Abstract
The utility of allogeneic hematopoietic stem cell transplantation is limited by graft-versus-host disease (GVHD), a significant cause of morbidity and mortality. Patients with GVHD exhibit cutaneous manifestations with histological features of interface dermatitis followed by scleroderma-like changes. JAK inhibitors represent a class of immunomodulatory drugs that inhibit signaling by multiple cytokines. Herein we report the effects of tofacitinib in a murine model of GVHD. Oral administration of tofacitinib prevented GVHD-like disease manifested by weight loss and mucocutaneous lesions. More importantly, tofacitinib was also effective in reversing established disease. Tofacitinib diminished the expansion and activation of murine CD8 T cells in this model, and had similar effects on IL-2-stimulated human CD8 T cells. Tofacitinib also inhibited the expression of IFN-γ-inducible chemoattractants by keratinocytes, and IFN-γ-inducible cell death of keratinocytes. Tofacitinib may be an effective drug for treatment against CD8 T-cell–mediated mucocutaneous diseases in patients with GVHD.
Introduction
Allogeneic hematopoietic stem cell transplantation has revolutionized the treatment of an array of disorders ranging from malignancy, autoimmune diseases, and primary immunodeficiency syndromes (Ringden and Le Blanc, 2005; Ikehara, 2010; Roifman, 2010). However, its utility is principally limited by the morbidity and mortality associated with graft-versus-host disease (GVHD; Ferrara et al., 2009; Blazar et al., 2012). Although recent developments have reduced the incidence of GVHD, there are only a few effective treatment modalities.
GVHD is a complex disorder that can be divided into acute and chronic forms. Skin is the most frequently affected organ. The rash seen in acute GVHD patients can be maculopapular, blistered, or ulcerated, reflecting histological features characterized by liquefaction degeneration of the basal epidermal layer (Ferrara et al., 2009). GVHD is a primarily T-cell–mediated complication that results from the activation of donor-derived T cells with a resultant production of proinflammatory cytokines (Coghill et al., 2011; Toubai et al., 2012). Earlier studies suggested that CD4 T cells are highly pathogenic, whereas CD8 T cells seemed to be less important in disease pathogenesis (Palathumpat et al., 1995; Chen et al., 2007; Yi et al., 2008). However, one clinical study reported that depletion of CD8 T cells prevented GVHD after bone-marrow transplantation (Maraninchi et al., 1988).
A variety of murine models of GVHD have provided insight into immunopathogenic mechanisms and served as vehicles for testing potential therapies (Schroeder and DiPersio, 2011). To dissect the specific roles of CD8 T cells in GVHD and other autoimmune skin diseases, we generated a transgenic mouse that involves the expression of chicken ovalbumin (OVA) in skin and mucosal epithelium under control of the keratin-14 promoter (keratin 14 promoter-membrane OVA transgenic (K14-mOVA) mouse). After adoptive transfer of transgenic T cells (OT-I cells) that express a TCR for the CD8-epitope of OVA (SIINFEKL), recipient K14-mOVA mice develop erosive skin and mucosal lesions along with weight loss, clinically resembling GVHD (Shibaki et al., 2004; Miyagawa et al., 2010). Thus, our model better represents the mucocutaneous features of GVHD rather than of full-blown systemic GVHD.
As various cytokines activate the intracellular signaling cascade in JAK/signal transducer and activator of transcription pathway leading to enhanced transcription of a large number of immunologically important molecules, JAKs have been viewed as potential therapeutic targets for the development of a class of immunosuppressive drugs (Pesu et al., 2008; O'Shea and Plenge, 2012). Tofacitinib is a first-generation JAK inhibitor targeting JAK1 and JAK3 and to a lesser extent JAK2 (Karaman et al., 2008), and is now approved in the USA and Japan for use in moderate to severe rheumatoid arthritis, and is being studied for use in UC and psoriasis (Boy et al., 2009; Kremer et al., 2012; Sandborn et al., 2012; Ports et al., 2013). JAK3 is linked to the IL-2 receptor common γ-chain-signaling pathway and mediates the actions of IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21 (Johnston et al., 1994; Ghoreschi et al., 2009). JAK1 is associated with receptors of type I/II IFNs and the gp130 subunit–utilizing cytokines (Muller et al., 1993; Rodig et al., 1998). A previous study showed that pre-treatment of mice with tofacitinib limited immunopathology mediated by the adoptive transfer of semiallogeneic bone marrow and CD4 T cells via the suppression of both proliferation and IFN-γ production by donor CD4 T cells (Park et al., 2010). In the present study, we sought to determine the effect of tofacitinib in a model of GVHD that uses the adoptive transfer of CD8 T cells. Importantly, we found that tofacitinib not only prevents GVHD-like disease in this murine model but that it also reverses disease in mice that already have GVHD-like symptoms. Tofacitinib had direct inhibitory effects on CD8 T cells and on target keratinocytes in this murine model of mucocutaneous disease and differs from the semiallogeneic bone marrow and CD4 T-cell–transferred model. Our data suggest that treatment with tofacitinib may thus have a role in the prevention and treatment of GVHD.
Results
Tofacitinib prevents GVHD-like disease
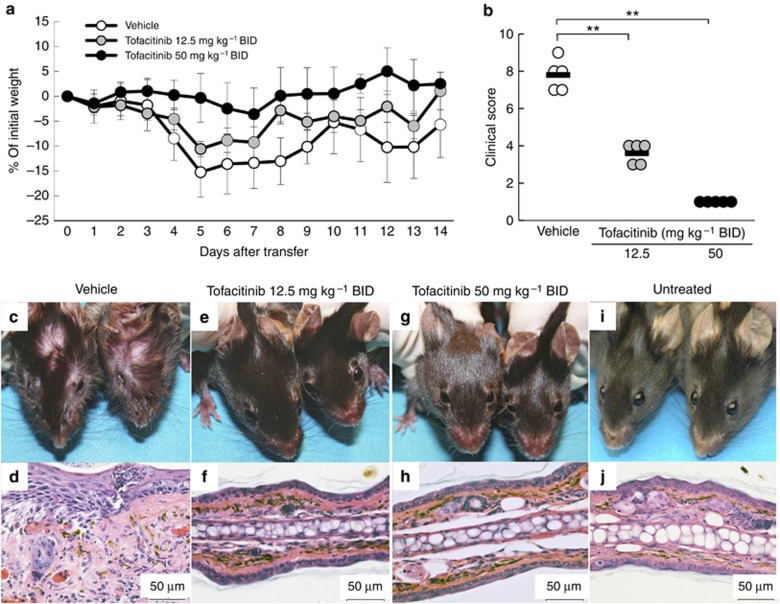
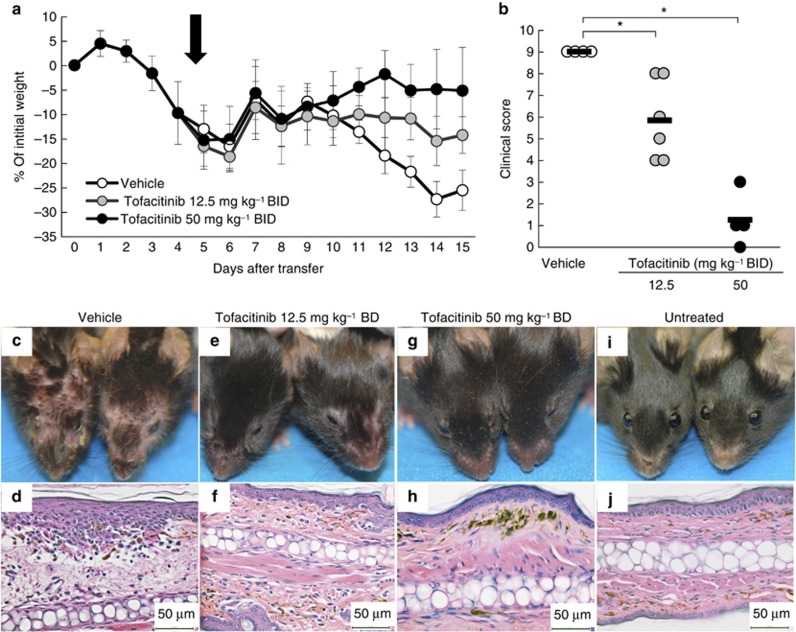
To determine whether tofacitinib prevented CD8 T-cell–mediated GVHD-like disease, K14-mOVA mice were treated with tofacitinib orally twice daily (bis in die (BID)) at 50 or 12.5 mg kg–1 beginning on the same day as OT-I cell transfer. As expected with this GVHD model, vehicle-treated control mice showed progressive weight loss starting 4 days after OT-I cell transfer and developed erosive skin and mucosal lesions. One mouse died in the vehicle-treated group. Although the higher dosage of tofacitinib (50 mg kg–1 BID) completely prevented weight loss (P<0.01; two-way analysis of variance (ANOVA) versus the vehicle group, Figure 1a), the lower dose (12.5 mg kg–1 BID) had only a partial effect on weight loss (P<0.01; two-way ANOVA vs. tofacitinib 50 mg kg–1 BID group and vehicle group, Figure 1a). Vehicle-treated mice developed crusted skin and mucosal lesions with histological changes including infiltrating inflammatory cells, exocytosis, and liquefaction degeneration of the basal epidermal layer 2 weeks after OT-I cell transfer (Figure 1c and d). Tofacitinib at both doses completely prevented the skin and mucosal lesions and the corresponding histological changes in the ears (Figure 1e–h).
Figure 1.
Tofacitinib prevents graft-versus-host disease (GVHD)-like disease. (a) The curves show the percentage of starting weight in mice treated with oral administration of tofacitinib daily at 50 or 12.5 mg kg–1 BID, or with vehicle control that was started on the same day as OT-I cell transfer (5–6 mice in each group). (b) Individual clinical scores of mice 14 days after OT-I cell transfer are plotted. The bars are mean scores of each group. **P<0.01, U-test. (c–i) Clinical and histological photographs of mice treated with vehicle control (c, d), or with tofacitinib daily at 12.5 (e, f) or 50 mg kg–1 BID (g, h) 14 days after OT-I cell transfer, and those of untreated mice (i, j). OT-I cells, transgenic T cells. Bar=50 μm.
Clinical scores assessed 2 weeks after OT-I transfer were also significantly reduced by tofacitinib in a dose-dependent manner (Figure 1b). The intermediate clinical scores in the group receiving tofacitinib 12.5 mg kg–1 BID are attributable to the significant weight loss that occurred in the absence of skin and mucosal lesions. Consistent with all of these findings, we found that the number of OT-I cells infiltrating the skin was markedly reduced when tofacitinib was given at either dosage (Supplementary Figure S1 online).
Tofacitinib inhibits OT-I cell activation and proliferation in skin-draining lymph nodes (SDLNs) of mice with GVHD-like disease
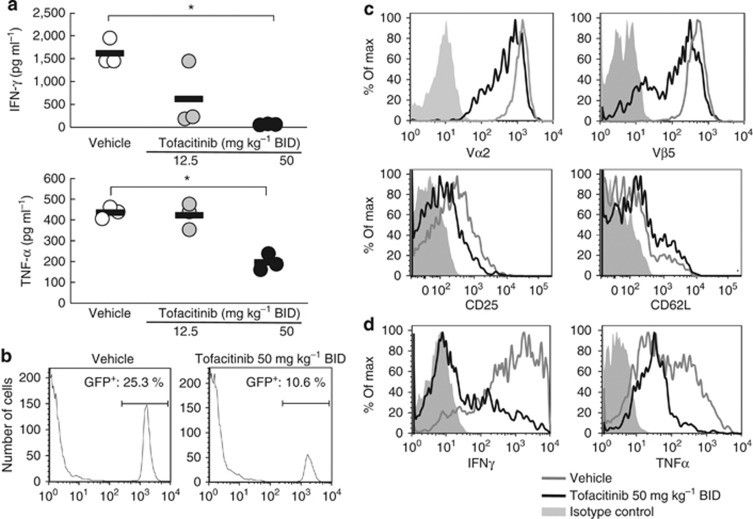
Serum levels for several cytokines, including IL-12, IL-4, IL-10, and GM-CSF, were not different in tofacitinib-treated mice as compared with vehicle-treated mice 5 days after OT-I cell transfer (data not shown), whereas serum levels of IFN-γ and tumor necrosis factor-α were reduced in tofacitinib-treated mice (Figure 2a). As OT-I cells in this murine model of GVHD are expected to be the main producers of IFN-γ and tumor necrosis factor-α, for tracking purposes we transferred green fluorescence protein transgenic+ (GFP+)OT-I cells into K14-mOVA mice that were treated with either tofacitinib 50 mg kg–1 BID or vehicle. We found that the frequencies of GFP+OT-I cells in the SDLNs from mice treated with tofacitinib 50 mg kg–1 BID were 50% less than those in vehicle-treated mice 4 days after GFP+OT-I cell transfer (Figure 2b). Total cell numbers in SDLNs were indistinguishable. Thus, tofacitinib selectively inhibited the proliferation of transferred OT-I cells.
Figure 2.
Tofacitinib inhibits OT-I cell activation in skin-draining lymph nodes (SDLNs) of mice with graft-versus-host disease (GVHD)-like disease. (a) The plots represent serum levels of IFN-γ and tumor necrosis factor (TNF)-α in mice treated with vehicle (white) or tofacitinib daily at 12.5 (grey) or 50 mg kg–1 BID (black) 5 days after cell transfer. The bars show means. *P<0.05, U-test. (b–d) FACS analysis of pooled SDLN cells taken 4 days after the transfer of green fluorescence protein transgenic+ (GFP+) OT-I cells into keratin 14 promoter-membrane OVA transgenic (K14-mOVA) mice (five mice in each group) show the proportions of recovered GFP+OT-I cells in SDLNs from vehicle-treated (left panel) or tofacitinib-treated (50 mg kg–1 ;right panel) mice (b). Expression of Vα2, Vβ5, CD25, and CD62L (c), and the production of IFN-γ and TNF-α (d) of GFP+OT-I cells in SDLNs from vehicle-treated mice or mice treated with tofacitinib 50 mg kg–1 BID. Data are representative of four separate experiments. OT-I cells, transgenic T cells.
Analysis of GFP-gated populations revealed reduced cell-surface expression of OVA-specific TCR (Vα2 and Vβ5) as well as CD25 and slightly enhanced the expression of CD62L on transferred OT-I cells in SDLNs from mice treated with tofacitinib 50 mg kg–1 BID compared with OT-I cells in vehicle-treated mice (Figure 2c). We also observed markedly reduced intracellular IFN-γ and tumor necrosis factor-α in the transferred OT-I cells in mice treated with tofacitinib 50 mg kg–1 BID versus vehicle-treated mice (Figure 2d). Taken together, these results indicate that tofacitinib impairs OT-I cell functions when administered beginning on the same day as the OT-I cell transfer.
Tofacitinib directly inhibits murine and human CD8 T-cell functions in vitro
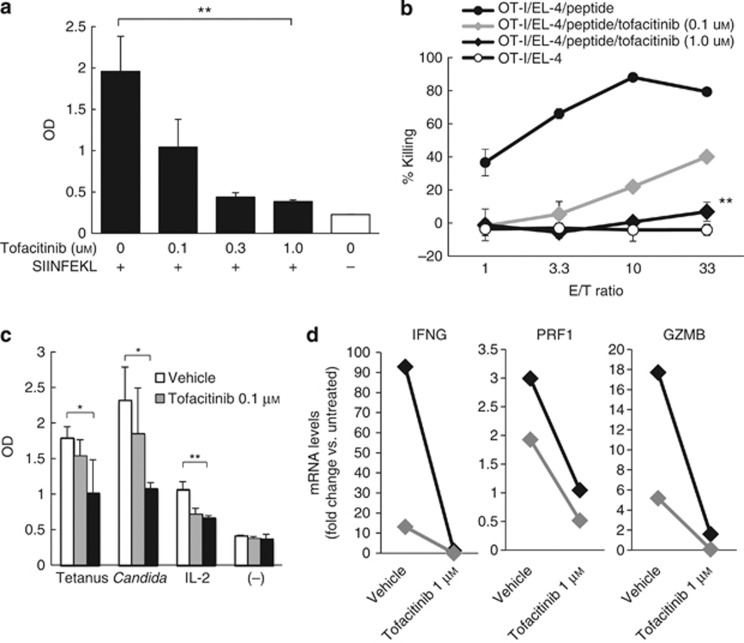
To determine whether tofacitinib had direct effects on OT-I cell proliferation, naive OT-I cells were co-cultured with SIINFEKL-pulsed B6 splenocytes. The addition of tofacitinib to culture wells inhibited the OT-I cell proliferation in a dose-dependent manner (Figure 3a). To assess the effects of tofacitinib on OT-I-mediated cytotoxicity, OT-I cells were stimulated with SIINFEKL-pulsed B6 splenocytes, IL-2, and IL-4 for 5 days with or without tofacitinib. The OT-I cells were subsequently cocultured with EL-4 target cells pulsed with a relevant peptide. We observed that activated OT-I cells were cytotoxic for the target EL-4 cells, but not for non-pulsed EL-4 cells. Tofacitinib inhibited the development of cytotoxic OT-I cells in a dose-dependent manner (Figure 3b).
Figure 3.
Tofacitinib inhibits CD8 T-cell activation in vitro in a dose-dependent manner. (a) WST-I assay was performed on OT-I cells stimulated with SIINFEKL in the presence of tofacitinib. The bars present OD means±SEM of three wells. (b) In the cytotoxicity assay using OT-I cells and SIINFEKL-pulsed EL-4, the curves show means±SEM (triplicate) of percentage killing of target cells by DMSO- and 0.1 or 1.0 μM tofacitinib treatment (black circles, gray or black diamonds) and the percentage killing of non-pulsed EL-4 (open circles). (c) The WST-I assay was performed on human CD8 T cells stimulated in various ways in the presence of tofacitinib. The bars present OD means±SEM (triplicate). (d) The quantitative real-time reverse-transcriptase–PCR array shows mRNA-fold changes for IL-2- and IL-2 plus tofacitinib-treated human CD8 T cells as compared with untreated cells. Black and gray diamonds represent separate experiments. All data represent duplicate experiments. *P<0.05, **P<0.01, t-test. GZMB, granzyme B; IFNG, IFN-γ OD, optical density; OT-I cells, transgenic T cells; PRF1, perforin.
Next, we performed in vitro assays using human peripheral CD8 T cells cocultured with tofacitinib. CD8 T cells purified from human blood were cultured with major histocompatibility complex (MHC) II+ peripheral blood cells pulsed with tetanus toxoid or Candida albicans protein, or with recombinant human IL-2. Human CD8 T cells proliferated vigorously in response to antigen-specific stimulation and to IL-2, and the proliferation was significantly inhibited by tofacitinib in a dose-dependent manner (Figure 3c). Quantitative real-time reverse-transcriptase–PCR arrays show that tofacitinib prevented the upregulation of mRNAs encoding IL-2-inducible and cytotoxic T-cell–produced activation markers, including IFN-γ (IFNG), perforin (PRF1), granzyme B (GZMB), and other molecules (Figure 3d, Supplementary Figure S2 online). These results suggested that tofacitinib inhibits the activation and proliferation of human and murine CD8 T cells.
Tofacitinib inhibits IFN-γ-induced activation and apoptosis of keratinocytes
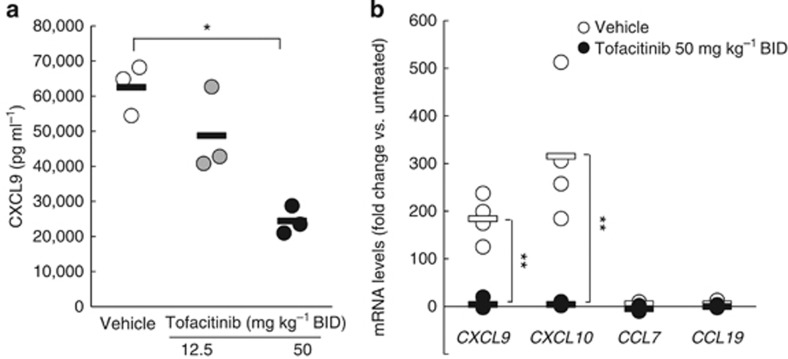
One of the most marked effects of tofacitinib administration in this GVHD model was the prevention of skin and mucosal lesions. Keratinocytes are the main components of the epidermis and secrete multiple chemokines, including CXCL9 and CXCL10, in response to IFN-γ from recruited immune cells such as CD8 T cells. In vivo studies demonstrated that serum levels of CXCL9, an IFNγ-inducible chemokine, were significantly reduced in tofacitinib-treated mice with GVHD-like disease in a dose-dependent manner (Figure 4a). Chemokine mRNA expression in ear epidermal keratinocytes of K14-mOVA mice 5 days after OT-I transfer was quantified using a quantitative real-time reverse-transcriptase–PCR array. The results normalized with internal control mRNAs are presented as fold-changes relative to those of mice without OT-I transfer. Quantitative real-time reverse-transcriptase–PCR revealed a markedly enhanced expression of IFN-γ-inducible chemokine mRNAs encoding CXCL9 and CXCL10 in vehicle-treated mice with GVHD-like disease, although non-IFN-γ-inducible chemokine mRNAs encoding CCL7 and CCL19 were unchanged. Tofacitinib 50 mg kg–1 BID treatment selectively inhibited the IFN-γ-inducible chemokine mRNA expression in the epidermis by >95% (Figure 4b).
Figure 4.
Tofacitinib inhibits IFN-γ-induced chemokine mRNA expression in keratinocytes in mice with graft-versus-host disease (GVHD)-like disease. (a) The plots represent serum levels of CXCL9 in K14-mOVA mice treated with vehicle (white), or with tofacitinib daily at 12.5 (gray), or with 50 mg kg–1 BID (black) 5 days after OT-I cell transfer. The bars show mean values. *P<0.05, U-test. (b) Quantitative real-time reverse-transcriptase–PCR (qRT-PCR) array for CXCL9, CXCL10, CCL7, and CCL19 mRNA expression was performed on RNA samples from ear epidermis of keratin 14 promoter-membrane OVA transgenic (K14-mOVA) mice treated with vehicle or tofacitinib daily at 50 mg kg–1 BID 5 days after OT-I cell transfer. Individual mRNA-fold changes for vehicle-treated mice (white) and tofacitinib (at 50 mg kg–1 BID)-treated mice (black) were compared with those of untreated mice. The bars express mean values. **P<0.01, U-test. OT-I cells, transgenic T cells.
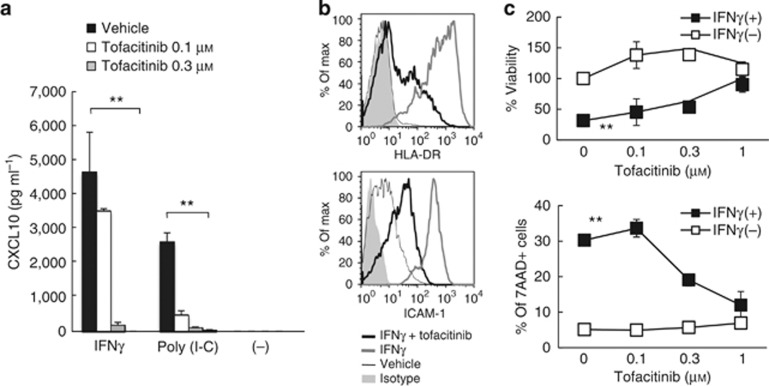
To determine whether tofacitinib has direct effects on keratinocytes, we incubated HaCaT cells, a human keratinocyte cell line, with IFN-γ or poly (I-C), a Toll-like receptor-3 ligand. We observed that tofacitinib inhibited CXCL10 production from IFN-γ-activated keratinocytes and from poly (I-C)-activated keratinocytes in a dose-dependent manner (Figure 5a). IFN-γ-activated HaCaT cells also upregulate human leukocyte antigen-DR and intercellular adhesion molecule-1 after a 24-hour stimulation. Upregulation was also inhibited by tofacitinib (Figure 5b). We also observed similar inhibitory effects of tofacitinib on poly (I-C)-activated HaCaT cells (data not shown). Taken together, we report that tofacitinib had effects on keratinocytes, limiting their activation by IFN-γ and a Toll-like receptor-3 ligand.
Figure 5.
Tofacitinib inhibits the activation and apoptosis of keratinocytes. (a) ELISA shows CXCL10 production by HaCaT cells stimulated with IFN-γ or poly (I-C) in the presence of tofacitinib. Bars express the mean±SEM of three wells. **P<0.01, t-test. (b) FACS analysis shows human leukocyte antigen-DR and intercellular adhesion molecule-1 (ICAM-1) expression on HaCaT cells treated with IFN-γ (gray lines), IFN-γ and tofacitinib (black lines), vehicle (fine black lines), and isotype control (gray solid). Data are representative of triplicate experiments. (c) The graphs show mean (three wells) of the percent viabilities of HaCaT cells (upper panel), which were calculated using the WST-I assay by comparing with non-treated cells, and percentage of 7AAD+ dead HaCaT cells (lower panel), when treated with IFN-γ and tofacitinib. **P<0.01, t-test.
It is well known that IFN-γ induces apoptotic cell death of keratinocytes. When compared with HaCaT cells treated with IFN-γ and vehicle, increasing doses of tofacitinib resulted in an augmented recovery of viable cells (Figure 5c). When viabilities were assessed by measuring the incorporation of the dye 7-AAD by flow cytometry, tofacitinib also inhibited IFN-γ-induced cell death (Figure 5c).
Tofacitinib effectively reverses GVHD-like disease
To determine whether tofacitinib was able to reverse established GVHD-like disease, we utilized the same animal model used in Figure 1, but drug administration was delayed until GVHD-like disease was evident. We initiated tofacitinib 50 or 12.5 mg kg–1 BID oral treatment 4 days after OT-I transfer, when all mice showed acute weight loss and skin and mucosal lesions. Tofacitinib 50 mg kg–1 BID treatment completely prevented the expected severe weight loss (P<0.01; two-way ANOVA vs. vehicle group), whereas the lower dose (tofacitinib 12.5 mg kg–1 BID) led to partial recovery (P<0.01 and P<0.05; two-way ANOVA vs. tofacitinib 50 mg kg–1 BID group and vehicle group, respectively; Figure 6a). Two of the six mice in the vehicle-treated group died, whereas none of the tofacitinib-treated mice died. Similarly, clinical scores assessed at 2 weeks after OT-I transfer were significantly reduced by tofacitinib in a dose-dependent manner (Figure 6b). Although the vehicle-treated mice developed severe skin and mucosal lesions with histological changes identical to those in Figure 1c and d (Figure 6c and d), mice treated with tofacitinib 50 mg kg–1 BID exhibited no skin or mucosal lesions, and mice treated with tofacitinib 12.5 mg kg–1 BID had only a few lesions near the eyes and nose (Figure 6e–h). From these experiments, we conclude that administration of tofacitinib can reverse established GVHD-like disease with complete normalization of both systemic and skin pathologies.
Figure 6.
Tofacitinib effectively reverses graft-versus-host disease (GVHD)-like disease. (a) Curves show the percentage of initial weight in mice treated with vehicle control, or oral tofacitinib at 12.5, or 50 mg kg–1 BID starting 4 days after OT-I cell transfer (arrow), the time of onset of GVHD-like disease. (b) Individual clinical scores of the mice 14 days after OT-I cell transfer are plotted. The bars are mean scores of each group. *P<0.05, U-test. (c–g) Clinical and histologic photographs of mice treated with vehicle control (c, d), or with tofacitinib daily (starting on day 4 after OT-I cell transfer) at 12.5 (e, f), or 50 mg kg–1 BID (g, h) 14 days after OT-I cell transfer, and those of untreated mice (i, j). OT-I cells, transgenic T cells. Bar=50 μm.
Discussion
In this study, we have shown that tofacitinib is effective not only in the prevention but also in the treatment of a CD8 T-cell–mediated murine model of GVHD via direct inhibition of CD8 T-cell proliferation and effector function. Furthermore, the JAK inhibitor blocked infiltration of CD8 OT-I cells and inhibited the proliferation and gene expression of cytotoxic molecules by human CD8 T cells in vitro. A previous study using blood from cynomolgus monkeys has shown that tofacitinib inhibited IL-15-induced CD69 expression in NK cells and also inhibited memory CD8 T-cell numbers (Conklyn et al., 2004). The effects of tofacitinib on activated CD8 T cells were not investigated. Previous studies using rodent disease models have focused mainly on CD4 T-cell–mediated autoimmune and/or inflammatory diseases. Tofacitinib attenuates semiallogenic CD4 T-cell–mediated GVHD (Park et al., 2010), Th1-mediated delayed-type hypersensitivity responses (Kudlacz et al., 2004), Th2-mediated pulmonary eosinophilia (Kudlacz et al., 2008), Th1- and/or Th17-mediated murine models of rheumatoid arthritis including collagen-induced arthritis (Milici et al., 2008; Tanaka et al., 2012), and Th17-mediated brain ischemia (Konoeda et al., 2010). Our results strongly suggest that tofacitinib may also be useful in the treatment of human CD8 T-cell–mediated diseases. Although the prevention and prophylaxis of GVHD with immunosuppressants is a strategy that is commonly used in patients, effective treatment of established GVHD remains an unmet need. Interestingly, as little as 1 week of tofacitinib administration prevented the weight loss and skin findings in mice that occur during the second week post adoptive transfer of OT-1 cells; hence, timing seems to be critical (Supplementary Figure S3 online). In the clinic, tofacitinib appears to have a good safety profile as an immunosuppressant, and for this reason tofacitinib could be used for this indication (Boy et al., 2009; Kremer et al., 2009; Sandborn et al., 2012; Ports et al., 2013).
In addition to effects on CD8 T cells, we demonstrated that tofacitinib inhibits IFN-γ-inducible chemokine production by keratinocytes in our murine model of GVHD and that tofacitinib directly inhibits IFN-γ and Toll-like receptor-3 stimulation–inducible production of chemokine (CXCL10) and expression of MHC class II and intercellular adhesion molecule-1 by human keratinocytes. The inhibitory effects of tofacitinib on keratinocytes suggest that tofacitinib may inhibit the recruitment of inflammatory cells to the skin. Perhaps related to the inhibitory effects of tofacitinib on IFN-γ-inducible apoptosis of HaCaT cells, treatment with tofacitinib completely inhibited the development of characteristic histological changes (liquefaction degeneration of the basal cell layers) that appear in GVHD. According to a ‘seed and soil' model, which proposes that effector T cells and target tissue cells work synergistically in the development of autoimmune diseases (Okiyama et al., 2012), tofacitinib has the significant advantage of inhibiting the activation of both the soil, keratinocytes, and the seeds, CD8 T cells.
Although we have no direct evidence that tofacitinib inhibits ‘interface dermatitis,' our results suggest that it may be effective in the treatment of IFN-γ-producing T-cell–mediated mucocutaneous diseases that exhibit histological changes with liquefaction degeneration, including those seen in skin lesions of acute GVHD, lupus erythematosus, dermatomyositis, Stevens–Johnson syndrome, and lichen planus (Sontheimer, 2009). A clinical trial of tofacitinib in psoriasis has shown tofacitinib to be efficacious: Psoriasis Area and Severity Index (PASI 75) response rates were significantly higher for tofacitinib twice-daily groups compared with placebo in a dose-dependent manner (Papp et al., 2012). Tofacitinib is thought to be effective in psoriasis via inhibition of CD4 T-cell activation with blockade of the JAK3 signaling pathway. Interestingly, topical tofacitinib has also recently been tested in psoriasis (Ports et al., 2013).
Although tofacitinib has effects on the signaling pathways of multiple cytokine receptors and on cells with various immunocyte lineages, we have focused on its effects on murine and human CD8 T-cell and keratinocyte activation and have shown its efficacy in CD8 T-cell–mediated GVHD-like disease. These results suggest that clinical trials of JAK inhibitors, including tofacitinib, in patients with acute GVHD merit strong consideration. In addition, the data suggest that JAK inhibitors may be useful in other diseases in which CD8 effectors are responsible for disease pathogenesis.
Materials and Methods
Mice
K14-mOVA mice were generated (Shibaki et al., 2004) by crossing Rag-1-deficient OT-I mice (Taconic, Hudson, NY) with human ubiqutin C promoter–GFP+ mice (The Jackson Laboratory, Bar Harbor, ME) and C57BL/6 (B6) mice to generate GFP+OT-I mice and OT-I mice, respectively. All animal studies were conducted with prior approval from the Animal Care and Use Committee of the NCI.
Cells
GFP+OT-I cells were purified from LNs of GFP+OT-I mice using CD8 columns (R&D Systems, Minneapolis, MN). Buffy coats were obtained from volunteers after informed consent and according to the institutional guidelines and the Declaration of Helsinki Principles, and CD8 T cells and MHC class II+ cells were purified by negative selection using a CD8 T-cell isolation kit and by positive selection using anti-MHC II microbeads (Miltenyi Biotec, Auburn, CA). HaCaT cells were kindly provided by Dr Stuart H Yuspa (NCI, NIH) and cultured in high-glucose DMEM (Life Technologies, Grand Island, NY) with 10% fetal bovine serum (Thermo, Wyman, MA), 1% GlutaMax (Life Technologies) with 20 ng ml–1 recombinant human IFN-γ (PeproTec, Rocky Hill, NJ) or 20 μg ml–1 poly (I-C) sodium salt (Sigma-Aldrich, St Louis, MO).
JAK inhibitor
Tofacitinib was provided by Pfizer Research Laboratories (Cambridge, MA) in 0.5% methylcellulose/0.025% Tween 20 (Sigma-Aldrich) for oral administration or in DMSO (Sigma-Aldrich) for in vitro use.
A murine model of GVHD
GFP+OT-I cells (1 × 106) were injected intravenously into K14-mOVA mice. The mice were given either vehicle control or varying doses of tofacitinib by gavage. Clinical scores were calculated for rash, alopecia, mucosal involvement, hunched appearance, and weight loss (Miyagawa et al., 2008).
Flow cytometry
SDLN cells from mice and cultured HaCaT cells were stained with phycoerythrin-conjugated anti-mouse Vβ5 (clone: MR9-4) and human CD54 (HA58), Per-CP5.5-conjugated anti-mouse CD8α (53-6.7) and mouse CD62L (MEL-14), allophycocyanin-conjugated anti-mouse Vα2 (B20.1), mouse CD25 (PC61), and human leukocyte antigen-DR (L243) monoclonal antibodies (BD Pharmingen, San Jose, CA). 7-AAD (BD Pharmingen) and LIVE/DEAD fixable violet dead-cell staining kit (Life Technologies) were used to detect dead cells. For intracellular cytokine staining, the SDLN cells were cultured in the presence of 2 μg ml–1 of SIINFEKL (Sigma-Aldrich) and 5 μg ml–1 of GolgiPlug (BD Pharmingen) for 5 hours, and then fixed and permeabilized in Cytofix/Cytoperm solution (BD Pharmingen). The cells were stained with phycoerythrin-conjugated anti-mouse IFN-γ (XMG1.2) or tumor necrosis factor-α (MP6-XT22) (BD Pharmingen). Isotype-matched antibodies were used as controls. Stained cells were analyzed on a LSR-II flow cytometer (BD Biosciences, San Jose, CA).
Serum cytokines and chemokines
Cytokine and chemokine levels in murine serum samples were measured with Bio-Plex ProTM Mouse Cytokine Th1/Th2 assay kits (Bio-Rad Laboratories, Hercules, CA).
Proliferation assay
Splenocytes from naive B6 mice were incubated with 1 μg ml–1 SIINFEKL and treated with 50 μg ml–1 mitomycin C (Sigma-Aldrich) for 1 hour. OT-I cells (2 × 105) were cultured with 2 × 105 SIINFEKL-pulsed splenocytes and tofacitinib in RPMI 1640 with 10% fetal bovine serum in 96-well flat-bottom plates. Human MHC class II+ cells were pulsed with 1 μg ml–1 Tetanus toxoid (Enzo Life Science, Farmingdale, NY) or 20 ng ml–1 Candida albicans protein (Fitzgerald Industries International, Acton, MA) for 2 hours, and then treated with mitomycin C. Human CD8 T cells (2 × 105) were cultured with the antigen-pulsed cells (2 × 105) or with 6 μg ml–1 recombinant human IL-2 (PeproTec) in RPMI 1640 with 10% human AB serum (Sigma-Aldrich) in 96-well flat-bottom plates for 2 days with tofacitinib. The WST-I assay was performed on the last day of culture (Clontech).
Cytotoxicity assay
Following previous articles (Khor et al., 2013; Miyagawa et al., 2013), splenocytes from OT-I mice were cultured with SIINFEKL, recombinant mouse IL-2 and IL-4 (PeproTech) in the presence of tofacitinib for 5 days. IFN-γ-stimulated and SIINFEKL-pulsed EL-4 cells (ATCC, Manassas, VA) were labeled with calcein AM fluorescence (Life Technologies). Effector OT-I cells were cocultured with 1.5 × 104 target EL-4 cells in calcium- and magnesium-free Hank's balanced salt solution with 5% fetal bovine serum in sealed 96-well round-bottom plates for 3 hours at 37 °C. Lysis buffer (50 mM sodium borate and 0.1% Triton X in distilled water) was used to induce maximum target cell lysis. The fluorescence release was measured on a fluorometer (excitation/emission=485 nm/535 nm). Percentage lysis was determined in the following manner: (experimental release−spontaneous release)÷(maximum release−spontaneous release) × 100.
Quantitative real-time reverse-transcriptase–PCR arrays
Human CD8 T cells were cultured with 6 μg ml–1 recombinant human IL-2 and tofacitinib for 2 days. mRNA was extracted from 1 × 107 cells using RNeasy Mini kits. To obtain epidermal sheets, mouse ears were incubated in 0.5 M ammonium thiocyanate (Sigma-Aldrich) for 20 min at 37 °C. mRNA was extracted from the epidermal sheets using RNeasy Fibrous Tissue kit (Qiagen, Gaithersburg, MD). Complementary DNA was synthesized from mRNA samples (40–600 ng) using an RT2 First Strand Kit (Qiagen). The complementary DNA samples mixed with RT2 SYBR Green PCR Master Mix were analyzed using RT2 Profiler PCR Arrays (Human T-Cell Anergy and Immune Tolerance and Mouse Inflammatory Cytokines and Receptors). All reagents were obtained from Qiagen.
Statistical analysis
Data were analyzed using a two-way ANOVA, the Mann–Whitney U-test, or a two-tailed Student's t-test. Values of P<0.05 were referred to as significant differences.
Acknowledgments
We thank Mark C Udey (NCI, NIH) for helpful discussions and Stuart H. Yuspa (NCI, NIH) and Pfizer Research Laboratories for materials.
Glossary
- BID
bis in die
- GFP
green fluorescence protein transgenic
- GVHD
graft-versus-host disease
- K14-mOVA
keratin 14 promoter-membrane OVA transgenic
- MHC
major histocompatibility complex
- OVA
chicken ovalbumin
- SDLN
skin-draining lymph node
YF, KG, MG, and JJO'S receive research funding from Pfizer under a Collaborative Research Agreement and Development Award. JJO'S receives royalties from a US Patent related to targeting JAKs.
Footnotes
SUPPLEMENTARY MATERIAL
Supplementary material is linked to the online version of the paper at http://www.nature.com/jid
Supplementary Material
References
- Blazar BR, Murphy WJ, Abedi M. Advances in graft-versus-host disease biology and therapy. Nat Rev Immunol. 2012;12:443–458. doi: 10.1038/nri3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boy MG, Wang C, Wilkinson BE, et al. Double-blind, placebo-controlled, dose-escalation study to evaluate the pharmacologic effect of CP-690,550 in patients with psoriasis. J Invest Dermatol. 2009;129:2299–2302. doi: 10.1038/jid.2009.25. [DOI] [PubMed] [Google Scholar]
- Chen X, Vodanovic-Jankovic S, Johnson B, et al. Absence of regulatory T-cell control of TH1 and TH17 cells is responsible for the autoimmune-mediated pathology in chronic graft-versus-host disease. Blood. 2007;110:3804–3813. doi: 10.1182/blood-2007-05-091074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coghill JM, Sarantopoulos S, Moran TP, et al. Effector CD4+ T cells, the cytokines they generate, and GVHD: something old and something new. Blood. 2011;117:3268–3276. doi: 10.1182/blood-2010-12-290403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conklyn M, Andresen C, Changelian P, et al. The JAK3 inhibitor CP-690550 selectively reduces NK and CD8+ cell numbers in cynomolgus monkey blood following chronic oral dosing. J Leukoc Biol. 2004;76:1248–1255. doi: 10.1189/jlb.0504282. [DOI] [PubMed] [Google Scholar]
- Ferrara JL, Levine JE, Reddy P, et al. Graft-versus-host disease. Lancet. 2009;373:1550–1561. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara S. The future of stem cell transplantation in autoimmune disease. Clin Rev Allergy Immunol. 2010;38:292–297. doi: 10.1007/s12016-009-8159-5. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Kawamura M, Kirken RA, et al. Phosphorylation and activation of the Jak-3 Janus kinase in response to interleukin-2. Nature. 1994;370:151–153. doi: 10.1038/370151a0. [DOI] [PubMed] [Google Scholar]
- Karaman MW, Herrgard S, Treiber DK, et al. A quantitative analysis of kinase inhibitor selectivity. Nat Biotechnol. 2008;26:127–132. doi: 10.1038/nbt1358. [DOI] [PubMed] [Google Scholar]
- Khor SS, Miyagawa T, Toyoda H, et al. Genome-wide association study of HLA-DQB1*06:02 negative essential hypersomnia. Peer J. 2013;1:e66. doi: 10.7717/peerj.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konoeda F, Shichita T, Yoshida H, et al. Therapeutic effect of IL-12/23 and their signaling pathway blockade on brain ischemia model. Biochem Biophys Res Commun. 2010;402:500–506. doi: 10.1016/j.bbrc.2010.10.058. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Bloom BJ, Breedveld FC, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60:1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- Kremer JM, Cohen S, Wilkinson BE, et al. A phase IIb dose-ranging study of the oral JAK inhibitor tofacitinib (CP-690,550) versus placebo in combination with background methotrexate in patients with active rheumatoid arthritis and an inadequate response to methotrexate alone. Arthritis Rheum. 2012;64:970–981. doi: 10.1002/art.33419. [DOI] [PubMed] [Google Scholar]
- Kudlacz E, Conklyn M, Andresen C, et al. The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia. Eur J Pharmacol. 2008;582:154–161. doi: 10.1016/j.ejphar.2007.12.024. [DOI] [PubMed] [Google Scholar]
- Kudlacz E, Perry B, Sawyer P, et al. The novel JAK-3 inhibitor CP-690550 is a potent immunosuppressive agent in various murine models. Am J Transplant. 2004;4:51–57. doi: 10.1046/j.1600-6143.2003.00281.x. [DOI] [PubMed] [Google Scholar]
- Maraninchi D, Mawas C, Guyotat D, et al. Selective depletion of marrow-T cytotoxic lymphocytes (CD8) in the prevention of graft-versus-host disease after allogeneic bone-marrow transplantation. Transplant Int. 1988;1:91–94. doi: 10.1007/BF00353826. [DOI] [PubMed] [Google Scholar]
- Milici AJ, Kudlacz EM, Audoly L, et al. Cartilage preservation by inhibition of Janus kinase 3 in two rodent models of rheumatoid arthritis. Arthritis Res Ther. 2008;10:R14. doi: 10.1186/ar2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa F, Gutermuth J, Zhang H, et al. The use of mouse models to better understand mechanisms of autoimmunity and tolerance. J Autoimmun. 2010;35:192–198. doi: 10.1016/j.jaut.2010.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa F, Okiyama N, Villarroel V, et al. Identification of CD3CD4CD8T cells as potential regulatory cells in an experimental murine model of graft vs. host skin disease (GvHD) J Invest Dermatol. 2013;133:2538–2545. doi: 10.1038/jid.2013.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyagawa F, Tagaya Y, Kim BS, et al. IL-15 serves as a costimulator in determining the activity of autoreactive CD8 T cells in an experimental mouse model of graft-versus-host-like disease. J Immunol. 2008;181:1109–1119. doi: 10.4049/jimmunol.181.2.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Briscoe J, Laxton C, et al. The protein tyrosine kinase JAK1 complements defects in interferon-alpha/beta and -gamma signal transduction. Nature. 1993;366:129–135. doi: 10.1038/366129a0. [DOI] [PubMed] [Google Scholar]
- O'Shea JJ, Plenge R. JAK and STAT signaling molecules in immunoregulation and immune-mediated disease. Immunity. 2012;36:542–550. doi: 10.1016/j.immuni.2012.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okiyama N, Sugihara T, Oida T, et al. T lymphocytes and muscle condition act like seeds and soil in a murine polymyositis model. Arthritis Rheum. 2012;64:3741–3749. doi: 10.1002/art.34629. [DOI] [PubMed] [Google Scholar]
- Palathumpat V, Dejbakhsh-Jones S, Strober S. The role of purified CD8+ T cells in graft-versus-leukemia activity and engraftment after allogeneic bone marrow transplantation. Transplantation. 1995;60:355–361. doi: 10.1097/00007890-199508270-00010. [DOI] [PubMed] [Google Scholar]
- Papp KA, Menter A, Strober B, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol. 2012;167:668–677. doi: 10.1111/j.1365-2133.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- Park HB, Oh K, Garmaa N, et al. CP-690550, a Janus kinase inhibitor, suppresses CD4+ T-cell-mediated acute graft-versus-host disease by inhibiting the interferon-gamma pathway. Transplantation. 2010;90:825–835. doi: 10.1097/TP.0b013e3181f24e59. [DOI] [PubMed] [Google Scholar]
- Pesu M, Laurence A, Kishore N, et al. Therapeutic targeting of Janus kinases. Immunol Rev. 2008;223:132–142. doi: 10.1111/j.1600-065X.2008.00644.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ports WC, Khan S, Lan S, et al. A randomised Phase 2a efficacy and safety trial of the topical Janus kinase inhibitor tofacitinib in the treatment of chronic plaque psoriasis. Br J Dermatol. 2013;169:137–145. doi: 10.1111/bjd.12266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringden O, Le Blanc K. Allogeneic hematopoietic stem cell transplantation: state of the art and new perspectives. APMIS. 2005;113:813–830. doi: 10.1111/j.1600-0463.2005.apm_336.x. [DOI] [PubMed] [Google Scholar]
- Rodig SJ, Meraz MA, White JM, et al. Disruption of the Jak1 gene demonstrates obligatory and nonredundant roles of the Jaks in cytokine-induced biologic responses. Cell. 1998;93:373–383. doi: 10.1016/s0092-8674(00)81166-6. [DOI] [PubMed] [Google Scholar]
- Roifman CM. Hematopoietic stem cell transplantation for profound T-cell deficiency (combined immunodeficiency) Immunol Allergy Clin North Am. 2010;30:209–219. doi: 10.1016/j.iac.2010.03.001. [DOI] [PubMed] [Google Scholar]
- Sandborn WJ, Ghosh S, Panes J, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367:616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- Schroeder MA, DiPersio JF. Mouse models of graft-versus-host disease: advances and limitations. Dis Model Mech. 2011;4:318–333. doi: 10.1242/dmm.006668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibaki A, Sato A, Vogel JC, et al. Induction of GVHD-like skin disease by passively transferred CD8(+) T-cell receptor transgenic T cells into keratin 14-ovalbumin transgenic mice. J Invest Dermatol. 2004;123:109–115. doi: 10.1111/j.0022-202X.2004.22701.x. [DOI] [PubMed] [Google Scholar]
- Sontheimer RD. Lichenoid tissue reaction/interface dermatitis: clinical and histological perspectives. J Invest Dermatol. 2009;129:1088–1099. doi: 10.1038/sj.jid.2009.42. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Maeshima Y, Yamaoka K. In vitro and in vivo analysis of a JAK inhibitor in rheumatoid arthritis. Ann Rheum Dis. 2012;71 Suppl 2:i70–i74. doi: 10.1136/annrheumdis-2011-200595. [DOI] [PubMed] [Google Scholar]
- Toubai T, Tanaka J, Paczesny S, et al. Role of cytokines in the pathophysiology of acute graft-versus-host disease (GVHD): are serum/plasma cytokines potential biomarkers for diagnosis of acute GVHD following allogeneic hematopoietic cell transplantation (Allo-HCT) Curr Stem Cell Res Ther. 2012;7:229–239. doi: 10.2174/157488812799859856. [DOI] [PubMed] [Google Scholar]
- Yi T, Zhao D, Lin CL, et al. Absence of donor Th17 leads to augmented Th1 differentiation and exacerbated acute graft-versus-host disease. Blood. 2008;112:2101–2110. doi: 10.1182/blood-2007-12-126987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.