TO THE EDITOR
Woolly hair nevus (WHN) is a mosaic disorder characterized by distinct patterns of tightly curled scalp hair which can appear concurrently with epidermal nevi (EN) at other sites (Peteiro et al., 1989; Venugopal et al., 2012). Woolly hair is also found in congenital disorders resulting from mutations affecting diverse cellular components including intermediate filament, adherens junction, and signal transduction proteins (Harel and Christiano, 2012).
Embryonic somatic mutation causes mosaic disorders which appear in patterns of ectodermal progenitor dorsovental migration. Somatic mutations causing mosaic disorders including Proteus syndrome (Lindhurst et al., 2011), port-wine stains (Shirley et al., 2013), and EN (Levinsohn et al., 2013; Sun et al., 2013) have been found using exome sequencing.
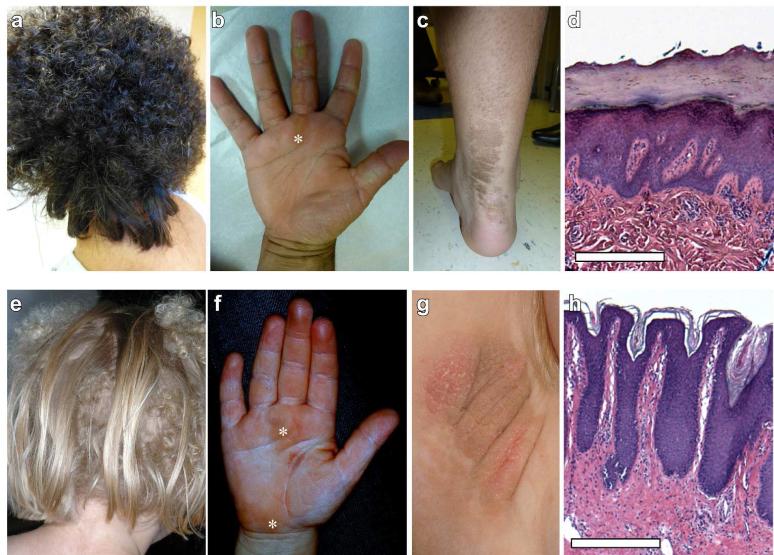
Recognizing that exome sequencing would permit identification of mutations causing WHN, we ascertained two cases. Our first (WHN100, Figure 1a-d) was a 10 year-old girl without history of developmental delay who had regions of slightly curly hair over her occipital scalp from infancy which progressively curled with no scalp surface change and lie alongside areas of straight hair. She has hyperpigmented patches on her neck, trunk, and arms, with more keratotic lesions on her distal extremities, and acanthosis nigricans in both axillae. There was linear palmar keratoderma (PPK) and hyperkeratosis over most metacarpophalangeal and some proximal interphalangeal joints. Given concurrent PPK and woolly hair, clinical concern for Naxos or Carvajal syndromes led to regular cardiology evaluations that found no abnormalities.
Figure 1.
Clinical features of index cases with woolly hair nevi. On the scalp, woolly hair nevus presents with a portion of the scalp exhibiting patches of curly, thin, hair intermixed with regions of normal, straight hair, as observed in WHN100 and WHN101. On the body, additional findings of linear palmoplantar keratoderma (b, f, asterisks) and epidermal nevi (c, g) are found. In WHN100, histology of linear palmar keratoderma (d) shows papillomatosis, hypergranulosis and compact hyperkeratosis, scale bar = 0.5 mm. In the epidermal nevus of WHN101, histology of the epidermal nevus (h) shows acanthosis, papillomatosis, and mild hyperkeratosis, scale = 0.25 mm.
Our second case (WHN101, Figure 1e-h) was a 6 year-old girl whose hair developed at age one and consisted of a mixture of poker-straight hair and curly, thin hair. In infancy, she developed linear dyspigmentation on the right arm and trunk, which became more raised and scaly on the distal extremities over time. She had normal development, with no cardiac or ophthalmic abnormalities found on routine physical examination, cardiac MRI and serial electrocardiograms. Clinical suspicion of mosaic Naxos or Caravajal syndrome motivated clinical sequencing of DSP, DSC1, DSG1, JUP, PKP2, and TMEM43; no mutations were found.
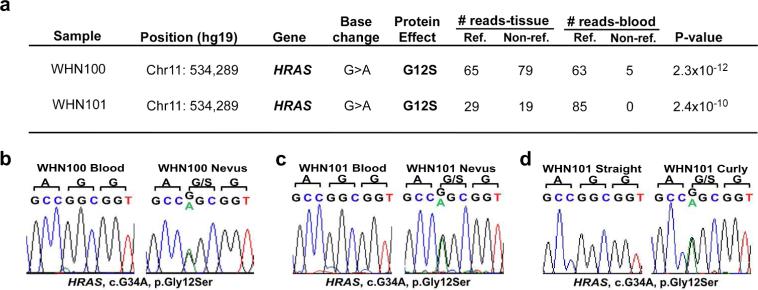
To determine the genetic basis of WHN, we performed paired whole exome sequencing of DNA isolated from affected tissue and blood in both cases (Supplementary Figure 2). Data was analyzed to identify somatic single nucleotide variants (SNVs), deletions and insertions (Supplementary Methods). A somatic heterozygous HRAS c.34G>A, p.G12S substitution was found in each (Figure 2a). There was no evidence of loss of heterozygosity (LOH) (Supplementary Figure 3) or secondary mutation somatic mutation, suggesting that HRAS mutation alone is sufficient to cause WHN. Sanger sequencing confirmed mutation presence in affected tissue (Figure 2b, c). To determine if this mutation causes woolly hair, we prepared DNA from hair bulbs of straight and curly hair obtained from affected individual WHN101, finding the HRAS p.G12S mutation in curly hair only (Figure 2d, Supplementary Figure 1).
Figure 2.
Somatic HRAS p.G12S mutation causes WHN. (a) In WHN100 and WHN101, exome sequencing of affected tissue and blood was performed. Tissue-specific SNVs are annotated bychromosome, position, base change, protein consequence, and numbers of reference and non-reference reads from affected tissue and genomic (blood) DNA. The p-values denoting the significance of the differences in reference and non-reference reads in tissue versus blood were calculated using a one-tailed Fisher's exact test. After filtering (Supplementary methods), only one SNV surpassed genome-wide significance (1.7 × 10<sup>-6</sup>), HRAS p.G12S in each case. (b, c) Sanger sequencing of blood and tissue of WHN100 and WHN101 confirmed this HRAS p.G12S mutation. (c) In WHN100, there is a small mutant allele fraction in blood demonstrated by exome (8% mutant reads) sequencing, but this mutation is enriched to the expected 50:50 ratio in tissue. (d) Sanger sequencing of HRAS in DNA from plucked straight and curly hair from WHN101 shows that HRAS p.G12S mutation is specific to curly hair.
Consistent with somatic mosaicism in an epidermal progenitor, prior cases of WHN have been reported with concurrent keratinocytic epidermal nevi (KEN). KEN result from somatic mutations in HRAS, KRAS, PIK3CA, FGFR3, and NRAS (Hafner et al., 2012) including the HRAS p.G12S mutation found in WHN (Hafner et al., 2011). Furthermore, Costello syndrome (CS), in which patients present with developmental delay, high birth weight, feeding difficulties, failure to thrive, cardiac anomalies, and curly hair, results from germline heterozygous HRAS mutations, including p.G12S (Gripp and Lin, 2012; Siegel et al., 2012). The timing of somatic mutation during embryonic development determines extent of cutaneous involvement and presence of other systemic abnormalities (Moss et al., 1993).
Notably, somatic activating HRAS mutations are found in most cases of nevus sebaceus (NS), a mosaic lesion which typically appears on the scalp and features alopecia, papillomatosis, and marked sebaceus hyperplasia (Groesser et al., 2012; Levinsohn et al., 2013; Sun et al., 2013). These features contrast with those of WHN in which hair is present but curly, and sebaceous hyperplasia is absent. Given that WHN and NS are both caused by somatic HRAS mutations, we hypothesize that their phenotypic divergence may derive from relative potency of the mutant allele with respect to MAP kinase activation. HRAS mutations in WHN and NS fall within the finger loop of HRAS, replacing glycine residues with larger amino acids which prevent GTP hydrolysis (Malumbres and Barbacid, 2003). Though comparison of the WHN p.G12S mutation and the common NS p.G13R mutation has not been performed, HRAS codon 12 serine substitutions have been shown to be less activating than arginine, aspartic acid or valine substitutions (Fasano et al., 1984).
To evaluate the frequency of HRAS mutation in NS, we screened 116 archival scalp NS lesions for HRAS and KRAS mutation. We found 88 HRAS and 9 KRAS mutations. HRAS p.G13R was present in 85 NS and p.G12S was not found (Supplementary Table 2). In prior reports, 64 additional samples were screened, and HRAS p.G12S mutations were not found (Levinsohn et al., 2013; Sun et al., 2013). In one report, 3 specimens with HRAS p.G12S mutations were identified; in 2 there was a concurrent HRAS p.G13R mutation, and in one, the lesion was on the ear, a site at which it could be difficult to distinguish EN and NS (Groesser et al., 2012). These data combined with evidence from CS suggest that more strongly activating RAS mutations may cause the alopecia and sebaceous hyperplasia found in NS, and the more mildly activating p.G12S mutation causes woolly hair phenotypes.
In summary, we find somatic HRAS c.34G>A, p.G12S mutation in affected tissue from two cases with mosaic woolly hair and EN. Consistent with reports of WHN and in KEN, the identified p.G12S mutation causes an EN phenotype on the body, but the finding of curly hair on the scalp suggests that WHN represents a mosaic RASopathy with phenotype determined by location, either due to distinct epidermal progenitor types or site-specific mesenchymal interactions. We hypothesize that in contrast to strongly activating RAS mutations found in NS which drive hair follicle progenitors toward sebocyte differentiation, the more weakly activating mutation found in WHN permits an intermediate phenotype with abnormal curly hair growth but without sebaceous hyperplasia.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Lynn Boyden and Young Lim for critical review of the manuscript, and Jing Zhou, Young Lim, Li Tian, Carol Nelson-Williams, Gerald Goh, and Samir Zaidi for technical assistance. This work was supported by a Doris Duke Charitable Foundation Clinical Scientist Development Award to KAC, and by the Yale Center for Mendelian Genomics (NIH U54 HG006504). JLL is a recipient of a Clinical Research Mentorship Award from the Doris Duke Charitable Foundation and is supported by the Medical Scientist Training Program (NIH NIGMS GM007205) at Yale University.
Abbreviations used
- WHN
woolly hair nevus
- KEN
keratinocytic epidermal nevus
- NS
nevus sebaceus
- CS
Costello syndrome
- SNV
single nucleotide variation
- LOH
loss of heterozygosity
- PPK
palmoplantar keratoderma
Footnotes
CONFLICT OF INTEREST
The authors claim no conflict of interest
REFERENCES
- Fasano O, Aldrich T, Tamanoi F, Taparowsky E, Furth M, Wigler M. Analysis of the transforming potential of the human H-ras gene by random mutagenesis. Proc Natl Acad Sci USA. 1984;81:4008–12. doi: 10.1073/pnas.81.13.4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gripp KW, Lin AE. Costello syndrome: a Ras/mitogen activated protein kinase pathway syndrome (rasopathy) resulting from HRAS germline mutations. Genet Med. 2012;14:285–92. doi: 10.1038/gim.0b013e31822dd91f. [DOI] [PubMed] [Google Scholar]
- Groesser L, Herschberger E, Ruetten A, Ruivenkamp C, Lopriore E, Zutt M, et al. Postzygotic HRAS and KRAS mutations cause nevus sebaceous and Schimmelpenning syndrome. Nat Genet. 2012;44:783–7. doi: 10.1038/ng.2316. [DOI] [PubMed] [Google Scholar]
- Hafner C, Toll A, Gantner S, Mauerer A, Lurkin I, Acquadro F, et al. Keratinocytic epidermal nevi are associated with mosaic RAS mutations. J Med Genet. 2012;49:249–53. doi: 10.1136/jmedgenet-2011-100637. [DOI] [PubMed] [Google Scholar]
- Hafner C, Toll A, Real FX. HRAS mutation mosaicism causing urothelial cancer and epidermal nevus. N Engl J Med. 2011;365:1940–2. doi: 10.1056/NEJMc1109381. [DOI] [PubMed] [Google Scholar]
- Harel S, Christiano AM. Genetics of structural hair disorders. J Invest Dermatol. 2012;132:E22–6. doi: 10.1038/skinbio.2012.7. [DOI] [PubMed] [Google Scholar]
- Levinsohn JL, Tian LC, Boyden LM, McNiff JM, Narayan D, Loring ES, et al. Whole-exome sequencing reveals somatic mutations in HRAS and KRAS, which cause nevus sebaceus. J Invest Dermatol. 2013;133:827–30. doi: 10.1038/jid.2012.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindhurst MJ, Sapp JC, Teer JK, Johnston JJ, Finn EM, Peters K, et al. A mosaic activating mutation in AKT1 associated with the Proteus syndrome. N Engl J Med. 2011;365:611–9. doi: 10.1056/NEJMoa1104017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Barbacid M. RAS oncogenes: the first 30 years. Nat Rev Cancer. 2003;3:459–65. doi: 10.1038/nrc1097. [DOI] [PubMed] [Google Scholar]
- Moss C, Larkins S, Stacey M, Blight A, Farndon PA, Davison EV. Epidermal mosaicism and Blaschko's lines. J Med Genet. 1993;30:752–5. doi: 10.1136/jmg.30.9.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peteiro C, Oliva NP, Zulaica A, Toribio J. Woolly-hair nevus: report of a case associated with a verrucous epidermal nevus in the same area. Pediatr Dermatol. 1989;6:188–90. doi: 10.1111/j.1525-1470.1989.tb00814.x. [DOI] [PubMed] [Google Scholar]
- Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–9. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel DH, Mann JA, Krol AL, Rauen KA. Dermatological phenotype in Costello syndrome: consequences of Ras dysregulation in development. Br J Dermatol. 2012;166:601–7. doi: 10.1111/j.1365-2133.2011.10744.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun BK, Saggini A, Sarin KY, Kim J, Benjamin L, LeBoit PE, et al. Mosaic activating RAS mutations in nevus sebaceus and nevus sebaceus syndrome. J Invest Dermatol. 2013;133:824–7. doi: 10.1038/jid.2012.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venugopal V, Karthikeyan S, Gnanaraj P, Narasimhan M. Woolly hair nevus: a rare entity. Int J Trichology. 2012;4:42–3. doi: 10.4103/0974-7753.96090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.