Abstract
Background
Understanding the mechanisms by which the immune system induces and controls allergic inflammation at the T cell epitope level is critical for the design of new allergy vaccine strategies.
Objective
To characterize allergen-specific T cell responses linked with allergy or peripheral tolerance and to determine how CD4+ T cell responses to individual allergen-derived epitopes change over allergen-specific immunotherapy (ASIT).
Methods
Timothy grass pollen (TGP) allergy was used as a model for studying grass pollen allergies. The breadth, magnitude, epitope hierarchy and phenotype of the DR04:01-restricted TGP-specific T cell responses in ten grass pollen allergic, five non-atopic and six allergy vaccine-treated individuals was determined using an ex vivo pMHCII-tetramer approach.
Results
CD4+ T cells in allergic individuals are directed to a broad range of TGP epitopes characterized by defined immunodominance hierarchy patterns and with distinct functional profiles that depend on the epitope recognized. Epitopes that are restricted specifically to either TH2 or TH1/TR1 responses were identified. ASIT was associated with preferential deletion of allergen-specific TH2 cells and without significant change in frequency of TH1/TR1 cells.
Conclusions
Preferential allergen-specific TH2-cells deletion after repeated high doses antigen stimulation can be another independent mechanism to restore tolerance to allergen during immunotherapy.
Keywords: Immunotherapy, allergy, epitope, pollen, T cells, CD4, peptide-MHC class II tetramer, peripheral tolerance, ex vivo
INTRODUCTION
Allergen-specific immunotherapy (ASIT) is the only disease-modifying treatment for allergy (1; 2). However, effective, limited understanding of the immunologic mechanisms underlying ASIT has hampered its broad applicability and the development of novel targeted vaccines with improved efficacy and safety. Many reports have described the pivotal role of T-helper (TH) cells in both the induction and regulation of allergic immune responses (3–6). However, the precise epitope identification and functional characterization of allergen-specific CD4+ T cells at the single epitope level are still lacking. Advancement in this area will facilitate the design of new allergy vaccine strategies and will help to understand how epitope recognition by T cells influences the magnitude, fate and quality of immune responses.
Timothy grass is one of the most prevalent types of grasses in the world and contains several allergens, of which Phl p 1, Phl p 5a and Phl p 5b are major allergens for grass pollen-sensitive patients (7) accounting for most of the IgE-binding capacity of crude pollen extracts. Immunotherapy with Timothy grass pollen (TGP) alone is effective for treating allergies caused by all grass species (8; 9). Therefore in this study we used Timothy grass (Phleum pratense) pollen allergy as a model for studying grass pollen allergies. We used an ex vivo pMHCII-tetramer approach to provide a complete description of the DR04:01-restricted TGP-specific CD4+ T cell responses both in allergic and non-atopic individuals, including the determination of the breadth, magnitude, epitope hierarchy and phenotype of response. We also assessed responses in ASIT-treated patients to correlate the induced T cell response with clinical benefit providing detailed information about the pathogenic and non-pathogenic responses in allergic and non-allergic individuals and the effects of conventional extract-based allergy vaccine on allergen-specific T cell responses. Results show that CD4+ T cells in allergic individuals are directed to a broad range of TGP epitopes characterized by defined immunodominance hierarchy patterns and with distinct functional profiles that depend on the epitope recognized. ASIT doesn’t specifically increase allergen-specific TH1/TR1 cell responses. Instead, we identified the preferential allergen-specific TH2-cell deletion as the main mechanism that drives the change in the TH1/TH2 allergen-specific T cell ratios and governs the restoration of tolerance to allergen during immunotherapy. Overall, these results elucidate what we believe to be a primary mechanism for ASIT that suggests new approaches for designing improved allergy vaccines.
METHODS
Subjects
Subjects with DR04:01 or DR07:01 haplotypes were recruited at the allergy clinic at Virginia Mason Medical Center (Seattle, WA) with written consent as part of an IRB approved study. TGP-allergic subjects (n=12) were selected based on their clinical symptoms, a positive skin prick test and positive IgE reactivity using the ImmunoCap test (Phadia AB, Uppsala, Sweden) with TGP extracts (test score ≥ 3). For subjects with no history of allergy (n=5), the non-atopic status was confirmed by a lack of IgE reactivity with grass pollen extracts (Supplemental Table EI). Patients that responded successfully to subcutaneous ASIT (n=6) were also recruited. These subjects had clinical history, positive skin prick test and IgE score to TGP before ASIT and then undergone ASIT for a minimum of 3 years. Treatment was considered efficacious when patients had a significant reduction in clinical symptoms and when their drug usage needs during pollen season decreased significantly.
Peptides and pMHCII tetramer reagents
A peptide library was generated based on the Phl p 1, Phl p 5a and Phl p 5b sequence. The library consisted of overlapping peptides spanning the entire allergen, each 20 amino acids long with a 12 amino acid overlap synthesized by Mimotopes (Clayton, Australia). Peptide loaded DR04:01 and DR07:01 proteins were generated as described (10) and subsequently conjugated as tetramers using R-PE streptavidin (Biosource International, Camarillo, CA). The Tetramer guided Epitope Mapping (TGEM) used to determine CD4+ T cell epitopes within TGP major allergens is described in the Methods section in this article’s Online repository at www.jacionline.org.
Ex vivo epitope-specific CD4+ T cell analysis
40 million PBMCs in culture medium at a concentration of 150 million/ml were treated with dasatinib (12) for 10 min at 37°C followed by staining with 20 µg/ml PE-labeled tetramers at room temperature for 100 min. After tetramer staining, cells were labeled with anti-PE magnetic beads and enriched using a magnetic column according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). Frequency was calculated as previously described (13). Magnetically enriched cells were next stained with antibodies against markers of interest or corresponding isotype-matched mAbs. Data acquisition was performed on a BD LSR II instrument and analyzed using FlowJo software (Treestar, Ashland, Ore).
Intracellular cytokine staining
Intracellular cytokine staining is described in the Methods section in this article’s Online repository at www.jacionline.org.
Statistical analysis
The nonparametric Mann-Whitney U test was used for unpaired comparisons between groups, whereas the nonparametric Wilcoxon matched pairs test was used for paired comparison. All statistical analysis was performed with the GraphPad Prism software 150 version 5.0a (GraphPad Software, La Jolla, CA).
RESULTS
Differences in the magnitude of the T cell responses to allergen are correlated with the allergic immune response
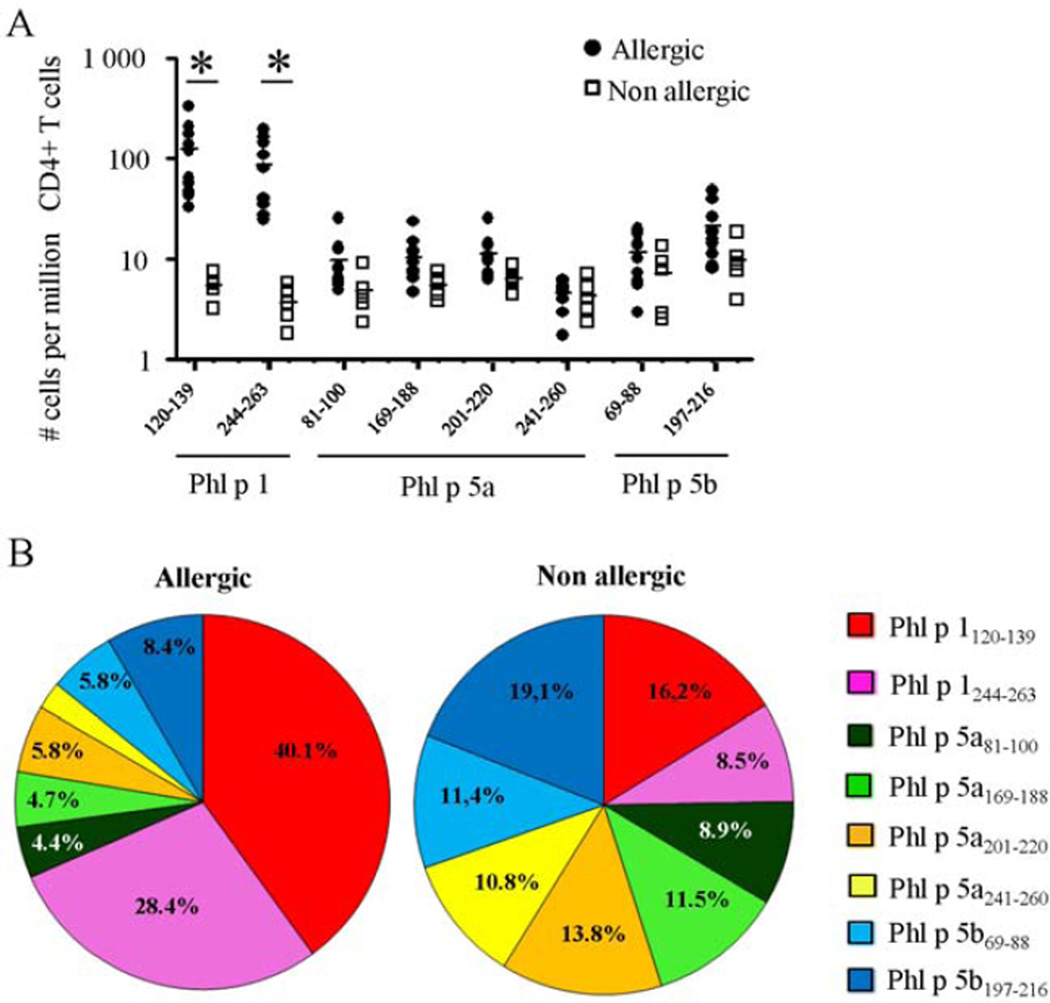
We used the TGEM approach to determine CD4+ T cell epitopes within TGP major allergens (Supplemental Fig. E1). These experiments focus on HLA-DRB1*04:01 because this allele was prevalent in our cohort of subjects with TGP allergy. We identified a total of eight immunogenic DR04:01-restricted CD4+ T-cell epitopes from group 1 (Phl p 1) and 5 (Phl p 5a and Phl p 5b) TGP major allergens. These epitopes are listed in Supplemental Table EII and were next loaded on pMHCII tetramers to track TGP allergen-specific CD4+ T cells ex vivo. We consistently detected DR04:01-restricted CD4+ T cells specific for all group 1 and group 5 TGP epitopes ex vivo using specific pMHCII tetramers (Supplemental Fig. E2). In non-atopic subjects, the magnitude of each TGP epitope-specific T cell response was very low, and no distinct immunodominance hierarchy of TGP-derived epitopes patterns was observed (Fig 1A,B). In the allergic group, Phl p 1120–139 and Phl p 1244–263 elicited a 20- to 30-fold greater T-cell immune response (average, 122.5 ± 95.9 and 86.3 ± 63.1 vs. 5.4 ± 1.6 and 3.7 ± 1.6 specific T cells per 106 CD4+ T cells, respectively, p<0.005) than in the healthy group (Fig 1A). Conversely, all group 5 TGP-derived epitopes remain poorly immunogenic ex vivo (average, 11.5 ± 8.7 specific T cells per 106 CD4+ T cells). Consequently, Phl p 1120–139- and Phl p 1244–263-specific CD4+ T cells dominate the DR04:01-restricted T cell repertoire in TGP allergic individuals, constituting about 70% of the global DR04:01 TGP-specific CD4+ T cell response (Fig 1B). Importantly, this epitope hierarchy was consistent among DR04:01-restricted allergic individuals and no difference was observed between the binding affinities of dominant epitopes Phl p 1120–139 and Phl p 1244–263 (75.9 and 232.3 nM, respectively) and non-dominant epitopes (range, 104 to 595 nM) to DR04:01 protein (Supplemental Table EII). This suggests that the efficiency of the pMHCII tetramer staining was equivalent for the different TGP epitopes tested and that this epitope hierarchy was not due to differences in binding affinities. To next explore whether Phl p 1 were intrinsically more immunogenic than Phl p 5a and Phl p 5b, we assessed the TGP-specific CD4+ T cell responses restricted by another HLA, HLA-DRB1*07:01, which was the second most prevalent allele in our cohort of allergic subjects. We observed that the DR07:01-restricted immunodominant T cell epitopes in allergic individuals are only derived from the group 5 TGP major allergens (Phl p 5a121–141 and Phl p 5b89–108 epitopes), whereas no immunogenic CD4+ T cell epitope was found in Phl p 1 for this allele (Supplemental Fig E1). Thus, different grass pollen allergens contain multiple T cell epitopes with different immunogenicity depending on HLA restriction, meaning that there will be differences in the allergen-derived peptides which are recognized by different patients’ T cells.
Figure 1.
A, Ex vivo frequencies of group 1 and group 5 TGP-specific T cells in allergic individuals (filled circles) and non-atopic subject (open squares). *P < 0.001. B, Contribution of each epitope to the global DR04:01-TGP allergen-specific T cell response in allergic individuals (n=10) and non-atopic subjects (n=5). Data are presented as the mean values from each group in pie charts.
The phenotypes of allergen-reactive T cells depend on the epitope they recognize
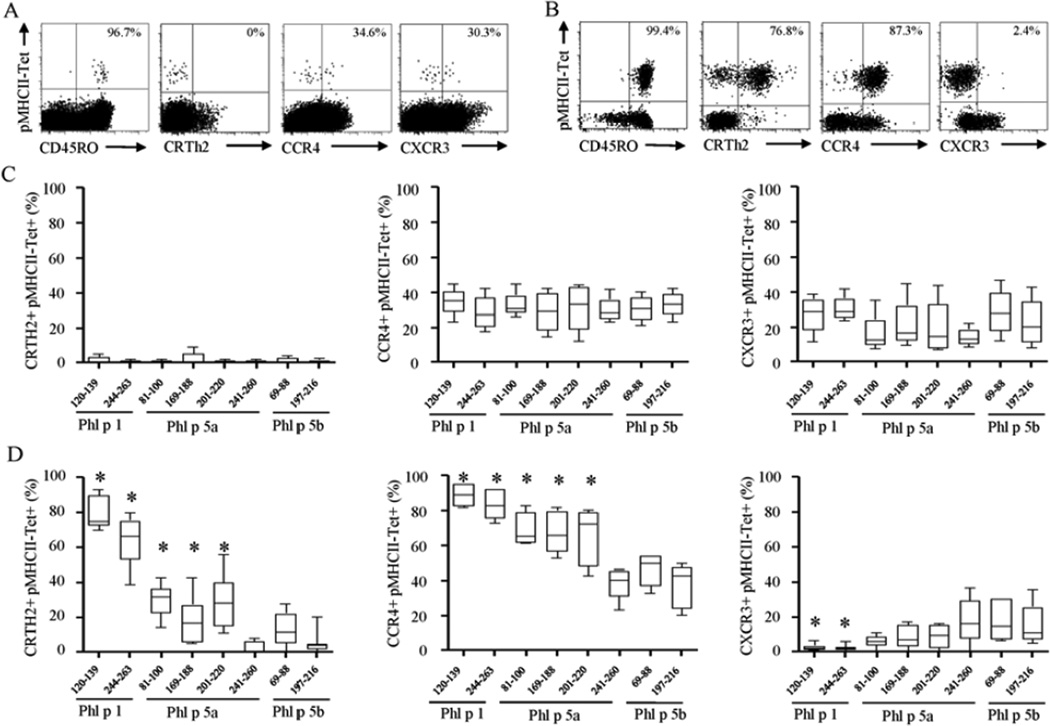
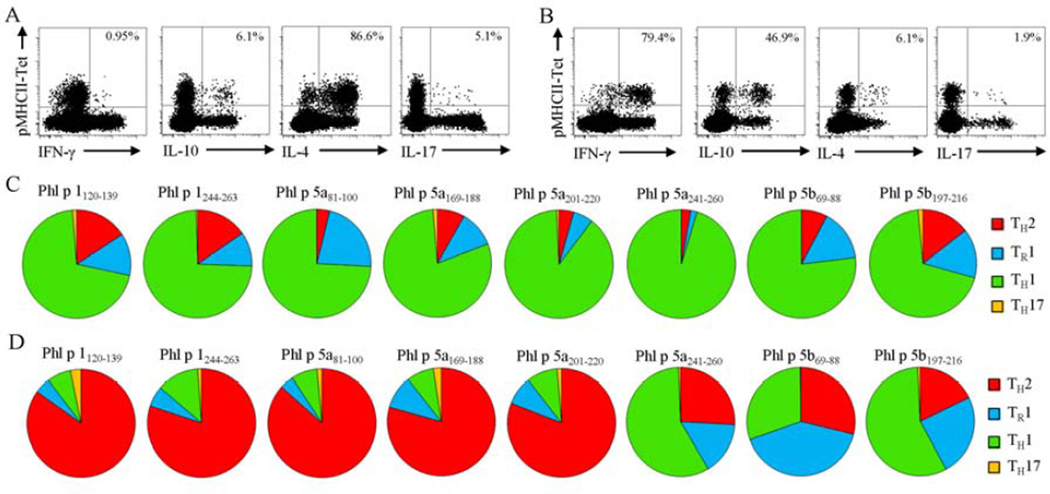
To investigate whether differences in epitope-specific T cell frequency correlate with differences in phenotype, we made a side-by-side comparison of the cytokine profile and the ex vivo surface marker expression of each DR04:01-restricted TGP epitope-specific T cells. In all allergic subjects and non-atopic individuals tested, TGP allergen-specific CD4+ T cells displayed a predominantly CD45RO+ memory phenotype implying that they are antigen-experienced T cells (Fig 2A and B). In non-atopic individuals, peripheral tolerance to all TGP-derived epitopes was associated with IFN-g producing CD4+ T cells (TH1 profile) and IL-10 producing CD4+ T cells (TR1 profile) that expressed the TH1-associated marker CXCR3 (14; 15) (average, 22.8 ± 12.7%) (Fig 2C and 3C). Conversely, in DR04:01-restricted allergic subjects certain epitopes elicited strong IL-4 responses, while other TGP-derived epitopes elicited IFN-γ and IL-10 responses in the same individual. Co-staining for cytokines in tetramer-positive cells show that IL-4 producing cells did not co-produce IFN-γ, IL-10 or IL-17, implicating these IL-4 producing cells were bona fide TH2 cells. Specifically, three basic patterns of surface marker expression can be discerned (Fig 2 and 3D). The first pattern is exemplified by the immunodominant epitopes Phl p 1120–139 and Phl p 1244–263. CD4+ T cells specific for these two epitopes highly expressed CRTH2 and CCR4, markers usually associated with TH2 cells (16,17) (average, 71.5 ± 13.7% and 85.9 ± 7.2%, respectively), which is in marked contrast to non-atopic individuals (average, 0.8 ± 1.2% and 31.6 ± 9.7%, respectively, p<0.0001) (Fig 2). Interestingly, these two epitopes also elicited distinct protective TH1/TR1 response in allergic individuals but at a dramatically lower proportion than pathogenic TH2 response (Fig 3). The second pattern is exemplified by the non-dominant epitopes Phl p 5a241–260, Phl p 5b69–88 and Phl p 5b197–216 and is characterized by a higher proportion of CXCR3 than cells of the first pattern (average, 16.9% vs. 2.4%, respectively, p<0.0001), low levels of CRTH2 and CCR4 (average, 6.3% and 40.8%, respectively), and dominant IFN-γ/IL-10 mediated immune response. Finally, a third pattern is exemplified by the non-dominant epitopes Phl p 5a81–100, Phl p 5a169–188 and Phl p 5a201–220, which preferentially elicit a TH2 response in allergic individuals but are characterized by intermediate expression of CRTH2 and CCR4 compared to the levels observed for first and second patterns. In Supplemental Table EII, we have categorized each of the TGP epitopes evaluated in this study as TH2 (allergy-associated) epitopes or TH1/TR1 epitopes.
Figure 2.
A and B, Representative dot plots showing ex vivo multicolor phenotyping of Phl p 1120–139-specific CD4+ T cells in DR04:01-restricted non-atopic (A) and allergic (B) individuals. C and D, Side by side comparison of the ex vivo phenotype of group 1 and group 5 TGP-specific CD4+ T cells in five non-atopic (C) and ten TGP-allergic (D) individuals. *P < 0.01.
Figure 3.
Cytokine expression of Phl p 1120–139-(A) and Phl p 5b197–216-(B) specific T cells in DR04:01-restricted allergic individual. Cand D, Cytokine profiles of each DR04:01-restricted TGP epitope-specific T cells in non-atopic (C) and in allergic (D) individuals. Data are representative for at least 5 individuals per group and are presented as the mean values from each group in pie charts.
Dominant allergen-derived epitope-specific CD4+ T cells are more sensitive to T cell deletion
Consistent with previous results in alder allergic individuals (18), we also observed that CRTH2 expression within allergen-specific CD4+ T cells coincided with the lack of CD27 expression in TGP allergic individuals (Supplemental Fig E3A). Interestingly, this is concomitant with a positive relationship between the frequency of allergen-specific CD4+ T cells elicited by an epitope and level of CRTH2 expression (Supplemental Fig E3B). Hence, the two immunodominant TH2 epitopes Phl p 1120–139 and Phl p 1244–263 elicited significantly greater proportions of terminally differentiated cells (i.e. CD7−, CCR7− and CD27−) compared to the TH1/TR1 epitopes Phl p 5a241–260, Phl p 5b69–88 and Phl p 5b197–216 (Fig 4A). Conversely, allergen-specific CD4+ T cells in healthy individuals were exclusively CD7+, CCR7+ and CD27+ (Fig 4B). Loss of CD27 expression has been associated with cells with a shorter life-span (19–21). To confirm the increased susceptibility to apoptosis of allergen-specific TH2 cells, we next investigated the expression of Bcl-2, a key inhibitor of apoptotic cell death, between CD27+ and CD27− allergen-specific CD4+ T cells. Along with the fact that IL-4 production was largely restricted to the allergen-specific CD4+ T cells lacking CD27 expression, those cells express less Bcl-2 than did the CD27+ subset of the same specificity (Fig 4C). Together, these results indicate that CD27− allergen-specific T cells are functionally connected with allergic disease and display classical features of short-lived cells.
Figure 4.
Ex vivo expression of CD27, CCR7 and CD7 of each DR04:01-restricted TGP epitope-specific T cells in ten allergic subjects (C) and five non-atopic individuals (D). *P < 0.01. e, IL-4, IFN-γ, Bcl-2 and T-bet expression by CD27+ (red histogram) and CD27− (blue histogram) Phl p 1120–139-specific T cells in allergic individuals. Data are representative of at least four DR04:01-restricted allergic individuals.
Allergen-specific TH2 cells represent the main subset affected by ASIT
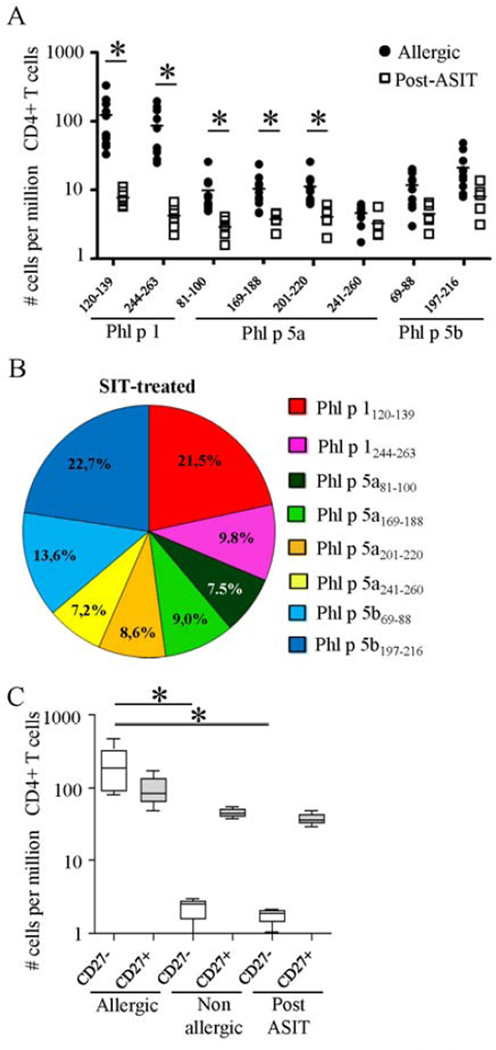
We next sought to determine the immunologic changes induced by grass pollen mixed extract ASIT by comparing the DR04:01-restricted TGP epitope-specific CD4+ T cell responses between allergic individuals and patients after successful ASIT. In post-ASIT patients, the mean numbers of total group 1 and group 5 TGP-reactive CD4+ T cells were significantly lower (32.4 ± 7.9) than those found in allergic individuals (280 ± 181.6 cells per 106 CD4+ T cells, p<0.001). Specifically, a 20- to 30-fold lower ex vivo frequency of Phl p 1120–139 and Phl p 1244-26-specific T cells was observed in all ASIT-treated patients (average, 7.7 ± 2.2 and 4.2 ± 1.6 vs. 122.5 ± 95.9 and 86.3 ± 63.1 cells per 106 CD4+ T cells, respectively, p<0.001) when compared to the allergic group (Fig 5A). In contrast, the frequencies of allergen-specific CD4+ T cells specific for non-dominant TH2 epitopes only decreased by 2-fold after ASIT, and those elicited by TH1/TR1 epitopes were unaltered (average; 12.1 ± 9.2 vs. 8.9 ± 5.7 cells per 106 CD4+ T cells, p>0.05) compared with allergic subjects. Thus, tolerance induction during ASIT was accompanied by a dramatic change in the TGP-allergen specific CD4+ T cell immunodominance hierarchy (Fig 5B). Notably, CD27− allergen-specific CD4+ T cells represent the main subset affected by ASIT, with no significant change in their CD27+ counterpart when compared with allergic subjects (Fig 5C). Consequently, the previously subdominant CD27-expressing allergen-specific CD4+ T cell subset becomes dominant as the allergen-specific CD4+ T cell subset lacking CD27 expression is deleted, and thereby changing the ratio of allergen-specific TH2/TH1 and TH2/ TR1 cells. Indeed, in contrast to the functional heterogeneity observed in allergic individuals, all DR04:01-restricted TGP epitope specific CD4+ T cell responses observed in post-ASIT patients were dominated by TH1 and TR1 cells (Fig 6A and Supplemental Fig E4). These results are also consistent with our ex vivo phenotyping showing minimal expression of CRTH2 and CCR4 along with proportionately higher expression of CXCR3 within DR04:01-restected TGP-specific T cells post-ASIT (Fig 6B). However, we did not observe any significant differences over the course of ASIT in the percentage of Foxp3-positive cells within allergen-specific CD4+ T cells (Data not shown). Together, our data indicate that allergen-specific TH2 cell deletion might be a crucial factor involved in the restoration of peripheral tolerance to allergen during ASIT.
Figure 5.
A, Ex vivo frequencies of each DR04:01-restricted TGP-specific CD4+ T cells in allergic subjects and in ASIT-treated patients. b, Contribution of each DR04:01-restricted TGP epitope to the global allergen-specific CD4+ T cell response in SIT-treated individuals. C, Overall frequencies of CD27− and CD27+ DR04:01-restricted TGP allergen-specific T cells in allergic subjects (n=10), non-atopic individuals (n=5) and patients post-ASIT (n=6).
Figure 6.
A, Cytokine profiles of each DR04:01-restricted TGP epitope-specific T cells in ASIT-treated patients. Data are representative of at least 4 individuals per group. B, Ex vivo phenotype of each DR04:01-restricted TGP epitope-specific CD4+ T cells in allergic individuals and in ASIT-treated patients. Differences between groups were analyzed by using the Mann-Whitney U test. *P < 0.01.
DISCUSSION
The design of optimal peptide based allergy vaccines is dependent on an accurate assessment of their ability to restore the protective immune responses. In the present study we utilized an ex vivo pMHCII tetramer approach to characterize the TGP-specific CD4+ T cell response at the epitope-specific level in DR04:01-restricted allergic individuals, ASIT-treated patients and non-allergic donors. Consistent with previous studies (22; 23), we found a broad range of TGP allergen-derived epitopes able to elicit DR04:01-restricted CD4+ T cell responses. Critical for clinically relevant peptides, we also found that DR04:01-restricted allergic individuals recognize exactly the same allergen-derived epitopes as allergen tolerant subjects. However, while all those epitopes strongly bind the DR04:01 molecules, our ex vivo approach reveals that the allergen-specific CD4+ T cell repertoire in allergic individuals falls into a highly reproducible immunodominance hierarchy which is absent in healthy controls. Notably, of the eight DR04:01 immuno-prevalent epitopes that drive the CD4+ T cell responses to group 1 and group 5 TGP allergens, only Phl p 1120–139 and Phl p 1244–263 elicit strong responses in allergic individuals. These responses are so robust that they dominate the T cell repertoire and suggest that the induction and regulation of allergic immune responses depend on a change in the magnitude, but not in the breadth of the T cell response towards allergen. Another finding of this study is that allergen-specific CD4+ T cells in allergic individuals have a range of phenotypes and frequencies that depend on the epitope they recognize. Conversely, all the allergen-specific T cells in non-atopic individuals are present at low frequencies and CD27 expressing TH1/TR1 cells are the dominant phenotype regardless of the epitopes they recognize.
Prior studies show that the cat allergen Fel d 1 has epitopes that preferentially elicit a TH1 cytokine pattern (24). More recently, pathogen-specific epitopes targeted by regulatory T cells have been described (25; 26). In agreement with this, we also found TGP epitopes (i.e. Phl p 5a241–260, Phl p 5b69–88 and Phl p 5b197–216) that preferentially elicit a TH1/TR1 cytokine pattern regardless of the allergic status of the donor. Alternately, we also found allergy-associated TGP epitopes (i.e. Phl p 1120–139 and Phl p 1244–263) that preferentially elicit a TH2 cytokine pattern in allergic individuals but not in non-atopic individuals. Of note, these epitopes remained able to elicit IFN-γ and IL-10-expressing CD4+ T cells in allergic individuals but at dramatically lower numbers than TH2 cells of the same specificity. Therefore, the effects of these concurrent responses in exacerbating or down regulating the immune response to an allergen seem to depend on their frequencies. These findings confirm the idea that the balance between pathogenic and protective T cells is decisive in the development of allergic disease or a healthy immune response (27).
The above observations raise questions about the mechanisms that cause epitopes that normally elicit low-frequency allergen-specific CD4+ T cell responses to become immunodominant and pathogenic in atopic individuals. It also remains to be determined why the phenotype displayed by allergen-specific CD4+ T cells can depend on both the atopic status and the allergen epitope. A combination of factors can shape immunodominance hierarchies, including some related to antigen processing and others related to CD4+ T cell dynamics (28; 29). Differences in the magnitude of ex vivo allergen-derived epitope-specific T cell responses between allergic and non-allergic individuals and within allergic subjects may arise from differences in antigen processing machinery. As a consequence, some epitopes may be more effectively processed and presented on the surface of the antigen-presenting cell, leading to better recruitment and memory expansion of the reactive CD4+ T cells that are present in the normal repertoire. Extrinsic factors such as danger signals and tissue specific inflammation may also be important (30). The quantity and strength of signal received upon cognate recognition of the TCR also may dictate in part the fate and function of CD4+ T cells (31; 32), and there is mounting evidence that CD27 is irreversibly lost from T cells only after repetitive antigenic stimulation (33; 34). Taken together with our observation that only terminally differentiated (CD27−) allergen-specific CD4+ T cells display a TH2 phenotype, this raises the possibility that repetitive TCR triggering biases the immune response in atopic individuals. Differences in the capacity to process and present peptide would be consistent with these observations and underlie the complexity of the cellular mechanisms that govern the allergic response.
It has been suggested that the primary mechanism of successful immunotherapy in atopic patients might be an increase in the number of CD4+ CD25+ Foxp3+ Treg cells and specific TR1 cells to suppress the allergic responses. Therefore, for clinical applications it has been speculated that vaccination strategies targeting TH1/TR1 cell target epitopes may most efficiently induce allergen tolerance. However, the absence of an increased number of CD4+ T cells directed to such non-dominant epitopes in the post-ASIT group argues against this. In addition, while the use of single peptides may bypass allergen-processing, epitopes that are not efficiently processed, like TH1/TR1 epitopes, may target T cells with a limited role in future adaptive immune responses, as they might not be efficiently recruited and activated during natural allergen exposure. With the assumption that the mechanisms underlying the efficacy of peptide-based allergy vaccine are similar to those for current extract-based ASIT, our results suggest that a combination of immunodominant allergy-associated (TH2) epitopes would result in better clinical efficacy during peptide based vaccine than non-dominant allergen-derived epitopes that selectively induce TH1 and TR1 cells. First, we found that successful ASIT was associated with a marked lower number of memory T cells reacting to previously immunodominant epitopes. Second, while we observed an increase in the allergen-specific TH1/TH2 and TR1/TH2 cell ratios, the overall frequencies of allergen-specific TH1/TR1 cells do not significantly increase when compared to allergic individuals. Third, we observed that allergen-specific TH2 cells express low levels of the survival protein Bcl-2 compared to allergen-specific TH1/TR1 cells of the same specificity. Hence, deletion of the previously dominant pathogenic TH2 response during ASIT is a crucial factor in the restoration of peripheral T cell tolerance to allergen allowing other responses to emerge. This is consistent with a recent study by Möbs et al (35) suggesting that allergen tolerance induced by ASIT is accompanied by long-term loss of allergen-specific TH2 cells.
Collectively, our study supports the notion that ASIT may not exclusively function by inducing allergen-specific regulatory T cells and suggests that preferential allergen-specific TH2-cells deletion after repeated high doses antigen stimulation can be another independent mechanism to restore tolerance to allergen during immunotherapy.
Supplementary Material
Key Messages.
Functional patterns of allergen-reactive T cells depend on the epitope they recognize.
Differences in the magnitude of the T cell responses to allergen-derived epitopes are correlated with the allergic immune response.
Allergen-specific TH2 cells deletion by prolonged high-dose stimulation is a crucial factor in the restoration of peripheral T cell tolerance to allergen.
ACKNOWLEDGEMENTS
We thank Jennifer Heaton for help with subject recruitment as well as D. Sorus for excellent secretarial assistance.
Declaration of all sources of funding: NIH contract HHSN272200700046C and NIH grant AI095074
Abbreviations
- Phl p
Phleum pratense
- HLA
Human histocompatibility leukocyte antigen
- MHC
Major histocompatibility complex
- PBMC
Peripheral blood mononuclear cell
- PE
Phycoerythrin
- PHA
Phytohemagglutinin
- pMHCII
Peptide-MHC class II
- Foxp3
Forkhead box P3
- ASIT
Allergen-specific immunotherapy
- TH
T helper
- TGP
Timothy grass pollen
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: The authors have declared that no conflict of interest exists.
REFERENCES
- 1.Durham SR, Walker SM, Varga EM, Jacobson MR, O'Brien F, Noble W, et al. Long-term clinical efficacy of grass-pollen immunotherapy. N Engl J Med. 1999 Aug 12;341(7):468–475. doi: 10.1056/NEJM199908123410702. [DOI] [PubMed] [Google Scholar]
- 2.Wilson DR, Lima MT, Durham SR. Sublingual immunotherapy for allergic rhinitis: systematic review and meta-analysis. Allergy. 2005 Jan;60(1):4–12. doi: 10.1111/j.1398-9995.2005.00699.x. [DOI] [PubMed] [Google Scholar]
- 3.Fujita H, Meyer N, Akdis M, Akdis CA. Mechanisms of immune tolerance to allergens. Chem Immunol Allergy. 2012;96:30–38. doi: 10.1159/000331868. [DOI] [PubMed] [Google Scholar]
- 4.Maggi E. T-cell responses induced by allergen-specific immunotherapy. Clin Exp Immunol. 2010 Jul 1;161(1):10–18. doi: 10.1111/j.1365-2249.2010.04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Akkoc T, Akdis M, Akdis CA. Update in the mechanisms of allergen-specific immunotheraphy. Allergy Asthma Immunol Res. 2011 Jan;3(1):11–20. doi: 10.4168/aair.2011.3.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larche M, Akdis CA, Valenta R. Immunological mechanisms of allergen-specific immunotherapy. Nat Rev Immunol. 2006 Oct;6(10):761–771. doi: 10.1038/nri1934. [DOI] [PubMed] [Google Scholar]
- 7.Vrtala S, Grote M, Duchene M, Van RR, Kraft D, Scheiner O, et al. Properties of tree and grass pollen allergens: reinvestigation of the linkage between solubility and allergenicity. Int Arch Allergy Immunol. 1993;102(2):160–169. doi: 10.1159/000236567. [DOI] [PubMed] [Google Scholar]
- 8.Dahl R, Kapp A, Colombo G, de Monchy JG, Rak S, Emminger W, et al. Efficacy and safety of sublingual immunotherapy with grass allergen tablets for seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006 Aug;118(2):434–440. doi: 10.1016/j.jaci.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Durham SR, Yang WH, Pedersen MR, Johansen N, Rak S. Sublingual immunotherapy with once-daily grass allergen tablets: a randomized controlled trial in seasonal allergic rhinoconjunctivitis. J Allergy Clin Immunol. 2006 Apr;117(4):802–809. doi: 10.1016/j.jaci.2005.12.1358. [DOI] [PubMed] [Google Scholar]
- 10.Novak EJ, Liu AW, Nepom GT, Kwok WW. MHC class II tetramers identify peptide-specific human CD4(+) T cells proliferating in response to influenza A antigen. J Clin Invest. 1999 Dec;104(12):R63–R67. doi: 10.1172/JCI8476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Novak EJ, Liu AW, Gebe JA, Falk BA, Nepom GT, Koelle DM, et al. Tetramer-guided epitope mapping: rapid identification and characterization of immunodominant CD4+ T cell epitopes from complex antigens. J Immunol. 2001 Jun 1;166(11):6665–6670. doi: 10.4049/jimmunol.166.11.6665. [DOI] [PubMed] [Google Scholar]
- 12.Lissina A, Ladell K, Skowera A, Clement M, Edwards E, Seggewiss R, et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J Immunol Methods. 2009 Jan 1;340(1):11–24. doi: 10.1016/j.jim.2008.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. J Allergy Clin Immunol. 2010 Jun;125(6):1407–1409. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007 Jun;8(6):639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 15.Bonecchi R, Bianchi G, Bordignon PP, D'Ambrosio D, Lang R, Borsatti A, et al. Differential expression of chemokine receptors and chemotactic responsiveness of type 1 T helper cells (Th1s) and Th2s. J Exp Med. 1998 Jan 5;187(1):129–134. doi: 10.1084/jem.187.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cosmi L, Annunziato F, Galli MIG, Maggi RME, Nagata K, Romagnani S. CRTH2 is the most reliable marker for the detection of circulating human type 2 Th and type 2 T cytotoxic cells in health and disease. Eur J Immunol. 2000 Oct;30(10):2972–2979. doi: 10.1002/1521-4141(200010)30:10<2972::AID-IMMU2972>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Mackay CR, Lanzavecchia A. Flexible programs of chemokine receptor expression on human polarized T helper 1 and 2 lymphocytes. J Exp Med. 1998 Mar 16;187(6):875–883. doi: 10.1084/jem.187.6.875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wambre E, Delong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. J Allergy Clin Immunol. 2012 Feb;129(2):544–551. 551. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wherry EJ. T cell exhaustion. Nat Immunol. 2011 Jun;12(6):492–499. doi: 10.1038/ni.2035. [DOI] [PubMed] [Google Scholar]
- 20.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nat Immunol. 2010 Jan;11(1):83–89. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hendriks J, Xiao Y, Borst J. CD27 promotes survival of activated T cells and complements CD28 in generation and establishment of the effector T cell pool. J Exp Med. 2003 Nov 3;198(9):1369–1380. doi: 10.1084/jem.20030916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Muller WD, Karamfilov T, Kahlert H, Stuwe HT, Fahlbusch B, Cromwell O, et al. Mapping of T-cell epitopes of Phl p 5: evidence for crossreacting and non-crossreacting T-cell epitopes within Phl p 5 isoallergens. Clin Exp Allergy. 1998 Dec;28(12):1538–1548. doi: 10.1046/j.1365-2222.1998.00432.x. [DOI] [PubMed] [Google Scholar]
- 23.Oseroff C, Sidney J, Kotturi MF, Kolla R, Alam R, Broide DH, et al. Molecular determinants of T cell epitope recognition to the common Timothy grass allergen. J Immunol. 2010 Jul 15;185(2):943–955. doi: 10.4049/jimmunol.1000405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reefer AJ, Carneiro RM, Custis NJ, Platts-Mills TA, Sung SS, Hammer J, et al. A role for IL-10-mediated HLA-DR7-restricted T cell-dependent events in development of the modified Th2 response to cat allergen. J Immunol. 2004 Mar 1;172(5):2763–2772. doi: 10.4049/jimmunol.172.5.2763. [DOI] [PubMed] [Google Scholar]
- 25.Shafiani S, Tucker-Heard G, Kariyone A, Takatsu K, Urdahl KB. Pathogen-specific regulatory T cells delay the arrival of effector T cells in the lung during early tuberculosis. J Exp Med. 2010 Jul 5;207(7):1409–1420. doi: 10.1084/jem.20091885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Zhao J, Fett C, Trandem K, Fleming E, Perlman S. IFN-gamma- and IL-10-expressing virus epitope-specific Foxp3(+) T reg cells in the central nervous system during encephalomyelitis. J Exp Med. 2011 Aug 1;208(8):1571–1577. doi: 10.1084/jem.20110236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. J Exp Med. 2004 Jun 7;199(11):1567–1575. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sercarz EE, Lehmann PV, Ametani A, Benichou G, Miller A, Moudgil K. Dominance and crypticity of T cell antigenic determinants. Annu Rev Immunol. 1993;11:729–766. doi: 10.1146/annurev.iy.11.040193.003501. [DOI] [PubMed] [Google Scholar]
- 29.Oseroff C, Peters B, Pasquetto V, Moutaftsi M, Sidney J, Panchanathan V, et al. Dissociation between epitope hierarchy and immunoprevalence in CD8 responses to vaccinia virus western reserve. J Immunol. 2008 Jun 1;180(11):7193–7202. doi: 10.4049/jimmunol.180.11.7193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinman RM. Dendritic cells in vivo: a key target for a new vaccine science. Immunity. 2008 Sep 19;29(3):319–324. doi: 10.1016/j.immuni.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 31.Margot CD, Ford ML, Evavold BD. Amelioration of established experimental autoimmune encephalomyelitis by an MHC anchor-substituted variant of proteolipid protein 139–151. J Immunol. 2005 Mar 15;174(6):3352–3358. doi: 10.4049/jimmunol.174.6.3352. [DOI] [PubMed] [Google Scholar]
- 32.Skokos D, Shakhar G, Varma R, Waite JC, Cameron TO, Lindquist RL, et al. Peptide-MHC potency governs dynamic interactions between T cells and dendritic cells in lymph nodes. Nat Immunol. 2007 Aug;8(8):835–844. doi: 10.1038/ni1490. [DOI] [PubMed] [Google Scholar]
- 33.Hintzen RQ, de JR, Lens SM, Brouwer M, Baars P, van Lier RA. Regulation of CD27 expression on subsets of mature T-lymphocytes. J Immunol. 1993 Sep 1;151(5):2426–2435. [PubMed] [Google Scholar]
- 34.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, et al. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997 Nov 3;186(9):1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mobs C, Ipsen H, Mayer L, Slotosch C, Petersen A, Wurtzen PA, et al. Birch pollen immunotherapy results in long-term loss of Bet v 1-specific T(H)2 responses, transient T(R)1 activation, and synthesis of IgE-blocking antibodies. J Allergy Clin Immunol. 2012 Sep 26; doi: 10.1016/j.jaci.2012.07.056. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.