Abstract
Objective
To study the effect of moderate stress on CRF components in the serotonergic midbrain region in a monkey model of FHA.
Design
After characterization of stress sensitivity, monkeys were moved to a novel room and given 20% less chow for 5 days prior to euthanasia.
Setting
University of Pittsburgh nonhuman primate facility.
Animals
Female cynomolgus macaques (Macaca fascicularis) characterized as highly stress resilient (HSR, n=5), medium stress resilient (MSR, N=4) or stress sensitive (SS, n=4).
Intervention
5 days of diet in a novel room with unfamiliar conspecifics.
Main Outcome Measures
Density of CRF axons in the serotonergic dorsal raphe nucleus; the number of UCN1 cells; the density of UCN1 axons; the expression of CRF-R1 and CRF-R2 in the dorsal raphe nucleus.
Results
CRF innervation was higher in HSR than SS animals; UCN1 cell number was higher in HSR than SS animals and UCN1 axon bouton density was not different, all opposite of non-stressed animals. CRF-R1 was not different between the sensitivity groups, but CRF-R2 was higher in HSR than SS animals. The relative expression of CRF-R1 and R2 was similar to non-stressed animals.
Conclusions
HSR animals respond to stress with an increase in CRF delivery to serotonin neurons. With stress, UCN1 transport decreases in HSR animals. CRF receptor expression was similar with or without stress. These changes may contribute to resilience in HSR animals.
Keywords: Stress, resilience, ovulation, amenorrhea, serotonin, CRF, macaques
Introduction
Exposure to stressful stimuli can lead to a variety of secondary diseases such as anxiety, depression, cardiovascular disease, and immune hyperactivity (1, 2). Reproductive dysfunction has been recently added to this growing list of stress-related disorders (3, 4). Functional Hypothalamic Amenorrhea (FHA) is a disorder that occurs in females with no identifiable organic cause who tend to score higher than average on psychometric tests for stress; who diet but do not qualify for anorexia or other eating disorders; who exercise regularly; and who have no menstrual cycles for over 6 months (5–7). FHA is also called Stress-Induced Amenorrhea, and it is clear that some individuals are very sensitive to stressors, while others are stress resilient. About 30 % of female amenorrhea is diagnosed as FHA or Stress-Induced Amenorrhea (8). Effective stress management and removal of metabolic stresses result in the restoration of fertility in most patients (9–11).
We have developed an experimental nonhuman primate model of FHA in which mild psychosocial stress combined with a mild diet, plus or minus a moderate exercise regimen, leads to suppression of reproductive function to different degrees that reverses upon stress removal (3, 12, 13). Female cynomolgus monkeys are either [1] highly stress-resilient (HSR) and maintain normal menstrual cyclicity when exposed to two cycles of combined stress, or [2] medium stress-resilient (MSR) and ovulate in the first stress cycle, but not in the second stress cycle, or [3] stress-sensitive (SS) and become anovulatory as soon as stress is initiated, similar to women with FHA (3, 12, 13). Two major CNS systems are thought to govern stress responses i.e., the CRF and serotonin systems (13, 14).
A reciprocal relationship between the CRF and serotonin systems exists (14). The dorsal and median raphe nuclei send serotonergic projections to the forebrain and diencephalon, including the PVN (15, 16) and there are CRF projections to the raphe nuclei (17). In contrast to rodent studies, our observations suggest that serotonin inhibits PVN-CRF production in primates (13, 18). Dysfunction of the CRF and serotonin systems is common in mood and anxiety disorders and both play critical roles in the stress response (19–22). In addition, the CRF and serotonin systems provide input into the hypothalamic-pituitary-gonadal axis and therefore may affect reproductive potential (23, 24). We have previously demonstrated that there are pivotal differences between HSR and SS animals in functional aspects of the serotonin and CRF systems. SS animals had lower release of serotonin after fenfluramine challenge (25), and lower gene expression of TPH2, SERT, 5-HT1A-receptors and Fev (serotonin developmental master gene) in the serotonergic dorsal raphe nucleus compared to HSR animals (26, 27). With regard to the CRF system, SS animals had higher CRF gene expression in the PVN and denser CRF axon staining in the serotonergic raphe nucleus and noradrenergic locus ceruleus than HSR animals (18, 28, 29).
We recently reported that after 5 days of moderate stress, the relative expression of the serotonin-related genes was similar to non-stressed conditions. That is, HSR animals still had higher expression of TPH2, SERT and 5HT1A mRNAs in the dorsal raphe than SS animals. In the locus ceruleus, HSR animals had denser serotonin axon innervation than SS animals, which was also the same with or without stress (29). Together, the observations suggested that the serotonin system did not markedly change with 5 days of moderate stress, but it continued to reflect stress sensitivity. However, the CRF innervation of the locus ceruleus reversed with stress. In the absence of stress, CRF axon density was lower in HSR than SS animals. In contrast, after 5 days of moderate stress, CRF innervation of the locus ceruleus was higher in HSR than SS animals. This study continues the investigation of the CRF in the raphe region. Here we report the expression of dorsal raphe CRF components, CRF, UCN1, CRF-R1 and CRF-R2 in HSR, MSR and SS animals after 5 days of moderate stress.
Methods and Materials
Animals and treatments
This experiment was approved by the Institutional Animal Care and Use Committee of the Oregon National Primate Research Center and conducted in accordance with the 2011 Eighth Edition of the National Institute of Health Guide for the Care and Use of Laboratory Animals.
Fifteen adult female cynomolgus monkeys (Macaca fascicularis) were utilized. The animals were 7–9 years of age with no prior pregnancies. The animals were imported and immediately housed in single cages in the same room. Cynomolgus macaques form social hierarchies in grouped housing with subordinates receiving more aggression. With single cages, the rank is not a variable. They are the same group of animals utilized in previous publications examining activity of the HPA axis in response to mild psychosocial and metabolic stress (30), LH pulses in response to a CRF-R1 antagonist (31) and serotonin gene expression (32). Housing, diet, food intake, and daily vaginal swabs for detecting menstruation have been described previously (33, 34). The monkeys were similar in weight, and there were no body weight changes throughout the characterization of stress sensitivity (30).
Assessment of Stress Sensitivity
For each monkey, sensitivity of the reproductive axis to stress was categorized by assessing changes in menstrual cycle length, ovulation, and reproductive hormone secretion when monkeys were exposed to a mild psychosocial and metabolic stressor. The stressor consisted of moving the monkeys to a novel room surrounded by unfamiliar conspecifics and reducing the available chow by 20%, as described previously (13). This study was performed after each monkey had been living in its home cage surrounded by familiar monkeys for several months. Monkeys that menstruated within 38 days subsequent to the initiation of stress were moved for a second stress cycle and remained on 20% lower calorie intake (13). Monkeys that did not mense were not moved a second time.
Animals were categorized as HSR if they presented a normal ovulatory menstrual cycle [25–38 days in length, peak E >200 pg/ml in follicular phase, peak P >2 ng/ml in luteal phase] in stress cycle 1 and again in stress cycle 2. MSR animals were defined as those animals that presented a normal ovulatory menstrual cycle in response to stress cycle 1, but failed to mense by day 38 of stress cycle 2. Animals that immediately suppressed normal menstrual cyclicity upon exposure to stress (i.e they failed to ovulate or mense within 60 days) were categorized as SS. Animals that exhibited disrupted menstrual cycles during this characterization of stress sensitivity (i.e MSR and SS monkeys) were allowed to recover normal menstrual cyclicity before further experiments were resumed. One HSR and one SS monkey were euthanized for clinical reasons prior to this study. Therefore, this examination of neural gene expression contained five animals categorized as HSR, four animals categorized as MSR, and four animals categorized as SS.
Short-term stress
Following a rest period and resumption of normal menstrual cycles, each animal was subjected to the combined moderate stress paradigm described above for 5 days and then euthanized. Hence, each animal was euthanized on day 5 of the follicular phase. From previous detailed examination ovarian steroids across control and stressed cycles, the serum concentrations of E and P at this time were similar in all of the animals (13).
Tissue Preparation
The monkeys were euthanized according to the procedures recommended by the Panel on Euthanasia of the American Veterinary Association. Each animal was sedated with ketamine, given an overdose of pentobarbital (25 mg/kg, i.v.), and exsanguinated by severance of the descending aorta. The details of the brain perfusion, dissection, post-fixation, cryoprotection, sectioning, mounting and storage were described in Bethea et al (32).
CRF and UCN1 Immunohistochemistry
The details of the immunohistochemistry assay protocols for CRF and UCN were reported in Weissheimer et al (28). The primary antibody to UCN1 was purchased from Sigma (Sigma, U4757).
In situ hybridization (ISH) assays for CRF-R1 and CRF-R2
Construction of the CRF-R1 and -R2 cDNA templates, transformation of plasmids, incorporation of digoxigenin into the reverse transcribed riboprobes and details of the ISH assays for CRF-R1 and CRF-R2 were published in Senashova et al (35).
Stereological Analysis
CRF stained sections (4/animal) were obtained at 2 rostral levels and 2 caudal levels of the dorsal raphe. CRF-R1 and CRF-R2 stained sections (7/animal) were obtained through the rostral-caudal extent of the dorsal raphe at 250 μm intervals. As previously described by May et al., (36), the UNC1-positive cell bodies were located adjacent to the Edinger-Westphal nucleus (EWN) in the supraocculomotor area (SOA), which is rostral to the raphe nucleus. Three anatomical levels of the SOA at 250 μm intervals were utilized for UCN1 cell body staining. UCN1 stained fibers were measured in a small consistent area between the SOA and the dorsal raphe at 3 anatomical levels 250μm apart. CRF, CRF-R1 and CRF-R2 montages were created with the Marianas Stereology Workstation and Slidebook 5.0 (Intelligent Imaging Innovations, Denver, CO). UCN1 images were captured with a Leica DFC290 camera mounted on a Leica brightfield microscope. All images were transferred to Image J (NIH). For each anatomical level, a square outline was placed over the area and the exact dimensions were recorded. The same size square was then used for all of the animals at that anatomical level. Image J software autosegmented the image into positive (stained) and negative (unstained) pixels. The program then provided the area of positive pixels, and with a filter applied, provided the number of cells or boutons in the defined area. The same procedure was applied at each anatomical level. Therefore, for each section the following data were obtained: total area measured, positive pixel area, and cell number. Further calculation yielded the average cell number and average positive pixel area for each animal. The animal means were subjected to statistical analysis.
Comparison Across Studies
It was of interest to compare earlier studies of animals that were euthanized in the absence of stress with this study of animals euthanized after 5 days of stress. The CRF fiber density, UCN1 cell number and UCN1 fiber density were previously examined in non-stressed HSR and SS animals with immunohistochemistry (28). CRF-R1 and -R2 expression were examined in a non-stressed placebo group from a previous study (35). To examine the relative pattern of expression for each gene, the ratio of HSR/SS for each gene in the presence and absence of stress was obtained. These groups represent the extremes of the population, which are the most informative for comparison.
Statistical Analysis of In Situ Hybridization and Immunohistochemistry signals
The section results were averaged across the levels, generating one overall average for each animal. The average of the individual animals in each group was subjected to statistical analysis, so the variance around the treatment group is due to animal-to-animal variation. The data were compared with analysis of variance (ANOVA) followed by Newman-Keuls post hoc pairwise comparison. For further interpretation, the HSR and SS groups were directly compared with Student’s t-test. All statistical analyses were conducted using the Prism Statistic Program 5.0 (GraphPad, San Diego, CA). A confidence level of p<0.05 was considered significant.
Results
CRF axon density in dorsal raphe
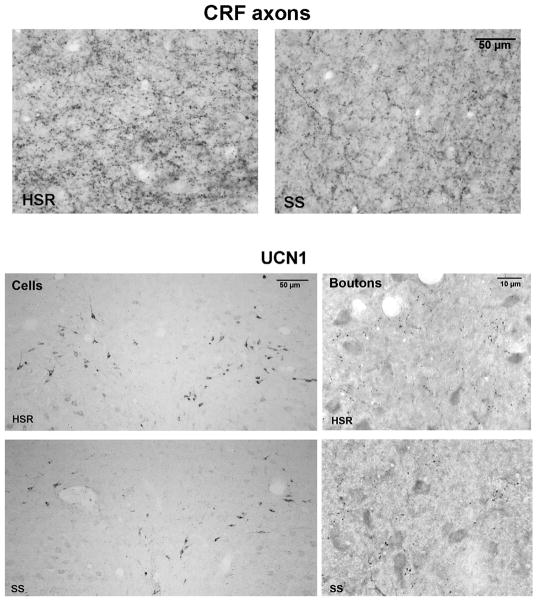
The CRF fiber immunostaining is illustrated in Figure 1, top panels, showing representative photomicrographs obtained with a Leica brightfield microscope. The difference in bouton density between the HSR and SS animal is apparent. The supplemental material contains figures illustrating the location of the area examined in the midbrain of an HSR animal.
Figure 1.
Figure 1, top. Photomicrographs of CRF axonal bouton staining in the dorsal raphe region of an HSR cynomolgus macaque obtained with a Leica brightfield microscope.
Left. CRF axonal bouton staining in an HSR animal.
Right. CRF axonal bouton staining in an SS animal.
Figure 1, bottom. Photomicrographs of UCN1 immunostained neurons and boutons at anatomically matching sections from representative HSR and SS monkeys obtained with a Leica brightfield microscope.
Left. The neurons are located in the supraocculomotor area (SOA) adjacent to the Edinger-Westfal nucleus, which is rostral to the raphe nucleus. There were more detectable neurons in the HSR monkey than the SS monkey, which was typical of the groups.
Right. The immunostained boutons are located caudal to the EWN and rostral to the raphe in a small consistent axonal cluster. There were similar numbers of UCN1 immunostained boutons in the HSR and SS groups, as observed in the representative animals shown here. Image subtraction was used in the bouton analysis and nonspecifically stained cells were removed to aid the threshold discrimination of boutons.
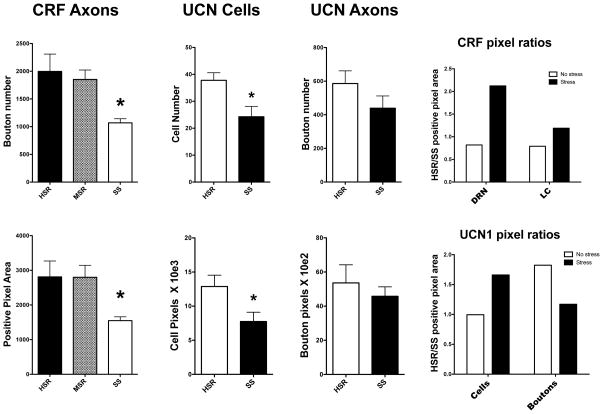
The CRF-positive bouton number and the CRF-positive pixel area were obtained for each animal at 4 anatomical levels. The average of the 4 levels yielded the animal result. Then, the average of the animals in each group was obtained. Figure 2, left panels illustrate the overall average CRF-positive cell boutons (top) and the overall average CRF-positive pixel area (bottom) in each group. There was a significant difference in average bouton number across the 3 groups (ANOVA, F [2,9]=5.66, p<0.025). The SS group was significantly lower than the HSR group (Newman-Keuls, p < 0.05). There was also a difference in the average CRF-positive pixel area across the 3 groups (ANOVA, F [2,9]=4.6, p<0.042). There was a significant difference between the HSR and SS groups in the average CRF-positive bouton number. The SS group had significantly fewer average CRF-positive boutons than the HSR group (t [6]=2.67, p<0.037).
Figure 2.
Histograms illustrating CRF immunostaining analysis in the raphe, the UCN1 cell analysis, the UCN1 axonal bouton analysis, and the HSR/SS ratios of the CRF and UCN1 positive pixel areas. *Significantly different from HSR by Newman-Keuls posthoc pairwise comparison or by t-test.
Left. Comparison of CRF axons.
Top. There was a significant difference in the number of CRF positive boutons between the groups (ANOVA F [2,9]=5.66, p<0.025). The SS group was significantly lower than the HSR and MSR groups with posthoc analysis (Newman-Keuls, p<0.05).
Bottom. There was a significant difference in the CRF positive pixel area between the groups (ANOVA F [2,9]=4.6, p<0.042). The SS group was significantly lower than the HSR group with direct comparison (t [6]=2.67, p<0.037).
Middle. Comparison of UCN1 cells.
Top. Direct comparison between HSR and SS groups found that the SS group had significantly fewer cells than the HSR group (t [7] = 2.90, p<0.023).
Bottom. Direct comparison between HSR and SS groups found that the SS group had significantly lower positive pixel area than the HSR group (t [7] = 2.31, p<0.05).
Right. Comparison of UCN1 boutons.
Top. ANOVA did not find a significant difference in UCN1 bouton number between the groups and t-test found no difference in a direct comparison of HSR and SS groups.
Bottom. ANOVA did not find a significant difference in UCN1 bouton pixel area between the groups and t-test found no difference in a direct comparison of HSR and SS groups.
Far Right. CRF and UCN1 Ratios.
Top. The HSR/SS ratio for CRF boutons was less than 1.0 in the absence of stress [extracted from 28] and was greater than 1.0 with stress, in both the dorsal raphe nucleus (DRN) and the locus ceruleus [extracted from 29]. This indicates that the HSR group was lower than the SS group with no stress, but the HSR group was higher than the SS group with stress.
Bottom. The HSR/SS ratio for UCN1 cell positive pixel area equaled 1.0 without stress and was greater than 1.0 with stress. This indicates that UNC1 cell pixel area was similar without stress, but the HSR group was markedly higher than the SS group with stress. In contrast, the HSR/SS ratio for UCN1 bouton positive pixel area was greater than 1.0 without stress, but equal to 1.0 with stress. This indicates that there was a decrease in UCN1 transport with moderate short-term stress. Primary data for UCN1 cell and bouton pixel area was extracted from Weissheimer et al (28).
UCN1 cells
Figure 1, bottom panels contain photomicrographs of UCN1 positive cells in the left panels and UCN1 positive boutons in the right panels of representative HSR and SS animals. It is clear that there are more cells in the HSR animal than in the SS animal. To distinguish UCN1 boutons in the fiber plexus area it was helpful to subtract non-specifically stained cells. This was accomplished in Image J by segmenting the image and capturing all items larger than 100μm to a mask. The mask was then subtracted from the original photomicrograph, leaving boutons and empty white spaces. The boutons could then be segmented and counted without interference. In the analysis, only objects between 0–4 pixels were counted.
The UCN1 cell number and positive pixel area were obtained at 3 anatomical levels of the supraoculomotor nucleus (SOA) for each animal. The average of the 3 levels yielded the animal result. Then, the average of the animals in each group was obtained. The number of UCN1 positive cells and the positive pixel area are shown in Figure 2, middle panels. In the comparison of cell number, there was no difference between the 3 groups (data not shown). However, direct comparison of the HSR and SS animals indicated that there were significantly fewer cells in the SS group than the HSR group (t [7] = 2.90, p<0.023). Similar results were obtained with the positive pixel area (t [7] = 2.31, p<0.05).
The UCN1 bouton number and positive pixel area were obtained at 3 anatomical levels in an area between the SOA and the dorsal raphe. The average of the 3 levels yielded the animal result. Then, the average of the animals in each group was obtained. The number of UCN1 boutons and positive pixel area are also shown in Figure 2, right panels. There was no difference between the 3 groups by ANOVA (data not shown); and there was no difference between HSR and SS groups with Student’s t-test. There appears to be a modest trend toward lower UCN1 fiber density in the SS group. It is possible that with more animals, this trend would reach statistical significance. Therefore, this could be a type 2 statistical error, which is always a concern with monkey studies containing small numbers of animals.
CRF and UCN1 comparisons with and without stress
In order to compare CRF fibers, UCN1 cells and UCN1 fibers to previous results with non-stressed animals, the ratio of HSR/SS groups was determined using pixel area and the results are illustrated in Figure 2, far left panels. CRF and UCN1 results from non-stressed animals were obtained from the measurements of placebo groups in an earlier experiment (28). Direct comparisons were not feasible due to the use of different imaging analysis systems in the different studies, which produce different ranges of outcomes. Thus, only the relative difference between the HSR and SS groups is available.
The CRF fiber ratio of HSR/SS changed markedly with stress. In the absence of stress, the ratio was less than 1.0, indicating that the SS group was higher than the HSR group. In contrast, with stress the ratio was patently greater than 1.0. This means that the HSR group had many more immunostained axonal boutons in the raphe than the SS group. Also shown for reader convenience are data illustrating the CRF innervation of the locus ceruleus, which were previously published (29). The CRF data in the raphe and locus ceruleus are consistent in that the SS group was higher than the HSR group in the absence of stress, but the HSR group was higher than the SS group in the presence of stress.
The UCN1 cell and fiber ratios of HSR/SS pixel area both reversed with the application of 5 days of moderate stress. There was no difference in UCN1 cells between HSR and SS groups without stress. However, with stress the HSR/SS ratio was greater than 1.0, indicating that there were more detectable cells in the HSR group. With respect to UCN1 axonal boutons, there was also a reversal with stress. In the absence of stress, the HSR/SS ratio was greater than 1.0, indicating that there were more boutons in the HSR group than the SS group. With stress, the ratio was nearly 1.0, signifying that the detection of axonal boutons was similar in HSR and SS groups.
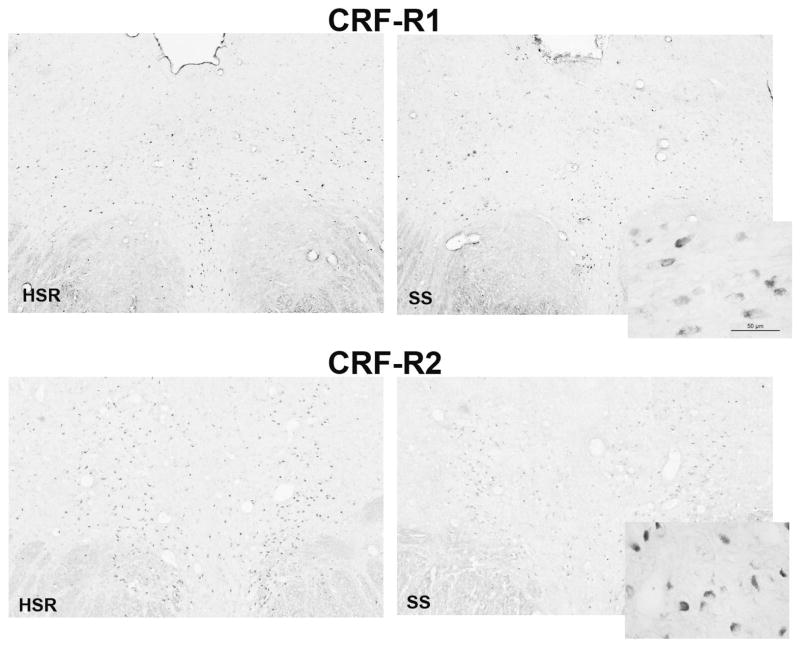
CRF-R1 and CRF-R2 in the dorsal raphe
The digoxigenin-ISH signals for CRF-R1 and –R2 in the dorsal raphe at a rostral level (CRF-R1) and a medial level (CRF-R2) from representative animals are shown in Figure 3. In the low power montages from the Marianas workstation, the positive cells appear as black and dark gray dots. The inserts illustrate the appearance of the signal at a higher power obtained with the Leica brightfield microscope. The number of CRF-R1 positive cells appeared similar between the groups. However, there was a perceptibly lower signal for CRF-R2 in the SS group compared to the HSR group. Please note that the background staining was similar between the groups.
Figure 3.
Photomicrographs of CRF-R1 and CRF-R2 digoxigenin in situ hybridization signals as detected with the Marianas Workstation and Slidebook 5. The inserts illustrate CRF-R1 and CRF-R2 digoxigenin ISH signals at higher magnification as observed with a Leica brightfield microscope.
Top. The signal for CRF-R1 expression appears similar between HSR and SS groups.
Bottom. The assay for CRF-R2 expression appears to detect more cells expressing detectable concentrations of CRF-R2 mRNA in the HSR group than the SS group.
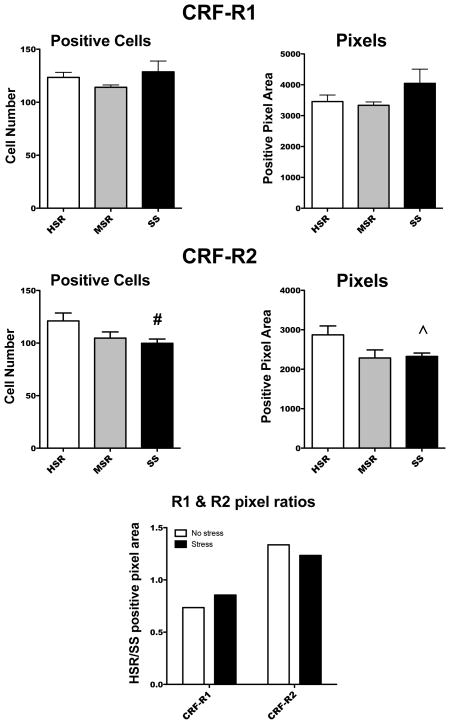
Upon quantification, there was no statistical difference between the groups in either the number of CRF-R1 positive cells or in the CRF-R1 positive pixel area (Figure 4). In reflection of the photomicrographs, the SS group had a significantly lower number of CRF-R2 positive cells (t [7] = 2.31, p<0.05) when directly compared to the HSR group. The SS group also had lower CRF-R2 positive pixel area compared to the HSR group although it did not reach statistical significance (Welsh’s t [5]= 2.25, p< 0.07).
Figure 4.
Histograms illustrating the analysis of CRF-R1 (top) and CRF-R2 (middle) positive cells and positive pixel area. The HSR/SS ratio for CRF-R1 and CRF-R2 in the presence and absence of stress is also shown (bottom). # Significantly different from HSR with t-test; ^ Difference from HSR not quite statistically significant.
Top. There was no difference in either the number of CRF-R1 positive cells or in the CRF-R1 positive pixel area.
Middle. There was a trend toward lower numbers of CRF-R2 positive cells as stress sensitivity increased (ANOVA F [2,10]=3.30, p<0.08). In a direct comparison of the HSR and SS groups, the SS group was significantly lower than the HSR group (t [7] = 2.31, p<0.05). There was a trend toward lower CRF-R2 positive pixel area in the SS group when directly compared to the HSR group (Welsh’s t [5]= 2.25, p< 0.07).
Bottom. The ratio of HSR/SS was similar for each receptor subtype with or without stress. The CRF-R1 ratio was less than 1.0, with or without stress, suggesting that the HSR group was lower than the SS group under each condition. However, this difference did not reach statistical significance in comparison of the primary data. The CRF-R2 ratio was greater than 1.0, with or without stress, signifying the HSR group was higher than the SS group under each condition. Primary data in the absence of stress was extracted from (35).
These results were compared to CRF-R1 and -R2 in non-stressed animals in Figure 4, bottom panel. The non-stressed animals used for comparison were the placebo group in a previous study (35). The bars represent the HSR/SS ratio in the presence and absence of stress. There was no change in the HSR/SS ratio of either CRF-R1 or CRF-R2 between the presence and absence of stress. First, CRF-R1 was less than 1.0, and similar, under both conditions. On the other hand, the CRF-R2 ratio was greater than 1.0, and similar, in the presence and absence of stress.
Discussion
CRF, UCN1, stress and serotonin
The hypothalamic-pituitary-adrenal (HPA) axix is well accepted as a first order responder to stress, and it consists largely of neuroendocrine neurons containing CRF that stimulate ACTH secretion, that in turn stimulates cortisol secretion from the adrenal. The brain ‘CRF system’ is comprised of several related ligands and receptors. The ligands are CRF and the urocortins 1, 2 and 3 (UCN1, UCN2, UCN3). CRF and urocortins mediate their effects by activating two known G-protein coupled receptors, CRF-R1 and CRF-R2 (37). CRF has a higher affinity for CRF-R1 than CRF-R2. In contrast, UCN1 binds CRF-R2 with higher affinity than it binds CRF-R1 (38, 39). It is thought that CRF-R1 mediates anxious behavior and the HPA axis response to stress, while CRF-R2 mediates stress-coping reactions (40–44).
We previously showed that CRF expression is elevated in the caudal PVN (18) and raphe (28) of non-stressed SS animals. However, it is important to recognize that the neuroendocrine CRF neurons are a small subset of CRF neurons that project to the hypothalamic median eminence. CRF neurons are widespread in the brain, and CRF neurons in the caudal PVN and amygdala project to the brainstem (45). Within the limbic system there is evidence that the CRF system modulates behavioral traits (46, 47). Indeed, we found that in stress-sensitive macaques, CRF mRNA was elevated in the subthalamic nucleus as well as the caudal PVN, and that CRF fiber density was greater in the central nucleus of the amgydala compared to stress-resilient macaques (18).
Together, these observations suggest that it may be possible to elevate CRF in non-neuroendocrine neurons and yet have little effect on the HPA axis. Support for this hypothesis comes from physiological studies of Dr. Judy L. Cameron showing that SS monkeys did not differ from MSR or HSR monkeys in any measurement of baseline HPA axis function even though elevated CRF was widespread in the brain of SS animals. However, MSR + SS monkeys secreted more cortisol than HSR monkeys after 24 hr exposure to move and diet (30). In addition, animals that developed abnormal menstrual cycles in response to stress (MSR + SS monkeys) suppressed LH pulse frequency in response to stress exposure. Antalarmin prevented the stress-induced suppression of LH secretion in these animals without blocking the stress-induced increase in cortisol secretion. These data suggest that non-neuroendocrine CRF regulates neurotransmitter systems other than the HPA axis and plays a role in causing stress-induced reproductive impairment in stress-sensitive individuals (31). Earlier studies indicated that daytime cortisol was elevated in women with FHA (48). The women with FHA are by definition stress-sensitive and under stress since they are not ovulating. These data are consistent with the cortisol elevation in SS monkeys after move and diet. In baboons, social isolation stress increases cortisol, but ovulation continues (49).
The urocortin system, which bears some homology with CRF, is also involved in the regulation of stress and anxiety (38) and both CRF and UCN1 fibers innervate the primate dorsal raphe nucleus (14, 50). UCN1 is involved in the stress adaptation response (51) and in stress-induced anxiety (52). Evidence from rodents suggests that, once released into the raphe, CRF and UCN1 bind to CRF-Receptor 1 (CRF-R1) and CRF-Receptor 2 (CRF-R2) respectively, and inversely regulate serotonin neurotransmission (53–56).
Administration of low dose CRF directly into the DRN inhibits serotonergic activity, and CRF-R1 antagonists block this effect (57). Conversely, the stimulatory effect of UCN and CRF-R2 is supported by increased 5-HT efflux in the basolateral amygdala (a projection region of the DRN) with intra-DRN administration of the CRF-R2 agonist, UCN2. This effect was completely blocked by antisauvagine-30 (ASV-30), a relatively selective CRF-R2 antagonist (58).
CRF detection after short-term stress in macaques
In this study, CRF axonal boutons were denser in HSR than SS animals after 5 days of moderate stress and this differed from animals that were not stressed (28). That is, with no stress the ratio of HSR/SS is less than 1.0 for the raphe. This means that CRF was higher in SS than HSR monkeys. With stress, the ratio increased markedly in the raphe, meaning that CRF was higher in HSR than SS monkeys. This was somewhat counterintuitive and one may have reasoned that the SS animals would increase production and delivery of this “stress peptide”. While it is clear that elevated CRF in a chronic manner has adverse physiological effects, there is also reason to believe that an acute elevation of CRF in response to a short-term stress may be beneficial. CRF has higher affinity for the anxiogenic R1 receptors than for the anxiolytic R2 receptors. However, if acute stress caused an inundation of CRF in the raphe, then the CRF concentration may have been sufficient to activate R2 receptors and initiate coping mechanisms. Indeed, Lukkes and colleges demonstrated an inverse dose response of CRF on serotonin release. While 100 ng of CRF significantly decreased serotonin release, 500 ng CRF significantly increased serotonin release in the nucleus accumbens terminal field (59). In addition, CRF injected into the rodent raphe blocks the response to uncontrollable stress (54). This may be the type of response that we observed in the HSR animals. That is, CRF innervation of the raphe is normally low in HSR animals, but upon stress it increased markedly and rapidly (5 days), maybe in a fashion that would stimulate CRF-R2. In contrast, the SS animals appear to have a somewhat elevated basal CRF innervation in the raphe, which does not change after 5 days of moderate stress. This pattern may chronically stimulate anxiolytic CRF-R1.
Based upon the earlier physiological experiments (30), it is likely that cortisol was elevated after 5 days of stress in the SS group. The stressed HSR group exhibited elevated CRF in the raphe, but did not exhibit elevated cortisol. It has been suggested that women with FHA are resistant to cortisol suppression of CRF (60). These data are consistent with our observations in macaques. That is, in the SS group, CRF fiber density in the raphe was similar in the presence and absence of stress or elevated cortisol. This may indicate that cortisol had little or no effect on the CRF projection to the raphe. However, there was no difference between FHA patients and controls in dexamethasone suppression tests (61). In future studies, the examination of different CRF populations in the brain of HSR and SS animals administered cortisol or placebo may approach this issue.
Peak ovarian steroid production differs between HSR and SS groups, which may impact the CRF system. This is discussed in the Supplemental Material.
Similar CRF results in the locus ceruleus
We found that the boutons of the CRF innervation entering the locus ceruleus also changed with stress. That is, CRF fibers were denser in the SS than the HSR group without stress, but the axonal bouton density was denser in the HSR than the SS group with stress in a manner similar to the dorsal raphe (29). In addition, it was clear that the SS groups had higher non-stressed CRF, which did not change with stress. Hence, the difference in axonal content of CRF appeared to be consistent between the raphe and the locus ceruleus.
UCN1 detection after short-term stress in macaques
Evidence from rodents suggests that, once released into the raphe, UCN1 binds to CRF-R2 and regulates serotonin neurotransmission (53, 56). In this study of monkeys with short-term moderate stress, we observed that there were more detectable UCN1 cells in HSR animals than in SS animals, whereas the axonal bouton density was similar with stress. This was the opposite of what was observed in the absence of stress. In the absence of stress, the HSR/SS ratio of UCN1 cell pixel area approaches 1.0, indicating the HSR and SS groups had similar amounts of cellular UCN1. With stress, the ratio increased, signifying that the UCN1 cells in the HSR monkeys had a higher positive pixel area than in the SS monkeys. With respect to UCN1 fibers, the positive pixel area also reversed. In the absence of stress, the HSR/SS ratio was greater than 1.0 meaning there was higher UCN1 in the fibers of the HSR animals compared to the SS animals. In the presence of stress, the ratio approached 1.0 indicating there was no difference in the UCN1 found in the boutons of HSR and SS groups. One interpretation of the data is that HSR animals generally synthesize more UCN1 than SS animals. In the absence of stress, the UCN1 is transported out from the cell body into the axons for transmission, as seen in the non-stressed HSR group with more boutons than SS animals (28). In the presence of stress, the HSR animals may continue to synthesize more UCN1, but it accumulates in the cell body rather than moving out to the axons. That is, stress reduces UCN1 transport. It would be fascinating to investigate the mechanism by which stress is translated to an action on neuronal transport.
CRF-R1 and CRF-R2 gene expression after short-term stress in macaques
Under the conditions of this study, there was no significant difference in CRF-R1 gene expression between the groups although SS animals trended higher, as previously observed in monkeys without stress (35). CRF-R2 gene expression was higher in HSR than SS animals, also as previously observed in monkeys without stress (35). The greater expression of CRF-R2 in HSR animals may indicate the system is ready to respond to an acute elevation of CRF with coping mechanisms, including stimulation of serotonin and containment of anxiety. This action may constitute part of the ability of a resilient individual to recover from stress better than a sensitive individual.
The relationship between HSR and SS groups in CRF-R1 and CRF-R2 gene expression was similar in the presence or absence of short-term stress as demonstrated by the HSR/SS ratios. One interpretation would be that sensitivity determines receptor expression, and application of stress does not. Different results may be obtained with a longer period of moderate stress. Another caveat is the possibility that any of the responses may have different ranges, which would not be reflected in the ratios. However, we did not visually observe a pronounced difference in the staining of any component. Under optimal circumstances, measurement of these endpoints would be executed at the same time, but many factors necessitated a longitudinal approach.
Conclusion
We now have a limited grasp of the function of the serotonin system, the manifestation of CRF system components in the raphe area, and the innervation and response of the locus ceruleus in macaques of high and low resilience, with and without short-term stress. We have demonstrated that there is little or no change in serotonin-related gene expression with application of short moderate stress and the HSR/SS ratio remains greater than 1.0. In compliment, there was little change in the serotonin innervation of the locus ceruleus, suggesting serotonin output has not changed after 5 days of stress. Nor was there a change in the HSR/SS ratio of CRF-R1 or CRF-R2 in the raphe. In contrast, CRF innervation of the raphe and locus ceruleus increased while UNC1 transport was reduced in HSR monkeys relative to SS monkeys under stress. Noradrenergic output of the locus ceruleus is higher in SS monkeys than HSR monkeys with no stress, and it increased markedly with stress in both HSR and SS animals (29).
We suggest that there is little adjustment in serotonin gene expression after 5 days of moderate stress. Nonetheless, there are differences observed in innervation of the raphe by systems known to impact serotonin neuron activity, and with longer stress the changes in norepinephrine, CRF and UCN1 may effect changes in serotonin. At this point, we hypothesize that the serotonin system reflects stress sensitivity, whereas the noradrenergic, CRF and UCN1 systems rapidly respond to stress, also shown in other models (60, 61). We further hypothesize that the relative function of these systems may determine whether an individual exhibits FHA and point toward the possibility of pharmacological intervention for FHA.
Supplementary Material
The location of the CRF axon plexus in the dorsal raphe nucleus that was subjected to image analysis and measurement of bouton number and bouton positive pixel area is shown below. Not shown is the pontine region, which is below the region in the photomicrograph and is located in the dorsal midbrain.
A. An image of the rostral raphe obtained with the Marianas Workstation and Slidebook 5.0. This image was transferred to Image J and cropped to the boxed area, which was then enlarged to the left. An SS animal is shown at the same magnification below the boxed area of the HSR animal.
B. Subregion of the HSR boxed area as observed in Image J.
C. Subregion of the same area from an SS animal for comparison to the HSR animal in ‘B’.
CC- central canal PAG-periaquaductal gray area
Acknowledgments
This study was supported by NIH grants HD62618 to JLC and CLB, P30-NS06180 to Sue Aicher, PhD and P51 OD011092 for the operation of the Oregon National Primate Research Center. The authors would like to thank Skyla Herod, PhD, Department of Biology and Chemistry, Azusa Pacific University, Los Angles, CA for the characterization of the monkeys and for performing the termination protocol and brain perfusion. In addition, we thank Aaron Kim for his careful sectioning of the dorsal raphe. The authors are indebted to the Division of Comparative Medicine at Oregon National Primate Research Center for the excellent animal husbandry provided for the monkeys in this study. The assistance of the Surgical Staff, Pathology Staff and the Endocrine Technology Laboratory at Oregon National Primate Research Center were also invaluable.
List of Abbreviations
- HSR
highly stress resilient
- MSR
medium stress resilient
- SS
stress sensitive
- CNS
central nervous system
- cDNA
copy DNA
- ISH
in situ hybridization
- IHC
immunohistochemistry
- ANOVA
analysis of variance
- FHA
Functional Hypothalamic Amenorrhea
- E
estradiol
- P
progesterone
- mRNA
messenger RNA
- CRF
corticotropin releasing factor
- CRF-R1
corticotropin releasing factor receptor 1
- CRF-R2
corticotropin releasing factor receptor 2
- UCN1
urocortin 1
- PVN
paraventricular nucleus of the hypothalamus
- TPH2
tryptophan hydroxylase 2, rate limiting enzyme for serotonin synthesis
- SERT
serotonin reuptake transporter
- 5HT1A
serotonin receptor, subtype 1A
- Fev
fifth ewing variant, master gene determines development of serotonin phenotype
- SOA
supraoccularmotor nucleus
Literature Cited
- 1.Golovatscka V, Ennes H, Mayer EA, Bradesi S. Chronic stress-induced changes in pro-inflammatory cytokines and spinal glia markers in the rat: a time course study. Neuroimmunomodulation. 2012;19:367–76. doi: 10.1159/000342092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Priyadarshini S, Aich P. Effects of psychological stress on innate immunity and metabolism in humans: a systematic analysis. PLoS One. 2012;7:e43232. doi: 10.1371/journal.pone.0043232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cameron JL. Reproductive dysfunction in primates, behaviorally induced. In: Fink G, editor. Encyclopedia of Stress. New York: Academic Press; 2000. pp. 366–72. [Google Scholar]
- 4.Xiao E, Xia-Zhang L, Ferin M. Stress and the menstrual cycle: short- and long-term response to a five-day endotoxin challenge during the luteal phase in the rhesus monkey. J Clin Endocrinol Metab. 1999;84:623–6. doi: 10.1210/jcem.84.2.5448. [DOI] [PubMed] [Google Scholar]
- 5.Giles DE, Berga SL. Cognitive and psychiatric correlates of functional hypothalamic amenorrhea: a controlled comparison. Fertil Steril. 1993;60:486–92. [PubMed] [Google Scholar]
- 6.Marcus MD, Loucks TL, Berga SL. Psychological correlates of functional hypothalamic amenorrhea. Fertil Steril. 2001;76:310–6. doi: 10.1016/s0015-0282(01)01921-5. [DOI] [PubMed] [Google Scholar]
- 7.Fioroni L, Fava M, Genazzani AD, Facchinetti F, Genazzani AR. Life events impact in patients with secondary amenorrhoea. J Psychosom Res. 1994;38:617–22. doi: 10.1016/0022-3999(94)90059-0. [DOI] [PubMed] [Google Scholar]
- 8.Reindollar RH, Novak M, Tho SP, McDonough PG. Adult-onset amenorrhea: a study of 262 patients. Am J Obstet Gynecol. 1986;155:531–43. doi: 10.1016/0002-9378(86)90274-7. [DOI] [PubMed] [Google Scholar]
- 9.Berga SL, Marcus MD, Loucks TL, Hlastala S, Ringham R, Krohn MA. Recovery of ovarian activity in women with functional hypothalamic amenorrhea who were treated with cognitive behavior therapy. Fertil Steril. 2003;80:976–81. doi: 10.1016/s0015-0282(03)01124-5. [DOI] [PubMed] [Google Scholar]
- 10.Berga SL, Loucks TL. Use of cognitive behavior therapy for functional hypothalamic amenorrhea. Ann N Y Acad Sci. 2006;1092:114–29. doi: 10.1196/annals.1365.010. [DOI] [PubMed] [Google Scholar]
- 11.Gordon CM. Clinical practice. Functional hypothalamic amenorrhea. N Engl J Med. 2010;363:365–71. doi: 10.1056/NEJMcp0912024. [DOI] [PubMed] [Google Scholar]
- 12.Williams NI, Berga SL, Cameron JL. Mild metabolic stress potentiates the suppressive effect of psychological stress on reproductive function in female cynomolgus monkeys. Endocrine Society Abstracts. 1997:PI–367. [Google Scholar]
- 13.Bethea CL, Centeno ML, Cameron JL. Neurobiology of stress-induced reproductive dysfunction in female macaques. Mol Neurobiol. 2008;38:199–230. doi: 10.1007/s12035-008-8042-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Linthorst AC. Interactions between corticotropin-releasing hormone and serotonin: implications for the aetiology and treatment of anxiety disorders. Handb Exp Pharmacol. 2005:181–204. doi: 10.1007/3-540-28082-0_7. [DOI] [PubMed] [Google Scholar]
- 15.Azmitia EC, Gannon PJ. The primate serotonergic system: a review of human and animal studies and a report on Macaca fascicularis. Advances in Neurology. 1986;43:407–468. [PubMed] [Google Scholar]
- 16.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiolog Rev. 1992;72:165–231. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 17.Ruggiero DA, Underwood MD, Rice PM, Mann JJ, Arango V. Corticotropic-releasing hormone and serotonin interact in the human brainstem: behavioral implications. Neuroscience. 1999;91:1343–54. doi: 10.1016/s0306-4522(98)00703-9. [DOI] [PubMed] [Google Scholar]
- 18.Centeno ML, Sanchez RL, Reddy AP, Cameron JL, Bethea CL. Corticotropin-releasing hormone and pro-opiomelanocortin gene expression in female monkeys with differences in sensitivity to stress. Neuroendocrinology. 2007;86:277–88. doi: 10.1159/000109877. [DOI] [PubMed] [Google Scholar]
- 19.Clark MS, Kaiyala KJ. Role of corticotropin-releasing factor family peptides and receptors in stress-related psychiatric disorders. Semin Clin Neuropsychiatry. 2003;8:119–36. doi: 10.1053/scnp.2003.50011. [DOI] [PubMed] [Google Scholar]
- 20.Reul JM, Holsboer F. Corticotropin-releasing factor receptors 1 and 2 in anxiety and depression. Curr Opin Pharmacol. 2002;2:23–33. doi: 10.1016/s1471-4892(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 21.Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–41. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Vaswani M, Linda FK, Ramesh S. Role of selective serotonin reuptake inhibitors in psychiatric disorders: a comprehensive review. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:85–102. doi: 10.1016/s0278-5846(02)00338-x. [DOI] [PubMed] [Google Scholar]
- 23.Li XF, Bowe JE, Kinsey-Jones JS, Brain SD, Lightman SL, O’Byrne KT. Differential role of corticotrophin-releasing factor receptor types 1 and 2 in stress-induced suppression of pulsatile luteinising hormone secretion in the female rat. J Neuroendocrinol. 2006;18:602–10. doi: 10.1111/j.1365-2826.2006.01450.x. [DOI] [PubMed] [Google Scholar]
- 24.Rivier C, Rivier J, Vale W. Stress-induced inhibition of reproductive functions: role of endogenous corticotropin-releasing factor. Science. 1986;231:607–9. doi: 10.1126/science.3003907. [DOI] [PubMed] [Google Scholar]
- 25.Bethea CL, Pau FK, Fox S, Hess DL, Berga SL, Cameron JL. Sensitivity to stress-induced reproductive dysfunction linked to activity of the serotonin system. Fertil Steril. 2005;83:148–55. doi: 10.1016/j.fertnstert.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 26.Lima FB, Centeno ML, Costa ME, Reddy AP, Cameron JL, Bethea CL. Stress sensitive female macaques have decreased fifth Ewing variant (Fev) and serotonin-related gene expression that is not reversed by citalopram. Neuroscience. 2009;164:676–91. doi: 10.1016/j.neuroscience.2009.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bethea CL, Streicher JM, Mirkes SJ, Sanchez RL, Reddy AP, Cameron JL. Serotonin-related gene expression in female monkeys with individual sensitivity to stress. Neuroscience. 2005;132:151–66. doi: 10.1016/j.neuroscience.2004.11.022. [DOI] [PubMed] [Google Scholar]
- 28.Weissheimer KV, Herod SM, Cameron JL, Bethea CL. Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorhea. Neuroendocrinology. 2010;92:224–34. doi: 10.1159/000319257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bethea CL, Kim A, Cameron JL. Function and innervation of the locus ceruleus in a macaque model of Functional Hypothalamic Amenorrhea. Neurobiol Dis. 2012;50C:96–106. doi: 10.1016/j.nbd.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Herod SM, Dettmer AM, Novak MA, Meyer JS, Cameron JL. Sensitivity to stress-induced reproductive dysfunction is associated with a selective but not a generalized increase in activity of the adrenal axis. Am J Physiol Endocrinol Metab. 2011;300:E28–36. doi: 10.1152/ajpendo.00223.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Herod SM, Pohl CR, Cameron JL. Treatment with a CRH-R1 antagonist prevents stress-induced suppression of the central neural drive to the reproductive axis in female macaques. Am J Physiol Endocrinol Metab. 2011;300:E19–27. doi: 10.1152/ajpendo.00224.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bethea CL, Phu K, Reddy AP, Cameron JL. The effect of short-term stress on serotonin gene expression in high and low resilient macaques. Prog Neuropsychopharmacol Biol Psychiatry. 2013;44:143–153. doi: 10.1016/j.pnpbp.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Williams NI, Helmreich DL, Parfitt DB, Caston-Balderrama A, Cameron JL. Evidence for a causal role of low energy availability in the induction of menstrual cycle disturbances during strenuous exercise training. J Clin Endocrinol Metab. 2001;86:5184–93. doi: 10.1210/jcem.86.11.8024. [DOI] [PubMed] [Google Scholar]
- 34.Williams NI, Berga SL, Cameron JL. Synergism between psychosocial and metabolic stressors: impact on reproductive function in cynomolgus monkeys. Am J Physiol Endocrinol Metab. 2007;293:E270–6. doi: 10.1152/ajpendo.00108.2007. [DOI] [PubMed] [Google Scholar]
- 35.Senashova O, Reddy AP, Cameron JL, Bethea CL. The effect of citalopram on midbrain CRF receptors 1 and 2 in a primate model of stress-induced amenorrhea. Reprod Sci. 2012;19:623–32. doi: 10.1177/1933719111430992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.May PJ, Reiner AJ, Ryabinin AE. Comparison of the distributions of urocortin-containing and cholinergic neurons in the perioculomotor midbrain of the cat and macaque. J Comp Neurol. 2008;507:1300–16. doi: 10.1002/cne.21514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dautzenberg FM, Hauger RL. The CRF peptide family and their receptors: yet more partners discovered. Trends Pharmacol Sci. 2002;23:71–7. doi: 10.1016/s0165-6147(02)01946-6. [DOI] [PubMed] [Google Scholar]
- 38.Vaughan J, Donaldson C, Bittencourt J, Perrin MH, Lewis K, Sutton S, et al. Urocortin, a mammalian neuropeptide related to fish urotensin I and to corticotropin-releasing factor. Nature. 1995;378:287–92. doi: 10.1038/378287a0. [DOI] [PubMed] [Google Scholar]
- 39.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–57. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 40.Timpl P, Spanagel R, Sillaber I, Kresse A, Reul JM, Stalla GK, et al. Impaired stress response and reduced anxiety in mice lacking a functional corticotropin-releasing hormone receptor 1. Nat Genet. 1998;19:162–6. doi: 10.1038/520. [DOI] [PubMed] [Google Scholar]
- 41.Muller MB, Zimmermann S, Sillaber I, Hagemeyer TP, Deussing JM, Timpl P, et al. Limbic corticotropin-releasing hormone receptor 1 mediates anxiety-related behavior and hormonal adaptation to stress. Nat Neurosci. 2003;6:1100–7. doi: 10.1038/nn1123. [DOI] [PubMed] [Google Scholar]
- 42.Keck ME, Ohl F, Holsboer F, Muller MB. Listening to mutant mice: a spotlight on the role of CRF/CRF receptor systems in affective disorders. Neurosci Biobehav Rev. 2005;29:867–89. doi: 10.1016/j.neubiorev.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Valdez GR, Inoue K, Koob GF, Rivier J, Vale W, Zorrilla EP. Human urocortin II: mild locomotor suppressive and delayed anxiolytic-like effects of a novel corticotropin-releasing factor related peptide. Brain Res. 2002;943:142–50. doi: 10.1016/s0006-8993(02)02707-5. [DOI] [PubMed] [Google Scholar]
- 44.Bailey JE, Papadopoulos A, Diaper A, Phillips S, Schmidt ME, van der Ark P, et al. Preliminary evidence of anxiolytic effects of the CRF1 receptor antagonist R317573 in the 7. 5% CO2 proof-of-concept experimental model of human anxiety. J Psychopharmacol. 2011;25:1199–206. doi: 10.1177/0269881111400650. [DOI] [PubMed] [Google Scholar]
- 45.Sakanaka M, Shibasaki T, Lederis K. Corticotropin releasing factor-like immunoreactivity in the rat brain as revealed by a modified cobalt-glucose oxidase-diaminobenzidine method. J Comp Neurol. 1987;260:256–98. doi: 10.1002/cne.902600209. [DOI] [PubMed] [Google Scholar]
- 46.Dunn AJ, Berridge CW. Physiological and behavioral responses to corticotropin-releasing factor administration: is CRF a mediator of anxiety or stress responses? Brain Res Brain Res Rev. 1990;15:71–100. doi: 10.1016/0165-0173(90)90012-d. [DOI] [PubMed] [Google Scholar]
- 47.Liebsch G, Landgraf R, Gerstberger R, Probst JC, Wotjak CT, Engelmann M, et al. Chronic infusion of a CRH1 receptor antisense oligodeoxynucleotide into the central nucleus of the amygdala reduced anxiety-related behavior in socially defeated rats. Regul Pept. 1995;59:229–39. doi: 10.1016/0167-0115(95)00099-w. [DOI] [PubMed] [Google Scholar]
- 48.Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertil Steril. 1997;67:1024–30. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- 49.O’Connor KA, Brindle E, Shofer J, Trumble BC, Aranda JD, Rice K, et al. The effects of a long-term psychosocial stress on reproductive indicators in the baboon. Am J Phys Anthropol. 2011;145:629–38. doi: 10.1002/ajpa.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vasconcelos LA, Donaldson C, Sita LV, Casatti CA, Lotfi CF, Wang L, et al. Urocortin in the central nervous system of a primate (Cebus apella): sequencing, immunohistochemical, and hybridization histochemical characterization. J Comp Neurol. 2003;463:157–75. doi: 10.1002/cne.10742. [DOI] [PubMed] [Google Scholar]
- 51.Kozicz T. Neurons colocalizing urocortin and cocaine and amphetamine-regulated transcript immunoreactivities are induced by acute lipopolysaccharide stress in the Edinger-Westphal nucleus in the rat. Neuroscience. 2003;116:315–20. doi: 10.1016/s0306-4522(02)00772-8. [DOI] [PubMed] [Google Scholar]
- 52.Skelton KH, Owens MJ, Nemeroff CB. The neurobiology of urocortin. Regul Pept. 2000;93:85–92. doi: 10.1016/s0167-0115(00)00180-4. [DOI] [PubMed] [Google Scholar]
- 53.Clark MS, McDevitt RA, Hoplight BJ, Neumaier JF. Chronic low dose ovine corticotropin releasing factor or urocortin II into the rostral dorsal raphe alters exploratory behavior and serotonergic gene expression in specific subregions of the dorsal raphe. Neuroscience. 2007;146:1888–905. doi: 10.1016/j.neuroscience.2007.03.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hammack SE, Pepin JL, DesMarteau JS, Watkins LR, Maier SF. Low doses of corticotropin-releasing hormone injected into the dorsal raphe nucleus block the behavioral consequences of uncontrollable stress. Behav Brain Res. 2003;147:55–64. doi: 10.1016/s0166-4328(03)00133-5. [DOI] [PubMed] [Google Scholar]
- 55.Hammack SE, Schmid MJ, LoPresti ML, Der-Avakian A, Pellymounter MA, Foster AC, et al. Corticotropin releasing hormone type 2 receptors in the dorsal raphe nucleus mediate the behavioral consequences of uncontrollable stress. J Neurosci. 2003;23:1019–25. doi: 10.1523/JNEUROSCI.23-03-01019.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pernar L, Curtis AL, Vale WW, Rivier JE, Valentino RJ. Selective activation of corticotropin-releasing factor-2 receptors on neurochemically identified neurons in the rat dorsal raphe nucleus reveals dual actions. J Neurosci. 2004;24:1305–11. doi: 10.1523/JNEUROSCI.2885-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Denihan A, Kirby M, Bruce I, Cunningham C, Coakley D, Lawlor BA. Three-year prognosis of depression in the community-dwelling elderly. Brit J Psychiatry. 2000;176:453–7. doi: 10.1192/bjp.176.5.453. [DOI] [PubMed] [Google Scholar]
- 58.Amat J, Tamblyn JP, Paul ED, Bland ST, Amat P, Foster AC, et al. Microinjection of urocortin 2 into the dorsal raphe nucleus activates serotonergic neurons and increases extracellular serotonin in the basolateral amygdala. Neuroscience. 2004;129:509–19. doi: 10.1016/j.neuroscience.2004.07.052. [DOI] [PubMed] [Google Scholar]
- 59.Lukkes JL, Forster GL, Renner KJ, Summers CH. Corticotropin-releasing factor 1 and 2 receptors in the dorsal raphe differentially affect serotonin release in the nucleus accumbens. Eur J Pharmacol. 2008;578:185–93. doi: 10.1016/j.ejphar.2007.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Brundu B, Loucks TL, Adler LJ, Cameron JL, Berga SL. Increased cortisol in the cerebrospinal fluid of women with functional hypothalamic amenorrhea. J Clin Endocrinol Metab. 2006;91:1561–5. doi: 10.1210/jc.2005-2422. [DOI] [PubMed] [Google Scholar]
- 61.Lindahl MS, Olovsson M, Nyberg S, Thorsen K, Olsson T, Sundstrom Poromaa I. Increased cortisol responsivity to adrenocorticotropic hormone and low plasma levels of interleukin-1 receptor antagonist in women with functional hypothalamic amenorrhea. Fertil Steril. 2007;87:136–42. doi: 10.1016/j.fertnstert.2006.06.029. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The location of the CRF axon plexus in the dorsal raphe nucleus that was subjected to image analysis and measurement of bouton number and bouton positive pixel area is shown below. Not shown is the pontine region, which is below the region in the photomicrograph and is located in the dorsal midbrain.
A. An image of the rostral raphe obtained with the Marianas Workstation and Slidebook 5.0. This image was transferred to Image J and cropped to the boxed area, which was then enlarged to the left. An SS animal is shown at the same magnification below the boxed area of the HSR animal.
B. Subregion of the HSR boxed area as observed in Image J.
C. Subregion of the same area from an SS animal for comparison to the HSR animal in ‘B’.
CC- central canal PAG-periaquaductal gray area