Abstract
We initially described the WHIM syndrome based on the combination of Warts, Hypogammaglobulinaemia, Infections and Myelokathexis (neutrophil retention in the bone marrow). Translational research led to the discovery that this rare immunodeficiency disease is caused by a heterozygous mutation in the CXCR4 gene. Recently, Plerixafor has been suggested as a treatment for WHIM syndrome due to its efficacy as a CXCR4 antagonist, closing the translational research loop. In this review, we will focus on the clinical manifestations, pathophysiology, diagnosis and possible therapies for this rare entity.
Keywords: CXCR4, myelokathexis, neutropenia, plerixafor, WHIM
Introduction
In 1964, myelokathexis (Zuelzer 1964) was used as a new term to describe chronic peripheral neutropenia in the presence of increased neutrophils of distinct morphology in the bone marrow. Myelokathexis was noted to possibly associate with infections and other clinical abnormalities (Plebani, et al 1988). In 1990, we described the combined clinical manifestations of warts (W), hypogammaglobulinaemia (H), bacterial infections (I), and myelokathexis (M) in a family including a father and his two daughters, and proposed that these features characterized a novel disorder that was coined the WHIM syndrome (Wetzler, et al 1990); this syndrome is now included in any congenital neutropenia work up (Badolato, et al 2004). Over the following two decades, data exploring this rare disease and finding its treatment has continued to accumulate.
Epidemiology
The incidence of WHIM is still unknown, perhaps because it is so rare; reports of this syndrome have surfaced in the literature from multiple regions of the globe, including USA (Palm, et al 2010), Japan (Ueda, et al 2009) and Europe (Beaussant Cohen, et al 2012, Gulino 2003, Krivan, et al 2010). WHIM syndrome affects both men and women; it is inherited in an autosomal dominant pattern; however autosomal recessive or sporadic cases have also been described (Gulino 2003). In spite of no male-to-male inheritance described to date; X- linked transmission has been ruled out by X chromosome studies (Gorlin, et al 2000).
Pathophysiology
Examining the bone marrow using light microscopy shows a hypercellular marrow with an increase in the proportion of mature myeloid cells, indicating a right shift in granulopoiesis. Neutrophils have cytoplasmic vacuoles and hypersegmented nuclei with dense pyknotic lobes (Wetzler, et al 1990), the so-called eyeglass-shaped or cloverleaf neutrophils (Figure 1) (Latger-Cannard, et al 2006, Liu, et al 2012). The retention of neutrophils in the bone marrow is designated “myelokathexis”. Although the reason for the odd morphology of the neutrophils was not known when WHIM was first described, today it is recognized that this morphology is typical for cells undergoing apoptosis (programmed cell death). The presence of apoptotic changes in the neutrophils is supported by electron microscopy studies showing membrane blebbing and hyperfragmented nuclei; in addition there is reduced expression of the anti-apoptotic protein bcl-x (BCL2L1) despite normal expression of Fas (FAS), Fas ligand (FASLG) and bcl-2 (BCL2) (Aprikyan, et al 2000, Taniuchi, et al 1999). In contrast to neutrophils, the morphology and proportion of lymphoid cells, erythroid cells and megakaryocytes are usually normal. Myelokathexis can occasionally be confused with other conditions, such as myelodysplastic, paraneoplastic or other congenital neutropenia syndromes (Maran, et al 1992, McDermott, et al 2010, Rassam, et al 1989).
Figure 1.
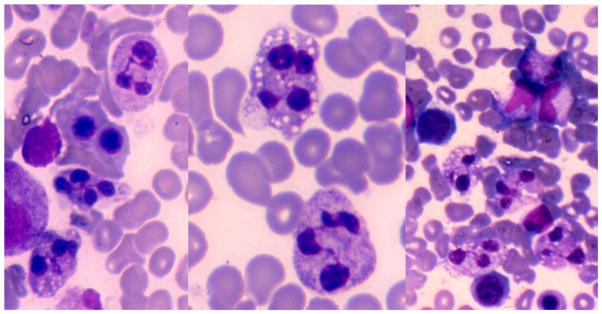
Bone marrow aspirate showing accumulation of mature neutrophils with cytoplasmic vacuoles and dense pyknotic nuclear lobes with interconnecting filaments (Wright Giemsa, original magnification ×400 for the left and right panels; ×1000 for the middle panel). It was only later when apoptosis was described that it was realized that myelokathexis is actually normal apoptotic changes.
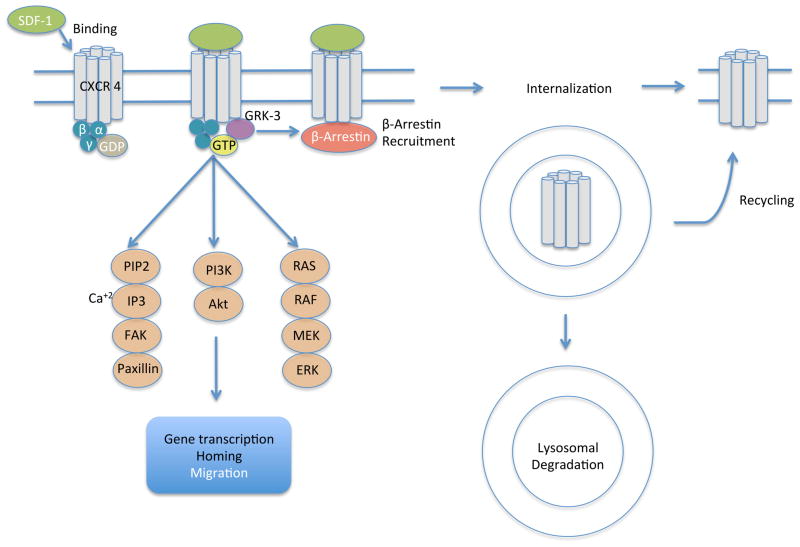
The reason behind the neutrophil retention/apoptosis in the bone marrow has been linked to aberrations in the chemokine receptor type-4 (CXCR4) (CD184) (Hernandez, et al 2003). This receptor was initially described because of its role as a co-receptor for the human immunodeficiency virus (Feng, et al 2011). CXCR4 is endowed with potent chemotactic properties for the lymphocytes. The CXCR4 ligand, stromal derived factor (SDF-1; also termed CXCL12) is important in haematopoietic stem cell homing to the bone marrow and in haematopoietic stem cell quiescence. CXCR4 is a G-protein-coupled receptor (GPCR) that has seven trans-membrane regions, an amino-terminal extracellular domain and a 45 amino acid intra-cytoplasmic carboxy-terminal tail (Busillo and Benovic 2007). It received its name, CXC, because it contains four distinctively conserved cysteine residues, and the first two cysteines are separated by one amino acid (Power and Wells 1996). CXCR4 is expressed on most human mature leucocyte subtypes and haematopoietic progenitor cells among other cells. When SDF-1 binds to CXCR4, signal transduction activates hetero-trimeric Gi proteins, which activate downstream effectors, such as AKT and extracellular signal-regulated kinases (Erk) 1/2 and eventually calcium flux to trigger adhesion and cell migration (Kucia, et al 2004). These processes are regulated by desensitization: GPCR kinase and protein C kinase mediate phosphorylation of the C-terminus of the cytoplasmic domain of CXCR4, and this leads to recruitment of β-arrestin to preclude further G protein activation, which further leads to receptor internalization and ubiquitination (Cheng, et al 2000, McCormick, et al 2009) (Figure 2). This signalling plays a critical role in bone marrow homing (Eash, et al 2010, McDermott, et al 2011) in addition to myelopoiesis and lymphopoiesis (the lineages most affected in WHIM) (Ma, et al 1998, Nagasawa, et al 1996, Tachibana, et al 1998, Zou, et al 1998), and B cell interaction with its niche (Egawa, et al 2001).
Figure 2.
CXCR4 signalling and regulation, SDF-1 binding to CXCR4 induces signal transduction pathways and gene expression. GRK- mediated phosphorylation leads to β Arrestin-mediated desensitization and internalization.
Abbreviations: ERK, extracellular signal-regulated kinase; FAK, focal adhesion kinase; GDP, guanosine diphosphate; GRK-3, G-protein-coupled receptor kinase-3; GTP, guanosine triphosphate; IP3, inositol triphosphate; MEK, mitogen-activated protein kinase kinase; PI3K, phsphoinositide 3-kinase; PIP2, phosphatidylinositol 4,5-biphosphate; RAF, rapidly accelerated fibrosarcoma; Ras, rat sarcoma; SDF-1, stromal-derived factor-1.
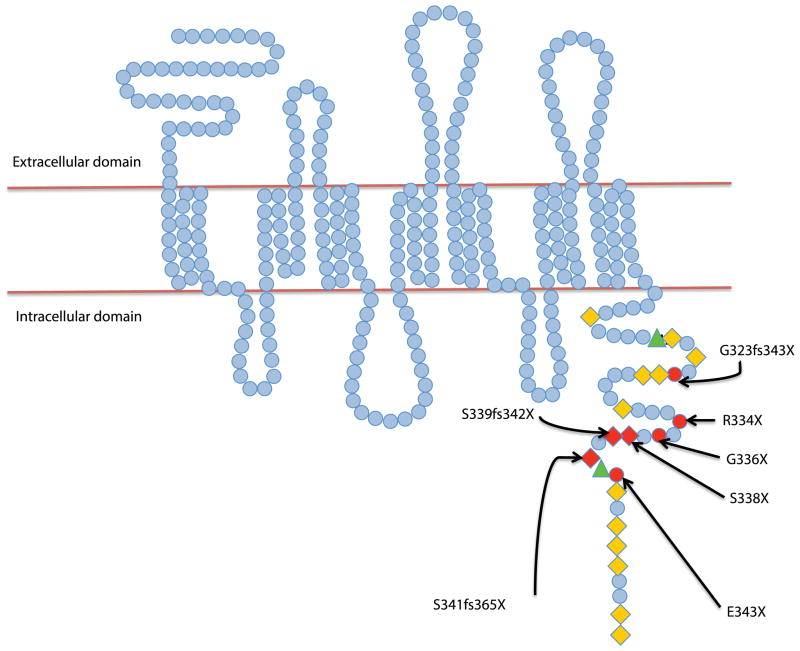
The aberrations responsible for the WHIM syndrome have been localized to chromosome 2q21; specifically, they are mutations in the gene encoding CXCR4 (Figure 3). Having a point mutation (for example R334X) leads to a premature stop codon, thus causing a truncation in the last 10–19 amino acids of the carboxy-tail of CXCR4 (Alapi, et al 2007, Beaussant Cohen, et al 2012, Hernandez, et al 2003, Tassone, et al 2009). Such a mutation results in enhanced production of inositol phosphate, and prolonged ligand-stimulated release of intracellular calcium flux in response to SDF-1 binding in cells from patients with WHIM syndrome (Haribabu, et al 1997). On the other hand, a different type of mutation (non-truncating gain of function mutation) (E343K) has also been described; it causes a change in the net charge of the CXCR-4 tail (Liu, et al 2012). This effect was demonstrated when the K562 cell line (that does not express native CXCR4 and does not respond to SDF-1) was transfected with a plasmid encoding CXCRE343K causing increased signal expression and calcium flux following SDF-1 stimulation (Liu, et al 2012). The picture becomes more complicated by mutations that were reported even in WHIM patients without a clear family history of the disease (Gulino, et al 2004) on the one hand, and, on the other hand, the existence of familial cases of WHIM syndrome in which CXCR4 mutations were absent. Intriguing studies of the latter patients suggest that their disease pathophysiology is based on, as of yet unknown, abnormalities that affect CXCR4 function in a similar fashion to the truncating mutations (Balabanian, et al 2005). So far, different mutations did not suggest a genotype-phenotype correlation. Even patients with recurrent R334X mutation could have clinical variability that could be due to modifier-genes polymorphism that are yet to be identified (Diaz 2005, Diaz and Gulino 2005). Research is ongoing to understand this area in depth by Dr. Jean Donadieu (CHU Paris Est - Hôpital d’Enfants Armand-Trousseau, Paris, France; orphanet identification number 58029).
Figure 3. The structure of CXCR4.
Amino acids are represented as blue circles. The cytoplasmic tail is rich in serine (orange diamonds) and threonine (green triangles) residues. Sites of mutations are highlighted in red. The respective reported mutations involved in the WHIM syndrome are marked with arrows.
Transgenic animal models were used to study the mutation’s effect on neutrophil dynamics. Interestingly, neutropenia and myelolathexis have been reproduced in a mouse model with a C-terminus truncated CXCR4 (Kawai, et al 2007). Also, a zebrafish model (Hickstein and West 2010, Walters, et al 2010) was used to observe neutrophil dynamics in-vivo and showed that mutated CXCR4WHIM failed to internalize following exposure to SDF-1. Even inflammation did not lead to neutrophil migration in the transgenic zebrafish model.
Additional steps in the CXCR4 signalling pathway have been postulated to be involved in the WHIM syndrome: GPCR kinase-3 (GRK3) plays a role as a regulator of SDF-1-promoted desensitization of CXCR4. When GRK3-silenced cells displayed enhanced chemotaxis, GRK3 over-expression in WHIM leucocytes restored desensitization and normalized chemotaxis, which provided a link between the GRK3 pathway and the CXCR4-related WHIM disorder (Balabanian, et al 2008). In the presence of truncating mutation of the C-terminal of CXCR4, enhanced binding of β-arrestin to other regions of the receptor paradoxically resulted in prolonged activation of the receptor and failure to internalize it via endocytosis (Lagane, et al 2008).
The Rac-2 (Ras-related C3 botulinum toxin substrate 2, RAC2) signalling pathway may also be involved (Deng, et al 2011), as Rac-2-deficient mice have increased neutrophil production in the bone marrow, yet defective host defences (Roberts, et al 1999). Rac-2 signalling is necessary for neutrophil retention, as was also shown in a WHIM zebrafish model with constitutive CXCR4 signalling that usually has peripheral neutrophilia and clustering of neutrophils in the rostral-ventral side of the fish head (where SDF-1 is expressed). This phenomenon was partially inhibited when Rac2D57N mutation was expressed in neutrophils from WHIM transgenic fish, consistent with the requirement of Rac2 in CXCR4-mediated chemotaxis (Deng, et al 2011). The sphingosine-1 phosphatase receptor-5 (S1P5) may also play a role in the WHIM syndrome; its expression increased independent of CXCR4 desensitization, to allow natural killer (NK) cells to exit the bone marrow into the blood (Mayol, et al 2011). Further investigation of this mechanism in neutrophil trafficking may be worthwhile.
Initially, most investigators focused on the bone marrow changes in WHIM models. The abnormalities in the rest of the immune system were poorly understood until the development of a mouse model with a heterozygous mutation in CXCR4 gene (CXCR+/1013) that engenders a desensitization-resistant receptor (as mentioned above, desensitization controls G protein-dependent signalling of chemokine receptors (Balabanian, et al 2012). The mouse developed pan-leucocytopenia with complex tissue findings. Neutropenia occurred in a context of normal bone marrow architecture and granulocyte lineage maturation, indicating that CXCR4-dependent signalling is not the sole factor in these processes. Both T and B lymphopoiesis appeared to be suppressed (with reduced B cell precursors without increased apoptosis, and normal mature B cells in the bone marrow), indicating a problem in lymphocyte trafficking. Furthermore, the thymus was deficient in T cells. The architecture in the secondary immune organs (the spleen and lymph nodes) was abnormal with reduced B cell follicles and normal to increased T cells in the nodes. Fewer lymphatic vessels were observed in the nodes, indicating a potential problem with lymphatic egress. In the spleen, naïve B cells were decreased. Treatment with CXCL12/CXCR4 antagonists transiently reversed blood anomalies. These mice provided a model to decipher the role of CXCR4 desensitization in the homeostasis of B and T cells and to investigate which manifestations of patients with WHIM syndrome may be overcome by dampening the gain of CXCR4 function.
Other studies also demonstrate that CXCR4 is integral to B-cell development and affinity maturation. By utilizing B cell spectratyping technique, a third complementarity-determining region of the rearranged heavy chain variable region was found, demonstrating B-cell oligoclonality in a family with the WHIM syndrome. Immunization with T-cell-dependent neo-antigen led to robust primary response but delayed and depressed isotype switch from IgM to IgG following secondary immunizations. This observation suggested an altered germinal centre microenvironment, lack of appropriate transit of B-cells into or within the germinal centre, and impaired secondary immune response in WHIM patients (Mc Guire, et al 2010).
On a different note, the tendency of patients with WHIM syndrome to develop human papilloma virus (HPV) infections seems to be disproportional, when compared to susceptibility to other viral infections. As wart keratinocytes up-regulate CXCR4 in both healthy skin and in WHIM patients, increased SDF-1 signalling is observed in HPV infected dermis (Balabanian, et al 2005, Chow, et al 2010), which is a host factor in facilitating HPV infection and could explain this pathognomonic presentation. Recent studies suggested that plasmacytoid dendritic cells (pDCs) might have a protective role against HPV by secreting the antiviral cytokine interferon (IFN) α. This function was investigated in patients with WHIM syndrome who were found to have a decrease in all subsets (myeloid and plasmacytoid) of dentritic cells in comparison to healthy subjects. More importantly, IFN-α secretion from pDCs was decreased after viral stimulation with herpes simplex virus 1 (HSV-1). Sections of warts from WHIM patients did not have dermal pDCs infiltrates and did not express the antiviral protein MxA (that usually results from IFN-α secretion), suggesting that pDCs cannot migrate to the skin or defend the host against HPV infection in these patients (Tassone, et al 2010).
It is known that there are numerous polyoma viruses (HPyV) that can affect human skin. Interestingly, Buck et al (2012) recently reported a tenth human polyoma virus HPyV10 that was previously unknown; it was extracted from the skin of a WHIM patient, which raises the possibility that those patients exert equally poor control over both HPV and HPyV infections.
Clinical manifestations and diagnosis
In our original report describing WHIM syndrome (Wetzler, et al 1990), we described warts, hypogammaglobulinaemia, infections and myelokathexis as the core WHIM syndrome findings. In regard to infections, patients were noted to have severe HPV infections, multiple verrucae vulgaris (generalized verrucosis if more than 20 lesions) (Sri, et al 2012) on the extremities, in addition to the presence of condylomata acuminata and cervical papillomatosis that can lead to cervical dysplasia and invasive cancer in female patients (Beaussant Cohen, et al 2012). Only a minority of patients remained warts free (Gulino 2003). Treatment of the neutropenia (discussed below) does not usually cause regression of those lesions although sporadic regression has been observed (Goddard, et al 1994). HPV positive oral squamous cell carcinoma has also been reported (Cipriani, et al 2010). Other viral infections, such as varicella zoster virus (Dotta, et al 2011) and HSV (Latger-Cannard, et al 2006), can also occur.
A spectrum of recurrent respiratory bacterial infections with encapsulated pathogens, such as Haemophilus influenza, Staphylococcus aureus and Proteus mirabilis (Wetzler, et al 1990) that could lead to bronchiectasis, has been described (Vinurel, et al 2008). In addition, recurrent otitis media since infancy (Hagan and Nguyen 2007, Wetzler, et al 1990) could lead to hearing loss and possibly to delayed speech. The infections could be mild, such as fungal vaginitis, cystitis (with Escherichia coli) (Naoum 2011, Wetzler, et al 1990), sinusitis and pharyngitis (Liu, et al 2012), but could also be more severe (deep soft tissue infections or meningitis) and even fatal due to disseminated mycobacterial avium or gordonae infection (Beaussant Cohen, et al 2012, Doncker, et al 2011). Most of the bacterial infections have a mild course which is attributed to the release of the retained neutrophils in response to the infectious process (Diaz and Gulino 2005).
Only a paucity of malignancies have been reported, such as one case of cutaneous B cell lymphoma (Chae, et al 2001) presenting as fleshy coloured facial nodules and treated successfully with CHOP (cyclophosphamide, adriamycin, vincristine and prednisone) therapy. Another case reported haemophagocytic syndrome in Epstein-Barr virus (EBV) positive T-cell lymphoproliferative disorder and EBV positive B-cell intestinal lymphoma (in the same patient) resulting in the patient’s demise (Imashuku, et al 2002). Unpublished data noted in a review (Diaz and Gulino 2005) revealed a death of a patient due to glioblastoma.
Only one report (Takaya, et al 2009) exists regarding the association of autoimmunity and WHIM in a young patient with type I diabetes mellitus and hypothyroidism. Finally, WHIM patients could have other congenital abnormalities, such as tetralogy of Fallot (Badolato, et al 2012), double aortic arch (Beaussant Cohen, et al 2012), radius hypoplasia, phalangeal agenesis or other skeletal dysmorphisms causing growth restriction (Plebani, et al 1988).
Regarding the diagnosis, patients with neutropenia associated with hypogammaglobulinaemia or lymphopenia should be suspected to have WHIM syndrome. Younger patients could only have warts or may have none. Obtaining the family history is essential, however sporadic cases could exist. Bone marrow biopsy is helpful to demonstrate myelokathexis. CXCR4 gene sequencing usually shows the known mutations in the cytoplasmic tail of the receptor.
Laboratory findings
Leucopenia is one of the main features of the syndrome; peripheral blood neutrophils are markedly depressed. However, they can transiently rise in response to stress, corticosteroids, growth factors or infections. Phagocytic performance, chemotaxis and bacterial killing are normal (Wetzler, et al 1990). Peripheral blood reveals lymphopenia with profoundly decreased absolute B and, to a lesser degree, T cells. Natural Killer (NK) cells are normal to increased in proportion to the absolute decrease in the other lymphocytes (Wetzler, et al 1990). Lymphocytes have impaired proliferative response to B and T cell mitogens (Wetzler, et al 1990). Memory CD27+ B cells are profoundly depressed, affecting both switched and unswitched CD19+ B lymphocytes (Gulino, et al 2004). Monocyte counts are usually low (Siedlar, et al 2008), however, their cytotoxic activity is increased, suggesting monocyte activation (Wetzler, et al 1990). Hypogammaglobulinaemia is another feature of the disease, where immunoglobulins levels are usually mildly to moderately depressed (and could be normal in some cases) (Dotta, et al 2011, Gorlin, et al 2000). The deficiency may affect IgG only or could extend to include IgA and/or IgM (Hord, et al 1997, Wetzler, et al 1990). The calculated mean value of available values in a cohort of eight patients showed an IgG of 6.22 .g/l (normal range 6.5–17.5 g/l), IgA 0.61 g/l (normal range 0.75–3.3 g/l) and IgM of 0.197 g/l for IgM (normal range 0.3–2.25 g/l) (Beaussant Cohen, et al 2012). Lack of anti-granulocytic antibodies and lack of anti-human leucocyte antigen antibodies rule out autoimmunity against neutrophils (Wetzler, et al 1990). Of note, patients are able to react to vaccinations as they can have normal protective antibody titres against pneumococcus, diphtheria, tetanus, rubella and hepatitis B, indicating that the defect in the humoral response is not complete (Wetzler, et al 1990). However, a history of clinical failure has also been documented for vaccines, e.g., influenza twice despite the annual seasonal vaccination, and clinical measles despite vaccination (Liu, et al 2012).
Treatment
As an immunodeficiency syndrome, intravenous immunoglobulins (IVIG) have been used (Wetzler, et al 1990) but are expensive and nonspecific for the disease. The first report of treatment targeting the neutrophils was the use of recombinant human granulocyte (G) colony-stimulating factor (CSF) (Weston, et al 1991) followed by the use of granulocyte-macrophage (GM) CSF (Wetzler, et al 1992). G-CSF exerts its effect by inducing proteases that inactivate SDF-1 and interrupt the continuous reuptake of leucocytes in the bone marrow (Gulino 2003). Interestingly, CXCR4 expression is higher on monocytes obtained from WHIM patients when compared to healthy controls, which translate into possible abnormal monocytes and granulocytes in the bone marrow and macrophages in the other tissues (Siedlar, et al 2008). Based on these data, GM-CSF may be superior to G-CSF in WHIM patients that are non-responsive to the latter via an increase in interleukin-1 and tumour necrosis factor-α or through other mechanisms (Sisson and Dinarello 1988).
Plerixafor (Mozobil, also known as AMD3100), a small molecule that acts as a CXCR4 antagonist, approved by the US Food and Drug Administration (FDA) for mobilizing haematopoietic stem cells from the bone marrow to the blood for autologous stem cell transplantation (in multiple myeloma and non-Hodgkin lymphoma) (DiPersio, et al 2009, Dugan, et al 2010), may be the optimal therapy for WHIM (Dale, et al 2011, McDermott, et al 2011, Sanmun, et al 2006) as it allows the egress of the neutrophils from the bone marrow, and attenuates the impact of the underlying CXCR4 mutation. The first study showing an effect for Plerixafor in WHIM (McDermott, et al 2011), was a phase I safety study where Plerixafor dose was escalated from 0.02 mg/kg subcutaneously on day 1 to 0.24 mg/kg on day 7 (the FDA approved dose for stem cell mobilization) with a stopping rule of an absolute neutrophil count (ANC) exceeding 4 × 109/l. Pharmacokinetic features of a single injection were similar to healthy volunteers. More importantly, even at dose of 0.02 mg/kg, a robust yet transient increase in white blood cell counts was noted, peaking at 3–12 h and lasting for 24 h from administration. The drug was well tolerated without cardiac, renal, hepatic or electrolytes abnormalities. Modest adverse events were documented and limited to hyperglycaemia, insomnia, headaches, nausea and mild injection site reactions. A second study (Dale, et al 2011) included 6 adult patients with the WHIM syndrome where Plexiafor was administered subcutaneously at 0.04–0.24 mg/kg as dose escalation every 2–4 days. Leucocytes peaked in a dose-dependant manner at 6–12 h and then declined within 24 h without any reported adverse events.
Other small CXCR4-neutralizing molecules, such as Chlacone-4, have shown activity in inhibiting the CXCR4 ligand, SDF-1, from binding to its receptor (Hachet-Haas, et al 2008). However, these molecules remain experimental.
Finally, umbilical cold blood transplantation has been reported with good outcome in one young woman with recurrent infections despite treatment with IVIG (Krivan, et al 2010). As HPV infection carries the risk of malignant transformation, Gardasil, a prophylactic HPV vaccine, has been used in a 12-year-old girl with WHIM syndrome (Handisurya, et al 2010) inducing HPV-specific antibodies and cellular immune response. Two other patients were vaccinated at 3 and 5 years of age and both of them were wart free at the time of a recent report, when aged 6.7 and 8.6 years (Beaussant Cohen, et al 2012). There is no available data on using the other HPV vaccine, Cervarix, in WHIM patients (Rezaei, et al 2011). Frequent and aggressive evaluation by a dermatologist is recommended for any suspicious skin lesions.
The use of prophylactic antibiotics has not been studied but expert opinions recommend them on the basis of extrapolating data from other immunodeficiency syndromes; trimethaprim-sulfamethoxazole is suggested for staphylococcal and encapsulated bacterial infections, while oral cephalosporins are an alternative in some cases (Kawai and Malech 2009).
Prognosis
Patients could have a shorter life span due to recurrent infections and increased risk of malignancy; the oldest reported age identified was a 75-year-old patient. In a report of 60 cases of WHIM syndrome, five deaths were identified (8.3%) aside from one case of medically aborted fetus with cardiac anomaly; two died of lymphoma when age 26 and 54 years, one died of mycobacteria-related liver failure (with underlying lymphoma) at 40 years old, one died of advanced HPV-related genital disease at the age of 39.5 years, and one due to bacterial meningitis when aged 31 years (Beaussant Cohen, et al 2012). The risk of malignancy in these cases was estimated at 30% by the fourth decade, with the onset being shortly beyond the third decade (Beaussant Cohen, et al 2012).
Improved quality and span of life could be expected with early recognition, controlling infections and preventing malignant transformation in addition to targeted and supportive treatments in order to decrease the fatality.
Conclusion
The abnormal CXCR4 and SDF-1 pathway impairs the regulation of both myeloid and lymphoid trafficking leading to abnormal immune surveillance against infections and possibly cancer. Diagnosis remains the main challenge as it could be delayed due to the lack of knowledge of this rare disease. Early diagnosis has a great impact on prognosis as appropriate prophylactic antibiotics, vaccination, haematopoietic growth factor support and cancer surveillance all play a critical role in improving the quality of life and survival of the WHIM patients. Hopefully, the ongoing research on Plerixafor and other CXCR4-antagonist small molecules could carry the hope for cure for a disease that could not be better described than by the word “whim”.
Acknowledgments
Supported partially by grants from the National Cancer Institute Grant CA 16056 (OAU, MW), the Leonard S. LuVullo Endowment for Leukemia Research (MW), the Nancy C. Cully Endowment for Leukemia Research (MW), the Babcock Family Endowment (MW) and the Heidi Leukemia Research Fund, Buffalo, NY (MW).
References
- Alapi K, Erdos M, Kovacs G, Marodi L. Recurrent CXCR4 sequence variation in a girl with WHIM syndrome. European Journal of Haematoly. 2007;78:86–88. doi: 10.1111/j.1600-0609.2006.00779.x. [DOI] [PubMed] [Google Scholar]
- Aprikyan AA, Liles WC, Park JR, Jonas M, Chi EY, Dale DC. Myelokathexis, a congenital disorder of severe neutropenia characterized by accelerated apoptosis and defective expression of bcl-x in neutrophil precursors. Blood. 2000;95:320–327. [PubMed] [Google Scholar]
- Badolato R, Fontana S, Notarangelo LD, Savoldi G. Congenital neutropenia: advances in diagnosis and treatment. Current Opinion in Allergy and Clinical Immunology. 2004;4:513–521. doi: 10.1097/00130832-200412000-00007. [DOI] [PubMed] [Google Scholar]
- Badolato R, Dotta L, Tassone L, Amendola G, Porta F, Locatelli F, Notarangelo LD, Bertrand Y, Bachelerie F, Donadieu J. Tetralogy of fallot is an uncommon manifestation of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome. Journal of Pediatrics. 2012;161:763–765. doi: 10.1016/j.jpeds.2012.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanian K, Lagane B, Pablos JL, Laurent L, Planchenault T, Verola O, Lebbe C, Kerob D, Dupuy A, Hermine O, Nicolas JF, Latger-Cannard V, Bensoussan D, Bordigoni P, Baleux F, Le Deist F, Virelizier JL, Arenzana-Seisdedos F, Bachelerie F. WHIM syndromes with different genetic anomalies are accounted for by impaired CXCR4 desensitization to CXCL12. Blood. 2005;105:2449–2457. doi: 10.1182/blood-2004-06-2289. [DOI] [PubMed] [Google Scholar]
- Balabanian K, Levoye A, Klemm L, Lagane B, Hermine O, Harriague J, Baleux F, Arenzana-Seisdedos F, Bachelerie F. Leukocyte analysis from WHIM syndrome patients reveals a pivotal role for GRK3 in CXCR4 signaling. Journal of Clinical Investigation. 2008;118:1074–1084. doi: 10.1172/JCI33187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanian K, Brotin E, Biajoux V, Bouchet-Delbos L, Lainey E, Fenneteau O, Bonnet D, Fiette L, Emilie D, Bachelerie F. Proper desensitization of CXCR4 is required for lymphocyte development and peripheral compartmentalization in mice. Blood. 2012;119:5722–5730. doi: 10.1182/blood-2012-01-403378. [DOI] [PubMed] [Google Scholar]
- Beaussant Cohen S, Fenneteau O, Plouvier E, Rohrlich PS, Daltroff G, Plantier I, Dupuy A, Kerob D, Beaupain B, Bordigoni P, Fouyssac F, Delezoide AL, Devouassoux G, Nicolas JF, Bensaid P, Bertrand Y, Balabanian K, Chantelot CB, Bachelerie F, Donadieu J. Description and outcome of a cohort of 8 patients with WHIM syndrome from the French Severe Chronic Neutropenia Registry. Orphanet Journal of Rare Diseases. 2012;7:71. doi: 10.1186/1750-1172-7-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Phan GQ, Raiji MT, Murphy PM, McDermott DH, McBride AA. Complete genome sequence of a tenth human polyomavirus. Journal of Virology. 2012;86:10887. doi: 10.1128/JVI.01690-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busillo JM, Benovic JL. Regulation of CXCR4 signaling. Biochimica et Biophysica Acta. 2007;1768:952–963. doi: 10.1016/j.bbamem.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chae KM, Ertle JO, Tharp MD. B-cell lymphoma in a patient with WHIM syndrome. Journal of American Academy of Dermatology. 2001;44:124–128. doi: 10.1067/mjd.2001.111337. [DOI] [PubMed] [Google Scholar]
- Cheng ZJ, Zhao J, Sun Y, Hu W, Wu YL, Cen B, Wu GX, Pei G. beta-arrestin differentially regulates the chemokine receptor CXCR4-mediated signaling and receptor internalization, and this implicates multiple interaction sites between beta-arrestin and CXCR4. Journal of Biological Chemistry. 2000;275:2479–2485. doi: 10.1074/jbc.275.4.2479. [DOI] [PubMed] [Google Scholar]
- Chow KY, Brotin E, Ben Khalifa Y, Carthagena L, Teissier S, Danckaert A, Galzi JL, Arenzana-Seisdedos F, Thierry F, Bachelerie F. A pivotal role for CXCL12 signaling in HPV-mediated transformation of keratinocytes: clues to understanding HPV-pathogenesis in WHIM syndrome. Cell Host and Microbe. 2010;8:523–533. doi: 10.1016/j.chom.2010.11.006. [DOI] [PubMed] [Google Scholar]
- Cipriani NA, Blair E, Taxy JB. WHIM syndrome and oral squamous cell carcinoma. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2010;109:105–108. doi: 10.1016/j.tripleo.2009.08.011. [DOI] [PubMed] [Google Scholar]
- Dale DC, Bolyard AA, Kelley ML, Westrup EC, Makaryan V, Aprikyan A, Wood B, Hsu FJ. The CXCR4 antagonist plerixafor is a potential therapy for myelokathexis, WHIM syndrome. Blood. 2011;118:4963–4966. doi: 10.1182/blood-2011-06-360586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q, Yoo SK, Cavnar PJ, Green JM, Huttenlocher A. Dual roles for Rac2 in neutrophil motility and active retention in zebrafish hematopoietic tissue. Developmental Cell. 2011;21:735–745. doi: 10.1016/j.devcel.2011.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz GA. CXCR4 mutations in WHIM syndrome: a misguided immune system? Immunological Reviews. 2005;203:235–243. doi: 10.1111/j.0105-2896.2005.00226.x. [DOI] [PubMed] [Google Scholar]
- Diaz GA, Gulino AV. WHIM syndrome: a defect in CXCR4 signaling. Current Allergy and Asthma Reports. 2005;5:350–355. doi: 10.1007/s11882-005-0005-0. [DOI] [PubMed] [Google Scholar]
- DiPersio JF, Micallef IN, Stiff PJ, Bolwell BJ, Maziarz RT, Jacobsen E, Nademanee A, McCarty J, Bridger G, Calandra G. Phase III prospective randomized double-blind placebo-controlled trial of plerixafor plus granulocyte colony-stimulating factor compared with placebo plus granulocyte colony-stimulating factor for autologous stem-cell mobilization and transplantation for patients with non-Hodgkin’s lymphoma. Journal of Clinical Oncology. 2009;27:4767–4773. doi: 10.1200/JCO.2008.20.7209. [DOI] [PubMed] [Google Scholar]
- Doncker AV, Balabanian K, Bellanne-Chantelot C, de Guibert S, Revest M, Bachelerie F, Lamy T. Two cases of disseminated Mycobacterium avium infection associated with a new immunodeficiency syndrome related to CXCR4 dysfunctions. Clinical Microbiology and Infection. 2011;17:135–139. doi: 10.1111/j.1469-0691.2010.03187.x. [DOI] [PubMed] [Google Scholar]
- Dotta L, Tassone L, Badolato R. Clinical and genetic features of Warts, Hypogammaglobulinemia, Infections and Myelokathexis (WHIM) syndrome. Current Molecular Medicine. 2011;11:317–325. doi: 10.2174/156652411795677963. [DOI] [PubMed] [Google Scholar]
- Dugan MJ, Maziarz RT, Bensinger WI, Nademanee A, Liesveld J, Badel K, Dehner C, Gibney C, Bridger G, Calandra G. Safety and preliminary efficacy of plerixafor (Mozobil) in combination with chemotherapy and G-CSF: an open-label, multicenter, exploratory trial in patients with multiple myeloma and non-Hodgkin’s lymphoma undergoing stem cell mobilization. Bone Marrow Transplant. 2010;45:39–47. doi: 10.1038/bmt.2009.119. [DOI] [PubMed] [Google Scholar]
- Eash KJ, Greenbaum AM, Gopalan PK, Link DC. CXCR2 and CXCR4 antagonistically regulate neutrophil trafficking from murine bone marrow. Journal of Clinicl Investigation. 2010;120:2423–2431. doi: 10.1172/JCI41649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egawa T, Kawabata K, Kawamoto H, Amada K, Okamoto R, Fujii N, Kishimoto T, Katsura Y, Nagasawa T. The earliest stages of B cell development require a chemokine stromal cell-derived factor/pre-B cell growth-stimulating factor. Immunity. 2001;15:323–334. doi: 10.1016/s1074-7613(01)00185-6. [DOI] [PubMed] [Google Scholar]
- Feng Y, Broder CC, Kennedy PE, Berger EA. Pillars article: HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996. 272: 872–877. Journal of Immunology. 2011;186:6076–6081. [PMC free article] [PubMed] [Google Scholar]
- Goddard EA, Hughes EJ, Beatty DW. A case of immunodeficiency characterized by neutropenia, hypogammaglobulinaemia, recurrent infections and warts. Clinical and Laboratory Haematology. 1994;16:297–302. doi: 10.1111/j.1365-2257.1994.tb00425.x. [DOI] [PubMed] [Google Scholar]
- Gorlin RJ, Gelb B, Diaz GA, Lofsness KG, Pittelkow MR, Fenyk JR., Jr WHIM syndrome, an autosomal dominant disorder: clinical, hematological, and molecular studies. American Journal of Medical Genetics. 2000;91:368–376. [PubMed] [Google Scholar]
- Gulino AV. WHIM syndrome: a genetic disorder of leukocyte trafficking. Current Opinion in Allergy and Clinical Immunology. 2003;3:443–450. doi: 10.1097/00130832-200312000-00005. [DOI] [PubMed] [Google Scholar]
- Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, Imberti L, Pirovano S, Notarangelo LD, Soresina R, Mazzolari E, Nelson DL, Notarangelo LD, Badolato R. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood. 2004;104:444–452. doi: 10.1182/blood-2003-10-3532. [DOI] [PubMed] [Google Scholar]
- Hachet-Haas M, Balabanian K, Rohmer F, Pons F, Franchet C, Lecat S, Chow KY, Dagher R, Gizzi P, Didier B, Lagane B, Kellenberger E, Bonnet D, Baleux F, Haiech J, Parmentier M, Frossard N, Arenzana-Seisdedos F, Hibert M, Galzi JL. Small neutralizing molecules to inhibit actions of the chemokine CXCL12. Journal of Biological Chemistry. 2008;283:23189–23199. doi: 10.1074/jbc.M803947200. [DOI] [PubMed] [Google Scholar]
- Hagan JB, Nguyen PL. WHIM syndrome. Mayo Clinic Proceeding. 2007;82:1031. doi: 10.4065/82.9.1031. [DOI] [PubMed] [Google Scholar]
- Handisurya A, Schellenbacher C, Reininger B, Koszik F, Vyhnanek P, Heitger A, Kirnbauer R, Forster-Waldl E. A quadrivalent HPV vaccine induces humoral and cellular immune responses in WHIM immunodeficiency syndrome. Vaccine. 2010;28:4837–4841. doi: 10.1016/j.vaccine.2010.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haribabu B, Richardson RM, Fisher I, Sozzani S, Peiper SC, Horuk R, Ali H, Snyderman R. Regulation of human chemokine receptors CXCR4. Role of phosphorylation in desensitization and internalization. Journal of Biological Chemistry. 1997;272:28726–28731. doi: 10.1074/jbc.272.45.28726. [DOI] [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nature Genetics. 2003;34:70–74. doi: 10.1038/ng1149. [DOI] [PubMed] [Google Scholar]
- Hickstein DD, West RR. A WHIM-sical zebrafish. Blood. 2010;116:2621–2622. doi: 10.1182/blood-2010-07-296426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hord JD, Whitlock JA, Gay JC, Lukens JN. Clinical features of myelokathexis and treatment with hematopoietic cytokines: a case report of two patients and review of the literature. Journal of Pediatric Hematology Oncology. 1997;19:443–448. doi: 10.1097/00043426-199709000-00007. [DOI] [PubMed] [Google Scholar]
- Imashuku S, Miyagawa A, Chiyonobu T, Ishida H, Yoshihara T, Teramura T, Kuriyama K, Imamura T, Hibi S, Morimoto A, Todo S. Epstein-Barr virus-associated T-lymphoproliferative disease with hemophagocytic syndrome, followed by fatal intestinal B lymphoma in a young adult female with WHIM syndrome. Warts, hypogammaglobulinemia, infections, and myelokathexis. Annals of Hematology. 2002;81:470–473. doi: 10.1007/s00277-002-0489-9. [DOI] [PubMed] [Google Scholar]
- Kawai T, Malech HL. WHIM syndrome: congenital immune deficiency disease. Current Opinion in Hematology. 2009;16:20–26. doi: 10.1097/MOH.0b013e32831ac557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai T, Choi U, Cardwell L, DeRavin SS, Naumann N, Whiting-Theobald NL, Linton GF, Moon J, Murphy PM, Malech HL. WHIM syndrome myelokathexis reproduced in the NOD/SCID mouse xenotransplant model engrafted with healthy human stem cells transduced with C-terminus-truncated CXCR4. Blood. 2007;109:78–84. doi: 10.1182/blood-2006-05-025296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivan G, Erdos M, Kallay K, Benyo G, Toth A, Sinko J, Goda V, Toth B, Marodi L. Successful umbilical cord blood stem cell transplantation in a child with WHIM syndrome. European Journal of Haematology. 2010;84:274–275. doi: 10.1111/j.1600-0609.2009.01368.x. [DOI] [PubMed] [Google Scholar]
- Kucia M, Jankowski K, Reca R, Wysoczynski M, Bandura L, Allendorf DJ, Zhang J, Ratajczak J, Ratajczak MZ. CXCR4-SDF-1 signalling, locomotion, chemotaxis and adhesion. Journal of Molecular Histology. 2004;35:233–245. doi: 10.1023/b:hijo.0000032355.66152.b8. [DOI] [PubMed] [Google Scholar]
- Lagane B, Chow KY, Balabanian K, Levoye A, Harriague J, Planchenault T, Baleux F, Gunera-Saad N, Arenzana-Seisdedos F, Bachelerie F. CXCR4 dimerization and beta-arrestin-mediated signaling account for the enhanced chemotaxis to CXCL12 in WHIM syndrome. Blood. 2008;112:34–44. doi: 10.1182/blood-2007-07-102103. [DOI] [PubMed] [Google Scholar]
- Latger-Cannard V, Bensoussan D, Bordigoni P. The WHIM syndrome shows a peculiar dysgranulopoiesis: myelokathexis. British Journal of Haematology. 2006;132:669. doi: 10.1111/j.1365-2141.2005.05908.x. [DOI] [PubMed] [Google Scholar]
- Liu Q, Chen H, Ojode T, Gao X, Anaya-O’Brien S, Turner NA, Ulrick J, DeCastro R, Kelly C, Cardones AR, Gold SH, Hwang EI, Wechsler DS, Malech HL, Murphy PM, McDermott DH. WHIM syndrome caused by a single amino acid substitution in the carboxy-tail of chemokine receptor CXCR4. Blood. 2012;120:181–189. doi: 10.1182/blood-2011-12-395608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Q, Jones D, Borghesani PR, Segal RA, Nagasawa T, Kishimoto T, Bronson RT, Springer TA. Impaired B-lymphopoiesis, myelopoiesis, and derailed cerebellar neuron migration in CXCR4- and SDF-1-deficient mice. Proceedings of the National Academy of Sciences U S A. 1998;95:9448–9453. doi: 10.1073/pnas.95.16.9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maran R, Mittelman M, Cohen AM, Djaldetti M. Myelokathexis and monocytosis in a patient with gastric cancer. Acta Haematologica. 1992;87:210–212. doi: 10.1159/000204770. [DOI] [PubMed] [Google Scholar]
- Mayol K, Biajoux V, Marvel J, Balabanian K, Walzer T. Sequential desensitization of CXCR4 and S1P5 controls natural killer cell trafficking. Blood. 2011;118:4863–4871. doi: 10.1182/blood-2011-06-362574. [DOI] [PubMed] [Google Scholar]
- Mc Guire PJ, Cunningham-Rundles C, Ochs H, Diaz GA. Oligoclonality, impaired class switch and B-cell memory responses in WHIM syndrome. Clinical Immunology. 2010;135:412–421. doi: 10.1016/j.clim.2010.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick PJ, Segarra M, Gasperini P, Gulino AV, Tosato G. Impaired recruitment of Grk6 and beta-Arrestin 2 causes delayed internalization and desensitization of a WHIM syndrome-associated CXCR4 mutant receptor. PLoS One. 2009;4:e8102. doi: 10.1371/journal.pone.0008102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, De Ravin SS, Jun HS, Liu Q, Priel DA, Noel P, Takemoto CM, Ojode T, Paul SM, Dunsmore KP, Hilligoss D, Marquesen M, Ulrick J, Kuhns DB, Chou JY, Malech HL, Murphy PM. Severe congenital neutropenia resulting from G6PC3 deficiency with increased neutrophil CXCR4 expression and myelokathexis. Blood. 2010;116:2793–2802. doi: 10.1182/blood-2010-01-265942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott DH, Liu Q, Ulrick J, Kwatemaa N, Anaya-O’Brien S, Penzak SR, Filho JO, Priel DA, Kelly C, Garofalo M, Littel P, Marquesen MM, Hilligoss D, Decastro R, Fleisher TA, Kuhns DB, Malech HL, Murphy PM. The CXCR4 antagonist plerixafor corrects panleukopenia in patients with WHIM syndrome. Blood. 2011;118:4957–4962. doi: 10.1182/blood-2011-07-368084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagasawa T, Hirota S, Tachibana K, Takakura N, Nishikawa S, Kitamura Y, Yoshida N, Kikutani H, Kishimoto T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- Naoum FA. A report of WHIM syndrome (myelokathexis) - clinical features and bone marrow morphology. Revista Brasileira de Hematologica e Hemoterapia. 2011;33:393–394. doi: 10.5581/1516-8484.20110105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palm MD, Tyring SK, Rady PL, Tharp MD. Human papillomavirus typing of verrucae in a patient with WHIM syndrome. Archives in Dermatology. 2010;146:931–932. doi: 10.1001/archdermatol.2010.184. [DOI] [PubMed] [Google Scholar]
- Plebani A, Cantu-Rajnoldi A, Collo G, Allavena P, Biolchini A, Pirelli A, Clerici Schoeller M, Masarone M. Myelokathexis associated with multiple congenital malformations: immunological study on phagocytic cells and lymphocytes. European Journal of Haematology. 1988;40:12–17. doi: 10.1111/j.1600-0609.1988.tb00790.x. [DOI] [PubMed] [Google Scholar]
- Power CA, Wells TN. Cloning and characterization of human chemokine receptors. Trends in Pharmacological Sciences. 1996;17:209–213. doi: 10.1016/0165-6147(96)10019-5. [DOI] [PubMed] [Google Scholar]
- Rassam SM, Roderick P, al-Hakim I, Hoffbrand AV. A myelokathexis-like variant of myelodysplasia. European Journal of Haematology. 1989;42:99–102. doi: 10.1111/j.1600-0609.1989.tb00255.x. [DOI] [PubMed] [Google Scholar]
- Rezaei N, Hedayat M, Aghamohammadi A, Nichols KE. Primary immunodeficiency diseases associated with increased susceptibility to viral infections and malignancies. Journal of Allergy and Clinical Immunology. 2011;127:1329–1341. e1322. doi: 10.1016/j.jaci.2011.02.047. quiz 1342–1323. [DOI] [PubMed] [Google Scholar]
- Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- Sanmun D, Garwicz D, Smith CI, Palmblad J, Fadeel B. Stromal-derived factor-1 abolishes constitutive apoptosis of WHIM syndrome neutrophils harbouring a truncating CXCR4 mutation. British Journal of Haematology. 2006;134:640–644. doi: 10.1111/j.1365-2141.2006.06240.x. [DOI] [PubMed] [Google Scholar]
- Siedlar M, Rudzki Z, Strach M, Trzyna E, Pituch-Noworolska A, Blaut-Szlosarczyk A, Bukowska-Strakova K, Lenart M, Grodzicki T, Zembala M. Familial occurrence of warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome. Archivum Immunologiae et Therapia Experimentalis (Warsz) 2008;56:419–425. doi: 10.1007/s00005-008-0046-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisson SD, Dinarello CA. Production of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor by human mononuclear cells stimulated with granulocyte-macrophage colony-stimulating factor. Blood. 1988;72:1368–1374. [PubMed] [Google Scholar]
- Sri JC, Dubina MI, Kao GF, Rady PL, Tyring SK, Gaspari AA. Generalized verrucosis: a review of the associated diseases, evaluation, and treatments. Journal of the American Academy of Dermatology. 2012;66:292–311. doi: 10.1016/j.jaad.2010.12.011. [DOI] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Takaya J, Fujii Y, Higashino H, Taniuchi S, Nakamura M, Kaneko K. A case of WHIM syndrome associated with diabetes and hypothyroidism. Pediatric Diabetes. 2009;10:484–486. doi: 10.1111/j.1399-5448.2009.00503.x. [DOI] [PubMed] [Google Scholar]
- Taniuchi S, Yamamoto A, Fujiwara T, Hasui M, Tsuji S, Kobayashi Y. Dizygotic twin sisters with myelokathexis: mechanism of its neutropenia. American Journal of Hematology. 1999;62:106–111. doi: 10.1002/(sici)1096-8652(199910)62:2<106::aid-ajh8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Tassone L, Notarangelo LD, Bonomi V, Savoldi G, Sensi A, Soresina A, Smith CI, Porta F, Plebani A, Notarangelo LD, Badolato R. Clinical and genetic diagnosis of warts, hypogammaglobulinemia, infections, and myelokathexis syndrome in 10 patients. Journal of Allergy and Clinical Immunology. 2009;123:1170–1173. 1173, e1171–1173. doi: 10.1016/j.jaci.2008.12.1133. [DOI] [PubMed] [Google Scholar]
- Tassone L, Moratto D, Vermi W, De Francesco M, Notarangelo LD, Porta F, Lougaris V, Facchetti F, Plebani A, Badolato R. Defect of plasmacytoid dendritic cells in warts, hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome patients. Blood. 2010;116:4870–4873. doi: 10.1182/blood-2010-03-272096. [DOI] [PubMed] [Google Scholar]
- Ueda K, Nakagawa S, Osono S, Inada H. Picture in clinical hematology no. 40: Infant case of neutropenia due to WHIM syndrome(myelokathexis) Rinsho Ketsueki Japanese Journal of Clinical Hematology. 2009;50:591. [PubMed] [Google Scholar]
- Vinurel H, Freymond N, Pacheco Y, Devouassoux G. The Whim syndrome: a rare cause of diffuse bronchiectasis. Immune defect of CXCR4 and chronic bronchial suppuration. Revue de Maladies Respiratoires. 2008;25:614–618. doi: 10.1016/s0761-8425(08)71621-8. [DOI] [PubMed] [Google Scholar]
- Walters KB, Green JM, Surfus JC, Yoo SK, Huttenlocher A. Live imaging of neutrophil motility in a zebrafish model of WHIM syndrome. Blood. 2010;116:2803–2811. doi: 10.1182/blood-2010-03-276972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weston B, Axtell RA, Todd RF, 3rd, Vincent M, Balazovich KJ, Suchard SJ, Boxer LA. Clinical and biologic effects of granulocyte colony stimulating factor in the treatment of myelokathexis. Journal of Pediatrics. 1991;118:229–234. doi: 10.1016/s0022-3476(05)80488-3. [DOI] [PubMed] [Google Scholar]
- Wetzler M, Talpaz M, Kleinerman ES, King A, Huh YO, Gutterman JU, Kurzrock R. A new familial immunodeficiency disorder characterized by severe neutropenia, a defective marrow release mechanism, and hypogammaglobulinemia. American Journal of Medicine. 1990;89:663–672. doi: 10.1016/0002-9343(90)90187-i. [DOI] [PubMed] [Google Scholar]
- Wetzler M, Talpaz M, Kellagher MJ, Gutterman JU, Kurzrock R. Myelokathexis: normalization of neutrophil counts and morphology by GM-CSF. Journal of the American Medical Association (JAMA) 1992;267:2179–2180. [PubMed] [Google Scholar]
- Zou YR, Kottmann AH, Kuroda M, Taniuchi I, Littman DR. Function of the chemokine receptor CXCR4 in haematopoiesis and in cerebellar development. Nature. 1998;393:595–599. doi: 10.1038/31269. [DOI] [PubMed] [Google Scholar]
- Zuelzer WW. “Myelokathexis”--a New Form of Chronic Granulocytopenia. Report of a Case. New England Journal of Medicine. 1964;270:699–704. doi: 10.1056/NEJM196404022701402. [DOI] [PubMed] [Google Scholar]