Abstract
Background
There has been little research published on the adaptation of diabetic exchange list diet approaches for the design of intervention diets in health research despite their clinical utility. The exchange list approach can provide clear and precise guidance on multiple dietary changes simultaneously. The objective of this study was to develop exchange list diets for Mediterranean and Healthy Eating, and to evaluate adherence, dietary intakes and markers of health risks with each counselling approach in 120 subjects at increased risk for developing colon cancer.
Methodology
A randomized clinical trial was implemented in the USA involving telephone counselling. The Mediterranean diet had ten dietary goals targeting increases in monounsaturated fats, n3 fats, whole grains and the amount and variety of fruits and vegetables. The Healthy Eating diet had five dietary goals that were based on the U.S. Healthy People 2010 recommendations.
Results
Dietary compliance was similar in both diet arms with 82–88% of goals being met at 6 months, but subjects took more time to achieve the Mediterranean goals than the Healthy Eating goals. The relatively modest fruit and vegetable goals in the Healthy Eating arm were exceeded, resulting in fruit and vegetable intakes of about 8 servings/day in each arm after six months. A significant (P<0.05) weight loss and a decrease in serum C-reactive protein concentrations were observed in the overweight/obese subgroup of subjects in the Mediterranean arm in the absence of weight loss goals.
Conclusions
Counselling for the Mediterranean diet may be useful for both improving diet quality and for achieving a modest weight loss in overweight or obese individuals.
Keywords: modified exchange lists, overweight, Mediterranean diet, telephone counseling
INTRODUCTION
Research into improving the health effects of specific dietary patterns is challenged by the availability of methods to elicit defined dietary changes. A large number of studies have designed interventions using group, school or worksite based approaches or electronic media, but these have generally resulted in very modest increases in fruit and vegetable intakes (Ammerman et al., 2002, Lin et al., 2010, Pomerleau et al., 2005, Harris et al., 2011). Studies that have utilized intensive one-on-one counselling combined with self-monitoring have generally shown larger dietary changes, and this includes two cancer prevention research studies that targeted increases in fruit, vegetable and fibre intakes combined with decreases in total fat (Newman et al., 2005, Lanza et al., 2001). None of these intervention studies has used an exchange list approach for improving diet quality.
The Exchange Lists for Meal Planning booklet was first developed by the American Dietetic Association and the American Diabetes Association as a tool for diabetic meal planning (Franz et al., 1987, Wheeler et al., 1996, Wheeler et al., 2008). The exchange lists have been modified for use in other countries, but there has been surprisingly little research done to evaluate their effectiveness (Wheeler et al., 1996, Ziegler et al., 1989, Shovic, 1994, Bawadi and Al-Sahawneh, 2008, Hung, 1989). Only a handful of studies have modified the exchange lists to achieve low-fat diets and/or diets that target increased variety of fruits and vegetable (Djuric et al., 2002, Djuric et al., 2009, Boyar and Loughridge, 1985). The exchange list approach is potentially a method to achieve the USDA dietary recommendations (U.S. Department of Agriculture and U.S. Department of Health and Human Services, 2010), but this has not been tested. The exchange list approach was used in this study to design two different diets that might be useful for colon cancer prevention.
Diet appears to plays a role in modulating risk of many cancers, and colon cancer is among the cancers for which diet has the biggest impact (American Institute for Cancer Research, 2011). One observational study of a large screening cohort found that persons who consumed diets with relatively more features of either the Mediterranean diet, the USDA Food Guide recommendations or the Dietary Approaches to Stop Hypertension (DASH) Eating Plan, all were preventive of colorectal adenomas in men, but only the USDA Food Guide pattern was preventive in women (Dixon et al., 2007).
Although research on USDA-recommended diets has been more limited, extensive international research on Mediterranean diets has indicated its’ cancer prevention potential (Simopoulos, 2004, Kontou et al., 2011, Verberne et al., 2010). Prior to the 1950’s, risk of colorectal cancer was low in Greece, but the incidence has increased with the westernization of the diet and incidence is higher among Greek immigrants to the U.S. and Australia (McMichael et al., 1980, Paspatis et al., 2001, Simopoulos, 2004, Fernandez et al., 2005). In the U.S., rates of colon cancer are among the highest in the world (World Cancer Research Fund/American Institute for Cancer Research, 2007). All the major components of the traditional Greek diet appear to be protective for colorectal cancer, including olive oil, fish, legumes, whole grains, and fruits and vegetables (Gallus et al., 2004). In comparison to the American diet, the Mediterranean diet has higher intakes of n-3 and n-9 fatty acids and lower intakes of n-6 polyunsaturated fats (Pauwels, 2011). The Mediterranean diet also contains much higher intakes of plant-based foods and monounsaturated fats (MUFA), and lower red meat intake (Pauwels, 2011).
In most of the Mediterranean diet intervention studies that have been done, the population being studied was living in southern Europe and a high MUFA food was provided. This is exemplified in two of the larger studies that were done with disease endpoints (de Lorgeril et al., 1998, Estruch et al., 2006). There have been relatively few intervention studies reported using a Mediterranean diet in American populations (Djuric et al., 2008a). Well-designed intervention studies can isolate and identify the effects of diet versus that of other lifestyle factors on health endpoints. Initially, we began studies to develop an exchange-list approach to elicit multiple dietary changes consistent with Mediterranean intakes (Djuric et al., 2008b). This approach was further expanded in the present study to include goals for dark green herbs (e.g. parsley, basil) and omega 3 fats obtained from fish and flax seeds to more fully mimic Greek-Mediterranean dietary intakes. In addition, an exchange list was devised to target Healthy People 2010 recommendations for fruits, vegetables, whole grains and saturated fats (Office of Disease Prevention and Health Promotion, 2005). With an exchange list, foods are classified into categories, and there are daily goals for consuming foods from each category. Any food within a category, in the specified serving size, can be used (or exchanged) to meet the daily intake goal for that category. Such an approach offers an individual flexibility in food choices for meeting dietary goals. The purpose of the present work was to demonstrate implementation of the exchange list counselling approach and to evaluate compliance to the Mediterranean diet versus compliance to a standard Healthy Eating diet in a randomized trial of persons at increased risk of colon cancer.
PARTICIPANTS AND METHODS
Participants and eligibility
The Healthy Eating for Colon Cancer Prevention Study was approved by the University of Michigan Institutional Review Board (HUM00007622). The study was listed on the ClinicalTrials.gov website maintained by the National Institutes of Health (registration number NCT00475722). A total of 120 subjects were recruited as previously described (Djuric et al., 2012). There were 61 participants in the Healthy Eating arm and 59 in the Mediterranean Diet arm, in the Ann Arbor, MI and surrounding areas from July 2007 to November 2010 (Djuric et al., 2012).
The overall objective of the Healthy Eating for Colon Cancer Prevention study was to design and evaluate implementation of novel exchange list diets that could be used in a biomarker study for individuals at high risk of colon cancer. The study collected blood and colon biopsy samples for investigation of cancer biomarker endpoints such as prostaglandins, epithelial proliferation and epithelial nuclear morphology. In a prevention study, one would target individuals at increased risk and it was therefore important to test the intervention in a high risk population. Subjects at increased risk of colon cancer were eligible for the study. Eligibility was defined as having one first-degree or two second-degree relatives with colon cancer or a personal history of adenomatous polyps or early stage colon cancer in the past if they were at least two years post cancer treatment. Other inclusion criteria included good, general health, being at least 21 years old, body mass index (BMI) at least 18.5 and less than 35 kg/m2. It was felt that it would be inappropriate to prescribe a diet that seeks to maintain current body weight in persons with class II obesity and higher. Exclusion criteria included being on a medically prescribed diet, following a diet that would require extensive counselling to correct nutritional deficiencies or taking supplements or medications that might interfere with the study such as vitamins and minerals above 250% of the Recommended Daily Allowance and high doses of other supplements with potential antioxidant function such as glucosamine and chondroitin.
Dietary eligibility criteria were designed to exclude persons already following a Mediterranean diet or a low-fat diet. Eligible diets were at least 23% calories from total fat with no more than 48% of fat as MUFA. Fruit and vegetable intakes to meet eligibility criteria were below two-thirds of the 2005 USDA recommended servings/day (Djuric et al., 2012). This was enumerated excluding white potatoes after the first serving and iceberg lettuce. These vegetables can be consumed in large quantities, but since they are low in carotenoids, subjects were not excluded from participation if intakes of fruits and vegetable were too high because consumption of these two foods.
Eligible participants were stratified into categories based on gender, body mass index (less than 25 versus at or above 25 kg/m2), regular use of non-steroidal anti-inflammatory drugs (yes/no), and colon cancer risk status (prior colon cancer, prior adenoma or a positive family history of colon cancer) prior to randomization to a Healthy Eating or Mediterranean diet for 6 months. Stratification was important to assure equal representation of participants with these characteristics in the two study arms. The full details of recruitment and retention to the Healthy Eating Study have been published elsewhere (Djuric et al., 2012).
Dietary Assessment
Dietary eligibility for recruitment to the study was assessed using two days of written records and one un-announced 24-hour recall. Subjects were given written and verbal instructions on how to maintain a complete food record with sufficient detail for analysis. If details were missing, staff called the subject to verify details of foods eaten. The ability to provide a complete and plausible food record was part of the eligibility determination. Dietary recalls and food records also were collected at baseline, 3 and 6 months. Food records were completed by subjects on a Sunday and Monday, and subjects were called for an un-unannounced 24-hour recall on one further weekday. All the dietary recalls were conducted using the 5-pass method (Conway et al., 2004). The recalls were done by trained staff but not by the study dietitian since it was felt that this would maximize objectivity in data collection. An additional 24-hour recall was obtained at the first study visit, and all four days were averaged to obtain an estimate of baseline diet. The same assessments were repeated at six months. At three months, two days of written records and one un-announced 24-hour recall were analysed before the visit to give each participant feedback on their progress. Mean nutrient intakes from the in-person recalls were similar to those calculated for the average of the three other days (Djuric et al., 2012). It should be noted that an average of at least three days is generally required for accurate estimation of energy intake, but even a single recall can provide estimates of energy, fat and fruit/vegetable servings that were not significantly different from that of four days of food records (Radakovich et al., 2006, Basiotis et al., 1987).
For 5% of the 406 records completed, one to two days of data was missing due to inability to obtain a recall or due to failure to collect a written record. The food records and recalls were analysed using the Nutrition Data System for Research (NDSR) software (version 2010, Nutrition Coordinating Center, University of Minnesota). Records entered with previous versions of the software (2007–2009) were re-analysed with the 2010 nutrient database at study completion. Double entry of a random sample of 30 records was done for quality control. and this revealed average differences of 10% or less for intakes of energy, vitamin E, vitamin C, calcium, percent of calories from fat, total carotenoids, and whole grain servings.
Questionnaires and Anthropometric Assessments
A study questionnaire designed for this study captured demographic characteristics of subjects. A Health-Update Questionnaire was used at 3 and 6 months to capture changes in medication use, health and physical activity levels. Physical activity was assessed using a validated questionnaire and metabolic equivalents (MET) were calculated (Johnson-Kozlow et al., 2007). This questionnaire asked respondents about time spent walking at various speeds and performing mild, moderate and strenuous activities.
Self-efficacy for making dietary changes was assessed in all subjects at baseline and 3 months using seven behaviors targeted by both interventions, and answers were given on a Likert-type 5-point scale (Likert, 1932). The seven items asked about confidence to find a way to eat a variety of fruits and vegetables, finding way to meet fat goals, finding time to buy needed foods, finding time to prepare foods, finding ways to stick to goals when others around you make it difficult, controlling the home environment, and meeting goals when eating out. Internal consistency of the scale was good with an overall Cronbach alpha of 0.85.
Anthropometric measures were obtained at baseline, 3 and 6 months by trained staff of the Michigan Clinical Research Unit using a written protocol. Body weight was measured in light clothing, without shoes and rounded to the nearest quarter pound with a Scale-Tronix model 5005 Stand On Scale (White Plains, NY). Height was measured to the nearest 0.1 cm with a stadiometer and BMI was calculated as kg/m2. Waist and hip circumference was measured to the nearest 0.1 cm. Blood pressure was measured using a sphygmomanometer by auscultation of the upper arm. All measures at baseline and 6 months were obtained in the morning after an overnight fast but the 3-month visits were scheduled at the subject’s convenience.
Dietary Interventions
The Mediterranean and Healthy Eating interventions were delivered using individualized counselling with a registered dietitian. The schedule for counselling was weekly for the first month, biweekly for the next two months and monthly for the last three months. The counselling at baseline and 3-months was done face to face, and the remained of the scheduled counselling was done by telephone calls that were structured to last about 20 minutes. All individual diet goals were based to maintain energy intake reported at baseline.
At the baseline visit, subjects were presented with exchange booklets written by study staff that listed foods in categories together with serving sizes, and their own individual goals were written in the booklet. The booklet information was also provided in an abbreviated form on a single, laminated page. Other printed materials provided were for buying fruits and vegetables, estimating portion sizes, and reading food labels. Subjects randomized to the Mediterranean diet treatment arm received study recipes, sample menus for seven days and flax recipes from the Flax Council of Canada. Subjects in the Mediterranean arm were asked to keep food diaries until they became adept at meeting exchange goals, as determined by the dietitian from review of self-monitoring records, after which they could use a checklist format to track exchanges consumed from each targeted food category. Subjects in the Healthy Eating diet arm received only checklists from the start. These checklists were available both in printed format and as excel files. Each group received a bimonthly newsletter written for that diet arm with news of the study progress, and information on seasonal foods and recipes‥
The dietary counselling used Bandura’s social cognitive theory that addresses self-efficacy, self- monitoring, social support, goal setting and developing problem solving strategies (Bandura, 1986). At every counselling session after baseline, a review of dietary intakes in the previous period was the main subject of discussion between the dietitians and the study participant, and this formed the basis for short-term goal setting. If a participant’s intake of any vitamin or mineral was <67% of Dietary Reference Intake values, however, they were given a list of foods that are rich in that nutrient to correct the deficiency.
Study participants were requested to keep self-monitoring records for 5–7 days before each counselling call and to mail them to the dietitian. The counselling session at by which a participant achieved all of their food exchange goals was recorded by the dietitian using a review of each participant’s self-monitoring logs. The number of goals met was also recorded at six months.
Dietary Goals
The goals for the Healthy Eating diet were based on the U.S. Healthy People 2010 recommendations (Office of Disease Prevention and Health Promotion, 2005). The specific dietary goals are shown in Table 1. The saturated fat goal was given in grams per day, based on baseline energy intake, and subjects enumerated grams of saturated fat in the foods that they consumed on the tracker. Reducing saturated fat intake resulted in a small decrease in total fat intake by study participants, and participants therefore were able to reduce total fat intake to less than 30% of calories without additional counseling for maintaining total fat intake to below 30% of calories. A food list of high salt foods, that participants should avoid was provided, but subjects were not asked to track sodium intake.
TABLE 1.
Summary of dietary goals that were tracked on self-monitoring forms in the two study arms.
| Diet Arm | Dietary Goal | Method of Enumeration |
|---|---|---|
| Healthy Eatinga | Saturated Fat < 10% of calories | Saturated Fat grams/day |
| Fruit | two servings/dayb | |
| Vegetables | two servings/day | |
| Dark green or orange vegetable | one serving/day | |
| Whole grains | at least three servings/day | |
| Mediterraneanc | High MUFA foods | 7–10 exchanges/day (5 g/exchange) |
| High omega 3 food | twice a week, 3 ounce serving size (with limits on fish with higher mercury) | |
| Dark green vegetable | one to two servings/day | |
| Orange and yellow vegetable | one to two servings/day | |
| Red vegetable | one to two servings/day | |
| Other vegetable | one to two servings/day | |
| Dark green culinary herbs | one serving/day, 1 TB fresh or 1 tsp. dried | |
| Allium vegetables | use liberally at least once a day | |
| Fruit | one serving/day Vitamin C Fruit and one serving/day Other Fruit | |
| Whole grains, at least 3 servings/d | at least three servings/day |
The exchange book for the Healthy Eating diet included a list of sodium content of various types of foods, but sodium intake was not tracked.
For both diets, one serving for fruits and vegetables was defined as 1 medium, 1 cup fresh, 2 cups leafy greens, ½ cup canned or cooked, ½ cup juice or ¼ cup dried. For grains, serving sizes were 1 ounce (12 chips or 6 crackers), 1 slice bread, ½ cup cooked grain, ¾ cup dry cereal, or 3 cups popcorn.
The exchange book for the Mediterranean diet included lists of foods high in omega 6 fats to either avoid, limit to twice a week or limit to twice a day and a high MUFA list. The total fruit and vegetable goal was 7–9 servings/day, depending on baseline energy intake, and variety was defined by use of five exchange groups for vegetables and two exchange groups for fruit.
The number of goals was greater in the Mediterranean arm (Table 1). The ‘fat’ goal was to maintain 30% of calories from fat while reducing PUFA and SFA intakes by about 50% and 30%, respectively, and increasing MUFA intake by about 50%. Subjects in this group were asked to consume foods high in omega 3 fatty acids at least twice a week. The ‘whole grain’ goal was the same as in the Healthy Eating treatment arm. ‘Fruit and vegetable’ goals were for consumption of at least 7–9 FDA servings per day, depending on energy intake, and to include culinary herbs and allium vegetables, as shown in Table 1.
Blood Sample Analyses
Blood samples were obtained at baseline and six months following after an overnight fast. Measures of total cholesterol, HDL, triacylglycerol (triglycerides) concentrations, were performed using a Cobas Mira Chemistry analyser from Roch Diagnostics Corporation (Indianapolis). High sensitivity C-reactive protein (CRP), were measured using a latex immunoturbimeteric assay. Glucose was measured using a hexaokinase colorimeteric assay. C-peptide, insulin-like growth factor 1, growth hormone and its binding protein 3 (IGF-bp3) were analysed using an Immulite chemiluminescent assay system purchased from Diagnostics Products Corporation (Los Angeles, CA). C-peptide is more stable biomarker than insulin and is secreted in equal amount as insulin (Nesbitt et al., 2006). All these laboratory analysis and all assays were done by the Michigan Diabetes Research and Training Center Core Chemistry Laboratory. LDL cholesterol was calculated from the Friedewald equation (Friedewald et al., 1972). The homeostasis model of assessment for insulin resistance (HOMA) was calculated from C-peptide and glucose using an online calculator from the University of Oxford (The HOMA Calculator version 2.2) (Menendez et al., 2005).
Statistical Analyses
Alcohol intake was calculated from the study questionnaire using USDA values for standard sizes of wine (15.4 g/glass), beer (13.9 g/beer) and spirits (15.9 g/drink). All analyses were done in SPSS version 18 (PASW Statistics, Rel 18.0.0, Chicago: IBM Corporation). Various aspects of dietary counselling were compared across the two dietary treatment arms using two-sample t-test or Fisher’s exact test depending on whether the variable of interest was continuous or categorical (Table 2). Linear regression was used to evaluate predictors of the percentage of goals met at the end of the trial (Table 3). To evaluate changes over time in the dietary intakes, regression analyses were carried out under a linear mixed models framework. Linear mixed model regression analysis is an intent-to-treat analysis that provides valid results in presence of drop-outs and incorporates all available data at every given time point. Separate models were used for each of the nutrients as outcome, with a 3-level variable time (baseline, 3 months, 6 months) as the primary within-subject factor and diet group assignment as the primary between-subject factor. The variable of interest was the group*time interaction that indicates any difference in the pattern of change over time across groups. Regression models were controlled for covariates that can affect dietary intakes including age, gender, and BMI. To isolate the effect of diet quality, energy intake was used as a time-dependent covariate for nutrient intakes. Residuals were checked for normality of the distribution and the outcome was appropriately transformed as needed prior to the final model fit (as indicated in the footnote of Table 4) on which the inference is based. Clustering within subjects was incorporated by means of an unstructured variance-covariance matrix. Models also were constructed using the data stratified by baseline weight status (normal or overweight/obese).
TABLE 2.
Compliance with dietary counselling for subjects who completed 6 months of study. Data shown is mean and SD, or number and percent for subjects who completed 6 months.
| Variable | Healthy Eating (n=46) |
Mediterranean (n=47) |
P-valuea |
|---|---|---|---|
| Number of Counselling Calls | 10.3, 0.6 | 10.6, 1.0 | 0.106 |
| Total minutes counsellingb | 212, 67 | 245, 45 | 0.008 |
| Number of sessions to meet goalsc | 5.2, 1.8 | 6.9, 2.2 | <0.001 |
| Record-Keepingd | 81%, 22% | 80%, 22% | 0.883 |
| Self-Efficacy score at baseline | 31, 4 | 31, 3 | 0.802 |
| Self-Efficacy score at 3 months | 31, 3 | 31, 3 | 0.399 |
| Percent of goals met at 6 months | 88%, 23% | 82%, 18% | 0.159 |
| Participants meeting ≥ 70% of goals at 6 months, number and percent | 41, 89% | 40, 85% | 0.759 |
| Participants meeting 100% of goals at 6 months, number and percent | 31, 67% | 15, 32%e | 0.001 |
Differences between arms were analysed by two-sample t-tests or by Fisher’s Exact test for proportions.
The sum total of minutes spent on counselling calls over six months. This does not include the in-person study visits at baseline and 3 months.
This excludes one subject in the Mediterranean arm who never met all goals. Goal attainment was judged by the study dietitian from review of self-monitoring records.
The percentage of self-monitoring records that were kept and returned to the study dietitian.
Only 2 of the twenty-five subjects who did not meet all Mediterranean goals at 6 months had never met those goals at any point in time while on study.
TABLE 3.
Predictors of dietary goal attainment at 6 months. The two factors shown accounted for 37% of the variance in goal attainment (p < 0.001). The model was controlled for diet arm assignment, gender, baseline age and baseline BMI status (normal weight or not), all of which were not significant predictors of goal attainment.
| Predictor of Dietary Goal Attainment |
Beta | P-value |
|---|---|---|
| Record-keeping | 0.476 | <0.001 |
| Self-efficacy at baseline | 0.342 | <0.001 |
TABLE 4.
Dietary intakes over time in the two study groups. Data shown is raw mean and SD for all available data.
| Nutrient or Food | Healthy Eating | Mediterranean | ||||
|---|---|---|---|---|---|---|
| Baseline (n=61) |
3 months (n=49) |
6 months (n=47) |
Baseline (n=59) |
3 months (n=50) |
6 months (n=47) |
|
| Energy kcal/daya | 2144, 649 | 1828, 509b | 1899, 454b | 2001, 574 | 2087, 665 | 2030, 657 |
| Total fat, % of energya,c | 35, 6 | 27, 6b | 28, 7b | 35, 6 | 36, 6 | 33, 6 |
| Total Protein, g/day | 84, 24 | 77, 24 | 81, 22 | 77, 22 | 84, 29 | 83, 24 |
| Carbohydrate, g/daya,c | 261, 83 | 260, 69b | 265, 71b | 247, 81 | 256, 93 | 261, 96 |
| Saturated fat, g/day | 30, 13 | 18, 10b | 18, 7b | 26, 9 | 19, 9b | 19, 8.5b |
| MUFA, g/daya,c | 32, 12 | 22, 10b | 24, 10b | 30, 12 | 46, 16b | 39, 18b |
| N6 PUFA, g/dayc,d | 15, 6 | 12, 5 | 13, 2 | 16.0, 7.6 | 13.2, 6.7b | 11.8, 4.7b |
| N3 PUFA, g/dayc,d | 1.8, 0.8 | 1.7, 1.1 | 1.8, 0.8 | 1.9, 1.1 | 2.9, 2.4b | 2.4, 1.7 |
| Long chain n3 fats, g/day | 0.13, 0.20 | 0.26, 0.52 | 0.24, 0.32 | 0.15, 0.26 | 0.41, 0.57 | 0.35, 0.43 |
| Trans fats, g/daya,c | 3.6, 2.0 | 2.3, 1.6b | 2.1, 1.4b | 3.6, 2.7 | 1.5, 1.4b | 1.9, 1.9b |
| Fruit/veg., serv./daya | 4.55, 1.84 | 7.64, 3.17b | 7.60, 3.43b | 4.47, 1.72 | 9.42, 3.12b | 8.20, 3.32b |
| Total Carotenoids, mg/day | 11.0, 6.0 | 22.3, 13.5b | 19.2, 8.6b | 11.2, 6.4 | 25.5, 13.4b | 22.1, 13.3b |
| Variety fruit/veg./day | 3.2, 1.2 | 4.5, 1.8b | 4.4, 1.8b | 3.4, 1.5 | 5.3, 1.4b | 4.9, 1.7b |
| Whole grains, serv./day | 1.8, 1.b | 3.3, 1.7b | 3.4, 1.6b | 1.9, 1.7 | 3.4, 1.8b | 3.4, 2.1b |
| Fibre, g/d day | 22, 8 | 29, 11b | 30, 10b | 22, 8 | 36, 16b | 33, 13b |
| Red meat, serv./day | 1.9, 1.6 | 1.4, 1.5 | 1.0, 1.2b | 1.5, 1.3 | 1.2, 1.6 | 0.9, 1.1b |
| Legumes, serv/day | 0.25, 0.38 | 0.16, 0.27 | 0.30, 0.44 | 0.20, 0.27 | 0.35, 0.49 | 0.41, 0.55 |
| Glycemic Loada,c | 200, 69 | 188, 54b | 189, 58 | 190, 73 | 168, 82b | 179, 79b |
| Sodium, g/daya,c | 3.48, 1.17 | 3.05, 1.19 | 3.04, 0.98 | 3.30, 1.13 | 2.77, 1.39b | 3.06, 1.12b |
| Calcium, mg/day | 934, 340 | 921, 376 | 978, 387 | 843, 36 | 1041, 403b | 1026, 331b |
A significant group*time interaction was present for indicated variables from mixed linear regression models using variables transformed to achieve normality, as described in methods. Covariates in the analyses were energy intake (except in the case of energy and percent fat), gender, baseline BMI status (normal weight or not) and baseline age. One subject in the Healthy arm who completed food records for the 6 month visit did not attend the 6 month study visit. The transformations used before analysis were log for saturated fat, PUFA, fibre, sodium, calcium, legumes and glycemic load; square root for whole grain servings, fruit and vegetable servings, and total carotenoids; fourth root for MUFA, and long chain omega 3 fats; and the reciprocal square root for energy.
Significantly different than baseline for that diet arm.
A significant fixed effect of group was present in the model.
The n6 PUFA intake was the sum of 18:2 and 20:4. The n3 PUFA intake was the NDS output variable “omega 3 fatty acids” which is the sum of 18:3, 18:4, 20:5, 22:6, and 22:5.
Significantly different than 3 months for that diet arm.
RESULTS
Study subjects
Recruitment and retention of subjects to this study was described previously (Djuric et al., 2012). Briefly, 59 subjects were randomized to the Mediterranean arm of the study and 60 subjects to the Healthy Eating arm. Most of the subjects were Caucasian (88%), mean age was 53 years and most were female (72%). Only one subject had a personal history of colon cancer and the rest of the subjects either had a strong family history of colon cancer (64%) or a previous adenoma (27%) or both (9%) (Djuric et al., 2012). None of these characteristics differed significantly between the two diet arms (Djuric et al., 2012). There were 93 participants of the original 120 who completed the whole 6 months of study participation (46 in the Healthy Eating arm and 47 in the Mediterranean arm).
Counselling adherence
Measures of compliance and dietary goal achievement are shown in Table 2. Subjects in both arms received a similar number of contacts over six months of study, as shown in Table 2. Compliance with the recordkeeping requirements were similar in both arms, with an average of 80% of the requested records being returned. The period that elapsed before the counselling session at which subjects were able to achieve all of their dietary goals, however, differed significantly by study arm (Table 2). The time required to meet goals was, on average, 48 days in the Healthy arm and 82 days in the Mediterranean arm.
At the 6-month time point, the food records and 24-hour recalls were used to assess the number of dietary goals met for each participant. The percent of goals met at 6 months in each arm was good, at about 80% in each arm, but the number of subjects meeting all goals at 6 months was considered by the team to be low, especially in the Mediterranean arm. This could be due in part to the fact that the omega 3 goal was for a weekly, not a daily, intake, making it difficult to discern goal- meeting from four days of diet data. There also was some deterioration of dietary intakes of target nutrients in the Mediterranean arm from 3 to 6 months. Since dietary goals were just being met by 2½ months, on average, better compliance to this diet might require a greater frequency of counselling contacts or more time for subjects to become adept at it.
Subjects of normal weight did not differ from subjects who were overweight or obese with regard to session number at which goals were reached, number of calls or minutes of counselling time (not shown). The percent of dietary goals met at 6 months was, however, greater for the 33 normal weight subjects (90% goal met, SD 13) versus the 60 overweight or obese subjects (82% goals met, SD 24%, p=0.036 by a two-sample t-test). Recordkeeping was also slightly greater in the normal weight versus overweight or obese subjects (86% versus 78%, respectively, p= 0.082). There were no significant differences by gender in counselling adherence, although the session number at which goals were reached in the Mediterranean arm was borderline different for men (5.9 sessions) versus women (7.3 sessions, p = 0.053 determined by the two-sample t-test).
A brief scale was used to measure self-efficacy for making dietary changes (see Methods). This scale was devised to measure the seven behaviors targeted by both interventions, and this revealed no significant differences in mean scores by diet arm (Table 2). In addition, there were no significant differences from baseline to 3 months in either diet arm, as determined from paired t-tests (not shown). Although self-efficacy for making dietary changes did not change appreciably over time, it was a significant predictor of reaching dietary goals in a linear regression model. Self-efficacy at baseline and record-keeping percentage over 6 months were significant predictors of goal attainment at six months (Table 3). Diet arm assignment, number of counselling calls, length of time spent on telephone counselling, gender, education, current smoking status, age, marital status, baseline intake of fruits and vegetables, baseline BMI, and baseline obesity were not significant predictors of meeting dietary goals at 6 months.
Food and nutrient intakes over time
Changes in nutrient intakes were evaluated using mixed linear regression models consistent with intention to treat principles (Table 4). Variables that exhibited significant fixed effects of diet group assignment and group*time interaction are annotated in Table 4. Significant fixed effects of BMI status (normal weight or overweight/obese) were evident for saturated fat, trans fats, carotenoids, fibre and calcium. Significant fixed effects of gender were evident for energy, saturated fat, n6 PUFA and fibre. There was a significant group*time interaction for several dietary variables. Energy was significantly decreased from baseline in only the Healthy Eating group and carbohydrate intakes were significantly increased only in the Mediterranean group. MUFA intake decreased in the Healthy group and increased in the Mediterranean group. Trans fats, total fruit and vegetable servings, glycemic load and sodium all changed in the same direction in both arms, but the pattern of change in the Mediterranean group was different over time, which resulted in a significant interaction effect (Table 4).
Subjects in the Healthy arm reported a reduction in total energy, percent of energy from fat and saturated fat intake over 6 months of intervention that was maintained quite well from 3 to 6 months. In the Mediterranean arm, there was some deterioration of diet in the last three months of study. It is interesting to note that MUFA intakes decreased in the Healthy Eating arm and increased in the Mediterranean arm. Intakes of n6 and n3 fatty acids also differed by diet arm, with significant interaction effects being present in each case. Although trans fats were not targeted by the intervention, there was a significant decrease in the Mediterranean arm only.
Both diet groups reported increased intakes of whole grains and fibre, and a decrease in red meat intake. Glycemic load decreased significantly in both diet arms. Carbohydrate intake increased in the Healthy arm significantly over time in the mixed regression model, although this was not reflected in the simple means of all available data shown in Table 4. The Mediterranean arm was unique in the significant decrease in sodium and the increase in calcium, even though these were not specifically targeted by the intervention. Sodium intake was not decreased in the Healthy arm.
The goal for consuming five servings of fruits and vegetables per day in the Healthy Eating arm was surpassed resulting in statistically similar fruit and vegetable intakes in the two study arms (7.6 vs. 8.2 servings/day in the Healthy and Mediterranean arms, respectively, at six months). The significant group* time interaction indicated that total fruit and vegetable intake changed over time differently in the two study arms, perhaps due to the decrease in the Mediterranean arm from 3 to 6 months. The somewhat higher total fruits and vegetable intakes in the Mediterranean arm were mainly due to vegetable intakes (not shown).
Variety of fruit and vegetable intakes was scored and assessed by adding one point for each different type of fruit or vegetable that was consumed in a quantity that was at least half of a serving/day. The variables included in the variety count were six kinds of fruit intake (citrus, citrus juice, other fruits, other fruit juice, avocado, and fried fruit) and eight different kinds of vegetable intake (deep green, deep yellow, tomato, white potato, other starchy vegetables, other vegetables, fried vegetables not including potatoes, and vegetable juice). Variety of fruit and vegetables intakes appeared to be similar between diet arms as well, but enumeration of allium vegetables and herb intakes was not available in the NDSR program. Increases in dark green and yellow vegetables were similar between the two arms and significant in each case, but citrus intake increased significantly only in the Mediterranean arm (data not shown). Tomato intakes did not differ significantly over time, although there was a trend for a decrease in the Healthy arm and an increase in the Mediterranean arm (not shown).
Changes in anthropometric variables and blood markers of health risks
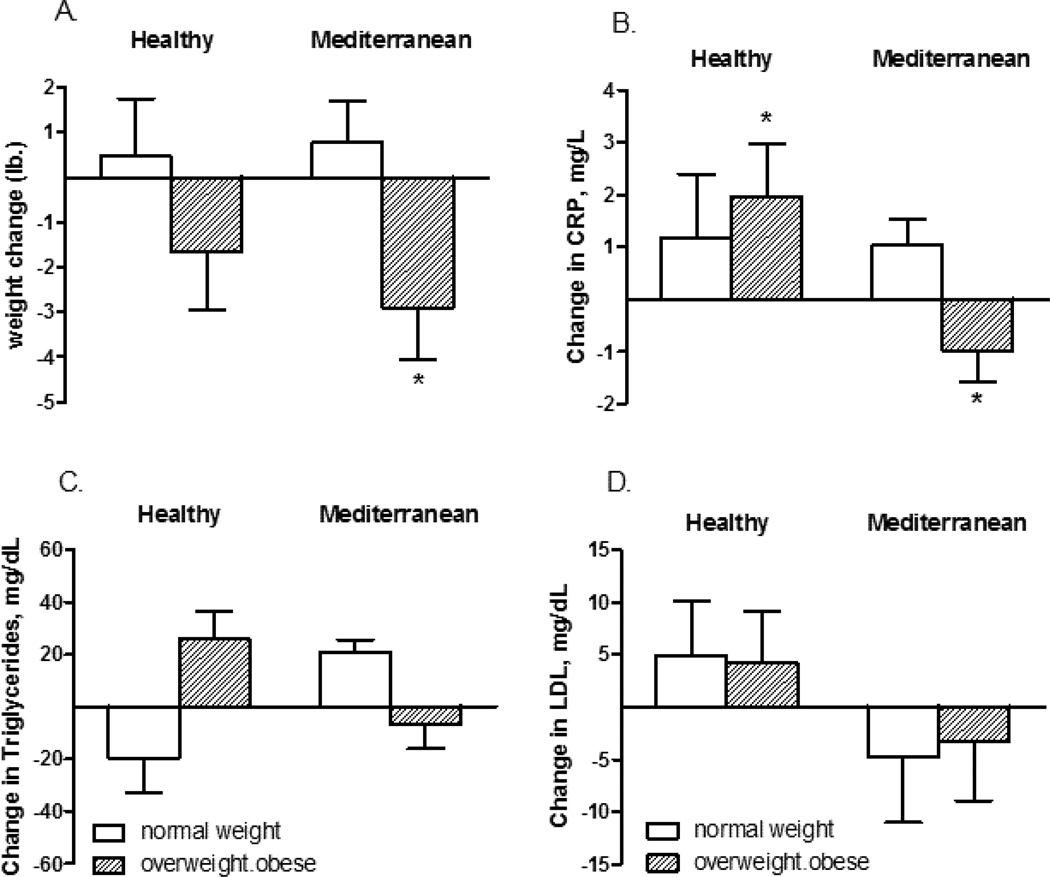
There was little change in anthropometric variables. There was a small mean weight loss in both arms, 0.92 and 1.58 kg in the Healthy and Mediterranean arms, respectively, but this was not statistically significant. In stratified analyses, there was a significant weight loss in overweight/obese subjects randomized to the Mediterranean arm, p<0.05 (Figure 1). Mean hip circumference decreased in the Mediterranean arm from 41.1 to 40.0 inches. Diastolic blood pressure decreased significantly in the Healthy arm (from 76 to 72 mm mercury).
FIGURE 1.
Change in A. body weight, B. C-reactive protein, C. triglycerides and D. low density lipoprotein (LDL) after 6 months in the Healthy Eating and Mediterranean arms in subjects who were either normal weight or overweight/obese at baseline. Data shown is the mean and SE. Mixed models regression indicated that the decrease in body weight and C-reactive protein in the Mediterranean arm for overweight/obese subjects (starred) was statistically significant after controlling for baseline age and gender (p<0.05).
There were no significant effects of either intervention on blood lipids, growth hormone or measures related to insulin status. In stratified analyses, HDL decreased and LDL did not change significantly Figure 1. C-reactive protein, however, decreased at 6 months in overweight/obese subjects randomized to the Mediterranean arm (Figure 1).
DISCUSSION
Dietary interventions that target the entire eating pattern as a whole have good potential for prevention of many cancers and can deliver a combination of preventive compounds. This may be important since interventions with single food components have not had consistently beneficial results (Ebrahimi et al., 2009, Peters et al., 2003, Bingham et al., 2003, Alberts et al., 1997). In the present study, exchange lists were derived to target either Healthy Eating and Mediterranean patterns. Goal attainment was reasonably good for participants on both diets and large dietary changes were observed in both study arms. However, it did take individuals more time to meet the dietary goals in the Mediterranean versus the Healthy arm, perhaps because the Mediterranean diet had more goals and therefore required larger changes from baseline (Table 2). Predictors of compliance to dietary goals were record-keeping and baseline self-efficacy for making dietary changes (Table 3). It therefore may be important to increase counselling efforts directed at self-efficacy and record-keeping to improve compliance. Compliance is always a concern in clinical trials. In the Polyp Prevention Trial, for example, there was no significant effect of a low-fat, high fibre intervention overall, but the subset of subjects with excellent adherence did have a lower polyp recurrence rate (Sansbury et al., 2009).
The study presented here yielded several unexpected results. The Mediterranean intervention resulted in an increase in calcium and decreases in both trans fats and sodium (Table 4). The latter could result from lower use of ready-made, foods products many of which have a dietary fat content that is not consistent with the Mediterranean goals. One of the other interesting results was that the higher goals for fruit and vegetable intakes in a greater variety in the Mediterranean diet arm did not result in significantly higher intakes than the more modest goals fruit and vegetable goals in the Healthy arm (Table 4). This indicates that the exchange list goals derived in this study for fruit and vegetable consumption that are consistent with Healthy People 2010 goals might be sufficient to increase both quantity and variety of intakes.
Given the similarity between the two diets arms in fruit and vegetable intakes, the major difference between the two interventions was found in dietary fat intakes. The Mediterranean intervention t uniquely increased mean dietary intakes of both MUFA and n-3 fats, with decreases in n-6 fats. This is potentially important since prostaglandin E2 (PGE2) is formed from arachidonic acid, n-6, and cyclooxygenase 2 is induced by high n6 fatty acid diets (Rao et al., 2001). PGE2 is strongly and positively associated with colon cancer risk (DuBois and Smalley, 1996). On the other hand, n-3 and n-9 fatty acids, the main types of fats found in Mediterranean diet, have protective effects and have been associated with decreased PGE2 levels and COX-2 expression (Singh et al., 1997, Broughton and Wade, 2002, Bartoli et al., 2000). In addition the mean, calcium intake was significantly increased only by the Mediterranean intervention, which is encouraging since a recent supplementation trial has indicated a preventive potential for calcium (Ahearn et al., 2011).
The other important difference between the two interventions was a significant weight loss in overweight or obese subjects randomized to the Mediterranean diet. This was achieved despite the fact that the dietary counselling was designed to maintain baseline weight. Mean reported energy intakes did not change significantly in the Mediterranean study arm (Table 4). The reasons for the observed weight loss with the Mediterranean diet are not clear. One of the factors might be related to increased post prandial oxidation of MUFA versus SFA, which would favor weight loss in the Mediterranean versus the Healthy diet (Piers et al., 2003, DeLany et al., 2000).
Other Mediterranean interventions were typically done with individuals who had cardiovascular or diabetes risks, and counselling for energy restriction was provided for individuals who were not of normal weight, such as the study of Esposito et al. (Esposito et al., 2004, Esposito et al., 2011). In the Medi-Ravage study, energy restriction was not used and there was a slight, non-significant weight loss (Vincent-Baudry et al., 2005). Our Mediterranean intervention was unique in that it provided more specific guidance for increasing the consumption of more categories of fruits and vegetables. Our study also achieved relatively large increases in dietary MUFA (Esposito et al., 2011, Djuric, 2011).
The subjects recruited for the present study were healthy, which could have limited effects of the dietary interventions in both arms of the study on blood measures of insulin resistance and plasma cholesterol concentrations. There was, however, a significant decrease in C-reactive protein in the overweight/obese subjects randomized to the Mediterranean arm, although we cannot determine if this was due to weight loss or to the change in dietary composition. Another limitation of the study is that persons at increased colon cancer risk might be more motivated than the general population. On the other hand, intensive interventions such as this may be most appropriate in populations with defined health risks such as that in this study. Strengths of the study include the randomized design and novel intervention methods with good participant compliance. Weaknesses include the reliance on self-report for dietary assessments and the fairly short time frame of intervention (6 months).
In conclusion, this study implemented two different exchange-list dietary intervention strategies in persons at increased risk of colon cancer. The intervention that was based on Healthy People 2010 goals resulted in similar increases in quantity and variety of fruits and vegetables as the more elaborate Mediterranean intervention, indicating that more modest goals for fruit and vegetables could be adequate. The Mediterranean intervention was unique in increasing intakes of MUFA and n-3 fatty acids. Self-monitoring and self-efficacy were key for goal attainment. The Mediterranean diet did not have weight loss goals, but it resulted in a significant weight loss and a decrease in serum C-reactive protein in the subjects who were overweight or obese at baseline. Given the difficulty in achieving and maintaining weight loss, the present results indicate that the Mediterranean exchange list approach should be more fully explored in studies of weight loss and weight loss maintenance.
ACKNOWLEDGMENTS
We thank all the individuals who volunteered their time to participate in the Healthy Eating Study. Students who assisted with nutritional assessments were Anna Arthur, Elizabeth Brown, Laura Glynn and Nancy Wener. Nora M. DiLaura, M.S., R.D. was a consulting dietitian for study design. Students who helped with data verification and organization were Tiffany Yang, Ofra Duchin, and Alexandra McCoy. Some of the questionnaire data was calculated by Gary Schneider and Megan Rook.
This study was supported by NIH grants RO1 CA120381, P30 CA130810 S1 and Cancer Center Support Grant P30 CA046592. The study used core resources supported by a Clinical Translational Science Award, NIH grant UL1RR024986 (the Michigan Clinical Research Unit), and by the Michigan Diabetes Research and Training Center funded by NIH grant 5P60 DK20572 (Chemistry Laboratory).
This trial was registered with the Clinical Trials.gov website maintained by the National Institutes of Health, registration number NCT00475722.
Footnotes
Author contributions: Elkhansa Sidahmed (wrote paper, data management, analyzed data), Maria Cornellier (conducted research, analyzed data), Leah Askew (constructed databases, analyzed data), Yiting Li (data management and statistical analysis), NizarTalaat (wrote paper, verified data), Mary Rapai (conducted research), Ananda Sen (designed research, analyzed data), Mack T. Ruffin (designed research), Dean Brenner (designed research), D. Kim Turgeon (designed research), and Zora Djuric (designed research, obtained funding, wrote paper, had primary responsibility for final content).
References
- Ahearn TU, Mccullough ML, Flanders WD, Long Q, Sidelnikov E, Fedirko V, Daniel CR, Rutherford RE, Shaukat A, Bostick RM. A randomized clinical trial of the effects of supplemental calcium and vitamin D3 on markers of their metabolism in normal mucosa of colorectal adenoma patients. Cancer Res. 2011;71:413–423. doi: 10.1158/0008-5472.CAN-10-1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alberts DS, Einspahr J, Ritenbaugh C, Aickin M, Rees-Mcgee S, Atwood J, Emerson S, Mason-Liddil N, Bettinger L, Patel J, Bellapravalu S, Ramanujam PS, Phelps J, Clark L. The effect of wheat bran fiber and calcium supplementation on rectal mucosal proliferation rates in patients with resected adenomatous colorectal polyps. Cancer Epidemiol Biomarkers Prev. 1997;6:161–169. [PubMed] [Google Scholar]
- American Institute for Cancer Research. Continuous Update Project: Colorectal cancer. Washington, D.C.: 2011. [Google Scholar]
- Ammerman AS, Lindquist CH, Lohr KN, Hersey J. The efficacy of behavioral interventions to modify dietary fat and fruit and vegetable intake: a review of the evidence. Prev Med. 2002;35:25–41. doi: 10.1006/pmed.2002.1028. [DOI] [PubMed] [Google Scholar]
- Bandura A. Social foundations of thought and action: A social cognitive theory. Englewood Cliffs, NJ: Prentice Hall; 1986. [Google Scholar]
- Bartoli R, Fernandez-Banares F, Navarro E, Castella E, Mane J, Alvarez M, Pastor C, Cabre E, Gassull MA. Effect of olive oil on early and late events of colon carcinogenesis in rats: modulation of arachidonic acid metabolism and local prostaglandin E(2) synthesis. Gut. 2000;46:191–199. doi: 10.1136/gut.46.2.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basiotis PP, Welsh SO, Cronin FJ, Kelsay JL, Mertz W. Number of days of food intake records required to estimate individual and group nutrient intakes with defined confidence. J Nutr. 1987;117:1638–1641. doi: 10.1093/jn/117.9.1638. [DOI] [PubMed] [Google Scholar]
- Bawadi HA, Al-Sahawneh SA. Developing a meal-planning exchange list for traditional dishes in jordan. J Am Diet Assoc. 2008;108:840–846. doi: 10.1016/j.jada.2008.02.016. [DOI] [PubMed] [Google Scholar]
- Bingham SA, Day NE, Luben R, Ferrari P, Slimani N, Norat T, Clavel-Chapelon F, Kesse E, Nieters A, Boeing H, Tjonneland A, Overvad K, Martinez C, Dorronsoro M, Gonzalez CA, Key TJ, Trichopoulou A, Naska A, Vineis P, Tumino R, Krogh V, Bueno-De-Mesquita HB, Peeters PH, Berglund G, Hallmans G, Lund E, Skeie G, Kaaks R, Riboli E. Dietary fibre in food and protection against colorectal cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC): an observational study. Lancet. 2003;361:1496–1501. doi: 10.1016/s0140-6736(03)13174-1. [DOI] [PubMed] [Google Scholar]
- Boyar AP, Loughridge JR. The Fat Portion Exchange List: a tool for teaching and evaluating low-fat diets. J Am Diet Assoc. 1985;85:589–594. [PubMed] [Google Scholar]
- Broughton KS, Wade JW. Total fat and (n-3):(n-6) fat ratios influence eicosanoid production in mice. J Nutr. 2002;132:88–94. doi: 10.1093/jn/132.1.88. [DOI] [PubMed] [Google Scholar]
- Conway JM, Ingwersen LA, Moshfegh AJ. Accuracy of dietary recall using the USDA five-step multiple-pass method in men: an observational validation study. J Am Diet Assoc. 2004;104:595–603. doi: 10.1016/j.jada.2004.01.007. [DOI] [PubMed] [Google Scholar]
- De Lorgeril M, Salen P, Martin JL, Monjaud I, Boucher P, Mamelle N. Mediterranean dietary pattern in a randomized trial: prolonged survival and possible reduced cancer rate. Arch Intern Med. 1998;158:1181–1187. doi: 10.1001/archinte.158.11.1181. [DOI] [PubMed] [Google Scholar]
- Delany JP, Windhauser MM, Champagne CM, Bray GA. Differential oxidation of individual dietary fatty acids in humans. Am J Clin Nutr. 2000;72:905–911. doi: 10.1093/ajcn/72.4.905. [DOI] [PubMed] [Google Scholar]
- Dixon LB, Subar AF, Peters U, Weissfeld JL, Bresalier RS, Risch A, Schatzkin A, Hayes RB. Adherence to the USDA Food Guide, DASH Eating Plan, and Mediterranean dietary pattern reduces risk of colorectal adenoma. J Nutr. 2007;137:2443–2450. doi: 10.1093/jn/137.11.2443. [DOI] [PubMed] [Google Scholar]
- Djuric Z. The Mediterranean diet: effects on proteins that mediate fatty acid metabolism in the colon. Nutr Rev. 2011;69:730–744. doi: 10.1111/j.1753-4887.2011.00439.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Poore KM, Depper JB, Uhley VE, Lababidi S, Covington C, Klurfeld DM, Simon MS, Kucuk O, Heilbrun LK. Methods to increase fruit and vegetable intake with and without a decrease in fat intake: compliance and effects on body weight in the nutrition and breast health study. Nutr Cancer. 2002;43:141–151. doi: 10.1207/S15327914NC432_4. [DOI] [PubMed] [Google Scholar]
- Djuric Z, Ren J, Blythe J, Vanloon G, Sen A. A Mediterranean dietary intervention in healthy American women changes plasma carotenoids and fatty acids in distinct clusters. Nutr Res. 2009;29:156–163. doi: 10.1016/j.nutres.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Ruffin MTT, Rapai ME, Cornellier ML, Ren J, Ferreri TG, Askew LM, Sen A, Brenner DE, Turgeon DK. A Mediterranean dietary intervention in persons at high risk of colon cancer: recruitment and retention to an intensive study requiring biopsies. Contemp Clin Trials. 2012;33:881–888. doi: 10.1016/j.cct.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Vanloon G, Radakovich K, Dilaura NM, Heilbrun LK, Sen A. Design of a Mediterranean exchange list diet implemented by telephone counseling. J Am Diet Assoc. 2008a;108:2059–2065. doi: 10.1016/j.jada.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuric Z, Vanloon G, Radakovich K, Dilaura NM, Heilbrun LK, Sen A. Design of a Mediterranean Exchange List Diet That Can Be Implemented by Telephone Counseling. J Amer. Diet. Assoc. 2008b;208:2059–2065. doi: 10.1016/j.jada.2008.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubois RN, Smalley WE. Cyclooxygenase, NSAIDs, and colorectal cancer. J Gastroenterol. 1996;31:898–906. doi: 10.1007/BF02358623. [DOI] [PubMed] [Google Scholar]
- Ebrahimi M, Ghayour-Mobarhan M, Rezaiean S, Hoseini M, Parizade SM, Farhoudi F, Hosseininezhad SJ, Tavallaei S, Vejdani A, Azimi-Nezhad M, Shakeri MT, Rad MA, Mobarra N, Kazemi-Bajestani SM, Ferns GA. Omega-3 fatty acid supplements improve the cardiovascular risk profile of subjects with metabolic syndrome, including markers of inflammation and auto-immunity. Acta Cardiol. 2009;64:321–327. doi: 10.2143/AC.64.3.2038016. [DOI] [PubMed] [Google Scholar]
- Esposito K, Kastorini CM, Panagiotakos DB, Giugliano D. Mediterranean diet and weight loss: meta-analysis of randomized controlled trials. Metab Syndr Relat Disord. 2011;9:1–12. doi: 10.1089/met.2010.0031. [DOI] [PubMed] [Google Scholar]
- Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'armiento M, D'andrea F, Giugliano D. Effect of a mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. Jama. 2004;292:1440–1446. doi: 10.1001/jama.292.12.1440. [DOI] [PubMed] [Google Scholar]
- Estruch R, Martinez-Gonzalez MA, Corella D, Salas-Salvado J, Ruiz-Gutierrez V, Covas MI, Fiol M, Gomez-Gracia E, Lopez-Sabater MC, Vinyoles E, Aros F, Conde M, Lahoz C, Lapetra J, Saez G, Ros E. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145:1–11. doi: 10.7326/0003-4819-145-1-200607040-00004. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Vecchia CL, Gonzales JR, Lucchini F, Negri E, Levi F. Coverging patterns of colorectal cancer mortality in Europe. European Journal of Cancer. 2005;41:430–437. doi: 10.1016/j.ejca.2004.11.014. [DOI] [PubMed] [Google Scholar]
- Franz MJ, Barr P, Holler H, Powers MA, Wheeler ML, Wylie-Rosett J. Exchange lists: revised 1986. J Am Diet Assoc. 1987;87:28–34. [PubMed] [Google Scholar]
- Friedewald WT, Levy RJ, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gallus S, Bosetti C, La Vecchia C. Mediterranean diet and cancer risk. Eur J Cancer Prev. 2004;13:447–452. doi: 10.1097/00008469-200410000-00013. [DOI] [PubMed] [Google Scholar]
- Harris J, Felix L, Miners A, Murray E, Michie S, Ferguson E, Free C, Lock K, Landon J, Edwards P. Adaptive e-learning to improve dietary behaviour: a systematic review and cost-effectiveness analysis. Health Technol Assess. 2011;15:1–160. doi: 10.3310/hta15370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hung CT. Food exchange list based on 80-kilocalorie rice unit. Taiwan Yi Xue Hui Za Zhi. 1989;88:595–600. [PubMed] [Google Scholar]
- Johnson-Kozlow M, Rock CL, Gilpin EA, Hollenbach KA, Pierce JP. Validation of the WHI brief physical activity questionnaire among women diagnosed with breast cancer. Am J Health Behav. 2007;31:193–202. doi: 10.5555/ajhb.2007.31.2.193. [DOI] [PubMed] [Google Scholar]
- Kontou N, Psaltopoulou T, Panagiotakos D, Dimopoulos MA, Linos A. The mediterranean diet in cancer prevention: a review. J Med Food. 2011;14:1065–1078. doi: 10.1089/jmf.2010.0244. [DOI] [PubMed] [Google Scholar]
- Lanza E, Schatzkin A, Daston C, Corle D, Freedman L, Ballard-Barbash R, Caan B, Lance P, Marshall J, Iber F, Shike M, Weissfeld J, Slattery M, Paskett E, Mateski D, Albert P. Implementation of a 4-y, high-fiber, high-fruit-andvegetable, low-fat dietary intervention: results of dietary changes in the Polyp Prevention Trial. Am J Clin Nutr. 2001;74:387–401. doi: 10.1093/ajcn/74.3.387. [DOI] [PubMed] [Google Scholar]
- Likert R. A Technique for the Measurement of Attitudes. Archives of Psychology. 1932;140:1–55. [Google Scholar]
- Lin JS, O'connor E, Whitlock EP, Beil TL, Zuber SP, Perdue LA, Plaut D, Lutz K. Preventive Services Task Force [Internet] 2011/05/20 ed. Rockville, MD: Agency for Healthcare Research and Quality (US); 2010. Behavioral Counseling to Promote Physical Activity and a Healthful Diet to Prevent Cardiovascular Disease in Adults: Update of the Evidence for the U.S. [PubMed] [Google Scholar]
- Mcmichael AJ, Mccall MG, Hartshorne JM, Woodings TL. Patterns of gastro-intestinal cancer in European migrants to Australia: the role of dietary change. Int J Cancer. 1980;25:431–437. doi: 10.1002/ijc.2910250402. [DOI] [PubMed] [Google Scholar]
- Menendez JA, Lupu R, Colomer R. Exogenous supplementation with omega-3 polyunsaturated fatty acid docosahexaenoic acid (DHA; 22:6n-3) synergistically enhances taxane cytotoxicity and downregulates Her-2/neu (c-erbB-2) oncogene expression in human breast cancer cells. Eur J Cancer Prev. 2005;14:263–270. doi: 10.1097/00008469-200506000-00011. [DOI] [PubMed] [Google Scholar]
- Nesbitt GS, Smye M, Sheridan B, Lappin TR, Trimble ER. Integration of local and central laboratory functions in a worldwide multicentre study: Experience from the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) Study. Clin Trials. 2006;3:397–407. doi: 10.1177/1740774506070695. [DOI] [PubMed] [Google Scholar]
- Newman VA, Thomson CA, Rock CL, Flatt SW, Kealey S, Bardwell WA, Caan BJ, Pierce JP. Achieving substantial changes in eating behavior among women previously treated for breast cancer--an overview of the intervention. J Am Diet Assoc. 2005;105:382–391. doi: 10.1016/j.jada.2004.12.008. quiz 488. [DOI] [PubMed] [Google Scholar]
- Office of Disease Prevention and Health Promotion. Healthy People 2010: Focus Area 19 Food and Drug Administration. National Institutes of Health; 2005. [Google Scholar]
- Paspatis GA, Papanikolaou N, Zois E, Michalodimitrakis E. Prevalence of polyps and diverticulosis of the large bowel in the Cretan population. An autopsy study. Int J Colorectal Dis. 2001;16:257–261. doi: 10.1007/s003840100304. [DOI] [PubMed] [Google Scholar]
- Pauwels EK. The protective effect of the Mediterranean diet: focus on cancer and cardiovascular risk. Med Princ Pract. 2011;20:103–111. doi: 10.1159/000321197. [DOI] [PubMed] [Google Scholar]
- Peters U, Sinha R, Chatterjee N, Subar AF, Ziegler RG, Kulldorff M, Bresalier R, Weissfeld JL, Flood A, Schatzkin A, Hayes RB. Dietary fibre and colorectal adenoma in a colorectal cancer early detection programme. Lancet. 2003;361:1491–1495. doi: 10.1016/S0140-6736(03)13173-X. [DOI] [PubMed] [Google Scholar]
- Piers LS, Walker KZ, Stoney RM, Soares MJ, O'dea K. Substitution of saturated with monounsaturated fat in a 4-week diet affects body weight and composition of overweight and obese men. Br J Nutr. 2003;90:717–727. doi: 10.1079/bjn2003948. [DOI] [PubMed] [Google Scholar]
- Pomerleau J, Lock K, Knai C, Mckee M. Interventions designed to increase adult fruit and vegetable intake can be effective: a systematic review of the literature. J Nutr. 2005;135:2486–2495. doi: 10.1093/jn/135.10.2486. [DOI] [PubMed] [Google Scholar]
- Radakovich K, Heilbrun LK, Venkatranamamoorthy R, Lababidi S, Klurfeld DM, Djuric Z. Women participating in a dietary intervention trial maintain dietary changes without much effect on household members. Nutr Cancer. 2006;55:44–52. doi: 10.1207/s15327914nc5501_6. [DOI] [PubMed] [Google Scholar]
- Rao CV, Hirose Y, Indranie C, Reddy BS. Modulation of experimental colon tumorigenesis by types and amounts of dietary fatty acids. Cancer Research. 2001;61:1927–1933. [PubMed] [Google Scholar]
- Sansbury LB, Wanke K, Albert PS, Kahle L, Schatzkin A, Lanza E. The effect of strict adherence to a high-fiber, high-fruit and -vegetable, and low-fat eating pattern on adenoma recurrence. Am J Epidemiol. 2009;170:576–584. doi: 10.1093/aje/kwp169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shovic AC. Development of a Samoan nutrition exchange list using culturally accepted foods. J Am Diet Assoc. 1994;94:541–543. doi: 10.1016/0002-8223(94)90219-4. [DOI] [PubMed] [Google Scholar]
- Simopoulos AP. The traditional diet of Greece and cancer. Eur J Cancer Prev. 2004;13:219–230. doi: 10.1097/01.cej.0000130011.99148.07. [DOI] [PubMed] [Google Scholar]
- Singh J, Hamid R, Reddy BS. Dietary fat and colon cancer: modulation of cyclooxygenase-2 by types and amount of dietary fat during the postinitiation stage of colon carcinogenesis. Cancer Res. 1997;57:3465–3470. [PubMed] [Google Scholar]
- U.S. Department of Agriculture & U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2010. 7th Edition. Washington, DC: U.S. Government Printing Ofice; 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verberne L, Bach-Faig A, Buckland G, Serra-Majem L. Association between the Mediterranean diet and cancer risk: a review of observational studies. Nutr Cancer. 2010;62:860–870. doi: 10.1080/01635581.2010.509834. [DOI] [PubMed] [Google Scholar]
- Vincent-Baudry S, Defoort C, Gerber M, Bernard MC, Verger P, Helal O, Portugal H, Planells R, Grolier P, Amiot-Carlin MJ, Vague P, Lairon D. The Medi-RIVAGE study: reduction of cardiovascular disease risk factors after a 3-mo intervention with a Mediterranean-type diet or a low-fat diet. Am J Clin Nutr. 2005;82:964–971. doi: 10.1093/ajcn/82.5.964. [DOI] [PubMed] [Google Scholar]
- Wheeler ML, Daly A, Evert A, Franz MJ, Geil P, Holzmeister LA, Kulkarni K, Loghmani E, Ross TA, Woolf P. Choose Your Foods: Exchange Lists for Diabetes, Sixth Edition, 2008: Description and Guidelines for Use. J. Amer. Diet. Assoc. 2008;108:883–888. [Google Scholar]
- Wheeler ML, Franz M, Barrier P, Holler H, Cronmiller N, Delahanty LM. Macronutrient and energy database for the 1995 Exchange Lists for Meal Planning: a rationale for clinical practice decisions. J Am Diet Assoc. 1996;96:1167–1171. doi: 10.1016/S0002-8223(96)00299-4. [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Food, Nutrition and Prevention of Cancer: A Global Perspective. Washington, D.C.: American Institute for Cancer Research; 2007. [Google Scholar]
- Ziegler VS, Sucher KP, Downes NJ. Southeast Asian renal exchange list. J Am Diet Assoc. 1989;89:85–92. [PubMed] [Google Scholar]