Abstract
A number of recent studies from as diverse fields as plant–pollinator interactions, analyses of caffeine as an environmental pollutant, and the ability of caffeine to provide protection against neurodegenerative diseases have generated interest in understanding the actions of caffeine in invertebrates. This review summarizes what is currently known about the effects of caffeine on behavior and its molecular mechanisms in invertebrates. Caffeine appears to have similar effects on locomotion and sleep in both invertebrates and mammals. Furthermore, as in mammals, caffeine appears to have complex effects on learning and memory. However, the underlying mechanisms for these effects may differ between invertebrates and vertebrates. While caffeine’s ability to cause release of intracellular calcium stores via ryanodine receptors and its actions as a phosphodiesterase inhibitor have been clearly established in invertebrates, its ability to interact with invertebrate adenosine receptors remains an important open question. Initial studies in insects and mollusks suggest an interaction between caffeine and the dopamine signaling pathway; more work needs to be done to understand the mechanisms by which caffeine influences signaling via biogenic amines. As of yet, little is known about whether other actions of caffeine in vertebrates, such as its effects on GABAA and glycine receptors, are conserved. Furthermore, the pharmacokinetics of caffeine remains to be elucidated. Overall behavioral responses to caffeine appear to be conserved amongst organisms; however, we are just beginning to understand the mechanisms underlying its effects across animal phyla.
Keywords: Drosophila, Honey bee, Insect, Methylxanthine, Adenosine receptor, Ryanodine receptor
Introduction
Caffeine may be the most widely consumed neuroactive compound on the planet, and it might not just be humans using it for a buzz. Caffeine can be synthesized by a number of species of plants, including those used to make coffee, tea, and maté [1]. Compounds such as caffeine and nicotine in plants have generally been regarded as defense mechanisms to reduce damage from herbivores. Thus, it is somewhat surprising to discover that caffeine also appears in the nectar from a number of species of plants [2–4], and recent work suggests plants may be using caffeine to manipulate the behavior of their invertebrate pollinators [4, 5]. These results suggest complex interactions between the caffeine produced by plants and the invertebrates that interact with them that may affect pollination levels and influence the coevolution of plants, pollinators, and herbivores.
Given its popularity, it is not surprising that caffeine occurs as a significant pollutant in wastewater. Even after sewage treatment, caffeine may be released into aquatic environments and present in the sludge, which may then contaminate soils via its use as a fertilizer [6, 7]. Although these concentrations are generally low, in the nanomolar range for treated effluent, little is known about the chronic effects of caffeine on aquatic invertebrates. Recent studies using environmentally relevant concentrations have shown that chronic caffeine exposure may be deleterious. Caffeine treatment leads to upregulation of Hsp-70 in the mussel Mytilus californianus [8] and to destabilization of lysosomal membranes in the clam Ruditapes philippinarum and the crab Carcinus maenas [9, 10], two indicators of cellular stress. Increasing our knowledge of how caffeine affects aquatic organisms, especially chronic exposure, is important for assessing the risks associated with caffeine contamination of the environment.
In addition, a number of studies show that caffeine consumption provides protection against neurodegenerative diseases, such as Parkinson’s and Alzheimer’s, as well as dementia [11–14]. However, little is known about the molecular mechanisms by which caffeine is providing protection. Invertebrate models have provided invaluable insight on the mechanisms through which drugs such as ethanol and cocaine affect the nervous system (for recent reviews see [15, 16]). For example, forward genetic approaches in the nematode, Caenorhabditis elegans, and the fruit fly, Drosophila melanogaster, have identified new targets for the actions of ethanol that were then verified as involved in responses to ethanol in mammals [17–19]. In addition, reverse genetic approaches using data from human or mouse genome-wide studies have shown that genes of interest can be studied in the simpler systems provided by invertebrates [20, 21]. A greater understanding of the mechanisms by which caffeine acts in invertebrates would allow the extensive genetic, behavioral and neurophysiological tools available in invertebrates to be used to examine the relationship between caffeine and neurodegeneration.
Thus, improving our understanding of the actions of caffeine is of growing interest from both an ecological and health perspective. The goal of this review is to provide an overview of what is known about the effects of caffeine on invertebrates and to highlight current questions.
Effects of caffeine on invertebrate behavior
Locomotion and sleep
In mammals, where its actions have been extensively studied in humans and rodent models, caffeine consumption is associated with increases in activity and alertness. Similarly, caffeine has been shown to increase locomotor activity in a variety of insects including hornets, Vespa orientalis [22], honey bees, Apis mellifera [22], the green scale insect, Coccus viridis [23] and flour beetles, Tribolium castaneum and Tribolium confusum [24, 25]. However, caffeine was also shown to inhibit swimming behavior in the jellyfish, Aurelia aurita [26], although the concentrations used in this case were very high.
The most detailed studies of caffeine on locomotion come from the analysis of its effects in the fruit fly where caffeine acts to increase activity, disrupt sleep patterns, and increase the amount of time flies spend awake [27–31]. Sleep behavior in flies has many of the characteristics of sleep in mammals including circadian and homeostatic regulation, and rebound effects after sleep deprivation [28, 30]. Both chronic and acute exposure to caffeine lead to a dose-dependent decrease in amount of time flies spent asleep during the night [27, 31]. Sleeping flies that have consumed caffeine are more likely to be woken by mechanical stimulation, suggesting that caffeine consumption leads to a higher level of arousal [31]. Furthermore, chronic consumption of caffeine led to a lengthening of the circadian period [31]. These results are consistent with the effects of caffeine consumption in humans, which can reduce the amount of time spent sleeping and affect how much time is spent in different sleep stages [32]. Indeed, the parallels between the effects of caffeine on sleep in mammals and the “sleep-like” behavior in flies was used to argue that a similar mechanism underlies sleep in flies and humans, and adds support to the idea that sleep is an important behavior conserved across animal phyla.
Learning and memory
In humans, whether or not caffeine acts as an actual cognitive enhancer beyond simply increasing alertness is unclear [33, 34]. However, there is clear evidence that caffeine affects learning and memory in invertebrates. When Drosophila are exposed to caffeine in their food for 20 h or more before conditioning, there is a significant reduction in their ability to associate a visual cue with an aversive stimulus [35, 36]. Similarly, honey bees fed an acute dose of caffeine, either in the reward solution during conditioning or prior to conditioning, showed a reduced level of acquisition during appetitive olfactory conditioning. However, the caffeine did not affect levels of recall during a 24-h memory test, suggesting that the honey bees had actually learned the association between odor and reward, even though they did not respond during the conditioning phase [37]. In contrast, a number of studies also suggest that caffeine increases learning in invertebrates. Honey bees fed caffeine in the reward solution performed better during massed olfactory training than those fed sucrose alone [4]. In addition, honey bees given caffeine performed better at visual learning using a delayed-match-to-sample learning paradigm [38]. In the common snail, Helix lucorum, injection of caffeine, either before or after a conditioning trial, increased the number of positive responses (closing of the pneumostoma) to the conditioned stimulus (tapping on the shell) during training such that the rate of acquisition was increased in snails given caffeine [39].
What might account for these different effects of caffeine on learning? One possible explanation is differences in the concentration of caffeine used. The experiments that resulted in a negative effect of caffeine on learning [35–37] used relatively high concentrations (10–50 mM) of caffeine, whereas the studies that showed positive effects on learning [4, 39] used lower concentrations (0.1–100 μM). In the visual learning experiments done in honey bees [38], a dose of 100 mM caffeine was given via the use of dimethylformamide to carry the compound across the cuticle, but the proportion of caffeine that actually crossed into the hemolymph is unknown making it difficult to compare these results. Another interesting factor is that, at least in honey bees, the studies that revealed a positive effect of caffeine were more difficult tasks. Difficult tasks are those that generally require more training trials to achieve the same level of response. For example, consumption of 100 μM caffeine in the reward during spaced training had no effect on learning whereas caffeine at this concentration enhanced learning during massed conditioning [4, 37]. Similarly, caffeine had no effect on learning during a Y-maze visual learning task, but the same concentration was shown to increase learning during the more difficult delayed-match-to-sample task [38]. This might suggest that the effects of caffeine on learning are somewhat subtle, and that more difficult learning tasks may be required to reveal caffeine’s influence.
There is also evidence that caffeine enhances recall of learned associations. Honey bees fed caffeine in the reward solution during massed olfactory training performed better on 24-h recall tests than those fed sucrose alone [4]. Furthermore, honey bees given caffeine and conditioned to a visual task using a Y-maze had better long-term memory recall [38]. Work from Perisse et al. [40] suggests that calcium signaling might play an important role in caffeine’s effect on long-term memory. In the honey bee, a single pairing of an odor with a sucrose reward does not produce a robust long-term memory. However, bees injected with caffeine before conditioning showed significantly higher levels of recall for a long-term memory task than control bees. Interestingly, this effect was mimicked by the uncaging of calcium just prior to conditioning, suggesting that caffeine’s effects on intracellular calcium may play a significant role in enhancing memory [40].
What are the molecular mechanisms through which caffeine acts?
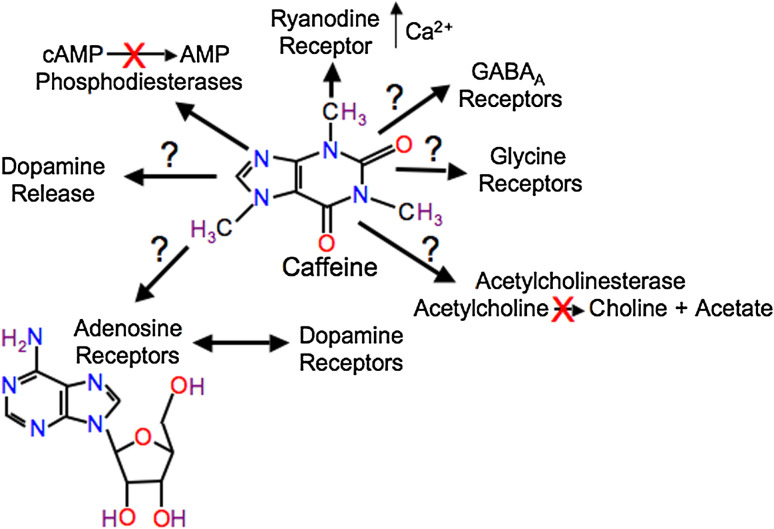
In Drosophila, increases in the concentration of cAMP were observed in the brain in response to caffeine consumption [36] or when caffeine was directly applied to the brain [31]. In addition, direct application of caffeine to the brains of honey bees results in an increase in intracellular calcium levels [40, 41]. However, exactly how caffeine causes increases in cAMP and calcium in invertebrates is still unknown. In vertebrate systems, caffeine has been shown to act through several mechanisms including increasing cAMP through the inhibition of phosphodiesterases, increasing intracellular calcium levels via release of intracellular stores through ryanodine receptors, and as an antagonist at adenosine receptors (Fig. 1). Because caffeine acts as an antagonist at A1 and A2A receptors at much lower concentrations than its other targets, it is expected that interactions with adenosine receptors is the main mechanism for caffeine’s action in mammals [42]. The mechanisms of caffeine’s action in invertebrates are much less clear.
Fig. 1.
The known actions of caffeine in vertebrates include: interaction with ryanodine receptors leading to intracellular calcium release; inhibition of phosphodiesterases leading to increased levels of cAMP or cGMP; inhibition of adenosine receptors; release of dopamine and the modulation of dopamine signaling via interaction between adenosine receptors and dopamine receptors; inhibition of the ligand-gated chloride channel GABAA and glycine receptors; and inhibition of acetylcholinesterase. Only two of these mechanisms, interaction with ryanodine receptors and inhibition of phosphodiesterases, have been confirmed in invertebrates
Binding of caffeine to ryanodine receptors
Ryanodine receptors are ligand-gated calcium channels that play important regulatory roles in many processes by releasing calcium from intracellular stores. Binding of caffeine to the receptor increases the affinity of the receptor for Ca2+, leading to activation of the channel at lower Ca2+ levels [43]. Whereas mammals have three genes encoding ryanodine receptors with distinct expression patterns and functional characteristics, invertebrates appear to only have one ryanodine receptor gene [44, 45]. Studies in nematodes [46], insects [47–49], mollusks [50], sea urchins [45], and crustaceans [51, 52] have shown that caffeine also interacts with invertebrate ryanodine receptors to release intracellular calcium stores. The concentrations of caffeine used in these studies are in the millimolar range (0.5–30 mM), similar to the concentrations of caffeine needed for calcium release via ryanodine receptors in mammals [42].
Caffeine inhibits phosphodiesterases
Caffeine also works as an inhibitor of phosphodiesterases. Cyclic nucleotide phosphodiesterases (PDE) act to regulate cGMP and cAMP levels by hydrolyzing them to 5′-GMP or 5′-AMP, respectively. Both mammals and invertebrates have multiple genes for PDEs, and post-transcriptional mechanisms lead to a variety of protein isoforms. Perhaps the best-known member of this protein family is the cAMP PDE II encoded by the dunce gene in Drosophila [53]. Given the well-established role of the Dunce protein in learning [54], it might be expected that as an inhibitor of PDEs, caffeine would also affect learning. In fruit flies, treatment with the PDE inhibitor IBMX mimics the effects of caffeine on sleep, and blocking cAMP signaling prevents caffeine from having an effect [31]. Studies on purified enzymes and cell homogenates demonstrated that caffeine acts as a competitive inhibitor of PDEs from a number of insects [55–62]. Similar to the case with the ryanodine receptors, the concentration required for 50 % inhibition of PDE activity by caffeine was in the millimolar range, from 0.5 to 10 mM. Although it is difficult to determine which specific form of PDE was being assayed in these studies, the overall trend suggests that concentrations above 0.5 mM would be required for significant inhibition of PDEs by caffeine.
Does caffeine act at invertebrate adenosine receptors?
Antagonism of adenosine receptors is believed to be the main molecular pathway for the effects of caffeine in mammals, as caffeine’s affinity for adenosine receptors suggests it may affect adenosine receptors at biologically relevant concentrations. For comparison, the caffeine concentration to which human neural tissues are exposed after consumption of a cup of coffee is estimated to be in the range of 1–10 μM [42], a much lower level than the caffeine concentrations associated with inhibition of phosphodiesterases or release of calcium via ryanodine receptors. Several adenosine receptor genes have been cloned from vertebrates with the receptors falling into four receptor families: A1, A2A, A2B, and A3. Caffeine binds with K D values in the micromolar range to all four of the human adenosine receptors, although the A1 and A2A receptors are the most likely targets for the actions of caffeine [42]. The A1 and A3 receptors are coupled to Gi leading to a reduction in cAMP levels, whereas the A2A and A2B receptors interact with Gs leading to the activation of adenylyl cyclase. A number of other G proteins may interact with the adenosine receptors as well leading to tissue-specific signaling cascades [63].
A number of pharmacological studies suggest that caffeine also interacts with adenosine receptors in invertebrates. Antagonists of mammalian A1 and A2 adenosine receptors mimic the behavioral effects of caffeine on sleep in Drosophila [27], whereas an A1 receptor agonist acts in the opposite manner as caffeine by increasing the amount of time flies spend sleeping [28]. Whole-cell recordings from honey bee Kenyon cells in the mushroom bodies also showed that the effects of caffeine were mimicked by a mammalian adenosine receptor antagonist [4]. In the mussel, Mytilus californianus, treatment with caffeine causes rounding up and a loss in the adhesion of the hemocytes that hold the mussel to the substratum; this effect of caffeine was reduced by the addition of adenosine or A2 receptor agonists, suggesting that the loss of adhesion was due to the interaction between caffeine and an adenosine receptor [64].
To date, adenosine receptors have only been cloned and functionally characterized from two invertebrates: the DmelAdoR receptor from Drosophila [31, 65] and the PminAdoR receptor from starfish [66]. Neither of these receptors have sequences that are closely conserved with the vertebrate adenosine receptor families (Fig. 2), with the vertebrate A2 receptor family being the most closely related to both the fly and starfish receptors. Interestingly, the fly and starfish receptors also share little sequence similarity between them (BLAST E value = 10−44, with a max identity of 36 %). When heterologously expressed in a Chinese hamster ovary cell line, stimulation of DmelAdoR with adenosine leads to increased levels of cAMP and Ca2+ [65]. However, the DmelAdoR receptor is endogenously expressed in a Drosophila neuroblast Bg2-c2 cell line, and treatment of these cells with adenosine leads to an increase in cAMP, but not calcium levels [67]. Thus, it is unclear whether the DmelAdoR receptor signals via Ca2+ under native conditions. Interestingly, in Bg2-c2 cells, caffeine does not antagonize the adenosine-dependent increase in cAMP [67]; furthermore, caffeine still affects sleep behavior in DmAdoR knockout flies [31]. In contrast to the pharmacological studies, these results suggest that caffeine may not act through adenosine receptors in invertebrates. One possible explanation for the conflicting results between the pharmacological and DmelAdoR studies is that another adenosine, or other type of caffeine sensitive receptor, is present in invertebrates. Further support for there being another adenosine receptor type in invertebrates comes from studies on the actions of adenosine in pond snail [68] and blowfly [69] where the pharmacology of the adenosine receptors involved does not match the pharmacological profile for the DmelAdoR receptor [67].
Fig. 2.
Invertebrate adenosine receptors share only low levels of similarity with their vertebrate counterparts. The amino acid sequences of the two functionally characterized invertebrate adenosine receptors (fruit fly, DmelAdoR, NM_001276172; starfish, PminAdoR, AF521907) are compared to the sequences of adenosine receptors from human (HsapA1, X68485; HsapA2a, X68486; HsapA2b, X68487; HsapA3, L22607), mouse (MmusA1, NM_001008533; MmusA2a, NM_009630; MmusA2b, NM_007413; MmusA3, NM_009631), chicken (GgalA1, U28380; GgalA2B, AY169692; GgalA3, AF115332) and zebrafish (DrerA1, XM_001345932; DrerA2a, AY945800; DrerA2b, AY945802; DrerA3, XM_694994). The sequence of the human M1 muscarinic receptor (HsapMus1, X52068) was used to root the tree. The phylogenetic tree was generated using ClustalW2 [91]
Interactions between caffeine and dopamine signaling
In mammals, caffeine has been shown to interact with the dopamine signaling pathway via three mechanisms: direct interactions between dopamine receptors and adenosine receptors, convergence of dopamine signaling pathways and adenosine signaling pathways on common second messengers (such as cAMP), and treatment with caffeine leading to dopamine release in the brain (for reviews see [70, 71]). Although little is known about the interaction between caffeine and dopamine signaling in invertebrates, several studies suggest that caffeine may act to influence dopamine signaling via one or more of these mechanisms. In the central ganglion of the pond snail, Planorbis corneus, caffeine potentiated the inhibitory postsynaptic potentials produced by a dopaminergic neuron [72]. This effect was mimicked by treatment with dibutyryl cAMP, suggesting that this effect was due to caffeine’s ability to raise cAMP levels, which could be due to its action as a phosphodiesterase inhibitor, its antagonism of adenosine receptors, or via other mechanisms. Caffeine treatment of honey bees led to an increase in mRNA expression levels of a D2-like dopamine receptor [73], suggesting an interaction between caffeine and dopamine receptors. In addition, expression of a D1-like dopamine receptor is necessary for the reduction in sleep due to caffeine consumption in Drosophila [27]. Taken together, these results support the hypothesis that caffeine interacts with the dopamine signaling pathway in invertebrates, but more studies are necessary to determine the underlying mechanisms.
Other possible mechanisms
Several other actions for caffeine have been proposed including the blockade of GABAA receptors [74–76], acting as a competitive inhibitor of glycine receptors [76, 77] and inhibition of acetylcholinesterase [78]. However, little information about the interactions between these targets and caffeine is known for invertebrates.
Caffeine pharmacokinetics
There is a dearth of information on the absorption and metabolism of caffeine in invertebrates. In mammals, caffeine is rapidly absorbed from the stomach and the gut, and it moves freely into fluids and tissues, including passage through the blood–brain barrier and the placenta [79]. Very little caffeine is excreted unchanged, instead it is metabolized to a number of different compounds, including theobromine, theophylline, and paraxanthine [79]. The ratio of the compounds into which caffeine is converted varies greatly even amongst mammals [80]. This is of particular interest as several of these metabolites are also biologically active, for example theophylline is a more potent inhibitor of mammalian adenosine receptors than caffeine [42]. Thus, determining the metabolic pathways for caffeine is necessary for a true understanding of its actions in invertebrates. A few steps in this direction have been made. In mammals, caffeine metabolism is mainly dependent on the cytochrome P450 family member CYP1A2 [81]. Initial studies in Drosophila [82, 83] and honey bees [73] show that genes for members of the cytochrome P450 family (CYP proteins) are upregulated in the presence of caffeine. In general, studies in invertebrates have assumed that caffeine will also move throughout the tissues, including passage into the brain. However, information regarding the absorption, tissue distribution, and metabolites of caffeine in invertebrates represents a significant gap in our understanding.
Future considerations
Clearly, one of the most important outstanding questions on the actions of caffeine in invertebrates is the role of adenosine receptors. Does caffeine act as an antagonist at invertebrate adenosine receptors? While the overall functions of G protein coupled receptors for particular ligands are well conserved between orthologous mammalian and invertebrate receptors, their pharmacological profiles can differ significantly (for example, see [84]). Thus, it is not completely unexpected that caffeine may not affect the DmelAdoR receptor, which is the only invertebrate adenosine receptor to be pharmacologically characterized so far. Given that a number of studies in invertebrates suggest that caffeine is acting through an adenosine receptor, it is also possible that there is another receptor for adenosine in invertebrates. A number of genes for “orphan” G protein coupled receptors in the Drosophila genome still remain to be characterized, and one (or more) of them could be the adenosine receptor affected by caffeine. If, on the other hand, caffeine is not acting through an adenosine receptor in invertebrates, then the parallels in the effects of caffeine on behavior in mammals and invertebrates suggests that caffeine may be acting on other pathways in mammals as well, and the assertion that its main function is as an adenosine receptor antagonist should be reexamined.
Secondly, the concentration of caffeine used in experiments is obviously important. In mammals, the behavioral responses to caffeine are biphasic with distinct effects at low versus high caffeine concentrations [33, 85–87]. This is most likely the case in invertebrates as well, for example, in the honey bee, low doses of caffeine (as low as 0.1 μM) increase memory retention, while higher doses (10 mM) reduce the levels of acquisition during appetitive olfactory conditioning [4, 37]. Since inhibition of phosphodiesterases and calcium release via ryanodine receptors require caffeine concentrations in the millimolar range, the ability of caffeine to affect behavior at lower concentrations suggests that there is a role for adenosine receptors or other, as of yet unknown, targets for caffeine in invertebrates. One issue with many of the invertebrate studies is that the actual amount of caffeine to which an individual subject is exposed is unknown. For example, many studies use caffeine added to the food source, which the animals then consume freely. Because caffeine has an aversive taste [37, 88–90], it is possible that higher caffeine concentrations lead to decreased food consumption, leading to a lower dose of caffeine than expected. A better understanding of the effective dose would strengthen our understanding of the targets on which caffeine is acting to produce specific changes in behavior.
Conclusions
In general, caffeine appears to have similar effects on the behavior of invertebrates as those observed in humans and rodent models. However, whether caffeine is acting via the same molecular mechanisms is still an open question. Even within the extremely large number of species encompassed by the invertebrate category, almost all of the work was done using the fruit fly and honey bee model systems. Questions regarding the interactions between plants, their herbivores and their pollinators, and issues concerning the ecotoxicity of caffeine suggest that we need a broader understanding of the effects of caffeine across animal phyla.
References
- 1.Ashihara H, Sano H, Crozier A. Caffeine and related purine alkaloids: biosynthesis, catabolism, function and genetic engineering. Phytochem. 2008;69(4):841–856. doi: 10.1016/j.phytochem.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Kretschmar JA, Baumann TW. Caffeine in Citrus flowers. Phytochem. 1999;52:19–23. doi: 10.1016/S0031-9422(99)00119-3. [DOI] [Google Scholar]
- 3.Naef R, Jaquier A, Velluz A, Bachofen B. From the linden flower to linden honey—volatile constituents of linden nectar, the extract of bee-stomach and ripe honey. Chem Biodivers. 2004;1:1870–1879. doi: 10.1002/cbdv.200490143. [DOI] [PubMed] [Google Scholar]
- 4.Wright GA, Baker DD, Palmer MJ, Stabler D, Mustard JA, Power EF, Borland AM, Stevenson PC. Caffeine in floral nectar enhances a pollinator’s memory of reward. Science. 2013;339(6124):1202–1204. doi: 10.1126/science.1228806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singaravelan N, Nee’man G, Inbar M, Izhaki I. Feeding responses of free-flying honeybees to secondary compounds mimicking floral nectars. J Chem Ecol. 2005;31(12):2791–2804. doi: 10.1007/s10886-005-8394-z. [DOI] [PubMed] [Google Scholar]
- 6.Deblonde T, Cossu-Leguille C, Hartemann P. Emerging pollutants in wastewater: a review of the literature. Int J Hyg Environ Health. 2011;214(6):442–448. doi: 10.1016/j.ijheh.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 7.Martin J, Camacho-Munoz D, Santos JL, Aparicio I, Alonso E. Occurrence of pharmaceutical compounds in wastewater and sludge from wastewater treatment plants: removal and ecotoxicological impact of wastewater discharges and sludge disposal. J Hazard Mater. 2012;239–240:40–47. doi: 10.1016/j.jhazmat.2012.04.068. [DOI] [PubMed] [Google Scholar]
- 8.del Rey ZR, Granek EF, Buckley BA. Expression of HSP70 in Mytilus californianus following exposure to caffeine. Ecotoxicology. 2011;20(4):855–861. doi: 10.1007/s10646-011-0649-6. [DOI] [PubMed] [Google Scholar]
- 9.Aguirre-Martinez GV, Buratti S, Fabbri E, DelVallis AT, Martin-Diaz ML. Using lysosomal membrane stability of haemocytes in Ruditapes philippinarum as a biomarker of cellular stress to assess contamination by caffeine, ibuprofen, carbamazepine and novobiocin. J Environ Sci. 2013;25(7):1408–1418. doi: 10.1016/S1001-0742(12)60207-1. [DOI] [PubMed] [Google Scholar]
- 10.Aguirre-Martinez GV, Del Valls TA, Martin-Diaz ML. Identification of biomarkers responsive to chronic exposure to pharmaceuticals in target tissues of Carcinus maenas . Mar Environ Res. 2013;87–88:1–11. doi: 10.1016/j.marenvres.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 11.Barberger-Gateau P, Lambert JC, Feart C, Peres K, Ritchie K, Dartigues JF, Alperovitch A. From genetics to dietetics: the contribution of epidemiology to understanding Alzheimer’s disease. J Alzheimers Dis. 2013;33(Suppl 1):S457–S463. doi: 10.3233/JAD-2012-129019. [DOI] [PubMed] [Google Scholar]
- 12.Cunha RA, Agostinho PM. Chronic caffeine consumption prevents memory disturbance in different animal models of memory decline. J Alzheimers Dis. 2010;20(Suppl 1):S95–S116. doi: 10.3233/JAD-2010-1408. [DOI] [PubMed] [Google Scholar]
- 13.Maia L, de Mendonca A. Does caffeine intake protect from Alzheimer’s disease? Eur J Neurol. 2002;9(4):377–382. doi: 10.1046/j.1468-1331.2002.00421.x. [DOI] [PubMed] [Google Scholar]
- 14.Ross GW, Abbott RD, Petrovitch H, Morens DM, Grandinetti A, Tung KH, Tanner CM, Masaki KH, Blanchette PL, Curb JD, Popper JS, White LR. Association of coffee and caffeine intake with the risk of Parkinson disease. JAMA. 2000;283(20):2674–2679. doi: 10.1001/jama.283.20.2674. [DOI] [PubMed] [Google Scholar]
- 15.Kaun KR, Devineni AV, Heberlein U. Drosophila melanogaster as a model to study drug addiction. Hum Genet. 2012;131(6):959–975. doi: 10.1007/s00439-012-1146-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scholz H, Mustard JA. Invertebrate models of alcoholism. Curr Top Behav Neurosci. 2013;13:433–457. doi: 10.1007/978-3-642-28720-6_128. [DOI] [PubMed] [Google Scholar]
- 17.Jee C, Lee J, Lim JP, Parry D, Messing RO, McIntire SL. SEB-3, a CRF receptor-like GPCR, regulates locomotor activity states, stress responses and ethanol tolerance in Caenorhabditis elegans . Genes Brain Behav. 2013;12(2):250–262. doi: 10.1111/j.1601-183X.2012.00829.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapfhamer D, Bettinger JC, Davies AG, Eastman CL, Smail EA, Heberlein U, McIntire SL. Loss of RAB-3/A in C. elegans and the mouse affects behavioral response to ethanol. Genes Brain Behav. 2008;7(6):669–676. doi: 10.1111/j.1601-183X.2008.00404.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasek AW, Giorgetti F, Berger KH, Tayor S, Heberlein U. Lmo genes regulate behavioral responses to ethanol in Drosophila melanogaster and the mouse. Alcohol Clin Exp Res. 2011;35(9):1600–1606. doi: 10.1111/j.1530-0277.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bhandari P, Hill JS, Farris SP, Costin B, Martin I, Chan CL, Alaimo JT, Bettinger JC, Davies AG, Miles MF, Grotewiel M. Chloride intracellular channels modulate acute ethanol behaviors in Drosophila, Caenorhabditis elegans and mice. Genes Brain Behav. 2012;11(4):387–397. doi: 10.1111/j.1601-183X.2012.00765.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joslyn G, Wolf FW, Brush G, Wu L, Schuckit M, White RL. Glypican gene GPC5 participates in the behavioral response to ethanol: evidence from humans, mice, and fruit flies. G3 (Bethesda) 2011;1(7):627–635. doi: 10.1534/g3.111.000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ishay JS, Paniry VA. Effects of caffeine and various xanthines on hornets and bees. Psychopharmacology. 1979;65(3):299–309. doi: 10.1007/BF00492219. [DOI] [PubMed] [Google Scholar]
- 23.Fernandes FL, Picanco MC, Fernandes ME, Queiroz RB, Xavier VM, Martinez HE. The effects of nutrients and secondary compounds of Coffea arabica on the behavior and development of Coccus viridis . Environ Entomol. 2012;41(2):333–341. doi: 10.1603/EN11003. [DOI] [PubMed] [Google Scholar]
- 24.Nakayama S, Sasaki K, Matsumura K, Lewis Z, Miyatake T. Dopaminergic system as the mechanism underlying personality in a beetle. J Insect Physiol. 2012;58(5):750–755. doi: 10.1016/j.jinsphys.2012.02.011. [DOI] [PubMed] [Google Scholar]
- 25.Nishi Y, Sasaki K, Miyatake T. Biogenic amines, caffeine and tonic immobility in Tribolium castaneum . J Insect Physiol. 2010;56(6):622–628. doi: 10.1016/j.jinsphys.2010.01.002. [DOI] [PubMed] [Google Scholar]
- 26.Schwab WE. The ontogeny of swimming behavior in the scyphozoan, Aurelia aurita. II The effects of ions and drugs. Biol Bull. 1977;152:251–262. doi: 10.2307/1540563. [DOI] [PubMed] [Google Scholar]
- 27.Andretic R, Kim YC, Jones FS, Han KA, Greenspan RJ. Drosophila D1 dopamine receptor mediates caffeine-induced arousal. Proc Natl Acad Sci USA. 2008;105(51):20392–20397. doi: 10.1073/pnas.0806776105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hendricks JC, Finn SM, Panckeri KA, Chavkin J, Williams JA, Sehgal A, Pack AI. Rest in Drosophila is a sleep-like state. Neuron. 2000;25(1):129–138. doi: 10.1016/S0896-6273(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 29.Lin FJ, Pierce MM, Sehgal A, Wu T, Skipper DC, Chabba R. Effect of taurine and caffeine on sleep-wake activity in Drosophila melanogaster . Nat Sci Sleep. 2010;2:221–231. doi: 10.2147/NSS.S13034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shaw PJ, Cirelli C, Greenspan RJ, Tononi G. Correlates of sleep and waking in Drosophila melanogaster . Science. 2000;287(5459):1834–1837. doi: 10.1126/science.287.5459.1834. [DOI] [PubMed] [Google Scholar]
- 31.Wu MN, Ho K, Crocker A, Yue Z, Koh K, Sehgal A. The effects of caffeine on sleep in Drosophila require PKA activity, but not the adenosine receptor. J Neurosci. 2009;29(35):11029–11037. doi: 10.1523/JNEUROSCI.1653-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roehrs T, Roth T. Caffeine: sleep and daytime sleepiness. Sleep Med Rev. 2008;12(2):153–162. doi: 10.1016/j.smrv.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 33.Angelucci ME, Vital MA, Cesario C, Zadusky CR, Rosalen PL, Da Cunha C. The effect of caffeine in animal models of learning and memory. Eur J Pharmacol. 1999;373(2–3):135–140. doi: 10.1016/S0014-2999(99)00225-3. [DOI] [PubMed] [Google Scholar]
- 34.Nehlig A. Is caffeine a cognitive enhancer? J Alzheimers Dis. 2010;20(Suppl 1):S85–S94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- 35.Folkers E, Spatz HC. Visual learning performance of Drosophila melanogaster is altered by neuropharmaca affecting phosphodiesterase activity and acetylcholine transmission. J Insect Physiol. 1984;30:957–965. doi: 10.1016/0022-1910(84)90074-X. [DOI] [Google Scholar]
- 36.Wang X, Liu L, Xia S, Feng C, Guo A. Relationship between visual learning/memory ability and brain cAMP level in Drosophila . Sci China C Life Sci. 1998;41:503–511. doi: 10.1007/BF02882888. [DOI] [PubMed] [Google Scholar]
- 37.Mustard JA, Dews L, Brugato A, Dey K, Wright GA. Consumption of an acute dose of caffeine reduces acquisition but not memory in the honey bee. Behav Brain Res. 2012;232(1):217–224. doi: 10.1016/j.bbr.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 38.Si A, Zhang SW, Maleszka R. Effects of caffeine on olfactory and visual learning in the honey bee (Apis mellifera) Pharmacol Biochem Behav. 2005;82(4):664–672. doi: 10.1016/j.pbb.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 39.Silant’eva DI, Gainutdinova T, Andrianov VV, Gainutdinov KhL. Electrophysiological studies of the effects of chronic administration of caffeine on the formation of a conditioned defensive reflex in the common snail. Neurosci Behav Physiol. 2009;39(4):403–407. doi: 10.1007/s11055-009-9136-4. [DOI] [PubMed] [Google Scholar]
- 40.Perisse E, Raymond-Delpech V, Neant I, Matsumoto Y, Leclerc C, Moreau M, Sandoz JC. Early calcium increase triggers the formation of olfactory long-term memory in honeybees. BMC Biol. 2009;7:30. doi: 10.1186/1741-7007-7-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rein J, Mustard JA, Strauch M, Smith BH, Galizia CG. Octopamine modulates activity of neural networks in the honey bee antennal lobe. J Comp Physiol A. 2013;199(11):947–962. doi: 10.1007/s00359-013-0805-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fredholm BB, Battig K, Holmen J, Nehlig A, Zvartau EE. Actions of caffeine in the brain with special reference to factors that contribute to its widespread use. Pharmacol Rev. 1999;51(1):83–133. [PubMed] [Google Scholar]
- 43.Pessah IN, Stambuk RA, Casida JE. Ca2+-activated ryanodine binding: mechanisms of sensitivity and intensity modulation by Mg2+, caffeine, and adenine nucleotides. Mol Pharmacol. 1987;31(3):232–238. [PubMed] [Google Scholar]
- 44.Sattelle DB, Cordova D, Cheek TR. Insect ryanodine receptors: molecular targets for novel pest control chemicals. Invert Neurosci. 2008;8(3):107–119. doi: 10.1007/s10158-008-0076-4. [DOI] [PubMed] [Google Scholar]
- 45.Shiwa M, Murayama T, Ogawa Y. Molecular cloning and characterization of ryanodine receptor from unfertilized sea urchin eggs. Am J Physiol Regul Integr Comp Physiol. 2002;282(3):R727–R737. doi: 10.1152/ajpregu.00519.2001. [DOI] [PubMed] [Google Scholar]
- 46.Robertson AP, Clark CL, Martin RJ. Levamisole and ryanodine receptors. I: a contraction study in Ascaris suum . Mol Biochem Parasitol. 2010;171(1):1–7. doi: 10.1016/j.molbiopara.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Collet C. Excitation-contraction coupling in skeletal muscle fibers from adult domestic honeybee. Pflugers Arch. 2009;458(3):601–612. doi: 10.1007/s00424-009-0642-6. [DOI] [PubMed] [Google Scholar]
- 48.Ebbinghaus-Kintscher U, Luemmen P, Lobitz N, Schulte T, Funke C, Fischer R, Masaki T, Yasokawa N, Tohnishi M. Phthalic acid diamides activate ryanodine-sensitive Ca2+ release channels in insects. Cell Calcium. 2006;39(1):21–33. doi: 10.1016/j.ceca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 49.Lehmberg E, Casida JE. Similarity of insect and mammalian ryanodine binding sites. Pestic Biochem Physiol. 1994;48:145–152. doi: 10.1006/pest.1994.1015. [DOI] [Google Scholar]
- 50.Woodward OM, Willows AO. Nervous control of ciliary beating by Cl(−), Ca(2+) and calmodulin in Tritonia diomedea . J Exp Biol. 2006;209(Pt 14):2765–2773. doi: 10.1242/jeb.02377. [DOI] [PubMed] [Google Scholar]
- 51.Györke S, Palade P. Calcium-induced calcium release in crayfish skeletal muscle. J Physiol. 1992;457:195–210. doi: 10.1113/jphysiol.1992.sp019373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Olivares E, Arispe N, Rojas E. Properties of the ryanodine receptor present in the sarcoplasmic reticulum from lobster skeletal muscle. Membr Biochem. 1993;10(4):221–235. doi: 10.3109/09687689309150270. [DOI] [PubMed] [Google Scholar]
- 53.Byers D, Davis RL, Kiger JA., Jr Defect in cyclic AMP phosphodiesterase due to the dunce mutation of learning in Drosophila melanogaster . Nature. 1981;289(5793):79–81. doi: 10.1038/289079a0. [DOI] [PubMed] [Google Scholar]
- 54.Nighorn A, Qiu Y, Davis RL. Progress in understanding the Drosophila dnc locus. Comp Biochem Physiol Biochem Mol Biol. 1994;108(1):1–9. doi: 10.1016/0305-0491(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 55.Fallon AM, Wyatt GR. Cyclic nucleotide phosphodiesterases in the cricket, Acheta domesticus . Biochim Biophys Acta. 1977;480(2):428–441. doi: 10.1016/0005-2744(77)90035-3. [DOI] [PubMed] [Google Scholar]
- 56.Filburn CR, Karn J, Wyatt GR. Cyclic nucleotide phosphodiesterases of Hyalophora cecropia silkmoth fat body. Biochim Biophys Acta. 1977;481(1):152–163. doi: 10.1016/0005-2744(77)90146-2. [DOI] [PubMed] [Google Scholar]
- 57.Morishima I. Cyclic nucleotide phosphodiesterase in silkworm: purification and properties. Biochim Biophys Acta. 1974;370(1):227–241. doi: 10.1016/0005-2744(74)90047-3. [DOI] [PubMed] [Google Scholar]
- 58.Morishima I. Cyclic nucleotide phosphodiesterase in silkworm. Characterization of cyclic GMP phosphodiesterase. Biochim Biophys Acta. 1975;410(2):310–317. doi: 10.1016/0005-2744(75)90233-8. [DOI] [PubMed] [Google Scholar]
- 59.Nathanson JA. Caffeine and related methylxanthines: possible naturally occurring pesticides. Science. 1984;226:184–187. doi: 10.1126/science.6207592. [DOI] [PubMed] [Google Scholar]
- 60.Odesser DB, Hayes DK, Schechter MS. Phosphodiesterase activity in pupae and diapausing and non-diapausing larvae of the European corn borer, Ostrinia nubilalis . J Insect Physiol. 1972;18:1097–1105. doi: 10.1016/0022-1910(72)90144-8. [DOI] [Google Scholar]
- 61.Rojakovick AS, March RB. Characteristics of cyclic 3′,5′-nucleotide phosphodiesterase from the brain of the Madagascar cockroach (Gromphadorhina portentosa) Comp Biochem Physiol B. 1974;47(1):189–199. doi: 10.1016/0305-0491(74)90103-5. [DOI] [PubMed] [Google Scholar]
- 62.Volmer H. Properties of cyclic nucleotide phosphodiesterase from the flight-muscles of Locusta migratoria . Insect Biochem. 1977;7:411–414. doi: 10.1016/S0020-1790(77)90017-8. [DOI] [Google Scholar]
- 63.Fredholm BB, Ijzerman AP, Jacobson KA, Klotz KN, Linden J. International Union of Pharmacology. XXV. Nomenclature and classification of adenosine receptors. Pharmacol Rev. 2001;53(4):527–552. [PMC free article] [PubMed] [Google Scholar]
- 64.Chen JH, Bayne CJ. Hemocyte adhesion in the California mussel (Mytilus californianus): regulation by adenosine. Biochim Biophys Acta. 1995;1268(2):178–184. doi: 10.1016/0167-4889(95)00074-3. [DOI] [PubMed] [Google Scholar]
- 65.Dolezelova E, Nothacker HP, Civelli O, Bryant PJ, Zurovec M. A Drosophila adenosine receptor activates cAMP and calcium signaling. Insect Biochem Mol Biol. 2007;37(4):318–329. doi: 10.1016/j.ibmb.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 66.Kalinowski RR, Jaffe LA, Foltz KR, Giusti AF. A receptor linked to a Gi-family G-protein functions in initiating oocyte maturation in starfish but not frogs. Dev Biol. 2003;253(1):139–149. doi: 10.1006/dbio.2002.0860. [DOI] [PubMed] [Google Scholar]
- 67.Kucerova L, Broz V, Fleischmannova J, Santruckova E, Sidorov R, Dolezal V, Zurovec M. Characterization of the Drosophila adenosine receptor: the effect of adenosine analogs on cAMP signaling in Drosophila cells and their utility for in vivo experiments. J Neurochem. 2012;121(3):383–395. doi: 10.1111/j.1471-4159.2012.07701.x. [DOI] [PubMed] [Google Scholar]
- 68.Malik A, Buck LT. Adenosinergic modulation of neuronal activity in the pond snail Lymnaea stagnalis . J Exp Biol. 2010;213(Pt 7):1126–1132. doi: 10.1242/jeb.033894. [DOI] [PubMed] [Google Scholar]
- 69.Magazanik LG, Fedorova IM. Modulatory role of adenosine receptors in insect motor nerve terminals. Neurochem Res. 2003;28(3–4):617–624. doi: 10.1023/A:1022893928104. [DOI] [PubMed] [Google Scholar]
- 70.Cauli O, Morelli M. Caffeine and the dopaminergic system. Behav Pharmacol. 2005;16(2):63–77. doi: 10.1097/00008877-200503000-00001. [DOI] [PubMed] [Google Scholar]
- 71.Xie X, Ramkumar V, Toth LA. Adenosine and dopamine receptor interactions in striatum and caffeine-induced behavioral activation. Comp Med. 2007;57(6):538–545. [PubMed] [Google Scholar]
- 72.Pentreath VW, Berry MS. Potentiation of dopaminergic transmission by phosphodiesterase inhibitors and cyclic nucleotides. J Pharm Pharmacol. 1976;28(12):874–877. doi: 10.1111/j.2042-7158.1976.tb04083.x. [DOI] [PubMed] [Google Scholar]
- 73.Kucharski R, Maleszka R. Microarray and real-time PCR analyses of gene expression in the honeybee brain following caffeine treatment. J Mol Neurosci. 2005;27(3):269–276. doi: 10.1385/JMN:27:3:269. [DOI] [PubMed] [Google Scholar]
- 74.Nistri A, Berti C. Caffeine-induced potentiation of GABA effects on frog spinal cord: an electrophysiological study. Brain Res. 1983;258(2):263–270. doi: 10.1016/0006-8993(83)91149-6. [DOI] [PubMed] [Google Scholar]
- 75.Taketo M, Matsuda H, Yoshioka T. Calcium-independent inhibition of GABA(A) current by caffeine in hippocampal slices. Brain Res. 2004;1016(2):229–239. doi: 10.1016/j.brainres.2004.05.008. [DOI] [PubMed] [Google Scholar]
- 76.Uneyama H, Harata N, Akaike N. Caffeine and related compounds block inhibitory amino acid-gated Cl− currents in freshly dissociated rat hippocampal neurones. Br J Pharmacol. 1993;109(2):459–465. doi: 10.1111/j.1476-5381.1993.tb13591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Duan L, Yang J, Slaughter MM. Caffeine inhibition of ionotropic glycine receptors. J Physiol. 2009;587(Pt 16):4063–4075. doi: 10.1113/jphysiol.2009.174797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Pohanka M, Dobes P. Caffeine inhibits acetylcholinesterase, but not butyrylcholinesterase. Int J Mol Sci. 2013;14(5):9873–9882. doi: 10.3390/ijms14059873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Arnaud MJ. Pharmacokinetics and metabolism of natural methylxanthines in animal and man. Handb Exp Pharmacol. 2011;200:33–91. doi: 10.1007/978-3-642-13443-2_3. [DOI] [PubMed] [Google Scholar]
- 80.Bonati M, Latini R, Tognoni G, Young JF, Garattini S. Interspecies comparison of in vivo caffeine pharmacokinetics in man, monkey, rabbit, rat, and mouse. Drug Metab Rev. 1984;15(7):1355–1383. doi: 10.3109/03602538409029964. [DOI] [PubMed] [Google Scholar]
- 81.Walton K, Dorne JL, Renwick AG. Uncertainty factors for chemical risk assessment: interspecies differences in the in vivo pharmacokinetics and metabolism of human CYP1A2 substrates. Food Chem Toxicol. 2001;39(7):667–680. doi: 10.1016/S0278-6915(01)00006-0. [DOI] [PubMed] [Google Scholar]
- 82.Bhaskara S, Dean ED, Lam V, Ganguly R. Induction of two cytochrome P450 genes, Cyp6a2 and Cyp6a8, of Drosophila melanogaster by caffeine in adult flies and in cell culture. Gene. 2006;377:56–64. doi: 10.1016/j.gene.2006.02.032. [DOI] [PubMed] [Google Scholar]
- 83.Willoughby L, Chung H, Lumb C, Robin C, Batterham P, Daborn PJ. A comparison of Drosophila melanogaster detoxification gene induction responses for six insecticides, caffeine and phenobarbital. Insect Biochem Mol Biol. 2006;36(12):934–942. doi: 10.1016/j.ibmb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 84.Mustard JA, Beggs KT, Mercer AR. Molecular biology of the invertebrate dopamine receptors. Arch Insect Biochem Physiol. 2005;59(3):103–117. doi: 10.1002/arch.20065. [DOI] [PubMed] [Google Scholar]
- 85.Brockwell NT, Eikelboom R, Beninger RJ. Caffeine-induced place and taste conditioning: production of dose-dependent preference and aversion. Pharmacol Biochem Behav. 1991;38(3):513–517. doi: 10.1016/0091-3057(91)90006-N. [DOI] [PubMed] [Google Scholar]
- 86.Nehlig A, Daval JL, Debry G. Caffeine and the central nervous system: mechanisms of action, biochemical, metabolic and psychostimulant effects. Brain Res Brain Res Rev. 1992;17(2):139–170. doi: 10.1016/0165-0173(92)90012-B. [DOI] [PubMed] [Google Scholar]
- 87.Patkina NA, Zvartau EE. Caffeine place conditioning in rats: comparison with cocaine and ethanol. Eur Neuropsychopharmacol. 1998;8(4):287–291. doi: 10.1016/S0924-977X(97)00086-2. [DOI] [PubMed] [Google Scholar]
- 88.Glendinning JI. Is chemosensory input essential for the rapid rejection of toxic foods? J Exp Biol. 1996;199(Pt 7):1523–1534. doi: 10.1242/jeb.199.7.1523. [DOI] [PubMed] [Google Scholar]
- 89.Hollingsworth RG, Armstrong JW, Campbell E. Caffeine as a repellent for slugs and snails. Nature. 2002;417(6892):915–916. doi: 10.1038/417915a. [DOI] [PubMed] [Google Scholar]
- 90.Sellier MJ, Reeb P, Marion-Poll F. Consumption of bitter alkaloids in Drosophila melanogaster in multiple-choice test conditions. Chem Senses. 2010;36(4):323–334. doi: 10.1093/chemse/bjq133. [DOI] [PubMed] [Google Scholar]
- 91.Goujon M, McWilliam H, Li W, Valentin F, Squizzato S, Paern J, Lopez R. A new bioinformatics analysis tools framework at EMBL-EBI. Nucleic Acids Res. 2010;38(Web Server issue):W695–W699. doi: 10.1093/nar/gkq313. [DOI] [PMC free article] [PubMed] [Google Scholar]