Abstract
Human fetal retinal pigment epithelium (hfRPE), when harvested by mechanical dissection and cultured initially under low calcium conditions, will proliferate and tolerate cryopreservation for future use. Cryopreserved cells can be subsequently thawed and cultured in standard calcium and in the presence of appropriate nutrients to a high state of differentiation, allowing recapitulation of multiple in vivo functions. In this review we briefly discuss some of our previous studies of the classical retinoid visual cycle and introduce current studies in our laboratory that involve two new areas of investigation; the dynamic response of the receptor for retinol binding protein, STRA6 to the addition of holo-retinol binding protein to the culture medium and the protective complement-based response of hfRPE to the ingestion of toxic byproducts of the visual cycle. This response is studied in the context of genotyped hfRPE expressing either predisposing or protective variants of complement factor H.
Keywords: retinoid visual cycle, retinal pigment epithelium, RPE cell culture, complement activation
1. Introduction
The retinal pigment epithelium, although post-mitotic in the adult eye, is capable of replication in vivo under appropriate conditions. It is also endowed with the ability to re-enter the cell cycle when removed from the eye and placed in cell culture. Thus, since the early 1970's, vision scientists have been studying the cell biology of this important cellular monolayer in vitro (Albert et al., 1972; Mannagh et al., 1973). These efforts produced mixed results for several decades. Common among the difficulties encountered was dedifferentiation of the RPE from its normal cobblestone appearance to a fusiform phenotype that was neither morphologically nor functionally normal. Immortalization of RPE cultures through the use of large T antigen (Nabi et al.1993) also produced cells that were less than optimal in appearance and function. Even RPE cells that transformed spontaneously (Dunn et al., 1996), although useful for certain studies, do not carry out the full repertoire of healthy RPE in vivo. However since 2001, the vision science community has had access to detailed RPE culture protocols that provide an opportunity to study the many facets of RPE in vivo function. An initial methods paper (Hu and Bok, 2001), followed by two modifications of human fetal RPE culture conditions (Maminishkis et al., 2006; Sonoda et al., 2009) have pushed the field forward. As far as we have ascertained, all three of these culture methods produce highly differentiated RPE that is useful for a variety of studies. Here we describe our method for producing large quantities of human RPE cells that can be readily frozen and used for multiple experiments. This also allows comparison of cell function among genotypic variants. We emphasize the utilization of these cultures for the study of the classical retinoid visual cycle and its toxic byproducts in a dish but also give examples of other processes that can be readily studied in vitro using these cultures.
2. Methods and Materials
2. 1 RPE culture
Our methods for the non-enzymatic harvesting of RPE from pairs of human fetal eyes, initial culture establishment and expansion of the cell population in low calcium medium (0.5mM), freezing, thawing, differentiation and use in selected experiments are fully documented in two publications (Hu and Bok, 2001; Hu and Bok, 2010). The latter reference more specifically addresses the use of these cultures for the study of the basolateral uptake of Vitamin A (retinol) from retinol-binding protein (RBP), intracellular transport and processing by retinoid binding proteins and enzymes and enhanced secretion of 11-cis retinal into the apical culture medium in the presence of interphotoreceptor retinoid-binding protein (IRBP). The protocols outlined in these two publications are suitable for all experiments described in this review. Therefore, the fine details will not be repeated here. In brief, human fetal RPE (hfRPE) is typically cultured in 12 mm Millicell™ HA chambers whose nitrocellulose support membrane has been coated with recombinant mouse laminin (Hu and Bok, 2010). The differentiation medium is Eagles Minimum Essential Medium containing 1.8 mM calcium and the special additives that we have shown to be important for the generation of highly differentiated cultures. These additives include bovine retinal extract, which enhances the transepithelial resistance essential for rigorous transport studies.
2.2 Retinol-binding protein incubation
Cultured RPE cells are grown for two months in Millicell™ HA culture wells. Apo-retinol-binding protein (apo-RBP) is generated by bleaching recombinant holo-retinol-binding protein (holo-RBP) at 328 nm in a spectrofluorometer (Hu and Bok 2010). The apo-RBP or holo-RBP are added at a concentration of 2.3 μM to the basal side of each culture well and incubation is carried out for various periods of time. In the example shown here, incubation proceeded for 3 hours.
2.3 Challenge of RPE cultures with wild type or Abca4-/- photoreceptor outer segments
Outer segments from BALB/c (wild type) and Abca4-/- mice (containing A2E precursors) are isolated from animals 3 to 6 months of age. Following dissection of the retinas, they are placed in Hanks' balanced salt solution (HBSS, Invitrogen) containing 45% sucrose and gently vortexed. The sheared outer segments are sedimented at 10,000× g for 10 minutes at 4°C, washed twice with HBSS and re-suspended in DMEM (Sigma). The outer segments (OS) from 2 BALB/c or 2 Abca4-/- mice are then added to genotyped, differentiated RPE cultures and incubation is continued for 15 hours. After removing the OS and washing with 37°C DMEM, full strength human serum is added to the basal compartment of the chambers for 2 hours. The cells are then washed once with HBSS, fixed, embedded in agarose, sectioned and stained with antibodies for confocal microscopy.
2.4 Vibratome sectioning
For confocal microscopy of monolayer cross-sections, the method of Hale and Matsumoto (2002) provides sections that are superior to cryosections. Following excision from its plastic chamber, the nitrocellulose support film and its attached cells are fixed at room temperature in 4% formaldehyde buffered with 0.1M phosphate. They are embedded in agarose (Type XI, low gelling temperature, Sigma-Aldrich, St. Louis, MO). 50 μm sections are cut in a vibratome (VT1000s, Leica Microsystems, Germany).
2.5 Antibodies
The following primary antibodies were used for images displayed in this review. Rabbit polyclonal to human STRA6 (1:100, Abcam, Cambridge, MA), mouse anti-RPE65 (1:250, Millipore), rabbit anti-LRAT polyclonal antibodies prepared by us (Ruiz et al., 1999) and mouse anti-C5b-9 antibodies (1:50 Dako).
2.6 Immunofluorescence
The fixed cells are permeabilized with TritonX-100 (0.1%) in phosphate buffered saline (PBS) for 10 minutes. After blocking with 5% goat serum and 1% bovine serum albumin, the cells are exposed to rabbit polyclonal anti human STRA6 antibodies (1:100, Abcam, Cambridge, MA) in blocking solution at 37°C for 2 hours. After washing three times with PBS, the cells are exposed to goat anti-rabbit IgG conjugated to Alexa-488 for 1 hour at room temperature. The cells and their supports are then mounted on glass slides and covered with 5% N-propyl gallate in glycerol to protect from fluorescence fading. Images are captured with an Olympus Fluoview FV1000 confocal scanning laser microscope.
3. Results and Discussion
3.1 Phenotype of differentiated, cultured human RPE
By two months in culture the monolayers are highly polarized with abundant apical microvilli facing the culture medium in the upper chamber of the Millicell (Fig. 1). The basal surface of the cell layer is tightly bound to the nitrocellulose support film, sometimes penetrating into the meshwork of the film by a micron or more. The cells are highly melanized and abundant mitochondria are mostly displaced toward the basal region of the cell. Intercellular junctional complexes are visible in appropriately aligned sections.
Figure 1.
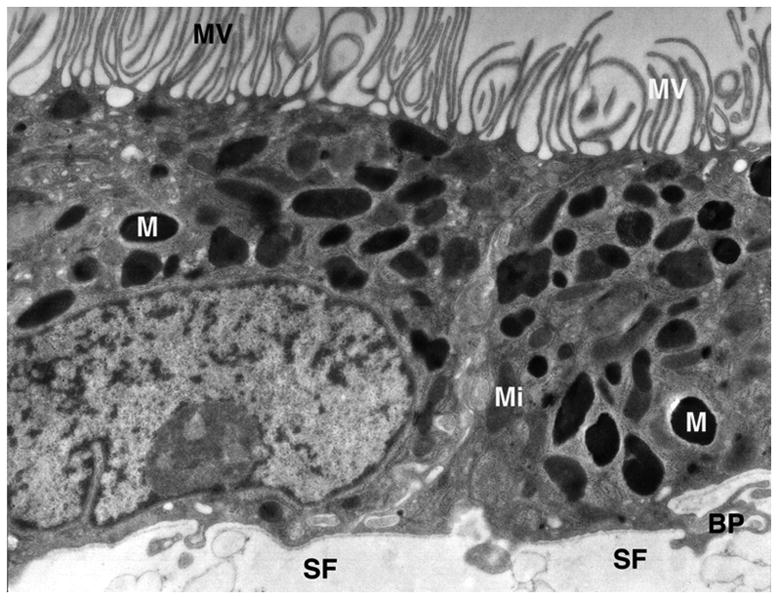
Electron micrograph of cultured RPE. The apical surface of the cell monolayer is at the top, where abundant microvilli (MV) project into the culture medium. Melanin granules (M) are abundant after 2 months of culture and mitochondria (Mi) are largely displaced toward the basal part of the cell, the natural position for these organelles in vivo. Basal processes (BP) project into the nitrocellulose support film (SF).
We know from studies of the RPE in vivo that a normal-appearing RPE phenotype does not necessarily guarantee normal function. For example, even in the presence of selected gene disruption, the RPE can be morphologically normal but lack the essential biosynthetic function of LRAT (Ruiz et al., 2007). Nonetheless, normal morphology is an essential starting point. Experimentally immortalized and spontaneously immortalized RPE cells uniformly lack essential morphological features such as abundant microvilli (Nabi et al., 1993; Davis et al., 1995; Dunn et al., 1996). RPE cells cultured under the conditions described here have all of the standard features observed in vivo. For example, our previous studies have shown that they sport a robust transepithelial resistance as do cells in vivo (Hu and Bok, 2001). They also express their Na,K ATPase appropriately on the apical surface (Hu and Bok, 2001), a unique feature of RPE and choroid plexus epithelium. And, of great importance, they process retinol into 11-cis retinal and secrete this essential visual chromophore into the apical culture medium when interphotoreceptor retinoid binding protein (IRBP) is present in the apical medium (Carlson and Bok, 1992). Of course, none of this would be possible in the absence of other essential retinoid binding proteins and processing enzymes, such as LRAT, RPE65 and others. Clearly, production of the final essential product, 11-cis retinal, enforces the fact that all of the players for the classical retinoid visual cycle are expressed in this culture system. In the following sections, we describe some new aspects of retinoid-related function that we have been able to observe in detail with the help of cultured hfRPE.
3.2 Analysis of the classical retinoid visual cycle in vitro
Because the classical retinoid visual cycle carried out by the RPE is a signature feature of that cell layer, we do not consider cultured RPE to be in possession of its full array of functions unless it is capable of secreting 11-cis retinal from its apical side, the normal route for delivery of the visual chromophore to rod and cone photoreceptor cells. To the best of our knowledge, this attribute has never been demonstrated in cell lines that have been immortalized by experimental means or by those that have arisen spontaneously as a distinct phenotype. Moreover, RPE cells that are harvested from healthy individuals of any age, but not cultured in the proper culture medium, are also incapable of producing the visual chromophore. The classical retinoid visual cycle is distinct from a second retinoid visual cycle mediated by Müller glial cells (Kaylor et al., 2013). The latter is thought to provide visual chromophore precursor in the form of 11-cis retinol to cones only, not rods. The cones are capable of oxidizing 11-cis retinol to 11-cis retinal, whereas rods are not.
To satisfy our criteria for retinoid processing, the RPE cells grown in Millicell™ chambers must be capable of taking up retinol from its physiological carrier protein, holo-RBP. The holoprotein is secreted by multiple tissues in the body, but its primary source is the liver. In healthy individuals, holo-RBP is found in the circulation at a concentration of about 5 mg/deciliter. In a typical experiment, holo-RBP is added to the basal medium at this concentration and retinol is taken up into the cells via STRA6, the receptor for RBP, which is localized exclusively to the basolateral membrane and is absent from the apical membrane of the RPE (Bok and Heller 1976, Kawaguchi et al., 2007). The retinol enters the cell through this receptor/channel and is subsequently passed along a brigade of binding proteins and enzymes, whereby it is processed into 11-cis retinal, the chromophore of rod and cone vision. It is then secreted by an unknown mechanism into the culture medium bathing the apical side of the RPE monolayer. This secretion is promoted exclusively by IRBP, which is thought to subsequently deliver it to rod and cone photoreceptor cells. As mentioned earlier, an alternative visual cycle is subserved by Müller glial cells, which produce and release 11-cis retinol (Kaylor et al., 2013). Again, IRBP is thought to deliver this retinoid to cone photoreceptors.
3.2.1 Localization of other proteins essential for the retinoid visual cycle
RPE cell culture conditions can be crucial for expression of proteins essential for the retinoid visual cycle. Early attempts at demonstrating RPE65 protein expression for example, were unsuccessful, even though there was measureable mRNA expression (Hamel et al., 1993). RPE65 is the critical enzyme required for isomerization of all-trans retinol to 11-cis retinol prior to its oxidation to 11-cis retinal. On the other hand, the expression of lecithin retinol acyltransferase, (LRAT) the enzyme that esterifies retinol through the addition of a fatty acid, usually palmitic acid, appears to be less demanding in terms of culture conditions when compared to RPE65 (Flood et al., 1983). Retinyl palmitate, the product of this esterification serves as the substrate for RPE65. Both LRAT and RPE65 are localized in the smooth endoplasmic reticulum of the RPE and hence their localization by laser confocal immunocytochemistry is very similar (Fig. 2A and 2B)
Figure 2.
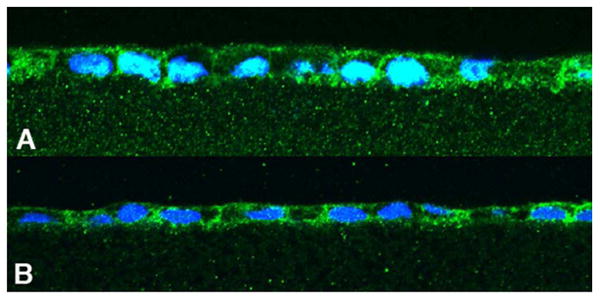
Cultured human fetal RPE immunofluorescence indicating LRAT (A) and RPE65 (B) expression. Since both of these proteins are localized to the smooth endoplasmic reticulum within the cell, the staining patterns are similar. The agarose section in A is sectioned somewhat obliquely, thereby giving the impression of a higher cell profile.
3.2.2 Incubation of Cultured human RPE in Holo and Apo Retinol-binding Protein
Here, we present preliminary data that reveal new and previously unpublished evidence regarding the membrane receptor, STRA6, which mediates the uptake of retinol from holo-RBP. They provide an additional and interesting example of the usefulness of highly differentiated RPE cultures in studies of this type and how new issues can be addressed in vitro.
We noted in our initial cloning and immunocytochemical localization of STRA6 (Kawaguchi et al., 2007), that STRA6 was localized not only to the basolateral plasma membrane of the RPE, but that there were also some Stra6-positive vesicles within the RPE (Fig 3A). The question that we are exploring currently is whether these vesicles contain RBP receptor that is trafficking to the plasma membrane or whether it represents RBP receptor that is being internalized from its normal functional location in the plasma membrane. To explore the dynamics of this process, we exposed the basal surface of the RPE cells to either holo-RBP or to apo-RBP in order to determine whether we could stimulate a change in position of these vesicles. The results were dramatic! RPE cells that are allowed to differentiate in culture for 2 months and provided with only the small amount of holo-RBP available in the 1% fetal calf serum added to the medium, are presumed to be in a relative state of quiescence with respect to retinoid uptake, processing and transport. Likewise, one would predict that RBP lacking its retinol cargo (apo-RBP) would not disturb this quiescence, and this was indeed the case. In contrast, following incubation for 3 hours in holo-RBP, there was a dramatic increase in the STRA6-positive vesicle population within the RPE (Fig. 3C), suggesting a rapid upregulation of STRA6 expression by cells that were confronted with the opportunity to take retinol into the cytoplasm from STRA6 that has bound to extracellular holo-RBP. We know from previous studies, that application of holo-RBP to the basolateral surface of cultured RPE results in the uptake of retinol, its processing into 11-cis retinal and its IRBP-promoted secretion into the apical culture medium (Carlson and Bok, 1993).
Figure 3.
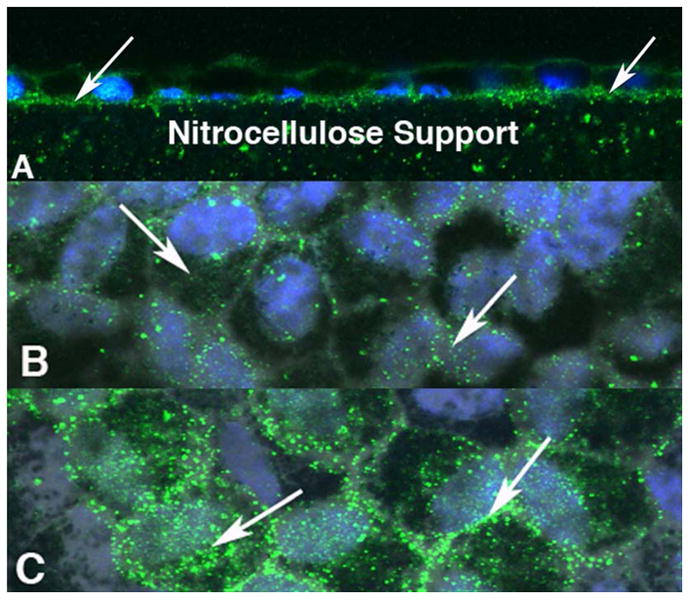
Scanning laser confocal microscope images of human RPE cells in which the retinol-binding protein receptor STRA6, is imaged by immunocytochemistry (green). RPE cell nuclei are blue. A is a cross section of the cell monolayer showing the localization of STRA6 to the basolateral membrane. As is the case in vivo, there are a lot of vesicular images (white arrows). Presumably these vesicles are trafficking the receptor to the cell surface, although this remains to be determined. When apo-RBP is added to the basal surface of the Millicell chamber, the vesicle population remains unchanged (B). However, the addition of holo-RBP appears to up-regulate the vesicle population (C) Vesicle density increased within the cell bodies (left arrow) and at cell margins (right arrow). The cells in B and C were exposed to apo and holo-RBP respectively for 3 hours prior to fixation.
3.3 Response by the RPE to a toxic byproduct of the visual cycle (A2E) and the role of toxic visual cycle byproducts in the etiology of inherited retinal disease
When a photon is absorbed by a rhodopsin or cone photopigment molecule, the prosthetic group, 11-cis retinal, undergoes an isomerization to all-trans retinal, which then dissociates from its aldimine linkage to a lysine residue. The all-trans retinal subsequently forms an aldimine bond with phosphatidyl ethanolamine in the lumenal leaflet of the photoreceptor disc lipid bilayer. In wild type outer segments, this N-retinyidene phosphatidyl ethanolamine (N-ret-PE) is then translocated (“flipped”) to the cytoplasmic leaflet of the disc membrane by the Abca4 protein, which resides in the rod and cone disc rims (“rim protein”). When Abca4 is mutated, this translocation rate is severely reduced and N-ret-PE accumulates in the disc bilayer, ultimately acquiring another all-trans retinal to form a bis-retinoid (Weng et al., 1999). This bis-retinoid is present in phagocytosed rod and cone outer segment discs, and is converted to A2E, the major chromophore of RPE lipofuscin. The conversion takes place in the acidic environment of the RPE phagolysosome. Zhou et al. (2006) have shown that, A2E can be converted to epoxides and endoperoxides that are more water-soluble than A2E and can therefore mobilize within the cell and enter the culture medium where they activate the C3 component of complement. We have evidence from our cell cultures that this activation could be detrimental to the RPE through inappropriate attack of the RPE basolateral surface. RPE carrying predisposing complement factor H (CFH) variants are less resistant to this attack as shown in an experiment reported here. We propose that this represents, in part, the mechanism for RPE cell compromise in patients with recessive Stargardt macular degeneration and in some patients with age-related macular degeneration (Radu et al., 2011).
The evidence for this hypothesis is derived from the behavior of cultured RPE cells in response to feeding of outer segments from wild type and recessive Stargardt mice (Fig. 4). When RPE expressing a protective haplotype of CFH (Hageman et al., 2005) is fed outer segments from Abca4-/- mice, there is very little evidence of membrane attack complex (MAC) on the basolateral surface of RPE cells that are homozygous for isoleucine at amino acid position 62 (II62) and homozygous for tyrosine at position 402 (YY402) (Fig. 4A). However, following a single feeding of outer segments to RPE cells that are homozygous for valine at position 62 (VV62) and homozygous for histidine at positon 402 (HH402) and therefore genetically predisposed to AMD (Hageman et al., 2005), there is abundant MAC on the basolateral surface facing the serum in the lower compartment of the Millicell (Fig. 4B). Thus, with these genotyped cultures, we have tested the hypothesis that a predisposing haplotype in CFH renders the RPE vulnerable to inappropriate complement attack, whereas the protective haplotype is indeed protective. This attack is apparently sublytic, because we have not detected evidence for cell death in these cultures. Thus it is likely that, individuals thus predisposed are able to benefit from functional RPE over many decades of life. However, over time, the lack of a proper defense mechanism likely leads to RPE cell compromise and death.
Figure 4.
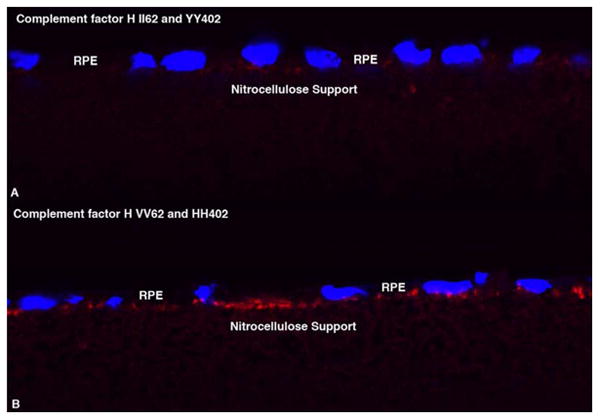
Cultured human RPE challenged with a single meal of outer segments from Abca4-/- mouse retinas 24 hours before fixation and staining for complement C5b-9 (Membrane Attack Complex, MAC). The protective phenotype II62/yy402 (A) is virtually free of MAC, whereas the basolateral surface of the predisposing phenotype, VV62/HH402 (B) is festooned with MAC.
From the results briefly presented here, it is clear that well differentiated, cultured RPE provides a highly useful tool for the study of normal and disease processes. We have now established over 80 genotyped RPE cell lines from fetal material that might otherwise have been discarded. Although not a renewable resource for standard methods, it is likely that cell lines that prove to be particularly useful in the study of normal function or disease processes could be reprogrammed into stem cells and thereby rendered renewable. Thus the future is bright for the study of normal and disease processes in a dish.
Highlights.
We established well differentiated RPE for in-vitro studies.
We study the visual cycle and its toxic byproducts in cultured RPE cells.
We showed the Importance of Cultured RPE cells in the study of normal and disease processes.
Acknowledgments
This work was supported by USPHS grants R01 EY00331 and R24 EY017404, and grants to DB from the Macula Vision Research Foundation and The Arnold and Mabel Beckman Initiative for Macular Research. DB is the Dolly Green Professor of Ophthalmology and Distinguished Research Professor in the Departments of Neurobiology and Ophthalmology at the David Geffen School of Medicine, University of California, Los Angeles.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albert DM, et al. In vitro growth of pure cultures of retinal pigment epithelium. Arch Ophthalmol. 1972;88:63–69. doi: 10.1001/archopht.1972.01000030065014. [DOI] [PubMed] [Google Scholar]
- Bok D, Heller J. Transport of retinol from the blood to the retina; An autoradiographic study of the pigment epithelial cell surface receptor for plasma retinol-binding protein. Exp Eye Res. 1976;22:395–402. doi: 10.1016/0014-4835(76)90177-9. [DOI] [PubMed] [Google Scholar]
- Carlson A, Bok D. Promotion of the release of 11-cis-retinal from cultured retinal pigment epithelium by interphotoreceptor retinoid-binding protein. Biochemistry. 1992;31:9056–9062. doi: 10.1021/bi00152a049. [DOI] [PubMed] [Google Scholar]
- Davis AA, et al. A human retinal pigment epithelial cell line that retains epithelial characteristics after prolonged culture. Invest Ophthalmol Vis Sci. 1995;36:955–964. [PubMed] [Google Scholar]
- Dunn KC, et al. ARPE-19, a human retinal pigment epithelial cell line with differentiated properties. Exp Eye Res. 1996;62:155–169. doi: 10.1006/exer.1996.0020. [DOI] [PubMed] [Google Scholar]
- Flood MT, et al. Vitamin A utilization in human retinal pigment epithelial cells in vitro. Invest Ophthalmol Vis Sci. 1983;24:1227–1235. [PubMed] [Google Scholar]
- Hageman GS, et al. A common haplotype in the complement regulatory gene factor H (HF1/CFH) predisposes individuals to age-related macular degeneration. Proc Natl Acad Sci USA. 2005;102:7227–7232. doi: 10.1073/pnas.0501536102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hale IL, Matsumoto B. Resolution of subcellular detail in thick tissue sections: immunohistochemical preparation and fluorescence confocal microscopy. Methods Cell Biol. 2002;70:301–335. doi: 10.1016/s0091-679x(02)70008-3. [DOI] [PubMed] [Google Scholar]
- Hamel CP, et al. Molecular cloning and expression of RPE65, a novel retinal pigment epithelium-specific microsomal protein that is post-translationally regulated in vitro. J Biol Chem. 1993;268:15751–15757. [PubMed] [Google Scholar]
- Hu J, Bok D. A cell culture medium that supports the differentiation of human retinal pigment epithelium into functionally polarized monolayers. Mol Vis. 2001;7:14–9. [PubMed] [Google Scholar]
- Hu J, Bok D. Culture of highly differentiated human retinal pigment epithelium for analysis of the polarized uptake, processing, and secretion of retinoids. In: Sun H, Travis GH, editors. Retinoids, Methods in Molecular Biology. Springer Science; 2010. pp. 55–73. [DOI] [PubMed] [Google Scholar]
- Kawaguchi R, et al. A membrane receptor for retinol binding protein mediates cellular uptake of Vitamin A. Science. 2007;315:820–825. doi: 10.1126/science.1136244. [DOI] [PubMed] [Google Scholar]
- Kaylor JJ, et al. Identification of DES1 as a vitamin A isomerase in Müller glial cells of the retina. Nat Chem Biol. 2013;9:30–36. doi: 10.1038/nchembio.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maminishkis A, et al. Confluent monolayers of cultured human fetal retinal pigment epithelium exhibit morphology and physiology of native tissue. Invest Ophthalmol Vis Sci. 2006;47:3612–3624. doi: 10.1167/iovs.05-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mannagh J, et al. Tissue culture of human retinal pigment epithelium. Invest Ophthalmol. 1973;12:52–64. [PubMed] [Google Scholar]
- Nabi IV, et al. Immortalization of polarized rat retinal pigment epithelium. J Cell Sci. 1993;104:37–49. doi: 10.1242/jcs.104.1.37. [DOI] [PubMed] [Google Scholar]
- Radu RA, et al. Complement system dysregulation and inflammation in the retinal pigment epithelium of a mouse model for Stargardt macular degeneration. J Biol Chem. 2011;286:18593–18601. doi: 10.1074/jbc.M110.191866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, et al. Molecular and biochemical characterization of lecithin retinol acyltransferase. J Biol Chem. 1999;274:3834–3841. doi: 10.1074/jbc.274.6.3834. [DOI] [PubMed] [Google Scholar]
- Ruiz A, et al. Somatic ablation of the Lrat gene in the mouse retinal pigment epithelium drastically reduces its retinoid storage. Invest Ophthalmol Vis Sci. 2007;48:5377–5387. doi: 10.1167/iovs.07-0673. [DOI] [PubMed] [Google Scholar]
- Sonoda S, et al. A protocol for the culture and differentiation of highly polarized human retinal pigment epithelial cells. Nat Protoc. 2009;4:662–673. doi: 10.1038/nprot.2009.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weng J, et al. Insights into the function of Rim protein in photoreceptors and etiology of Stargardt's disease from the phenotype in ABCR knockout mice. Cell. 1999;98:13–23. doi: 10.1016/S0092-8674(00)80602-9. [DOI] [PubMed] [Google Scholar]
- Zhou J, et al. Complement activation by photooxidation products of A2E, a lipofuscin constituent of the retinal pigment epithelium. Proc Natl Acad Sci USA. 2006;103:16182–16187. doi: 10.1073/pnas.0604255103. [DOI] [PMC free article] [PubMed] [Google Scholar]


