Abstract
Tumor-associated macrophages (TAMs) play essential roles in tumor progression and metastasis. Tumor cells recruit myeloid progenitors and monocytes to the tumor site, where they differentiate into TAMs; however, this process is not well studied in humans. Here we show that human CD7, a T cell and NK cell receptor, is highly expressed by monocytes and macrophages. Expression of CD7 decreases in M-CSF differentiated macrophages and in Melanoma-conditioned Medium Induced Macrophages (MCMI/Mϕ) in comparison to monocytes. A ligand for CD7, SECTM1 (Secreted and transmembrane protein 1), is highly expressed in many tumors, including melanoma cells. We show that SECTM1 binds to CD7 and significantly increases monocyte migration by activation of the PI3K pathway. In human melanoma tissues, tumor-infiltrating macrophages expressing CD7 are present. These melanomas, with CD7-positive inflammatory cell infiltrations, frequently highly express SECTM1, including an N-terminal, soluble form, which can be detected in the sera of metastatic melanoma patients but not in normal sera. Taken together, our data demonstrate that CD7 is present on monocytes and tumor macrophages, and that its ligand, SECTM1, is frequently expressed in corresponding melanoma tissues, possibly acting as a chemoattractant for monocytes to modulate the melanoma microenvironment.
Introduction
Tumor-associated macrophages (TAMs) are a major component of tumor stroma, and they modulate the tumor microenvironment by increasing tumor initiation and growth, remodeling the extracellular matrix, promoting angiogenesis and suppressing anti-tumor immunity. High numbers of macrophages are associated with a poor prognosis in a variety of cancers, including breast cancer and colon cancer (Qian and Pollard, 2010; Solinas et al., 2009; Torisu et al., 2000). In melanoma, it has been reported that macrophages are essential for ultraviolet radiation-induced melanomagenesis in a neonatal mouse model (Handoko et al., 2013; Zaidi et al., 2011). Furthermore, macrophages produce many factors that suppress T cell functions, and increase tumor angiogenesis, invasion and metastasis (Gazzaniga et al., 2007; Varney et al., 2005; Wang et al., 2012a). More importantly, it has been reported that the numbers of macrophages in melanoma tissues correlate with poor prognosis in early stage melanomas (stage I, II) (Jensen et al., 2009).
Macrophages have been classified into M1 and M2 macrophages depending on the environmental cues. M1 macrophages are polarized by LPS and IFN-γ, and produce high levels of IL-12 and low levels of IL-10, while M2 macrophages are polarized by IL-4, and produce low levels of IL-12 and high levels of IL-10. It is generally accepted that TAMs resemble M2 macrophages and exert pro-tumor activity. However, recent studies indicated that TAMs are highly heterogeneous, and may have mixed phenotypes of M1 and M2 macrophages. Our previous study demonstrated that Melanoma-Conditioned Medium Induced Macrophages (MCMI/Mϕ) express both M1 and M2 macrophage markers, but exert M2 macrophage functions, such as immune suppression and enhancement of melanoma cell invasion (Wang et al., 2012a).
Macrophages are differentiated from monocytes. Tumor cells can promote this by elaborating many factors, including M-CSF and CCL-2, that recruit blood monocytes and contribute to their differentiation to macrophages (Duluc et al., 2007; Pixley and Stanley, 2004; Roca et al., 2009). However, other unknown factors may also play roles in monocyte migration.
CD7 is a 40 kDa type I transmembrane glycoprotein, which is found on T cells, natural killer (NK) cells, myeloid precursor cells, plasmacytoid dendritic cells (DCs), as well as a variety of leukemia cells (Milush et al., 2009; Sempowski et al., 1999; Stillwell and Bierer, 2001). Despite extensive analysis, no dominant biological process or disease has been discovered that results from CD7 loss of function or overstimulation in humans. Loss of CD7 in a murine model system showed only a mild effect, mainly a reduction of regulatory T cells and a resistance to LPS induced septic shock. CD7 is also expressed by early hematopoietic precursors, including the common lymphoid progenitor, but it has no defined role in blood cell formation. It has been reported that CD7 expression is lost when cells differentiate from myeloid precursor cells to monocytes (Wells et al., 2003).
A potentially important role for CD7 is activation by its ligand SECTM1 in the co-stimulation and proliferation of T and NK cells (Lyman et al., 2000; Wang et al., 2012b). SECTM1 is a 27 kDa type I transmembrane protein whose soluble form is a ligand for CD7. SECTM1 is expressed by a number of normal cell types, including neutrophils, dendritic cells and epithelial cells. It is also highly expressed by breast and prostate cancer cell lines, and by some myeloid leukemias (Lam et al., 2005; Slentz-Kesler et al., 1998; Wang et al., 2012b). However, the biological significance of SECTM1 expression in tumors is unknown. The expression of SECTM1 can be up-regulated by IFN-γ in many cell types, including thymic epithelial cells, monocytes and DCs, as well as some types of tumor cells (Huyton et al., 2011; Lam et al., 2005; Wang et al., 2012b)
Anecdotal evidence has suggested that CD7 might be expressed by monocytes. We have performed microarray analyses of monocytes and different types of macrophages, which demonstrated definitively that CD7 is expressed in human monocytes but is down-regulated in MCMI/Mϕ (Wang et al., 2012a). In this study, we confirmed that CD7 is expressed in human monocytes at both the RNA and protein levels and to a lesser extent in M-CSF differentiated macrophages (M-CSF/Mϕ) and in MCMI-Mϕ. CD7 is expressed only at a very low level in GM-CSF differentiated macrophages (GM-CSF/Mϕ).
Our analysis of the expression of the ligand for CD7 has shown that SECTM1 is highly expressed in melanoma cell lines compared to normal melanocytes, and is induced by interferon-α and Velcade. We demonstrated that SECTM1 activates the PI3 kinase/AKT signaling pathway in monocytes and increases monocyte migration in a CD7-dependent manner. SECTM1 is not only expressed at high levels in melanoma tissues but also is detectable by western blot in sera from metastatic melanoma patients, but not in normal sera. Since melanoma tissues contain a significant number of CD7-positive macrophages, SECTM1 may have a role in either attracting or activating monocytes in melanomas. Therefore, the interaction of CD7 and SECTM1 may have a significant biological role in melanoma and may affect the efficacy of melanoma therapies.
Results and Discussion
CD7 is expressed in monocytes and macrophages
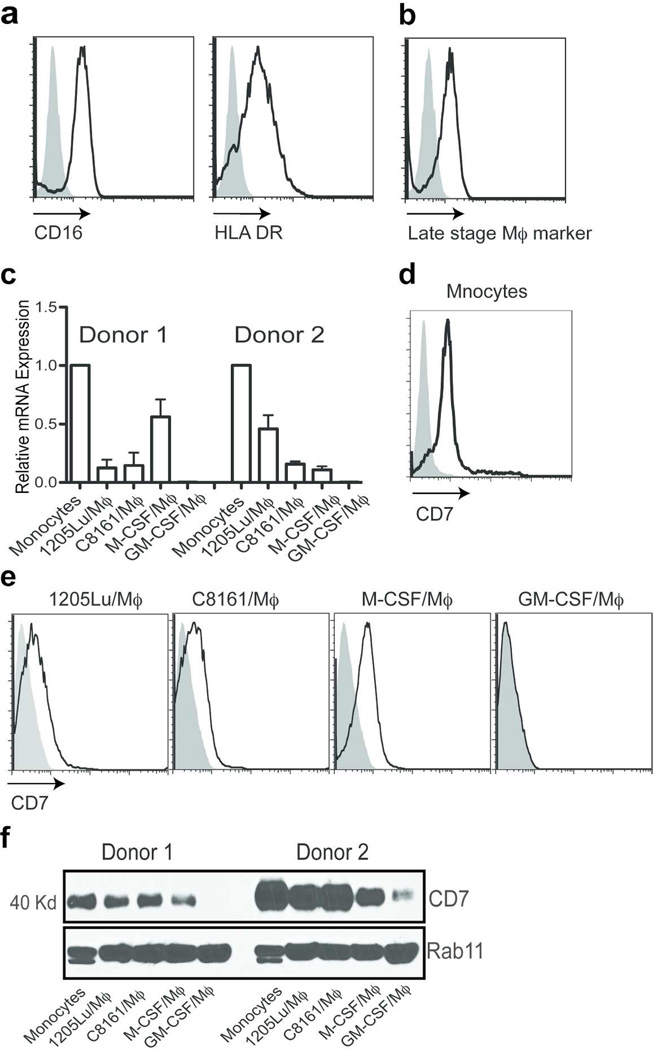
We have reported an in vitro system to differentiate monocytes to macrophages using melanoma-conditioned media (Wang et al., 2012a). Because it has been shown that myeloid-derived suppressor cells (MDSC) express many markers similar to macrophages and share many similar functions with tumor-associated macrophages in human tumors (Nagaraj and Gabrilovich, 2010), we further characterized MCMI/Mϕ to exclude the possibility of MDSC contamination. We found that MCMI/Mϕ express CD16 and HLA-DR, two markers that are negative for MDSC (Figure 1a). MCMI/Mϕ also express a late-stage macrophage marker (Figure 1b). Together, these data and our previous work indicate that MCMI/ϕ are highly similar to the tumor-associated macrophages.
Figure 1. Expression of CD7 by monocytes and macrophages.
Expression of CD16, HLA DR (a) and the late stage maker (b) was analyzed by flow cytometry. 1205Lu-Mϕ) were stained with the fluorescence-conjugated anti-CD16, HLA DR and late stage macrophage marker. (c) Real-time PCR was used to analyze the expression of CD7 in monocytes, GM-CSF, M-CSF differentiated macrophages, and C8161 (C68161-Mϕ) and 1205Lu (1205Lu-Mϕ) melanoma conditioned media differentiated Mϕ. Samples were normalized to GAPDH. Monocytes (d) M-CSF/Mϕ, M-CSF/M(|), C8161/Mϕ) and 1205Lu/Mϕ) (e) were stained with the anti-human CD7 monoclonal antibody, 3A1, following by FITC-conjugated anti-mouse IgG staining for FACS analysis. Filled: Isotype control, black line: anti-CD7. (f) Western blot analysis expression of CD7 in monocytes, GM-CSF/Mϕ), M-CSF/Mϕ, and C8161/Mϕ and 1205Lu/Mϕ. RAb11 was used as a loading control.
To confirm the expression of CD7 in monocytes and macrophages, we performed real-time PCR for CD7 on monocytes, MCMI/Mϕ, M-CSF/Mϕ and GM-CSF/Mϕ (Hume and MacDonald, 2012). We found that mRNA levels for CD7 were expressed at a high level in monocytes, while expression of CD7 was expressed at a lower level in M-CSF/Mϕ and in MCMI/Mϕ. A much lower level of CD7 expression was detected in GM-CSF/Mϕ (Figure 1c). Next, we examined the expression of CD7 at the protein level in monocytes, M-CSF/Mϕ, GM-CSF/Mϕ and in MCMI-Mϕ by flow cytometric analysis with the anti-CD7 antibody, 3A1, which also was used to stain for cell surface expression of CD7 in T cells. Corresponding to the RNA expression studies, CD7 was also expressed in monocytes (Figure 1d), M-CSF/Mϕ, C8161/Mϕ and 1205Lu/Mϕ, but not in GM-CSF/Mϕ (Figure 1e). Finally, we conducted western blot analysis with a novel rabbit anti-human CD7 monoclonal antibody, which recognizes the C-terminal 25 amino acids of the CD7 molecule. This peptide was used to produce the antibody because it contains no homologous sequence in other human molecules, and staining of cells known to be negative for CD7 expression by RT-PCR supports the specificity of this antibody (data not shown). Consistent with the flow cytometric analysis results, western blot analysis of purified monocytes and macrophages with this antibody revealed an anticipated 40 kDa band, with the highest level of CD7 expression in monocytes and with lowest levels in GM-CSF/Mϕ (Figure 1e).
Despite the widespread use of anti-CD7 antibodies, there has not been a definitive study demonstrating CD7 expression on monocytes and macrophages. In order to better understand this, we used several other commercially available CD7 monoclonal antibodies to look for the expression of CD7 on these cell types and, contrary to our results described above, these antibodies did not detect the expression of CD7 in monocytes. It is possible that those antibodies recognize different epitopes or have a lower affinity than the CD7 antibodies used in this study. However, similar differences in results with different anti-CD7 antibodies were also seen in studies to detect the expression of CD7 in T cells (Haynes, 1981). It is also possible that different epitopes of CD7 are present in monocytes compared to T cells due to modification(s) or clustering with other molecules. For example, an anti-CD7 monoclonal antibody, clone 3D9, does not recognize intestinal intraepithelial lymphocytes (IEL), whereas most other anti-CD7 antibodies recognize them (Russell et al., 1994). Nonetheless, our data definitively demonstrate that CD7 is expressed in monocytes, M-CSF/Mϕ and MCMI/Mϕ and at much lower levels or undetectable levels in GM-CSF/Mϕ, suggesting that CD7 might play roles in modulating the functions of monocytes and macrophages within the tumor microenvironment.
SECTM1 is highly expressed in melanomas
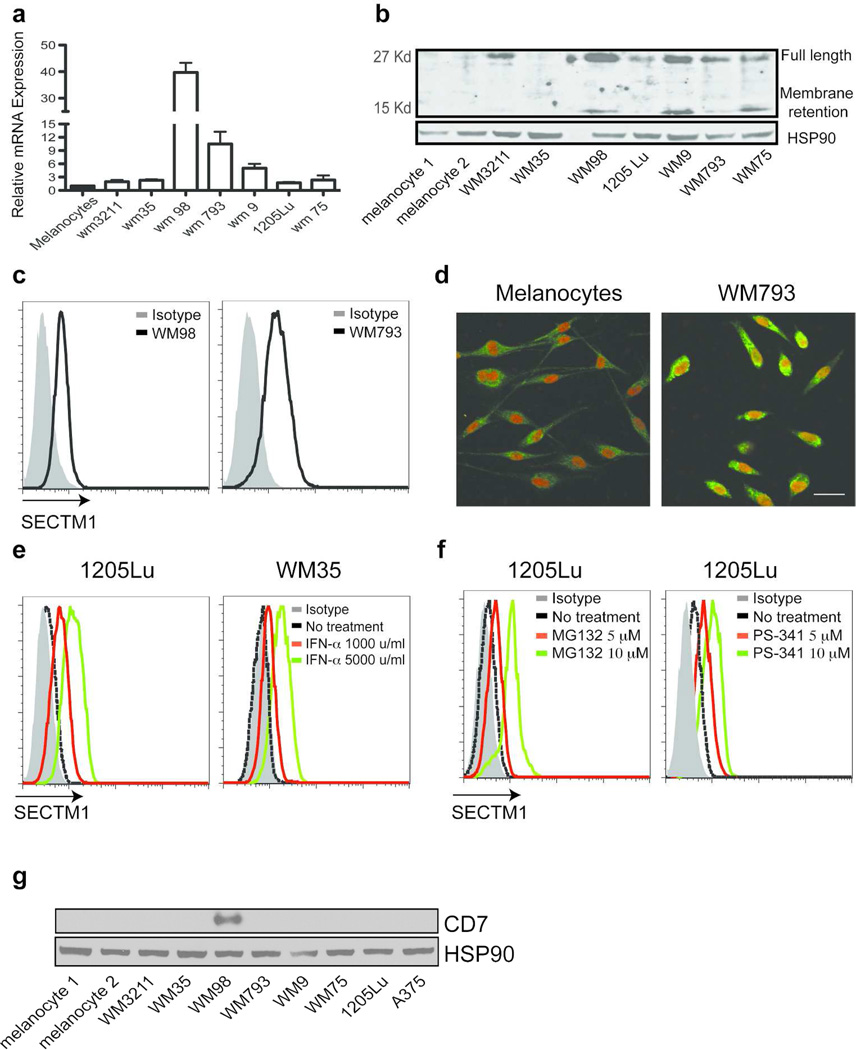
We previously reported that SECTM1 is expressed in breast cancer and in prostate cancer cell lines (Slentz-Kesler et al., 1998). Bioinformatics analysis of the Gene Expression Omnibus (GEO) database indicates that SECTM1 is also expressed in other types of cancers, including melanomas (data not shown). We further characterized the expression of SECTM1 in melanoma cell lines and in cultured melanocytes by realtime PCR. SECTM1 is highly expressed in many of the melanoma cell lines analyzed and is particularly highly expressed in WM793, WM98 and WM9 cells in comparison with normal melanocytes (Figure 2a). Analysis of protein expression of SECTM1 by western blot was generally consistent with the mRNA analysis, with expression of SECTM1 (full length and the membrane retained segment) being higher in melanoma cells than in melanocytes (Figure 2b). Flow cytometric analysis indicated that SECTM1 can be detected in WM98 and WM793 melanoma cells by intracellular staining, but not by surface staining (Figure 2c, and data not shown). Furthermore, confocal microscopy analysis confirmed that SECTM1 was expressed more highly in melanoma cells than in melanocytes, with the pattern being perinuclear, but not on the cell surface (Figure 2d). This expression pattern is similar to that of SECTM1 seen in primary breast cancer lines (Slentz-Kesler et al., 1998). Interestingly, we found that CD7 is not expressed in melanocytes, but is expressed in one of eight melanoma cell lines, WM98 (Figure 2g). Furthermore, this cell line also expresses a high level of SECTM1 compared to other melanoma cell lines tested, indicating the potential for autocrine interaction between SECTM1 and CD7 in melanoma cells.
Figure 2. SECTM1 is highly expressed in melanoma.
(a) Relative amounts of SECTM1 mRNA expression were determined by real-time RT-PCR in melanoma cells and melanocytes. Samples were normalized to GAPDH. (b) Western blot analysis of SECTM1 expression in melanocytes and in melanoma cells. The upper band is the 27 kD full length SECTM1, the lower band is the membrane retention part of SECTM1. HSP90 was used as the loading control. (c) Expression of SECTM1 analyzed by flow cytometry. WM98 and WM793 cells were intracellularly stained with an anti-SECTM1 antibody. (d) Confocal analysis of SECTM1 expression (green) in melanocytes and in 1205Lu melanoma cells. Topro-3 was used for nuclear staining (Red color). Scale bar = 30 µm. (e) WM35 and 1205Lu melanoma cells were treated with increased concentrations of interferon-α for 6 hr. Intracellular staining was performed to measure the expression of SECTM1 by FACS analysis. (f) 1205Lu cells were stimulated with the indicated concentration of proteasome inhibitors, MG-132 (left panel) and Velcade (PS-341, right panel) for 4 hr. Intracellular staining was performed to measure the expression of SECTM1. (g) Cell lysates from melanocytes and melanoma cell lines were loaded and analyzed by western blot using a rabbit monoclonal anti-CD7 antibody. HSP90 was used as a loading control.
Expression of SECTM1 is induced by interferon-α and proteasome inhibitors
Our previous work demonstrated that the expression of SECTM1 is induced by interferon-γ in a STAT1 transcription factor dependent manner (Wang et al., 2012b). Since interferon-α has been used as an immune adjuvant therapy for melanoma therapy, and many genes that are regulated by interferon-γ are also induced by interferon-α through the activation of STAT1, we investigated whether SECTM1 was also induced in melanoma cells by interferon-α. As expected, flow cytometric analysis showed that SECTM1 was strongly induced in 1205Lu and WM35 melanoma cell lines (Figure 2e).
It was noted that the pattern of expression of SECTM1 protein in melanoma cells was not fully consistent with the mRNA levels (Figure 2a, b), which suggested that the expression of SECTM1 might also be regulated at the post-transcriptional level. Indeed, we demonstrated using flow cytometric analysis that SECTM1 was significantly induced after WM35 melanoma cells were treated with proteasome inhibitors, MG-132 or Velcade (PS-341). Western blot analysis also indicated that MG132 induces SECTM1 expression in WM35 cells in a dose and time dependent manner (Figure S1). Velcade is a proteasome inhibitor that is FDA approved for treating multiple myeloma, and has been used in clinical trials for treating melanomas and breast cancer (Figure 2f). These drugs would likely have an effect on SECTM1 expression and could have a clinical effect on these tumors.
SECTM1 increases monocyte migration via binding to CD7
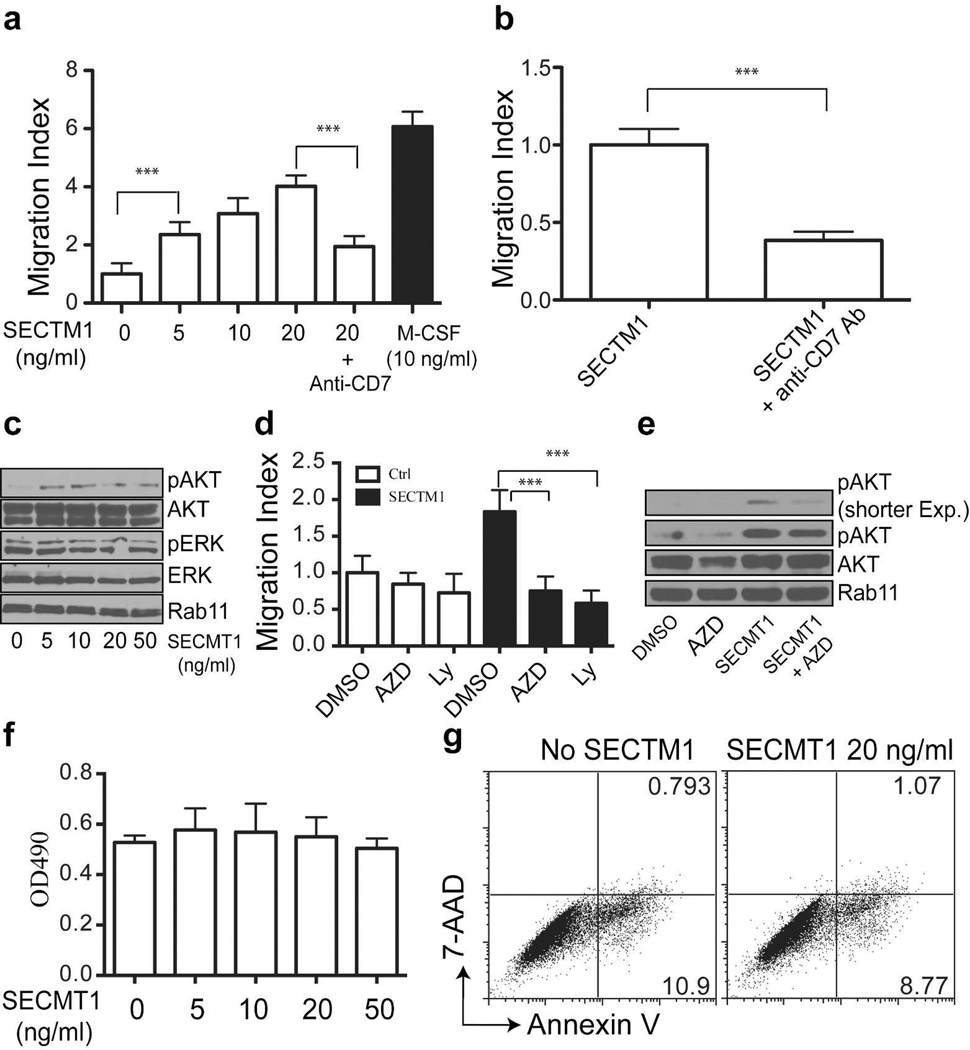
The high levels of expression of CD7 in monocytes and the expression of SECTM1 in melanoma cells suggest that SECTM1 may play a role in modulating the functions of monocytes. We therefore performed a transwell assay to determine the effects of SECTM1 on monocyte migration. As shown in Figure 3a, the recombinant soluble extracellular SECTM1 protein was a potent chemoattractant for monocytes, though less effective compared with M-CSF, a major chemoattactant for monocytes. SECTM1-induced monocyte migration was significantly blocked by the anti-CD7 monoclonal antibody 3A1 (Figure 3a). We examined the ability of the anti-CD7 monoclonal antibody to block the monocyte migration induced by supernatant from C8161 cells engineered to over-express the soluble form of SECTM1 (sSECTM1) (Figure S2). As shown in Figure 3b, blockade of CD7 significantly inhibited melanoma cell derived SECTM1-induced monocyte migration. Finally, we used conditioned media from WM98 cells (SECTM1 positive) and C8161 cells (SECTM1 negative) to conduct a monocyte migration assay. We did not find a significant difference in monocyte migration (data not shown). By themselves, these data comparing the SECTM1 expressing and non-expressing cell lines contribute little to our understanding of the role of SECTM1 since we do not know all the factors expressed in these cells that may make them chemoattractant. Also, the comparison of the naturally expressing SECTM1 with the cell lines engineered to express SECTM1 is difficult to interpret because we know that cells that naturally express SECTM1 do not demonstrate surface expression, whereas those engineered to express SECTM1 usually can be detected with antibody to SECTM1 (data not shown). Cells engineered to express SECTM1 usually express a higher level and this high amount of SECTM1 may be needed to augment monocyte chemoattraction in the face of existing potent factors (Figure 3a). Since SECTM1 is significantly induced by IFN-α and Velcade, SECTM1 induced monocyte migration may be most noticeable during exposure to those inducing agents. Therefore, our data indicate that SECTM1 is a monocyte chemoattractant mediated by binding to CD7, and may be particularly active under conditions when it is expressed at high levels.
Figure 3. SECTM1 increases monocyte migration through activation of the PI3K pathway.
(a) SECTM1 increases monocyte migration in a transwell assay. Monocytes were added to the upper chamber, and increasing concentrations of recombinant SECTM1 protein were added to the lower chamber. M-CSF was used as a positive control and an anti-CD7 monoclonal Ab (10 µg/ml) was used to block migration. Migration was evaluated after 18 hr of incubation by counting stained migrating cells on the lower side of the filter. (b) Monocytes from a healthy donor were added to the upper chamber of the transwell, and conditioned medium from SECTM1 over-expressing WM35 cells were used as the chemoattractant. Anti-CD7 antibodies (10 µg/ml) were used to block SECTM1-induced monocyte migration. Migration was evaluated after 18 hr of incubation by counting stained migrating cells on the lower side of the filter. Results are reported as migration index (fold increase over control). Data represent means ± SD of four independent experiments. ** P<0.01. (c) Monocytes were seeded in serum-free RPMI1640 medium for 16 hours and were then stimulated with the indicated amount of SECTM1 for 20 min. Cells were lysed for western blot analysis. (d) SECTM1-induced monocyte migration is dependent on the MAPK and PI3K pathways. Migration assay was conducted as in a. PI3K inhibitor AZD8055 (AZD, 2 µM), LY3940002 (Ly, 5 µM) were used to block the PI3K pathway. (e) Monocytes were seeded in serum-free RPMI1640 medium for 16 hours and were then stimulated with SECTM1 (20 ng/ml) and AZD8055 (2 µM) for 20 min. Cells were lysed for western blot analysis. (f) Monocytes were cultured in the indicated concentration of SECTM1 for 3 days. MTS assay was used to determine cell growth. (g) Monocytes were cultured with or without SECTM1 (20 ng/ml) for 3 days. Flow cytometric analysis of Annexin V and 7-AAD staining was used to determine cell death.
SECTM1-induced monocyte migration is dependent on the activation of the PI3K pathway, but not the MAPK pathway
Ligation of CD7 results in activation of the PI3K pathway in T cells (Stillwell and Bierer, 2001; Ward et al., 1995). We investigated the downstream signaling pathway activated by SECTM1. We found that SECTM1 strongly activated AKT signaling, but not ERK signaling (Figure 3c). To examine the relevance of activating the PI3K/AKT pathway on SECTM1-induced monocyte migration, we examined the chemoattractive effect of SECTM1 in the presence of a MEK inhibitor, GSK110212, and two PI3K/AKT inhibitors, LY294002 and AZD8055, to block the kinase cascade at a dose that can inhibit signaling, but does not induce monocyte death (data not shown). We found that blocking the AKT pathway was able to inhibit SECTM1-induced monocyte migration, while no significant effect was observed with the MEK inhibitors (Figure 3d, data not shown). Consistent with these migration results, blockade of the AKT pathway with AZD8055 diminished SECTM1-induced activation of AKT signaling (Figure 3e). Our data suggest that SECTM1 activates the PI3K pathway to increase monocyte migration in a PI3K/AKT dependent manner. Since the AKT signaling pathway is also important for monocyte growth and survival, and SECTM1 has been shown to increase T and NK cell proliferation, we tested whether this also occurred in monocytes. MTS proliferation assay indicated that SECTM1 induced no significant increase in monocyte proliferation (Figure 3f). Furthermore, SECTM1 stimulation did not affect cell death by flow cytometric analysis of Annexin V and 7-AAD staining (Figure 3g). Our finding that CD7 is expressed in monocytes, TAMs, M-CSF/Mϕ and TCIM-Mϕ, but not in GM-CSF/Mϕ, and the broad expression of its ligand SECTM1 suggests that SECTM1 activation via CD7 may play a significant role in the tumor microenvironment, and may have a role in other diseases associated with monocytes and macrophages. Also, activation of the PI3K pathway modulates many functions of monocytes/macrophages (Bhatt et al., 2002; Olieslagers et al., 2011), suggesting a role for SECTM1/CD7 interaction in other monocyte/macrophage functions.
CD7 is expressed in patient melanoma tissues
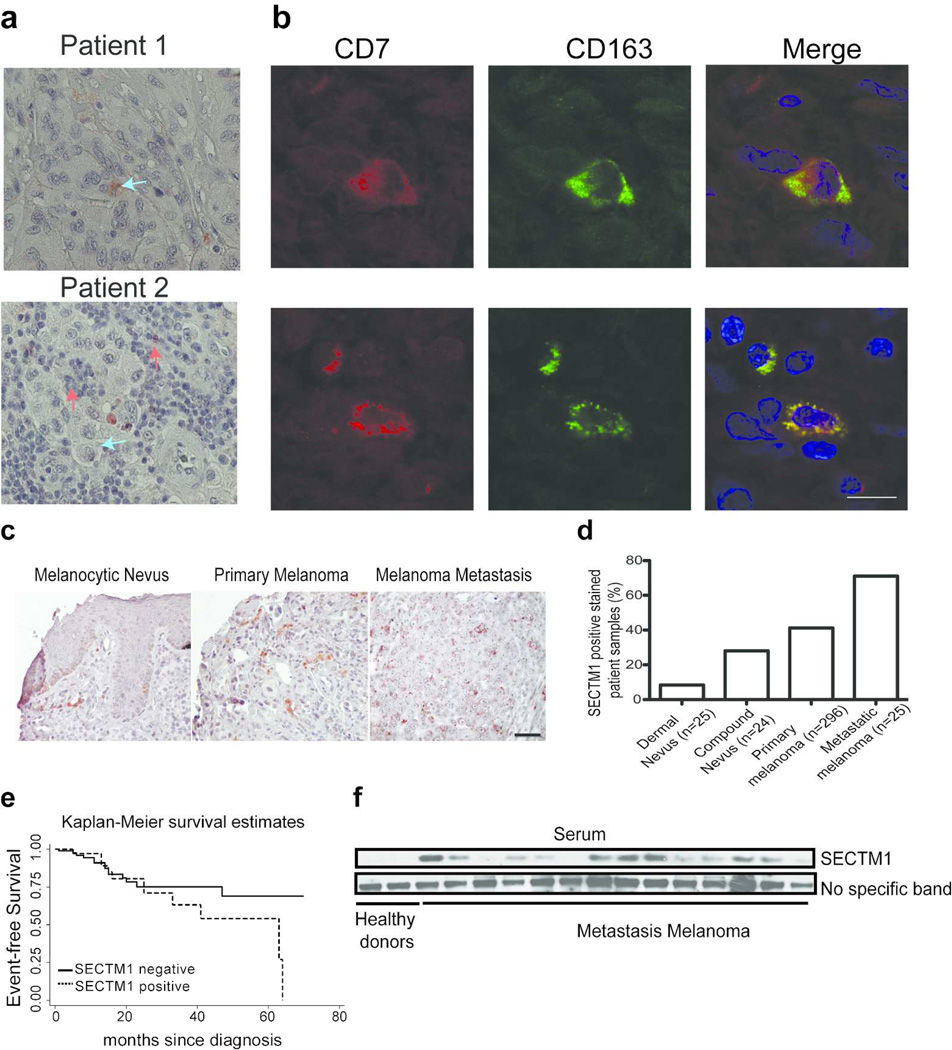
The co-expression of SECTM1 and CD7 in tumor tissues would provide additional evidence of the potential for a significant biological interaction. Therefore we examined melanoma tissues for CD7 expression by TAMs with the use of immunohistochemistry. We found that some CD7-positive cells had typical macrophage morphology, with an abundant, slightly foamy cytoplasm and bland centrally localized nuclei (upper panel, Figure 4a). We also found that CD7 was expressed in lymphoid cells (lower panel, Figure 4a). Co-focal analysis indicated that CD7-positive cells expressed a specific TAM marker, CD163 (Figure 4b). Since TAMs have been demonstrated to be a marker for a poor prognosis in melanomas (Jensen et al., 2009; Torisu et al., 2000), it is likely that CD7 positive macrophages also can be used as a prognostic marker for melanoma patients.
Figure 4. Expression of CD7 and SECTM1 melanoma tissues.
(a) Immunohistochemical analysis of CD7 expression in melanoma lesions. Arrow indicates CD7-positive macrophages. Red arrows: macrophage-positive cells. Blue arrows: lymphocyte-positive cells. Scale bar = 50 µm. (b) Confocal analysis of the co-expression of CD7 with CD163 in melanoma lesions. Red color: CD7-positive cells. Green color: CD163-positive cells. Blue color: DAPI was used to stain cell nuclei. Scale bar = 100 µm. (c) Representative images of expression of SECTM1 in melanocytic nevus, primary melanoma and melanoma metastasis lesion as noted. Immunohistochemical analysis was conducted to analyze SECTM1 expression in paraffin-embedded sections of the human tissue microarray. Scale bar = 50 µm. (d) Summary of the percent of SECTM1-positive samples in melanocytic nevus, primary melanoma and melanoma metastasis lesion. (e) Kaplan-Meier plot of event-free survival in melanomas with or without expression of SECTM1. Log rank test P-value = 0.14. (f) Serum samples from healthy donors and metastatic melanoma patients were used for detecting SECTM1 expression by western blot analysis. A non-specific band was used as a loading control.
SECTM1 is highly expressed in patient melanoma tissues and can be detected in sera from metastatic melanoma patients
We have demonstrated that SECTM1 expression can be induced in cell lines. To better understand the expression of SECTM1 in tumor tissues, we compared the expression of SECTM1 in primary human melanoma specimens with that seen in melanoma cell lines. We performed immunohistochemical analysis of melanoma samples in a tissue array consisting of 296 human primary melanomas of different tumor stages, 25 dermal and 24 compound melanocytic nevi, and 35 cutaneous metastases from melanomas. Clinically normal dermal nevi with little or no inflammatory cell infiltrate showed particularly low SECTM1 expression (92% negative), while many non-inflammatory cells in compound nevi that had high levels of inflammatory cell infiltration expressed SECTM1 (72% negative). We found that 37% of the primary melanoma samples had SECTM1 expression, while 75% of cutaneous metastatic samples were SECTM1-positive (Figure 4c, d). We did not detect expression of SECTM1 in normal skin tissues (Figure S4). These data demonstrate that expression of SECTM1 is increased in primary melanomas and to a greater degree in metastatic lesions compared to benign nevus lesions. We further investigated the clinical implication of expression of SECTM1 in melanomas. Kaplan-Meier methods and Cox regression analysis were used to analyze the relationships between patient’s event-free survival (EFS) and the expression of SECTM1 in early stage (UICC stage I, II) melanomas (N=136, SECTM1 (+) = 40, SECTM1 (-) = 96). After adjusting for UICC stage and depth of the tumor, our data suggested that patients with SECTM1 expression had shorter EFS compared to those without SECTM1 expression, but it did not reach statistical significance (hazards ratio=1.98, 95% CI (0.81,4.41), p=0.14) (Fig. 4e). Interestingly, we observed a considerable increase in SECTM1 expression at sites where melanoma tumors had been infiltrated by immune cells. We observed that 64.5% of the 172 melanoma samples with inflammatory infiltrations showed significant SECTM1 signals (Figure S5a). A similar expression pattern was seen in primary breast cancer tissues (Figure S5b). This is likely due to SECTM1 being up-regulated by interferon-γ or interferon-α secreted by inflammatory cancer stromal cells, such as T cells (Huyton et al., 2011; Lam et al., 2005; Wang et al., 2012b).
Since the N-terminus of SECTM1 is expressed as a soluble form (Slentz-Kesler et al., 1998; Wang et al., 2012b), we examined the expression of SECTM1 in sera from healthy donors and from metastatic melanoma patients. We detected only very low, if any, SECTM1 expression in sera from healthy donors, but found that SECTM1 was detected in all 14 metastatic melanoma patient serum samples (Figure 4f). Considering the broad expression pattern of SECTM1, we cannot exclude that SECTM1 is derived from immune cells that may be stimulated by growth factors from tumor cells rather than the tumor cells alone. Regardless, SECTM1 potentially may be used as a prognostic biomarker for melanoma patients. The ability to detect the soluble form of SECTM1 from a variety of tumors broadens its potential as a biomarker for cancers. In fact, the soluble form of SECTM1 is also detected in serum samples from ovarian cancer patients, and is one of the top five candidate biomarkers for ovarian cancers (Kulasingam et al., 2010).
In this study, we have shown conclusively that CD7, one of the most commonly used T and NK cell markers, is also expressed in the mature monocyte/macrophage lineage and is a chemoattractant receptor for its ligand SECTM1, which is highly expressed in melanoma cells. SECTM1 is also induced by interferon-α and by proteasome inhibitors. The ligation of CD7 by SECTM1 strongly activates the PI3K pathway, which is sufficient for SECTM1-induced monocyte migration. We further demonstrate that CD7 is expressed in melanoma TAMs and SECTM1 is highly expressed in metastatic melanoma tissues, raising the possibility that the interaction between SECTM1 and CD7 might play critical roles in modulating the tumor microenvironment by attracting monocytes or macrophages. Blocking their interaction may be a potential novel strategy for melanoma therapy. Finally, we show that SECTM1 can be detected in sera from melanoma patients but not normal donors, raising the possibility that SECTM1 can be a useful serum biomarker for melanoma.
Materials and Methods
Reagents
The recombinant human secreted form of SECTM1 (sSECTM1) was from Abpro Corporation. Anti-SECTM1 A1 monoclonal and anti-human CD7 3A1 hybridomas were gifts from Dr. Barton F. Haynes (Duke University). Antibodies were made by the Wistar Hybridoma core facility. The rabbit anti-human SECTM1 2816 antibody was made in our lab using full length SECTM1 as the antigen, and can recognize the three forms of SECTM1 (full length, soluble and the membrane retained segment). Anti-phospho-ERK, total ERK, phospho-AKT, total AKT, phospho-RSK90, Rab11, HSP90 and vinculin antibodies were from Cell Signaling. M-CSF, GM-CSF and Interferon-alpha were from R&D Systems. MG132 and Velcade were from Calbiochem. MTS [3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt] was from Sigma. Annexin-PE apoptosis detection kit was from BD Biosciences.
Cell cultures
The culture conditions for melanocytes and melanoma cells were as previously described (Wang et al., 2012a).
Differentiation of monocytes to macrophages
Monocytes were from the AIDS Research Human Immunology Core at the University of Pennsylvania. To generate M-CSF/Mϕ, GM-CSF/Mϕ or MCMI-Mϕ, monocytes were incubated in the presence of complete RPMI1640 medium with 10 ng/ml M-CSF or 10 ng/ml GM-CSF or melanoma conditioned media from 1205Lu and C8161 melanoma cells for 7 days. Half of the medium in each culture was replaced with fresh medium at day 3. See details in (Wang et al., 2012a).
Monocyte proliferation and apoptosis
To assess the effect of SECTM1 on monocyte proliferation and survival, 5×104 monocytes were cultured in RPMI 1640 medium containing 5% fetal bovine serum (FBS) with or without SECTM1 for 3 days. Cell proliferation was determined by MTS assay. Flow cytometric analysis of Annexin V-PE, and 7-AAD staining was used to determine cell death according to the manufacturer’s instructions.
Real-time PCR
Total RNAs were isolated using an RNeasy Mini kit (Qiagen). cDNAs were prepared using oligo(dT) primers and Superscript reverse transcriptase (Invitrogen). Real-time RT-PCR was conducted using an ABI Prism 7000 using SYBR Green PCR Master Mix (Applied Biosystems). For SECTM1 expression, real-time PCR was conducted using Taqman assay according to the company’s product instructions. All reagents were from Applied Biosystems.
For CD7 expression, real-time PCR was conducted using a SYBR Green Kit (Applied Biosystems) with the following primers: Human CD7: Forward, 5′-ACAGAAGGACCACCAGTAGCCA-3′, and reverse, 5′-GGTGCTTCATAAAGTCCTGGACC-3′. GAPDH: Forward, 5′-GGCTGAGAACGGGAAGCTTGTCA-3′, and reverse, 5′-CGGCCATCACGCCACAGTTTC-3’. The primers were designed according to software for quantitative real-time PCR (Roche). All real-time PCR was performed using an initial reaction at 95°C for 5 min, followed by 40 cycles of 95°C for 30 s, 58°C for 30 s, 72°C for 30 s, and then extension for 10 min at 72°C.
Flow cytometry
Monocytes and macrophages were stained with the anti-human CD7 3A1 mAb followed by FITC-conjugated anti-mouse IgG antibody for detection of membrane CD7. Intracellular staining of SECTM1 was done as described previously (Wang et al., 2012b) . Monocytes were cultured in 10% FBS Apoptosis assay were conducted using Annexin V and 7-AAD staining according to the manufacturer’s instructions (BD Biosciences).
Western blot analysis
Cell lysates were separated on 4–20% Tris-glycine gels, and were transferred to nitrocellulose membranes. A rabbit anti-CD7 monoclonal antibody from Epitomics was used for detection of CD7 in monocytes and macrophages. A rabbit anti-SECTM1 antibody was used for detection of SECTM1 in melanocytes and melanoma cells. Rab11, HSP90 or vinculin were used as loading controls.
Monocytes were incubated in serum-free RPMI medium for 2 hr. Cells were then stimulated by SECTM1 for 20 min and were lysed to detect phospho-AKT, total AKT, phospho-ERK and total ERK. Rab11 was used as a loading control.
Induction of SECTM1 by interferon-α and Velcade
1205Lu and/or WM35 melanoma cells were stimulated with Velcade or interferon-α for 6 hr. Intracellular staining of SECTM1 was analyzed by flow cytometry (Wang et al., 2012b).
Generation of the sSECTM1 over-expressing C8161 cell line
sSECTM1 was cloned into the phCMV3 vector (Genlantis). The primers of sSECTM1 used for cloning were as follows: Forward primer, CCCAAGCTTACCATGCAGACCT GCCCCCTGGCA; reverse primer, CGCGGATCCCCCAGTGTCAGGGGCGGAC. The cloning vector was verified by DNA sequencing at The Wistar Genomic Core Facility. For generating stable cell lines, C8161 melanoma cells were transfected with sSECTM1 using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The transfected C8161 cells were selected with G418 for 1 week, and stable cell lines were verified by western blot analysis with a rabbit anti-human SECTM1 polyclonal antibody.
Monocyte migration
The migration assay was conducted with 24-well Transwell inserts (5 µm pore size; Corning). Freshly purified monocytes in 1% FBS RPMI1640 medium were added in the upper chamber. The lower chamber contained 0 (control), 5, 10 and 20 ng/ml sSECTM1. After overnight incubation, cells that had migrated in the bottom chambers were counted for each transwell. The migration index was calculated as the ratio of migrating cell numbers with SECTM1 divided by those without SECTM1.
For the blocking assay using the CD7 antibody, monocytes were preincubated with 10 µg/ml anti-CD7 3A1 mouse antibody or an isotype control for 10 min at room temperature. Migration was then conducted as described above.
For the blocking assays using MEK inhibitor, GSK1120212 (0.5 µM Selleck) PI3K inhibitors, LY200094 (5 µM, Sigma) and AZD8055 (2 µM Selleck) were added to the Transwell. Migration was then conducted as described above.
Human tissue samples
Obtainment of archived formalin-fixed paraffin-embedded (FFPE) material for preparation of TMAs was approved by the Internal Review Board of the Regensburg University Medical Center in adherence to the declaration of Helsinki. According to statement E, II, 8 (2003, ‘further use of human tissue’) of the German Central Ethical Committee, informed consent was not required because archived and anonymized tissue was used that remained from surgery after completion of essential diagnostic procedures. Melanoma tissues were obtained from the University of Pennsylvania. The protocol was approved by the institutional review board at the University of Pennsylvania in adherence to the Helsinki Guidelines. Discarded surgical specimens that are without identifying information or way to trace it back to the subject were used in the experiments. As per NIH guideline, patient consent for experiments was not required to use discarded material. Patient sera were obtained under institutional review board-approved studies at the University of Pennsylvania. All patients provided informed written consent. The breast cancer tissue array was from IMGENE (commercial). The Wistar Institute Institutional Review Board approved the use of all the above-obtained tissues.
For immunohistochemical staining of SECTM1 in melanoma and breast cancer tissues, human melanoma and breast cancer tissues were deparaffinized through a series of xylene and graded alcohols, Boiled in 10 mM Citrate Buffer (pH 6.0) for 10 min at 92°C–95°C and equilibrated in PBS for antigen retrieval. Subsequently, the slides were incubated with the rabbit anti-human SECTM1 polyclonal antibody (1:100) or anti-human CD7 rabbit monoclonal antibody (1:100, Epitomics) overnight at 4°C in a humidified chamber, followed with a Vectastain Elite ABC Kit and Vector AEC substrate (Vector Laboratories).
Melanocytes and melanoma cells staining of SECTM1
Cells were fixed with 4% formaldehyde, and incubated with a rabbit anti-human SECTM1 antibody (clone 2816) followed by incubation with Alexa Fluor 488 secondary antibodies (Molecular Probes). Vectashield mounting media containing DAPI nuclear stain (Vector Laboratories) was used to mount the slides with coverslips. Cells were counterstained with TO-PRO3 (Invitrogen Life Technologies) and examined using a Leica LSC P2 confocal microscope.
For immunofluorescence staining to examine the expression of SECTM1 and the melanocyte marker HMB45, paraffin embedded human foreskins derived from circumcisions of newborns were antigen retrieved as described above. Tissues were incubated with the anti-human SECTM1 antibody followed by incubation with Alexa Fluor 488 secondary antibodies (Molecular Probes). The slides were subsequently incubated with the mouse anti-HMB45 (Abcam) antibody diluted in 0.5% BSA PBS for 2 hr at room temperature, followed by Alex 568-conjugated anti-mouse IgG mAb. Slides were counterstained with TO-PRO3 (Invitrogen Life Technologies) and examined using a Leica LSC P2 confocal microscope.
For immunofluorescence co-staining to examine the expression of CD7 and CD163, primary melanoma tissues were antigen retrieved as described above. After incubation with the rabbit anti-CD7 monoclonal antibody (1:200) at 4°C following Alex 568-anti-rabbit antibody for 30 min at room temperature in a humidified chamber, the slides were subsequently incubated with the mouse anti-CD163 (Abcam) antibody diluted in 0.5% BSA PBS for 2 hr at room temperature, followed by Alex 488-conjugated anti-mouse IgG mAb. Slides were counterstained with DAPI (Invitrogen Life Technologies) and examined using a LSC P2 confocal microscope.
Statistics
Descriptive statistics were computed for study variables. Kaplan-Meier curves, log-rank test, and Cox proportion hazards regression were used to analyze the relationships between event-free survival (in month) and SECTM1 expression in melanomas. See detailed patient information in references (Meyer et al., 2012; Meyer et al., 2009; Meyer et al., 2010).
Supplementary Material
Acknowledgments
We thank Luis Montaner and Jose R Conejo-Garcia for discussion and manuscript preparation; the Wistar Institute Cancer Center Microscopy Core Facility for imaging and figures; the Genomics and Bioinformatics Core Facility for helping with analysis of SECTM1 expression using the existing microarray database, and the Flow Cytometry Core Facility for helping with instrument setup and data analysis.
This work was supported by grants from the National Institutes of Health (5P30CA 010815-42) and the Commonwealth Universal Research Enhancement Program of the Pennsylvania Department of Health for R.E.K., The Wistar Institute Intramural grants for R.E.K and T.W., National Institutes of Health grants for M.H. (CA047159, CA025874, CA114046), and The WW Smith Foundation to REK.
Footnotes
Disclosure of Conflicts of Interest
The authors declare no conflict of interest.
Authorship
Contributions: T.W., designed, performed research and drafted the manuscript; Y.G, X.M., A.L-C., C.H., L.L. P.A., M.L., E.B., R.L., performed experiments; A.R and T.V. provided tissue microarray analysis; X.X. provided melanoma tissues; W-T.H. performed the statistical analysis; M.H helped organize the manuscript; R.E.K. designed research and drafted the manuscript.
References
- Bhatt NY, Kelley TW, Khramtsov VV, Wang Y, Lam GK, Clanton TL, et al. Macrophage-colony-stimulating factor-induced activation of extracellular-regulated kinase involves phosphatidylinositol 3-kinase and reactive oxygen species in human monocytes. J Immunol. 2002;169:6427–6434. doi: 10.4049/jimmunol.169.11.6427. [DOI] [PubMed] [Google Scholar]
- Duluc D, Delneste Y, Tan F, Moles MP, Grimaud L, Lenoir J, et al. Tumor-associated leukemia inhibitory factor and IL-6 skew monocyte differentiation into tumor-associated macrophage-like cells. Blood. 2007;110:4319–4330. doi: 10.1182/blood-2007-02-072587. [DOI] [PubMed] [Google Scholar]
- Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, et al. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. 2007;127:2031–2041. doi: 10.1038/sj.jid.5700827. [DOI] [PubMed] [Google Scholar]
- Handoko HY, Rodero MP, Boyle GM, Ferguson B, Engwerda C, Hill G, et al. UVB-Induced Melanocyte Proliferation in Neonatal Mice Driven by CCR2-Independent Recruitment of Ly6cMHCII Macrophages. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.9. [DOI] [PubMed] [Google Scholar]
- Haynes BF. Human T lymphocyte antigens as defined by monoclonal antibodies. Immunol Rev. 1981;57:127–161. doi: 10.1111/j.1600-065x.1981.tb00445.x. [DOI] [PubMed] [Google Scholar]
- Hume DA, MacDonald KP. Therapeutic applications of macrophage colony-stimulating factor-1 (CSF-1) and antagonists of CSF-1 receptor (CSF-1R) signaling. Blood. 2012;119:1810–1820. doi: 10.1182/blood-2011-09-379214. [DOI] [PubMed] [Google Scholar]
- Huyton T, Gottmann W, Bade-Doding C, Paine A, Blasczyk R. The T/NK cell co-stimulatory molecule SECTM1 is an IFN "early response gene" that is negatively regulated by LPS in Human monocytic cells. Biochim Biophys Acta. 2011;1810:1294–1301. doi: 10.1016/j.bbagen.2011.06.020. [DOI] [PubMed] [Google Scholar]
- Jensen TO, Schmidt H, Moller HJ, Hoyer M, Maniecki MB, Sjoegren P, et al. Macrophage markers in serum and tumor have prognostic impact in American Joint Committee on Cancer stage I/II melanoma. J Clin Oncol. 2009;27:3330–3337. doi: 10.1200/JCO.2008.19.9919. [DOI] [PubMed] [Google Scholar]
- Kulasingam V, Pavlou MP, Diamandis EP. Integrating high-throughput technologies in the quest for effective biomarkers for ovarian cancer. Nature reviews Cancer. 2010;10:371–378. doi: 10.1038/nrc2831. [DOI] [PubMed] [Google Scholar]
- Lam GK, Liao HX, Xue Y, Alam SM, Scearce RM, Kaufman RE, et al. Expression of the CD7 ligand K-12 in human thymic epithelial cells: regulation by IFN-gamma. J Clin Immunol. 2005;25:41–49. doi: 10.1007/s10875-005-0356-5. [DOI] [PubMed] [Google Scholar]
- Lyman SD, Escobar S, Rousseau AM, Armstrong A, Fanslow WC. Identification of CD7 as a cognate of the human K12 (SECTM1) protein. J Biol Chem. 2000;275:3431–3437. doi: 10.1074/jbc.275.5.3431. [DOI] [PubMed] [Google Scholar]
- Meyer S, Fuchs TJ, Bosserhoff AK, Hofstadter F, Pauer A, Roth V, et al. A seven-marker signature and clinical outcome in malignant melanoma: a large-scale tissue-microarray study with two independent patient cohorts. PLoS One. 2012;7:e38222. doi: 10.1371/journal.pone.0038222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Vogt T, Landthaler M, Berand A, Reichle A, Bataille F, et al. Cyclooxygenase 2 (COX2) and Peroxisome Proliferator-Activated Receptor Gamma (PPARG) Are Stage-Dependent Prognostic Markers of Malignant Melanoma. PPAR Res. 2009:848645. doi: 10.1155/2010/848645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer S, Wild PJ, Vogt T, Bataille F, Ehret C, Gantner S, et al. Methylthioadenosine phosphorylase represents a predictive marker for response to adjuvant interferon therapy in patients with malignant melanoma. Experimental dermatology. 2010;19:e251–e257. doi: 10.1111/j.1600-0625.2010.01072.x. [DOI] [PubMed] [Google Scholar]
- Milush JM, Long BR, Snyder-Cappione JE, Cappione AJ, 3rd, York VA, Ndhlovu LC, et al. Functionally distinct subsets of human NK cells and monocyte/DC-like cells identified by coexpression of CD56, CD7, and CD4. Blood. 2009;114:4823–4831. doi: 10.1182/blood-2009-04-216374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagaraj S, Gabrilovich DI. Myeloid-derived suppressor cells in human cancer. Cancer J. 2010;16:348–353. doi: 10.1097/PPO.0b013e3181eb3358. [DOI] [PubMed] [Google Scholar]
- Olieslagers S, Pardali E, Tchaikovski V, ten Dijke P, Waltenberger J. TGF-beta1/ALK5-induced monocyte migration involves PI3K and p38 pathways and is not negatively affected by diabetes mellitus. Cardiovasc Res. 2011;91:510–518. doi: 10.1093/cvr/cvr100. [DOI] [PubMed] [Google Scholar]
- Pixley FJ, Stanley ER. CSF-1 regulation of the wandering macrophage: complexity in action. Trends Cell Biol. 2004;14:628–638. doi: 10.1016/j.tcb.2004.09.016. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roca H, Varsos ZS, Sud S, Craig MJ, Ying C, Pienta KJ. CCL2 and interleukin-6 promote survival of human CD11b+ peripheral blood mononuclear cells and induce M2-type macrophage polarization. J Biol Chem. 2009;284:34342–34354. doi: 10.1074/jbc.M109.042671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell GJ, Parker CM, Cepek KL, Brenner MB, Bhan AK. Evidence for a structural difference in the CD7 polypeptide on human thymocytes and intraepithelial lymphocytes defined by a new monoclonal antibody, 3D9. Cell Immunol. 1994;154:153–165. doi: 10.1006/cimm.1994.1065. [DOI] [PubMed] [Google Scholar]
- Sempowski GD, Lee DM, Kaufman RE, Haynes BF. Structure and function of the CD7 molecule. Crit Rev Immunol. 1999;19:331–348. [PubMed] [Google Scholar]
- Slentz-Kesler KA, Hale LP, Kaufman RE. Identification and characterization of K12 (SECTM1), a novel human gene that encodes a Golgi-associated protein with transmembrane and secreted isoforms. Genomics. 1998;47:327–340. doi: 10.1006/geno.1997.5151. [DOI] [PubMed] [Google Scholar]
- Solinas G, Germano G, Mantovani A, Allavena P. Tumor-associated macrophages (TAM) as major players of the cancer-related inflammation. J Leukoc Biol. 2009;86:1065–1073. doi: 10.1189/jlb.0609385. [DOI] [PubMed] [Google Scholar]
- Stillwell R, Bierer BE. T cell signal transduction and the role of CD7 in costimulation. Immunologic research. 2001;24:31–52. doi: 10.1385/ir:24:1:31. [DOI] [PubMed] [Google Scholar]
- Torisu H, Ono M, Kiryu H, Furue M, Ohmoto Y, Nakayama J, et al. Macrophage infiltration correlates with tumor stage and angiogenesis in human malignant melanoma: possible involvement of TNFalpha and IL-1alpha. Int J Cancer. 2000;85:182–188. [PubMed] [Google Scholar]
- Varney ML, Johansson SL, Singh RK. Tumour-associated macrophage infiltration, neovascularization and aggressiveness in malignant melanoma: role of monocyte chemotactic protein-1 and vascular endothelial growth factor-A. Melanoma research. 2005;15:417–425. doi: 10.1097/00008390-200510000-00010. [DOI] [PubMed] [Google Scholar]
- Wang T, Ge Y, Xiao M, Lopez-Coral A, Azuma R, Somasundaram R, et al. Melanoma-derived conditioned media efficiently induce the differentiation of monocytes to macrophages that display a highly invasive gene signature. Pigment Cell Melanoma Res. 2012a;25:493–505. doi: 10.1111/j.1755-148X.2012.01005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Huang C, Lopez-Coral A, Slentz-Kesler KA, Xiao M, Wherry EJ, et al. K12/SECTM1, an interferon-gamma regulated molecule, synergizes with CD28 to costimulate human T cell proliferation. J Leukoc Biol. 2012b;91:449–459. doi: 10.1189/jlb.1011498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward SG, Parry R, LeFeuvre C, Sansom DM, Westwick J, Lazarovits AI. Antibody ligation of CD7 leads to association with phosphoinositide 3-kinase and phosphatidylinositol 3,4,5-trisphosphate formation in T lymphocytes. Eur J Immunol. 1995;25:502–507. doi: 10.1002/eji.1830250229. [DOI] [PubMed] [Google Scholar]
- Wells DA, Benesch M, Loken MR, Vallejo C, Myerson D, Leisenring WM, et al. Myeloid and monocytic dyspoiesis as determined by flow cytometric scoring in myelodysplastic syndrome correlates with the IPSS and with outcome after hematopoietic stem cell transplantation. Blood. 2003;102:394–403. doi: 10.1182/blood-2002-09-2768. [DOI] [PubMed] [Google Scholar]
- Zaidi MR, Davis S, Noonan FP, Graff-Cherry C, Hawley TS, Walker RL, et al. Interferon-gamma links ultraviolet radiation to melanomagenesis in mice. Nature. 2011;469:548–553. doi: 10.1038/nature09666. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.