Background: Endothelial glycocalyx degradation contributes to the pathogenesis of critical illness.
Results: Mechanically ventilated subjects exhibited plasma glycocalyx breakdown signatures (glycosaminoglycan fragments) characteristic of direct versus indirect etiologies of respiratory failure.
Conclusion: Circulating glycosaminoglycans provide insight into respiratory failure pathophysiology.
Significance: This is the first study to characterize circulating glycosaminoglycans during critical illness, offering insight into the mechanisms underlying respiratory failure.
Keywords: Chondroitin Sulfate, Glycobiology, Heparan Sulfate, Hyaluronate, Lung Injury, Sepsis, Endothelial Glycocalyx, Respiratory Failure
Abstract
Systemic inflammatory illnesses (such as sepsis) are marked by degradation of the endothelial glycocalyx, a layer of glycosaminoglycans (including heparan sulfate, chondroitin sulfate, and hyaluronic acid) lining the vascular lumen. We hypothesized that different pathophysiologic insults would produce characteristic patterns of released glycocalyx fragments. We collected plasma from healthy donors as well as from subjects with respiratory failure due to altered mental status (intoxication, ischemic brain injury), indirect lung injury (non-pulmonary sepsis, pancreatitis), or direct lung injury (aspiration, pneumonia). Mass spectrometry was employed to determine the quantity and sulfation patterns of circulating glycosaminoglycans. We found that circulating heparan sulfate fragments were significantly (23-fold) elevated in patients with indirect lung injury, while circulating hyaluronic acid concentrations were elevated (32-fold) in patients with direct lung injury. N-Sulfation and tri-sulfation of heparan disaccharides were significantly increased in patients with indirect lung injury. Chondroitin disaccharide sulfation was suppressed in all groups with respiratory failure. Plasma heparan sulfate concentrations directly correlated with intensive care unit length of stay. Serial plasma measurements performed in select patients revealed that circulating highly sulfated heparan fragments persisted for greater than 3 days after the onset of respiratory failure. Our findings demonstrate that circulating glycosaminoglycans are elevated in patterns characteristic of the etiology of respiratory failure and may serve as diagnostic and/or prognostic biomarkers of critical illness.
Introduction
The endothelial glycocalyx is a layer of proteoglycans and associated glycosaminoglycans (including heparan sulfate (HS),2 hyaluronic acid (HA), and chondroitin sulfate (CS)) that lines the vascular lumen (1). In vivo, the glycocalyx forms a thick endothelial surface layer (ESL) that contributes to the regulation of endothelial permeability, leukocyte adhesion, and nitric oxide production (2–4). Accordingly, degradation of the ESL has been implicated in the pathogenesis of critical illnesses (e.g. sepsis, major trauma) characterized by vascular hyperpermeability, inflammation, and aberrant vascular tone (5, 6). Increasing attention has therefore been dedicated to understanding the fate of glycocalyx/ESL integrity during the course of critical illness. Glycocalyx protection is increasingly a goal of resuscitation strategies in intensive care unit (ICU) patients (7).
To date, human studies of glycocalyx/ESL degradation during critical illness have primarily relied upon either intravital microscopy of vascular beds of uncertain clinical relevance (e.g. the sublingual microcirculation (8, 9)) or the detection of circulating glycocalyx fragments by immunoassay (6, 9–13). These techniques offer little insight into the mechanisms underlying ESL loss across different vascular beds during critical illness. As glycosaminoglycan composition varies across tissues (14), different pathophysiologic insults may impart unique circulating glycosaminoglycan signatures during critical illness. Furthermore, different mechanisms of glycocalyx degradation (i.e. enzymatic cleavage versus nonspecific destruction (2, 15)) could impart characteristic glycosaminoglycan fragment patterns. Novel mass spectrometry techniques offer both precise quantification and characterization of circulating glycosaminoglycans (16), allowing for the detection of signatures of ESL degradation that may have relevance as biomarkers of critical illness.
Specific structural characteristics of circulating glycosaminoglycans may additionally indicate a functional importance of glycocalyx/ESL fragments. Highly sulfated HS motifs (spanning five to eight saccharides in length) can exert biologic activity by binding to antithrombin (imparting a heparin-like effect) or promoting growth factor-receptor interactions (17, 18). These signaling cascades may significantly impact the progression of and/or recovery from critical illness. Furthermore, fragmented HA can function as a damage-associated molecular pattern inducing tissue inflammation and injury (19).
We hypothesized that the circulating glycosaminoglycan signatures of adult, mechanically ventilated patients would vary according to the etiology of respiratory failure. We collected plasma from healthy donors as well as three groups of patients with respiratory failure: those mechanically ventilated for altered mental status (e.g. ischemic brain injury, intoxication), those with indirect injury to the lung (e.g. nonpulmonary sepsis, pancreatitis), and those with direct lung injury (e.g. pneumonia, aspiration). We now demonstrate that the quantity of circulating HS and HA as well as the sulfation patterns of HS vary according to inciting pulmonary insult. Circulating HS was additionally correlated with ICU length of stay, a measure of patient outcome. These findings suggest that circulating glycosaminoglycan fragments may serve as both diagnostic and prognostic biomarkers in critical illnesses such as acute respiratory failure.
EXPERIMENTAL PROCEDURES
Patient Enrollment
From June 2010 to March 2011, as part of a study of ventilator-associated pneumonia (ClinicalTrials.gov, NCT00938002), we collected plasma from mechanically ventilated adult subjects admitted to the Denver Health Medical Center (Denver, CO) Medical Intensive Care Unit. Inclusion criteria included mechanical ventilation for <72 h, with an expectation for at least 48 h of additional ventilatory support. Exclusion criteria included therapeutic anticoagulation, bronchiectasis/cystic fibrosis, pregnancy, participation in another clinical trial, or a moribund state (expectation of <14 day survival). We obtained written and informed consent from proxy decision makers; after subjects regained decision-making capacity, they were informed about their participation and reconsented according to Denver Health Medical Center policies. We collected plasma immediately after consent was obtained (day 0) using EDTA-lined tubes. Persistently ventilated subjects had an additional blood sample drawn 3 days later (day 3). The etiology of respiratory failure was defined as the primary insult leading to the need for mechanical ventilation, as identified by the clinical care team. We measured severity of illness by APACHE II (Acute Physiology and Chronic Health Evaluation II), renal function by serum creatinine and blood urea nitrogen, and hepatic function by total bilirubin and INR (international normalized ratio, a marker of liver synthetic function). We determined patient outcomes by measuring ICU length of stay and number of ventilator-free days (i.e. days alive and without mechanical ventilation) in the first 28 days after enrollment. C. Silliman (Bonfils Blood Bank, Denver, CO) generously provided plasma from healthy donors. The Colorado Multiple Institutions Review Board approved all human protocols.
Glycosaminoglycan Isolation and Purification
Isolation of glycosaminoglycans from media samples has been described previously (20, 21). The plasma samples were lyophilized and defatted and then individually subjected to proteolysis at 55 °C with 10% (w/v) of actinase E (20 mg/ml in HPLC grade water, Kaken Biochemicals, Tokyo, Japan) for 20 h. After proteolysis, particulates were removed from the resulting solutions by passing each through a 0.22-μm membrane syringe filter. Samples were then concentrated using Microcon YM-3 [YM-3] centrifugal filter units (3 kDa molecular mass cut-off, Millipore) by centrifugation at 12,000 × g and washed with 15 ml of distilled water to remove peptides. The retentate was collected and lyophilized and then purified or fractionated. Samples were dissolved in 0.5 ml of 8 m urea containing 2% CHAPS (pH 8.3). A Vivapure Mini Q H spin column (Viva Science, Edgewood, NJ) was prepared by equilibrating with 200 μl of 8 m urea containing 2% CHAPS (pH 8.3). To remove any remaining proteins, the clarified, filtered samples were loaded onto and run through the equilibrated Vivapure Mini Q H spin columns under centrifugal force (700 × g). The columns were then washed with 200 μl of 8 m urea containing 2% CHAPS at pH 8.3, followed by five washes with 200 μl of 200 mm NaCl. Total glycosaminoglycans were released from the spin column by washing three-times with 200 μl of 2.0 m NaCl. The glycosaminoglycans were collected, desalted using YM-3 spin columns, and finally lyophilized.
Enzymatic Digestion of Glycosaminoglycans
The recovered glycosaminoglycans (per 1 ml of plasma) were next completely depolymerized using polysaccharides lyases. Chondroitin lyase ABC (15 m-units) and chondroitin lyase ACII (6-m units) in 10 μl of 0.1% BSA were added to the glycosaminoglycan sample in 75 μl of 50 mm Tris solution containing 60 mm sodium acetate at pH 8.0, and incubated at 37 °C for 10 h. The enzymatic products were recovered by centrifugal filtration at 12,000 × g. CS/dermatan sulfate (a CS stereoisomer) disaccharides that passed through the filter were freeze-dried for LC-MS analysis. Glycosaminoglycans remaining in the retentate were collected by reversing the filter and spinning at 12,000 × g, followed by either purification by mini Q H spin column (for PAGE analysis) or incubation with 10-m units of heparin lyase I, II, and III at 35 °C for 10 h. The products were recovered by centrifugal filtration using a YM-3 spin column, and the disaccharides were collected in the flow-through and freeze-dried. Cloning, overexpression in Escherichia coli, and purification of the recombinant heparin lyase I (EC 4.2.2.7), heparin lyase II (no EC assigned), and heparin lyase III (EC 4.2.2.8) from Flavobacterium heparinum were all performed as described previously (22, 23).
Derivatization of Unsaturated Disaccharides with 2-Aminoacridone (AMAC)
The freeze-dried biological sample containing glycosaminoglycan-derived disaccharides (∼5 μg) or a mixture of eight HS disaccharide standards or one HA and eight CS/dermatan sulfate disaccharide standards (5 μg per each disaccharide) was added to 10 μl of 0.1 m AMAC solution in acetic acid/dimethyl sulfoxide (3:17, v/v) and mixed by vortexing for 5 min. Next, 10 μl of 1 m NaBH3CN was added to the reaction mixture and incubated at 45 °C for 4 h (24). Finally, the AMAC-tagged disaccharide mixtures were diluted to various concentrations (0.5–100 ng) using 50% (v/v) aqueous dimethyl sulfoxide, and LC-MS analysis was performed.
LC-MS Disaccharide Composition Analysis of CS, HS, and HA
LC-MS analyses of CS, HS, and HA disaccharides were performed on an Agilent 1200 LC/MSD instrument (Agilent Technologies, Inc. Wilmington, DE) equipped with a 6300 ion-trap and a binary pump, as described previously (16). Briefly, the column used was a Poroshell 120 C18 column (3.0 × 150 mm, 2.7 μm, Agilent) at 45 °C. Eluent A was 80 mm ammonium acetate solution, and eluent B was methanol. Solution A and 15% solution B were flowed (150 μl/min) through the column for 5 min followed by linear gradients from 15–30% solution B from 5 to 30 min. The column effluent entered the ESI-MS source for continuous detection by MS. The electrospray interface was set in negative ionization mode with a skimmer potential of −40.0 V, a capillary exit of −40.0 V, and a source temperature of 350 °C, to obtain the maximum abundance of the ions in a full-scan spectrum (150–1200 Da). Nitrogen (8 liters/min, 40 psi) was used as a drying and nebulizing gas (16).
Quantitative analysis of AMAC-labeled disaccharides was performed using calibration curves constructed by separation of increasing amounts of unsaturated disaccharide standards (0.1, 0.5, 1, 5, 10, 20, 50, and 100 ng/each). Linearity was assessed based on amount of disaccharide and peak intensity in extracted ion chromatography.
Measurement of HS Degradation Activity
HS degradation activity was measured in plasma according to the manufacturer's instructions (Genway Biotech, San Diego, CA), as described previously (2).
Assessment of Circulating Glycan Size
Purified HS fragments isolated from plasma samples were analyzed by native PAGE using 0.75 mm × 6.8 cm × 8.6 cm mini gels cast from 22% T resolving gel monomer solution and 5% T stacking gel monomer solution. Bovine lung heparin partially digested by heparinase 1, 2, and 3 was used as molecular markers. The 22% mini gels were subjected to electrophoresis at a constant 220 V for 100 min, the 15% mini gels were subjected to electrophoresis at a constant and 150 V for 40 min, and then visualized with 0.5% (w/v) Alcian blue in 2% (v/v) aqueous acetic acid solution. Molecular weight analysis was performed with the aid of UNSCANIT software (Silk Scientific, Orem, UT) using the logarithmic relationship between the glycosaminoglycan molecular weight and its migration distance.
Statistical Analysis
Data are represented as scatter plots with superimposed means ± S.E. We performed multiple comparisons by analysis of variance (parametric data) or Kruskal-Wallis testing with Dunn's post hoc testing (for nonparametric data). We compared glycosaminoglycan concentrations to clinical outcomes by two-tailed Spearman (nonparametric) correlation. Comparisons of day 0 and day 3 samples were performed within individual subjects using a Wilcoxon matched-pairs signed rank test. Differences were statistically significant if p < 0.05. We performed all calculations using Prism (GraphPad, San Diego, CA).
RESULTS
Between June 2010 and March 2011, we collected plasma from 17 patients with respiratory failure (Table 1). The etiologies of respiratory failure fell into one of three general categories: respiratory failure due to altered mental status (i.e. patients endotracheally intubated for hypoventilation and/or airway protection), respiratory failure due to indirect lung injury (i.e. nonpulmonary illnesses that induce pulmonary dysfunction such as sepsis and pancreatitis), and respiratory failure due to direct lung injury (e.g. pneumonia or witnessed gastric aspiration). Four normal samples were collected from volunteer, de-identified donors. Although there was a trend toward older age in subjects with altered mental status-associated respiratory failure, there were no statistically significant differences between groups with regards to age, time from intubation to blood draw, indices of renal/hepatic function, or APACHE II score. Four subjects (nos. 5, 7, 18, and 21) died during their ICU stay.
TABLE 1.
Clinical characteristics of subjects providing plasma for glycosaminoglycan analysis
Only gender data are available for normal plasma donors. All information was determined at the time of initial plasma collection for glycosaminoglycan analysis (day 0). Analyses of variance were performed to determine baseline differences between groups. INR, international normalized ratio.
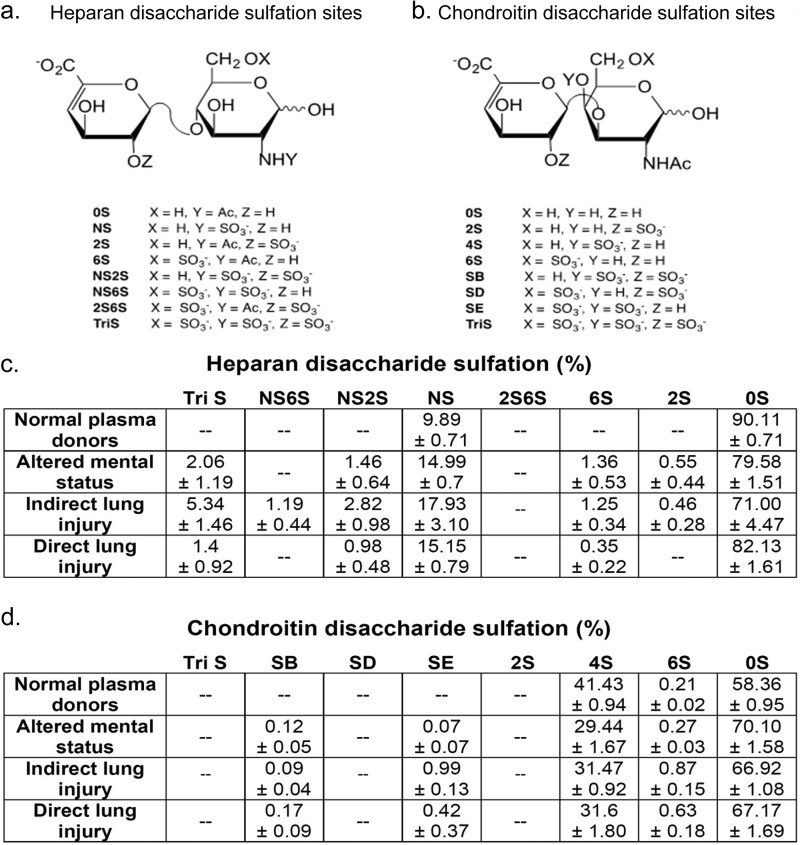
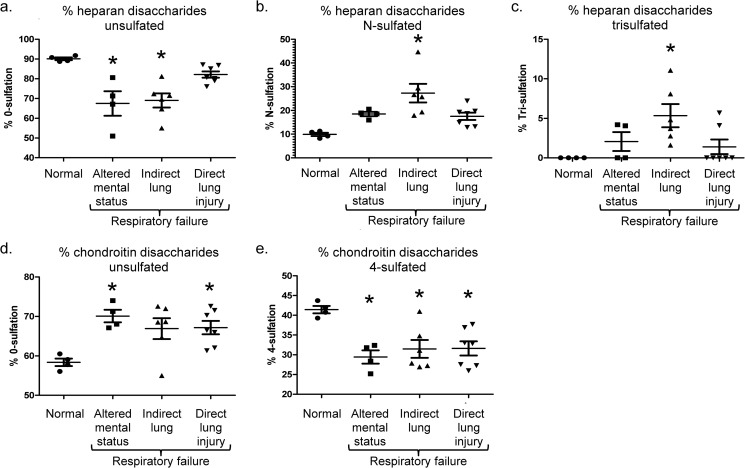
LC-MS analyses (including extracted ion chromatography, Fig. 1) revealed that circulating glycosaminoglycan concentrations varied according to the etiology of respiratory failure. Plasma HS levels were significantly elevated (in comparison to normal donors) in indirect lung injury (Fig. 2a), whereas elevated HA levels reached statistical significance in patients with direct lung injury (Fig. 2b). This statistically significant difference persisted even after exclusion of a high-HS concentration outlier (subject no. 9). Chondroitin sulfate concentrations did not vary between groups (Fig. 2c). Sulfation analyses of glycosaminoglycan disaccharides revealed patterns reflective of the etiology of respiratory failure (Fig. 3). In contrast to the largely unsulfated HS disaccharides in normal plasma, heparan sulfation increased in patients with altered mental status- or indirect lung injury-induced respiratory failure (Fig. 4a). Patients with indirect lung injury had increased levels of N-sulfated (Fig. 4b) and tri-sulfated (Fig. 4c) disaccharides. As above, these statistically significant differences persisted even when excluding a high-sulfation outlier (subject no. 9). Chondroitin sulfation was suppressed in all patients with respiratory failure (Fig. 4, d and e). Given its unsulfated structure, HA sulfation analyses could not be performed. Hematologic indices (e.g. white blood cell or platelet counts) had no association with quantity or sulfation of circulating glycosaminoglycan.
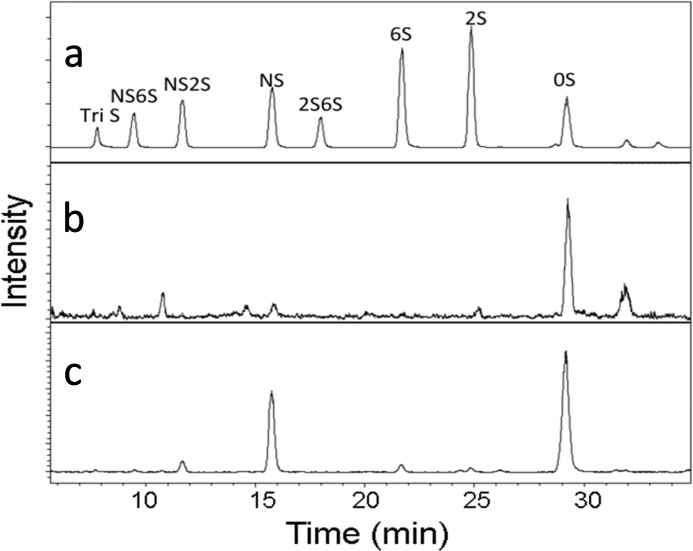
FIGURE 1.
Extracted ion chromatography (EIC) of AMAC-tagged disaccharide analysis of HS fragments isolated from human plasma. a, disaccharide standards; b, HS disaccharides from normal human plasma; c, HS disaccharides from the plasma of a patient (no. 14) with severe acute pancreatitis.
FIGURE 2.
Plasma glycosaminoglycan concentrations in normal donors or patients with respiratory failure due to altered mental status, indirect pulmonary injury, or direct lung injury. *, p < 0.05 compared with normal donors.
FIGURE 3.
Heparan and chondroitin sulfate disaccharide compositional analyses. Heparan sulfate (a) and chondroitin sulfate (b) disaccharides may be modified at several sulfation sites. c and d, after isolation from human plasma, glycosaminoglycans are fragmented into constituent disaccharides, allowing for determination of sulfation patterns across patient groups. Data are reported as percentage of detected disaccharides (means ± S.E.).
FIGURE 4.
Heparan and chondroitin sulfate disaccharide compositional analyses in normal donors or patients with respiratory failure due to altered mental status, indirect pulmonary injury, or direct lung injury. *, p < 0.05 compared with normal donors.
The increased concentrations of highly sulfated HS fragments detected in subjects with indirect lung injury are suggestive of increased activation of heparanase, a glucuronidase that (when acting upon the extracellular matrix/glycocalyx) releases highly sulfated HS fragments (25–27). We have previously demonstrated that patients with nonpulmonary sepsis (subject nos. 11–14) had increased plasma heparanase activity (1.72-fold increase in HS degradation activity as compared with normal donors (2)). We expanded upon these findings by measuring plasma HS degradation activity in patients with pancreatitis (subject nos. 9 and 10, not reported previously). These patients similarly demonstrated a 2.38- and 1.14-fold respective increase in HS degradation activity as compared with normal donors. When reconciled with our previously reported data, these findings indicate that patients with indirect lung injury have increased systemic heparanase activity, leading to elaboration of circulating HS fragments.
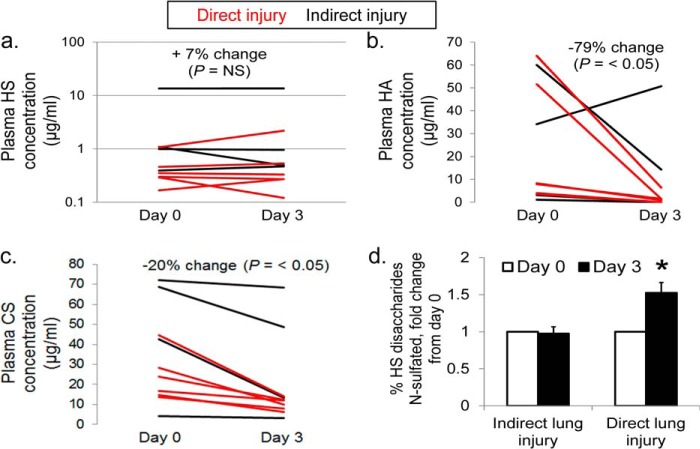
Ten patients (four with indirect lung injury (nos. 9, 11–13) and six with direct injury (nos. 16–21)) had a second plasma sample collected 72 h after study enrollment. These samples demonstrate persistent plasma concentrations of circulating HS fragments (Fig. 5a) in both patient groups, contrasted by a decline in circulating HA and CS (Fig. 5, b and c). Given that study enrollment (and day 0 sample collection) could occur up to 72 h after respiratory failure (Table 1), these findings indicate a striking prolongation of elevated plasma HS during indirect lung injury-induced respiratory failure. Interestingly, day 3 plasma collected from patients with direct lung injury demonstrated a 1.5-fold increase in HS disaccharide N-sulfation (Fig. 5d), approximating the ∼30% N-sulfation observed at both time points in patients with indirect lung injury (Fig. 4b). No differences in CS sulfation were observed between day 0 and 3 samples.
FIGURE 5.
Serial analyses of plasma HS (a), HA (b), and CS (c) concentrations at days 0 and 3. Lines represent serial plasma samples collected from the same subject. Red, direct lung injury; black, indirect lung injury. d, % N-sulfation of HS disaccharides from direct and indirect lung injury patients, normalized to day 0 value from the same patient. *, p < 0.05 compared with matched day 0 samples.
As biologic activity of heparan oligosaccharides is dependent not only on the degree of sulfation but also oligosaccharide size (17, 18), we performed PAGE analyses of HS polysaccharide fragments isolated from plasma to estimate circulating glycosaminoglycan fragment mass (Fig. 6). All groups of patients displayed high molecular weight bands. Interestingly, a smaller weight band was noted in HS fragments from plasma pooled from sepsis patients (patient nos. 11–14). The average molecular mass of this small fraction was 1246.8 Da, as derived by extrapolating the linear standard curve obtained by using the available heparin standards log (molecular weight) as a function of migration time (y = −0.0011x + 4.4367, r2 = 0.9984). This weight corresponds roughly to an octasaccharide, which is the minimal HS size necessary for activation of fibroblast growth factor signaling. Due to limited sample availability, we were unable to further resolve the structure of this putative octasaccharide.
FIGURE 6.
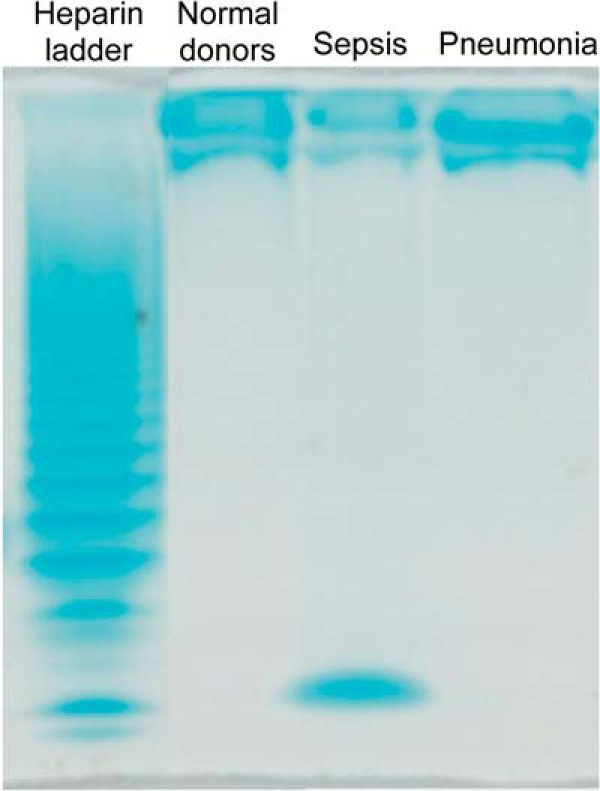
Alcian blue-stained polyacrylamide gel electrophoresis of HS polysaccharide fragments isolated from pooled normal plasma as well as plasma pooled from patients with non-pulmonary sepsis or pneumonia. Bovine lung heparin digested by a mixture of heparinase I, II, and III serves as a molecular marker.
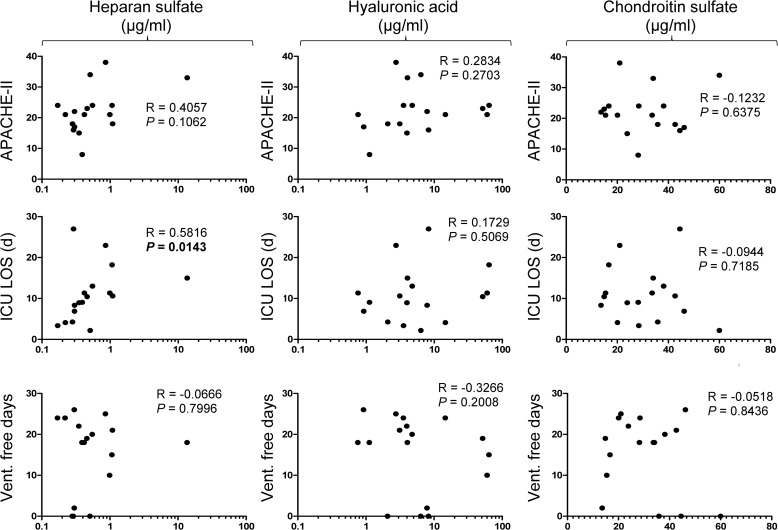
We sought to determine whether the circulating glycosaminoglycan signature was associated with clinical outcomes. Glycosaminoglycan levels (HS, HA, CS) did not correlate with severity of illness (APACHE II) at the time of plasma collection (Fig. 7). Total HS plasma concentrations correlated with ICU length of stay, suggesting that HS fragmentation may have prognostic significance in critical illness. HS or CS sulfation patterns did not correlate with severity of illness or measures of patient outcomes (data not shown).
FIGURE 7.
Two-tailed Spearman correlation analyses of plasma glycosaminoglycan concentrations (columns) with indices of severity of illness (APACHE II, first row) or ICU outcomes (length of stay, LOS; ventilator (vent.)-free days).
DISCUSSION
Endothelial glycocalyx degradation has been increasingly recognized as a major contributor to the pathophysiology of critical illness (5–7). Detection of glycocalyx breakdown products may consequently have value as diagnostic and/or prognostic ICU biomarkers. Although previous studies have detected glycosaminoglycan fragments in the blood of critically ill patients, our work is the first to define the precise structural characteristics of these fragments. By performing high-sensitivity mass spectrometry analyses of plasma collected from mechanically ventilated patients, we now identify that circulating glycosaminoglycan patterns vary according to the insult leading to acute respiratory failure. This precise characterization of circulating fragments allows for insight into the pathogenesis of respiratory failure and may improve precision as a biomarker of critical illness.
A major challenge in identifying glycosaminoglycan signatures in human subjects is the relatively low level of plasma glycosaminoglycans, especially HS, and the high level of interfering substances in such samples, including salts, metabolites, lipids, and proteins. We have developed a highly specific analytical methodology for the analysis of glycosaminoglycan-derived disaccharides that is based on specific polysaccharide lyase digestion, AMAC-labeling of the disaccharides at their hemiacetal reducing ends, and LC-MS detection (16). This results in improved signal to noise over previous methods (28) and affords a detection limit as low as 100 pg of disaccharide in a plasma sample. The high-resolution chromatographic separation of all the labeled disaccharides, derived from CS, HS, and HA, is essential for their analysis in biological samples, as this avoids interfering substances, which can potentially cause false positive results.
Using these analyses, patients with respiratory failure arising from an indirect pulmonary insult (i.e. indirect lung injury) had increased plasma concentrations of circulating (Fig. 2a), highly sulfated (Fig. 4, a–c) heparan sulfate fragments. HS fragmentation in these patients may occur via the action of endothelial heparanase, an HS-specific glucuronidase that we have previously implicated in the pathogenesis of sepsis-induced acute lung injury (2). Indeed, animals with endogenous or transgenic heparanase overexpression are characterized by the accumulation of highly sulfated HS oligosaccharides (25, 26), reflecting the preferential release of these fragments by heparanase action upon the extracellular matrix (27). The involvement of heparanase is further supported by increased plasma HS degradation activity of patients with indirect lung injury, as demonstrated previously in sepsis (2) and (in this study) in patients with severe acute pancreatitis. Conversely, patients with direct lung injury or altered mental status-induced respiratory failure had a relative absence of highly sulfated circulating HS fragments (Fig. 4, b and c), consistent with the low plasma HS degradation activity previously observed in these groups (2).
As plasma HS fragment concentrations have been employed as a noninvasive measure of glycocalyx integrity (10), our findings suggest quantitative differences in glycocalyx degradation between direct and indirect mechanisms of respiratory failure. However, we are unable to exclude additional non-glycocalyx sources of HS fragmentation. Endothelial heparanase could act upon basement membrane HS (29), potentially contributing to the elaboration of circulating fragments. Furthermore, heparanase from other cell types (e.g. mast cells) could contribute to septic HS degradation, particularly given the known contribution of mast cells to sepsis outcomes (30). Despite these limitations, however, our findings remain consistent with the involvement of systemic heparanase activation in the pathogenesis of indirect lung injury-associated respiratory failure.
Interestingly, the release of highly sulfated HS fragments in subjects with indirect lung injury may have biologic consequence. Highly sulfated fragments of sufficient length (pentasaccharides or larger) can bind and activate ligands such as antithrombin or fibroblast growth factors (17, 18). The presence and physiologic impact of activation of these pathways by ESL fragments are unexplored. Using polyacrylamide gel electrophoresis (and Alcian blue staining), we were able to identify large glycan fragments in the blood of patients with respiratory failure. In sepsis, an octasaccharide fragment was identified; due to the paucity of biological material available, we were unable to resolve structural characteristics (including sulfation) of this fragment. Further mechanistic animal and human studies will be required to further define the size and biologic impact of HS shed from a degraded ESL during critical illness.
In contrast to indirect lung injury, patients with direct lung injury had statistically significant elevations in circulating HA. Although our experimental approaches are unable to precisely determine HA fragment size, our findings are consistent with the known role of HA fragmentation in the pathophysiology of several animal models of direct lung injury (15). HA degradation in these models may arise from a nonspecific, oxidant-mediated fragmentation of extracellular matrix structures, not necessarily limited to the endothelial glycocalyx (31).
Elevations in circulating glycosaminoglycans persisted for several days after the onset of respiratory failure, as evident by the detection of signatures in plasma collected up to 71 h after the initiation of mechanical ventilation (Table 1). The persistence of elevated plasma HS concentrations (Fig. 5) in patients with respiratory failure may not only reflect ongoing glycocalyx degradation but also could indicate an impairment of glycosaminoglycan clearance. Although hepatic or renal dysfunction may delay clearance of circulating glycosaminoglycans (32–34), the relative homogeneity of indices of renal function (blood urea nitrogen, creatinine) and hepatic function (bilirubin, international normalized ratio) across groups (Table 1) suggests that the observed glycosaminoglycan signatures are not the primary consequence of aberrant liver or kidney clearance. Our data, however, cannot exclude subtle abnormalities in renal and/or hepatic function that are not captured by these common indices of organ function. Regardless, the prolonged course of glycosaminoglycan elevation supports its practical relevance as a diagnostic or prognostic biomarker of acute respiratory failure.
Our findings promote appreciation for the complexity of acute respiratory failure pathogenesis. Indirect and direct causes of lung injury are presumably based upon highly disparate pathophysiologic processes: indeed, lung injury caused by pancreatitis is intuitively different from injury caused by gastric acid inhalation (35). Despite these substantial differences, acute lung injury is typically treated as a homogenous disease state: the “acute respiratory distress syndrome.” The longstanding inability to identify an efficacious, pathophysiology-targeted treatment for acute respiratory distress syndrome may in part reflect this false homogenization of distinct pathophysiologic mechanisms that ultimately converge into the common final outcome of acute respiratory distress syndrome. Our work provides evidence that indirect and direct forms of lung injury are mechanistically distinct and identifies a potential biomarker to stratify future clinical investigations of this highly morbid critical illness.
Although our study is based upon a small cohort of patients, our novel results strongly support additional, large glycomic studies of critical illness. Larger cohorts and evolving glycomic techniques may allow additional insight into the diagnostic and prognostic importance of circulating glycosaminoglycans in severe diseases such as acute respiratory failure.
This work was supported by National Institutes of Health/NHLBI Grant K08 HL105538 and Colorado Clinical and Translational Sciences Institute Grants UL1 RR025780 (to E. P. S.) and GM38060 (to R. J. L.).
- HS
- heparan sulfate
- HA
- hyaluronic acid
- CS
- chondroitin sulfate
- ESL
- endothelial surface layer
- ICU
- intensive care unit
- APACHE II
- acute physiology and chronic health evaluation II
- AMAC
- 2-aminoacridone
- COPD
- chronic obstructive pulmonary disease.
REFERENCES
- 1. Weinbaum S., Tarbell J. M., Damiano E. R. (2007) The Structure and Function of the Endothelial Glycocalyx Layer. Annu. Rev. Biomed. Eng. 9, 121–167 [DOI] [PubMed] [Google Scholar]
- 2. Schmidt E. P., Yang Y., Janssen W. J., Gandjeva A., Perez M. J., Barthel L., Zemans R. L., Bowman J. C., Koyanagi D. E., Yunt Z. X., Smith L. P., Cheng S. S., Overdier K. H., Thompson K. R., Geraci M. W., Douglas I. S., Pearse D. B., Tuder R. M. (2012) The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Curry F. E., Adamson R. H. (2012) Endothelial Glycocalyx: Permeability Barrier and Mechanosensor. Ann. Biomed. Eng. 40, 828–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Florian J. A., Kosky J. R., Ainslie K., Pang Z., Dull R. O., Tarbell J. M. (2003) Heparan sulfate proteoglycan is a mechanosensor on endothelial cells. Circ. Res. 93, e136–e142 [DOI] [PubMed] [Google Scholar]
- 5. Burke-Gaffney A., Evans T. (2012) Lest we forget the endothelial glycocalyx in sepsis. Crit. Care 16, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Johansson P. I., Stensballe J., Rasmussen L. S., Ostrowski S. R. (2011) A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 254, 194–200 [DOI] [PubMed] [Google Scholar]
- 7. Myburgh J. A., Mythen M. G. (2013) Resuscitation Fluids. N. Engl. J. Med. 369, 1243–1251 [DOI] [PubMed] [Google Scholar]
- 8. Nieuwdorp M., Meuwese M. C., Mooij H. L., van Lieshout M. H., Hayden A., Levi M., Meijers J. C., Ince C., Kastelein J. J., Vink H., Stroes E. S. (2009) Tumor necrosis factor-α inhibition protects against endotoxin-induced endothelial glycocalyx perturbation. Atherosclerosis 202, 296–303 [DOI] [PubMed] [Google Scholar]
- 9. Donati A., Damiani E., Domizi R., Romano R., Adrario E., Pelaia P., Ince C., Singer M. (2013). Alteration of the sublingual microvascular glycocalyx in critically ill patients. Microvasc. Res. 10.1016/j.mvr.2013.08.007 [DOI] [PubMed] [Google Scholar]
- 10. Steppan J., Hofer S., Funke B., Brenner T., Henrich M., Martin E., Weitz J., Hofmann U., Weigand M. A. (2011) Sepsis and Major Abdominal Surgery Lead to Flaking of the Endothelial Glycocalix. J. Surg. Res. 165, 136–141 [DOI] [PubMed] [Google Scholar]
- 11. Nelson A., Berkestedt I., Schmidtchen A., Ljunggren L., Bodelsson M. (2008) Increased levels of glycosaminoglycans during septic shock: relation to mortality and the antibacterial actions of plasma. Shock. 30, 623–627 [DOI] [PubMed] [Google Scholar]
- 12. Yagmur E., Koch A., Haumann M., Kramann R., Trautwein C., Tacke F. (2012) Hyaluronan serum concentrations are elevated in critically ill patients and associated with disease severity. Clin. Biochem. 45, 82–87 [DOI] [PubMed] [Google Scholar]
- 13. Sallisalmi M., Tenhunen J., Yang R., Oksala N., Pettilä V. (2012) Vascular adhesion protein-1 and syndecan-1 in septic shock. Acta Anaesthesiol Scand 56, 316–322 [DOI] [PubMed] [Google Scholar]
- 14. Ledin J., Staatz W., Li J. P., Götte M., Selleck S., Kjellén L., Spillmann D. (2004) Heparan Sulfate Structure in Mice with Genetically Modified Heparan Sulfate Production. J. Biol. Chem. 279, 42732–42741 [DOI] [PubMed] [Google Scholar]
- 15. Jiang D., Liang J., Noble P. W. (2011) Hyaluronan as an Immune Regulator in Human Diseases. Physiol. Rev. 91, 221–264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Yang B., Chang Y., Weyers A. M., Sterner E., Linhardt R. J. (2012) Disaccharide analysis of glycosaminoglycan mixtures by ultra-high-performance liquid chromatography/mass spectrometry. J Chromatogr A 1225, 91–98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esko J. D., Lindahl U. (2001) Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Goetz R., Mohammadi M. (2013) Exploring mechanisms of FGF signalling through the lens of structural biology. Nat. Rev. Mol. Cell Biol. 14, 166–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jiang D., Liang J., Fan J., Yu S., Chen S., Luo Y., Prestwich G. D., Mascarenhas M. M., Garg H. G., Quinn D. A., Homer R. J., Goldstein D. R., Bucala R., Lee P. J., Medzhitov R., Noble P. W. (2005) Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med 11, 1173–1179 [DOI] [PubMed] [Google Scholar]
- 20. Yang B., Weyers A., Baik J. Y., Sterner E., Sharfstein S., Mousa S. A., Zhang F., Dordick J. S., Linhardt R. J. (2011) Ultra-performance ion-pairing liquid chromatography with on-line electrospray ion trap mass spectrometry for heparin disaccharide analysis. Anal. Biochem. 415, 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Zhang F., Sun P., Muñoz E., Chi L., Sakai S., Toida T., Zhang H., Mousa S., Linhardt R. J. (2006) Microscale isolation and analysis of heparin from plasma using an anion-exchange spin column. Anal. Biochem. 353, 284–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shaya D., Tocilj A., Li Y., Myette J., Venkataraman G., Sasisekharan R., Cygler M. (2006) Crystal Structure of Heparinase II from Pedobacter heparinus and Its Complex with a Disaccharide Product. J. Biol. Chem. 281, 15525–15535 [DOI] [PubMed] [Google Scholar]
- 23. Yoshida E., Arakawa S., Matsunaga T., Toriumi S., Tokuyama S., Morikawa K., Tahara Y. (2002) Cloning, sequencing, and expression of the gene from bacillus circulans that codes for a heparinase that degrades both heparin and heparan sulfate. Biosci Biotechnol. Biochem. 66, 1873–1879 [DOI] [PubMed] [Google Scholar]
- 24. Kitagawa H., Kinoshita A., Sugahara K. (1995) Microanalysis of Glycosaminoglycan-Derived Disaccharides Labeled with the Fluorophore 2-Aminoacridone by Capillary Electrophoresis and High-Performance Liquid Chromatography. Anal. Biochem. 232, 114–121 [DOI] [PubMed] [Google Scholar]
- 25. Escobar Galvis M. L., Jia J., Zhang X., Jastrebova N., Spillmann D., Gottfridsson E., van Kuppevelt T. H., Zcharia E., Vlodavsky I., Lindahl U., Li J. P. (2007) Transgenic or tumor-induced expression of heparanase upregulates sulfation of heparan sulfate. Nat Chem Biol 3, 773–778 [DOI] [PubMed] [Google Scholar]
- 26. Sandwall E., Bodevin S., Nasser N. J., Nevo E., Avivi A., Vlodavsky I., Li J. P. (2009) Molecular Structure of Heparan Sulfate from Spalax. J. Biol. Chem. 284, 3814–3822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peterson S. B., Liu J. (2013). Multi-faceted substrate specificity of heparanase. Matrix Biol. 32, 223–227 [DOI] [PubMed] [Google Scholar]
- 28. Wei W., Miller R. L., Leary J. A. (2013) Method development and analysis of free HS and HS in proteoglycans from pre- and postmenopausal women: evidence for biosynthetic pathway changes in sulfotransferase and sulfatase enzymes. Anal. Chem. 85, 5917–5923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parish C. R., Hindmarsh E. J., Bartlett M. R., Staykova M. A., Cowden W. B., Willenborg D. O. (1998) Treatment of central nervous system inflammation with inhibitors of basement membrane degradation. Immunol. Cell Biol. 76, 104–113 [DOI] [PubMed] [Google Scholar]
- 30. Seeley E. J., Matthay M. A., Wolters P. J. (2012) Inflection points in sepsis biology: from local defense to systemic organ injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 303, L355–L363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gao F., Koenitzer J. R., Tobolewski J. M., Jiang D., Liang J., Noble P. W., Oury T. D. (2008) Extracellular superoxide dismutase inhibits inflammation by preventing oxidative fragmentation of hyaluronan. J. Biol. Chem. 283, 6058–6066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Berg S. (1997) Hyaluronan turnover in relation to infection and sepsis. J. Int. Med. 242, 73–77 [DOI] [PubMed] [Google Scholar]
- 33. McKee R. F., Hodson S., Dawes J., Garden O. J., Carter D. C. (1992) Plasma concentrations of endogenous heparinoids in portal hypertension. Gut 33, 1549–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. de Ru M. H., van der Tol L., van Vlies N., Bigger B.W., Hollak C. E., Ijlst L., Kulik W., van Lenthe H., Saif M.A., Wagemans T., van der Wal W.M., Wanders R.J., Wijburg F.A. (2013) Plasma and urinary levels of dermatan sulfate and heparan sulfate derived disaccharides after long-term enzyme replacement therapy (ERT) in MPS I: correlation with the timing of ERT and with total urinary excretion of glycosaminoglycans. J. Inherit. Metab. Dis. 36, 247–255 [DOI] [PubMed] [Google Scholar]
- 35. Pelosi P., D'Onofrio D., Chiumello D., Paolo S., Chiara G., Capelozzi V. L., Barbas C. S., Chiaranda M., Gattinoni L. (2003) Pulmonary and extrapulmonary acute respiratory distress syndrome are different. Eur. Respir. J. Suppl. 42, 48s–56s [DOI] [PubMed] [Google Scholar]