Abstract
Background
While obesity increases risk and negatively impacts survival for many malignancies, the prognostic implications in squamous cell carcinoma (SCC) of the oral tongue, a disease often associated with pre-diagnosis weight loss, are unknown.
Methods
Patients with T1–T2 oral tongue SCC underwent curative-intent resection in this single-institution study. All patients underwent nutritional assessment prior to surgery. Body mass index (BMI) was calculated from measured height and weight and categorized as obese (≥30 kg/m2), overweight (25 to 29.9 kg/m2), or normal (18.5 to 24.9 kg/m2). Clinical outcomes including disease specific survival (DSS), recurrence free survival (RFS), and overall survival (OS), were compared by BMI group using Cox regression.
Results
From 2000–2009, 155 patients (90 men, 65 women) of median age 57 (range 18 to 86) were included. Baseline characteristics were similar by BMI group. Obesity was significantly associated with adverse DSS compared with normal weight in univariable (hazard ratio [HR] = 2.65, 95% confidence interval [CI], 1.07 to 6.59; P = .04) and multivariable analyses (HR = 5.01; 95% CI, 1.69 to 14.81; P = .004). A consistent association was seen between obesity and worse RFS (HR =1.87; 95% CI, .90 to 3.88) and between obesity and worse OS (HR=2.03; 95% CI, .88 to 4.65) though without reaching statistical significance (P = .09 and P = .10 respectively) in multivariable analyses.
Conclusions
In this retrospective study, obesity was an adverse independent prognostic variable. This association may not have been previously appreciated due to confounding by multiple factors including pre-diagnosis weight loss.
Keywords: obesity, tongue neoplasms, prognosis, body mass index, head and neck neoplasms, squamous cell carcinoma of the head and neck
Introduction
Malignancies of the oral cavity and oropharynx remain one of the top ten leading cancers in the United States (U.S.) with an estimated 41,380 new cases and 7,890 deaths expected in 2013 alone.1 Despite overall improvements in surgical and medical management, clinical outcomes in patients with squamous cell carcinoma (SCC) of the oral tongue, a subsite of the oral cavity, have remained largely unchanged.2–4 Efforts to identify additional prognostic variables beyond tumor histology and TNM stage have yielded mixed results. Notably, carcinogenic strains of the human papilloma virus (HPV) have been associated with better outcomes in patients with oropharynx malignancies.5–8 In contrast, however, HPV has not been associated with oral tongue SCC.3, 6, 9, 10
The relationships between oral tongue SCC and dietary habits, nutritional status, and body mass index (BMI) have also been explored.11–20 Given the association between obesity and worse outcomes for several common cancers including breast, colon, esophagus, and others, BMI is of significant general interest.21–27 Hyperadiposity has been associated with metabolic dysfunction, including insulin resistance and altered adipokine levels, which promotes tumor cell proliferation and survival.21 In parallel with the increasing incidence of oral tongue SCC, obesity rates are also on the rise with predicted rates as high as 65% of the population of several regions of the United States by 2030.28 However, it is unknown whether elevated BMI specifically affects oral tongue cancer-related outcomes. Pre-diagnosis weight loss and low BMI have been reported in some studies to be associated with diminished survival in patients with oral cancers.18–20 However, these observations are possibly reflective of leanness associated with comorbid habits (i.e. smoking and alcohol use) and weight loss as a result of tumor-related malnutrition and/or treatment.
Investigations of BMI and survival in oral cancers have thus been confounded by several factors. Given the association between obesity and adverse outcomes for many other malignancies, we explored the impact of obesity on the survival of patients with T1 or T2 SCC of the oral tongue.
Patients and Methods
Study Cohort
This retrospective study was approved by the Institutional Review Board of Memorial Sloan-Kettering Cancer Center (MSKCC). Adult patients with early tumor stage, pathologically confirmed SCC of the oral tongue who underwent curative-intent resection at MSKCC between January 1, 2000 and December 31, 2009 were included. In limiting the cohort to patients with T1 or T2 disease (tumor less than 4 cm in greatest dimension) and analyzing BMI at the time of diagnosis, we sought to minimize the influence of disease- and treatment-associated weight loss and thereby isolate a potential obesity effect. Cases prior to 2009 were included to ensure adequate follow-up for survival endpoints. Patients were selected from the prospectively maintained MSKCC Oral Cancer Clinical Database, which registers all patients undergoing surgery at MSKCC. Clinical data were systematically extracted by research staff and physicians (AK, NMI, PGM, AP), and independent data review was carried out for quality assurance. All data entry was reviewed twice for accuracy independently by 2 physicians (NMI and PGM). The cohort included a total of 155 patients who met all inclusion criteria and no patients were excluded (Figure 1).
Figure 1.
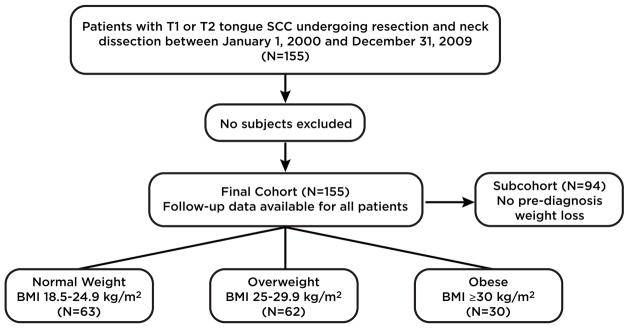
Consolidated Standards of Reporting Trials (CONSORT) diagram of current study. Abbreviations: BMI; body mass index.
Collection of Anthropometric and Clinical Data
Demographic and clinicopathologic data including age, gender, race, alcohol and tobacco use, comorbid conditions, medications, and tumor stage were prospectively recorded. All patients undergoing presurgical evaluation at MSKCC routinely have height and weight measured and documented in the electronic medical record (EMR) by a clinician. These measurements were used to calculate BMI as weight (in kilograms) divided by height squared (square meters). Patients completed a written history form and underwent nutritional assessment by clinical staff both at initial surgical consultation and during the inpatient postoperative course. Medical and nutritional histories were verified at the time of initial surgical consultation by the surgeon performing the resection. The written history form, nutritional assessment, and surgical consultation note were digitized and archived in the EMR.
Data for this retrospective analysis were extracted from the prospectively maintained database and BMI was verified for each patient by recalculation. Additionally, preoperative weight loss, postoperative chemotherapy and/or radiation, follow-up, and survival data were obtained from the EMR. When unavailable, date and cause of death were obtained from the Social Security Death Index.
P16 immunostaining
Paraffin embedded tissue sections from the primary tumor were subjected to immunostaining for p16 using the pre-diluted mouse monoclonal antibody (CINtec p16) from Ventana (Tucson, AZ) according to the manufacturer’s recommendations. A carcinoma was considered positive for p16 if strong and diffuse nuclear and cytoplasmic staining were seen in ≥ 70% of the tumor cells.29
Clinical End Points and Statistical Analysis
The primary outcome variable of the study was disease specific survival (DSS), defined as time from surgery to death as a direct result of oral tongue SCC. Data were censored at the date of death from causes not related to oral tongue cancer or at the date of last follow-up. Secondary outcomes included recurrence free survival (RFS), defined as time from surgery to first recurrence of the primary cancer or death, and overall survival (OS), defined as time from surgery to all-cause mortality. World Health Organization BMI ranges were used to categorize patients as obese (BMI ≥ 30.0 kg/m2), overweight (BMI 25.0 – 29.9 kg/m2), and normal weight (18.5 – 24.9 kg/m2). Smoking status was self-reported and stratified as patients who never smoked (never smokers), quit smoking at any time prior to diagnosis (former smokers), or smoked at the time of diagnosis (current smokers).
Statistical differences across BMI groups were evaluated by the Kruskal-Wallis test followed by Wilcoxon rank sum test for pairs of comparison groups for continuous variables and Fisher’s exact or χ2 test for categorical variables, where appropriate. For the primary study endpoint, DSS outcomes by BMI group were compared using the log-rank test and the Kaplan-Meier curves were plotted. Similar analyses were conducted for the secondary endpoints (RFS and OS). A Cox proportional hazard model was used to examine the association between each covariate and survival in univariate analysis. Hazard ratios between each group and the reference group for categorical variables, and for each unit increase for continuous variables, were reported with 95% CIs and two-tailed P-values. A P value ≤ .05 was considered statistically significant. Variables of interest were defined as having a P value < .25 in univariate analysis and were included in multivariate analysis using Cox proportional hazard models. Covariates included in multivariate analysis for DSS were age, T stage, tumor grade, perineural invasion, vascular invasion, diagnosis of diabetes mellitus, presence of lymph node metastases, use of post-operative radiation, and black race. Additionally, smoking status, a known prognostic variable, was included in multivariate models. Analyses were conducted using R (R Core Team 2013, R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org).
Results
Study Population
A total of 155 patients, median age 57 years (range 18–86), with T1 or T2 SCC of the oral tongue who underwent resection at MSKCC were included (Table 1). The majority of patients were male (58%), had T1 tumors (70%) and pathologically node-negative disease (65%). All patients underwent neck dissection and the median number of lymph nodes resected was 27 (range 0–71). Most tumors were located at the lateral tongue (90%) while the remainder were located at the ventral tongue (10%). Baseline clinicopathologic characteristics and use of adjuvant therapies are listed in Table 1. Of the patients who underwent combined modality adjuvant therapy (N = 8), radiation was generally administered concurrently with a single agent or a combination of agents including platinum, taxane, fluorouracil, or cetuximab.
Table 1.
Baseline characteristics of patients (N = 155)
| Clinicopathological Variables | All (N = 155) | Normal Weight (N= 63) | Overweight (N = 62) | Obese (N = 30) | P |
|---|---|---|---|---|---|
| Age, years | |||||
| Median (range) | 57 (18 to 86) | 53 (18 to 82) | 59 (20 to 86) | 59 (23 to 80) | .07 |
| Gender | |||||
| Female | 65 (42%) | 31 (49%) | 22 (35%) | 12 (40%) | |
| Male | 90 (58%) | 32 (51%) | 40 (65%) | 18 (60%) | .29 |
| Race | |||||
| Asian | 21 (14%) | 11 (17%) | 8 (13%) | 2 (7%) | |
| Black | 4 (3%) | 3 (5%) | 1 (2%) | 0 (0%) | |
| Hispanic or Latino | 1 (1%) | 1 (2%) | 0 (0%) | 0 (0%) | |
| White | 129 (83%) | 48 (76%) | 53 (85%) | 28 (93%) | .46 |
| Alcohol | |||||
| Never | 47 (30%) | 16 (25%) | 19 (31%) | 12 (40%) | |
| Former | 10 (6%) | 5 (8%) | 2 (3%) | 3 (10%) | |
| Current | 98 (63%) | 42 (67%) | 41 (66%) | 15 (50%) | .34 |
| Smoking | |||||
| Never | 65 (42%) | 23 (37%) | 28 (45%) | 14 (47%) | |
| Former | 48 (31%) | 20 (32%) | 17 (27%) | 11 (37%) | |
| Current | 42 (27%) | 20 (32%) | 17 (27%) | 5 (17%) | .54 |
| Diabetes mellitus | |||||
| No | 144 (93%) | 60 (95%) | 59 (95%) | 25 (83%) | |
| Yes | 11 (7%) | 3 (5%) | 3 (5%) | 5 (17%) | .10 |
| Pre-diagnosis Weight Loss | |||||
| No | 94 (61%) | 36 (57%) | 38 (61%) | 20 (67%) | |
| Yes | 44 (28%) | 20 (32%) | 18 (29%) | 6 (20%) | .57 |
| Unknown | 17 (11%) | 7 (11%) | 6 (10%) | 4 (13%) | .85 |
| T stage | |||||
| T1 | 109 (70%) | 45 (71%) | 43 (69%) | 21 (70%) | |
| T2 | 46 (30%) | 18 (29%) | 19 (31%) | 9 (30%) | .97 |
| Tumor Thickness, cm | |||||
| Median (range) | .65 (.05 to 3.1) | .6 (.1 to 2.5) | .7 (.05 to 1.9) | .73 (.1 to 3.1) | .40 |
| Tumor Grade | |||||
| 1 | 39 (25%) | 16 (25%) | 18 (29%) | 5 (17%) | |
| 2 | 103 (66%) | 43 (68%) | 39 (63%) | 21 (70%) | |
| 3 | 10 (6%) | 3 (5%) | 4 (6%) | 3 (10%) | .67 |
| Missing data | 3 (2%) | 1 (2%) | 1 (2%) | 1 (3%) | .61 |
| Perineural Invasion | |||||
| No | 73 (47%) | 26 (41%) | 31 (50%) | 16 (53%) | |
| Yes | 62 (40%) | 29 (46%) | 22 (35%) | 11 (37%) | .43 |
| Missing data | 20 (13%) | 8 (13%) | 9 (15%) | 3 (10%) | .91 |
| Vascular Invasion | |||||
| No | 111 (72%) | 41 (65%) | 48 (77%) | 22 (73%) | |
| Yes | 24 (15%) | 14 (22%) | 5 (8%) | 5 (17%) | .10 |
| Missing data | 20 (13%) | 8 (13%) | 9 (15%) | 3 (10%) | .91 |
| Lymph Node Involvement | |||||
| No | 100 (65%) | 40 (64%) | 41 (66%) | 19 (63%) | |
| Yes | 55 (36%) | 23 (37%) | 21 (34%) | 11 (37%) | .95 |
| Final Surgical Margins | |||||
| Negative | 145 (94%) | 59 (94%) | 57 (92%) | 29 (97%) | |
| Positive | 10 (6%) | 4 (6%) | 5 (8%) | 1 (3%) | .84 |
| Postoperative Chemotherapy | |||||
| No | 146 (94%) | 58 (92%) | 59 (95%) | 29 (97%) | |
| Yes | 9 (6%) | 5 (8%) | 3 (5%) | 1 (3%) | .74 |
| Postoperative Radiation | |||||
| No | 96 (62%) | 38 (60%) | 39 (63%) | 19 (63%) | |
| Yes | 59 (38%) | 25 (70%) | 23 (37%) | 11 (37%) | .95 |
Archived paraffin embedded tissue was available from 61 patients. Tumors were evaluated for the cyclin-dependent kinase inhibitor p16, a known biomarker of HPV oncoprotein function.5 All primary tumor samples tested were p16 negative.
Height and weight at surgery were available for all patients. At time of surgery, 63 (41%) patients were normal weight, 62 (40%) were overweight, and 30 (19%) were obese. Obese patients were generally older than those of normal weight (Table 1) although this difference was not statistically significant (P=.07). Additionally, of 11 patients with diabetes mellitus, 8 (73%) were obese/overweight and 3 were normal weight (27%). Pre-diagnosis weight was stable for 94 (61%) patients, whereas 44 (28%) patients reported antecedent weight loss. The occurrence of pre-diagnosis weight change was unknown for 17 (11%) patients. In patients who did lose weight prior to tongue cancer diagnosis, median amount of weight loss was 4.5 kg (range 0–23 kg). The distribution of patients who experienced weight loss was similar across all BMI groups (P=0.57). Of the 94 patients who did not lose weight, 20 (21%) were obese, 38 (40%) were overweight, and 36 (38%) were normal weight at time of surgery.
Of the 155 patients, 87 (56%) had no evidence of disease at last follow-up, 13 (8%) were alive with disease, 32 (21%) died of disease, 11 (7%) died of other causes, and 12 (8%) died of unknown causes. Of the 13 patients alive with disease, 12 (92%) had locoregional recurrence and 1 (8%) had a second primary tongue cancer. Of the 32 patients who died of disease, 25 (78%) had locoregional recurrence and 7 (22%) had distant recurrence. Known prognostic factors including age (hazard ratio [HR] = 1.03; 95% confidence interval [CI], 1.00 to 1.06; P = .02), T stage (HR = 2.06; 95% CI, 1.02 to 4.19; P = .05), tumor thickness (HR = 1.88; 95% CI, 1.08 to 3.27; P = .03), tumor grade (grade 2: HR 3.63; 95% CI, 1.09 to 12.11; P = .04; grade 3: HR 6.32; 95% CI, 1.40 to 28.57; P = .02), perineural invasion (HR = 4.97; 95% CI, 2.00 to 12.34; P = .001), vascular invasion (HR = 3.67; 95% CI, 1.68 to 8.05; P = .001), the presence of lymph node metastases (HR = 2.69; 95% CI, 1.33 to 5.42; P = .01), and black race (HR = 5.87; 95% CI, 1.76 to 19.55; P = .004) were associated with worse DSS (Table 2) in univariate analyses. Furthermore, diabetes was associated with worse RFS (HR = 2.65; 95% CI, 1.25 to 5.60; P = .01) and worse OS (HR = 2.69; 95% CI, 1.21 to 5.98; P = .02) in univariate analyses.
Table 2.
Disease specific survival in univariate analysis and multivariate models
| Clinicopathologic Variables | Univariable | Multivariable | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| HR | 95% CI | P | HR | 95%CI | P | |
| Age (per year increase) | 1.03 | 1.00 to 1.06 | .02 | 1.00 | .97 to 1.04 | .98 |
| Race | ||||||
| Non-Black | Ref. | Ref. | ||||
| Black | 5.87 | 1.76 to 19.55 | .004 | 5.59 | .84 to 37.38 | .08 |
| BMI | ||||||
| Normal | Ref. | Ref. | ||||
| Overweight | 1.34 | .59 to 3.06 | .49 | 1.82 | .64 to 5.22 | .26 |
| Obese | 2.65 | 1.07 to 6.59 | .04 | 5.01 | 1.69 to 14.81 | .004 |
| Smoking | ||||||
| Never | Ref. | Ref. | ||||
| Former | 1.24 | .55 to 2.78 | .60 | 2.00 | .74 to 5.38 | .17 |
| Current | .97 | .40 to 2.34 | .94 | .67 | .17 to 2.66 | .57 |
| Diabetes Mellitus | ||||||
| No | Ref. | Ref. | ||||
| Yes | 2.47 | .86 to 7.09 | .09 | .80 | .23 to 2.81 | .73 |
| T stage | ||||||
| T1 | Ref. | Ref. | ||||
| T2 | 2.06 | 1.02 to 4.19 | .05 | 1.73 | .71 to 4.25 | .23 |
| Tumor Grade | ||||||
| 1 | Ref. | Ref. | ||||
| 2 | 3.63 | 1.09 to 12.11 | .04 | 3.14 | .67 to 14.68 | .15 |
| 3 | 6.32 | 1.40 to 28.57 | .02 | 4.35 | .74 to 25.45 | .10 |
| Perineural Invasion | ||||||
| No | Ref. | Ref. | ||||
| Yes | 4.97 | 2.00 to 12.34 | .001 | 3.44 | 1.21 to 9.76 | .02 |
| Vascular Invasion | ||||||
| No | Ref. | Ref. | ||||
| Yes | 3.67 | 1.68 to 8.05 | .001 | 2.42 | .86 to 6.80 | .09 |
| Lymph Node | ||||||
| Involvement | ||||||
| No | Ref. | Ref. | ||||
| Yes | 2.69 | 1.33 to 5.42 | .006 | 2.73 | 0.77 to 9.69 | .12 |
| Postoperative | ||||||
| Radiation | ||||||
| No | Ref. | Ref. | ||||
| Yes | 1.58 | .78 to 3.17 | .20 | .46 | .12 to 1.69 | .24 |
Abbreviations: BMI, body mass index; Ref, reference (HR = 1.0)
BMI and DSS
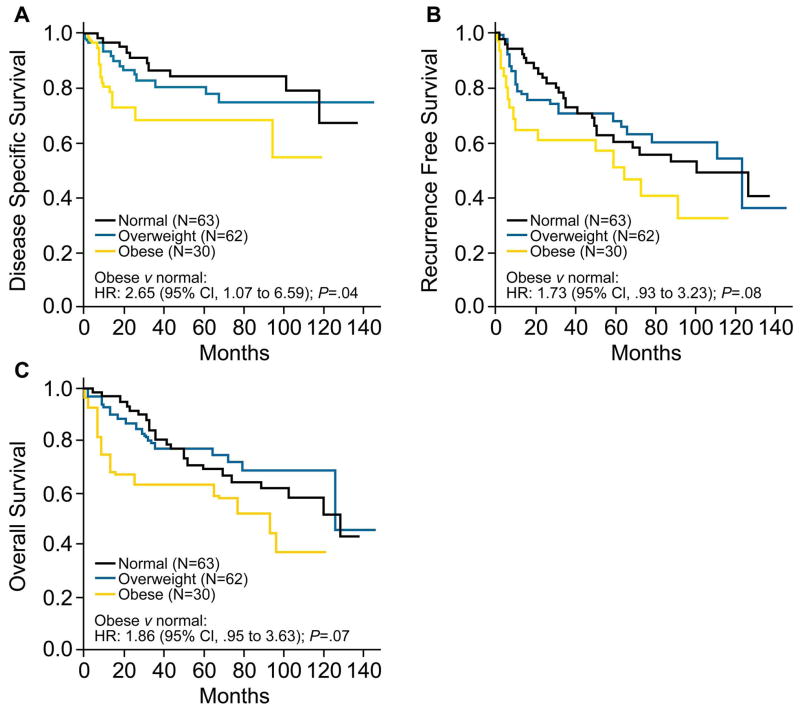
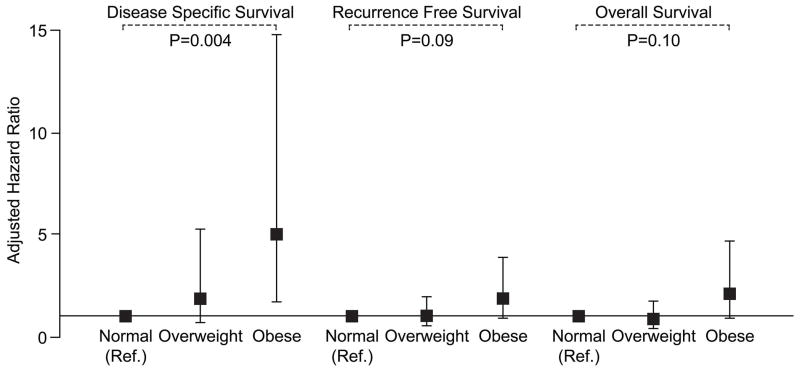
Obese patients had significantly shorter DSS as compared with normal weight patients in univariate analysis (HR = 2.65; 95% CI, 1.07 to 6.59; P = .04; Fig 2A). In addition, compared to normal weight, overweight subjects appeared to have shorter DSS (HR = 1.34; 95% CI, .59 to 3.06, P = .49) although a larger sample size is needed to confirm this observation. The 3-year DSS rates for obese, overweight, and normal weight patients are 68% (95% CI, 52% to 89%), 81% (95% CI, 71% to 92%), and 87% (95% CI, 78% to 96%), respectively (Figure 2A). In multivariable analysis after adjusting for known prognostic variables, obesity remained significantly associated with adverse DSS (HR = 5.01; 95% CI, 1.69 to 14.81; P = .004; Table 2, Figure 3).
Figure 2.
A. Disease specific survival after curative resection and neck dissection for oral tongue squamous cell carcinoma according to body mass index at time of surgery (N = 155). B. Recurrence free survival (N = 155). C. Overall survival (N=155). Abbreviations: HR, hazard ratio; CI, confidence interval.
Figure 3.
Multivariate hazard ratios (HRs) stratified by body mass index for disease specific survival, recurrence free survival, and overall survival in patients who underwent curative resection and neck dissection for oral tongue SCC (N = 155). Covariates include age, black race, smoking history, diabetes, T stage, tumor grade, perineural invasion, vascular invasion, lymph node metastases, and use of post-operative radiation.
Additionally, patients were then stratified by lymph node metastases status. For those without lymph node involvement (n=100), the association between obesity and shorter DSS remained significant HR = 29.5; 95% CI, 3.06 to 285.5; P = .003). Conversely, in patients with lymph node involvement (n=55), no association between obesity and DSS was seen (HR = .97; 95% CI, .16 to 5.86; P = .97). Based on these results, we went on to examine whether the differential associations could be quantified through interaction between the presence of lymph node metastases and BMI categories in a multivariate model. However, in this model, the interaction between the presence of lymph node metastases and BMI categories was not statistically significant.
BMI and Other Survival Outcomes
Compared with normal weight subjects, obese patients had shorter RFS (HR = 1.73; 95% CI, .93 to 3.23) although this association did not reach statistical significance (P = .08 Figure 2B). Similarly, overweight status tended to confer worse RFS compared with normal weight (HR = 1.74; 95% CI, .85 to 3.55), though without reaching statistical significance (P = .13). Furthermore, obese patients had shorter OS (HR = 1.86; 95% CI .95 to 3.63) although again this result was not statistically significant (P = .07, Figure 2C). Among patients who did not lose weight prior to tongue cancer diagnosis (N = 94), obesity was associated with worse OS as compared with normal weight (HR = 2.70; 95% CI, 1.12 to 6.54; P = .03) in univariate analysis. Consistent results were obtained for these outcomes when multivariable models were used.
Discussion
Identifying prognostic features for oral tongue SCC is of significant interest given its rising incidence despite falling tobacco use and lack of association with HPV infection. Because of the association of obesity with inferior outcomes for several common malignancies, we examined its association with prognosis in a relatively large single institution cohort of patients with early T stage oral tongue SCC who underwent curative resection and neck dissection. We found that obesity, compared to normal weight, was associated with a five-fold increase in risk of death (DSS) from oral tongue SCC. As obesity is associated with increased all-cause mortality,30 the association between excess BMI and DSS is critical in identifying obesity as an independent prognostic factor for patients with oral tongue SCC. Notably, although our cohort included only 4 black patients, black race was also associated with adverse DSS in univariate analysis, consistent with published data.31
To our knowledge, this report is the first to suggest that elevated BMI is a poor prognostic variable in patients with SCC of the oral tongue. Several prior reports have suggested that leanness may be associated with adverse outcomes after diagnosis for patients with head and neck malignancies. Additionally, low BMI has been reported to be associated with elevated head and neck cancer risk.11–20, 32 These study populations, however, may be confounded by the inclusion of a variety of tumor sites (each with potentially unique risk factors) and disease stages, thus making interpretation of these previous reports difficult. For example, Liu et al reported a higher probability of death from oral cancer in Taiwanese patients with a preoperative BMI < 22.8 kg/m2 (relative risk, RR = 1.29; 95% CI, 1.04 to 1.61; P = .02).18 However, patients in this study, unlike ours, had a variety of tumor sites in the oral cavity and oropharynx with a variety of tumor sizes from small to obstructive. Importantly, malnutrition occurs commonly in patients with head and neck cancers, and preoperative weight loss has been associated with worse prognosis.19, 20 In a small retrospective analysis including 64 patients with malignancies of various sites in the head and neck, men with preoperative weight loss of greater than 5% had a significantly increased risk of death (RR = 43; 95% CI, 6 to 324; P = .02).19 In another retrospective review of 97 patients with recurrent tumors of the oral cavity and oropharynx, weight loss was the strongest predictor of mortality.20 Thus weight loss is a critical prognostic variable, and has not been consistently considered in studies of BMI and head and neck cancers. Accounting for disease and treatment related weight loss has been a key challenge in understanding the consequences of obesity in other cancers.33
We sought to minimize such confounding through a number of methods. We limited the study population to a single primary site – the oral tongue. Additionally, T stage, determined by tumor size, is a key consideration in the prognostic evaluation of patients with oral tongue SCC. Larger tumors, and thereby more advanced T stage, may often result in partial obstruction of the upper digestive tract leading to diminished oral intake, poor nutrition, and weight loss. Accordingly, we sought to limit confounding due to advanced T stage and tumor-related weight loss by including only patients with T1 or T2 oral tongue SCC. As a result, the majority of patients in our study population did not report preoperative weight loss and in those who did, the degree of weight loss was relatively small. In addition to study design, the accuracy of our data is supported by several findings. First, we confirmed the lack of a prognostic contribution by HPV status in our study as none of the available tumor samples (N=61) were found to express p16. Second, known prognostic variables including histologic features and lymph node involvement, were reproduced in our study population. Additionally, the association between obesity and worse DSS was further strengthened in multivariate analysis after adjusting for these known prognostic features. Finally, stratifying patients by nodal metastasis status supported findings from our multivariate model and suggested possible differential effects of obesity on outcomes for patients with versus without lymph node metastasis, though our study was not adequately powered to examine this interaction.
The mechanisms by which obesity contributes to disease progression and worse outcomes are complex. Hyperadiposity has been linked with several consequences, including dysregulated energy metabolism and increased levels of proinflammatory mediators, which can promote tumor cell survival, proliferation, and invasion.21, 34–41 Alterations of adipokine levels, including elevated leptin and decreased adiponectin, are known to occur in obesity and have direct effects on several signal transduction pathways involved in cell survival.21 Similarly, obesity-associated insulin resistance, characterized by hyperinsulinemia, has been shown to promote cell proliferation and inhibit apoptosis.34 Additionally, excess BMI is associated with release of free fatty acids leading to macrophage activation and release of several proinflammatory mediators including tumor necrosis factor-α, interleukin-6 and prostaglandin E2.36 Furthermore, we have previously shown that inflammation of breast white adipose tissue may play a critical role in the link between obesity and breast cancer.37–39 Having now described a prognostic impact of obesity in oral tongue SCC, it will be important to investigate the role of local adipose depots, such as neck and tongue fat, in the progression of oral tongue SCC. Notably, our results raise the possibility that interventions including weight reduction, exercise and pharmacological therapies, e.g., anti-inflammatory drugs could improve the outcomes of obese patients with early stage oral tongue cancers. Certainly, such approaches are being considered in patients with breast and other cancers.42
Our study is strengthened by a homogenous study population in terms of primary site, T stage, and management, which was attainable due to the higher number of oral tongue SCC patients treated at our institution. Both nutritional assessment and BMI were prospectively collected. Despite the retrospective design, the validity of our data is preserved as clinicopathologic data and study variables were ascertained by two independent physicians and subjected to internal audit.
Nonetheless, our study remains limited by its retrospective design and it will be important to validate our findings in an independent cohort. Furthermore, our findings are not necessarily generalizable to advanced T stages or other sites of head and neck malignancy, although the consistency with other diseases suggests the existence of a common pathophysiological link.
In conclusion, we report for the first time that obesity is an independent predictor of increased risk of death from disease in patients with early stage oral tongue cancer. The prognostic impact of obesity may not have previously been recognized due to pre-diagnosis weight loss related to comorbid habits and tumor stage. Further mechanistic studies are needed to elucidate the biological underpinnings of this association. Our findings are clinically relevant given both the rising oral tongue SCC and obesity rates worldwide.
Acknowledgments
Funding Sources: This work was supported by NIH UL1TR000457 (N.M. Iyengar), UL1RR024996 (X.K. Zhou), and NIDCD T32 000027 (A. Kochhar).
Footnotes
Financial Disclosures: None
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Depue RH. Rising mortality from cancer of the tongue in young white males. N Engl J Med. 1986;315:647. doi: 10.1056/NEJM198609043151013. [DOI] [PubMed] [Google Scholar]
- 3.Salem A. Dismissing links between HPV and aggressive tongue cancer in young patients. Ann Oncol. 2010;21:13–17. doi: 10.1093/annonc/mdp380. [DOI] [PubMed] [Google Scholar]
- 4.Saba NF, Goodman M, Ward K, et al. Gender and ethnic disparities in incidence and survival of squamous cell carcinoma of the oral tongue, base of tongue, and tonsils: a surveillance, epidemiology and end results program-based analysis. Oncology. 2011;81:12–20. doi: 10.1159/000330807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dahlgren L, Dahlstrand HM, Lindquist D, et al. Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer. 2004;112:1015–1019. doi: 10.1002/ijc.20490. [DOI] [PubMed] [Google Scholar]
- 7.Chaturvedi AK, Engels EA, Anderson WF, Gillison ML. Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol. 2008;26:612–619. doi: 10.1200/JCO.2007.14.1713. [DOI] [PubMed] [Google Scholar]
- 8.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–720. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 9.D’Souza G, Kreimer AR, Viscidi R, et al. Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med. 2007;356:1944–1956. doi: 10.1056/NEJMoa065497. [DOI] [PubMed] [Google Scholar]
- 10.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the International Agency for Research on Cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–1783. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 11.Gaudet MM, Olshan AF, Chuang SC, et al. Body mass index and risk of head and neck cancer in a pooled analysis of case-control studies in the International Head and Neck Cancer Epidemiology (INHANCE) Consortium. Int J Epidemiol. 2010;39:1091–1102. doi: 10.1093/ije/dyp380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreimer AR, Randi G, Herrero R, et al. Diet and body mass, and oral and oropharyngeal squamous cell carcinomas: analysis from the IARC multinational case-control study. Int J Cancer. 2006;118:2293–2297. doi: 10.1002/ijc.21577. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez T, Altieri A, Chatenoud L, et al. Risk factors for oral and pharyngeal cancer in young adults. Oral Oncol. 2004;40:207–213. doi: 10.1016/j.oraloncology.2003.08.014. [DOI] [PubMed] [Google Scholar]
- 14.Nieto A, Sanchez MJ, Martinez C, et al. Lifetime body mass index and risk of oral cavity and oropharyngeal cancer by smoking and drinking habits. Br J Cancer. 2003;89:1667–1671. doi: 10.1038/sj.bjc.6601347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Franceschi S, Dal Maso L, Levi F, Conti E, Talamini R, La Vecchia C. Leanness as early marker of cancer of the oral cavity and pharynx. Ann Oncol. 2001;12:331–336. doi: 10.1023/a:1011191809335. [DOI] [PubMed] [Google Scholar]
- 16.Kabat GC, Chang CJ, Wynder EL. The role of tobacco, alcohol use, and body mass index in oral and pharyngeal cancer. Int J Epidemiol. 1994;23:1137–1144. doi: 10.1093/ije/23.6.1137. [DOI] [PubMed] [Google Scholar]
- 17.D’Avanzo B, La Vecchia C, Talamini R, Franceschi S. Anthropometric measures and risk of cancers of the upper digestive and respiratory tract. Nutr Cancer. 1996;26:219–227. doi: 10.1080/01635589609514478. [DOI] [PubMed] [Google Scholar]
- 18.Liu SA, Tsai WC, Wong YK, et al. Nutritional factors and survival of patients with oral cancer. Head Neck. 2006;28:998–1007. doi: 10.1002/hed.20461. [DOI] [PubMed] [Google Scholar]
- 19.van Bokhorst-de van der S, van Leeuwen PA, Kuik DJ, et al. The impact of nutritional status on the prognoses of patients with advanced head and neck cancer. Cancer. 1999;86:519–527. [PubMed] [Google Scholar]
- 20.Nguyen TV, Yueh B. Weight loss predicts mortality after recurrent oral cavity and oropharyngeal carcinomas. Cancer. 2002;95:553–562. doi: 10.1002/cncr.10711. [DOI] [PubMed] [Google Scholar]
- 21.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18:2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 22.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348:1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 23.Yoon HH, Lewis MA, Shi Q, et al. Prognostic impact of body mass index stratified by smoking status in patients with esophageal adenocarcinoma. J Clin Oncol. 2011;29:4561–4567. doi: 10.1200/JCO.2011.37.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sinicrope FA, Foster NR, Sargent DJ, O’Connell MJ, Rankin C. Obesity is an independent prognostic variable in colon cancer survivors. Clin Cancer Res. 2010;16:1884–1893. doi: 10.1158/1078-0432.CCR-09-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sinicrope FA, Foster NR, Yoon HH, et al. Association of obesity with DNA mismatch repair status and clinical outcome in patients with stage II or III colon carcinoma participating in NCCTG and NSABP adjuvant chemotherapy trials. J Clin Oncol. 2012;30:406–412. doi: 10.1200/JCO.2011.39.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyerhardt JA, Tepper JE, Niedzwiecki D, et al. Impact of body mass index on outcomes and treatment-related toxicity in patients with stage II and III rectal cancer: findings from Intergroup Trial 0114. J Clin Oncol. 2004;22:648–657. doi: 10.1200/JCO.2004.07.121. [DOI] [PubMed] [Google Scholar]
- 27.Meyerhardt JA, Ma J, Courneya KS. Energetics in colorectal and prostate cancer. J Clin Oncol. 2010;28:4066–4073. doi: 10.1200/JCO.2009.26.8797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levi J, Segal LM, St Laurent R, Lang A, Rayburn J. [accessed 09/28/2012, 2012];F as in Fat: How Obesity Threatens America’s Future. 2012 Available from URL: http://healthyamericans.org/assets/files/TFAH2012FasInFatFnlRv.pdf.
- 29.Bishop JA, Westra WH. Human papillomavirus-related small cell carcinoma of the oropharynx. Am J Surg Pathol. 2011;35:1679–1684. doi: 10.1097/PAS.0b013e3182299cde. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Calle EE, Thun MJ, Petrelli JM, Rodriguez C, Heath CW., Jr Body-mass index and mortality in a prospective cohort of U.S. adults. N Engl J Med. 1999;341:1097–1105. doi: 10.1056/NEJM199910073411501. [DOI] [PubMed] [Google Scholar]
- 31.Ragin CC, Langevin SM, Marzouk M, Grandis J, Taioli E. Determinants of head and neck cancer survival by race. Head Neck. 2011;33:1092–1098. doi: 10.1002/hed.21584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nieto A, Sanchez MJ, Quintana MJ, et al. BMI throughout life, intake of vitamin supplements and oral cancer in Spain. IARC Sci Publ. 2002;156:259–261. [PubMed] [Google Scholar]
- 33.Jeon JY, Meyerhardt JA. Energy in and energy out: what matters for survivors of colorectal cancer? J Clin Oncol. 2012;30:7–10. doi: 10.1200/JCO.2011.39.6374. [DOI] [PubMed] [Google Scholar]
- 34.Pollak MN, Schernhammer ES, Hankinson SE. Insulin-like growth factors and neoplasia. Nat Rev Cancer. 2004;4:505–518. doi: 10.1038/nrc1387. [DOI] [PubMed] [Google Scholar]
- 35.Tilg H, Moschen AR. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6:772–783. doi: 10.1038/nri1937. [DOI] [PubMed] [Google Scholar]
- 36.Olefsky JM, Glass CK. Macrophages, inflammation, and insulin resistance. Annu Rev Physiol. 2010;72:219–246. doi: 10.1146/annurev-physiol-021909-135846. [DOI] [PubMed] [Google Scholar]
- 37.Subbaramaiah K, Howe LR, Bhardwaj P, et al. Obesity is associated with inflammation and elevated aromatase expression in the mouse mammary gland. Cancer Prev Res (Phila) 2011;4:329–346. doi: 10.1158/1940-6207.CAPR-10-0381. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 38.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Subbaramaiah K, Morris PG, Zhou XK, et al. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2:356–365. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 40.Kekatpure VD, Boyle JO, Zhou XK, et al. Elevated Levels of Urinary Prostaglandin E Metabolite Indicate a Poor Prognosis in Ever Smoker Head and Neck Squamous Cell Carcinoma Patients. Cancer Prev Res (Phila Pa) 2009;20:20. doi: 10.1158/1940-6207.CAPR-09-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Morris PG, Zhou XK, Milne GL, et al. Increased Levels of Urinary PGE-M, a Biomarker of Inflammation, Occur in Association with Obesity, Aging, and Lung Metastases in Patients with Breast Cancer. Cancer Prev Res (Phila) 2013 doi: 10.1158/1940-6207.CAPR-12-0431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ligibel JA, Goodwin PJ. NEW and RENEW: Building the Case for Weight Loss in Breast Cancer. J Clin Oncol. 2012;30:2294–2296. doi: 10.1200/JCO.2012.42.5496. [DOI] [PubMed] [Google Scholar]