Abstract
Insomnia is among the most prevalent and costly of all sleep-related disorders. To characterize the neural mechanisms underlying subjective dysfunction in insomnia, we examined brain activity in 17 female insomniacs and 17 female healthy controls using simultaneous functional magnetic resonance imaging (fMRI) and electroencephalography (EEG) while they were resting and while they were trying to fall asleep. In examining the dynamic regional activity within intrinsic brain networks, we found that, compared with controls, insomniacs had greater involvement of the anterior insula with salience networks, as well as insula BOLD correlation with EEG gamma frequency power during rest in insomniacs. This increased involvement of the anterior insula was associated with negative affect in insomniacs. Aberrant activation of the insula, which integrates temporal and bodily states, in arousal networks may underlie the misperception of sleep quality and subjective distress in insomnia.
Keywords: insomnia, fMRI, EEG, resting state, insula, salience networks
Introduction
Insomnia is a disorder of all-day impairment from sleep-related distress that involves a perceived difficulty falling asleep, staying asleep, or obtaining refreshing sleep. Afflicting up to 10% of the population (Ohayon, 2002), insomnia may persist for months or years and predicts the development of other disorders, such as Major Depressive Disorder (Ford & Kamerow, 1989). Researchers have proposed multiple psychological and biological explanations for the symptoms of insomnia (Harvey & Tang, 2012), including dysfunction in neural circuitry like the brainstem systems controlling sleep-wake (Lu et al., 2006), faulty sleep drive (Krystal and Edinger, 2010), psychological factors, or multiple factors (Riemann et al., 2009).
An important framework for understanding insomnia is ‘hyperarousal,’ or the posited heightened activity of neural, metabolic, electrophysiological, and neuroendocrine systems in insomniacs (Bonnet & Arand, 2010). Importantly, however, a key aspect of insomnia is the subjective reporting of more sleep dysfunction, such as increased sleep latency, than is recorded by ‘objective’ measures such as polysomnography. Thus, the diagnosis of insomnia is based on the subjective report of psychological distress, particularly during the sleep-to-wake transition. This suggests a limitation of polysomnography for capturing a neural phenotype of insomnia. Alternative imaging methods may elucidate the neural basis of hyperarousal, and one of the few studies to examine neural activity in individuals diagnosed with insomnia reported anomalies in both wakefulness-promoting regions and regions that underlie the neural response to stress (Nofzinger et al., 2004). Using positron emission tomography, these investigators found that insomniacs failed to reduce activation in limbic system structures, particularly in the medial temporal cortex, amygdala, insula, and anterior cingulate cortex. Notably, there were no differences between insomniacs and healthy controls in EEG measures of sleep, including sleep onset latency, sleep efficiency, and spectral characteristics of sleep.
Psychological states during the sleep-to-wake transition are challenging to assess, as are the brain systems underlying these states. Task-based functional magnetic resonance imaging (fMRI), in which participants respond to external cues or process information, is counterproductive to the quiescent process of sleep onset that is disrupted in insomnia. In contrast, intrinsic network imaging, which does not require a specific task or even participant engagement or alertness, is particularly well suited to provide novel insights concerning dynamic brain functions underlying psychological processes in insomnia. This method can provide a dynamic portrait of brain networks even in the absence of a guided task (Raichle et al., 2001). In intrinsic network imaging, the blood-oxygen level dependent (BOLD) signal in the brain is organized into networks of regions with coherent activity. Although the study of these networks and their relation to cognitive and affective states is still nascent, these intrinsic network analyses are promising methods for determining regions with aberrant coactivation with canonical networks in neurological and psychiatric disorders (Sheline, Price, Yan, & Mintun, 2010). These regions with aberrant coactivation may elucidate the underlying neural basis for neurological and psychiatric disorders.
Intrinsic network imaging offers a powerful tool to investigate brain regions and networks involved in insomnia without disrupting an individual’s current mental state with more intrusive or invasive methods. This method also enables targeting of specific networks putatively involved in arousal and insomnia. In the present study, we examined late-night, intrinsic network fMRI in 17 female adults diagnosed with insomnia and 17 female healthy-sleeping controls. To assess sleep-onset dysfunction in insomniacs, we imaged participants in two conditions: resting-state and ‘fall asleep,’ in which participants were asked to let themselves fall asleep. We focused specifically on the role of affective regions within resting-state networks that include arousal-promoting structures that have been implicated in insomnia (Nofzinger et al., 2004).
Methods
Participants
We recruited females, ages 18-40, who self-reported insomnia or healthy sleep. Participants were excluded for any past or present DSM-IV Axis I disorder, any past or present sleep disorder except insomnia, current use of prescription psychotropic or hypnotic medication, BMI greater than 30, and any exclusionary criteria for the MRI environment. We recruited only females because they have a higher prevalence of insomnia than do males (Ohayon, 2002), as well as to increase the homogeneity of the sample and the power of this study.
Eligible participants were administered the Structured Clinical Interview for Diagnosis of DSM-IV-TR Axis I disorders (First et al., 1997) and the Duke Structured Interview for Sleep Disorders (Edinger et al., 2001; Stepanski et al., 2004). No participant met any criteria for any DSM-IV-TR Axis I disorder or any sleep disorder, other than insomnia in insomniacs: DSM-IVTR insomnia, ICSD-2 psychophysiological insomnia, or ICSD-2 idiopathic insomnia. Insomniacs had to retrospectively report at least 30 total minutes of sleep difficulty at least 3 times a week for at least 2 months, along with subjective distress. These criteria were selected to balance DSM-IV-TR and ICSD-2 criteria (Ohayon and Reynolds III, 2009), while reflecting evolving nosologies of insomnia (Edinger et al., 2011). Participants then completed demographic information, the Beck Depression Inventory II (BDI-II) (Beck et al., 1996), Beck Anxiety Inventory (BAI) (Beck et al., 1988), the Pittsburgh Sleep Quality Index (PSQI) (Buysse et al., 1989), the Dysfunctional Beliefs and Attitudes about Sleep scale (DBAS-16) (Morin et al., 2007), the Insomnia Severity Index (ISI) (Bastien et al., 2001), the Ford Insomnia Response to Stress scale (FIRST) (Drake et al., 2004), the Fatigue Severity Scale (FSS) (Krupp et al., 1989), and specific information about current (within the last month) and past (past six months) sleep.
Several factors suggest that this is a viable clinical group. Differences between the two groups in scores on the insomnia severity index (ISI), Ford Insomnia Response to Stress scale (FIRST), and Pittsburgh Sleep Quality Index (PSQI) clearly indicate that the insomnia group experiences greater subjective sleep distress than does the control group. Indeed, all but one of the insomnia group participants had at least subthreshold insomnia based on the ISI (Bastien et al., 2001); interestingly, this is not the same individual who reported less than 30 minutes of sleep latency. More than half (8 of 17) of the insomnia participants reported at least clinically severe levels of insomnia, based on the ISI.
fMRI acquisition
Eligible participants were instructed to abstain from using over-the-counter medications that may affect sleep for a week prior to the scan and to limit the consumption of caffeinated beverages on the day of the scan. At midnight, participants completed a high-resolution SPGR anatomical scan and two 20-minute spiral-in/out scans: a resting-state scan, with the instruction to “rest quietly with your eyes closed,” and a ‘fall asleep’ scan, with the instruction to “rest quietly with your eyes closed and let yourself fall asleep.” Following each scan, participants rated using a button box both their alertness during the previous scan and their post-scan alertness on a modified version of the Karolinska sleepiness scale (Kaida et al., 2006); ratings on this scale ranged from 1 to 9, with 1 corresponding to “wide awake,” and 9 corresponding to “in deep sleep.” High-resolution anatomical scans were obtained with an SPGR sequence with a resolution of 0.859mm × 0.859mm × 1mm. Resting-state and ‘fall asleep’ scans were whole-brain spiral-in/out scans (Glover and Law, 2001), with 30 oblique axial slices with a thickness of 4mm (1mm skip) and an in-plane voxel size of 3.4375mm × 3.4375mm (TE=30ms, FOV=22cm, flip angle = 80°, and TR=2.04s) and 600 time frames for each scan for a total time per scan of 20 minutes, 24 seconds. Before and after the session, participants completed the PANAS (Watson et al., 1988).
fMRI preprocessing
For the two spiral-in/out scans, we used modified NITRC (NITRC.org) and custom-designed scripts to preprocess data. RETROICOR (Glover et al., 2000) was used to remove time-locked cardiac and respiratory artifacts, and RVHRCOR (Chang et al., 2009) was used to remove low-frequency heart rate and respiratory volume artifacts. We discarded the first 6 TRs because of T1 equilibrium effects. We then applied slice timing correction, motion correction, skull-stripping, and linear and quadratic detrending. Functional scans were registered to the MNI152 average brain template (Mazziotta et al., 1995). Motion files were used to ‘censor’ (remove) TRs in which the derivative value of any of six motion parameters (x-shift, y-shift, z-shift, rotation, pitch, yaw) exceeded a Euclidean norm of 1.2. Insomniacs and healthy controls did not differ in the number of TRs removed during the rest scan, t (32) =0.397, or the ‘fall asleep’ scan, t (32) =1.792, both p>0.05.
Nuisance signal timecourses in spiral-in/out volumes arising from white-matter, and CSF were calculated from segmented anatomical scans and were regressed from spiral-in/out volumes along with the 6 motion parameters. The demeaned residuals were then subjected to Multivariate Exploratory Linear Optimized Decomposition into Independent Components (MELODIC) using FSL. We initially used the Laplace approximation to the Bayesian evidence of the model order to determine the number of components, but the length and resolution of the scans produced hundreds of components that proved impractical for analysis, as noted previously (Yourganov et al., 2011). Consequently, we selected 25 components for resting and ‘fall asleep’ scans based on previous dual regression studies (Filippini et al., 2009).
Visually identified components corresponding to known noise and artifacts resulting from scanner noise, movement, residual white matter or CSF signal, or residual physiological noise were filtered from the resulting volumes (Kelly et al., 2010). Given the size of the volumes and lengths of the scan, multiple noise components persisted after filtering; consequently, this procedure was repeated a total of three times on each scan session. The MELODIC component of dual-regression requires equivalent length data, thus excluding the use of motion-censored data blocks. Subsequent analyses that were later conducted on the original non-de-noised datasets indicated that the statistical contrasts did not differ from analyses conducted on de-noised datasets. Insomniacs and healthy controls did not differ in the number of noise components removed, t (32) =1.44, p>0.05.
fMRI analyses
All individual de-noised datasets from each scan were concatenated and decomposed into 25 spatiotemporal components for each of the two scan types. Components of interest were analyzed by dual regression (Filippini et al., 2009; Zuo et al., 2010). Briefly, the spatial maps derived from the temporal concatenation ICA were used to produce a timeseries for each component for each individual. Next, these timeseries were used to produce spatial maps of the corresponding component for each individual. A z-statistic of this resulting spatial map was subjected to non-parametric permutation testing, with 5,000 permutations and a variance smoothing equal to the FWHM. The result of the permutation analysis is a test of between-group differences in each of the 25 component maps. Thresholding of group statistics was based on threshold-free cluster enhancement. Results are presented for clusters that reach a family-wise error corrected value of p<0.05; uncorrected values of p<0.001 are also shown for illustrative purposes.
EEG acquisition and preprocessing
EEG was acquired using a MRI-compatibile EGI HydroCel 256-electrode dense-array Geodesic Sensor Net at a sampling rate of 250hz. No signal quality decline was observed during the scan session. Using NetStation, the TR marker was used to filter out the MR artifact using a moving average of 5 TRs. Bad channels were visually identified and replaced with a spline interpolation. The resulting file was imported into the EEGlab toolbox in Matlab (R2011b). The first 6 and last 5 TRs, which remain contaminated with MR-related artifacts, were censored. The first three harmonics of the slice frequency (14.6hz, 29.3hz, 44.0hz) were removed using a finite impulse response (FIR) notch filter in Matlab. Using PPG markers, the ballistocardiographic artifact was removed using a principal components method (Niazy et al., 2005) , with an optimal basis set of 4 components. The resulting file was resampled to 125hz, re-referenced to average, and segmented using the TR trigger marker; the result is a file with 589 epochs of 2.04 seconds each across 256 channels.
EEG/fMRI analysis
For each epoch, signals were averaged from (10-20 system) Fp1, Fp2, F7, F8, F3, F4, Fz, C3, C4, P3, P4, Pz T3, T4, T5, T6, O1, O2. Epochs in which extreme values occurred (signal > 300μV) were censored. A fast-Fourier transform (FFT) was applied to the square root power of the averaged electrode signal and output in 0.5hz frequency bins to calculate power spectra. EEG/fMRI timecourses were constructed using a finite impulse response bandpass filter. The total power of a TR of each EEG frequency was calculated using a Teager Energy Operator. This timeseries was then log-transformed and fitted to the data from each fMRI voxel using ordinary least squares regression as implemented by 3dDeconvolve in AFNI. The ratio of the power in a particular band to the total EEG power of all bands at any given TR was calculated (de Munck et al., 2009), as this was more robust against noise-related broadband amplitude modulation. This timeseries was regressed against the fMRI data using 3dDeconvolve to produce z-statistic maps. TRs with excessive motion or extreme EEG values were censored from analysis (Jansen et al., 2012). EEG/fMRI results for the frequency band correlations were determined at the voxel-wise p<0.001, cluster-wise p<0.05, while the exploratory analyses of insomniacs and healthy controls are presented at a voxel-wise p<0.005 level.
Results
fMRI
The 17 insomniacs and 17 healthy controls were equivalent in age and education level (Table 1). As expected, insomniacs reported more sleep dysfunction than did controls as measured by the PSQI, ISI, and FIRST, and also reported greater sleep onset latency, less overall sleep, and more impaired sleep function (Table 1). Sleep dysfunction was also present before the scan session (Supplementary Results). Insomniacs had higher BDI and BAI scores than did healthy controls, but scores for both groups were well below the clinical cut-offsfor these questionnaires. Participants completed two 20-minute, task-free fMRI scans beginning at 12:35AM and 1:00AM, which was close to their habitual bedtimes (Table 1). Insomniacs and healthy controls did not significantly differ in bedtimes or waketimes.
Table 1. Mean values, effect sizes, and p-values for group differences of demographic variables of insomniacs (INSM) and healthy controls (CTL). Standard deviations are presented in parentheses. Abbreviations: Insomnia Severity Index (ISI), Dysfunctional Beliefs and Attitudes about Sleep scale (DBAS-16), Ford Insomnia Response to Stress scale (FIRST), Fatigue Severity Scale (FSS), Pittsburgh Sleep Quality Index (PSQI), Beck Anxiety Inventory (BAI), Beck Depression Inventory II (BDI-II).
Demographic, sleep, and affective characteristics
| CTL mean | INSM mean | Cohen’s d |
p-value | |
|---|---|---|---|---|
|
|
||||
| Age in years | 27.56 (6.83) | 27.16 (6.67) | 0.06 | .865 |
| Education level | 6.18 (1.63) | 6.24 (1.71) | 0.04 | .919 |
| Sleep onset latency (last month) | 11.82 (6.96) | 46.71 (42.67) | 1.14 | .002 |
| Wake after sleep onset (last month) | 2.41 (3.87) | 43.23 (76.42) | 0.75 | 0.035 |
| Total sleep time (last month) | 7.63 (0.60) | 6.18 (1.06) | 1.69 | <0.001 |
| Bedtime | 11:42PM (56.71) | 11:54PM (96.12) | 0.16 | 0.645 |
| Waketime | 7:51AM (41.80) | 8:18AM (108.25) | 0.33 | 0.345 |
| ISI | 1.76 (2.77) | 15.00 (3.77) | 4.00 | <0.001 |
| FIRST | 14.71 (3.82) | 24.24 (5.07) | 2.12 | <0.001 |
| PSQI | 1.94 (1.48) | 9.24 (3.38) | 2.79 | <0.001 |
| BAI | 0.82 (0.81) | 3.88 (2.67) | 1.55 | <0.001 |
| BDI-II | 1.82 (3.13) | 4.59 (4.51) | 0.71 | .046 |
| PANAS negative affect | 16.18 (2.38) | 15.88 (1.11) | 0.16 | 0.674 |
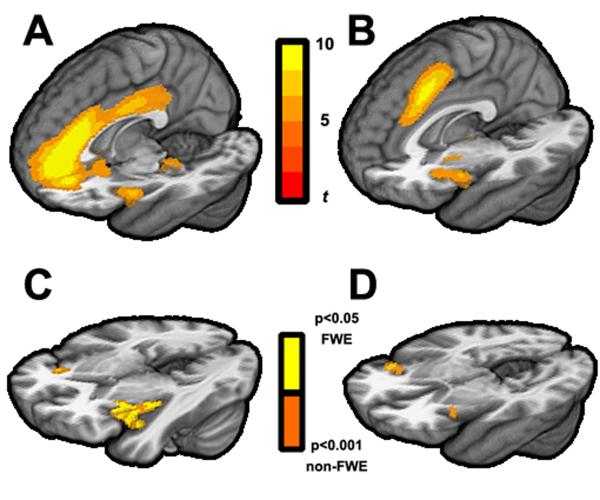
Using data from all participants, we conducted independent components analysis (ICA) to extract maps of brain networks for rest and ‘fall asleep’ scans that were specific to this study but that corresponded to previously described intrinsic networks. Among these networks, we were particularly interested in ‘salience’ networks (Deen et al., 2011) , which include structures implicated in arousal and insomnia, such as the anterior cingulate cortex and insular cortex. Examining the spatially distinct salience networks maps generated from data combined across both groups, we found evidence in both the rest and the ‘fall asleep’ scans of three previously-reported salience networks (Deen et al., 2011) :ventral anterior insula (vAI) salience network (Fig. 1A), dorsal anterior insula (dAI) salience network (Fig. 1B), and posterior insula salience network. We then used a dual regression approach (Filippini et al., 2009) to examine whether insomniacs and healthy control participants exhibited different patterns of brain regions in which BOLD activity was either more or less strongly correlated with these salience networks. Briefly, for each scan, we used salience network maps from the combined group-level ICA to derive network timecourses, from which individual subject maps were derived; these subject maps were then analyzed using permutation-based statistical inference to test for group differences in spatial regions with more or less coactivation with the salience networks.
Figure 1.
fMRI BOLD networks derived from independent components analyses in the ‘fall asleep’ scan. These include the ventral anterior insula salience network (A) and dorsal anterior insula salience network (B) templates. Dual-regression analyses of regions that show increased coactivation in insomniacs in the ventral anterior insula salience network (C), as well as regions that show increased coactivation with dorsal anterior insula salience network (D), with colors corresponding to p<0.05 family-wise error corrected and p<0.001 uncorrected using non-parametric permutation testing.
For the rest scan, we found no differences between insomniacs and healthy control participants in brain regions that coactivated with any of the salience networks. For the ‘fall asleep’ scan, we found increased coactivation of the anterior insula in insomniacs compared to healthy control participants with the vAI salience network (Fig. 1C) and the dAI network (Fig. 1D), but not with the posterior insula salience network. Thus, while attempting to fall asleep, insomniacs had greater coactivation of the anterior insula with the dAI and vAI salience networks than did healthy controls.
Because the fMRI analyses described above are dependent on the networks derived from the resting or ‘fall asleep’ scans, they do not allow direct comparisons of the overall effect of scan type (resting vs. ‘fall asleep’), the overall effect of group (insomniacs vs. healthy controls, across both scan types), or the interaction of group and scan type. To test for these effects, we concatenated data from both groups in both resting and ‘fall asleep’ scans and conducted an ICA network extraction and dual-regression analysis as described above (Fig. S1A; insomniacs had greater insula coactivation with vAI salience network than did healthy controls (Fig. S1B). There were not, however, overall significant group effects in the dAI salience network, nor was there a significant effect of scan or a significant interaction of group and scan in any salience network. Thus, while insomniacs show heightened insula coactivation with the vAI salience network, this coactivation does not appear to be exclusive to the ‘fall asleep’ scan.
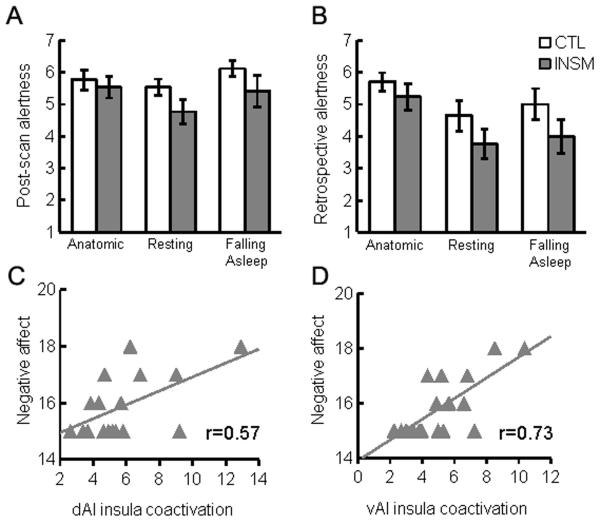
Insomniacs did not differ from healthy controls in their subjective ratings of their alertness following the anatomic, rest, or ‘fall asleep’ scans, F(1,32)=2.91, p>0.05; moreover, self-rated post-scan alertness after these scans did not decrease throughout the session, F(2,31)=2.28, p>0.05 (Fig. 2A). Insomniacs also did not differ from healthy controls in their retrospective ratings of alertness during any scan, F(1,32)=2.821, p>0.05, although in both groups there was a significant decrease of alertness across scans, F(2,31)=10.18, p<0.001 (Fig. 2B). There were no interactions of group and time for either current ratings after scans, F(2,31)=0.76, p>0.05, or retrospective ratings of the scans, F(2,31)=0.42, p>0.05. Insomniacs and healthy controls also did not differ in negative affect, t(32)=0.462, p>0.05, or positive affect, t(32)=0.951, p>0.05. Exploratory analyses of the correlation between coactivation and affect within the insomnia group revealed that insula coactivation was significantly correlated with post-scan PANAS negative affect scores in both dAI, p=0.018 (Fig. 2C), and vAI networks, p<0.001 (Fig. 2D) but not PANAS positive affect scores, p>0.05. Conducting these analyses including healthy controls or within the healthy control group alone did not yield significant correlations.
Figure 2.
Self-rated subjective alertness and fMRI BOLD network coactivation. Post-scan (A) and retrospective (B) alertness, as rated on a scale of 1 (deeply asleep) to 9 (wide awake) ± SEM. is moderately correlated with insula coactivation z-scores in dorsal anterior insula (C) and ventral anterior insula (D) salience networks. Retrospective alertness is not correlated with insula coactivation with dorsal anterior insula salience network (E) but is moderately correlated with insula coactivation with ventral anterior insula salience network (F). Insula coactivation in both dorsal (G) and ventral (H) anterior insula salience networks is significantly correlated with post-scan PANAS negative affect scores.
EEG
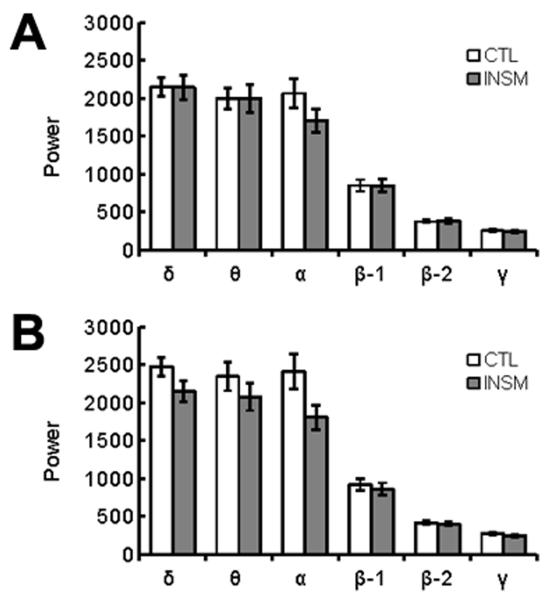
Next, we examined EEG measures in insomniacs and healthy control participants during the rest and ‘fall asleep’ scans. We did not observe obvious sleep episodes from visual inspection of the EEG. Overall power spectra were calculated for rest (Fig. 3A) and ‘fall asleep’ scans (Fig. 3B) in 0.5hz frequency bins. We examined overall frequency power in delta (δ: 0.5-4hz), theta (θ: 4-8hz), alpha (α: 8-14hz), beta-1 (β-1: 14-20hz), beta-2 (β-2: 20-35hz), or gamma (γ: 35-50hz) power bands using a repeated-measures multivariate analysis of variance (MANOVA; scan by frequency band by group). This analysis yielded a significant effect of scan, F(1,32)=10.270, p=0.003, a significant effect of frequency band, F(1,32)=111.151, p<0.001;the main effect of group was not significant, F(1,32)=1.352, p>0.05, nor was the interaction of group and frequency band, F(5,28)=1.546, p>0.05. There was a significant interaction of scan and frequency band, F(5,28)=3.415, p=0.016, and a significant interaction of group and scan, F(1,32)=4.800, p=0.036. Relative to healthy controls, insomniacs had reduced EEG power across multiple frequency bands during the ‘fall asleep’ scan compared to the resting scan. Post-hoc analyses of individual frequencies reveal that α is the only individual band with significant differences between the two groups during the ‘fall asleep’ scan, t(32)=2.14, p=0.040, uncorrected. Notably, there were no significant differences between the two groups in the bands associated with non-REM sleep, δ and θ.
Figure 3.
EEG power of healthy controls (CTL) and insomniacs (INSM). EEG during rest (A) and ‘fall asleep’ (B), by power frequency ± SEM.
EEG/FMRI
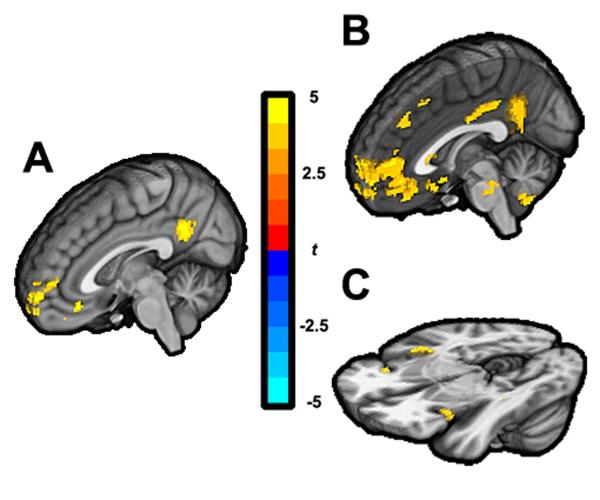
We then constructed timeseries from each frequency band to examine whether insomniacs and healthy controls differed in BOLD correlates of EEG power. The full results for each power band are presented in Table S1. While there were few significant group differences in BOLD signal associated with lower frequency bands of EEG, we found for the resting state scan that healthy controls showed significantly greater BOLD signal associated with γ power ratio in the posterior cingulate (PCC) and medial prefrontal cortex (mPFC) than did insomniacs (Fig. 4A). Given the involvement of these structures in canonical resting state networks, specifically in default mode networks, we examined each group separately in an exploratory analysis to determine the BOLD signal associated with γ power ratio. In healthy controls, the pattern of BOLD signal includes PCC and mPFC (Fig. 4B); in contrast, in insomniacs, the pattern of BOLD signal includes bilateral insula, resembling the spatial pattern of the vAI network (Fig. 4C). We also examined the dorsal and ventral default mode networks in rest and ‘fall asleep’ using dual-regression, and found no differences between insomniacs and healthy controls in coactivations with these networks. There were no significant correlations in any participant between any EEG frequency band power timeseries and ICA-derived network timeseries.
Figure 4.
EEG γ band power associated BOLD signal. Differences between healthy controls and insomniacs (A), p<0.001, cluster corrected p<0.05, and BOLD correlated with γ band in healthy controls (B) and insomniacs (C), both at uncorrected voxel-wise p<0.005.
Discussion
The present study is the first to characterize resting-state networks in insomniacs and healthy sleeping controls using combined EEG and fMRI. Using a dual regression approach, we found increased bilateral anterior insula BOLD coactivation with vAI and dAI salience networks, as well as insula-associated high-frequency γ power during rest in insomniacs. Notably, the insula is a key hub in the salience network itself, and the difference in coactivation between groups suggests a role for the salience network in insomnia. Indeed the aberrant insula contribution to salience networks is consistent with previous studies of insomnia in rodents (Cano et al., 2008) and humans (Nofzinger et al., 2004), suggesting that these networks, and in particular the anterior insula, contribute to the neural circuitry underlying insomnia. Anterior insula and nearby orbitofrontal cortex gray matter density have been previously implicated in insomnia (Stoffers et al., 2012). The insula has been posited to be a source of the slow waves that characterize deeper stages of sleep (Murphy et al., 2009), and the increased coactivation of the left insula with salience networks may interfere with the progressive generation of low-frequency EEG waves as part of the transition to sleep.
Whereas some studies have found few differences between insomniacs and controls in polysomnography-measured sleep (Rosa and Bonnet, 2000), others have documented persistent high-frequency activity during sleep in insomniacs (Krystal et al., 2002; Perlis et al., 2001) . We did not observe increased high-frequency activity in insomniacs, although it is possible that the scanning environment impaired N1 sleep. Healthy controls had greater PCC and mPFC signal associated with γ power ratio than did insomniacs. Whereas the γ ratio power in healthy controls was associated with BOLD signal in PCC and mPFC, spatially similar to DMN networks, the γ ratio power in insomniacs was associated with BOLD signal in anterior insula, similar to salience networks. While these associations were not evident in the rest scan, the γ ratio power BOLD associations with anterior insula might be indicative of impairments occurring outside attempted sleep onset in insomniacs. While there was a significant group by scan interaction in overall EEG spectra, there were only differences in α power between groups during the ‘fall asleep’ scan. Previous studies of EEG power in insomnia have found that insomniacs have reduced alpha power (Lamarche and Ogilvie, 1997), combined with a failure to reduce alpha power during the sleep onset period (Staner et al., 2003) and wakefulness (Freedman, 1986). It is not clear whether alpha power differences extend into the sleep period.
The insula is thought to play a role in affect, for example the anticipation of negative stimuli in anxious individuals (Simmons et al., 2011). Insomnia is associated with psychiatric conditions, including anxiety and major depression, and in this sample insomniacs have increased anxiety and depression scores compared to healthy controls, albeit below clinical thresholds. The increased insula coactivation observed could be indicative of this subthreshold anxiety, worry and rumination, or a signature of insufficient gating of the sensory stimuli of the fMRI environment (Hairston et al., 2010). However, another intriguing mechanism by which the insula may contribute to insomnia is through the subjective perception of sleep distress. DSM IV, ICSD-9, and ICD-10 all define insomnia based on subjective distress, rather than on an objective measure of sleep disturbance, as insomniacs may misperceive the quantity or quality of their sleep relative to polysomnography (American Academy of Sleep Medicine, 2005; Association and DSM-IV, 2000; “WHO | International Classification of Diseases (ICD),” n.d.) . We propose that the insula coactivation with salience networks underlies this subjective disturbance; indeed we found positive correlations in insomniacs between the degree of insula coactivation with salience networks and with self-reported alertness and negative affect. The insula has been proposed to integrate a variety of information, including interoceptive awareness, time perception, and emotional salience ( (Bud) Craig, 2009) . Among other functions, the insula has been proposed to underlie facets of self-awareness, time dilation, and subjective salience, all of which have been proposed to play a role in insomnia (Harvey and Tang, 2012) . Increased insula coactivation with salience networks may contribute specifically to the misperception of sleep and wakefulness. This misperception, whether a misestimate of time or some qualia of sleep satisfaction, may outweigh the fulfillment of homeostatic sleep need in insomnia.
Intrinsic network imaging has several advantages over task-based imaging for studying insomnia. Specifically, the lack of externally guided task—with accompanying visual or auditory stimuli—better simulates the mental state prior to sleep onset that is posited to be dysfunctional in insomnia. Furthermore, the focus on specific networks permits the identification of not only aberrant activity in a brain region but also the possible role of this brain region within a network of structures. Because intrinsic network imaging uses the same pulse sequences as task-based imaging and requires no additional setup, this is an important tool for the study of insomnia and other psychological disorders that may not be amenable to traditional task-based fMRI.
There are limitations of the current study that should be addressed in future investigations. For example, even though the insomnia group did report sleep dysfunction at the time of the scan (see Supplementary Results), a two-week sleep diary combined with actigraphy would be instructive in confirming the diagnosis of chronic insomnia. Currently, diagnoses of insomnia rely on subjectively reported distress, not on actigraphy; indeed, insomniacs who have objective short sleep may represent a different phenotype than do those whose sleep disturbance is primarily subjective (Krystal et al., 2002b). Similarly, it is possible that the fMRI network findings we describe will vary as a function of individual differences in sleep variables as measured with traditional polysomnography. Certainly the fMRI environment is not an ideal sleep environment, especially combined with the ‘first-night effect’ reported in polysomnography studies. Importantly, in present study there were no significant differences between the insomniac and control groups in δ or θ bands, suggesting that the group differences we observed in neural coactivation were not due to differences in sleep. Nevertheless, the present findings must be interpreted in the context of the study conditions. We did not use polysomnography to diagnose insomnia, and scoring sleep stage during scans may yield important information in future fMRI/EEG studies of this disorder.
In this study we have identified a role for the anterior insula in arousal-promoting BOLD signal networks in insomnia. This structure, as part of a network of structures involved in hyperarousal in insomnia, may be an important target for novel therapies for this disorder. In this context, it is noteworthy that administration of benzodiazepines has been found to reduce regional cerebral blood flow to multiple limbic system structures, including the anterior insula (Kajimura, 2004). Future studies could focus on directly altering BOLD signal in this structure using real-time fMRI or other interventions for the treatment of chronic insomnia.
Supplementary Material
Highlights.
Insomnia is characterized by sleep-related psychological distress, often in the absence of ‘objective’ sleep disturbance.
We used intrinsic network imaging methods and dual regression to non-invasively assess brain networks of insomniacs and healthy controls.
We found that insomniacs have increased insula coactivation with brain networks that are associated with salience and arousal.
These findings suggest that dysfunction in insomnia is related to aberrant brain network functioning.
Acknowledgments
We thank Arkadiy Maksimovskiy, Rebecca Sacks, and Sarah Victor for their assistance with participant recruitment and data collection. This work was supported by a Stanford Graduate Research Opportunity Grant, an American Psychological Association Dissertation Research Award, and a Norman H. Anderson Research Grant awarded to M.C.C., who was also supported by National Institute of Mental Health grant T32 MH019956.
Footnotes
Financial disclosure The authors report no financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- (Bud) Craig AD. How do you feel [mdash] now? The anterior insula and human awareness. Nat Rev Neurosci. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- American Academy of Sleep Medicine . The International Classification of Sleep Disorders: diagnostic and coding manual. 2nd ed American Academy of Sleep Medicine; Westchester, IL: 2005. [Google Scholar]
- Association, A.P. DSM-IV, A.P.A.T.F. on . Diagnostic and statistical manual of mental disorders: DSM-IV-TR. American Psychiatric Association; Washington, DC: 2000. [Google Scholar]
- Bastien CH, Vallières A, Morin CM. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2:297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for beck depression inventory II (BDI-II) Psychology Corporation; San Antonio, TX: 1996. [Google Scholar]
- Bonnet MH, Arand DL. Hyperarousal and insomnia: State of the science. Sleep Medicine Reviews. 2010;14:9–15. doi: 10.1016/j.smrv.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cano G, Mochizuki T, Saper CB. Neural Circuitry of Stress-Induced Insomnia in Rats. J. Neurosci. 2008;28:10167–10184. doi: 10.1523/JNEUROSCI.1809-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C, Cunningham JP, Glover GH. Influence of heart rate on the BOLD signal: the cardiac response function. Neuroimage. 2009;44:857–869. doi: 10.1016/j.neuroimage.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Munck JC, Gonçalves SI, Mammoliti R, Heethaar RM, Lopes da Silva FH. Interactions between different EEG frequency bands and their effect on alpha-fMRI correlations. Neuroimage. 2009;47:69–76. doi: 10.1016/j.neuroimage.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Deen B, Pitskel NB, Pelphrey KA. Three Systems of Insular Functional Connectivity Identified with Cluster Analysis. Cereb. Cortex. 2011;21:1498–1506. doi: 10.1093/cercor/bhq186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake C, Richardson G, Roehrs T, Scofield H, Roth T. Vulnerability to stress-related sleep disturbance and hyperarousal. Sleep. 2004;27:285–291. doi: 10.1093/sleep/27.2.285. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wohlgemuth WK, Radtke RA, Marsh GR, Quillian RE. Cognitive behavioral therapy for treatment of chronic primary insomnia: a randomized controlled trial. JAMA. 2001;285:1856–1864. doi: 10.1001/jama.285.14.1856. [DOI] [PubMed] [Google Scholar]
- Edinger JD, Wyatt JK, Stepanski EJ, Olsen MK, Stechuchak KM, Carney CE, Chiang A, Crisostomo MI, Lineberger MD, Means MK, Radtke RA, Wohlgemuth WK, Krystal AD. Testing the reliability and validity of DSM-IV-TR and ICSD-2 insomnia diagnoses. Results of a multitrait-multimethod analysis. Arch. Gen. Psychiatry. 2011;68:992–1002. doi: 10.1001/archgenpsychiatry.2011.64. [DOI] [PubMed] [Google Scholar]
- Filippini N, MacIntosh BJ, Hough MG, Goodwin GM, Frisoni GB, Smith SM, Matthews PM, Beckmann CF, Mackay CE. Distinct patterns of brain activity in young carriers of the APOE-epsilon4 allele. Proc. Natl. Acad. Sci. U.S.A. 2009;106:7209–7214. doi: 10.1073/pnas.0811879106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- First M, Gibbon M, Spitzer R, Williams J. Structured Clinical Interview for DSM-IV Axis I Disorders. American Psychiatric Press; Washington DC: 1997. [Google Scholar]
- Ford DE, Kamerow DB. Epidemiologic Study of Sleep Disturbances and Psychiatric Disorders: An Opportunity for Prevention? JAMA. 1989;262:1479–1484. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- Freedman RR. EEG power spectra in sleep-onset insomnia. Electroencephalography and Clinical Neurophysiology. 1986;63:408–413. doi: 10.1016/0013-4694(86)90122-7. [DOI] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magnetic Resonance in Medicine. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Glover GH, Li TQ, Ress D. Image-based method for retrospective correction of physiological motion effects in fMRI: RETROICOR. Magn Reson Med. 2000;44:162–167. doi: 10.1002/1522-2594(200007)44:1<162::aid-mrm23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- Hairston IS, Talbot LS, Eidelman P, Gruber J, Harvey AG. Sensory gating in primary insomnia. Eur. J. Neurosci. 2010;31:2112–2121. doi: 10.1111/j.1460-9568.2010.07237.x. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Tang NKY. (Mis)perception of sleep in insomnia: a puzzle and a resolution. Psychol Bull. 2012;138:77–101. doi: 10.1037/a0025730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen M, White TP, Mullinger KJ, Liddle EB, Gowland PA, Francis ST, Bowtell R, Liddle PF. Motion-related artefacts in EEG predict neuronally plausible patterns of activation in fMRI data. NeuroImage. 2012;59:261–270. doi: 10.1016/j.neuroimage.2011.06.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaida K, Takahashi M, Akerstedt T, Nakata A, Otsuka Y, Haratani T, Fukasawa K. Validation of the Karolinska sleepiness scale against performance and EEG variables. Clinical Neurophysiology. 2006;117:1574–1581. doi: 10.1016/j.clinph.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Kajimura N. Deactivation by Benzodiazepine of the Basal Forebrain and Amygdala in Normal Humans During Sleep: A Placebo-Controlled [15O]H2O PET Study. American Journal of Psychiatry. 2004;161:748–751. doi: 10.1176/appi.ajp.161.4.748. [DOI] [PubMed] [Google Scholar]
- Kelly RE, Alexopoulos GS, Wang Z, Gunning FM, Murphy CF, Morimoto SS, Kanellopoulos D, Jia Z, Lim KO, Hoptman MJ. Visual inspection of independent components: Defining a procedure for artifact removal from fMRI data. J Neurosci Methods. 2010;189:233–245. doi: 10.1016/j.jneumeth.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The fatigue severity scale. Application to patients with multiple sclerosis and systemic lupus erythematosus. Arch. Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD. Sleep EEG Predictors and Correlates of the Response to Cognitive Behavioral Therapy for Insomnia. Sleep. 2010;33:669–677. doi: 10.1093/sleep/33.5.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002a;25:630–640. [PubMed] [Google Scholar]
- Krystal AD, Edinger JD, Wohlgemuth WK, Marsh GR. NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep. 2002b;25:630–640. [PubMed] [Google Scholar]
- Lamarche CH, Ogilvie RD. Electrophysiological changes during the sleep onset period of psychophysiological insomniacs, psychiatric insomniacs, and normal sleepers. Sleep. 1997;20:724–733. [PubMed] [Google Scholar]
- Lu J, Sherman D, Devor M, Saper CB. A putative flip-flop switch for control of REM sleep. Nature. 2006;441:589–594. doi: 10.1038/nature04767. [DOI] [PubMed] [Google Scholar]
- Mazziotta JC, Toga AW, Evans A, Fox P, Lancaster J. A probabilistic atlas of the human brain: theory and rationale for its development. The International Consortium for Brain Mapping (ICBM) Neuroimage. 1995;2:89–101. doi: 10.1006/nimg.1995.1012. [DOI] [PubMed] [Google Scholar]
- Morin CM, Vallières A, Ivers H. Dysfunctional beliefs and attitudes about sleep (DBAS): validation of a brief version (DBAS-16) Sleep. 2007;30:1547–1554. doi: 10.1093/sleep/30.11.1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Riedner BA, Huber R, Massimini M, Ferrarelli F, Tononi G. Source modeling sleep slow waves. Proc. Natl. Acad. Sci. U.S.A. 2009;106:1608–1613. doi: 10.1073/pnas.0807933106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niazy RK, Beckmann CF, Iannetti GD, Brady JM, Smith SM. Removal of FMRI environment artifacts from EEG data using optimal basis sets. NeuroImage. 2005;28:720–737. doi: 10.1016/j.neuroimage.2005.06.067. [DOI] [PubMed] [Google Scholar]
- Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ. Functional Neuroimaging Evidence for Hyperarousal in Insomnia. Am J Psychiatry. 2004;161:2126–2128. doi: 10.1176/appi.ajp.161.11.2126. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Medicine Reviews. 2002;6:97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Ohayon MM, Reynolds CF., III Epidemiological and clinical relevance of insomnia diagnosis algorithms according to the DSM-IV and the International Classification of Sleep Disorders (ICSD) Sleep Medicine. 2009;10:952–960. doi: 10.1016/j.sleep.2009.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlis ML, Smith MT, Andrews PJ, Orff H, Giles DE. Beta/Gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep. 2001;24:110–117. doi: 10.1093/sleep/24.1.110. [DOI] [PubMed] [Google Scholar]
- Raichle ME, MacLeod AM, Snyder AZ, Powers WJ, Gusnard DA, Shulman GL. A default mode of brain function. Proc. Natl. Acad. Sci. U.S.A. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riemann D, Spiegelhalder K, Feige B, Voderholzer U, Berger M, Perlis M, Nissen C. The hyperarousal model of insomnia: A review of the concept and its evidence. Sleep Med Rev. 2009 doi: 10.1016/j.smrv.2009.04.002. [DOI] [PubMed] [Google Scholar]
- Rosa RR, Bonnet MH. Reported Chronic Insomnia Is Independent of Poor Sleep as Measured by Electroencephalography. Psychosom Med. 2000;62:474–482. doi: 10.1097/00006842-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Price JL, Yan Z, Mintun MA. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proceedings of the National Academy of Sciences. 2010;107:11020–11025. doi: 10.1073/pnas.1000446107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons AN, Stein MB, Strigo IA, Arce E, Hitchcock C, Paulus MP. Anxiety positive subjects show altered processing in the anterior insula during anticipation of negative stimuli. Human Brain Mapping. 2011;32:1836–1846. doi: 10.1002/hbm.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staner L, Cornette F, Maurice D, Viardot G, Le Bon O, Haba J, Staner C, Luthringer R, Muzet A, Macher J-P. Sleep microstructure around sleep onset differentiates major depressive insomnia from primary insomnia. J Sleep Res. 2003;12:319–330. doi: 10.1046/j.0962-1105.2003.00370.x. [DOI] [PubMed] [Google Scholar]
- Stepanski EJ, Edinger JD, Bonnet MH, Bootzin RR, Doghramji K, Dorsey CM, Espie CA, Jamieson AO, McCall WV, Morin CM. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27:1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- Stoffers D, Moens S, Benjamins J, van Tol M-J, Penninx BWJH, Veltman DJ, Van der Wee NJA, Van Someren EJW. Orbitofrontal gray matter relates to early morning awakening: a neural correlate of insomnia complaints? Front Neurol. 2012;3:105. doi: 10.3389/fneur.2012.00105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology. 1988;54:1063–1070. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- WHO [accessed 10.30.09];International Classification of Diseases (ICD) [WWW Document] n.d. URL http://www.who.int/classifications/icd/en/
- Yourganov G, Chen X, Lukic AS, Grady CL, Small SL, Wernick MN, Strother SC. Dimensionality estimation for optimal detection of functional networks in BOLD fMRI data. Neuroimage. 2011;56:531–543. doi: 10.1016/j.neuroimage.2010.09.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X-N, Kelly C, Adelstein JS, Klein DF, Castellanos FX, Milham MP. Reliable intrinsic connectivity networks: Test-retest evaluation using ICA and dual regression approach. NeuroImage. 2010;49:2163–2177. doi: 10.1016/j.neuroimage.2009.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.