Background: A non-catalytic vitamin B6 biosynthesis protein (PDX1.2) exists in plants, but its role is unknown.
Results: PDX1.2 interacts with its catalytic counterparts, enhancing their activity and forming a dodecameric complex induced under abiotic stress.
Conclusion: PDX1.2 is a pesudoenzyme that regulates vitamin B6 biosynthesis under abiotic stress.
Significance: This work presents a model for regulation of vitamin B6 biosynthesis and the existence/significance of pseudoenzymes in plants.
Keywords: Antioxidants, Biosynthesis, Enzyme Catalysis, Plant Biochemistry, Pyridoxal Phosphate, Stress Response
Abstract
Vitamin B6 is an indispensable compound for survival, well known as a cofactor for numerous central metabolic enzymes and more recently for playing a role in several stress responses, particularly in association with oxidative stress. Regulatory aspects for the use of the vitamin in these roles are not known. Here we show that certain plants carry a pseudoenzyme (PDX1.2), which is involved in regulating vitamin B6 biosynthesis de novo under stress conditions. Specifically, we demonstrate that Arabidopsis PDX1.2 enhances the activity of its catalytic paralogs by forming a heterododecameric complex. PDX1.2 is strongly induced by heat as well as singlet oxygen stress, concomitant with an enhancement of vitamin B6 production. Analysis of pdx1.2 knockdown lines demonstrates that boosting vitamin B6 content is dependent on PDX1.2, revealing that this pseudoenzyme acts as a positive regulator of vitamin B6 biosynthesis during such stress conditions in plants.
Introduction
All organisms require vitamin B6 because it is an essential cofactor in its form as pyridoxal 5′-phosphate (PLP)3 for more than 140 metabolic enzymes related primarily to amino acid as well as glucose and lipid metabolism. Whereas humans must take the vitamin in their diet, microorganisms and plants perform biosynthesis de novo of vitamin B6. Two exclusive biosynthesis pathways are now known to exist (1, 2). In most organisms, biosynthesis de novo involves only two enzymes, PDX1 and PDX2 (1, 3–5), whereas a seven-enzyme pathway is employed by Escherichia coli and a few other microorganisms (reviewed in Ref. 2). In bacteria, PDX1 and PDX2 form an ornate 24-subunit complex displaying glutamine amidotransferase activity for the direct production of PLP from three substrates, ribose 5-phosphate (R5P), glyceraldehyde 3-phosphate, and glutamine (6–9). PDX1 acts as the synthase with a remarkable polymorphic catalytic ability, culminating in the production of PLP, while PDX2 is the glutaminase that supplies ammonia for transfer to PDX1 (6, 7, 10, 11). In the course of unraveling the molecular details of this pathway, several investigations revealed a relationship between vitamin B6 and cellular stress responses (1, 4, 12–17). The demonstration in vitro that vitamin B6 is a potent antioxidant served to propagate an unrecognized function of this vitamin and its ability to quench reactive oxygen species, singlet oxygen in particular (1, 4, 18). It is noteworthy that several forms of the vitamin coexist, namely pyridoxine, pyridoxal, pyridoxamine, and their respective phosphorylated derivatives as well as glycosylated forms (19). Although PLP is the cofactor form of the vitamin, it is not known if the other forms that are present in relative abundance (20–22) and at levels equivalent to other antioxidants (e.g. glutathione) (20) perform independent functions related to the response to stress. Indeed, insight into the regulatory mechanisms behind the use of the vitamin as a cofactor or antioxidant is currently not available.
As a result of the genomic era, it is becoming increasingly apparent that several enzyme families can include inactive homologs (23). A characteristic of several such homologs is that it is precisely the residues known to be required for catalysis that are not conserved. Although many of these non-active or assumed dead enzymes have been overlooked, it is now emerging that these proteins may function as biological regulators. Notably, the corresponding genes are expressed, distinguishing them from pseudogenes, and they have instead become known as pseudoenzymes (24). Important examples from humans related to kinases, proteases, and ubiquitin-conjugating enzymes have been illustrated recently (25). In this context, it is intriguing that such a seemingly inactive homolog of PDX1 (annotated as PDX1.2 in Arabidopsis) is present among its catalytic counterparts in certain plant species. Moreover, this paralog is known to interact and form complexes with its catalytic paralogs (26, 27). However, the precise nature of these complexes and whether PDX1.2 has any function have remained elusive to date.
Although PDX1.2 homologs are highly conserved with their catalytic counterparts (13), we have noted that they form a distinct group that harbor the characteristics of pseudoenzymes. In this study, we report a detailed characterization of PDX1.2 from Arabidopsis and show that it forms a heterododecameric protein complex with its catalytically competent paralogs. Surprisingly, complexation with PDX1.2 substantially increases the rate of vitamin B6 biosynthesis. In vitro studies indicate that modification of a single alanine residue at the N terminus of PDX1.2 may play a role in the modulation of the catalytic activity. Furthermore, although the PDX1.2 transcript is present at very low levels (13) and not detectable at the protein level under normal growth conditions, it is strongly up-regulated upon heat stress, in particular, as well as singlet oxygen stress in Arabidopsis concomitant with an increase in vitamin B6 production. This feature is not observed in pdx1.2 knockdown lines, demonstrating that PDX1.2 is a positive regulator of vitamin B6 biosynthesis, most likely for its use as an antioxidant under such abiotic stress conditions, and thereby uncovering the presence of an intriguing class of pseudoenzymes in plants.
EXPERIMENTAL PROCEDURES
Expression and Purification of Recombinant Homomeric and Heteromeric Protein Complexes
The constructs pET-PDX1.1, pET-PDX1.2, and pET-PDX1.3 described previously (28) were used in this study. Site-directed mutagenesis to generate native versions of PDX1.1 and PDX1.3 by adding a stop codon at the end of each sequence was carried out using the QuikChange® XL site-directed mutagenesis kit (Stratagene). The respective forward and reverse oligonucleotides used for mutagenesis were as follows: 5′-GCTAGTCGTTCTGAGTAACTCGAGCACCACCAC-3′ and 5′-GTGGTGGTGCTCGAGTTA-CTCAGAACGACTAGC-3′ for PDX1.1; 5′-GCTAATCGCTCCGAGTGACTCGAGCACCACCACC-3′ and 5′-GGTGGTGGTGCTCGAG-TCACTCGGAGCGATTAGC-3′ for PDX1.3. The mutant PDX1.2 A2P was generated using the QuikChange® XL site-directed mutagenesis kit (Stratagene) using 5′-GAAGGAGATATACATATGCCGGATCAAGCTATGACG-3′ and 5′-CGTCATAGCTTGATCCGGCATATGTATATCTCCTTC-3′ as forward and reverse oligonucleotides, respectively, and with pET-PDX1.2 as a template. For co-expression experiments, E. coli strain BL21 or BL21-RIL was first transformed with pET-PDX1.2 or pET-PDX1.2 A2P. Independent transformants were then made chemically competent for co-transformation with PDX1.1 (BL21) or PDX1.3 (BL21-RIL). Expression was induced by the addition of 0.2 mm isopropyl-1-β-galactopyranoside, followed by growth for 6 h at 28 °C. The proteins were purified by Ni-NTA chromatography (Qiagen) using the nondenaturing protocol described by the manufacturer in the presence of 1 mm phenylmethylsulfonyl fluoride and 5 mm dithiothreitol. The purification was monitored by SDS-PAGE on 15% polyacrylamide gels and staining with Coomassie Blue. The concentration of protein was determined by the method of Bradford using bovine serum albumin as a standard (29).
Size Exclusion Chromatography and Multiangle Light Scattering Experiments
PDX1 complexes (100 μl at 1 mg/ml) were first separated by size exclusion chromatography on a Superdex 200 10/300 GL column (GE Healthcare) using 50 mm potassium phosphate, pH 7.5, containing 50 mm potassium chloride and 0.01% sodium azide. A miniDAWN TREOS light scattering instrument (Wyatt Technologies) was connected immediately downstream of the separation media for light scattering analysis. The data were analyzed using the software ASTRA (Wyatt Technologies). Absorption coefficients used for the calculation of the weight average molecular mass for the different enzymes and complexes were as follows: 224 ml g−1 cm−1 for PDX1.1; 175 ml g−1 cm−1 for PDX1.2; 178 ml g−1 cm−1 for PDX1.3; 200 ml g−1 cm−1 for PDX1.1-PDX1.2; 177 ml g−1 cm−1 for PDX1.3-PDX1.2 and PDX1.1-PDX1.2; 197 ml g−1 cm−1 for PDX1.1-PDX1.2 A2P; and 176 ml g−1 cm−1 for PDX1.3-PDX1.2 A2P.
Electrospray Ionization Mass Spectrometry (ESI-MS) and MALDI-TOF MS/MS Analysis of PDX1 Complexes
Each protein sample (100 μl of 10 pm) was dialyzed in 0.2% formic acid overnight at 4 °C and analyzed by ESI-MS (Waters quadrupole time-of-flight Ultima API). For peptide analysis, PDX1.2 was digested with 1 μg of trypsin in 50 mm Tris-Cl, pH 7.5, at 37 °C overnight, and the peptide mass fingerprint was determined using MALDI-TOF (Bruker Autoflex). MALDI-TOF/TOF (Bruker Ultraflex II) analyses were performed on peptide 1 (Ala2–Lys26, 2571.19 Da) of PDX1.2 and its modified form (2613.2 Da).
Enzyme Assays
The rate of glutaminase hydrolysis by PDX2 was measured as described previously (7, 30) by following the absorbance at 363 nm in 96-well plates. For determination of the kinetic constants, a 4 μm concentration of each enzyme was used. The PLP biosynthetic activity of PDX1 complexes was monitored by UV-visible spectrophotometry in 96-well plates, where the appearance of a maximum at 414 nm indicated enzymatic formation of PLP (7). Reactions were carried out using 20 μm catalytic protein and 20 mm ammonium sulfate (7).
Plant Material, RNA Extraction, and Quantitative Real-time RT-PCR (qPCR) Analysis
The conditional flu mutant (31) was kindly donated by Klaus Apel (Cornell University, Ithaca, NY). The RNAi constructs used to generate the PDX1.2 knockdown lines targeted the unique nucleotide sequence at the N terminus of the gene, composed of a sense and an antisense fragment (bp 3–460 and bp 3–303, respectively) amplified from the cDNA sequence of PDX1.2. For the sense sequence, the oligonucleotides 5′-CATGCCATGGGGCGGATCAAGCTATGACG-3′ (forward) and 5′-GCACTAGTCTCCTGTATCACGGCAACC-3′ (reverse) were used, incorporating SpeI and NcoI restriction sites as indicated in italic type. The antisense fragment was amplified using 5′-GCACTAGTCGTGCCATCACAGGTACAG-3′ and 5′-CAGGGTAACCGGCGGATCAAGCTATGACG-3′ as forward and reverse primers as well as introducing a SpeI and a BstEII restriction site, respectively. The two fragments were separated by a linker sequence (ACTAGT), allowing the formation of a hairpin structure, and cloned in the binary vector pCAMBIA2301 for plant expression. The construct was transformed either into Arabidopsis wild-type or the Arabidopsis flu mutant line through Agrobacterium tumefaciens-mediated transformation by the floral dip method (32). Homozygous lines carrying a single copy of the construct were selected using the respective antibiotic, and independent lines displaying the lowest levels of PDX1.2 expression, as analyzed by qPCR, were used for further analyses. All plant lines were cultivated on half-strength MS medium without vitamins (33) containing 0.8% agar and 1% sucrose under continuous light (120 μmol photons m−2 s−1) and at 22 °C. For the flu mutant experiments, 10–12-day-old seedlings were either transferred to darkness for 8 h and afterward transferred back to light (DL-shift) or left to grow in continuous light (LL). For the heat stress experiments, 7-day-old seedlings were subjected to 45 °C for the indicated time periods and then transferred back to 22 °C, employing a plant growth chamber from Percival (model I-30BL4/D). Samples (100 mg) for qPCR and vitamin B6 content were harvested either before or after the stress treatment at the indicated time points. The survival rate was measured 5 days after the seedlings were returned to 22 °C by scoring (n = 100, 3–4 biological replicates of each line). Total RNA was extracted using the Nucleospin RNA kit from Macherey-Nagel and was reverse transcribed with SuperScriptTM II RNaseH−, using oligo(dT) primers 12–18 according to the manufacturer's recommendations (Invitrogen) starting with 2 μg of total RNA for the synthesis. qPCR was performed using SYBR® Green (Power Sybr® Green PCR master mix, Applied Biosystems) with a 7900HT Fast Real-Time PCR System (Applied Biosystems). The GAPDH gene (At1g13440) was employed as a control with forward and reverse oligonucleotides: 5′-TTGGTGACAACAGGTCAAGCA-3′ and 3′-AAACTTGTCGCTCAATGCAATC-5′, respectively. All values were normalized to the time 0 values, which were set to 1. The oligonucleotides used for amplification of the PDX1 genes were as follows: PDX1.1, 5′-GGGAGGTTACCGGTGG-3′ (forward) and 5′-CCTTAGCCCTCTTCACC-3′ (reverse); PDX1.2, 5′-GATGCAGCTAGGTTGTGATGG-3′ (forward) and 5′-TCCATTGCATTCTCCAATCC-3′ (reverse); PDX1.3, 5′-GGTCGTCTTCCTGTAGTC-3′ (forward) and 5′-CACGTGCACGACGAGCT-3′ (reverse).
Determination of Vitamin B6
Plant material taken from flu seedlings 10–12 days after germination following a DL-shift was used for determining the levels of vitamin B6 by HPLC as described (22). Briefly, ∼100 mg of tissue was ground to a powder and resuspended in 200 μl of 50 mm ammonium acetate buffer, pH 4.0. After centrifugation for 20 min at 11,000 × g, the supernatant was heated at 100 °C for 3 min, left to cool down to room temperature (22–24 °C), and centrifuged at 11,000 × g for 10 min. The resulting supernatant was used for analysis of vitamin B6 on an Agilent Technologies 1200 Series HPLC system using a SunfireTM C18 reverse phase column (3.5 μm, 4.6 × 150 mm; Waters). The vitamin B6 content of the plant material generated in the heat stress experiments was measured using a microbiological assay as described previously (16).
RESULTS
PDX1.2 Displays the Hallmarks of a Pseudoenzyme
Whereas bacteria have only one homolog of PDX1 and PDX2, yeast, certain fungi and plants can have several copies of PDX1 in particular (based on a BLAST search). The extensive structural and biochemical analyses that have been completed on PDX1 in recent years have facilitated the annotation of essential catalytic residues (8, 9, 11, 30, 34–36). Notably, PDX1 has two active sites annotated as P1 and P2 (8, 9) corresponding to the binding sites of bound substrates or products (Fig. 1A). Key catalytic residues include the lysine and aspartate residues of the R5P binding site (site P1) as well as the lysine residue that coordinates the product PLP (site P2) (Fig. 1, A and C). In this context, a closer look at those organisms that carry several copies of PDX1 allowed us to predict those expected to be catalytically active (where not already functionally characterized). Interestingly, we noted that homologs are found in certain plant species (but not in lower organisms), in which it is predominantly the catalytic residues that are not conserved. Indeed, similar to an earlier study on the Arabidopsis PDX1 homologs (13), a phylogenetic analysis of currently sequenced genomes from plant species established that these non-catalytic homologs form a distinct subfamily within the family of PDX1s (Fig. 2, red lines). For example, members of the Brassicaceae (e.g. Arabidopsis, At3g16050), Euphorbiaceae (cassava 4.1_034454m), and Salicaceae (e.g. poplar, POPTR_0001s18240), among others, harbor such “dead” enzymes (Fig. 2). Intriguingly, it emerged from this analysis that the monocot species analyzed only carry active homologs (e.g. Oryza sativa (rice) has three homologs, each of which harbor the catalytic residues) (Fig. 2, green lines). On the other hand, notable exceptions within the eudicota are Glycine max (soybean) and Phaseolus vulgaris (common bean), which have two and one PDX1 homolog, respectively, all predicted to be active (Fig. 2). Because this non-catalytic homolog appears to be exclusive to the plant lineage, this could represent a case of neofunctionalization, whereby this subfamily functionally diverged from its catalytic counterparts. Because the Arabidopsis non-catalytic homolog has been annotated as PDX1.2, we will use this notation for this subfamily from here on, and we focus on a characterization of Arabidopsis PDX1.2, in particular. A comparison of the predicted structure of the Arabidopsis PDX1.2 with the known structure of a bacterial homolog (8) indicates that the overall fold of the protein is likely to be preserved but highlights the non-conservation of catalytic residues (Fig. 1B). We have previously demonstrated that although Arabidopsis PDX1.2 is expressed, it is not catalytic in vitamin B6 biosynthesis (11, 28). However, it is important to note that PDX1.2 has the same subcellular localization (i.e. cytosolic) as its catalytic paralogs (28). Strikingly, the above features are all hallmarks of the newly defined class of proteins referred to as pseudoenzymes (24).
FIGURE 1.
A distinct subfamily of PDX1s in plants displaying the hallmarks of a pseudoenzyme. A and B, ribbon structures of PDX1 from Bacillus (PDB code 2NV1) and a model of Arabidopsis PDX1.2 generated by I-Tasser (54). Key catalytic residues are shown in stick mode in Bacillus PDX1 as well as the corresponding residues in the Arabidopsis PDX1.2 model. C, alignment of the deduced amino acid sequences of Arabidopsis PDX1.1, PDX1.2, PDX1.3, and Bacillus PDX1 generated by Geneious version 5.3.6, using the log expectation algorithm (MUSCLE). The asterisks highlight key catalytic residues, and structural motifs denoted from Bacillus PDX1 (PDB code 2NV2) are shown in red.
FIGURE 2.

PDX1.2 is absent from monocots and only found in eudicot species. Sequences and annotations of PDX1 protein sequences were obtained from Phytozome. The phylogenetic tree was constructed using the Phylogeny Web service (55). The values given at internal nodes represent the p values for clustering (0–100%) using the Shimodaira-Hasegawa test (only values above 50% were considered). The scale bar signifies evolutionary distances. The accession numbers of PDX1 homologs are as follows: Arabidopsis thaliana At2g38230 (c), At3g16050 (nc), At5g01410 (c) (PDX1.1, PDX1.2 and PDX1.3, respectively); Brassica rapa Br021157 (nc), Br017133 (c), Br005678 (c), Br028901 (c); Carica papaya Cp43142 (nc), Cp11144 (c); Cucumis sativus Cs176360 (c), Cs359110 (c), Cs139260 (nc); Fragaria vesca Fv07903 (c), Fv18342 (c), Fv20470 (nc); G. max Gm13g29700 (c), Gm15g09350 (c); Manihot esculenta cassava4.1_012402m (c), cassava4.1_012325m (c), cassava4.1_034454m (nc), cassava4.1_020927m (nc); Medicago truncatula Mt2g017520 (c), Mt4g012860 (nc); Oryza sativa Os07g01020 (c), Os10g01080 (c), Os11g48080 (c); Phaseolus vulgaris Pv6g171100 (c); Populus trichocarpa Pt16g116400 (c), Pt1g182100 (nc), Pt6g100500 (c); Sorghum bicolor Sg2g000720 (c); Solanum lycopersicum Sl6g081980 (c), Sl3g120090 (nc); Solanum tuberosum St3DMG400030359 (c), St3DMG400005690 (nc); Theobroma cacao Tc1EG028738 (nc), Tc1EG021693 (c); Vitis vinifera GSVIVG01024735001 (c), GSVIVG01017087001 (nc); Zea mays Zm2g120652 (c), Zm5g850015 (c) (where catalytic and non-catalytic homologs are designated as (c) and (nc), respectively). Red and black lines indicate eudicots, whereas green lines indicate monocots. The dashed black lines indicate homologs where at least one catalytic residue is conserved but not all.
PDX1.2 Forms a Stable Dodecameric Heterocomplex with either PDX1.1 or PDX1.3
Given the emerging importance of pseudoenzymes (25), we were intrigued to define the functionality of PDX1.2. Previous observations based on yeast two-hybrid and split YFP data indicated that the Arabidopsis PDX1 proteins can form heteromers, but the nature of these complexes and a physiological reason for their existence were not established (26, 27). Therefore, we were prompted to investigate the PDX1 proteins in more detail and our own hypothesis concerning the possible function of PDX1.2 as a regulator of vitamin B6 biosynthesis. In an attempt to independently confirm that PDX1.2 can interact with the two other paralogs (PDX1.1 and PDX1.3) as shown previously by Leuendorf et al. (26), we co-expressed and purified the recombinant proteins. For this analysis, PDX1.2 was expressed with a C-terminal polyhistidine affinity tag, whereas PDX1.1 and PDX1.3 were expressed without a tag. Co-expression of PDX1.2 with either PDX1.1 or PDX1.3 followed by nickel nitrilotriacetic acid (Ni-NTA) chromatography led to the co-elution of either of the respective untagged PDX1 paralogs with PDX1.2 (i.e. the heterocomplexes PDX1.1-PDX1.2 and PDX1.3-PDX1.2) (Fig. 3A). Fortunately, the lower mobility of PDX1.2 on SDS-PAGE enabled us to distinguish this protein from the two catalytic paralogs (Fig. 3A) and confirmed an interaction between PDX1.2 and either PDX1.1 or PDX1.3. It is noteworthy that, at least in our hands, mixing of the individual purified proteins did not lead to the formation of heteromeric complexes; coexpression was necessary. This point is relevant in that PDX1.2 is of much lower abundance than either PDX1.1 or PDX1.3 under normal growth conditions in Arabidopsis (13, 17), and we explore it further under “Discussion.” PDX1 from different sources appears to exist as combinations of hexamers. In particular, bacterial homologs exist in a hexameric-dodecameric equilibrium, the yeast homolog SNZ1 as a hexamer, and there is evidence that Plasmodium falciparum PDX1 can form fibers or a cylinder of hexamers (8, 35, 37, 38). However, although size exclusion chromatography has been performed on Arabidopsis plant extracts ectopically expressing the individual PDX1s, indicating higher order complexes (26), the precise oligomeric state of a plant homolog has not been determined. With the Arabidopsis heteromeric and homomeric complexes in hand, it was therefore of interest to determine the oligomeric nature of these complexes. This analysis was carried out employing size exclusion chromatography coupled to static light scattering. Interestingly, the determined sizes of the PDX1.1-PDX1.2 (383,000 ± 1000 Da) and the PDX1.3-PDX1.2 (370,000 ± 10,000 Da) heterocomplexes closely corresponded to the expected size of a dodecamer (395,000–419,000 Da) (Fig. 3B, black and gray traces, respectively). The estimated sizes of the homomeric PDX1.1 or PDX1.2 complexes (369,000 ± 1000 and 410,000 ± 2000 Da) also closely correspond to a dodecameric state (expected sizes 395,000 and 419,000, respectively) (Fig. 3C, black and dark gray traces, respectively). Although the dodecamer form of the homomeric proteins was anticipated, the finding of a dodecamer for both of the heteromeric (i.e. PDX1.1-PDX1.2 and PDX1.3-PDX1.2) complexes is intriguing. Moreover, we noted that the retention time of PDX1.3 alone, although approaching a dodecamer (expected size 411,000 Da) was longer than for the other paralogs, with a broad trailing shoulder (Fig. 3C, light gray trace). This asymmetric peak is indicative that PDX1.3 is less stable than the two other paralogs under the conditions used. Interestingly, in the presence of PDX1.2, the peak of the heterocomplex is symmetric and monodisperse (Fig. 3B), corresponding to a dodecamer, suggesting that PDX1.3 is more stable in the presence of PDX1.2 under these conditions.
FIGURE 3.
The Arabidopsis PDX1s form dodecameric homomers and heteromers. A, the three individual PDX1s (PDX1.1, PDX1.2, and PDX1.3) can be purified to homogeneity (lanes 1–3, respectively). Coexpression of hexahistidine-tagged PDX1.2 with either untagged PDX1.1 or untagged PDX1.3 results in their co-elution, demonstrating the formation of heteromeric complexes (lanes 4 and 5, respectively). B and C, size exclusion chromatography coupled to multiangle light scattering analysis of the heteromeric complexes of PDX1.1-PDX1.2 and PDX1.3-PDX1.2 and the individual homomeric PDX1 proteins, respectively. In B, the combination of size exclusion chromatography and multiangle light scattering reveals a predominant peak corresponding to dodecameric forms of PDX1.1-PDX1.2 (black line) or PDX1.3-PDX1.2 (gray line). In C, PDX1.1 (black line) and PDX1.2 (gray line) elute as a dodecamer. On the other hand, a higher retention time and a broad trailing shoulder are observed for PDX1.3 (light gray line), indicating the presence of lower order oligomeric states and/or degradation products. The molar mass and the UV absorbance were plotted in an elution volume-dependent manner.
PDX1.2 Positively Affects the Catalytic Activity of PDX1.1 and PDX1.3
We next compared the enzymatic activity of the heteromeric versus the homomeric complexed proteins for the formation of PLP. In the first instance, the relative amount of the catalytic protein (i.e. PDX1.1 or PDX1.3) in the respective heterocomplexes was estimated by a semiquantitative analysis employing ESI-MS (Fig. 4). In this context, it is important to know that at least in the case of the Bacillus subtilis PDX1 homolog, intermediates between the protein and substrates/products along the multistep reaction coordinate have been observed by mass spectrometry (30) and corroborated by absorbance spectrophotometry and nuclear magnetic resonance spectroscopy studies (7, 30, 39–41). Indeed, the latter recombinant protein as isolated is a heterogeneous population of the apoprotein as well as some of these intermediates. It could be concluded from these studies that one of the first steps in the formation of PLP is the binding of the R5P substrate in the P1 active site of PDX1, leading to the formation of a Schiff's base between an internal lysine in PDX1 and R5P. This intermediate can be observed by ESI-MS as a peak with an additional mass of 212 Da compared with that of the apoprotein (30). Furthermore, another intermediate subsequent to the Schiff's base formation and resulting from the loss of water and phosphate is the so-called chromophoric adduct (30), which displays an ESI-MS peak with an additional mass of 95 Da compared with the apoprotein. Both of these reaction intermediate peaks can be observed with the Arabidopsis PDX1.1 and PDX1.3 proteins but not with PDX1.2 (Fig. 4, A and B). Co-purification of the heterocomplexes was done in triplicate, resulting in similar observations each time. Based on the total height of the ESI-MS peaks corresponding to the masses of the respective proteins, the stoichiometric ratios calculated for the heterocomplexes could be calculated as 1.45 ± 0.04:1 for PDX1.1/PDX1.2 and 0.44 ± 0.01:1 for PDX1.3/PDX1.2 (Fig. 4, A and B). Notably, the total peak height corresponding to apo and intermediate forms of PDX1.1 and PDX1.3 was used to calculate these ratios. If however, only the apoprotein heights are used, the ratio is 0.93 ± 0.05:1 for PDX1.1/PDX1.2 and 0.28 ± 0.02:1 for PDX1.3/PDX1.2. We used the stoichiometric ratio based on total peak height to estimate the enzymatic activities below.
FIGURE 4.

Semiquantitative analysis of catalytic PDX1 complexed to PDX1.2 and evidence for N-terminal modification of PDX1.2. A and B, ESI-mass spectra of PDX1.1-PDX1.2 and PDX1.3-PDX1.2, respectively. The masses given correspond to native PDX1.1 (32,732.2 Da); PDX1.1 with the chromophoric adduct bound (32,827.3 Da); PDX1.1 with ribose 5-phosphate bound (32,944.8 Da); PDX1.2 (34,771.3 Da) and N-terminally modified PDX1.2 (34,813.3 Da); native PDX1.3 (33,219.4 Da); PDX1.3 with the chromophoric adduct bound (33,314.5 Da); PDX1.3 with ribose 5-phosphate bound (33,431.5 Da); and PDX1.2 (34,772.5 Da) and N-terminally modified PDX1.2 (34,814.2 Da), respectively. C, ESI-mass spectrum of PDX1.2 alone (34,769.4 Da) and the corresponding N-terminal modified form (34,810.8 Da). These experiments were repeated with at least three biological replicates, and similar results were obtained each time.
PDX1 is essential for induction of glutaminase activity in PDX2 resulting from a conformational change in the PDX2 active site coordinated by its interaction with PDX1 (8). The ability of PDX1.1 and PDX1.3 to initiate the glutaminase activity of PDX2 was monitored by the addition of a stoichiometric amount of catalytic PDX1 protein (i.e. an equal amount of either PDX1.1 or PDX1.3 within the heterocomplex based on the calculated ratios as described above) to PDX2 (4 μm). Both PDX1.1 and PDX1.3 were able to activate the glutaminase activity of PDX2 to a similar extent, independently of their complexation with PDX1.2 (Table 1 and Fig. 5A). Because PDX2 has been shown not to interact with PDX1.2 (11, 27), this validates the mass spectrometric quantitation of the amount of catalytic PDX1 present in the heteromeric complexes. Moreover, it demonstrates that the complexation of PDX1.2 with either PDX1.1 or PDX1.3 does not affect their interaction with PDX2 or its activity. The effect of PDX1.2 on the glutaminase activity of PDX2 was assessed as a control, and no activity was observed (Fig. 5A), corroborating our previous analyses (11). However, the kinetic parameters for PLP formation with the catalytic PDX1 proteins changed significantly in the presence of PDX1.2. Notably, the kcat for PLP biosynthesis increased 1.8-fold when PDX1.2 was bound to PDX1.3, whereas a 1.3-fold change was observed when PDX1.2 was complexed with PDX1.1 (Fig. 5, B and C, and Table 2). Interestingly, a considerable decrease in the Km for R5P was observed for the two heterocomplexes compared with the homomeric proteins alone. No significant change was observed in the Km for the second substrate, glyceraldehyde 3-phosphate (Table 2). Overall, this leads to a 5-fold increase in the specificity constant (kcat/Km) for R5P, in particular, in the case of the PDX1.1-PDX1.2 heterocomplex and a 9-fold increase for the PDX1.3-PDX1.2 heterocomplex (Table 2).
TABLE 1.
Kinetic constants for the glutaminase activity of PDX2 in the presence of PDX1 complexes
Assays were performed at 37 °C in 50 mm Tris-Cl, pH 7.5.
| PDX1 complex | Km Gln | kcat | kcat/Km Gln |
|---|---|---|---|
| mm | min−1 | min−1 mm−1 | |
| PDX1.1 | 0.79 ± 0.06 | 1.11 ± 0.02 | 1.4 |
| PDX1.1-PDX1.2 | 0.71 ± 0.05 | 1.00 ± 0.02 | 1.4 |
| PDX1.3 | 0.79 ± 0.09 | 1.18 ± 0.04 | 1.5 |
| PDX1.3-PDX1.2 | 0.72 ± 0.07 | 1.10 ± 0.03 | 1.5 |
FIGURE 5.

PDX1.2 positively affects the catalytic activity of PDX1.1 and PDX1.3. A, glutaminase activity of PDX1.1-PDX1.2 (▾), PDX1.3-PDX1.2 (▵), PDX1.1 (●), PDX1.3 (○), or PDX1.2 (■) as a function of glutamine. In each case, 4 μm catalytic PDX1 and 4 μm PDX2 was used. B and C, PLP synthase activity of PDX1.3-PDX1.2 (○) compared with PDX1.3 alone (●) (B) and PLP synthase activity of PDX1.1-PDX1.2 (○) compared with PDX1.1 alone (●) (C) as a function of ribose 5-phosphate and in the presence of ammonium sulfate as a nitrogen source. In each case, 20 μm catalytic PDX1 was used. Fitting of the data was according to the Michaelis-Menten equation, f(x) = a × x/b + x. The values are the mean of three technical and two biologically independent experiments with error bars indicating S.D.
TABLE 2.
Kinetic constants for the PLP synthase activity of PDX1 complexes
Assays were performed at 37 °C in 50 mm Tris-Cl, pH 7.5. R5P, ribose 5-phosphate; G3P, glyceraldehyde 3-phosphate.
| PDX1 complex | Km R5P | Km G3P | kcat | kcat/Km R5P | kcat/Km G3P |
|---|---|---|---|---|---|
| mm | mm | min−1 | min−1 mm−1 | min−1 mm−1 | |
| PDX1.1 | 0.180 ± 0.023 | 0.24 ± 0.02 | 0.028 ± 0.001 | 0.16 | 0.12 |
| PDX1.1-PDX1.2 | 0.045 ± 0.006 | 0.23 ± 0.03 | 0.036 ± 0.001 | 0.80 | 0.16 |
| PDX1.1-PDX1.2 A2P | 0.062 ± 0.011 | 0.24 + 0.03 | 0.027 ± 0.001 | 0.44 | 0.12 |
| PDX1.3 | 0.057 ± 0.007 | 0.22 ± 0.01 | 0.025 ± 0.003 | 0.44 | 0.12 |
| PDX1.3-PDX1.2 | 0.012 ± 0.004 | 0.22 ± 0.07 | 0.045 ± 0.002 | 3.8 | 0.20 |
| PDX1.3-PDX1.2 A2P | 0.026 ± 0.003 | 0.24 ± 0.02 | 0.021 ± 0.001 | 0.85 | 0.11 |
In Vitro Evidence that an N-terminal Modification in PDX1.2 Contributes to Enhancing PDX1 Catalytic Activity
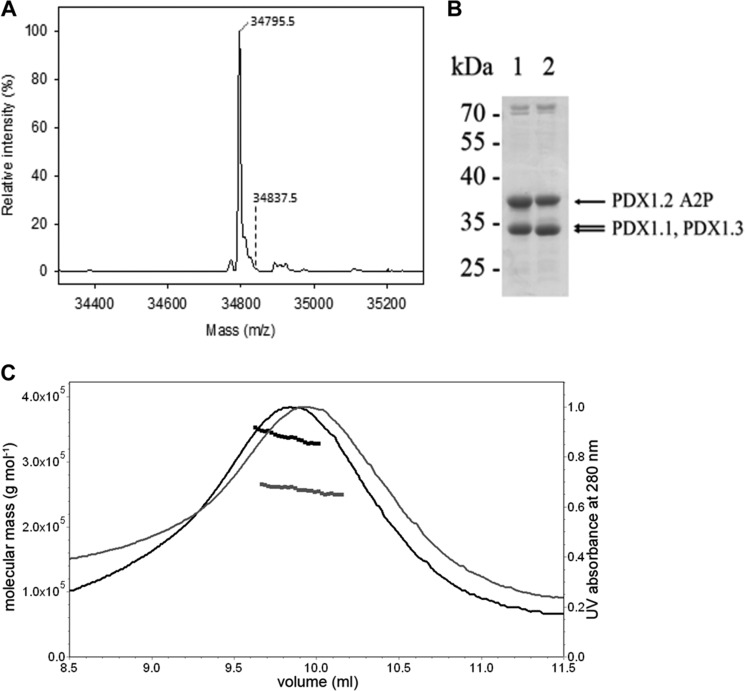
In all ESI-MS experiments of PDX1.2, in addition to the peak (34,771.3 Da) corresponding to the expected mass of the protein (34,769.47 Da), an extra peak with an additional mass of ∼42 Da was observed (34,813.3 Da), whereas no such peak was detected for PDX1.1 and PDX1.3 (Fig. 4). To localize the site of modification of PDX1.2, a tryptic digest of the protein followed by MALDI-TOF mass spectrometry analysis was performed. The peptide masses observed covered 85% of the whole protein sequence with the N-terminal peptide carrying the extra mass of 42 Da (Fig. 6A). MALDI-TOF/TOF tandem mass spectrometry confirmed that it is the N-terminal peptide of PDX1.2 (ADQAMTDQDQGAVTLYSGTAITDAK) that carries the additional mass of 42 Da (Fig. 6, B and C). A comparison of the unmodified (m/z 2571.19) and modified precursor ions (m/z 2613.2) yielded a b-fragmentation pattern clearly identifying AMTD, which is unique to this peptide within the protein sequence (Fig. 6, B and C). Because the observed b-fragmentation pattern of the modified peptide already carries the additional mass, it must be assumed that a residue on or preceding Ala-5 has the modification. It is important to note that the N-terminal methionine is cleaved off of this protein. Site-directed mutagenesis of the terminal alanine (Ala2) residue to a proline and ESI-MS of the resulting protein revealed only a single peak (34,795.5 Da) corresponding to the expected mass of the unmodified protein (34795.5 Da). No peak with an additional mass of 42 Da was observed with this PDX1.2 A2P mutein, corroborating that it is the N-terminal alanine residue that is modified (Fig. 7A). Notably, PDX1.1 also has an alanine residue at the N terminus, but it is not modified (Fig. 4). An additional mass of 42 Da could correspond to either acetylation or trimethylation, two well established protein modifications (42). N-terminal acetylation is one of the most common modifications occurring in cytosolic eukaryotic proteins (up to 98% (43)). In particular, proteins in which the terminal methionine has been processed, exposing an alanine residue, are prone to this modification (44, 45). Although this modification is rare in prokaryotic proteins, it has been shown to occur during production of eukaryotic recombinant proteins in E. coli and, moreover, was proven to be relevant to functionality in its normal host (46). It is noteworthy that trimethylation of the terminal alanine residue can also occur but is rare and has mainly been observed on ribosomal proteins (47). Therefore, because PDX1.2 is of extremely low abundance in Arabidopsis under normal growth conditions (13, 17), we thought it worthwhile to probe this modification in more detail with the recombinant protein. First, co-transformation, expression, and purification of the PDX1.2 A2P mutein with either PDX1.1 or PDX1.3 resulted in the co-elution of either PDX1.1 or PDX1.3 with PDX1.2 A2P, as had been observed with the wild-type PDX1.2 protein (Fig. 7B). Notably, size exclusion chromatography coupled to static light scattering established that both PDX1.1-PDX1.2 A2P and the PDX1.3-PDX1.2 A2P heterocomplexes are dodecameric species (Fig. 7C, black and gray traces, respectively). Next, we estimated the enzymatic activity for the formation of PLP by the heteromeric complexes, each time adding an equivalent amount of catalytic protein based on the ratios estimated from mass spectrometry, as for the wild-type proteins. In the presence of PDX1.2 A2P, the kcat for PLP formation by either PDX1.1 or PDX1.3 was similar to that of the individual homomeric enzymes and did not reach the levels observed in the presence of wild-type PDX1.2 (Table 2). Although a decrease in the Km value for R5P was still observed for both PDX1.1 and PDX1.3 in the presence of PDX1.2 A2P, it was to a lesser extent than that observed with wild-type PDX1.2 (Table 2). No effect on the Km for glyceraldehyde 3-phosphate was observed (Table 2), and moreover, no change in glutaminase activity was observed for PDX1.2 A2P complexed with either PDX1.1 or PDX1.3, compared with the wild-type enzyme complex (data not shown). Therefore, it appears that the modified form of PDX1.2 at least contributes to the enhanced kinetics observed for the catalytic PDX1s. It is noteworthy that the observed modified form of PDX1.2 represents only a small fraction of this protein as produced in E. coli (Fig. 4). Thus, the catalytic effect may be much greater in planta, if a larger fraction of the PDX1.2 is present in the modified form. The enhancement in PDX1 kinetics may also have a contribution from the stabilizing effect of PDX1.2 because a dodecameric complex is still formed with the PDX1.2 A2P mutein (Fig. 7C), possibly accounting for the incomplete reversion of the Km to that observed with the catalytic PDX1 homomeric complexes.
FIGURE 6.

PDX1.2 is modified in vitro at the N terminus. A, MALDI-TOF analysis of a tryptic digest of PDX1.2. The mass corresponding to the unmodified (m/z 2571.19 Da) and modified (m/z 2613.2 Da) N-terminal peptide alanine 2-lysine 26 is indicated. B, MALDI-TOF/TOF analysis of the modified and unmodified precursor ions m/z 2571.19 and m/z 2613.2, yielding a fragmentation pattern matching the predicted profile for the N-terminal peptide ADQAMTDQDQGAVTLYSGTAITDAK. Observed b ions are indicated.
FIGURE 7.
Alanine 2 of PDX1.2 carries the modification, but its removal still permits complexation with PDX1.1 or PDX1.3. A, ESI-mass spectrometry analysis of PDX1.2 A2P (34,795.5 Da). No peak corresponding to N-terminal modification (expected mass, 34,837.5 Da) is observed. B, coexpression of hexahistidine-tagged PDX1.2 A2P with either untagged PDX1.1 or untagged PDX1.3 results in their co-elution, demonstrating maintenance of the formation of heteromeric complexes (lanes 1 and 2, respectively). C, size exclusion chromatography coupled to static light scattering analysis of the heteromeric complexes of PDX1.1-PDX1.2 A2P (black line) and PDX1.3-PDX1.2 A2P (gray line) reveals a predominant peak in each case, which corresponds to their respective dodecameric forms. The molar mass and the UV absorbance were plotted in an elution volume-dependent manner.
Up-regulation of PDX1.2 under Oxidative Stress Conditions Concomitant with an Increase in Vitamin B6 Levels
As mentioned above, vitamin B6 has been implicated as an antioxidant with a particular potency against singlet oxygen (1, 4, 15, 18, 48). This, together with the observation that PDX1.2 is the most sensitive PDX1 paralog to oxidative stress (13), prompted us to investigate the expression of the three PDX1 paralogs in Arabidopsis after a burst of singlet oxygen. For these studies, we made use of the Arabidopsis conditional fluorescent (flu) mutant (31). The FLU gene codes for a negative regulator of chlorophyll biosynthesis and is particularly related to the step involving conversion of the chlorophyll precursor protochlorophyllide (Pchlide) to chlorophyllide. The latter step is light-dependent such that in dark-grown plants, chlorophyll biosynthesis is inhibited after reaching a critical level of Pchlide. The flu mutant lacks this regulation step and therefore accumulates Pchlide when grown in the dark (31, 49). Because the Pchlide molecule is a photosensitizer, upon shifting plants incubated in the dark to the light, a burst of singlet oxygen is observed (31). A long incubation in the dark (e.g. 8 h) results in a build-up of Pchlide in the flu mutant, which causes singlet oxygen to be produced in excessive amounts when this mutant is switched back to light (49). We analyzed the abundance of the three PDX1 genes over a 24-h period in 12-day-old seedlings of the flu mutant using qPCR following an 8-h incubation in the dark followed by a shift to light (referred to as DL-shift) (Fig. 8A). The flu mutant grown under continuous light (LL) was used as a control (Fig. 8B). Interestingly, we observed an up-regulation of the PDX1.2 transcript (∼2.5-fold) already 1 h after the DL-shift in the flu mutant, whereas no significant change was observed for PDX1.1 or PDX1.3 (Fig. 8A). Notably, this peak was transient because it decreased to basal levels 2–6 h after the DL-shift. No corresponding change in abundance was observed in the seedlings grown under continuous light (Fig. 8B). The level of vitamin B6 was also measured and increased 2 h after the DL-shift but then returned to basal levels (Fig. 8D), whereas no significant change was observed with plants grown under continuous light (Fig. 8E). In addition, we generated a knockdown line of PDX1.2 by RNA interference in the flu background. We measured the vitamin B6 level in two independent lines that had a considerably lower level of expression of PDX1.2 compared with the flu mutant alone (Fig. 8C). No change in the expression of either PDX1.1 or PDX1.3 was observed in these lines (data not shown). Neither of the two double mutant lines (i.e. flu pdx1.2) showed the transient increase in vitamin B6 content that is observed with the flu mutant alone (Fig. 8, D and E). We have noted that the total vitamin B6 content is higher in plants grown under continuous light compared with plants subjected to the DL-shift (Fig. 8, compare D and E), indicating that vitamin production may occur more efficiently during the light period, compared with a dark period. This latter result is corroborated by the previously observed light induction of PDX1.1 and PDX1.3 (17).
FIGURE 8.
PDX1.2 is up-regulated under oxidative stress conditions and is required for the observed increase in vitamin B6 levels. A, qPCR analysis of the transcript levels of PDX1.1, PDX1.2, and PDX1.3 in the Arabidopsis flu mutant in response to a burst of singlet oxygen. Seedlings were first cultivated under continuous light (100 μmol photons m−2 s−1) at 22 °C. 12 days after germination, the seedlings were transferred to the dark for 8 h before placing them back to light (DL-shift). B, seedlings cultivated under continuous light (LL). For A and B, transcript abundance was related to the time point 0 of the same line (set to 1). The data are the mean of three biological and four technical replicates. Error bars, S.E.; *, statistically significant differences for p < 0.05 (ANOVA, Tukey test). C, qPCR analyses of PDX1.2 expression in the flu mutant as well as two independent flu:pdx1.2 RNAi lines (L1 and L2). Lines were grown under continuous light (100 μmol photons m−2 s−1) at 22 °C for 12 days following germination. The data are the mean of three biological and two technical replicates. The levels were normalized using the levels of glyceraldehyde 3-phosphate dehydrogenase (At1g13440) as a control. Error bars, S.E.; *, significant values from a pairwise comparison (p < 0.05; ANOVA, Tukey test)). D and E, time course of vitamin B6 levels after a DL shift (D) or in seedlings kept under continuous light (LL) (E). For D and E, the data are from at least four biological replicates. Error bars, S.E.; *, statistically significant differences for p < 0.05 (ANOVA, Tukey test).
PDX1.2 Expression Is Rapidly and Strongly Up-regulated upon Heat Stress in Arabidopsis
Whereas the response to singlet oxygen was rather subtle, we probed for other stress conditions that may influence PDX1.2 expression. During the course of these studies, we observed a particularly dramatic response of PDX1.2 to heat stress. Specifically, exposure of Arabidopsis seedlings to 45 °C for up to 120 min increased PDX1.2 expression ∼100-fold (Fig. 9A). Notably, this increase was observed already after 15 min of exposure to heat stress (Fig. 9A). This is a level of induction similar to that seen in heat-shock proteins (50). There was no significant change in the expression of PDX1.1 or PDX1.3 upon the same treatment (Fig. 9B). Moreover, the level of vitamin B6 increased almost 2-fold in these seedlings compared with the control (Fig. 9C). Plant lines knocked down in the expression of PDX1.2 (Fig. 9D) generated by RNA interference (as for the flu mutant background above) did not show a similar increase in vitamin B6 levels under the same conditions (Fig. 9C). The lack of correlation between the dramatic increase in PDX1.2 transcript and subtle increase in total vitamin B6 content at the seedling level leads us to hypothesize that the response is local to the area affected by exposure to heat and thus is diluted upon analysis of the entire seedling. Nonetheless, the pdx1.2 mutant lines are more sensitive to heat stress than wild-type under the same conditions, because less survived upon exposure to heat stress (45 °C for 120 min) compared with wild type (Fig. 9E).
FIGURE 9.
PDX1.2 is rapidly and strongly up-regulated under heat stress conditions and is required for the concomitant increase in vitamin B6 levels and seedling survival. A, qPCR analysis of the relative transcript levels of PDX1.2 in Arabidopsis upon exposure to heat stress at 45 °C for the indicated times. Seedlings were first cultivated for 7 days under standard long day conditions (100 μmol photons m−2 s−1 for 16 h at 22 °C followed by 8 h of darkness at 18 °C). PDX1.2 is strongly induced even after 15 min of exposure to heat stress. *, statistically significant differences for p < 0.001 (ANOVA, Tukey test) compared with the control (time 0). B, transcript abundance of PDX1.1 and PDX1.3 under the same conditions as in A. No significant differences in expression were found. C, time course of vitamin B6 levels after exposure of 7-day-old seedlings to heat stress (90 min at 45 °C). Control (unheated) samples were compared with samples taken at the end of the exposure to heat (HS+0h) and 2 h after the heat stress (HS+2h). The data are from at least three biological replicates. Error bars, S.E.; *, statistically significant differences for p < 0.01 (ANOVA, Tukey test). D, qPCR analyses of PDX1.2 expression in two independent pdx1.2 RNAi lines (L1 and L2) compared with wild-type expression. The data are the mean of three biological and three technical replicates. The levels were normalized using the levels of glyceraldehyde 3-phosphate dehydrogenase (At1g13440) as a control. Error bars, S.E.; *, significant values from a pairwise comparison (p < 0.05; ANOVA, Tukey test). E, percentage survival of pdx1.2 and wild-type seedlings after exposure to heat stress (120 min at 45 °C followed by 5 days at 22 °C). The data are from at least three biological replicates, each consisting of 100 seedlings. Error bars, S.E.; * and **, statistically significant differences for p < 0.05 and p < 0.01 (ANOVA, Tukey test), respectively.
DISCUSSION
It is becoming increasingly clear that previously assumed “dead” enzymes (i.e. expressed but unable to catalyze a chemical reaction) are gainfully employed in organisms, many of which may have important regulatory functions. In this study, we provide unequivocal evidence for the existence of a pseudoenzyme (PDX1.2) in certain plant species that regulates vitamin B6 biosynthesis. The use of vitamin B6 both as a cofactor for numerous metabolic enzymes and as an antioxidant would appear to justify the need for such regulation. We show that the expression of the non-catalytic PDX1.2 is strongly up-regulated under heat stress as well as under singlet oxygen stress, whereas its catalytic paralogs are not. Indeed, the rapid induction of PDX1.2 expression parallels the response observed with heat-shock proteins under similar conditions (50). The time course of PDX1.2 expression is concomitant with an increase in vitamin B6 levels that is abolished in its absence, providing indirect evidence that PDX1.2 is associated with this response in plants. Isolation of the recombinant PDX1 protein heterocomplexes and demonstration of an enhancement in vitamin B6 production in the presence of PDX1.2 corroborate this statement. Moreover, we provide evidence that modification of an N-terminal amino acid of PDX1.2 may be involved in mediating the response.
The mechanism behind the functionality of PDX1.2 as a positive regulator of vitamin B6 biosynthesis under the conditions tested is of paramount importance, given that it may be specific to conditions of abiotic stress. Here we show that PDX1.2 can form heteromeric complexes with PDX1.1 and PDX1.3 in vitro. This corroborates previous yeast two-hybrid and split YFP analyses that demonstrated interaction in vivo but did not determine the nature of the interaction and the physiological relevance (26, 27). Significantly, we demonstrate that PDX1.2 enhances the specificity constant for R5P in particular, pinpointing its mechanism of action to the early steps of the reaction coordinate of PLP biosynthesis (30). Intriguingly, the PDX1.2 heterocomplexes with either PDX1.1 or PDX1.3 are dodecameric. However, the positive effect on catalysis observed in the presence of PDX1.2 is more pronounced with PDX1.3 than PDX1.1. In this context, an important observation is that PDX1.3 is apparently unstable while in a homomeric complex, but a stable dodecamer is formed with PDX1.2 (Fig. 3). All PDX1 homologs that have been studied exhibit at least a hexameric architecture, with the majority having been shown to be in a hexamer-dodecamer equilibrium (8, 37). Although a structural determination of the precise architecture of the Arabidopsis heteromeric complexes is beyond the scope of this study, the most intuitive assembly would involve a single hexamer of each protein type in the respective dodecameric complex. Indeed, the high sequence similarity of the region required for interdigitation of the hexamers (i.e. helices α6, α6′, and α6″) (8, 9, 37) over the three PDX1 paralogs suggests that this region is similarly folded for PDX1.1, PDX1.2, and PDX1.3, facilitating the assembly of heterohexamers. Of course, intrahexamer assembly (i.e. monomer mixtures in a single hexamer) must also be considered. However, it has been demonstrated that the C-terminal region of the individual catalytic PDX1 subunits is essential for activity and moreover the positive cooperativity for R5P observed within the complex (34, 36). Notably, the latter is orchestrated by the interaction of a PDX1 protomer with its neighboring protomer within a hexameric ring (i.e. in trans). Furthermore, a hexamer is required for formation of the covalent adduct with R5P (51). Because PDX1.2 does not form the latter adduct, whereas PDX1.1 and PDX1.3 do even in complex with PDX1.2 (Fig. 4), and moreover because PDX1.2 does not show high conservation in the C-terminal region (Fig. 1C), we are more in favor of an interhexamer (i.e. one hexamer stacks and interdigitates with the other, as shown in the scheme in Fig. 10) rather than an intrahexamer dodecameric assembly of the catalytic PDX1s with PDX1.2.
FIGURE 10.

Proposed model for heterocomplex formation with PDX1.3 and PDX1.2, in particular. PDX1.3 can exist in an hexamer/dodecamer equilibrium as depicted by the blue rings. PDX1.3 is subject to ubiquitination (Ub) and degradation through the proteasome pathway. During localized oxidative stress (e.g. singlet oxygen), PDX1.2 is induced (depicted as an amber ring) and can form a heterocomplex with PDX1.3. The pale blue star denotes the N-terminal modification of the PDX1.2 protein that is required for enhancement of PLP biosynthesis by PDX1.3.
Although PDX1.3 has been shown to be the most abundant PDX1 in Arabidopsis (17), its level appears to be tightly regulated, and it cannot be overexpressed at the protein level (16). Indeed, PDX1.3 has been found to be a ubiquitination target (52), suggesting degradation through the proteasome pathway. The interaction of PDX1.2 with PDX1.3 in vivo would represent a strategy to preserve the latter from degradation and maintain production of vitamin B6 at a time when more is needed, such as under conditions of environmental stress. It should be noted that the pdx1.3 mutant, in particular, is highly sensitive to abiotic stress conditions (17, 20). We hypothesize that this response is likely to be concentrated locally, occurring in the vicinity of damaged tissue. Future studies targeting analysis of damaged tissue will help to amplify the response and unravel the precise mechanism. PDX1.1 is likely to be similarly regulated under conditions specific to its spatial and temporal expression. Although this study and independent transcriptional analyses have shown that PDX1.2 is the most sensitive of the PDX1s to abiotic stress (13), the response viewer program of the Arabidopsis microarray database, Genevestigator (53), further corroborates these observations. In particular, PDX1.2 is hyperresponsive to stress conditions, such as high light (induces singlet oxygen production as a result of chlorophyll excitation), ozone, boron accumulation, and heat stress (data not shown). Although PDX1.1 appears to be down-regulated under some of these conditions, PDX1.3 does not change, with the exception of heat stress where it is up-regulated. This provides support to the hypothesis that PDX1.2 serves to enhance and stabilize an already existing PDX1 complex in the cell, as depicted in Fig. 10.
In summary, this study has opened up avenues to further unravel the role of an intriguing pesudoenzyme, PDX1.2, in Arabidopsis and establishes a model for numerous plants harboring this paralog. Moreover, this provides further support for the hypothesis on the importance of these previously assumed dead enzymes. Furthermore, the study suggests a newly established mechanism for the response of certain plants against abiotic stress, mediated by vitamin B6, whose role as an antioxidant has been recently reported.
Acknowledgments
We thank Serge Chensov, René Brunisholz, and Yolanda Auchli for assistance with mass spectrometry analyses at the Functional Genomics Center Zürich. We thank Michael Moulin and Nicolas Szydlowski (University of Geneva) for setting up the HPLC method for vitamin B6 quantification and for assistance during the experiments. We are grateful to Klaus Apel (Cornell University) for kindly providing seeds of the flu mutant.
This work was supported by Swiss National Science Foundation Grants PPOOA_1191186 and 31003A-141117/1 (to T. B. F.) and the University of Geneva.
- PLP
- pyridoxal 5′-phosphate
- R5P
- ribose 5-phosphate
- ESI
- electrospray ionization
- qPCR
- quantitative real-time RT-PCR
- ANOVA
- analysis of variance.
REFERENCES
- 1. Ehrenshaft M., Bilski P., Li M. Y., Chignell C. F., Daub M. E. (1999) A highly conserved sequence is a novel gene involved in de novo vitamin B6 biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 96, 9374–9378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fitzpatrick T. B., Amrhein N., Kappes B., Macheroux P., Tews I., Raschle T. (2007) Two independent routes of de novo vitamin B6 biosynthesis: not that different after all. Biochem. J. 407, 1–13 [DOI] [PubMed] [Google Scholar]
- 3. Ehrenshaft M., Daub M. E. (2001) Isolation of PDX2, a second novel gene in the pyridoxine biosynthesis pathway of eukaryotes, archaebacteria, and a subset of eubacteria. J. Bacteriol. 183, 3383–3390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Osmani A. H., May G. S., Osmani S. A. (1999) The extremely conserved pyroA gene of Aspergillus nidulans is required for pyridoxine synthesis and is required indirectly for resistance to photosensitizers. J. Biol. Chem. 274, 23565–23569 [DOI] [PubMed] [Google Scholar]
- 5. Sakai A., Kita M., Katsuragi T., Ogasawara N., Tani Y. (2002) yaaD and yaaE are involved in vitamin B6 biosynthesis in Bacillus subtilis. J. Biosci. Bioeng. 93, 309–312 [DOI] [PubMed] [Google Scholar]
- 6. Burns K. E., Xiang Y., Kinsland C. L., McLafferty F. W., Begley T. P. (2005) Reconstitution and biochemical characterization of a new pyridoxal-5′-phosphate biosynthetic pathway. J. Am. Chem. Soc. 127, 3682–3683 [DOI] [PubMed] [Google Scholar]
- 7. Raschle T., Amrhein N., Fitzpatrick T. B. (2005) On the two components of pyridoxal 5′-phosphate synthase from Bacillus subtilis. J. Biol. Chem. 280, 32291–32300 [DOI] [PubMed] [Google Scholar]
- 8. Strohmeier M., Raschle T., Mazurkiewicz J., Rippe K., Sinning I., Fitzpatrick T. B., Tews I. (2006) Structure of a bacterial pyridoxal 5′-phosphate synthase complex. Proc. Natl. Acad. Sci. U.S.A. 103, 19284–19289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Zein F., Zhang Y., Kang Y.-N., Burns K., Begley T. P., Ealick S. E. (2006) Structural insights into the mechanism of the PLP synthase holoenzyme from Thermotoga maritima. Biochemistry 45, 14609–14620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Belitsky B. R. (2004) Physical and enzymological interaction of Bacillus subtilis proteins required for de novo pyridoxal 5′-phosphate biosynthesis. J. Bacteriol. 186, 1191–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tambasco-Studart M., Tews I., Amrhein N., Fitzpatrick T. B. (2007) Functional analysis of PDX2 from Arabidopsis thaliana, a glutaminase involved in vitamin B6 biosynthesis. Plant Physiol. 144, 915–925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen H., Xiong L. (2005) Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stresses. Plant J. 44, 396–408 [DOI] [PubMed] [Google Scholar]
- 13. Denslow S. A., Rueschhoff E. E., Daub M. E. (2007) Regulation of the Arabidopsis thaliana vitamin B6 biosynthesis genes by abiotic stress. Plant Physiol. Biochem. 45, 152–161 [DOI] [PubMed] [Google Scholar]
- 14. Denslow S. A., Walls A. A., Daub M. E. (2005) Regulation of biosynthetic genes and antioxidant properties of vitamin B6 vitamers during plant defense responses. Physiol. Mol. Plant Pathol. 66, 244–255 [Google Scholar]
- 15. Knöckel J., Müller I. B., Butzloff S., Bergmann B., Walter R. D., Wrenger C. (2012) The antioxidative effect of de novo generated vitamin B6 in Plasmodium falciparum validated by protein interference. Biochem. J. 443, 397–405 [DOI] [PubMed] [Google Scholar]
- 16. Raschke M., Boycheva S., Crèvecoeur M., Nunes-Nesi A., Witt S., Fernie A. R., Amrhein N., Fitzpatrick T. B. (2011) Enhanced levels of vitamin B6 increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J. 66, 414–432 [DOI] [PubMed] [Google Scholar]
- 17. Titiz O., Tambasco-Studart M., Warzych E., Apel K., Amrhein N., Laloi C., Fitzpatrick T. B. (2006) PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J. 48, 933–946 [DOI] [PubMed] [Google Scholar]
- 18. Bilski P., Li M. Y., Ehrenshaft M., Daub M. E., Chignell C. F. (2000) Vitamin B6 (Pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem. Photobiol. 71, 129–134 [DOI] [PubMed] [Google Scholar]
- 19. Fitzpatrick T. B., Basset G. J., Borel P., Carrari F., DellaPenna D., Fraser P. D., Hellmann H., Osorio S., Rothan C., Valpuesta V., Caris-Veyrat C., Fernie A. R. (2012) Vitamin deficiencies in humans. Can plant science help? Plant Cell 24, 395–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Havaux M., Ksas B., Szewczyk A., Rumeau D., Franck F., Caffarri S., Triantaphylidès C. (2009) Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 9, 130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Leuendorf J. E., Genau A., Szewczyk A., Mooney S., Drewke C., Leistner E., Hellmann H. (2008) The Pdx1 family is structurally and functionally conserved between Arabidopsis thaliana and Ginkgo biloba. FEBS J. 275, 960–969 [DOI] [PubMed] [Google Scholar]
- 22. Szydlowski N., Bürkle L., Pourcel L., Moulin M., Stolz J., Fitzpatrick T. B. (2013) Recycling of pyridoxine (vitamin B6) by PUP1 in Arabidopsis. Plant J. 75, 40–52 [DOI] [PubMed] [Google Scholar]
- 23. Pils B., Schultz J. (2004) Inactive enzyme homologues find new function in regulatory processes. J. Mol. Biol. 340, 399–404 [DOI] [PubMed] [Google Scholar]
- 24. Adrain C., Freeman M. (2012) New lives for old. Evolution of pseudoenzyme function illustrated by iRhoms. Nat. Rev. Mol. Cell Biol. 13, 489–498 [DOI] [PubMed] [Google Scholar]
- 25. Leslie M. (2013) “Dead” enzymes show signs of life. Science 340, 25–27 [DOI] [PubMed] [Google Scholar]
- 26. Leuendorf J. E., Osorio S., Szewczyk A., Fernie A. R., Hellmann H. (2010) Complex assembly and metabolic profiling of Arabidopsis thaliana plants overexpressing vitamin B biosynthesis proteins. Mol. Plant 3, 890–903 [DOI] [PubMed] [Google Scholar]
- 27. Wagner S., Bernhardt A., Leuendorf J. E., Drewke C., Lytovchenko A., Mujahed N., Gurgui C., Frommer W. B., Leistner E., Fernie A. R., Hellmann H. (2006) Analysis of the Arabidopsis rsr4-1/pdx1-3 mutant reveals the critical function of the PDX1 protein family in metabolism, development, and vitamin B6 biosynthesis. Plant Cell 18, 1722–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tambasco-Studart M., Titiz O., Raschle T., Forster G., Amrhein N., Fitzpatrick T. B. (2005) Vitamin B6 biosynthesis in higher plants. Proc. Natl. Acad. Sci. U.S.A. 102, 13687–13692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 30. Raschle T., Arigoni D., Brunisholz R., Rechsteiner H., Amrhein N., Fitzpatrick T. B. (2007) Reaction mechanism of pyridoxal 5′-phosphate synthase: detection of an enzyme bound chromophoric intermediate. J. Biol. Chem. 282, 6098–6105 [DOI] [PubMed] [Google Scholar]
- 31. Meskauskiene R., Nater M., Goslings D., Kessler F., op den Camp R., Apel K. (2001) FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci. U.S.A. 98, 12826–12831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Clough S. J., Bent A. F. (1998) Floral dip. A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16, 735–743 [DOI] [PubMed] [Google Scholar]
- 33. Murashige T., Skoog F. (1962) A revised medium for rapid growth of bioassays with tobacco tissue culture. Physiol. Plant. 15, 473–497 [Google Scholar]
- 34. Moccand C., Kaufmann M., Fitzpatrick T. B. (2011) It takes two to tango. Defining an essential second active site in pyridoxal 5′-phosphate synthase. PLoS One 6, e16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neuwirth M., Strohmeier M., Windeisen V., Wallner S., Deller S., Rippe K., Sinning I., Macheroux P., Tews I. (2009) X-ray crystal structure of Saccharomyces cerevisiae Pdx1 provides insights into the oligomeric nature of PLP synthases. FEBS Lett. 583, 2179–2186 [DOI] [PubMed] [Google Scholar]
- 36. Raschle T., Speziga D., Kress W., Moccand C., Gehrig P., Amrhein N., Weber-Ban E., Fitzpatrick T. B. (2009) Intersubunit cross-talk in pyridoxal 5′-phosphate synthase, coordinated by the C terminus of the synthase subunit. J. Biol. Chem. 284, 7706–7718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Guédez G., Hipp K., Windeisen V., Derrer B., Gengenbacher M., Böttcher B., Sinning I., Kappes B., Tews I. (2012) Assembly of the eukaryotic PLP-synthase complex from Plasmodium and activation of the Pdx1 enzyme. Structure 20, 172–184 [DOI] [PubMed] [Google Scholar]
- 38. Matsuura A., Yoon J. Y., Yoon H. J., Lee H. H., Suh S. W. (2012) Crystal structure of pyridoxal biosynthesis lyase PdxS from Pyrococcus horikoshii. Mol. Cells 34, 407–412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hanes J. W., Burns K. E., Hilmey D. G., Chatterjee A., Dorrestein P. C., Begley T. P. (2008) Mechanistic studies on pyridoxal phosphate synthase: the reaction pathway leading to a chromophoric intermediate. J. Am. Chem. Soc. 130, 3043–3052 [DOI] [PubMed] [Google Scholar]
- 40. Hanes J. W., Keresztes I., Begley T. P. (2008) Trapping of a chromophoric intermediate in the Pdx1-catalyzed biosynthesis of pyridoxal 5′-phosphate. Angew. Chem. Int. Ed. Engl. 47, 2102–2105 [DOI] [PubMed] [Google Scholar]
- 41. Hanes J. W., Keresztes I., Begley T. P. (2008) 13C NMR snapshots of the complex reaction coordinate of pyridoxal phosphate synthase. Nat. Chem. Biol. 4, 425–430 [DOI] [PubMed] [Google Scholar]
- 42. Zhang K., Yau P. M., Chandrasekhar B., New R., Kondrat R., Imai B. S., Bradbury M. E. (2004) Differentiation between peptides containing acetylated or tri-methylated lysines by mass spectrometry: an application for determining lysine 9 acetylation and methylation of histone H3. Proteomics 4, 1–10 [DOI] [PubMed] [Google Scholar]
- 43. Arnesen T., Van Damme P., Polevoda B., Helsens K., Evjenth R., Colaert N., Varhaug J. E., Vandekerckhove J., Lillehaug J. R., Sherman F., Gevaert K. (2009) Proteomics analyses reveal the evolutionary conservation and divergence of N-terminal acetyltransferases from yeast and humans. Proc. Natl. Acad. Sci. U.S.A. 106, 8157–8162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Arnesen T. (2011) Towards a functional understanding of protein N-terminal acetylation. PLoS Biol. 9, e1001074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Starheim K. K., Gevaert K., Arnesen T. (2012) Protein N-terminal acetyltransferases: when the start matters. Trends Biochem. Sci. 37, 152–161 [DOI] [PubMed] [Google Scholar]
- 46. Charbaut E., Redeker V., Rossier J., Sobel A. (2002) N-terminal acetylation of ectopic recombinant proteins in Escherichia coli. FEBS Lett. 529, 341–345 [DOI] [PubMed] [Google Scholar]
- 47. Webb K. J., Lipson R. S., Al-Hadid Q., Whitelegge J. P., Clarke S. G. (2010) Identification of protein N-terminal methyltransferases in yeast and humans. Biochemistry 49, 5225–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ehrenshaft M., Jenns A. E., Chung K. R., Daub M. E. (1998) SOR1, a gene required for photosensitizer and singlet oxygen resistance in Cercospora fungi, is highly conserved in divergent organisms. Mol. Cell 1, 603–609 [DOI] [PubMed] [Google Scholar]
- 49. op den Camp R. G., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg E., Göbel C., Feussner I., Nater M., Apel K. (2003) Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell 15, 2320–2332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Sung D. Y., Vierling E., Guy C. L. (2001) Comprehensive expression profile analysis of the Arabidopsis Hsp70 gene family. Plant Physiol. 126, 789–800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Derrer B., Windeisen V., Guédez Rodríguez G., Seidler J., Gengenbacher M., Lehmann W. D., Rippe K., Sinning I., Tews I., Kappes B. (2010) Defining the structural requirements for ribose 5-phosphate-binding and intersubunit cross-talk of the malarial pyridoxal 5-phosphate synthase. FEBS Lett. 584, 4169–4174 [DOI] [PubMed] [Google Scholar]
- 52. Manzano C., Abraham Z., López-Torrejon G., Del Pozo J.-C. (2008) Identification of ubiquitinated proteins in Arabidopsis. Plant Mol. Biol. 68, 145–158 [DOI] [PubMed] [Google Scholar]
- 53. Hruz T., Laule O., Szabo G., Wessendorp F., Bleuler S., Oertle L., Widmayer P., Gruissem W., Zimmermann P. (2008) Genevestigator V3: a reference expression database for the meta-analysis of transcriptomes. Adv. Bioinformatics 2008, 420747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Roy A., Kucukural A., Zhang Y. (2010) I-TASSER. A unified platform for automated protein structure and function prediction. Nat. Protoc. 5, 725–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.-F., Guindon S., Lefort V., Lescot M., Claverie J.-M., Gascuel O. (2008) Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36, W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]