Abstract
Background
Previous studies of very preterm (VPT) infants have shown a wide range of seizure prevalence and association with intraventricular hemorrhage (IVH), white matter injury (WMI) and death. However, the impact of seizures on neurodevelopment is not well known. We hypothesized that seizures in the first three days after VPT birth would be associated with increased radiographic brain injury and later neurodevelopmental risk.
Methods
For 72 hours after birth 95 VPT infants underwent aEEG monitoring. High and low seizure burdens were related to radiographic brain injury, death in the neonatal period and children’s Bayley III performance at 2 years corrected age in a subgroup of 59 infants.
Results
The overall incidence of seizures in this sample was 48%. High seizure burden was associated with increased risk of IVH on day 1; IVH, WMI and death on day 2 and high grade IVH on day 3. The presence of seizures on any day was associated with decreased language performance at age 2, even after controlling for family social risk.
Conclusions
Seizures during the first three days after birth are common and are associated with an increased risk of IVH, WMI and death. They were also associated with poorer early language development.
Introduction
The incidence of electrographic seizures in the preterm infant varies widely from 4% (1) to 48% (2). There is some suggestion that seizures may be under-recognized in this population since a higher incidence of seizures tends to be reported in studies where infants are prospectively monitored compared to studies where EEGs are obtained after a clinical event thought to be a seizure. Seizures in the neonatal period are often brief; usually last less than two minutes and often occur without overt clinical signs (3–5). Multiple studies of both term and preterm infants have demonstrated poor sensitivity for seizure detection by observation alone, with only 13% to 44% of seizures having an obvious clinical correlate at the time of an electrographic seizure, and many having periodic sub-clinical seizures interspersed with clinically observable episodes (2,6–9). Nevertheless, electrographic seizures in the preterm infant have been associated with adverse outcomes including intraventricular hemorrhage (IVH) (1,2,10), white matter injury (WMI) (10), and death (1,2,11) in the neonatal period and moderate to severe cognitive impairment on follow-up (10).
Though the prospective use of continuous EEG affords a higher seizure detection rate, conventional EEG requires 24-hour neurophysiology interpretation and challenging lead placement on the small surface area of the preterm scalp, further complicated by the fragile skin of these infants. Amplitude-integrated EEG (aEEG), which allows for limited channel and time compressed continuous EEG recording has been validated for the detection of seizures, and when used concurrently with simultaneous raw EEG has sensitivity of up to 76–78% in term neonates (3,12). aEEG monitors are also simple to set up and can be interpreted successfully by neurologists and neonatologists (4).
In this study, we quantified electrographic seizures using two-channel aEEG data collected during the first three days after very preterm birth, and examined associations between seizure timing and severity and a range of outcomes, including the presence and severity of IVH on cranial ultrasound (CUS), WMI on term equivalent MRI and infant neurodevelopmental outcome by 2 years corrected age. We hypothesized that the presence and the extent of seizure burden in the first 72 hours would be associated with increased risks of a) IVH due to altered cerebral perfusion, b) WMI due to neuronal stress and apoptosis, and in turn, c) poorer neurodevelopmental outcomes.
Materials and Methods
Participants
Between 2008 and 2010, infants born between 24 and 30 weeks gestational age were prospectively recruited from the NICU at St. Louis Children’s Hospital for aEEG monitoring.
Procedure
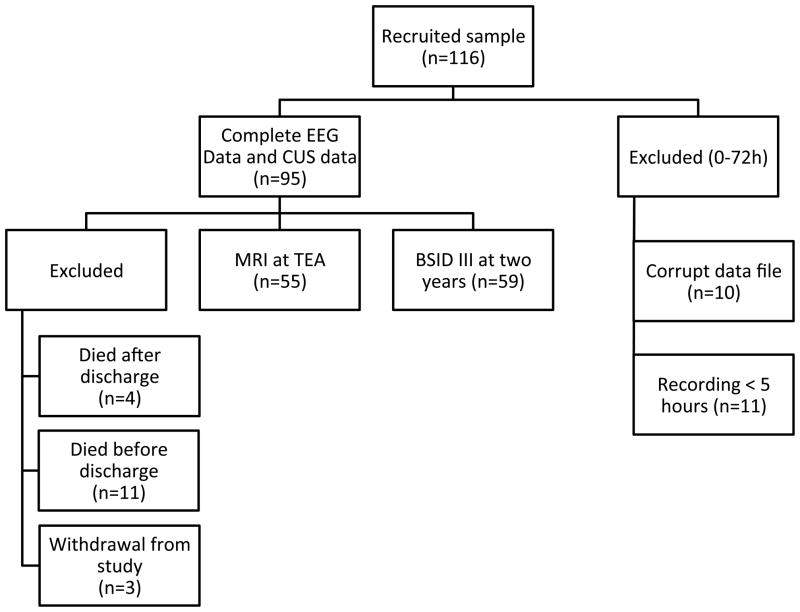
All research protocols were approved by the Washington University School of Medicine institutional review board. Written informed consent was obtained from all legal guardians. Following consent, study infants were monitored with a two-channel (C3-P3, C4-P4 configuration) aEEG using the BRM2 monitor (Natus Medical Incorporated, San Carlos, CA) and hydrogel electrodes. Monitoring began as soon as the infant was stabilized and lead placement was possible. Recording continued uninterrupted until 72 hours after birth. Study participants also underwent CUS imaging following routine clinical practice during the first three days after birth. At term-equivalent age, excluding 3 children whose families withdrew from the study, all surviving infants (n=84/92) had an MRI scan. Imaging data was of sufficient quality for analysis for 55/92 (60%) of these infants. Finally at age 2 years corrected age, excluding deaths post discharge (n=4), 59 (66%) of all eligible children were assessed using the Bayley Scales of Infant development (BSID III). Figure 1 provides an overview of the study design and measures used. Also shown are the numbers of children with data on key measures and the reason for sample loss.
Figure 1. Overview of study design.
Overview of study design depicting number of participants for each stage of analysis and the reason for exclusion.
Measures
Infant clinical characteristics
Information about infant gestational age, sex, ventilator status, antenatal steroid exposure and CRIB II scores (13) was obtained from their medical records.
Family social background
Five measures of family social background were also collected including infant ethnicity, single mother, maternal illicit drug use during pregnancy, early motherhood (<20 years) and family socioeconomic status defined using insurance type (public, private) as a proxy for family income (14). These dichotomous (yes/no) measures were then summed to form an overall index of family social risk (15).
Seizures
A multimodal approach was used to assess for the presence of seizures. Initial screening was undertaken by assessing for a sudden change in the upper and lower margin (8) of the time-compressed tracing or for marks placed by an automatic seizure detection algorithm. The raw trace was then inspected at those time periods to assess whether the finding was artifactual or not based on the appearance of the raw trace and by reading time-stamped notations by nursing staff indicating patient handling during that time period. The entire raw trace was then manually inspected for seizure activity not detected by the automated algorithm or by changes in the baseline. Due to variation in age at recruitment and length of recording, aEEG data files were available for 69, 90 and 94 subjects on days one, two and three after birth respectively. The recordings were started at an average of 18.5 (SD=12.7) hours after birth and had an average duration of 66 hours (SD=21.8).
Seizures were defined in the manner described by Scher et al. (1), namely a series of sharp waves, at least 10 seconds in length, which evolve in frequency, amplitude and morphology and are clearly distinguishable from the background or artifact. Cumulative daily seizure burden was recorded for each day after birth. Data was not collected in a fashion that allowed for calculation of inter-rater reliability, but EEG readings were supervised closely, by two senior investigators with substantial experience in reading aEEG traces (TI and AM).
Seizure incidence and median seizure burden were calculated for each day after birth as well as for the entire 72-hour period. Those infants with a seizure burden exceeding the 90th percentile were identified as a separate high-risk group.
Intraventricular Hemorrhage
The presence and grade of IVH was diagnosed exclusively by CUS studies done during the first 72 hours after birth. CUS studies were evaluated for IVH using the classification system originally described by Papile (16).
White Matter Injury
The presence and severity of WMI was assessed based on term MRI, with scans assessed qualitatively for the presence or absence of any type of WMI and specifically for cystic WMI by one individual (TI).
Two Year Neurodevelopmental Outcome
The BSID-III provides a standardized neurodevelopmental assessment of children’s motor, cognitive and language functioning at corrected age 2 years. Although the expectation was that the score distribution for the BSID-III in the general population would be normally distributed with a mean of 100 and standard deviation of 15, there is growing awareness that current test norms overestimate performance by approximately 15 points.
Statistical Analysis
All analyses were conducted with R version 3.0 (R Project for Statistical Computing, Vienna, Austria). Data analysis was conducted in four steps. First, clinical and socioeconomic background characteristics of all infants were described and comparisons made between those infants who had seizures in the first 3 days and those who did not using either student’s T-test for continuous variables or the chi-squared test of independence for categorical variables. Second, the proportion of infants experiencing seizures and the severity of seizure burden was examined across each of the three days of monitoring. Of particular interest was the identification of those infants subject to high seizure burden defined as a burden greater than the 90th percentile of the sample for each study day. Third, the extent to which the presence of early seizures and/or high seizure burden placed infants at increased risk of any IVH, high grade IVH, WMI, cystic WMI and death were then examined for each day and all three days. Finally, associations between early seizure risk and seizure burden and later motor, cognitive and language outcomes were examined before and after statistical adjustment for family social risk.
Results
A total of 116 study infants were initially recruited. Of these, 21 (18%) were subsequently excluded due to insufficient recording time (<5 hours, n=11) or corrupt data (n=10) leaving a total sample of 95 infants. No significant differences were found between those infants included and excluded from this analysis in terms of infant clinical characteristics or family socioeconomic background. Table 1 provides a descriptive profile of the sample. It is important to note that the infants with seizures were more immature and possessed greater severity of illness (CRIB).
Table 1.
Sample Clinical and Family Social Background Characteristics
| Characteristic | No Seizures (N=49) | Seizures (N=46) | p |
|---|---|---|---|
| Infant clinical | |||
| Gestational age at birth, M ± SD, weeks | 26.9 ± 1.7 | 25.8 ± 1.8 | 0.001 |
| Male, n (%) | 24 (49) | 23 (50) | 1.0 |
| CRIB II score, M ± SD | 2.8 ± 2.9 | 5.6 ± 3.9 | < 0.001 |
| Antenatal steroids | |||
| Full course, n (%) | 21 (43) | 18 (39) | |
| Partial, n (%) | 20 (41) | 23 (50) | |
| None, n (%) | 8 (16) | 5 (11) | 0.60 |
| Extubated at 72 hours of life, n (%) | 32 (65) | 17 (37) | 0.01 |
| Any Intraventricular hemorrhage, n (%) | 6 (12) | 18 (39) | 0.005 |
| High grade intraventricular hemorrhage, n (%) | 3 (6) | 7 (15) | 0.27 |
| Any white matter injury, n (%) | 8 (16) | 9 (20) | 0.85 |
| Cystic white matter injury, n (%) | 2 (4) | 3 (7) | 0.92 |
| Family Social background | |||
| Minority status, n (%) | 30 (61) | 24 (52) | 0.50 |
| Low socioeconomic status (“Public” insurance), n (%) | 37 (76) | 28 (61) | 0.19 |
| Single parent household, n (%) | 31 (63) | 24 (52) | 0.38 |
| Illicit drug use during pregnancy, n (%) | 2 (4) | 4 (9) | 0.62 |
| Young (<20 years) motherhood, n (%) | 10 (20) | 12 (26) | 0.68 |
Examination of seizure frequency and pattern by day after birth
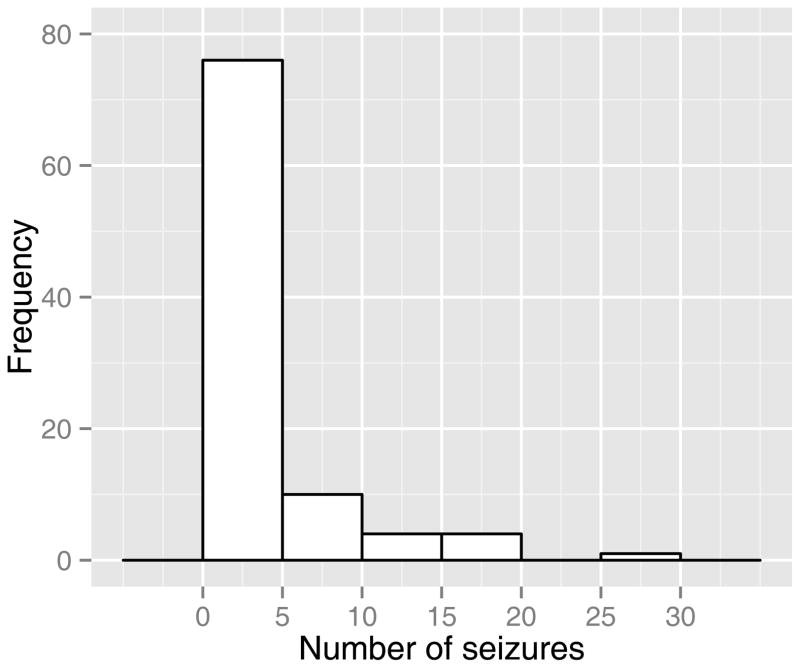
At least one seizure was noted in 48% (46/95) of the infants in the first 72 hours after birth. Of those who experienced seizures, a median of four ictal episodes occurred (range 1–28). The frequency distribution of seizure episodes is shown in Figure 2. Three infants had status epilepticus (defined as a single ictal episode with a duration greater than 30 minutes) (17,18).
Figure 2. Frequency distribution of seizures.
Histogram depicting cumulative number of seizures during the first 72 hours after birth.
As shown in Table 2, during the first 24 hours after birth, seizures were noted in a 33% (23/69) of infants. Among the 23 infants with seizures, the median seizure burden was 71 seconds and those in the upper decile of seizure burden had greater than 121 seconds of seizures. During the second 24 hours after birth, seizures were noted in 42% (38/90) of infants. Among those 38 subjects with seizures, the median seizure burden was 66 seconds and those in the upper decile of seizure burden had greater than 201 seconds of seizures. During the third 24 hours after birth, seizures were noted in 34% (32/94) of the subjects. Among those 32 infants with seizures, the median seizure burden was 44 seconds and those in the upper decile of seizure burden had greater than 91 seconds of seizures.
Table 2.
Seizure characteristics by monitoring period
| Seizure characteristic | 0–24 hours (N=69) | 24–48 hours (N=90) | 48–72 hours (N=94) | 0–72 hours (N=95) |
|---|---|---|---|---|
| Incidence, n (%) | 23 (33) | 38 (42) | 32 (34) | 46 (48) |
| Burden, Mdn (Range), sec | 71 (10–2395) | 66 (10–9827) | 44 (10–8250) | 104 (10–18169) |
| 90th percentile burden threshold, sec | > 121 | > 201 | > 91 | n/a |
Note: Variation in sample size for each study day due to differences in age at time of recruitment and length of recording.
Seizures in first 24 hours after birth
Table 3 examines the effects of high seizure burden by day after birth on risks of IVH, WMI and death. For those infants who experienced at least one seizure during the first 24 hours after birth, there was no increase in the relative risk for IVH of any grade, WMI, cystic WMI or death. In contrast, high seizure burden during the first 24 hours after birth was associated with an increased risk of IVH of any type (RR 2.9, 95% CI 1.4–5.8). No association was found with high-grade IVH, WMI, cystic WMI or death.
Table 3.
Relative risk for intraventricular hemorrhage, white matter injury and death in infants with 90th percentile seizure burden on each day after birth
| 0–24 hours (N=69) | 24–48 hours (N=90) | 48–72 hours (N=94) | |
|---|---|---|---|
| Any Intraventricular hemorrhage | 2.9 (1.4–5.8) | 2.6 (1.4–4.8) | 3.1 (1.9–5.3) |
| High grade intraventricular hemorrhage (Grade III & IV) | 2.2 (0.4–12.1) | 2.5 (0.8–8.3) | 4.0 (1.5–10.8) |
| Any white matter injury† | 2.3 (0.8–6.9) | 3.0 (1.3–6.6) | 1.4 (0.4–5.9) |
| Cystic white matter injury† | - | - | - |
| Death | 1.7 (0.6–5.2) | 2.7 (1.1–6.7) | 1.6 (0.5–4.8) |
Note: 95% confidence interval given in parantheses.“-”denotes insufficient data to calculate. Variation in sample size for each study day due to differences in age at time of recruitment and length of recording.
MRI analysis performed on a smaller subsample (n=55).
Seizures in the second 24 hours after birth
For those infants who experienced at least one seizure during the second 24 hours after birth, there was no increased relative risk for IVH of any grade, WMI, cystic WMI or death compared to those who did not. Whereas, in contrast, infants with high seizure burden in the second 24 hours after birth were at increased risk for IVH of any type (RR 2.6, 95% CI 1.4–4.8), WMI (RR 3.0, 95% CI 1.3–6.6) and death (RR 2.7, 95% CI 1.1–6.7) but not high-grade IVH and cystic WMI compared to those who did not.
Seizures in the third 24 hours after birth
For those infants who experienced at least one seizure during the third 24 hours after birth, there was no increase in the relative risk for IVH of any grade, WMI, cystic WMI or death compared to those who did not. Those subjects who had high seizure burden on day 3, had an increased relative risk for IVH of any type (RR 3.1, 95% CI 1.9–5.3) and high-grade IVH (RR 4.0, 95% CI 1.5–10.8) but not WMI, cystic WMI and death compared to those who did not.
Seizures and Neurodevelopmental Outcomes
Table 4 examines the extent to which seizures in the first 3 days after birth placed very preterm infants at an increased risk of poorer motor, cognitive and language scores at age 2. As shown, there was a general tendency for infants with seizures detected to have poorer motor, cognitive and language scores. After statistical adjustment for the effects of family social risk, a statistically significant difference was noted in the language scale (6 point difference, p=0.04). Extending on this analysis, we then examined whether high seizure burden on each of the first three days might influence later risk. As reported in Table 5, no association was found between high seizure burden on either day 1, 2 or 3 and children’s subsequent motor, cognitive and language scores at age 2.,
Table 4.
Neurodevelopmental outcomes of infants with and without seizures
| Neurodevelopmental outcome, Age 2 years | No Seizures (N=30) | Seizures (N=29) | p | Adj.* P |
|---|---|---|---|---|
| Cognition, M ± SD | 86 ± 9 | 83 ± 9 | 0.15 | 0.18 |
| Motor, M ± SD | 88 ± 10 | 85 ± 10 | 0.23 | 0.21 |
| Language, M ± SD | 86 ± 11 | 80 ± 11 | 0.09 | 0.04 |
Note:
Adjusted for social risk index
Table 5.
Neurodevelopmental outcome by seizure burden percentile and time after birth
| Neurodevelopmental outcome, Age 2 years (M ± SD) | 0–24 hours (N =43) | 24–48 hours (N =55) | 48–72 hours (N=59) | ||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| p | Adj.* p | p | Adj.* p | p | Adj.* p | ||||
| Cognition | |||||||||
| < 90th percentile | 85 ± 9 | 85 ± 9 | 85 ± 9 | ||||||
| > 90th percentile | 85 ± 6 | 0.38 | 0.90 | 85 ± 8 | 0.36 | 0.97 | 84 ± 9 | 0.42 | 0.98 |
| Motor | |||||||||
| < 90th percentile | 87 ±12 | 86 ± 11 | 87 ± 11 | ||||||
| > 90th percentile | 89 ± 6 | 0.75 | 0.76 | 89 ± 2 | 0.44 | 0.62 | 87 ± 4 | 0.51 | 0.73 |
| Language | |||||||||
| < 90th percentile | 84 ± 11 | 84 ± 10 | 83 ± 11 | ||||||
| > 90th percentile | 83 ± 11 | 0.70 | 0.75 | 78 ± 9 | 0.36 | 0.32 | 79 ± 4 | 0.40 | 0.35 |
Note: Variation in sample size for each study day due to differences in age at time of recruitment and length of recording.
Adjusted for social risk index
Clinically detected seizures
Of the 46 infants who were found to have evidence of electrographic seizure activity, only three (7%) had a clinically apparent correlate. As the aEEG traces were reviewed offline, information about electrographic seizure activity was not available to the clinical team and the diagnosis was made using clinical criteria alone. All three subjects with clinically detected seizures were treated with phenobarbital. There were no subjects who were clinically suspected to have seizures without electrographic correlate and two of the three infants with clinically detected seizures died during the first 72 hours after birth.
Discussion
The incidence of seizures in our preterm cohort is high and similar to that noted by other studies that have used prospective monitoring, particularly those done by Hellström-Westas et al. and Wikström et al. (2,19). Infants who had seizures were more premature and sicker that those who did not seize. In addition, we provide further evidence to the likelihood that even experienced clinicians frequently fail to detect seizures using clinical observation in this population.
The seizures observed in our very preterm cohort demonstrated a similar evolution in timeline to the seizures of a term infant suffering from hypoxic-ischemic encephalopathy (HIE). Our very preterm infants had the highest median seizure burden during the 0–24 hour period, similar to term infants with HIE (20). Of note, somewhat in contrast to term infants, the greatest proportion of very preterm infants had seizures during the second day after birth with many preterm infants displaying seizures persisting into the third day of life. This may represent a mixed antenatal and perinatal insult, similar to term ischemic brain injury (21) followed by disordered transition (22) or loss of cerebral auto-regulation (23) associated with postnatal hemorrhagic-ischemic cerebral insults.
Excitotoxic neuroapoptosis caused by excessive glutamate receptor activation in the setting of excessive hypoxic or seizure-induced oxidative stress, proposed by Jensen (24) and later demonstrated in murine models (25), may explain our findings. Though the mere presence of seizures for any given participant was not associated with an increased risk of WMI, those infants with the greatest burden of seizures during the second day after birth had a 3-fold greater risk than other infants who had no or low levels of seizure burden. This suggests that there may be a threshold, potentially even on the order of 2–3 minutes (the lower bounds of the 90th percentile) over which the cumulative excitotoxicity in the setting of potential ischemia may contribute to the development of WMI.
In contrast to MRI findings of WMI demonstrated months after the seizures, the emergence of IVH on cranial ultrasound was contemporaneous with the monitoring period and was associated with seizures on each day after birth and for the monitoring period overall. Cerebral hemorrhage has been recognized as a major risk factor for symptomatic seizures throughout the lifespan (1). The association of high-grade IVH with seizure burden on the third day after birth is likely related to further extension of hemorrhages from the first two days after birth, rather than isolated high-grade hemorrhages suddenly emerging.
In contrast to the findings reported by Wikström, our analysis provides some suggestion that seizures in the first three days after birth may increase risks for poorer language development. The presence of any seizure activity was related to lower language scores, particularly after adjustment for social risk, which may represent a threshold effect of neuronal injury that can impact later outcomes. In contrast, the extent of seizure activity was not related to later outcomes. This is more challenging to interpret but is masked by infants with the most severe brain injury dying in the neonatal period and the impact of other factors on neurodevelopmental outcome. The factors that influence neurodevelopmental outcome are complex, heavily intertwined and occur both during the hospital course in the NICU as well as after discharge. Examples include the association between late-onset sepsis and necrotizing enterocolitis, both of which occur after our aEEG capture period (4/95 infants in this cohort), and poor neurodevelopmental outcome (26,27). Additionally, it is important to note that a number of participants either died or were lost to follow-up likely weakening the statistical association of seizures and neurodevelopmental outcome.
The importance of prompt treatment of seizures continues to be debated in the literature, despite animal model evidence showing deleterious effects of prolonged seizures (20). One must balance the apparent self-limited nature of seizures for the majority of infants in our cohort, the risks associated with indiscriminate treatment (28) and the potential long-term influence of the seizures on neurodevelopmental outcome. Furthermore, even for those infants with the most significant seizure burdens, our data suggest seizures are highly associated with underlying clinical instability, which may be better targeted to reduce morbidity or even seizure burden. This is further evidenced by the lack of impact of early administration of phenobarbital on the risk for IVH in preterm infants given to sedate infants and reduce “fighting on the ventilator” as a risk factor for IVH (29).
It is important to note that this study has several notable limitations. The reliability of aEEG recordings for accurate recognition of seizures has been previously investigated. Shellhaas et al. demonstrated in 2007 that neonatologists correctly identify 22–57% of known electrographic seizures using aEEG recordings (30). Similarly, Shah et al. noted that 27–56% of seizures were detectable using aEEG alone (12). The use of aEEG with simultaneous raw EEG analysis has an improved sensitivity of 76% (12). Automated algorithms using wave analysis have shown the potential for superior sensitivity to aEEG analysis with or without raw EEG trace analysis, with reported sensitivities of 45–88% (31), 83–95% (32), 96–99% (33). To our knowledge, no prior studies have evaluated the sensitivity of seizure detection using the combination of simultaneous analysis of aEEG and raw EEG traces and an automated seizure detection algorithm. Secondly, although there was a significant association between IVH and seizures, the lack of standardized CUS timing or a protocol to obtain imaging immediately at the onset of ictal discharges makes it impossible to decipher what is cause and what is effect. Furthermore, as the MRI data were obtained at term-equivalent age, it is difficult to say conclusively that abnormal findings were the result of acute injury in the immediate post-natal period rather than the result of chronic recurrent insults that frequently mark the hospital course of premature infants including sepsis, necrotizing enterocolitis or apnea and bradycardia of prematurity.
Our findings suggest that there may be a role for active cerebral monitoring in very preterm infants, particularly during this important early transition phase, with real-time simultaneous data capturing both the physiology of blood flow to the brain and monitoring of functional changes using aEEG. This may provide more insight into the inciting events of brain injury in the premature infant and allow for the developmental of more granular strategies to prevent adverse outcomes in a targeted population. Replication of this study with a larger sample followed to older ages is needed to full understand the effects of neonatal seizures on longer-term neurodevelopmental outcomes.
Acknowledgments
Statement of financial support: The work reported herein was supported by the National Institutes of Health (P30 HD062171 and R01 HD057098) and the Intellectual and Developmental Disabilities Research Center (IDDRC) at Washington University (NIH/NICHD P30 HD062171)
Footnotes
Disclosure: None of the authors of this manuscript have any conflicts of interest or financial disclosures to report
References
- 1.Scher MS, Aso K, Beggarly ME, Hamid MY, Steppe DA, Painter MJ. Electrographic seizures in preterm and full-term neonates: clinical correlates, associated brain lesions, and risk for neurologic sequelae. Pediatrics. 1993;91:128–34. [PubMed] [Google Scholar]
- 2.Hellström-Westas L, Rosén I, Svenningsen NW. Cerebral function monitoring during the first week of life in extremely small low birthweight (ESLBW) infants. Neuropediatrics. 1991;22:27–32. doi: 10.1055/s-2008-1071411. [DOI] [PubMed] [Google Scholar]
- 3.Shellhaas RA, Clancy RR. Characterization of neonatal seizures by conventional EEG and single-channel EEG. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2007;118:2156–61. doi: 10.1016/j.clinph.2007.06.061. [DOI] [PubMed] [Google Scholar]
- 4.Frenkel N, Friger M, Meledin I, et al. Neonatal seizure recognition--comparative study of continuous-amplitude integrated EEG versus short conventional EEG recordings. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2011;122:1091–7. doi: 10.1016/j.clinph.2010.09.028. [DOI] [PubMed] [Google Scholar]
- 5.Murray DM, Boylan GB, Ali I, Ryan CA, Murphy BP, Connolly S. Defining the gap between electrographic seizure burden, clinical expression and staff recognition of neonatal seizures. Arch Dis Child Fetal Neonatal Ed. 2008;93:F187–191. doi: 10.1136/adc.2005.086314. [DOI] [PubMed] [Google Scholar]
- 6.Eyre JA, Oozeer RC, Wilkinson AR. Diagnosis of neonatal seizure by continuous recording and rapid analysis of the electroencephalogram. Arch Dis Child. 1983;58:785–90. doi: 10.1136/adc.58.10.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Connell J, Oozeer R, de Vries L, Dubowitz LM, Dubowitz V. Continuous EEG monitoring of neonatal seizures: diagnostic and prognostic considerations. Arch Dis Child. 1989;64:452–8. doi: 10.1136/adc.64.4_spec_no.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hellström-Westas L, Rosén I, Swenningsen NW. Silent seizures in sick infants in early life. Diagnosis by continuous cerebral function monitoring. Acta Paediatr Scand. 1985;74:741–8. doi: 10.1111/j.1651-2227.1985.tb10024.x. [DOI] [PubMed] [Google Scholar]
- 9.Clancy RR, Legido A, Lewis D. Occult neonatal seizures. Epilepsia. 1988;29:256–61. doi: 10.1111/j.1528-1157.1988.tb03715.x. [DOI] [PubMed] [Google Scholar]
- 10.Davis AS, Hintz SR, Van Meurs KP, et al. Seizures in extremely low birth weight infants are associated with adverse outcome. J Pediatr. 2010;157:720–725. e1–2. doi: 10.1016/j.jpeds.2010.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shah DK, Zempel J, Barton T, Lukas K, Inder TE. Electrographic seizures in preterm infants during the first week of life are associated with cerebral injury. Pediatr Res. 2010;67:102–6. doi: 10.1203/PDR.0b013e3181bf5914. [DOI] [PubMed] [Google Scholar]
- 12.Shah DK, Mackay MT, Lavery S, et al. Accuracy of bedside electroencephalographic monitoring in comparison with simultaneous continuous conventional electroencephalography for seizure detection in term infants. Pediatrics. 2008;121:1146–54. doi: 10.1542/peds.2007-1839. [DOI] [PubMed] [Google Scholar]
- 13.Parry G, Tucker J, Tarnow-Mordi W. CRIB II: an update of the clinical risk index for babies score. Lancet. 2003;361:1789–91. doi: 10.1016/S0140-6736(03)13397-1. [DOI] [PubMed] [Google Scholar]
- 14.Whitehead NS. The relationship of socioeconomic status to preterm contractions and preterm delivery. Matern Child Health J. 2012;16:1645–56. doi: 10.1007/s10995-012-0948-4. [DOI] [PubMed] [Google Scholar]
- 15.Foster-Cohen SH, Friesen MD, Champion PR, Woodward LJ. High prevalence/low severity language delay in preschool children born very preterm. J Dev Behav Pediatr JDBP. 2010;31:658–67. doi: 10.1097/DBP.0b013e3181e5ab7e. [DOI] [PubMed] [Google Scholar]
- 16.Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. J Pediatr. 1978;92:529–34. doi: 10.1016/s0022-3476(78)80282-0. [DOI] [PubMed] [Google Scholar]
- 17.Wusthoff CJ. Diagnosing neonatal seizures and status epilepticus. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2013;30:115–21. doi: 10.1097/WNP.0b013e3182872932. [DOI] [PubMed] [Google Scholar]
- 18.Abend NS, Wusthoff CJ. Neonatal seizures and status epilepticus. J Clin Neurophysiol Off Publ Am Electroencephalogr Soc. 2012;29:441–8. doi: 10.1097/WNP.0b013e31826bd90d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wikström S, Pupp IH, Rosén I, et al. Early single-channel aEEG/EEG predicts outcome in very preterm infants. Acta Paediatr Oslo Nor 1992. 2012;101:719–26. doi: 10.1111/j.1651-2227.2012.02677.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Volpe JJ. Neurology of the newborn. 5. Philadelphia, PA: Saunders/Elsevier; 2008. [Google Scholar]
- 21.Perlman JM. Intrapartum hypoxic-ischemic cerebral injury and subsequent cerebral palsy: medicolegal issues. Pediatrics. 1997;99:851–9. doi: 10.1542/peds.99.6.851. [DOI] [PubMed] [Google Scholar]
- 22.Evans N, Kluckow M. Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed. 1996;75:F183–186. doi: 10.1136/fn.75.3.f183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O’Leary H, Gregas MC, Limperopoulos C, et al. Elevated cerebral pressure passivity is associated with prematurity-related intracranial hemorrhage. Pediatrics. 2009;124:302–9. doi: 10.1542/peds.2008-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jensen FE. Developmental factors regulating susceptibility to perinatal brain injury and seizures. Curr Opin Pediatr. 2006;18:628–33. doi: 10.1097/MOP.0b013e328010c536. [DOI] [PubMed] [Google Scholar]
- 25.Dommergues MA, Patkai J, Renauld JC, Evrard P, Gressens P. Proinflammatory cytokines and interleukin-9 exacerbate excitotoxic lesions of the newborn murine neopallium. Ann Neurol. 2000;47:54–63. [PubMed] [Google Scholar]
- 26.Hintz SR, Kendrick DE, Stoll BJ, et al. Neurodevelopmental and growth outcomes of extremely low birth weight infants after necrotizing enterocolitis. Pediatrics. 2005;115:696–703. doi: 10.1542/peds.2004-0569. [DOI] [PubMed] [Google Scholar]
- 27.Rees CM, Pierro A, Eaton S. Neurodevelopmental outcomes of neonates with medically and surgically treated necrotizing enterocolitis. Arch Dis Child Fetal Neonatal Ed. 2007;92:F193–198. doi: 10.1136/adc.2006.099929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farwell JR, Lee YJ, Hirtz DG, Sulzbacher SI, Ellenberg JH, Nelson KB. Phenobarbital for febrile seizures--effects on intelligence and on seizure recurrence. N Engl J Med. 1990;322:364–9. doi: 10.1056/NEJM199002083220604. [DOI] [PubMed] [Google Scholar]
- 29.Perlman JM, Volpe JJ. Prevention of neonatal intraventricular hemorrhage. Clin Neuropharmacol. 1987;10:126–42. doi: 10.1097/00002826-198704000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Shellhaas RA, Soaita AI, Clancy RR. Sensitivity of amplitude-integrated electroencephalography for neonatal seizure detection. Pediatrics. 2007;120:770–7. doi: 10.1542/peds.2007-0514. [DOI] [PubMed] [Google Scholar]
- 31.Gotman J, Flanagan D, Zhang J, Rosenblatt B. Automatic seizure detection in the newborn: methods and initial evaluation. Electroencephalogr Clin Neurophysiol. 1997;103:356–62. doi: 10.1016/s0013-4694(97)00003-9. [DOI] [PubMed] [Google Scholar]
- 32.Navakatikyan MA, Colditz PB, Burke CJ, Inder TE, Richmond J, Williams CE. Seizure detection algorithm for neonates based on wave-sequence analysis. Clin Neurophysiol Off J Int Fed Clin Neurophysiol. 2006;117:1190–203. doi: 10.1016/j.clinph.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 33.Liu A, Hahn JS, Heldt GP, Coen RW. Detection of neonatal seizures through computerized EEG analysis. Electroencephalogr Clin Neurophysiol. 1992;82:30–7. doi: 10.1016/0013-4694(92)90179-l. [DOI] [PubMed] [Google Scholar]