Abstract
Intensive research has demonstrated that extracellular matrix (ECM) molecules and growth factors (GF) collaborate at many different levels. The ability of ECM to modulate GF signals has important implications in tissue formation and homeostasis as well as novel therapies for acute and chronic wounds. Recently, a number of GF binding sites were identified in fibronectin and were shown to provide another layer of regulation on GF signaling. Here, we review these new findings on fibronectin interaction with GF in context of general ways ECM molecules regulate GF signaling.
Keywords: Extracellular matrix, growth factor, growth factor receptor, growth factor-binding domain, fibronectin, matrikine, tissue engineering
1 Introduction
ECM is composed of secreted molecules (glycoproteins, collagens, glycosaminoglycans and proteoglycans) that constitute the cell microenvironment (Hynes, 2009). ECM provides structural support and segregation for different tissues in vivo. In the skin, it is essential to the tensile strength and flexibility in the dermis and basement membrane (Kim et al., 2011). The basement membrane also acts as a mechanical barrier to cell migration into and out of the epidermis and as a filter of fluid and solute exchange.
However, ECM provides much more than just mechanical and structural support. Multiple specific domains in ECM can bind interacting partners such as other ECM molecules, GFs, cell ECM receptors, e.g. integrins, and other cell surface receptors including some GF receptors. Through such domains, ECM regulates the nature, intensity, and duration of GF signaling. This in turn determines cell behavior, polarity, migration, differentiation, proliferation, and survival (Ivaska and Heino, 2011; Legate et al., 2009).
Many clinical trials in GF-based tissue engineering have been largely disappointing. Recently preclinical studies have shown benefit when GF carriers, such as ECM GF-binding domains, were included in tissue-engineering constructs (Discher et al., 2009; Ghosh et al., 2006; Martino et al., 2013; Martino and Hubbell, 2010). These data underline the importance of spatial and temporal regulation of GF signaling to achieve tangible therapeutic effects. Thus, it is critical to understand mechanisms by which ECM can regulate GF signaling in both normal and pathological conditions. Here we review some new findings on fibronectin (FN) interactions with GF in the context of general ways ECM can modulate GF signaling. A more detailed description of ECM and GF interactions can be found in an earlier review by our group (Macri et al., 2007).
2 ECM can regulate GF signaling based on ECM-GF binding
An increasing number of GFs, including IGF, FGF, TGF-β, HGF, and PDGF have been found to associate with the ECM through growth factor-binding sites (Hynes, 2009). Some of these growth factor-binding sites are conserved across different ECM proteins, e.g. VWC domain in collagen II binds TGF-β1 and BMP-2; heparin II domain in FN binds FGF, VEGF and PDGF (Martino and Hubbell, 2010; Wijelath et al., 2006); and the FN first type III repeat (FNIII1) binds PDGF-BB (Lin et al., 2011). A generally held view is that ECM acts as a sink or reservoir for GFs and may assist in establishing stable gradients of GFs bound to ECM. In addition, GFs bound to ECM (as a solid-phase ligand) can generate different signals compared to their soluble form (Mohammadi et al., 2005). Proteolytic processing of ECM can release matrix-sequestered GFs during injury or inflammation (Arroyo and Iruela-Arispe, 2010) inducing rapid and localized changes in the activity of these GFs. Some of these growth factor-binding domains are cryptic and will only be exposed upon proteolytic processing or by the tension force generated by cells (Lin and Clark, unpublished observations).
2.1 ECM as reservoir for GF
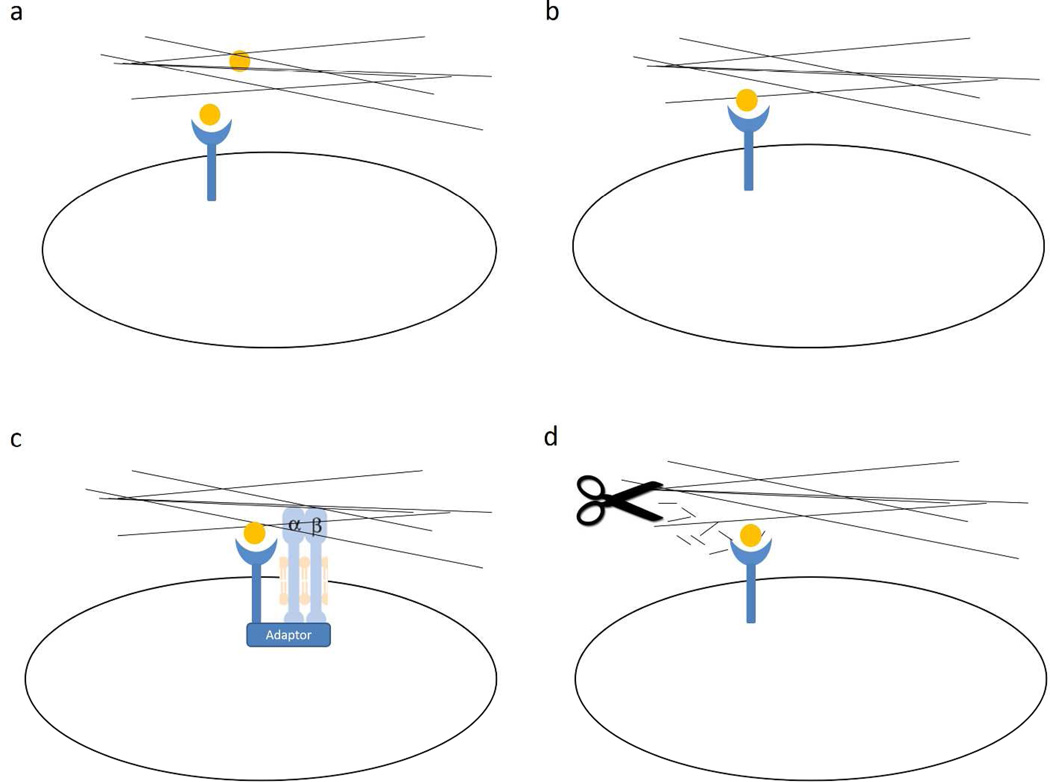
There is increasing evidence for specific, direct binding of GFs to ECM in general (Macri et al., 2007) and to multiple growth factor-binding domains in FN (Lin et al., 2011; Martino and Hubbell, 2010; Wijelath et al., 2006). The ECM can serve as a GF reservoir and increase its local bioavailability (Figure 1a). Hepatocyte growth factor (HGF) binds both FN and vitronectin and forms complexes with HGF receptor and integrins, leading to enhanced cell migration (Rahman et al., 2005). Similarly VEGF binds to specific FN type III domains in both FN and tenascin-C. Using recombinant FN domains, the C-terminal heparin-II domain of FN (FNIII13–14) was identified as a key VEGF-binding site. Mutation of the heparin-binding residues on FNIII13–14 abolished VEGF binding, and peptides corresponding to the heparin-binding sequences in FNIII13-14 inhibited VEGF binding to FN. These ECM associations with VEGF synergize to promote cell proliferation (Ishitsuka et al., 2009; Wijelath et al., 2006). More recently it was found that FN III12–14 binds most of the GFs from the platelet-derived growth factor (PDGF)/VEGF and FGF families and some GFs from the transforming growth factor-β and neurotrophin families (Martino and Hubbell, 2010). In fibrin matrices functionalized with FN III12–14, PDGF-BB-induced smooth muscle spheroid sprouting was greatly enhanced. Furthermore, enzymatic release of ECM-sequestered GFs during inflammatory processes, including wound healing, can release such GFs (Arroyo and Iruela-Arispe, 2010). Thus, rapid and local growth factor-mediated activation of cellular functions can occur without de novo synthesis (Cox and Erler, 2011).
Figure 1. ECM regulation of GF signaling dependent on ECM-GF binding.
a) ECM serves as a reservoir of GF to spatially regulate its bioavailability. b) GF tethered to ECM through growth factor-binding domain is presented as solid phase ligand that generates protracted signaling. c) The juxtaposition of growth factor-binding domain and integrin binding site in ECM facilitates the formation of adhesion complexes, the structural basis for the synergistic signaling of ECM and GF. d) Proteolytic processing not only releases ECM sequestered GF; it also generates bioactive peptides that can directly bind GF and enhance GF signaling.
2.2 Solid-phase presentation of GF
When tethered to a solid substrate, EGF is more potent than soluble EGF in eliciting DNA synthesis (Kuhl and Griffith-Cima, 1996). EGF presented in solid-phase is not internalized and degraded, but instead provides sustained signaling. For example, tethered EGF promoted mesenchymal stem cell (MSC) survival by causing sustained cell surface EGFR activation (Rodrigues et al., 2013). Furthermore, the ability of tethered EGF to restricted signaling to the cell surface promoted osteogenic differentiation of multipotent marrow stromal cells (Platt et al., 2009). Most interesting, addition of soluble EGF to cells cultured on tethered EGF substrata reduced osteogenic differentiation. Furthermore, soluble EGF down-regulated EGFR and HER2 demonstrating that soluble EGF can modulate tethered EGF/EGFR interactions.
In a similar fashion FN-growth factor-binding domains (FNIII1, FNIII13–14, and IIICS) enhanced PDGF-BB survival signals in FN-null fibroblasts only when they were surface-bound (Lin et al., 2011). These FN domains contain sequence similarities that bind PDGF-BB (Lin et al., 2013) as well as VEGF, FGF-2, and TGF-β1 (Lin et al., manuscript in preparation). Therefore, ECM tethered GFs are fine-tuned to generate enhanced and/or distinct signals from their soluble counterparts (Figure 1b).
2.3 Formation of adhesion complexes
GFR and integrin have been shown to form signaling complexes through various mechanisms (Fujita et al., 2012; Motegi et al., 2011). Thus, it is also possible that growth factor-binding domains in ECM proteins can serve as the link that brings together GFR and integrin (Figure 1c) as proposed previously by our group (Lin et al., 2011). For example, the α5β1 cell-binding domain (FNIII9–10/FNIII8–11) and a growth factor-binding domain (either FNIII1 or FNIII12–14/FNIII12–15) only demonstrated synergistic effects when the binding domains for α5β1 and GF were coupled or presented on the same surface - presentation on two separate surfaces did not suffice (Lin et al., 2011; Martino et al., 2011; Wijelath et al., 2006). For example, FN-null fibroblasts cultured on recombinant FNIII8–11 without a FN-growth factor-binding domain (FNIII1 or FNIII12–15) demonstrated minimal metabolism and underwent autophagy at 24 hours even in the presence of PDGF-BB (Lin et al., 2011). In contrast, FN-null fibroblasts plated on FNIII8–11 contiguous with a FN-growth factor-binding domain survived without, and proliferated with, PDGF-BB. These results suggest that the mechanism of GF/FN synergism is mediated outside of the cell by the formation of a GF/FN complex requiring both the cell-binding and VEGF-binding domains linked in a single molecular unit. Since such proximity is important, ECM molecules, by virtue of their ordered-domain organization, could act to organize focal adhesion complexes that contain not only integrins, but also growth factor receptors, in the plane of the membrane as previously suggested by results from the Ingber (Plopper et al., 1995) and Yamada (Miyamoto et al., 1996) groups. Such complexes could enhance membrane-proximal regulation among receptors and promote integration of transduced signals (Hynes, 2009; Lin et al., 2011).
2.4 Tension force by cell exposes cryptic domain in ECM
ECM is not an inanimate, unchanging assemblage of collagens, proteoglycans, and glycoproteins. Rather the ECM undergoes constant remodeling, most obviously during development, wound healing, and other repair processes. Furthermore, many ECM molecules are flexible and extendable. Mechanical tension studies on FN suggest that while eliminating normally exposed sites, cell-driven extension of FN can expose cryptic sites that are not available in its native state (Vogel, 2006). Indeed, FN fibrillogenesis requires strain on FNIII1 and FNIII10 to expose cryptic sites (Zhong et al., 1998). Such cell-driven strain on FN requires α5β1-binding to the FN central cell-binding domain (FNIII9–10) (Mosher et al., 1992) that is normally exposed on FN in a relaxed state (Vogel, 2006). Two classes of VEGF binding sites on FN have been identified (Mitsi et al., 2006). One is constitutively available whereas the availability of the other was modulated by the conformational state of FN. Atomic force microscopy studies revealed that heparin and hydrophilic substrates promoted an extended conformation of FN, leading to increased VEGF binding. Treatment of the complex endothelial ECM with heparin also increased VEGF binding, suggesting that heparin/heparan sulfate might regulate VEGF interactions within the ECM by controlling the structure and organization of FN matrices. Recent results from our laboratory also strongly support the need of cell tension on FN to expose a cryptic FN peptide for optimal cell survival on intact FN (Lin and Clark, unpublished observations).
2.5 ECM peptides as co-factor of GF
In venous thrombus resolution, proteolytic fragments of FN, which contain a VEGF binding site, are expressed in a distinct spatial and temporal pattern (Evans et al., 2012). Thus, FN may influence capillary morphogenesis by generation of fragments that modulate VEGF stimulated proliferation, migration, and protease activation (Figure 1d). An analysis of FN fragments (Fnf) generated by proteolytic processing from matrix metalloprotease-2 (MMP-2) showed that Fnf of 30-kD and 120-kDa size positively affected proliferation of microvascular cells but not macrovascular cells (Grant et al., 1998). Furthermore, a 45-kDa gelatin-binding fragment of FN inhibited retinal endothelial cell proliferation but stimulated pericyte and smooth muscle cell proliferation. Our group has recently identified a bioactive FN peptide P12 from FNIII1 (Lin et al. 2013, in press) that is theoretically released during proteolysis as part of a larger fragment. Soluble P12 binds PDGF-BB with high affinity (KD in the nanomolar range) and can synergistically promote human dermal fibroblast cell survival by augmenting PDGFR activation and survival signals, i.e. Akt phorphorylation. Moreover, P12 can also affect the PDGF-BB/PDGFR route of endocytosis (Zhu et al., 2013). When P12 was present, GFP-PDGFR co-localized with a macropinosome marker (70kD-dextran). Results suggested that P12 shifted entry of PDGF-BB from clathrin/caveolin-mediated endocytosis to a macropinocytosis-like pathway and thereby enhanced PDGFR retention before lysosomal degradation. This introduces another layer of interaction/synergism between ECM and growth factor signaling: peptides generated by proteolytic processing of ECM can regulate GF signaling by affecting GF endocytosis.
3 ECM can regulate GF signaling independent of ECM-GF binding
All animal cells express receptors for GFs and ECM molecules (Ivaska and Heino, 2011). GFs have cell-surface receptors that are linked to cellular signaling machinery while adhesion receptors link the cell cytoskeleton to their surroundings, i.e. ECM or other cells, and concomitantly activate signaling pathways. Therefore, the fate of a cell is dependent on signals generated by both receptor systems and the integration between them. In this section we discuss how ECMs with different chemical and physical properties regulate GF signaling independent of ECM-GF binding.
3.1 ECM and GF collaborate to activate the same pathway
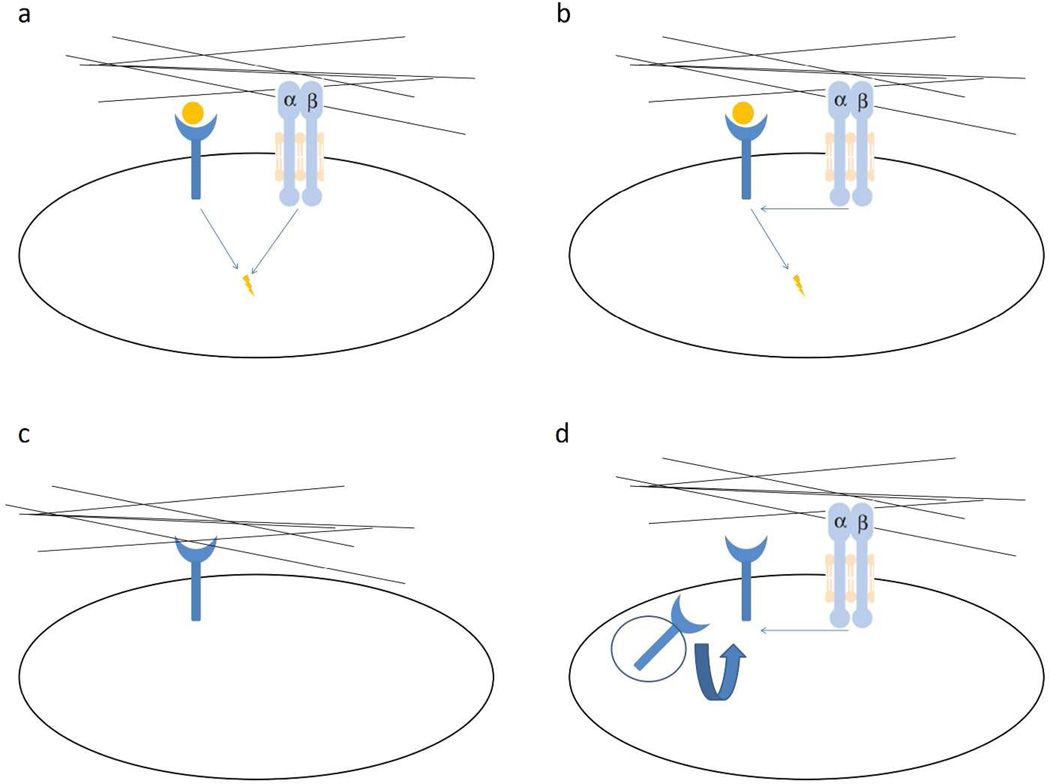
Signals triggered by cell adhesion receptors, such as integrin, are distinct but overlap with GFR signaling pathways (Figure 2a). Signals from both receptor types can converge on the same signaling pathways, e.g. Ras-MAPK pathway, PI3K-Akt pathway, Rho family GTPases (Schwartz and Ginsberg, 2002; Yamada and Even-Ram, 2002). One of the converging points between ECM and GF signaling is focal adhesion kinase (FAK) (Ilic et al., 1997). It was well established that integrin-mediated cell attachment to ECM triggers FAK activation that is required for integrin-stimulated signaling (Sieg et al., 1999). However, FAK is required to integrate, at least some, integrin and GF signals (Sieg et al., 2000). Cells deprived of FAK are refractory to motility signals from EGF while re-expression of FAK rescues these defects. At the same time, efficient EGF-stimulated migration also requires FAK for integrin-receptor clustering. In another scenario, interaction between integrin and PDGFR was demonstrated to be tissue transglutaminase-dependent (Zemskov et al., 2009).
Figure 2. ECM regulation of GF signaling independent of ECM-GF binding.
a) ECM and GF generate signals that activate the same pathway synergistically. b) The type or stiffness of ECM pre-conditions the cell and regulates the outcome of GF signaling. c) ECM can also activate GFR in the absence of GF either through integrin or direct binding to GFR. d) ECM generates signals through integrin to regulate the expression level (recycling) of GFR or GF.
3.2 ECM conditions cells for GF signaling
Anchorage dependence refers to a cell's requirement of adhesion for cell survival (Hadden and Henke, 2000; Ruoslahti and Pierschbacher, 1987). It is widely appreciated that adherent cells depend on integrin-mediated adhesion for responsiveness to GFs (Figure 2b). Loss of adhesion in normal cells results in growth arrest or anoikis owing to impaired signaling from GFs and cytokines (Danen and Yamada, 2001; Schwartz and Assoian, 2001). For example, PDGF stimulated ERK2 activation in fibroblasts is dependent on attachment to FN (Renshaw et al., 1997). However, recent reports suggest that GF signaling can occur in anchorage-independent conditions. For example, IGF1 signals were more clearly detectable in anchorage-independent conditions (poly 2-hydroxyethyl methacrylate-coated plates) than in anchorage-dependent conditions. IGF signaling required αvβ3 integrin expression, and mutant IGF that cannot bind αvβ3 was defective in inducing signals in polyHEMA-coated plates. These results suggest that αvβ3-IGF1 interaction, not αvβ3-ECM interaction, is essential for IGF signaling (Fujita et al., 2012; Fujita et al., 2013).
Besides anchorage dependence, different ECM can condition the cell to generate different responses to the same GF. For example, ligation of α5β1 integrin to FN results in Rac activation and cell-cycle progression, whereas α2β1-mediated adhesion to laminin in the same cells results in growth arrest (Cailleteau et al., 2010; Mettouchi et al., 2001). As another example, primary mammary epithelial cells treated with insulin are protected from cell death when plated on laminin, tenascin C or collagen IV, but not on collagen I (Merlo et al., 1995; Streuli et al., 1995). The protective effects of insulin are dependent on the ability of the integrin to promote activation of Akt/PKB in collaboration with insulin receptors.
The physical property of ECM can also condition cells to generate different responses (Discher et al., 2005). For example, ECM stiffness has been recognized to provide critical clues for stem cell differentiation (Discher et al., 2009; Geiger et al., 2009). Cellular responses to mechanical signals include differentiation, migration, proliferation, and alterations in cell–cell and cell–matrix adhesion. This “stiffness sensing” has been demonstrated in a variety of cell types including endothelial cells (Califano and Reinhart-King, 2010), smooth muscle cells (Engler et al., 2004), transformed cells (Levental et al., 2009) and stem cells (Engler et al., 2007). Stiff substrates increase both focal adhesion and cytoskeletal organization (Engler et al., 2004; Genes et al., 2004; Wang, 2009; Yeung et al., 2005) and conditions cells to GF stimulation. For example, matrix stiffening sensitizes epithelial cells to EGF and enables loss of contact inhibition (Kim and Asthagiri, 2011). A moderate (4.5-fold) stiffening of matrix reduces threshold amount of EGF needed to override contact inhibition. ECM stiffness also primes the TGFβ pathway to promote chondrocyte differentiation (Allen et al., 2012).
3.3 ECM can activate GFR in the absence of GF
In the absence of GFs, some ECMs can activate GFR on their own through integrin signaling. For example, FNIII9–10 (the RGD-containing central cell-binding domain) mediates FN-binding to cells and generates important survival signals either through α5β1 integrin (Zhang et al., 1995) or by activating GFR (Shen and Kramer, 2004). Furthermore, cell adhesion to FN induces α5β1 -dependent phosphorylation of PDGFR-β in the absence of GF stimulation (Veevers-Lowe et al., 2011). Similarly, GF-independent activation of c-Met by FN binding to α5β1 occurs in various systems (Mitra et al., 2011; Wang et al., 2001). In another system, activation of integrin α5/β1 by FN could also activate EGFR independent of EGF (Kuwada and Li, 2000). This EGFR transactivation was proposed to be mediated by Src dependent Tyr-845 phosphorylation on EGFR (Balanis and Carlin, 2012). In addition, some bioactive domains within other ECM molecules (matrikines) can activate GFR independent of integrin binding (Tran et al., 2005) (Figure 2c). For example, EGF-like domains from laminin (Schenk et al., 2003) or tenascin (Iyer et al., 2007) presented as soluble ligands can bind to EGFR and stimulate signaling.
3.4 ECM signaling can regulate GF and GFR expression level
As an example of ECM regulation of GF level (Figure 2d), the extra domain A (EDA) of FN increases VEGF-C expression in colorectal carcinoma (Xiang et al., 2012). Furthermore, blocking β1-integrin signaling with antibody caused a down-regulation of EGFR expression level in mammary epithelial cells cultured in three-dimensional basement membrane gels (Wang et al., 1998). This does not happen when cells were cultured in two-dimensional matrix, indicating that GFR and integrin couple in a three-dimensional environment. EGFR expression can be regulated by β1 integrin signaling and is reduced when cells are detached from ECM (Reginato et al., 2003). Also, cell adhesion to ECM inhibits PDGFR degradation thus increases PDGFR (Baron and Schwartz, 2000) and a low concentration of RGD-mimetic promote VEGF stimulated angiogenesis by increasing VEGFR-2 recycling (Ghosh et al., 2006).
4. Conclusion and future perspectives
Intensive research has demonstrated that ECM and GF signaling collaborate at many different levels. ECM signaling often synergizes with GF signaling by activating the same pathways. The type and stiffness of ECM condition the cell and regulate the outcome of GF signaling. Some ECM can activate GFR in the absence of GF. The expression level of GFR is regulated by ECM signaling.
Recently, many growth factor-binding domains have been discovered in FN, introducing a new layer of interaction between ECM and GF. First, FN acts as a reservoir of GF and thereby forms GF gradients. Proteolytic processing of FN can release GF deposited in FN while at the same time generate bioactive growth factor-binding peptides. FN tethered GFs are presented as solid phase ligand that retards GFR internalization regulating the duration and outcome of GF signaling. Also, juxtaposition of growth factor-binding domain and integrin binding site in FN facilitates formation of adhesion complex, a structural basis of ECM-GF synergistic signaling. Cells exert tension force on FN that can expose cryptic sites that activate GFR or bind GF. Recently our lab demonstrated FN peptide could regulate GF/GFR trafficking thus affecting GF signaling.
Difficult to heal or chronic wounds exhibit FN deficits and GF abnormalities that likely contribute to their stalled progression. Wound healing strategies that incorporate both FN or other ECM and GF may be beneficial for these wound types and, indeed, therapies of only one type or the other have generally proved disappointing (Agren and Werthen, 2007). Future research designed to understand ECM regulation of GF signaling in normal wound healing process may provide important insights in GF/ECM-based tissue engineering (Schultz and Wysocki, 2009).
Acknowledgements
This work was support by the Armed Forces Institute of Regenerative Medicine (RAFC) and NIH/AR063445 (RAFC).
Abbreviations
- ECM
extracellular matrix
- GF
growth factor
- GFR
growth factor receptor
- FN
fibronectin
- PDGF
platelet-derived growth factor
- EGF
epidermal growth factor
- VEGF
vascular endothelial growth factor
Footnotes
Conflict of Interest:
Richard Clark co-discovered P12, a cryptic peptide in FN that binds and enhances PDGF-BB as well as other GF. Richard Clark is President and Founder of NeoMatrix Formulations, a biotechnology company engaged in preclinical studies for P12 treatment of burns.
REFERENCES
- Agren MS, Werthen M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int J Low Extrem Wounds. 2007;6:82–97. doi: 10.1177/1534734607301394. [DOI] [PubMed] [Google Scholar]
- Allen JL, Cooke ME, Alliston T. ECM stiffness primes the TGFbeta pathway to promote chondrocyte differentiation. Mol Biol Cell. 2012;23:3731–3742. doi: 10.1091/mbc.E12-03-0172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arroyo AG, Iruela-Arispe ML. Extracellular matrix, inflammation, and the angiogenic response. Cardiovasc Res. 2010;86:226–235. doi: 10.1093/cvr/cvq049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balanis N, Carlin CR. Mutual cross-talk between fibronectin integrins and the EGF receptor: Molecular basis and biological significance. Cell Logist. 2012;2:46–51. doi: 10.4161/cl.20112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron V, Schwartz M. Cell adhesion regulates ubiquitin-mediated degradation of the platelet-derived growth factor receptor beta. J Biol Chem. 2000;275:39318–39323. doi: 10.1074/jbc.M003618200. [DOI] [PubMed] [Google Scholar]
- Cailleteau L, Estrach S, Thyss R, Boyer L, Doye A, Domange B, Johnsson N, Rubinstein E, Boucheix C, Ebrahimian T, Silvestre JS, Lemichez E, Meneguzzi G, Mettouchi A. alpha2beta1 integrin controls association of Rac with the membrane and triggers quiescence of endothelial cells. J Cell Sci. 2010;123:2491–2501. doi: 10.1242/jcs.058875. [DOI] [PubMed] [Google Scholar]
- Califano JP, Reinhart-King CA. Substrate Stiffness and Cell Area Predict Cellular Traction Stresses in Single Cells and Cells in Contact. Cell Mol Bioeng. 2010;3:68–75. doi: 10.1007/s12195-010-0102-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox TR, Erler JT. Remodeling and homeostasis of the extracellular matrix: implications for fibrotic diseases and cancer. Dis Model Mech. 2011;4:165–178. doi: 10.1242/dmm.004077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danen EH, Yamada KM. Fibronectin, integrins, and growth control. J Cell Physiol. 2001;189:1–13. doi: 10.1002/jcp.1137. [DOI] [PubMed] [Google Scholar]
- Discher DE, Janmey P, Wang YL. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005;310:1139–1143. doi: 10.1126/science.1116995. [DOI] [PubMed] [Google Scholar]
- Discher DE, Mooney DJ, Zandstra PW. Growth factors, matrices, and forces combine and control stem cells. Science. 2009;324:1673–1677. doi: 10.1126/science.1171643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler A, Bacakova L, Newman C, Hategan A, Griffin M, Discher D. Substrate compliance versus ligand density in cell on gel responses. Biophys J. 2004;86:617–628. doi: 10.1016/S0006-3495(04)74140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engler AJ, Sweeney HL, Discher DE, Schwarzbauer JE. Extracellular matrix elasticity directs stem cell differentiation. J Musculoskelet Neuronal Interact. 2007;7:335. [PubMed] [Google Scholar]
- Evans CE, Humphries J, Waltham M, Saha P, Mattock K, Patel A, Ahmad A, Wadoodi A, Modarai B, Rahman S, Patel Y, Smith A. Protein fragments from the VEGF binding domain of fibronectin are expressed in distinct spatial and temporal patterns during venous thrombus resolution. Thromb Res. 2012;130:281–284. doi: 10.1016/j.thromres.2012.05.006. [DOI] [PubMed] [Google Scholar]
- Fujita M, Ieguchi K, Davari P, Yamaji S, Taniguchi Y, Sekiguchi K, Takada YK, Takada Y. Cross-talk between integrin alpha6beta4 and insulin-like growth factor-1 receptor (IGF1R) through direct alpha6beta4 binding to IGF1 and subsequent alpha6beta4-IGF1-IGF1R ternary complex formation in anchorage-independent conditions. J Biol Chem. 2012;287:12491–12500. doi: 10.1074/jbc.M111.304170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Takada YK, Takada Y. Insulin-like growth factor (IGF) signaling requires alphavbeta3-IGF1-IGF type 1 receptor (IGF1R) ternary complex formation in anchorage independence, and the complex formation does not require IGF1R and Src activation. J Biol Chem. 2013;288:3059–3069. doi: 10.1074/jbc.M112.412536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger B, Spatz JP, Bershadsky AD. Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol. 2009;10:21–33. doi: 10.1038/nrm2593. [DOI] [PubMed] [Google Scholar]
- Genes NG, Rowley JA, Mooney DJ, Bonassar LJ. Effect of substrate mechanics on chondrocyte adhesion to modified alginate surfaces. Arch Biochem Biophys. 2004;422:161–167. doi: 10.1016/j.abb.2003.11.023. [DOI] [PubMed] [Google Scholar]
- Ghosh K, Ren XD, Shu XZ, Prestwich GD, Clark RA. Fibronectin functional domains coupled to hyaluronan stimulate adult human dermal fibroblast responses critical for wound healing. Tissue Eng. 2006;12:601–613. doi: 10.1089/ten.2006.12.601. [DOI] [PubMed] [Google Scholar]
- Grant MB, Caballero S, Bush DM, Spoerri PE. Fibronectin fragments modulate human retinal capillary cell proliferation and migration. Diabetes. 1998;47:1335–1340. doi: 10.2337/diab.47.8.1335. [DOI] [PubMed] [Google Scholar]
- Hadden HL, Henke CA. Induction of lung fibroblast apoptosis by soluble fibronectin peptides. Am J Respir Crit Care Med. 2000;162:1553–1560. doi: 10.1164/ajrccm.162.4.2001015. [DOI] [PubMed] [Google Scholar]
- Hynes RO. The extracellular matrix: not just pretty fibrils. Science. 2009;326:1216–1219. doi: 10.1126/science.1176009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilic D, Damsky CH, Yamamoto T. Focal adhesion kinase: at the crossroads of signal transduction. J Cell Sci. 1997;110(Pt 4):401–407. doi: 10.1242/jcs.110.4.401. [DOI] [PubMed] [Google Scholar]
- Ishitsuka T, Ikuta T, Ariga H, Matsumoto K. Serum tenascin-X strongly binds to vascular endothelial growth factor. Biol Pharm Bull. 2009;32:1004–1011. doi: 10.1248/bpb.32.1004. [DOI] [PubMed] [Google Scholar]
- Ivaska J, Heino J. Cooperation between integrins and growth factor receptors in signaling and endocytosis. Annu Rev Cell Dev Biol. 2011;27:291–320. doi: 10.1146/annurev-cellbio-092910-154017. [DOI] [PubMed] [Google Scholar]
- Iyer AK, Tran KT, Borysenko CW, Cascio M, Camacho CJ, Blair HC, Bahar I, Wells A. Tenascin cytotactin epidermal growth factor-like repeat binds epidermal growth factor receptor with low affinity. J Cell Physiol. 2007;211:748–758. doi: 10.1002/jcp.20986. [DOI] [PubMed] [Google Scholar]
- Kim JH, Asthagiri AR. Matrix stiffening sensitizes epithelial cells to EGF and enables the loss of contact inhibition of proliferation. J Cell Sci. 2011;124:1280–1287. doi: 10.1242/jcs.078394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Turnbull J, Guimond S. Extracellular matrix and cell signalling: the dynamic cooperation of integrin, proteoglycan and growth factor receptor. J Endocrinol. 2011;209:139–151. doi: 10.1530/JOE-10-0377. [DOI] [PubMed] [Google Scholar]
- Kuhl PR, Griffith-Cima LG. Tethered epidermal growth factor as a paradigm for growth factor-induced stimulation from the solid phase. Nat Med. 1996;2:1022–1027. doi: 10.1038/nm0996-1022. [DOI] [PubMed] [Google Scholar]
- Kuwada SK, Li X. Integrin alpha5/beta1 mediates fibronectin-dependent epithelial cell proliferation through epidermal growth factor receptor activation. Mol Biol Cell. 2000;11:2485–2496. doi: 10.1091/mbc.11.7.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legate KR, Wickstrom SA, Fassler R. Genetic and cell biological analysis of integrin outside-in signaling. Genes Dev. 2009;23:397–418. doi: 10.1101/gad.1758709. [DOI] [PubMed] [Google Scholar]
- Levental KR, Yu H, Kass L, Lakins JN, Egeblad M, Erler JT, Fong SF, Csiszar K, Giaccia A, Weninger W, Yamauchi M, Gasser DL, Weaver VM. Matrix crosslinking forces tumor progression by enhancing integrin signaling. Cell. 2009;139:891–906. doi: 10.1016/j.cell.2009.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ren XD, Pan Z, Macri L, Zong WX, Tonnesen MG, Rafailovich M, Bar-Sagi D, Clark RA. Fibronectin growth factor-binding domains are required for fibroblast survival. J Invest Dermatol. 2011;131:84–98. doi: 10.1038/jid.2010.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Zhu J, Tonnesen MG, Taira BR, McClain SA, Singer AK, Clark RAF. Novel fibronectin peptides bind PDGF-BB and enhance cell and tissue survival under stress. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.420. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macri L, Silverstein D, Clark RA. Growth factor binding to the pericellular matrix and its importance in tissue engineering. Adv Drug Deliv Rev. 2007;59:1366–1381. doi: 10.1016/j.addr.2007.08.015. [DOI] [PubMed] [Google Scholar]
- Martino MM, Briquez PS, Ranga A, Lutolf MP, Hubbell JA. Heparin-binding domain of fibrin(ogen) binds growth factors and promotes tissue repair when incorporated within a synthetic matrix. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:4563–4568. doi: 10.1073/pnas.1221602110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino MM, Hubbell JA. The 12th–14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. FASEB J. 2010;24:4711–4721. doi: 10.1096/fj.09-151282. [DOI] [PubMed] [Google Scholar]
- Martino MM, Tortelli F, Mochizuki M, Traub S, Ben-David D, Kuhn GA, Muller R, Livne E, Eming SA, Hubbell JA. Engineering the growth factor microenvironment with fibronectin domains to promote wound and bone tissue healing. Sci Transl Med. 2011;3:100ra189. doi: 10.1126/scitranslmed.3002614. [DOI] [PubMed] [Google Scholar]
- Merlo GR, Basolo F, Fiore L, Duboc L, Hynes NE. p53-dependent and p53-independent activation of apoptosis in mammary epithelial cells reveals a survival function of EGF and insulin. J Cell Biol. 1995;128:1185–1196. doi: 10.1083/jcb.128.6.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettouchi A, Klein S, Guo W, Lopez-Lago M, Lemichez E, Westwick JK, Giancotti FG. Integrin-specific activation of Rac controls progression through the G(1) phase of the cell cycle. Mol Cell. 2001;8:115–127. doi: 10.1016/s1097-2765(01)00285-4. [DOI] [PubMed] [Google Scholar]
- Mitra AK, Sawada K, Tiwari P, Mui K, Gwin K, Lengyel E. Ligand-independent activation of c-Met by fibronectin and alpha(5)beta(1)-integrin regulates ovarian cancer invasion and metastasis. Oncogene. 2011;30:1566–1576. doi: 10.1038/onc.2010.532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsi M, Hong Z, Costello CE, Nugent MA. Heparin-mediated conformational changes in fibronectin expose vascular endothelial growth factor binding sites. Biochemistry. 2006;45:10319–10328. doi: 10.1021/bi060974p. [DOI] [PubMed] [Google Scholar]
- Miyamoto S, Teramoto H, Gutkind JS, Yamada KM. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: roles of integrin aggregation and occupancy of receptors. J Cell Biol. 1996;135:1633–1642. doi: 10.1083/jcb.135.6.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadi M, Olsen SK, Goetz R. A protein canyon in the FGF-FGF receptor dimer selects from an a la carte menu of heparan sulfate motifs. Curr Opin Struct Biol. 2005;15:506–516. doi: 10.1016/j.sbi.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Mosher DF, Sottile J, Wu C, McDonald JA. Assembly of extracellular matrix. Curr. Opin. Cell Biol. 1992;4:810–818. doi: 10.1016/0955-0674(92)90104-k. [DOI] [PubMed] [Google Scholar]
- Motegi S, Garfield S, Feng X, Sardy M, Udey MC. Potentiation of platelet-derived growth factor receptor-beta signaling mediated by integrin-associated MFG-E8. Arterioscler Thromb Vasc Biol. 2011;31:2653–2664. doi: 10.1161/ATVBAHA.111.233619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt MO, Roman AJ, Wells A, Lauffenburger DA, Griffith LG. Sustained epidermal growth factor receptor levels and activation by tethered ligand binding enhances osteogenic differentiation of multi-potent marrow stromal cells. J Cell Physiol. 2009;221:306–317. doi: 10.1002/jcp.21854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper GE, McNamee HP, Dike LE, Bojanowski K, Ingber DE. Convergence of integrin and growth factor receptor signaling pathways within the focal adhesion complex. Mol Biol Cell. 1995;6:1349–1365. doi: 10.1091/mbc.6.10.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman S, Patel Y, Murray J, Patel KV, Sumathipala R, Sobel M, Wijelath ES. Novel hepatocyte growth factor (HGF) binding domains on fibronectin and vitronectin coordinate a distinct and amplified Met-integrin induced signalling pathway in endothelial cells. BMC Cell Biol. 2005;6:8. doi: 10.1186/1471-2121-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginato MJ, Mills KR, Paulus JK, Lynch DK, Sgroi DC, Debnath J, Muthuswamy SK, Brugge JS. Integrins and EGFR coordinately regulate the pro-apoptotic protein Bim to prevent anoikis. Nat Cell Biol. 2003;5:733–740. doi: 10.1038/ncb1026. [DOI] [PubMed] [Google Scholar]
- Renshaw MW, Ren XD, Schwartz MA. Growth factor activation of MAP kinase requires cell adhesion. EMBO J. 1997;16:5592–5599. doi: 10.1093/emboj/16.18.5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues M, Blair H, Stockdale L, Griffith L, Wells A. Surface tethered epidermal growth factor protects proliferating and differentiating multipotential stromal cells from FasL-induced apoptosis. Stem Cells. 2013;31:104–116. doi: 10.1002/stem.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruoslahti E, Pierschbacher MD. New perspectives in cell adhesion: RGD and integrins. Science. 1987;238:491–497. doi: 10.1126/science.2821619. [DOI] [PubMed] [Google Scholar]
- Schenk S, Hintermann E, Bilban M, Koshikawa N, Hojilla C, Khokha R, Quaranta V. Binding to EGF receptor of a laminin-5 EGF-like fragment liberated during MMP-dependent mammary gland involution. J Cell Biol. 2003;161:197–209. doi: 10.1083/jcb.200208145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound Repair Regen. 2009;17:153–162. doi: 10.1111/j.1524-475X.2009.00466.x. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Assoian RK. Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J Cell Sci. 2001;114:2553–2560. doi: 10.1242/jcs.114.14.2553. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Shen X, Kramer RH. Adhesion-mediated squamous cell carcinoma survival through ligand-independent activation of epidermal growth factor receptor. Am J Pathol. 2004;165:1315–1329. doi: 10.1016/S0002-9440(10)63390-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Ilic D, Klingbeil CK, Schaefer E, Damsky CH, Schlaepfer DD. FAK integrates growth-factor and integrin signals to promote cell migration. Nat Cell Biol. 2000;2:249–256. doi: 10.1038/35010517. [DOI] [PubMed] [Google Scholar]
- Sieg DJ, Hauck CR, Schlaepfer DD. Required role of focal adhesion kinase (FAK) for integrin-stimulated cell migration. J Cell Sci. 1999;112(Pt 16):2677–2691. doi: 10.1242/jcs.112.16.2677. [DOI] [PubMed] [Google Scholar]
- Streuli CH, Schmidhauser C, Bailey N, Yurchenco P, Skubitz AP, Roskelley C, Bissell MJ. Laminin mediates tissue-specific gene expression in mammary epithelia. J Cell Biol. 1995;129:591–603. doi: 10.1083/jcb.129.3.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran KT, Lamb P, Deng JS. Matrikines and matricryptins: Implications for cutaneous cancers and skin repair. J Dermatol Sci. 2005;40:11–20. doi: 10.1016/j.jdermsci.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Veevers-Lowe J, Ball SG, Shuttleworth A, Kielty CM. Mesenchymal stem cell migration is regulated by fibronectin through alpha5beta1-integrin-mediated activation of PDGFR-beta and potentiation of growth factor signals. J Cell Sci. 2011;124:1288–1300. doi: 10.1242/jcs.076935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel V. Mechanotransduction involving multimodular proteins: converting force into biochemical signals. Annu Rev Biophys Biomol Struct. 2006;35:459–488. doi: 10.1146/annurev.biophys.35.040405.102013. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, Larabell CA, Dedhar S, Briand P, Lupu R, Bissell MJ. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci U S A. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R, Ferrell LD, Faouzi S, Maher JJ, Bishop JM. Activation of the Met receptor by cell attachment induces and sustains hepatocellular carcinomas in transgenic mice. J Cell Biol. 2001;153:1023–1034. doi: 10.1083/jcb.153.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YL. Traction forces and rigidity sensing of adherent cells. Conf Proc IEEE Eng Med Biol Soc. 2009;2009:3339–3340. doi: 10.1109/IEMBS.2009.5333200. [DOI] [PubMed] [Google Scholar]
- Wijelath ES, Rahman S, Namekata M, Murray J, Nishimura T, Mostafavi-Pour Z, Patel Y, Suda Y, Humphries MJ, Sobel M. Heparin-II domain of fibronectin is a vascular endothelial growth factor-binding domain: enhancement of VEGF biological activity by a singular growth factor/matrix protein synergism. Circ Res. 2006;99:853–860. doi: 10.1161/01.RES.0000246849.17887.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang L, Xie G, Ou J, Wei X, Pan F, Liang H. The extra domain A of fibronectin increases VEGF-C expression in colorectal carcinoma involving the PI3K/AKT signaling pathway. PLoS One. 2012;7:e35378. doi: 10.1371/journal.pone.0035378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada KM, Even-Ram S. Integrin regulation of growth factor receptors. Nat Cell Biol. 2002;4:E75–E76. doi: 10.1038/ncb0402-e75. [DOI] [PubMed] [Google Scholar]
- Yeung T, Georges PC, Flanagan LA, Marg B, Ortiz M, Funaki M, Zahir N, Ming W, Weaver V, Janmey PA. Effects of substrate stiffness on cell morphology, cytoskeletal structure, and adhesion. Cell Motil Cytoskeleton. 2005;60:24–34. doi: 10.1002/cm.20041. [DOI] [PubMed] [Google Scholar]
- Zemskov EA, Loukinova E, Mikhailenko I, Coleman RA, Strickland DK, Belkin AM. Regulation of platelet-derived growth factor receptor function by integrin-associated cell surface transglutaminase. J Biol Chem. 2009;284:16693–16703. doi: 10.1074/jbc.M109.010769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Vuori K, Reed JC, Ruoslahti E. The alpha 5 beta 1 integrin supports survival of cells on fibronectin and up-regulates Bcl-2 expression. Proc Natl Acad Sci U S A. 1995;92:6161–6165. doi: 10.1073/pnas.92.13.6161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong C, Chrzanowska-Wodnicka M, Brown J, Shaub A, Belkin AM, Burridge K. Rho-mediated contractility exposes a cryptic site in fibronectin and induces fibronectin matrix assembly. J Cell Biol. 1998;141:539–551. doi: 10.1083/jcb.141.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J, Lin F, Brown D, RAF Clark. A fibronectin peptide redirects PDGF-BB/PDGFR complexes to macropinocytosis-like internalization and augments PDGF-BB survival signals. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.463. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]