Abstract
Protein kinase D (PKD) is a family of stress-responsive serine/threonine kinases implicated in the regulation of diverse cellular functions including cell growth, differentiation, apoptosis, and cell motility. Although all three isoforms are expressed in keratinocytes, their role in skin biology and pathology is poorly understood. We recently identified a critical role for PKD1 during reversal of keratinocyte differentiation in culture, suggesting a potential pro-proliferative role in epidermal adaptive responses. Here, we generated mice with targeted deletion of PKD1 in epidermis to evaluate the significance of PKD1 in normal and hyperplastic conditions. These mice displayed a normal skin phenotype indicating that PKD1 is dispensable for skin development and homeostasis. Upon wounding however, PKD1-deficient mice exhibited delayed wound re-epithelialization correlated with a reduced proliferation and migration of keratinocytes at the wound edge. In addition, the hyperplastic and inflammatory responses to topical phorbol ester were significantly suppressed suggesting involvement of PKD1 in tumor promotion. Consistently, when subjected to two-stage chemical skin carcinogenesis protocol, PKD1-deficient mice were resistant to papilloma formation when compared to control littermates. These results revealed a critical pro-proliferative role for PKD1 in epidermal adaptive responses, suggesting a potential therapeutic target in skin wound and cancer treatment.
Keywords: protein kinase D, epidermis, skin tumor, wound healing, carcinogenesis
INTRODUCTION
Protein kinase D is a family of stress-responsive serine/threonine kinases, involved in the regulation of diverse biological and pathological processes including, cell proliferation, differentiation, adhesion, migration, stress-induced cardiac hypertrophy, pathological angiogenesis, tumor cell proliferation and metastasis (Rozengurt, 2011). PKDs are effectors of diacylglycerol (DAG) and PKCs and are activated by a variety of stimuli, including growth factors, neuropeptides, hormones, and phorbol esters (Fu and Rubin, 2011). PKD isoforms (PKD1, PKD2 and PKD3) share highly homologous regulatory subdomains and can be activated by the same stimuli, however, they also have distinct functions based on their level of expression, tissue specificity and their interacting proteins (Fu and Rubin, 2011).
Despite recent progress in understanding the biological functions of PKD enzymes and their involvement in disease processes, the role of PKD in skin biology and pathology is poorly understood. PKD1 is the most studied member of the family. Earlier studies have shown correlation of PKD expression with proliferation status of cultured keratinocytes suggesting a pro-proliferative role for PKD1 in normal keratinocytes (Ernest Dodd et al., 2005; Rennecke et al., 1999). In addition, PKD expression was shown to be up-regulated in mouse carcinomas and human basal cell carcinomas, although the functional significance of PKD activation or up-regulation in these processes was not determined (Rennecke et al., 1999; Ristich et al., 2006). However, these biochemical and immunohistochemical studies have been difficult to interpret because of the presence of antibody cross-reactivity and a failure to distinguish between individual isoforms.
We have recently shown a critical pro-proliferative role for PKD1 in differentiated cultures of epidermis during de-differentiation in response to a low calcium switch (Jadali and Ghazizadeh, 2010). Specific knockdown of PKD1 to 20% of its normal level by RNA interference was sufficient to block re-initiation of proliferation and reversal of differentiation without affecting normal proliferation and differentiation of mouse keratinocytes (Jadali and Ghazizadeh, 2010). Notably, neither PKD2 nor PKD3 could compensate for the loss of PKD1 function in this process, suggesting a major role for PKD1 in stress-induced responses in keratinocytes.
Although there is compelling evidence in cell culture demonstrating a unique and critical role for PKD1 in keratinocyte de-differentiation, the in vivo relevance of these findings and the physiological role of PKD1 in skin remain to be determined. In the present study, we generated a conditional knockout of PKD1 in mouse stratified epithelia in order to characterize unique functions of PKD1 in skin. Our results identified a crucial role for PKD1 in wound healing, phorbol ester-induced hyperplasia and skin tumor formation.
RESULTS
Epidermal PKD1 is dispensable for mouse skin homeostasis
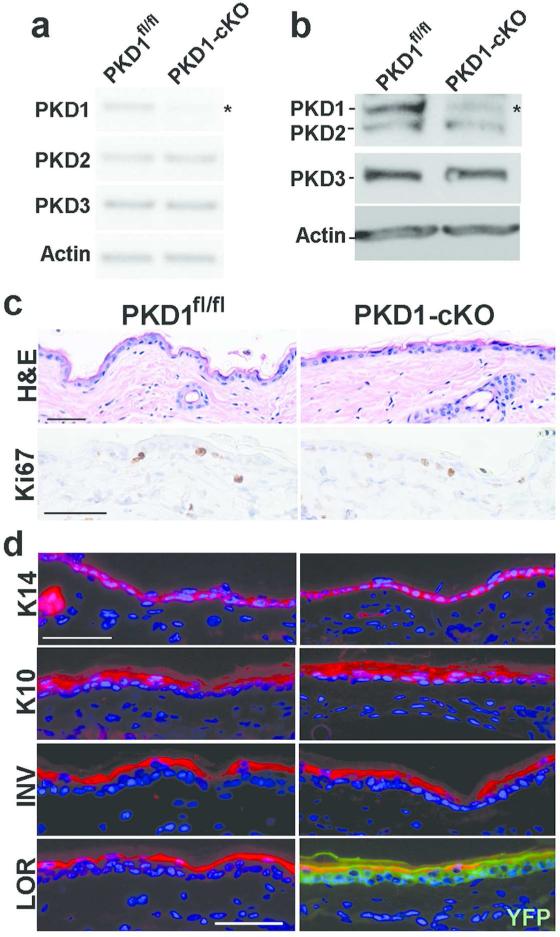
Disruption of the mouse pkd1 gene causes embryonic lethality (Fielitz et al., 2008), therefore to investigate the role of PKD1 in skin epithelia, mice with targeted disruption of PKD1 in keratinocytes (PKD1-cKO) were generated. These mice were carrying three genetic modifications: (i) homozygously floxed pkd1 allele (PKD1fl/fl), (ii) Keratin 14 (K14)-Cre, and (iii) a Cre reporter, flox-STOP-flox-ROSA26-YFP. PKD1-cKO mice were born at expected Mendelian ratio and appeared indistinguishable from their PKD1fl/fl littermates. As shown in Figure 1, K14-regulated recombination in PKD1-cKO mice was highly efficient and specific resulting in uniform YFP expression in skin epithelia (Figure 1d-lower panel). Analysis of transcript and protein levels of three PKD isoforms confirmed efficient and specific loss of PKD1 in PKD1-CKO keratinocytes (Figure 1a-b). A lack of alteration in the expression of PKD2 and PKD3 indicated no compensatory upregulation of these closely related isozymes in the absence of PKD1. The observed residual PKD1 mRNA and protein in PKD1-cKO epidermis is most likely reflective of PKD1 expression in melanocytes and fibroblasts contaminating the primary epidermal cultures.
Figure 1. PKD1 is dispensable for mouse skin homeostasis.
(a) Primary cultures of epidermal cells isolated from PKDfl/fl or PKD1-cKO mice and cultured for 5 days were analyzed for expression of PKD isozymes by semi-quantitative RT-PCR using isozyme-specific primers at 32 cycles for PKD1, 28 cycles for PKD2 and PKD3, and 22 cycles for Actin; (b) Western blot analysis of cell lysates described in (a) using an antibody cross-reacting to PKD1/PKD2 or one specific to PKD3. Actin served as loading control. Shown is representative of at least three experiments. Asterisks in (a and b) indicate residual PKD1 expression likely contributed by melanocytes and fibroblasts contaminating the primary epidermal cultures. (c) Skin sections prepared from adult PKD1fl/fl or PKD1-cKO mice were stained with either hemotoxylin and eosin (H&E) for histology or immunohistochemical staining with proliferation marker Ki67 (peroxidase, brown nuclear staining). (d) Immunofluorescent staining of frozen skin sections with antibody against basal cell marker (K14) or markers of early (K10), intermediate (INV) and late (LOR) epidermal differentiation followed by Alexa-594 conjugated secondary antibody (red). Sections were counterstained with dapi (blue nuclear staining). YFP expression (green) in LOR panel included to show efficient and specific Cre-mediated recombination in keratinocytes. Scale bars=50 um.
Previous studies have suggested a pro-proliferative and/or anti-differentiation role for PKD1 in normal keratinocytes (Ernest Dodd et al., 2005). Histological analysis of dorsal skin of adult mice showed no significant difference in the skin and hair morphology, or in the number of proliferating epithelial cells (Ki67 staining) between PKD1-cKO and PKD1fl/fl mice (Figures 1c and 3d). Furthermore, analysis of K14, an epidermal basal cell marker and that of differentiation markers including K10, involucrin (INV) and loricrin (LOR) by either immunofluorescent (Figure 1d) or westen blot analysis (data not shown) did not indicate any alteration in epidermal proliferation and differentiation . Overall, these data indicated that under normal conditions PKD1 is dispensable for skin development and homeostasis.
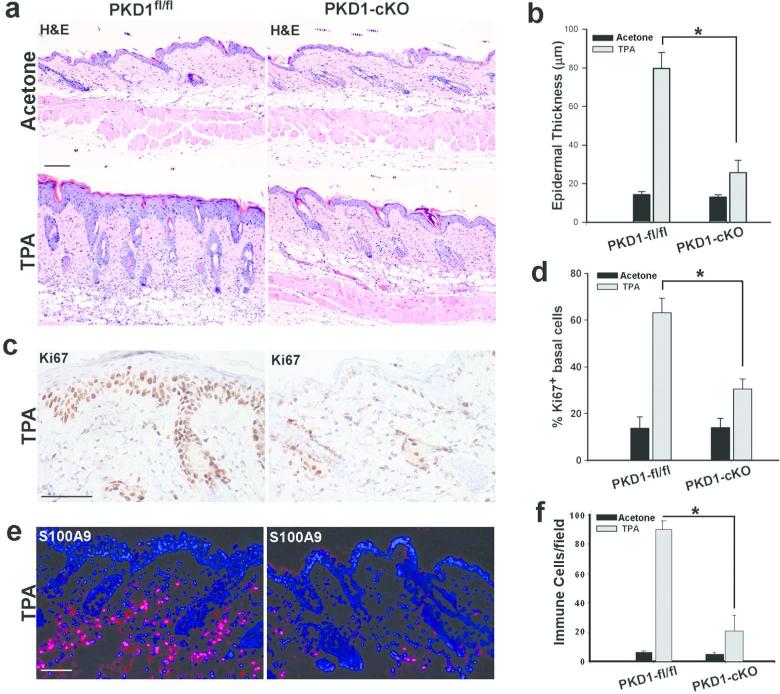
Figure 3. Suppression of TPA-induced responses in PKD1-cKO mouse skin.
PKD1fl/fl or PKD1-cKO dorsal skin treated with a single dose of acetone (carrier control) or TPA (5 nmole/100 ul aceton) and 48 hrs later skin biopsies were taken and processed for histological analysis. Skin sections were stained with (a) H&E for histology, (c) antibody against proliferation marker Ki67 (peroxidase, dark brown staining) or (e) antibody against leukocyte marker S100A9 followed by Alexa-594 conjugated secondary antibody (red). Dapi (nuclear blue staining) was used as a counterstain. A representative of 6 mice from two separate experiments is shown. Scale Bars=100 μm. Graphs show the average thickness of epidermis (b), the number of Ki67-positive basal keratinocytes (d) and, the number of inflammatory S100A9-positive cells (f) in skin sections. Six different regions in sections prepared from three different mice were quantified. Values represent mean +SEM (n=3); * P< 0.001 TPA-treated PKD1-cKO versus TPA-treated control.
Impaired wound healing by PKD1-deficiant keratinocytes.
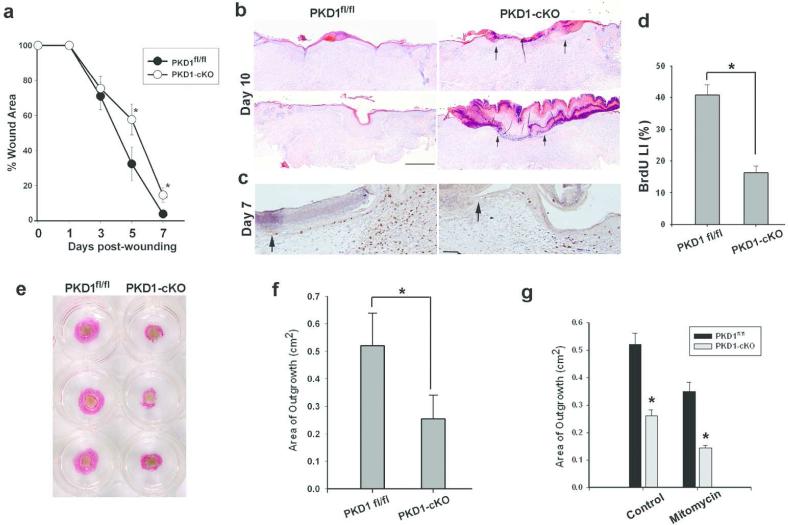
PKD1 is a stress-responsive kinase and has been implicated in cell proliferation and motility suggesting a potential role during wound healing (Olayioye et al., 2013). To investigate the role of epidermal PKD1 in wound healing, the dorsal skin of PKD1-cKO and age-and sex-matched PKD1fl/fl mice were wounded with one 6 mm circular, splinted, full-thickness excisional wounds, and monitored daily. As shown in Figure 2a, the kinetics of wound healing in PKD1-cKO mice was slightly but significantly slower than control. Histological analysis of wounds at 10 days post-wounding indicated the presence of a migrating tongue (Figure 2b, arrows) with a gap averaging 0.96±0.47 mm (n=3) in PKD1-deficient wounds, while control wounds were completely re-epithelialized (Figure 2b). Immunohistochemical analysis of 7-day-old wounds when epidermal hyperplasia and a migrating tongue of keratinocytes are present in both groups (Figure 2c, arrows), demonstrated a lower proliferation rate (BrdU labeling index) for PKD1-deficient keratinocytes at the wound edge when compared to the control (Figure 2c-d). These data indicated a correlation between delayed wound healing and reduced proliferative response of keratinocytes in PKD1-cKO mice.
Figure 2. Impaired wound healing and re-epithelialization in PKD1-deficient mice and skin explants.
(a) A 6 mm circular excisional wound was generated in the upper back of PKD1-cKO or control mice (n=6) and wound closure was monitored daily. Digital images were obtained and wound area was quantified and expressed as percent area of original wound remained open at the indicated time. Values represent mean+SEM (n=6), * P<0.05 PKD1-cKO versus the control. (b) Healing wounds of PKD1-cKO or control mice were biopsied, sectioned at the center and stained with H&E for histology. Sections from two representative mice are shown with arrows indicating wound margins in PKD1-cKO. Scale bar = 200 μm. (c-d) Sections of 7-day-old wounds when a migrating tongue is present in both groups were stained with anti-BrdU antibody (dark brown nuclei). The graph in (d) shows the mean percentage of BrdU positive keratinocytes in the wound edge and the migrating tongue. Values represent mean+SEM (n=3), * P< 0.001 null versus control. (e) Representative PKD1fl/fl and PKD1-cKO skin explants (light brown area in the center) grown in culture for 7 days and stained for K14 (pink staining) to show keratinocyte outgrowth. (f) K14-positive areas were measured and results are shown as mean+SEM (n=48), P<0.01 versus the control (g) Graph shows area of explant outgrowth grown with or without mitomycin C treatment .Values represent mean+SEM (n=24 explants from 4 mice). * P< 0.001 PKD1-cKO versus PKD1fl/fl.
To confirm the involvement of PKD1 in wound re-epithelialization independent of wound inflammation and contraction, a skin explant culture assay that mimics the behavior of keratinocytes at the edge of skin wounds was used (Mazzalupo et al., 2002). As shown in Figure 2e-f, the areas of keratinocyte outgrowths were significantly smaller in PKD1-cKO explants (25±8 mm2) when compared to that of PKDfl/fl (52±12 mm2), confirming a role for PKD1 in wound re-epithelialization. Re-epithelialization of skin wounds results from increases in both mitotic activity and migration of keratinocytes at the wound edge (Gurtner et al., 2008). To determine if keratinocyte migration was affected by the loss of PKD1, control or PKD1-cKO explants were treated at 48 hours post-seeding with mitomycin C to irreversibly block mitosis, and the area of outgrowth was measure 5 days later. As shown in Figure 2g, although mitomycin treatment resulted in a significant reduction in the outgrowth area in both control and PKD1-deficient explants, the effects were more pronounced on the latter (45% in PKD1-deficient vs. 37% in control) indicating a defect in migration of PKD1-deficient keratinocytes. These data supported pro-proliferative and pro-migratory roles for PKD1 during wound healing.
PKD1 is a major mediator of TPA-induced epidermal hyperplasia and inflammation
To determine the significance of PKD1 in epidermal hyperplastic responses to other stimuli, the responses of PKD1-deficient epidermis to a tumor promoter, 12-O-tetradecanoylphorbol–13-acetate (TPA) was examined. TPA is a DAG analogue and a known inducer of PKD activation in mouse keratinocytes (Ernest Dodd et al., 2005; Jadali and Ghazizadeh, 2010). Topical application of TPA is known to induce hyperplasia and inflammation, and is necessary for skin tumor development in two-stage chemical carcinogenesis (Rundhaug and Fischer, 2010). To determine the potential role of PKD1 in TPA-induced mitogenic responses in skin, PKD1-cKO and PKDfl/fl were treated with a single dose of TPA or acetone (control), and analyzed 48 hours later. As shown in Figure 3, a single dose of TPA in PKD1fl/fl skin induced a robust proliferative response leading to a four-fold increase in the number of Ki67-positive keratinocytes and more than a five-fold increase in the epidermal thickness. In PKD1-cKO mice however, these responses were blunted with only a two-fold increase in proliferating basal keratinocytes and in the epidermal thickness. In addition, analysis of skin sections showed a marked suppression of TPA-induced inflammation in PKD1-cKO mice (Figure 3a). Immuofluorescent analysis of skin sections for S100A9, which is constitutively expressed on monocytes and neutrophils (Lagasse and Weissman, 1992), showed a five-fold reduction in the number of infiltrating leukocytes in PKD1-cKO mice (Figure 3e-f). These data indicate a critical role for PKD1 as a positive regulator of epidermal hyperplasia and inflammation in response to phorbol esters, suggesting a role in tumor promotion.
PKD1-deficient mice are resistance to tumor formation in two-stage chemically-induced skin carcinogenesis
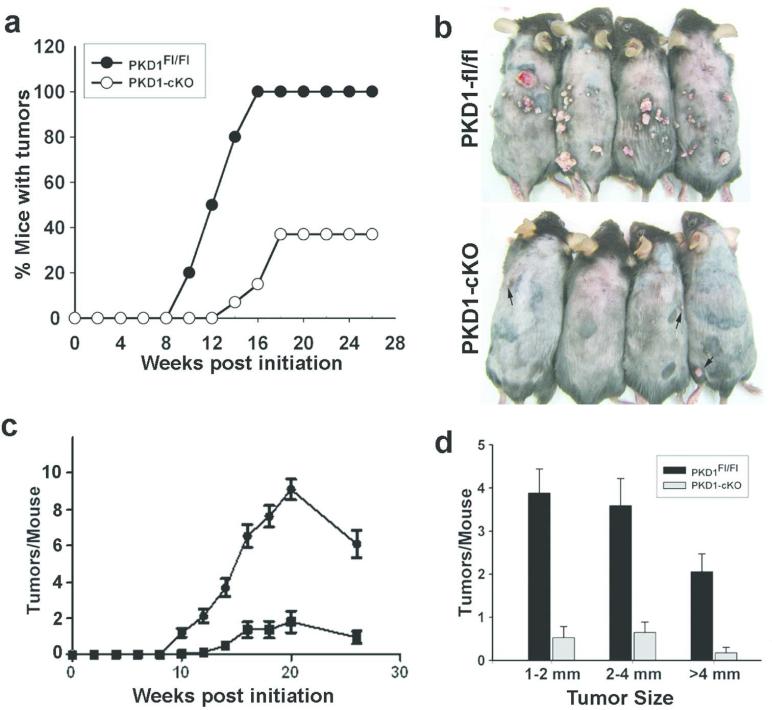
To examine the potential effects of epidermal PKD1 in tumor promotion, two-stage chemical carcinogenesis experiments were carried out in PKD1-cKO and their normal littermates. The use of 7,12-dimethylbenz[α]anthracene (DMBA), to introduce oncogenic mutations primarily on the Hras1 gene, and TPA as a tumor promoter, to allow selective outgrowth of initiated cells, is a well-established chemical carcinogenic treatment that leads primarily to papilloma formation in the skin (Abel et al., 2009). Groups of 15 mice at 7-8 weeks of age were treated with DMBA followed a week later by twice weekly TPA for 20 weeks. Animals were examined weekly to determine tumor incidence and multiplicity. Six weeks after the last TPA treatment, tumors were quantified, harvested and analyzed. As shown in Figure 4, mice lacking PKD1 in the epidermis were refractory to papilloma formation. While all control mice develop tumors by 16 weeks of promotion, more than 60% of PKD1-deficient mice did not develop any tumor during the entire 26 weeks of observation (Figure 4a-b). In addition, the average number and size of tumors in tumor-bearing PKD1-cKO mice were markedly reduced (Figure 4 c-d). Histological analysis of tumors revealed that most of the tumors formed in both groups were benign papillomas and keratoacanthomas (data not shown). At 26 weeks, the frequency of malignant conversion of these benign tumors was less than 3% and was restricted to the control mice. Malignant conversion in PKD1-cKO mice however, may have remained undetected because of the lower total number of tumors developed in these mice. The PKD1-cKO mice resistance to carcinogen-induced tumorigenesis was not the result of increased apoptosis in PKD1-deficient keratinocytes as the number of apoptotic keratinocytes following 24 hrs of DMBA treatment was comparable between the two groups (data not shown). These data identified PKD1 as the key transducer of the tumor promoting effects of TPA in chemically-induced skin carcinogenesis.
Figure 4. Reduced skin tumor formation in PKD1-deficient mice.
(a) The incidence of tumors in groups of PKD1-cKO and control mice (n=15/group) subjected to DMBA/TPA protocol and monitored weekly for tumors >1.5 mm in diameter. (b) Representative mice from each group showing reduced tumor number and volume in PKD1-cKO at 26 weeks. Arrows in the lower panel indicate small tumors developed in PKD1-deficient mice. (c) Kinetics of tumor multiplicity using 15 mice per genotype. P<0.0001 for overall differences and P<0.001 for differences between PKD1-cKO mice versus PKD1fl/fl at each week. (d) Graph showing average size of tumors developed per mouse. Error bars represent standard error of the mean. P<0.001 PKD1-cKO mice versus PKD1fl/fl.
DISCUSSION
Most of the functions assigned to PKD1 have been characterized in cell culture systems and their in vivo relevance has yet to be determined. Using a conditional knockout of PKD1 targeted to stratified epithelia, we investigated the non-redundant role of PKD1 in epidermis. Although PKD1 was found to be dispensable for skin development and homeostasis, our study identified a critical role for this enzyme during wound healing and in the TPA-induced hyperplastic/inflammatory responses that are necessary for tumor development. Our findings are consistent with the PKD function as a stress-responsive kinase and provide direct genetic evidence supporting a pro-proliferation role for PKD1 in skin tumor development. PKD isoforms share high sequence homology and all isoforms could be activated by TPA (Fu and Rubin, 2011). Despite expression of all three PKD isoforms in mouse keratinocytes (Jadali and Ghazizadeh, 2010), disruption of PKD1 gene alone resulted in marked reduction in TPA-induced responses and tumor promotion. This indicated that PKD2 and PKD3 cannot fully compensate for the loss of PKD1 function during this process. The TPA-induced responses in PKD1-cKO mice however, were not completely blocked and may reflect some redundant functions of PKD2 and PKD3 during tumor promotion.
Previous studies using primary cultures of mouse keratinocytes have suggested a pro-proliferative and/or anti-differentiation role for PKD1 in normal keratinocytes (Ernest Dodd et al., 2005). The normal skin architecture and unaltered expression of proliferation and differentiation markers in PKD1-deficient epidermis did not support this hypothesis. Moreover, the growth and differentiation of primary cultures of PKD1-deficient keratinocytes was comparable to that of control littermates, arguing against a general pro-proliferation/anti-differentiation role for PKD1 in keratinocytes (data not shown). This is consistent with our previous studies using small inhibitory RNA to knock down PKD1 in mouse keratinocytes (Jadali and Ghazizadeh, 2010). Although there was no compensatory up-regulation of PKD2 and PKD3 in PKD1-null keratinocytes (Figure 1), the possible functional redundancy of PKDs or other stress-responsive kinases acting on common targets during normal growth and differentiation of keratinocytes cannot be excluded.
Using two complementary ex vivo and in vivo approaches we showed that disruption of PKD1 impaired re-epithelialization during wound healing. PKD1 has been implicated as an inhibitor or a promoter of directed cell migration depending on the cell type and the experimental condition (Olayioye et al., 2013). Consistent with our studies, PKD1 has been shown to be involved in regulation of hemidesmosome dynamics through direct phosphorylation of integrin β4 on its signaling domain, a process important in promoting keratinocyte migration and proliferation (Frijns et al., 2012; Nikolopoulos et al., 2005). In addition to the PKD1 regulation of hemidesomosomes in the basal keratinocytes, PKD1 has been shown to play a distinct pro-proliferation function during reversal of differentiation in keratinocytes (Jadali and Ghazizadeh, 2010). It is plausible to assume that PKD1 activation in differentiated cells in vivo, may increase the size of proliferative cell pool during wound healing where normal differentiation process may be reversed (Morasso and Tomic-Canic, 2005).
PKDs are involved in a diverse set of signaling pathways important to tumor development and cancer progression and have been shown to be dysregulated in several cancer types (LaValle et al., 2010; Sundram et al., 2011). Our study underlines a role for PKD1 in skin tumor formation. The two-stage chemical carcinogenesis is widely used to study the mechanism of epithelial carcinogenesis (Rundhaug and Fischer, 2010). Our data identified PKD1 as a major downstream target of TPA/DAG, and a key mediator of skin tumor promotion. TPA is known to activate PKDs via a PKC-dependent mechanism. The PKCs, directly bind, phosphorylate and activate PKDs, although classical PKCs specifically PKCα can also activate PKDs (Rozengurt et al., 2005). δ, ε, η isoforms are expressed in mouse keratinocytes, however, δ and η isoforms are thought to be anti-tumorigenic, and the role of PKCε appears to be more complex (Rundhaug and Fischer, 2010). Mice overexpressing PKCε have been shown to be resistant to papilloma formation but develop papilloma-independent metastatic carcinomas independent of TPA treatment (Reddig et al., 2000). These studies suggest, at least in part, distinct roles for PKCε and PKD1 in tumor formation. Another highly expressed PKC in epidermis, PKCα is a major target for TPA in differentiated keratinocytes (Dlugosz et al., 1994). Similar to PKD1, disruption of PKCα gene in mice have been shown to result in impaired TPA- and wound-induced epidermal hyperplasia. However, PKCα-null mice were more susceptible to tumor formation (Hara et al., 2005). The apparent divergence of PKC and PKD1 signaling in response to TPA may be explained by distinct substrate-specificity of PKCs and PKDs or, by the time and context dependent activation of PKD1 by PKC-dependent and –independent mechanisms as previously described (Jacamo et al., 2008; Rybin et al., 2009). Clearly, further studies are necessary to delineate the mechanism by which PKD1 mediates its pro-proliferative effects in epidermis.
In summary, the results presented here underlines the importance of PKD signaling in epidermal adaptive responses including wound healing and skin carcinogenesis, suggesting another therapeutic target to alter wound healing or suppress skin tumor formation. Consistent with our findings, PKD1 signaling has been suggested as a target for the cancer-preventive activity of green tea- constituents in mouse skin (Chiou et al., 2013).
MATERILAS AND METHODS
Mice
PKD1-cKO mice were carrying three genetic modifications: (i) homozygously floxed pkd1 allele from exon 12 to 14 encoding part of the catalytic domain of PKD1 which is essential for kinase function and PKD mRNA stability (Fielitz et al., 2008), (ii) K14-Cre which targets Cre recombinase to keratinocytes (Dassule et al., 2000), and (iii) a Cre reporter, flox-STOP-flox-ROSA26-YFP (Srinivas et al., 2001) to identify PKD1-cKO mice from PKD1fl/fl littermates. The specific knockdown of PKD1 in primary cultures of epidermis was verified with semi-quantitative RT-PCR and immunoblotting. Total RNA was isolated using Trizol Reagent (Life Technologies, Grand Island, NY) and analyzed by One Step RT-PCR kit (Qiagen ,Valencia, CA). The following primers were used: PKD1, 5’-CACTGTGACCTCAAGCCAGA-3’ and 5’-CCAACAGACCACATGTCCAG-3’; PKD2, 5’-AGAGTGCTCTCCATGCCAGT-3’ and 5’-GACAGCGGGATTTCCTTGTA-3’; PKD3, 5’-AATGTGCAGGGTCAAAGTCC-3’ and 5’-CCCCTACTGCCATCACTGTT-3’. RNA levels of the target genes were normalized against the β-actin transcript levels. PKD1 and PKD2 protein levels in 30 μg of protein lysates were analyzed by immunoblotting using antibodies against PKD1/2 (CS-2052), PKD3 (CS-5655) (Cell signaling Technologies, Danvers, MA), and β-actin (SC-1615) as a loading control. PKD1-cKO and control mice were maintained on a mixed 129/Sv × C57BL/6 background. Animals were housed under standard conditions and all animal experiments were performed in accordance with institutional guidelines set forth by the State University of New York.
Wounding healing Analysis
For in vivo analysis, a well-established and reproducible, excisional wound healing model was used (Galiano et al., 2004). Dorsal hair of 7- to 9-week-old mice (age- and sex-matched) was clipped and a full thickness 6 mm circular wound was generated in the upper back. A 10 mm circular splint was placed around the wound perimeter and secured with Krazy glue and 6 interrupted sutures to fix the splint to the skin. Wounds were covered with sterile Tegaderm dressings (3M Healthcare, St. Paul, MN) which were changed every other day until wounds were closed. Digital images were obtained at the time of dressing changes. Wound area was quantified using the splint to normalize the wound size. Wound area was calculated as percent area of the original wound. Representative wounds were biopsied following a 2-hr BrdU pulse (50 μg/g body weight), bisected and fixed in 10% formalin for routine histological processing and immunostaining.
For ex vivo analysis, the quantitative explant outgrowth assay of mouse skin was used as previously described (Mazzalupo et al., 2002). Briefly, dorsal skin of 2-day-old pups were removed, and 4 mm punch biopsies were cultured for 7 days. To assess keratinocyte outgrowth, explants were immunostained using an antibody against K14 and Supersensitive IHC Detection kit (Biogenex Laboratories, San Ramon, CA). Plates were photographed and the total area of outgrowth was measured using NIH-image J software. A subset of explants were treated with mitomycin C (5 ug/ml for 2 hrs; Sigma-Aldrich) or PBS (as controls) at 48 hours post-seeding and analyzed 5 days later as described above.
TPA-induction of epidermal hyperplasia
The dorsal skin of 7-9-week old male mice were shaved and the next day were treated with either a single dose of 5 nmole TPA (LC laboratories, Woburn, MA) in 100 μl acetone or 100 μl acetone (carrier control). Two days later, mice were euthanized, the treated skin was biopsies and fixed for histological processing. Skin samples were analyzed following H&E staining or immunostaining using antibodies specific for Ki67 (proliferation marker; Novocastra, New Castle, UK) or S100A9 (leukocyte marker; Axxzel Biosystem LLC, Houston, TX). The epidermal thickness was measured in sections stained with H&E at a minimum of 6 different regions in sections prepared from three different mice.
Two stage chemical carcinogenesis experiments
A dorsal area of 7-8-week old mice (10 males and 5 females/group) was shaved, and a day later treated with a single application of DMBA (100 μg in 200 μl acetone; Sigma Aldrich, St. Louis, MO) as an initiating agent. A week later, mice were treated with TPA (20 nmole/200 μl acetone) twice weekly for 20 weeks to promote tumor formation. There was no significant difference between the average number of tumors developed in male and females. Tumor defined as raised lesions with a minimum of 1.5 mm in diameter, were assessed weekly for 26 weeks. At this time tumor were harvested for further analysis.
Statistical Analysis
Statistical analysis was performed using the GraphPad Prism version 5.0 (GraphPad Software). Student's t-test was used for comparing two groups of data. For analysis of tumor incidence, comparison of the curves showing the mice with tumors was performed using log-rank χ2 test. Tumor multiplicity was analyzed using repeated measures analysis of variance (ANOVA) for overall differences between the two groups and Mann–Whitney test for comparing differences at each week between PKDfl/fl and PKD1-cKO. Only values with p<0.05 were accepted as significant.
ACKNOWLEDGEMENTS
We are grateful to Drs Marcia Simon and David Owens for constructive discussion of the study; Dr. Olson for providing PKD1fl/fl mice; and Mr. Trivikram Gaddapara for technical support. This work was supported by grants to S.G. from NIH (AR056013).
Abbreviations
- DMBA
7,12-dimethylbenz[α]anthracene
- TPA
12-O-decanoyl-phorbol-13-acetate
- PKD
protein kinase D
- cKO
conditional knockout
- K
keratin
- YFP
Yellow florescent protein
- BrdU
5-bromo-2'-deoxyuridine
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Abel EL, Angel JM, Kiguchi K, et al. Multi-stage chemical carcinogenesis in mouse skin:Fundamentals and applications. Nat Protocols. 2009;4:1350–62. doi: 10.1038/nprot.2009.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiou Y-S, Sang S, Cheng K-H, et al. Peracetylated (−)-epigallocatechin-3-gallate (AcEGCG) potently prevents skin carcinogenesis by suppressing the PKD1-dependent signaling pathway in CD34+skin stem cells and skin tumors. Carcinogenesis. 2013;34:1315–22. doi: 10.1093/carcin/bgt042. [DOI] [PubMed] [Google Scholar]
- Dassule HR, Lewis P, Bei M, et al. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development. 2000;127:4775–85. doi: 10.1242/dev.127.22.4775. [DOI] [PubMed] [Google Scholar]
- Dlugosz AA, Cheng C, Williams EK, et al. Alterations in murine keratinocyte differentiation induced by activated rasHa genes are mediated by protein kinase C-alpha. Cancer Res. 1994;54:6413–20. [PubMed] [Google Scholar]
- Ernest Dodd M, Ristich VL, Ray S, et al. Regulation of protein kinase D during differentiation and proliferation of primary mouse keratinocytes. J Investig Dermatol. 2005;125:294–306. doi: 10.1111/j.0022-202X.2005.23780.x. [DOI] [PubMed] [Google Scholar]
- Fielitz J, Kim M-S, Shelton JM, et al. Requirement of protein kinase D1 for pathological cardiac remodeling. Proc Natl Acad Sci USA. 2008;105:3059–63. doi: 10.1073/pnas.0712265105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frijns E, Kuikman I, Litjens S, et al. Phosphorylation of threonine 1736 in the C-terminal tail of integrin β4 contributes to hemidesmosome disassembly. Mol Biol Cell. 2012;23:1475–85. doi: 10.1091/mbc.E11-11-0957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y, Rubin CS. Protein kinase D: coupling extracellular stimuli to the regulation of cell physiology. EMBO Rep. 2011;12:785–96. doi: 10.1038/embor.2011.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galiano RD, Michaels Jt, Dobryansky M, et al. Quantitative and reproducible murine model of excisional wound healing. Wound Rep Regene. 2004;12:485–92. doi: 10.1111/j.1067-1927.2004.12404.x. [DOI] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, et al. Wound repair and regeneration. Nature. 2008;453:314–21. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- Hara T, Saito Y, Hirai T, et al. Deficiency of protein kinase Cα in mice results in impairment of epidermal hyperplasia and enhancement of tumor formation in two-stage skin carcinogenesis. Cancer Res. 2005;65:7356–62. doi: 10.1158/0008-5472.CAN-04-4241. [DOI] [PubMed] [Google Scholar]
- Jacamo R, Sinnett-Smith J, Rey O, et al. Sequential protein kinase C (PKC)-dependent and PKC-independent protein kinase D catalytic activation via Gq-coupled receptors: differential regulation of activation loop Ser744 and Ser748 phosphorylation. J Biol Chem. 2008;283:12877–87. doi: 10.1074/jbc.M800442200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadali A, Ghazizadeh S. Protein kinase D is implicated in the reversible commitment to differentiation in primary cultures of mouse keratinocytes. J Biol Chem. 2010;285:23387–97. doi: 10.1074/jbc.M110.105619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. Mouse MRP8 and MRP14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992;79:1907–15. [PubMed] [Google Scholar]
- LaValle CR, George KM, Sharlow ER, et al. Protein kinase D as a potential new target for cancer therapy. Biochimica et Biophysica Acta (BBA) - Reviews on Cancer. 2010;1806:183–92. doi: 10.1016/j.bbcan.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzalupo S, Wawersik MJ, Coulombe PA. An ex vivo assay to assess the potential of skin keratinocytes for wound epithelialization. J Invest Dermatol. 2002;118:866–70. doi: 10.1046/j.1523-1747.2002.01736.x. [DOI] [PubMed] [Google Scholar]
- Morasso MI, Tomic-Canic M. Epidermal stem cells: the cradle of epidermal determination, differentiation and wound healing. Biol Cell. 2005;97:173–83. doi: 10.1042/BC20040098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulos SN, Blaikie P, Yoshioka T, et al. Targeted deletion of the integrin β4 signaling domain suppresses laminin-5-dependent nuclear entry of mitogen-activated protein kinases and NF-κB, causing defects in epidermal growth and migration. Mol Cell Biol. 2005;25:6090–102. doi: 10.1128/MCB.25.14.6090-6102.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olayioye MA, Barisic S, Hausser A. Multi-level control of actin dynamics by protein kinase D. Cell Signal. 2013;25:1739–47. doi: 10.1016/j.cellsig.2013.04.010. [DOI] [PubMed] [Google Scholar]
- Peterburs P, Heering J, Link G, et al. Protein kinase D regulates cell migration by direct phosphorylation of the cofilin phosphatase slingshot 1 like. Cancer Res. 2009;69:5634–8. doi: 10.1158/0008-5472.CAN-09-0718. [DOI] [PubMed] [Google Scholar]
- Reddig PJ, Dreckschmidt NE, Zou J, et al. Transgenic mice overexpressing protein kinase Cε in their Epidermis exhibit reduced papilloma burden but enhanced carcinoma formation after tumor promotion. Cancer Res. 2000;60:595–602. [PubMed] [Google Scholar]
- Rennecke J, Rehberger PA, Furstenberger G, et al. Protein-kinase-Cmu expression correlates with enhanced keratinocyte proliferation in normal and neoplastic mouse epidermis and in cell culture. Int J Cancer. 1999;80:98–103. doi: 10.1002/(sici)1097-0215(19990105)80:1<98::aid-ijc19>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Protein kinase D signaling: Multiple biological functions in health and disease. Physiology. 2011;26:23–33. doi: 10.1152/physiol.00037.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E, Rey O, Waldron RT. Protein kinase D signaling. J Biol Chem. 2005;280:13205–8. doi: 10.1074/jbc.R500002200. [DOI] [PubMed] [Google Scholar]
- Rundhaug JE, Fischer SM. Molecular mechanisms of mouse skin tumor promotion. cancers (Basel) 2010;2:436–82. doi: 10.3390/cancers2020436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rybin VO, Guo J, Steinberg SF. Protein kinase D1 autophosphorylation via distinct mechanisms at Ser744/Ser748 and Ser916. J Biol Chem. 2009;284:2332–43. doi: 10.1074/jbc.M806381200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivas S, Watanabe T, Lin CS, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Deve Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundram V, Chauhan SC, Jaggi M. Emerging roles of protein kinase D1 in cancer. Mol Cancer Res. 2011;9:985–96. doi: 10.1158/1541-7786.MCR-10-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]