaching the prospective 3d or 21d of respiratory support. By this definition, overall success was ~75%. For the 3d studies, the sample size is five. Seven preterm lambs were necessary because two male lambs developed respiratory distress secondary to uncontrolled air leaks that occurred within the first 12h of life. Both lambs died shortly thereafter and thus were excluded. All five preterm lambs that were weaned to HFNV survived. For the 21d studies, the sample size is four. Seven preterm lambs were necessary because three lambs (2 male; 1 female) developed respiratory distress secondary to uncontrolled air leaks or renal failure within week of life 3, before 21d, and therefore were excluded. The male lamb required re-intubation and IMV during week of life 3 (day 15) and could not be weaned back to HFNV.
Demographic characteristics are summarized in Table 1. Gestational age, birth weight, and sex were the same within and between the 3d and 21d groups. However, body weight at 3d or 21d was significantly greater in HFNV group compared to the corresponding intubation and IMV group (p<0.05).
Table 1.
Demographic characteristics of preterm lambs managed by invasive or non-invasive respiratory support (mean ± SD)
| Parameter | Preterm 3d (n=5 each) | Preterm 21d (n=4 each) | ||
|---|---|---|---|---|
| Intubation and IMV | HFNV | Intubation and IMV | HFNV | |
| Gestational age (d) | 131 ± 1 | 132 ± 1 | 132 ± 1 | 133 ± 1 |
| Weight at delivery (Kg) | 4.3 ± 0.4 | 4.7 ± 0.3 | 4.5 ± 0.5 | 4.1 ± 0.9 |
| Weight at study end (Kg) | 3.9 ± 0.4 | 4.6 ± 0.5* | 3.9 ± 0.4 | 4.5 ± 0.4* |
| Sex | 3 female / 2 male | 3 female / 2 male | 2 female / 2 male | 2 female / 2 male |
Statistically different from IMV group by unpaired t-test, p<0.05.
The radiographic shadow of the uncuffed true Murphy tube is visible in the midline of the head (Figure 1a). Postmortem, the nasal cavity was opened for all five 3d HFNV lambs to measure the location of the tip from the nostril. The tip was ~5 cm into the nasal cavity (~10 cm long; data not shown).
Figure 1.
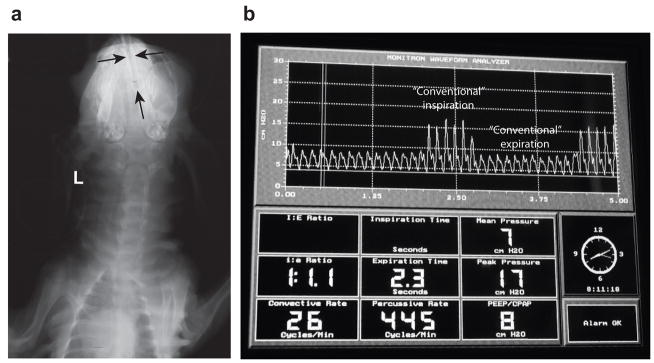
HFNV support preparation and airway pressure tracings. Panel a: Head radiograph of a preterm lamb supported by HFNV for 3d (posterior-anterior view; L, left). Black arrows highlight the uncuffed tube in the nasal cavity. The vertical black arrow identifies the tube’s tip, which is ~5 cm along the length of the nose (~10 cm). Panel b: View of the display panel of the Percussionaire VDR4 ventilator, showing phasic trace of intra-tracheal pressure and numeric data for respiratory values. The intra-tracheal pressure trace has continuous high-frequency percussions (jagged contours), including through ‘conventional inspiration’. The convective rate is 26, PIP is 17 cmH2O, and PEEP is 8 cmH2O. High-frequency rate is ~7 Hz (445 cycles/min) and mean pressure is 7 cmH2O.
A segment of an intra-tracheal pressure trace in a lamb supported by HFNV is shown in Figure 1b. The Percussionaire ventilator was configured to provide high-frequency, low amplitude pressure fluctuations during both inspiration and expiration (Figure 1b). Also evident are two background low-frequency breaths (pressure-limited at 17 cmH2O).
Pressure measurements at the ventilator, connector piece from the ventilator circuit to the nasal tube, in the nasopharynx, and intra-tracheally are summarized in Table 2. We tested two peak pressure settings: high PIP (~27 cmH2O) or moderate PIP (~14 cmH2O). Regardless of PIP setting, the corresponding peak and mean pressures persisted at the junction of the connector to the nasal tube. However, peak and mean pressures were ~60% less at the nasopharynx (1–2 cm cranial to the vocal cords), as well as in the trachea (~10 cm caudal to the vocal cords).
Table 2.
Airway pressure profile at 3d of HFNV support of preterm lambs (mean ± SD; n=3)
| Location | High Peak Inspiratory Pressure (cmH2O) | Moderate Peak Inspiratory Pressure (cmH2O) | ||
|---|---|---|---|---|
| Peak | Mean | Peak | Mean | |
| Ventilator | 27 ± 1 | 9 ± 1 | 14 ± 1 | 6 ± 1 |
| Connector | 27 ± 1 | 9 ± 1 | 14 ± 1 | 6 ± 1 |
| Nasopharynx | 10 ± 2 | 4 ± 2 | 3 ± 2 | 1 ± 1 |
| Intra-tracheal | 10 ± 2 | 4 ± 2 | 3 ± 2 | 1 ± 1 |
Daily gas exchange and respiratory support results for the 3d groups of ventilated preterm lambs are summarized in Table 3 (middle columns). By design, the two groups (n=5 each) had the same targeted range for PaO2 (73–85 mmHg) and targeted range for PaCO2 (44–64 mmHg) at days of life 1, 2, and 3. The physiologically targeted PaO2 range was attained using ~30% lower FiO2 on day of life 3 (p<0.05) for the HFNV group compared to the intubation and IMV group. Likewise, the physiologically targeted PaCO2 range was attained using ~40% lower PIP (p<0.05) at each day-of-life for the HFNV group. Paw was ~50% lower (p<0.05). PEEP at the ventilator was the same between the two groups at each day of life. Intra-tracheal pressure (ITP) measured in situ was ~90% lower (p<0.05) at each day of life for the HFNV group compared to the intubation and IMV group.
Table 3.
Respiratory gas exchange parameters in preterm lambs managed by invasive or non-invasive respiratory support (mean ± SD, except as noted)
| Parameter | Preterm 3d (n=5 each) | Preterm 21d (n=4 each) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day of life | Intubation and IMV | HFNV | Week of life | Intubation and IMV | HFNV | |
| PaO2 (mmHg) | 1 | 73 ± 17 | 73 ± 11 | 1 | 76 ± 13 | 75 ± 12 |
| 2 | 85 ± 9 | 81 ± 10 | 2 | 76 ± 3 | 73 ± 14 | |
| 3 | 73 ± 8 | 77 ± 16 | 3 | 74 ± 6 | 68 ± 3 (n=3) | |
| FiO2 | 1 | 0.33 ± 0.03 | 0.32 ± 0.03 | 1 | 0.42 ± 0.11 | 0.30 ± 0.04 |
| 2 | 0.35 ± 0.04 | 0.31 ± 0.03 | 2 | 0.36 ± 0.04 | 0.26 ± 0.03* | |
| 3 | 0.38 ± 0.04 | 0.27 ± 0.03* | 3 | 0.33 ± 0.05 | 0.23 ± 0.02* | |
| PaCO2 (mmHg) | 1 | 49 ± 7 | 50 ± 16 | 1 | 46 ± 7 | 52 ± 4 |
| 2 | 44 ± 5 | 55 ± 8 | 2 | 49 ± 4 | 45 ± 5 | |
| 3 | 53 ± 10 | 64 ± 11 | 3 | 47 ± 8 | 42 ± 6 (n=3) | |
| PIP (cmH2O)a | 1 | 22 ± 2 | 13 ± 4* | 1 | 14 ± 1 | 18 ± 3 |
| 2 | 20 ± 2 | 13 ± 4* | 2 | 15 ± 2 | 10 ± 4 | |
| 3 | 25 ± 2 | 13 ± 2* | 3 | 19 ± 5 | 4 ± 1* | |
| ITP (cmH2O) | 1 | 22 ± 2 | 2 ± 2* | 1 | ND | ND |
| 2 | 20 ± 2 | 2 ± 3* | 2 | ND | ND | |
| 3 | 25 ± 2 | 2 ± 2* | 3 | ND | ND | |
| Paw (cmH2O)a | 1 | 11 ± 1 | 6 ± 1* | 1 | 10 ± 1 | 9 ± 3 |
| 2 | 12 ± 2 | 6 ± 1* | 2 | 10 ± 1 | 5 ± 1* | |
| 3 | 12 ± 1 | 7 ± 1* | 3 | 10 ± 1 | 4 ± 1* | |
| PEEP (cmH2O)a | 1 | 6 ± 1 | 5 ± 1 | 1 | 7 ± 2 | 7 ± 3 |
| 2 | 7 ± 1 | 6 ± 2 | 2 | 7 ± 1 | 4 ± 1* | |
| 3 | 7 ± 1 | 6 ± 3 | 3 | 7 ± 1 | 4 ± 1* | |
| pH | 1 | 7.34 ± 0.05 | 7.35 ± 0.09 | 1 | 7.34 ± 0.10 | 7.33 ± 0.06 |
| 2 | 7.43 ± 0.07 | 7.37 ± 0.03 | 2 | 7.30 ± 0.04 | 7.43 ± 0.06* | |
| 3 | 7.37 ± 0.14 | 7.34 ± 0.08 | 3 | 7.35 ± 0.07 | 7.45 ± 0.04 (n=3) | |
| Oxygenation Index [Median (IQR)] | 1 | 5.7 (0.8) | 2.3 (0.5)† | 1 | 5.0 (2.7) | 1.9 (0.9)† |
| 2 | 4.6 (1.8) | 2.2 (0.2)† | 2 | 4.8 (0.9) | 4.0 (1.6)† | |
| 3 | 5.6 (1.7) | 2.5 (1.3)† | 3 | 3.2 (0.8) | 1.4 (0.1)† (n=3) | |
| A-a gradient (mmHg) [Median (IQR)] | 1 | 65 (14) | 47 (15) | 1 | 83 (124) | 33 (40) |
| 2 | 55 (24) | 20 (26)† | 2 | 70 ( 48) | 53 (20)† | |
| 3 | 90 (47) | 9 (11)† | 3 | 40 ( 53) | 18 (10)† (n=3) | |
| PaO2/FiO2 ratio [Median (IQR)] | 1 | 209 (24) | 243 (59) | 1 | 212 (102) | 261 (133) |
| 2 | 243 (58) | 284 (53) | 2 | 220 ( 49) | 233 ( 50) | |
| 3 | 184 (62) | 262 (88)† | 3 | 256 (121) | 294 ( 23) (n=3) | |
Statistically different from IMV group by unpaired t-test, p<0.05.
Statistically different from IMV group by Mann-Whitney U-test, p<0.05.
Pressure at the ventilators.
ND, not determined.
IQR, interquartile range.
Oxygenation Index = (Paw × FiO2)/PaO2.
A-a gradient = [(FiO2) × (640−47)] − (PaCO2/0.8) − PaO2; Barometric pressure at Salt Lake City (~5,000 feet above sea level) is ~640 mmHg.
We calculated oxygenation index (OI), Alveolar-arterial gradient of O2 (A-a gradient), and PaO2/FiO2 (P/F) ratio (Table 3). Median OI was ~60% lower at each day of life (p<0.05) for the HFNV group compared to the intubation and IMV group. The difference was even larger when we substituted mean ITP measured in situ for Paw in the formula for OI. Using ITP in the formula, median OI was ~80% lower at each day of life (p<0.05) for the HFNV group compared to the intubation and IMV group. Median A-a gradient was ~60 to 90% lower at days of life 2 and 3 for the HFNV group. The calculation of A-a gradient took into account the lower barometric pressure at Salt Lake City (~5,000 feet above sea level). Conversely, median P/F ratio was ~50% higher at day-of-life 3 for the HFNV group of lambs supported for 3d.
Prolonged HFNV for 21d also provided acceptable gas exchange and respiratory system pressures (Table 3; right-most columns). A caveat is one lamb in the HFNV group developed a blood clot in its arterial catheter. Therefore, arterial blood could not be sampled during its final week of life. Thus, sample size became 3 for just arterial blood gas values at week of life 3 for the HFNV group. We continued to measure O2 saturation, which remained between 88% and 94%, and therefore was within the same range as all of the other lambs in the 21d groups (data not shown).
For the 21d groups, targeted arterial PaO2 range was attained using ~30% lower FiO2 at weeks of life 2 and 3 (p<0.05) for the HFNV group compared to the same weeks for the matched intubation and IMV group. The targeted PaCO2 range was attained using ~75% lower respiratory system pressures for the HFNV group (p<0.05). Both Paw and PEEP were ~50% lower at weeks of life 2 and 3 (p<0.05) compared to the matched weeks of life for the intubation and IMV group. At weeks of life 2 and 3, pH was significantly higher (p<0.05) for the HFNV group. We did not measure ITP in situ in the 21d HFNV group because that would have required sedating and intubating the lambs, which could have affected the physiological results. For this reason, OI was calculated using Paw for the 21d groups. Median OI was ~20 to 60% lower at each week of life (p<0.05) for the HFNV group compared to the intubation and IMV group. Median A-a gradient was ~25 to 50% lower at weeks of life 2 and 3 (p<0.05) for the HFNV group. Median P/F ratio, while larger for the HFNV group at each week of life, was not different between the two 21d groups. These physiological improvements for the HFNV group were not influenced by initial differences in ventilator settings during the first 3h of IMV, before weaning to HFNV (see the table in online supplemental material).
We found time effects and group-time interactions between the HFNV group and the intubation and IMV group for the 21d study, using linear mixed effects models. Time effects were detected overall for both PIP (β = −5.6, t(16) = −6.0, p<0.05) and Paw (β = −2.4, t(16) = −5.3, p<0.05). PIP and Paw decreased from week-of-life 1 to 3 for the HFNV group, whereas PIP increased slightly and Paw remained constant during the same time period for the intubation and IMV group. These group differences were corroborated by group-time interactions (PIP: β = 7.5, t(16) = 6.0, p<0.05; Paw: (β= 2.4, t(16) = 4.0, p<0.05). The models also showed that FiO2 tended to decrease from week-of-life 1 to 3; however, the trend was not statistically significant (β = −0.04, t(16) = 1.8, p=0.09).
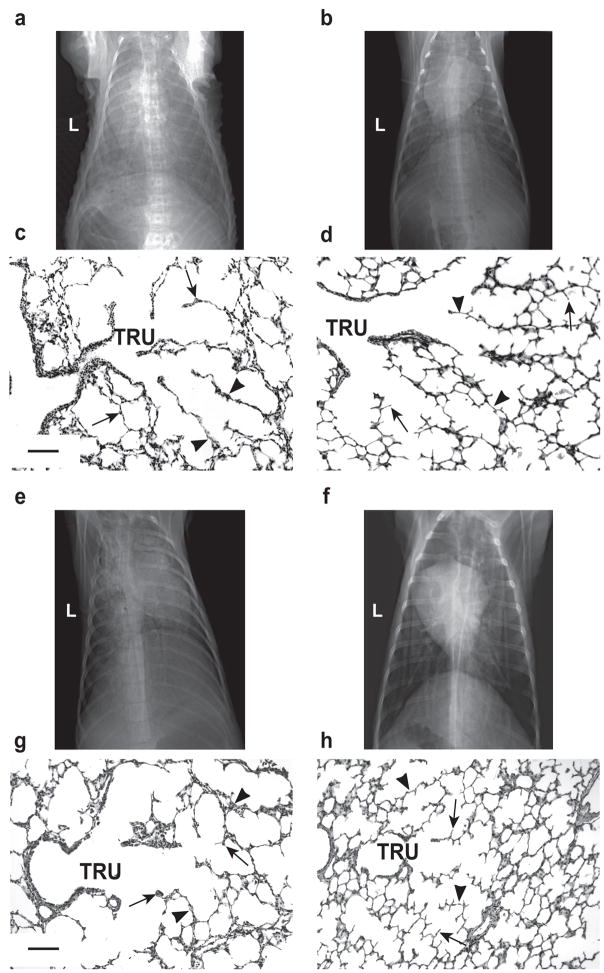
The physiologically better outcomes for the HFNV groups at 3d and 21d were associated with better radiographic and histologic appearances of the lungs (Figure 2). Radiographically, the lung fields were more translucent and lung volumes appeared larger for all five preterm lambs at 3d of HFNV and all four preterm lambs at 21d of HFNV compared to the matched number of preterm lambs per period of intubation and IMV. Also, air bronchograms were less obvious radiographically for all of the preterm lambs in the two HFNV groups. Histologically, terminal respiratory units appeared more developed in the HFNV group than the matched intubation and IMV groups. That is, the lung’s parenchymal appearance in the HFNV groups included distal airspaces that had more uniform and homogeneous size and shape. These observations were borne out by quantitative histology (Figure 3). Morphometry and stereology showed that at 21d of ventilation support, the HFNV group had greater radial alveolar count (p<0.05) and volume density for alveolar secondary septa (p<0.05), and narrower distal airspace walls (p<0.05), compared to the IMV group.
Figure 2.
Chest radiographic and histologic appearance of lungs. Invasive support is endotracheal intubation and IMV (left column). Non-invasive support is HFNV (right column). The top two rows juxtapose radiographic and histologic images for the 3d groups. The bottom two rows juxtapose radiographic and histologic images for the 21d groups. Panels a versus b; and e versus f: Chest radiographs show better aeration and larger lung volume in the HFNV groups compared to the corresponding intubation and IMV groups (posterior-anterior view; L, left). Panels c versus d; and g versus h: Terminal respiratory units (TRU) have more uniform distal airspaces, thinner distal airspace walls (arrowheads), and more and thinner alveolar secondary septa (arrows) in the HFNV groups compared to corresponding intubation and IMV groups (the scale bar is 100 μm in length).
Figure 3.
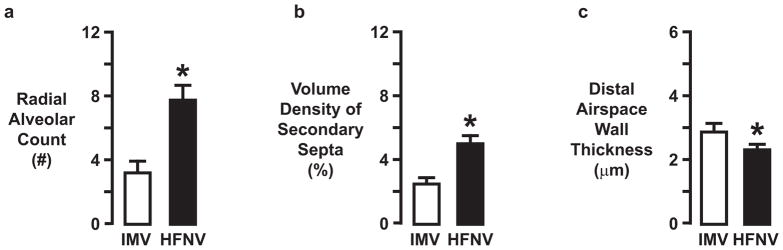
Summary of morphometric measurements (mean ± SD) made in lung tissue sections from preterm lambs managed by intermittent mandatory ventilation (IMV; white bars) or high-frequency nasal ventilation (HFNV; black bars) (n=4/group). Radial alveolar count (panel a) and volume density of secondary septa (panel b) were significantly greater, whereas distal airspace wall thickness (panel c) was significantly narrower, in the HFNV group than in the IMV group. * different from the corresponding IMV group by unpaired t-test (p<0.05).
We compared the feeding volume between HFNV and IMV groups because of the potential for differences in nutrition to influence lung development. For the 3d study, the daily volume of colostrum was the same between the HFNV group and the intubation and IMV group (Table 4). For the 21d study, by comparison, the weekly volume of colostrum/milk increased for the HFNV group, whereas the weekly volume decreased for the intubation and IMV group (Table 4). At week of life 3, the volume of milk delivered was 3.5-fold larger for the HFNV group. Nonetheless, plasma glucose remained physiological in between HFNV and IMV groups (Table 4).
Table 4.
Feeding and sedation parameters in preterm lambs managed by invasive or non-invasive respiratory support (mean ± SD)
| Parameter | Preterm 3d (n=5 each) | Preterm 21d (n=4 each) | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Day of life | Intubation and IMV | HFNV | Week of life | Intubation and IMV | HFNV | |
| Plasma glucose (gm/dL) | 1 | 101 ± 75 | 118 ± 46 | 1 | 84 ± 20 | 95 ± 10 |
| 2 | 96 ± 32 | 109 ± 30 | 2 | 81 ± 20 | 105 ± 7 | |
| 3 | 83 ± 25 | 93 ± 13 | 3 | 84 ± 7 | 99 ± 6 | |
| Enteral feeding (mL/Kg/d)a | 1 | 13 ± 9 | 9 ± 7 | 1 | 106 ± 10 | 107 ± 10 |
| 2 | 35 ± 22 | 24 ± 15 | 2 | 81 ± 55 | 149 ± 59 | |
| 3 | 51 ± 35 | 52 ± 31 | 3 | 53 ± 23 | 188 ± 70* | |
| Pentobarbital (mg/Kg/d) | 1 | 7 ± 4 | 4 ± 4 | 1 | 24 ± 20 | 1 ± 1* |
| 2 | 2 ± 2 | 1 ± 1 | 2 | 29 ± 31 | 1 ± 1 | |
| 3 | 4 ± 2 | 2 ± 1 | 3 | 73 ± 64 | 1 ± 1* | |
| Buprenorphine (mcg/Kg/d) | 1 | 0.04 ± 0.01 | 0.04 ± 0.01 | 1 | 0.06 ± 0.02 | 0.03 ± 0.03* |
| 2 | 0.04 ± 0.03 | 0.05 ± 0.01 | 2 | 0.06 ± 0.01 | 0.03 ± 0.03* | |
| 3 | 0.05 ± 0.01 | 0.05 ± 0.01 | 3 | 0.05 ± 0.01 | 0.01 ± 0.01* | |
Ewe’s colostrum (days of life 1–3) followed by ewe’s maturing milk (day of life 4 and thereafter).
Statistically different from IMV group by unpaired t-test, p<0.05.
We also compared the dosages of pentobarbital and buprenorphine that were given to the HFNV and IMV groups (Table 4). For the 3d study, the daily dosages of pentobarbital and buprenorphine were comparable between the HFNV group and the intubation and IMV group. However, for the 21d study, weekly dosages of pentobarbital and buprenorphine were significantly lower for the HFNV group (p<0.05).
Discussion
Our study has two principal results for chronically ventilated preterm lambs. The first principal result is physiological arterial blood gas values are maintained for 21d of HFNV, with significantly lower applied O2 and respiratory system pressures, compared to intubation and IMV. Secondly, alveolar formation progressed during 21d of HFNV, whereas alveolar simplification occurred during 21d of intubation and IMV. Together, these principal results show that non-invasive HFNV leads to prolonged improvement in functional and structural development of the lung of preterm lambs. Conversely, prolonged invasive ventilation evolves into neonatal CLD through persistent disruption of both functional and structural development of the parenchyma of the immature lung.
The driver for non-invasive respiratory support was a Percussionaire ventilator (Sand Point, ID). This ventilator allows for a combined low-frequency conventional/high-frequency percussive approach, whereby a conventional pressure-limited, time-cycled breath provided at low background frequency is coupled with an underlying high-frequency pattern of small, percussive pressures that deliver sub-physiological tidal volumes (24). Thus, both conventional and high-frequency gas exchange principles were configured. This combination of principles is similar to combining conventional pressure-limited breaths with high-amplitude bubble nasal CPAP, which augments volume delivery and reduces work of breathing (25). Coupling creates “biologic noise” (also called stochastic resonance) in the total waveform that is postulated to contribute to recruitment of lung volume (26–28). The Percussionaire ventilator, when configured to provide low-frequency conventional breaths, is unique in its ability to continuously provide sub-physiologic tidal volume, high-frequency breaths throughout both inspiratory and expiratory phases of respiration.
We used low-frequency conventional and high-frequency percussive configuration because preterm lambs that were supported by bubble nasal CPAP for more than 3–4h had progressive deterioration of respiratory gas exchange (9). We initially tried bubble nasal CPAP because it is associated with less abundance of pro-inflammatory mediators (18) and more production of surfactant in the lung (29). However, we found that during prolonged bubble nasal CPAP, PaCO2 and pH became unphysiological even though the lambs were treated with antenatal corticosteroids to promote lung development, exogenous surfactant to enhance lung compliance, and caffeine citrate to stimulate respiratory drive. While the cause of progressive deterioration of respiratory gas exchange remains unknown, we suspect that the long nose and neck of lambs, which increase anatomic dead space, may be contributory. Our results suggest that long-term use of HFNV as applied in this study may be a more effective approach to non-invasive respiratory support than either nasal CPAP or nasal IMV (30–33).
Use of short-term non-invasive ventilation support, with supra-physiological respiratory frequency through a nasopharyngeal tube, is reported for preterm infants. Two studies used an Infant Star HFV ventilator in its HFV mode. Van der Hoeven and colleagues (34) used nasal HFV in 21 premature infants within the first 20 d of life; 72% of infants were started on nasal HFV within 36 hours of age. Nearly 80% of infants showed improved ventilation and oxygenation, with a median of 36 hours support via nasal HFV. Colaizy and coworkers (35) used the same type of ventilator to support 14 stable preterm infants for 2h. Their results showed that nasal HFV significantly reduced PaCO2 and raised pH. However, the Infant Star ventilator is no longer made. Furthermore, the Infant Star ventilator was limited by maximum amplitude that it could generate, which limited the tidal volume that was delivered. A third study used a Draeger Babylog 8000 ventilator for 24h after extubation of a 760 gm preterm infant. Hoehn and Krause (36) showed that the infant’s PaCO2 remained in physiological range, thereby avoiding reintubation. A fourth study used the VDR3 Percussionaire, which is what we used. Dumas De La Roque (37) used HFNV in a prospective, unmasked, randomized, controlled clinical trial for 46 eligible newborn infants who were hospitalized for transient tachypnea of the newborn. HFNV reduced the period of transient tachypnea by 50%. However, those studies did not test long-term efficacy. Our experimental animal model’s results show that 21d of HFNV, as we used it, facilitates physiological arterial blood gas values at lower FiO2 and respiratory pressures. Whether our results in preterm lambs are translatable will require testing HFNV in high-risk preterm infants. This test will be important because of anatomic differences of the nose and neck between lambs and infants.
Only one of the lambs failed to reach 21d of HFNV. Failure was related to progressive respiratory failure. Another observation of our study is that air accumulated in the stomach of some of the lambs that were supported by HFNV. This observation, also common among preterm infants on nasal ventilation, was easily managed by vented orogastric tube and did not impair feeding tolerance.
An important physiological point made by our study is that intra-tracheal pressure is very low during HFNV. The in situ measurements show that intra-tracheal pressure during HFNV of spontaneously breathing preterm lambs is about 1/10th that during intubation and IMV (Table 3). The location where pressure is attenuated is after the connector piece, in the nasopharynx. Attenuation is ~60% of the pressure setting at the ventilator (Table 2). Peak and mean airway pressures were respectively constant within the nasopharynx and trachea. The in situ measurements also reveal that HFNV maintains positive mean intra-tracheal pressure of 1–2 cmH2O, therefore low PEEP. In our model, substantial opportunity exists for leak from the nasal-oropharyngeal cavity. In the presence of substantial leak, others also demonstrated, through direct or indirect measurements, more significant reduction in distal airway pressure than expected based on the measured or set pressure at the device interface (38, 39). During intubation and IMV support, by comparison, airway pressures reflected the pressure settings at the ventilator (Table 3). The latter finding is consistent with the results of a study showing that intratracheal pressure during invasive mechanical ventilation is comparable to ventilator pressure (40).
Low peak and mean intra-tracheal pressures during HFNV, combined with more uniform inflation seen radiographically, may contribute to the better structural development of the lung’s parenchyma. Indeed, studies using chronically ventilated preterm baboons demonstrate that early nasal CPAP promotes alveolar formation compared to intubation and IMV (20, 21). We recently reported that progression of alveolar formation at the end of 3d of HFNV is associated with more apoptosis, and less proliferation, among mesenchymal cells in the walls of the distal airspaces compared to intubation and IMV (9). This level of balance between apoptosis and proliferation of mesenchymal cells is related to thinning of the distal airspace walls, which is necessary for efficient respiratory gas exchange. We are in the process of quantifying apoptosis and proliferation of mesenchymal cells in preterm lambs that were supported by HFNV versus IMV for 21d, and will report the results separately.
A potential confounding element of our study design is that all of the preterm lambs were endotracheally intubated and supported by IMV during the ~3h transition period leading to HFNV support. However, no differences in the initial ventilator settings or respiratory physiological results occurred (refer to supplemental online data), suggesting that the 3h transition period of IMV did not confound the outcomes for the HFNV groups.
Better pulmonary outcomes at 21d of HFNV may be related to better enteral feeding and weight gain (Table 4) (41, 42). Better enteral feeding and weight gain, in turn, may be impacted by less sedation (Table 4) because sedation decreases gastrointestinal motility and absorption, as well as growth and development of the lung (43). In new studies, we are determining the separate effects of enteral feeding versus sedation on lung structural and functional outcomes. We are assessing the impact of matched-feeding (mL (calories)/Kg/d) versus matched-sedation (mg/Kg/d) in separate groups of preterm lambs on HFNV. Matching is done at the daily level for intubated preterm lambs on IMV. Initial results suggest that only matched enteral feeding shifts lung outcomes toward the poorer outcomes that occur in intubated lambs on IMV.
We conclude that early non-invasive respiratory support via HFNV as we applied using a high-frequency flow interrupter ventilator, coupled with maternal antenatal steroids, prophylactic surfactant replacement, and postnatal caffeine therapy, effectively maintains gas exchange at lower O2 levels and respiratory pressures out to 21d. In addition, the quantitative histological results indicate that HFNV out to 21d improves alveolarization. Our study’s results raise the possibility that long-term HFNV may be an alternative approach to acceptable respiratory support for preterm infants. Our observation that lung development is occurring raises the possibility that HFNV may reduce neonatal CLD (17). Translating our results from preterm lambs to preterm infants will require clinical studies.
Acknowledgments
Supported by National Institutes of Health (NIH) grants HL110002, HL062875, HL007744, the Division of Neonatology, and the Children’s Health Research Center in the Department of Pediatrics, and the University of Utah Study Design and Biostatistics Center, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, NIH grant 8UL1 TR000105 (formerly UL1 RR025764).
We thank the large number of undergraduate students and medical students who tended the chronically ventilated preterm lambs. We also thank Ronald S. Bloom, MD for suggestions.
Footnotes
The authors have no financial ties to products used in this study.
References
- 1.Van Marter LJ, Allred EN, Pagano M, et al. Do clinical markers of barotrauma and oxygen toxicity explain interhospital variation in rates of chronic lung disease? The Neonatology Committee for the Developmental Network. Pediatrics. 2000;105:1194–1201. doi: 10.1542/peds.105.6.1194. [DOI] [PubMed] [Google Scholar]
- 2.Henderson-Smart DJ, Cools F, Bhuta T, Offringa M. Elective high frequency oscillatory ventilation versus conventional ventilation for acute pulmonary dysfunction in preterm infants. Cochrane Database Syst Rev. 2007:CD000104. doi: 10.1002/14651858.CD000104. [DOI] [PubMed] [Google Scholar]
- 3.Yoder BA, Harrison M, Clark RH. Time-related changes in steroid use and bronchopulmonary dysplasia in preterm infants. Pediatrics. 2009;124:673–679. doi: 10.1542/peds.2008-2793. [DOI] [PubMed] [Google Scholar]
- 4.Gortner L, Misselwitz B, Milligan D, et al. Rates of bronchopulmonary dysplasia in very preterm neonates in Europe: results from the MOSAIC cohort. Neonatology. 2011;99:112–117. doi: 10.1159/000313024. [DOI] [PubMed] [Google Scholar]
- 5.Laughon MM, Langer JC, Bose CL, et al. Prediction of bronchopulmonary dysplasia by postnatal age in extremely premature infants. Am J Respir Crit Care Med. 2011;183:1715–1722. doi: 10.1164/rccm.201101-0055OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Delemos RA, Coalson JJ, Gerstmann DR, et al. Ventilatory management of infant baboons with hyaline membrane disease: the use of high frequency ventilation. Pediatr Res. 1987;21:594–602. doi: 10.1203/00006450-198706000-00018. [DOI] [PubMed] [Google Scholar]
- 7.Carlton DP, Cummings JJ, Scheerer RG, Poulain FR, Bland RD. Lung overexpansion increases pulmonary microvascular protein permeability in young lambs. J Appl Physiol. 1990;69:577–583. doi: 10.1152/jappl.1990.69.2.577. [DOI] [PubMed] [Google Scholar]
- 8.Davis JM, Dickerson B, Metlay L, Penney DP. Differential effects of oxygen and barotrauma on lung injury in the neonatal piglet. Pediatr Pulmonol. 1991;10:157–163. doi: 10.1002/ppul.1950100305. [DOI] [PubMed] [Google Scholar]
- 9.Reyburn B, Li M, Metcalfe DB, et al. Nasal ventilation alters mesenchymal cell turnover and improves alveolarization in preterm lambs. Am J Respir Crit Care Med. 2008;178:407–418. doi: 10.1164/rccm.200802-359OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han B, Lodyga M, Liu M. Ventilator-induced lung injury: role of protein-protein interaction in mechanosensation. Proc Am Thorac Soc. 2005;2:181–187. doi: 10.1513/pats.200501-008AC. [DOI] [PubMed] [Google Scholar]
- 11.Harding R, Hooper SB. Regulation of lung expansion and lung growth before birth. J Appl Physiol. 1996;81:209–224. doi: 10.1152/jappl.1996.81.1.209. [DOI] [PubMed] [Google Scholar]
- 12.Kitterman JA. The effects of mechanical forces on fetal lung growth. Clin Perinatol. 1996;23:727–740. [PubMed] [Google Scholar]
- 13.Albertine KH. Progress in understanding the pathogenesis of BPD using the baboon and sheep models. Seminars in perinatology. 2013;37:60–68. doi: 10.1053/j.semperi.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Buczynski BW, Maduekwe ET, O’Reilly MA. The role of hyperoxia in the pathogenesis of experimental BPD. Seminars in perinatology. 2013;37:69–78. doi: 10.1053/j.semperi.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Finer NN, Carlo WA, Walsh MC, et al. Early CPAP versus surfactant in extremely preterm infants. NEJM. 2010;362:1970–1979. doi: 10.1056/NEJMoa0911783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Levesque BM, Kalish LA, LaPierre J, Welch M, Porter V. Impact of implementing 5 potentially better respiratory practices on neonatal outcomes and costs. Pediatrics. 2011;128:e218–226. doi: 10.1542/peds.2010-3265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlo WA. Gentle ventilation: the new evidence from the SUPPORT, COIN, VON, CURPAP, Colombian Network, and Neocosur Network trials. Early Hum Dev. 2012;88 (Suppl 2):S81–83. doi: 10.1016/S0378-3782(12)70022-1. [DOI] [PubMed] [Google Scholar]
- 18.Jobe AH, Kramer BW, Moss TJ, Newnham JP, Ikegami M. Decreased indicators of lung injury with continuous positive expiratory pressure in preterm lambs. Pediatr Res. 2002;52:387–392. doi: 10.1203/00006450-200209000-00014. [DOI] [PubMed] [Google Scholar]
- 19.Hillman NH, Moss TJ, Nitsos I, Jobe AH. Moderate tidal volumes and oxygen exposure during initiation of ventilation in preterm fetal sheep. Pediatr Res. 2012;72:593–599. doi: 10.1038/pr.2012.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thomson MA, Yoder BA, Winter VT, et al. Treatment of immature baboons for 28 days with early nasal continuous positive airway pressure. Am J Respir Crit Care Med. 2004;169:1054–1062. doi: 10.1164/rccm.200309-1276OC. [DOI] [PubMed] [Google Scholar]
- 21.Thomson MA, Yoder BA, Winter VT, Giavedoni L, Chang LY, Coalson JJ. Delayed extubation to nasal continuous positive airway pressure in the immature baboon model of bronchopulmonary dysplasia: lung clinical and pathological findings. Pediatrics. 2006;118:2038–2050. doi: 10.1542/peds.2006-0622. [DOI] [PubMed] [Google Scholar]
- 22.Albertine KH, Jones GP, Starcher BC, et al. Chronic lung injury in preterm lambs. Disordered respiratory tract development. Am J Respir Crit Care Med. 1999;159:945–958. doi: 10.1164/ajrccm.159.3.9804027. [DOI] [PubMed] [Google Scholar]
- 23.Albertine KH, Dahl MJ, Gonzales LW, et al. Chronic lung disease in preterm lambs: effect of daily vitamin A treatment on alveolarization. Am J Physiol Lung Cell Mol Physiol. 2010;299:L59–L72. doi: 10.1152/ajplung.00380.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lucangelo U, Fontanesi L, Antonaglia V, et al. High frequency percussive ventilation (HFPV) Principles and technique Minerva Anestesiol. 2003;69:841–848. [PubMed] [Google Scholar]
- 25.Diblasi RM, Zignego JC, Tang DM, et al. Noninvasive respiratory support of juvenile rabbits by high-amplitude bubble continuous positive airway pressure. Pediatr Res. 2010;67:624–629. doi: 10.1203/PDR.0b013e3181dcd580. [DOI] [PubMed] [Google Scholar]
- 26.Suki B, Alencar AM, Sujeer MK, et al. Life-support system benefits from noise. Nature. 1998;393:127–128. doi: 10.1038/30130. [DOI] [PubMed] [Google Scholar]
- 27.Pillow JJ, Travadi JN. Bubble CPAP: is the noise important? An in vitro study Pediatr Res. 2005;57:826–830. doi: 10.1203/01.PDR.0000157721.66812.07. [DOI] [PubMed] [Google Scholar]
- 28.Pillow JJ, Hillman N, Moss TJ, et al. Bubble continuous positive airway pressure enhances lung volume and gas exchange in preterm lambs. Am J Respir Crit Care Med. 2007;176:63–69. doi: 10.1164/rccm.200609-1368OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mulrooney N, Champion Z, Moss TJ, Nitsos I, Ikegami M, Jobe AH. Surfactant and physiologic responses of preterm lambs to continuous positive airway pressure. Am J Respir Crit Care Med. 2005;171:488–493. doi: 10.1164/rccm.200406-774OC. [DOI] [PubMed] [Google Scholar]
- 30.Friedlich P, Lecart C, Posen R, Ramicone E, Chan L, Ramanathan R. A randomized trial of nasopharyngeal-synchronized intermittent mandatory ventilation versus nasopharyngeal continuous positive airway pressure in very low birth weight infants after extubation. J Perinatol. 1999;19:413–418. doi: 10.1038/sj.jp.7200205. [DOI] [PubMed] [Google Scholar]
- 31.Khalaf MN, Brodsky N, Hurley J, Bhandari V. A prospective randomized, controlled trial comparing synchronized nasal intermittent positive pressure ventilation versus nasal continuous positive airway pressure as modes of extubation. Pediatrics. 2001;108:13–17. doi: 10.1542/peds.108.1.13. [DOI] [PubMed] [Google Scholar]
- 32.Barrington KJ, Bull D, Finer NN. Randomized trial of nasal synchronized intermittent mandatory ventilation compared with continuous positive airway pressure after extubation of very low birth weight infants. Pediatrics. 2001;107:638–641. doi: 10.1542/peds.107.4.638. [DOI] [PubMed] [Google Scholar]
- 33.De Paoli AG, Davis PG, Lemyre B. Nasal continuous positive airway pressure versus nasal intermittent positive pressure ventilation for preterm neonates: a systematic review and meta-analysis. Acta Paediatr. 2003;92:70–75. doi: 10.1111/j.1651-2227.2003.tb00472.x. [DOI] [PubMed] [Google Scholar]
- 34.van der Hoeven M, Brouwer E, Blanco CE. Nasal high frequency ventilation in neonates with moderate respiratory insufficiency. Arch Dis Child Fetal Neonatal Ed. 1998;79:F61–63. doi: 10.1136/fn.79.1.f61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Colaizy TT, Younis UM, Bell EF, Klein JM. Nasal high-frequency ventilation for premature infants. Acta Paediatr. 2008;97:1518–1522. doi: 10.1111/j.1651-2227.2008.00900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hoehn T, Krause MF. Effective elimination of carbon dioxide by nasopharyngeal high-frequency ventilation. Respir Med. 2000;94:1132–1134. doi: 10.1053/rmed.2000.0889. [DOI] [PubMed] [Google Scholar]
- 37.Dumas De La Roque E, Bertrand C, Tandonnet O, et al. Nasal high frequency percussive ventilation versus nasal continuous positive airway pressure in transient tachypnea of the newborn: A pilot randomized controlled trial ( NCT00556738) Pediatr Pulmonol. 2010 doi: 10.1002/ppul.21354. [DOI] [PubMed] [Google Scholar]
- 38.Saslow JG, Aghai ZH, Nakhla TA, et al. Work of breathing using high-flow nasal cannula in preterm infants. J Perinatol. 2006;26:476–480. doi: 10.1038/sj.jp.7211530. [DOI] [PubMed] [Google Scholar]
- 39.Kahn DJ, Courtney SE, Steele AM, Habib RH. Unpredictability of delivered bubble nasal continuous positive airway pressure: role of bias flow magnitude and nares-prong air leaks. Pediatr Res. 2007;62:343–347. doi: 10.1203/PDR.0b013e318123f702. [DOI] [PubMed] [Google Scholar]
- 40.Sondergaard S, Karason S, Hanson A, et al. Direct measurement of intratracheal pressure in pediatric respiratory monitoring. Pediatr Res. 2002;51:339–345. doi: 10.1203/00006450-200203000-00013. [DOI] [PubMed] [Google Scholar]
- 41.Massaro D, Massaro GD, Baras A, Hoffman EP, Clerch LB. Calorie-related rapid onset of alveolar loss, regeneration, and changes in mouse lung gene expression. Am J Physiol Lung Cell Mol Physiol. 2004;286:L896–L906. doi: 10.1152/ajplung.00333.2003. [DOI] [PubMed] [Google Scholar]
- 42.Dias CM, Passaro CP, Cagido VR, et al. Effects of undernutrition on respiratory mechanics and lung parenchyma remodeling. J Appl Physiol. 2004;97:1888–1896. doi: 10.1152/japplphysiol.00091.2004. [DOI] [PubMed] [Google Scholar]
- 43.Paugam-Burtz C, Molliex S, Lardeux B, et al. Differential effects of halothane and thiopental on surfactant protein C messenger RNA in vivo and in vitro in rats. Anesthesiology. 2000;93:805–810. doi: 10.1097/00000542-200009000-00030. [DOI] [PubMed] [Google Scholar]