Background: Proximal tubule kidney epithelial cells differentiate into a “loose” epithelium by unknown mechanisms.
Results: Deleting integrin β1 converts proximal tubule cells from a “loose” to a “tight” epithelium.
Conclusion: Integrin β1 regulates the composition and function of tight and adherens junctions that define paracellular transport properties of proximal tubule epithelial cells.
Significance: Integrins might regulate terminal differentiation of polarized epithelial cells.
Keywords: Adherens Junction, E-cadherin, Epithelial Cell, Renal Physiology, Tight Junctions
Abstract
Epithelial cells lining the gastrointestinal tract and kidney have different abilities to facilitate paracellular and transcellular transport of water and solutes. In the kidney, the proximal tubule allows both transcellular and paracellular transport, while the collecting duct primarily facilitates transcellular transport. The claudins and E-cadherin are major structural and functional components regulating paracellular transport. In this study we present the novel finding that the transmembrane matrix receptors, integrins, play a role in regulating paracellular transport of renal proximal tubule cells. Deleting the integrin β1 subunit in these cells converts them from a “loose” epithelium, characterized by low expression of E-cadherin and claudin-7 and high expression of claudin-2, to a “tight” epithelium with increased E-cadherin and claudin-7 expression and decreased claudin-2 expression. This effect is mediated by the integrin β1 cytoplasmic tail and does not entail β1 heterodimerization with an α-subunit or its localization to the cell surface. In addition, we demonstrate that deleting the β1 subunit in the proximal tubule of the kidney results in a major urine-concentrating defect. Thus, the integrin β1 tail plays a key role in regulating the composition and function of tight and adherens junctions that define paracellular transport properties of terminally differentiated renal proximal tubule epithelial cells.
Introduction
The mammalian kidney is formed by an intricate network of nephrons consisting of a glomerulus (the filtering unit) followed by tubules lined by a monolayer of polarized epithelial cells. The tubules are formed by anatomically distinct segments; namely the proximal tubule, the loop of Henle, the thick ascending limb, the connecting segment, and the collecting duct. The primary function of the tubules is to reabsorb and secrete solute and water from filtrate to form concentrated urine. The proximal tubule mainly functions as a bulk transporter of water and solute, and it accounts for ∼70% of all glomerular filtrate reabsorption. As the renal tubule becomes more distal, it reabsorbs and secretes less but with higher fidelity. In fact, the collecting ducts only regulate about 3% of solute reabsorption, and their reabsorption of water is hormonally regulated. To perform these diverse functions, the various tubular segments have distinct molecular and morphological characteristics. The proximal tubule is lined by a “loose” epithelium that allows both transcellular and paracellular transport, while the tubules forming the distal nephron and the collecting system are lined by a “tight” epithelium that primarily facilitates transcellular transport. These transport characteristics are primarily regulated by the adherens (AJ)3 and tight (TJ) junctions between the epithelial cells.
The TJ is the most apical adhesion complex in polarized renal epithelial cells. TJs regulate both paracellular permeability across epithelial cell sheets and also serve as a barrier to intramembrane diffusion of components between apical and basolateral membrane domains (1). TJs consist primarily of members from three transmembrane protein families; the occludins, junctional adhesion molecules, and claudins. Occludins directly interact with zonula occludens proteins, the actin cytoskeleton, and junctional adhesion molecules and are proposed to regulate signaling events at the TJ (1). JAMs are single transmembrane spanning members of the immunoglobulin superfamily that participate in cell adhesion through homophilic interactions (1). Claudins (of which there are 27 members) are the backbone of the TJ complex. They have 4 transmembrane domains, two extracellular loops, and three intracellular domains. They form a barrier-like structure by forming cis-interactions with other claudins in the same cell and trans-interactions with claudins in adjacent cells (2). Multiple claudins are expressed in different segments of the nephron, and they determine the paracellular permeability properties or “tightness” of the seal between these cells. There are two major forms of claudins: the barrier claudins, which increase transepithelial resistance (TER) when overexpressed in leaky cell lines and the pore claudins, which decrease TER in most cell lines. Claudin-2, which is a pore claudin, is highly expressed in the loose epithelium of the proximal tubules. In contrast, a number of the barrier claudins, including claudin-7, are expressed in the distal nephron and collecting ducts. These two claudins have been used as markers of loose and tight epithelia, respectively (2).
AJs, which are also key components of epithelial cell-cell junctions, are protein complexes located more basally than TJs. They consist of cadherin/catenin complexes anchored to the cytoskeleton and the microtubules as well as the nectin/afadin complexes, which are also anchored to the actin cytoskeleton (3–5). The transmembrane-spanning cadherins bind homotypically with cadherins on opposite cells to form trans-bonds across the cell contact in a Ca2+-dependent manner. E-cadherin is the major AJ protein expressed in epithelial cells and is highly expressed in the distal nephron and the collecting ducts, where it plays a role in decreasing paracellular permeability (6). Interestingly, E-cadherin is expressed in low abundance in the loose epithelium of the proximal tubule, whereas N-cadherin, which does not regulate paracellular permeability, is the major cadherin (7).
In addition to cell-cell junctions, cell-extracellular matrix (ECM) interactions play a key role in sustaining epithelial architecture. Integrins are the principal transmembrane receptors whereby cells bind to ECM. They exist as αβ heterodimers formed from 18 α- and 8 β-subunits, each of which exhibits different ligand binding and signaling properties (8). Each integrin subunit consists of an extracellular domain, which determines the ligand binding properties, a transmembrane domain, and a short cytoplasmic tail that binds to multiple cytosolic and transmembrane proteins to form focal adhesions (9). Integrins are primarily thought of as adhesive molecules; however, they also act as signaling hubs for numerous cellular processes as they allow cells to sense and respond to their microenvironment. β1 is the most abundantly expressed β integrin subunit and is found in almost all cell types in the body, including the kidney tubule epithelium. The integrin β1 cytoplasmic tail regulates integrin functions by binding to signaling- and actin-binding proteins. Two well-defined NPXY motifs found in the integrin β1 cytoplasmic domain play a key role in regulating multiple integrin-dependent functions by interacting with proteins such as talins and kindlins (10).
In this study, we propose a new role for integrins in the context of epithelial cell biology. We show that the integrin β1 regulates the permeability properties or tightness of renal proximal tubule cells in vitro by regulating the transcription of claudins and cadherins. This function is mediated by the β1 cytoplasmic domain and does not require the integrin subunit to be expressed at the cell surface or interact with ECM. In addition, we show that deleting the β1 integrin subunit in the proximal tubule results in a significant abnormality in the ability of the kidney to concentrate urine. These data suggest a novel mechanism whereby integrins regulate the composition and function of TJs and AJs in highly terminally differentiated polarized epithelial cells. Furthermore they suggest that integrin β1 expression regulates the absorptive characteristics of the proximal tubule of the kidney in vivo.
EXPERIMENTAL PROCEDURES
Reagents
All chemicals were of analytical grade and were purchased from ThermoFisher Scientific, Waltham, MA or Sigma. Mouse anti E-cadherin antibody was purchased from BD Biosciences, San Jose, CA, mouse anti-claudin-2 and claudin-7 were from Sigma, and rabbit anti ZO-1 was from Invitrogen, Grand Island, NY.
Generation of Integrin β1−/− Proximal Tubule Cells
We generated proximal tubule cells (PTC) from β1flox/flox mice crossed with mice containing the Immortomouse transgene (H-2Kb-tsA58) using a previously modified protocol (11). Briefly, cortices were isolated from 6-week-old mice, digested with collagenase, passed through a 70-micron filter, and separated on a Percoll gradient by centrifugation into four bands. The bottom (F4) band was removed, washed, and plated with DMEM/F12 media containing 2.5% FBS, 50 ng/ml hydrocortisone, 5 μg/ml insulin/transferrin/selenium, 6.5 ng/ml triiodothyronine, 92 μg/ml d-valine, and penicillin/streptomycin. PTCs were incubated at 33 °C with 10 ng/ml interferon-γ because the large tumor antigen of the Immortomouse transgene is thermolabile and interferon-inducible. Two weeks before experiments, PTCs in passages two to eight were transferred to 37 °C, and interferon-γ was removed.
The β1 integrin subunit was deleted by infecting the cells with an adenocre virus in vitro. To verify adequate deletion of β1 integrin, the cells were subjected to flow cytometry as previously described (12). The β1−/− PTCs were reconstituted with full-length human β1 integrin, β1 integrin truncated at Glu-769, β1 integrin carrying a cytosolic domain YY/AA mutation (13), the Tac-β1 chimera (14), and the GFP-tagged cytoplasmic domain of β1 integrin. To ensure equal surface expression of the WT or mutant β1 integrin subunits, they were selected using a fluorescence-activated cell sorter (FACS) and antibody AIIB2, a monoclonal antibody directed against the extracellular domain of the human integrin β1 (primary) and an anti-rat phycoerythrin (PE) (secondary) or GFP. The cells expressing Tac-β1 were sorted using a monoclonal antibody directed against the extracellular domain of the IL2 receptor.
Generation of Kidney Interstitial Cells
Mouse renal interstitial fibroblasts were isolated as previously described (15). Renal cortex was minced and digested in a solution of 0.2% type I collagenase. To remove contaminating glomeruli and tubules, the digests were passed through a 36-μm mesh. Renal fibroblasts were cultured in DMEM containing 10% FBS. Isolated cells were identified as interstitial fibroblasts by their fibroblastic morphology and immunocytochemical staining (positive for the mesenchymal marker, α-smooth muscle actin, and negative for an epithelial marker, E-cadherin).
Measurement of Transepithelial Resistance, Inulin Flux, and the Calcium Switch
The degree of tightness of the TJ was evaluated by measuring the TER across the cells as previously described (16). Cell monolayers were grown to confluency on 24-well transwell dishes, and TER was measured using an EVOM epithelial voltmeter from World Precision Instruments, Sarasota, FL. The resistance was represented as ohms/cm2.
Inulin flux assays were performed on PTC monolayers grown on transwell dishes, as described previously (16). 5 μg/ml fluorescein isothiocyanate-conjugated inulin were added to the apical layer, and 100 μl from the basal and 50 μl from the apical layers were taken and read in the Fluoroscan plate reader at various time points. The inulin flux into the basal well was represented as flux/hr./cm2.
The calcium switch assay was carried out as previously described (17). Briefly, cell monolayers were treated with 4 mm EGTA in both the apical and basal compartments until the TER was reduced to about 15–17% that of basal values. The cells were then quickly washed three times with DMEM to remove all traces of EGTA and incubated in regular DMEM containing calcium for varying times. The integrity of TJ was analyzed by measuring TER.
Quantitative RT-PCR
RNA was isolated from cells using Trizol (Invitrogen) as per the supplier's protocol. RNA was quantitated prior to cDNA synthesis. Primers for E-cadherin, claudin-2, and claudin-7 were used to synthesize respective cDNAs, using the iScript kit from Bio-Rad. Quantitative RT-PCR was run on the Bio-Rad CFX96 instrument, using the sybr green 2stepAmp+ melt curve protocol.
Immunofluorescence
Cells were grown to confluency on 12-mm diameter, 0.4-μm pore size transwell inserts. The membranes were cut from the transwell support, washed twice with PBS, fixed with 4% paraformaldehyde, blocked with 3% nonfat milk in Tris-buffered saline (TBST), and incubated with appropriate primary antibodies followed by the secondary antibodies. The membranes were then mounted on glass slides using Vectashield from Vector Laboratories Inc.
Cell Fractionation Protocols
Triton-insoluble (actin-rich) and Triton-soluble fractions of PTCs were prepared as described previously (18). Briefly, PTCs were incubated for 5 min with lysis buffer-CS (50 mm Tris/HCl, pH 7.4, 1.0% Triton X-100, 5 mm EGTA, and 10 μg/ml protease inhibitor mixture). Cell lysates were centrifuged at 15,600 × g for 5 min at 4 °C to sediment the high density actin-rich fraction. The pellet was suspended in 200 μl of lysis buffer D (0.3% SDS in 20 mm Tris/HCl buffer, pH 7.4, and 10 μg/ml protease inhibitor mixture). Fractionation of nuclear and cytoplasmic proteins was performed using the NE-PER kit from Thermo Scientific as per their protocol.
Immunoblotting
Cells were washed with cold PBS, lysed with cell lysis buffer (Cell Signaling Technology, Danvers, MA), after which the cell lysate was clarified by centrifugation, and the protein was estimated using the Pierce BCA protein assay kit. Equal amounts of protein were loaded on SDS-polyacrylamide gels and electrophoresed. The proteins were transferred onto PVDF membranes and blocked with either 5% nonfat milk or BSA. The membranes were then immunoblotted with specific primary antibodies and appropriate secondary antibodies conjugated with HRP. The immunoreactive signals were detected by using the Amersham Biosciences's ECL reagent (GE Healthcare, Pittsburgh, PA).
Generation of γgtcre:β1flox/flox Mice
All procedures were approved by the Institutional Animal Care and Use Committee of Vanderbilt University and conducted according to the National Institutes of Health Guide for the Care and Use of Laboratory Animals. β1flox/flox mice (gift from Elaine Fuchs) (19) were crossed with mice containing cre under control of the γgt promoter (gift from Eric Neilson) (20).
Metabolic Studies
Mice undergoing metabolic studies were acclimatized to metabolic cages (Hatteras, Cary, NC) for 2 days. Water intake and urine output were measured for 24 h after the mice were acclimatized to the cages. For acute water loading, mice were injected with 2 ml of water intraperitoneally followed by a second injection of 2 ml, 18 h after the first. Subsequent to the second injection, the mice were fluid restricted, and urine was collected at 2-h intervals. Urine osmolality was determined using freezing point depression (FPD) as measured by an Advanced Instruments Osmometer, Model 3320 (Advanced Instruments).
Statistical Analysis
The Student's t test for comparisons between two groups and analysis of variance to assess statistical differences between multiple groups were carried out using Sigma-Stat software. A p value of </= 0.05 was considered statistically significant.
RESULTS
Deleting Integrin β1 in Proximal Renal Tubule Cells Decreases Claudin-2 Expression
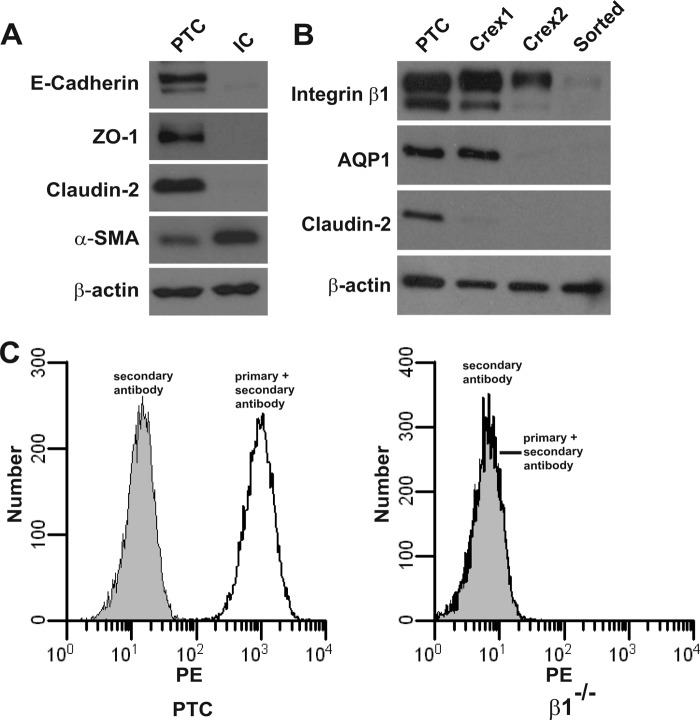
We generated PTCs from β1flox/flox mice and verified the cells were derived from this nephron segment and were epithelial in nature, as they expressed E-cadherin, ZO-1, and claudin-2 a tight junction protein localized to the proximal tubule (Fig. 1A). By contrast, renal interstitial cells, which are of mesenchymal origin, expressed large amounts of α-smooth muscle actin relative to the PTCs. PTCs lacking β1 expression (β1−/−) were produced by infecting β1flox/flox PTCs with adeno-Cre. When we checked the cre efficiency, we noted that with decreasing expression of integrin β1 there was loss of expression of claudin-2 and the water channel aquaporin 1 (AQP1), another protein that is highly expressed in PTCs (Fig. 1B). A pure β1−/− PTC population sorted by flow cytometry no longer expressed either claudin-2 or AQP1 (Fig. 1, B and C), suggesting that integrin β1 regulated the expression of proteins that are specifically found in PTCs.
FIGURE 1.
Integrin β1−/− PTCs lose expression of aquaporin 1 and claudin-2. A, Western blot analysis of cell lysates obtained from kidney PTCs and interstitial cells (IC) demonstrating that PTCs express the epithelial cell markers E-cadherin, ZO-1, and claudin-2, while ICs express smooth muscle actin (SMA). β-Actin was run as a loading control. B and C, PTCs obtained from β1fl/fl mice were infected twice with adeno cre virus to delete β1 integrin. To obtain a pure β1−/− population, they were sorted via FACS with antibodies directed to the extracellular domain of integrin β1. B, immunoblotting of cell lysates demonstrated that integrin β1 expression decreased with sequential adeno-cre infections, and there was no expression in the β1−/− PTC population. Immunoblotting for aquaporin 1 (AQP1) and claudin-2 demonstrates that their expression decreases as the β1 integrin expression decreases. C, left panel demonstrates high surface expression of integrin β1 in β1fl/fl PTCs, whereas the right panel shows there is no β1 surface expression in β1−/− PTCs.
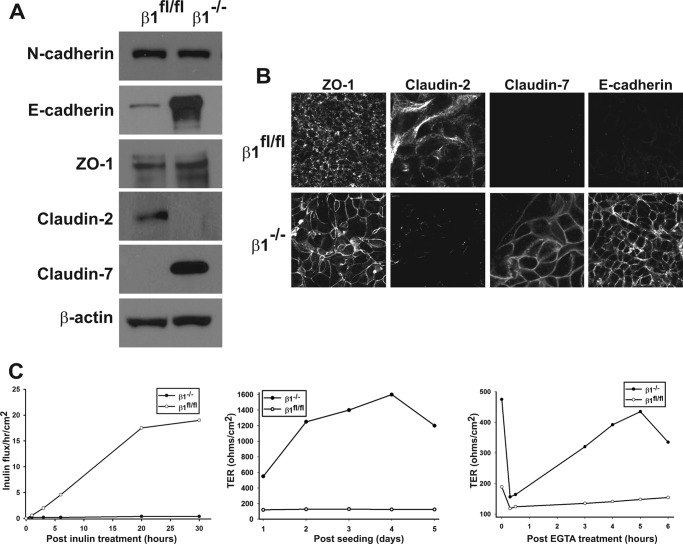
Deleting Integrin β1 in PTCs Increases E-cadherin and Claudin-7 Expression and Decreases Transcellular Permeability
The observation that deleting integrin β1 resulted in altered expression of PTC-specific proteins prompted us to characterize the β1flox/flox and β1−/− PTC populations in detail. ZO-1, as well as N-cadherin, which is ubiquitously expressed in all renal tubule cells (7), was expressed equally in both cell populations (Fig. 2A). Interestingly, there was a marked increase in E-cadherin and claudin-7 and decreased claudin-2 expression in the β1−/− PTC populations, suggesting these cells acquired the characteristics of tight epithelial cells found in the distal nephron or collecting ducts. We verified changes of expression of these proteins by performing immunofluorescence on PTCs grown on transwells. While there were comparable amounts of ZO-1 expression in the two cell populations, ZO-1 was localized to the cell membrane of β1−/− PTCs, but not β1flox/flox PTCs (Fig. 2B). Claudin-2 was expressed on the cell surface of the β1flox/flox PTCs, but its expression was undetectable in β−/− PTCs. In contrast, markedly increased expression of E-cadherin and claudin-7 was detectable only on the cell surface of the β1−/− PTCs (Fig. 2B). The increased E-cadherin and claudin-7 expression by β1−/− PTCs, typical of tight epithelial cells, suggested these cells would have diminished paracellular transport and TER. To test this possibility, we measured inulin flux across monolayers of β1flox/flox and β1−/− PTCs grown on transwells. There was negligible flux across β1−/− PTCs, while a large amount fluxed across β1flox/flox PTCs (Fig. 2C). We next measured the TER in these different cell populations and showed approximately a 16-fold increase in TER in β1−/− relative to β1flox/flox PTCs by day 4 when the cells reached confluence (Fig. 2C). The β1flox/flox PTCs never increased their TER above 100 ohms/cm2 irrespective of their density. We verified that the high TER observed in the β1−/− PTCs was at least in part due to the high surface expression of E-cadherin, because there was marked TER loss and reconstitution in the calcium switch assay, which measures the ability of E-cadherins to form calcium-dependent homophilic interactions (Fig. 2C). Taken together, these results show that deleting integrin β1 changes renal PTCs from low to high TER and changes the expression profile of AJ and TJ proteins that support a tight phenotype.
FIGURE 2.
Deleting integrin β1 alters E-cadherin and claudin expression as well as inulin flux and TER. A, cell lysates from β1fl/fl and β1−/− PTCs were analyzed for expression of AJ (E-cadherin and N-cadherin) and TJ (ZO-1 and claudins 2 and 7) proteins by Western blot analysis. B, β1fl/fl and β1−/− PTCs were grown to confluence on transwell inserts and immunostained for ZO-1, E-cadherin, claudin-2, and claudin-7. C, β1fl/fl and β1−/− PTCs were grown on transwell inserts. Inulin clearance (left panel), transepithelial resistance (TER) (middle panel), and reassembly of tight junctions as measured by the calcium switch assay (right panel) were assessed as described under “Experimental Procedures.” An example from a single assay is shown. At least three assays were performed with similar results.
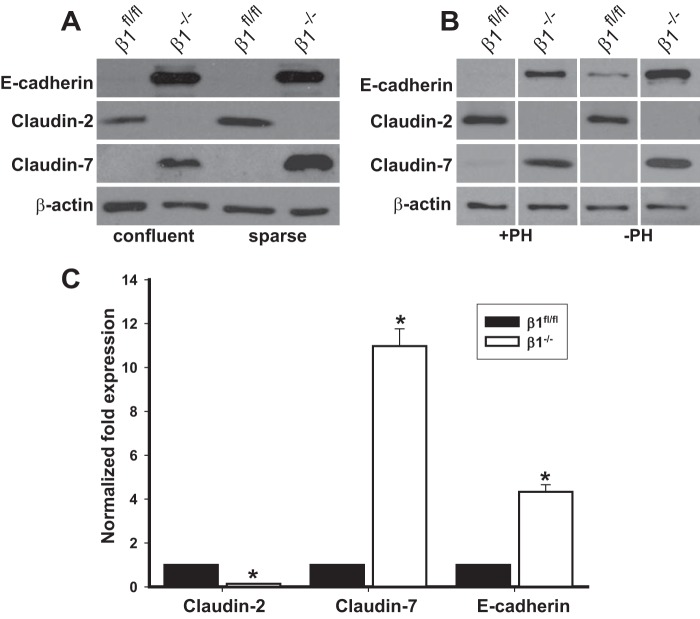
Claudins and E-cadherin Expression Is Transcriptionally Regulated by Integrin β1 and Is Independent of Confluency and Anchorage
The confluency of cultured PTCs can regulate the expression of TJ and AJ proteins. We, therefore, investigated whether the unexpected differences in E-cadherin, claudin-2, or claudin-7 expression was related to cell confluence. This was not the case as under both sparse and confluent conditions increased E-cadherin and claudin-7 and decreased claudin-2 expression were seen in β1−/− PTCs (Fig. 3A). The principal function of integrins is to promote cell adhesion to extracellular matrix and deleting integrin β1 from PTCs caused a severe adhesion defect on multiple extracellular matrices (data not shown). We therefore determined whether cell adhesion was required for this change in phenotype by growing cells in polyhema (2-hydroxyethyl methacrylate), which prevents cells from adhering to cell culture plates. As shown in Fig. 3B, E-cadherin, claudin-2, and claudin-7 expression was similar under both conditions, suggesting that integrin-dependent cell adhesion was not required for integrin β1 to induce these changes on PTCs.
FIGURE 3.
Claudin and E-cadherin expression is transcriptionally regulated by integrin β1 and is independent of confluency and anchorage. A, cell lysates from β1fl/fl and β1−/− PTCs either sparsely grown or grown to confluency were immunoblotted for the proteins shown in the figure. B, β1fl/fl and β1−/− PTCs were grown in the presence or absence of polyhema (PH), and the amount of E-cadherin and claudin-2 and -7 was analyzed by Western blotting. The lanes are separated by lines to demarcate samples run on the same gel but not in the same order as shown in the figure. C, quantitative RT-PCR analyses for claudin-2, claudin-7, and E-cadherin was performed on cDNA synthesized from RNA isolated from β1fl/fl and β1−/− PTCs. The results were normalized to expression by β1fl/fl PTCs. Mean measurements of three independent experiments are shown; *, p ≤ 0.05 between β1fl/fl and β1−/− PTCs.
We next determined whether the change in expression of the AJ and TJ proteins between the β1flox/flox and β1−/− PTCs was regulated at the transcriptional level. Quantitative RT-PCR showed that there was a significant decrease in claudin-2 and increase in E-cadherin and claudin-7 message in β1−/− PTCs (Fig. 3C). Addition of the ubiquitin inhibitor lactacystin or chloroquin, which inhibits the lysosomal degradative pathway, did not change the amount of protein in either of the cell populations (data not shown). Together, these results suggest that integrin β1 transcriptionally regulates the expression levels of claudin-2, claudin-7, and E-cadherin in PTCs.
Transducing β1−/− PTCs with Integrin β1 Reestablishes a Loose Epithelium and AQP1 Expression
We next confirmed that the unexpected result of increased TER in integrin β1−/− PTCs occurred as a direct consequence of the loss of integrin β1 by transfecting them with a full-length human integrin β1 cDNA and sorting for a pure cell population of PTCs expressing integrin β1 on the cell surface (Fig. 4A). The reconstituted (RC) PTCs had increased claudin-2 as well as AQP1 and decreased E-cadherin and claudin-7 expression compared with β1−/− PTCs (Fig. 4B). Consistent with these alterations in expression of AJ and TJ proteins the RC PTCs reverted to an epithelium with low TER (Fig. 4C). These results confirm that the change in the phenotype of β1−/− PTCs was directly due to the loss of integrin β1 expression.
FIGURE 4.

Transducing β1−/− PTCs with integrin β1 reestablishes a loose epithelium. A, human integrin β1 cDNA was transfected into the β1−/− PTCs, after which they were sorted to obtain a pure population of high expressing cells (RC). Surface expression of integrin β1 was verified by flow cytometry. B, equal amounts of whole cell lysate from β1−/− and RC PTCs were electrophoresed and immunoblotted for E-cadherin, claudin-2, and claudin-7. C, TER was measured on β1−/− and RC PTCs grown to confluency on transwell inserts for 3 days. Mean measurements of three independent experiments are shown; *, p ≤ 0.05 between β1−/− and RC PTCs.
The Cytoplasmic Tail of Integrin β1 Is Sufficient for Changes in Regulation of TER, E-cadherin, Claudin-2, and Claudin-7 in PTCs
Our results showing that integrin-dependent adhesion to ligand was not required for integrin β1 to regulate the tightness of PTCs suggested these effects were mediated by the integrin β1 cytoplasmic tail. We tested this hypothesis by transfecting the β1−/− PTCs with cDNA encoding the Tacβ1 chimeric integrin, which consists of the IL2 receptor extracellular and transmembrane domain and the integrin β1 cytoplasmic domain (Fig. 5A) (14), or with an IL2 receptor control vector. The PTCs were sorted using an anti-IL2 antibody to obtain a population where comparable levels of IL2 and Tacβ1 were expressed on the cell surface (data not shown). The Tacβ1 expressing β1−/− PTCs cells had the same phenotype as β1−/− PTCs reconstituted with the full-length integrin β1 subunit, with decreased expression of E-cadherin and claudin-7 and increased expression of claudin-2 and AQP1 relative to the β1−/− PTCs transfected with the IL2 receptor only (Fig. 5B). Consistent with the altered expression levels of E-cadherin and the claudins, the Tacβ1 PTCs had a low TER (Fig. 5C). These data suggest that the cytoplasmic domain of integrin β1 integrin tail is sufficient for β1 to regulate the PTC tightness.
FIGURE 5.
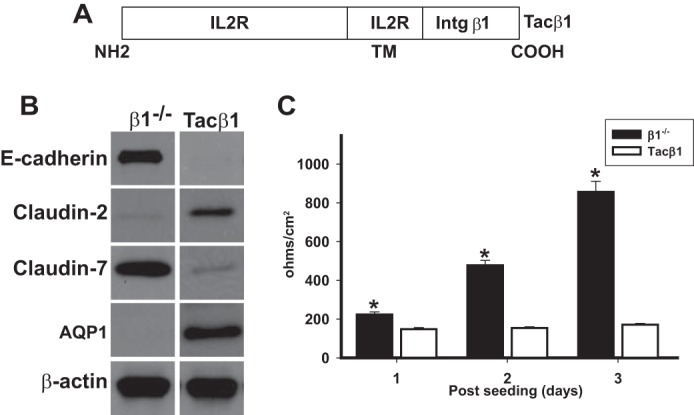
The cytoplasmic tail of integrin β1 is sufficient for changes in regulation of TER, E-cadherin, claudin-2, and claudin-7 in PTCs. A, schematic of the Tacβ1 construct. The extracellular and transmembrane domain of this chimera is from the Tac receptor of human interleukin 2 (IL2R), and the cytoplasmic domain is from the β1 integrin. B, equal amounts of whole cell lysate from PTCs stably expressing Tacβ1 or the IL2R (β1−/−) cells were electrophoresed and immunoblotted for E-cadherin, claudin-2, or claudin-7. Protein loading was controlled for by immunoblotting for β-actin. C, TER was measured on β1−/− and Tacβ1 PTCs grown to confluency on transwell inserts for 3 days. Mean measurements of three independent experiments are shown; *, p ≤ 0.05 between Tacβ1 and β1−/− PTCs.
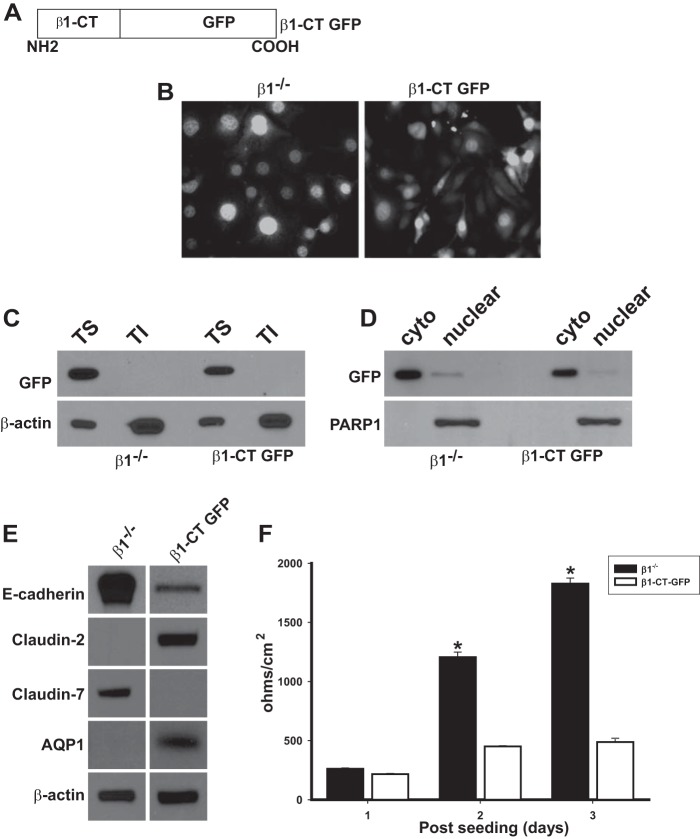
Free Cytoplasmic Integrin β1 Cytoplasmic Domains Are Sufficient to Regulate AJ and TJ Composition of PTCs
Our data thus far suggested that the integrin β1 tail exerts its effects in a ligand-independent manner and does not require heterodimerization with α-subunits. We therefore defined whether expression of non-cell membrane-associated free integrin β1 cytoplasmic domains could alter the composition of PTC AJs and TJs as well as their TER. We expressed a free integrin β1 cytoplasmic domain (β1-CT) fused to GFP in β1−/− PTCs (Fig. 6A) and used β1−/− PTCs transfected with GFP only as control. Both cell populations were sorted for medium expression of GFP by flow cytometry. The distribution of GFP was very similar in both GFP and β1CT-GFP PTCs as verified by fluorescent microscopy (Fig. 6B). We confirmed the β1CT-GFP was not expressed on the cell membrane by performing an immunoblot of Triton-soluble and -insoluble fractions of the transfected PTCs and found that GFP was only present in the soluble fraction (Fig. 6C). We established that the β1CT-GFP did not accumulate in the nucleus by generating nuclear and cytosolic fractions and showing that GFP was only expressed in cytosolic fractions (Fig. 6D). The β1CT-GFP PTCs expressed low amounts of E-cadherin as well as claudin-7 but expressed claudin-2 and AQP1 (Fig. 6E). Consistent with these expression patterns, β1CT-GFP PTCs had a consistently low TER (Fig. 6F). Thus the phenotype of the β1CT-GFP PTCs was identical to that of β1flox/flox PTCs, confirming that localization of the integrin β1 cytoplasmic domain to the cell surface is not required for PTCs to form a “leaky” epithelium characterized by low levels of E-cadherin and high levels of claudin-2 expression. Furthermore, the fact that the CT is not found in the nucleus means that integrin β1 does not directly interact with the transcriptional machinery.
FIGURE 6.
Free cytoplasmic integrin β1 cytoplasmic domains are sufficient to regulate AJ and TJ composition of PTCs. A, schematic of the integrin β1cytoplasmic tail GFP construct (β1-CT GFP) where the cytoplasmic domain of integrin β1 was cloned in-frame with GFP in the pEGFP-N2 vector. B, β1−/− PTCs transfected with either an empty GFP vector (β1−/−) or β1-CT GFP were sorted via FACS to collect cell populations expressing equal levels of GFP. GFP levels and localization were analyzed in the cells by placing the cells under an epifluorescence microscope. Cells were incubated with DAPI to visualize nuclei. C, equal amounts of Triton-soluble (TS) and insoluble (TI) fractions from β1−/− and β1-CT GFP PTCs were analyzed by Western blot for levels of and localization of GFP. D, equal amounts of cytoplasmic (cyto) and nuclear fractions of β1−/− and β1-CT GFP PTCs were analyzed by Western blot using anti-GFP antibodies. GFP was only detected only in the cytoplasm and not the nucleus in both cell lines. PARP1 was used to verify the nuclear fraction. E, equal amounts of whole cell lysate from β1−/− and β1-CT GFP PTCs were analyzed by Western blot for levels of E-cadherin, claudin-2, or claudin-7. β-Actin was used to verify equal loading. F, TER was measured on stably expressing β1−/− and β1-CT GFP PTCs grown to confluency on transwell inserts for 3 days. Mean measurements of three independent experiments are shown; *, p ≤ 0.05 between β1-CT GFP and β1−/− PTCs.
E-cadherin, Claudin-2, and Claudin-7 Expression Is Regulated by Specific Domains of the Integrin β1 Tail
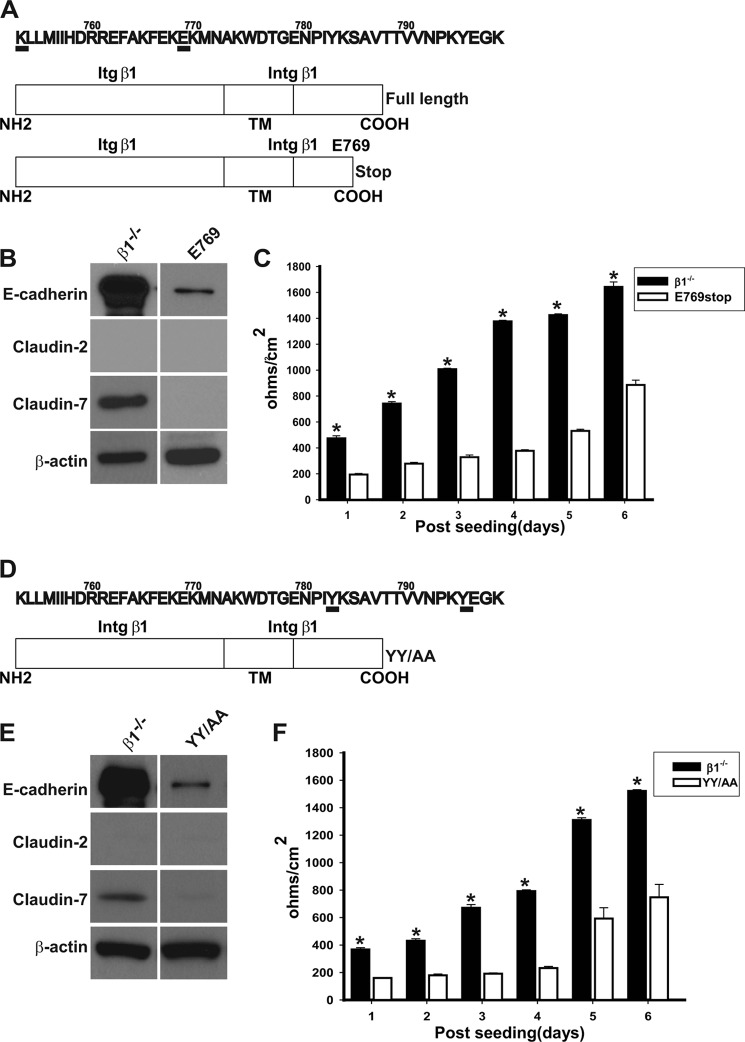
We next investigated which part of the integrin β1 tail was required to regulate the looseness of the PTCs by transfecting β1−/− PTCs with deletion mutants of the integrin β1 tail. We deleted the entire 47 amino acid of the β1 tail (K752-K798) as well as the last 29 amino acids immediately after Glu-769 (Lys-770 to Lys-798) (Fig. 7A). We were unable to get surface expression of the construct lacking the β1 tail; however, we obtained a population that expressed the Glu-769 mutant on the cell surface at the same level as PTCs reconstituted with full-length integrin β1 (RC PTP) (data not shown). The β1E769 PTCs (Fig. 7B) had decreased expression of E-cadherin just like the RC PTCs (Fig. 4B); however, they did not express claudin-2 or claudin-7. Unlike RC PTCs, where the TER was unchanged over time (Fig. 4C), in β1E769 PTCs TER increased to about half of that seen in β1−/− PTCs by 6 days (Fig. 7C). These data suggested that the decreased expression of E-cadherin in PTCs was regulated by the membrane proximal region of the integrin β1 cytoplasmic tail, while increased expression of claudin-2 was modulated by the distal tail. Interestingly, decreased claudin-2 expression correlated with a moderate increase in TER in these PTCs, suggesting that TER was in part regulated by claudin-2 expression.
FIGURE 7.
E-cadherin, claudin-2, and claudin-7 expression is regulated by specific domains of the integrin β1 tail. A, schematic of the full-length and deletion mutant of β1 integrin. A stop codon was introduced after Glu-769 to obtain the Glu-769 construct. B, equal amounts of whole cell lysate from PTCs stably expressing Glu-769 or control vector (β1−/−) were electrophoresed and immunoblotted for E-cadherin, claudin-2, or claudin-7. Protein loading was controlled for by immunoblotting for β-actin. C, TER was measured on PTCs stably expressing the Glu-769 truncation mutant or control vector (β1−/−) grown to confluency on transwell inserts for 6 days. Mean measurements of three independent experiments are shown; *, p ≤ 0.05 between Glu-769 and β1−/− PTCs. D, schematic of the YY/AA mutant. The 2 tyrosine residues Tyr-783 and Tyr-795 in the highly conserved NPXY motifs were mutated to alanine in integrin β1. E, equal amounts of whole cell lysate from PTCs stably expressing the YY/AA mutant or control vector (β1−/−) were electrophoresed and immunoblotted for E-cadherin, claudin-2, or claudin-7. Protein loading was controlled for by immunoblotting for β-actin. F, TER was measured on PTCs stably expressing the YY/AA or control vector (β1−/−) grown to confluency on transwell inserts for 6 days. Mean measurements of three independent experiments are shown; *, p ≤ 0.05 between YY/AA and β1−/− PTCs.
There are two NPXY motifs in the integrin β1 cytoplasmic tail that regulate numerous integrin-dependent functions by binding cytosolic proteins such as talins and kindlins (10). Mutating the tyrosines in both these motifs to alanines inactivates the integrin, resulting in decreased cell adhesion and signaling of renal epithelial cells (13). We assessed the role of the NPXY motifs in regulating the TJ and AJ composition in PTCs by reconstituting β1−/− PTCs with integrin β1 where the tyrosines in 2 NPXY motifs were mutated to alanines (YY/AA) (Fig. 7D). The β1YY/AA PTCs were sorted to obtain a cell population that expressed the same amount of β1 integrin on the cell surface as the RC PTCs (data not shown). Like RC or β1flox/flox PTCs, the YY/AA PTCs had decreased expression of E-cadherin; however, they did not express claudin-2 or claudin-7 (Fig. 7E). The TER in the YY/AA PTCs increased over time to about half of that seen in β1−/− PTCs (Fig. 7F). Thus the phenotypes of the YY/AA and Glu-769 PTCs were similar. These data suggest that the two NPXY motifs in the integrin β1 cytoplasmic tail are required for increased claudin-2 expression found in PTCs.
Deleting β1 Integrin in the Proximal Tubule Results in Abnormal Urine Concentration
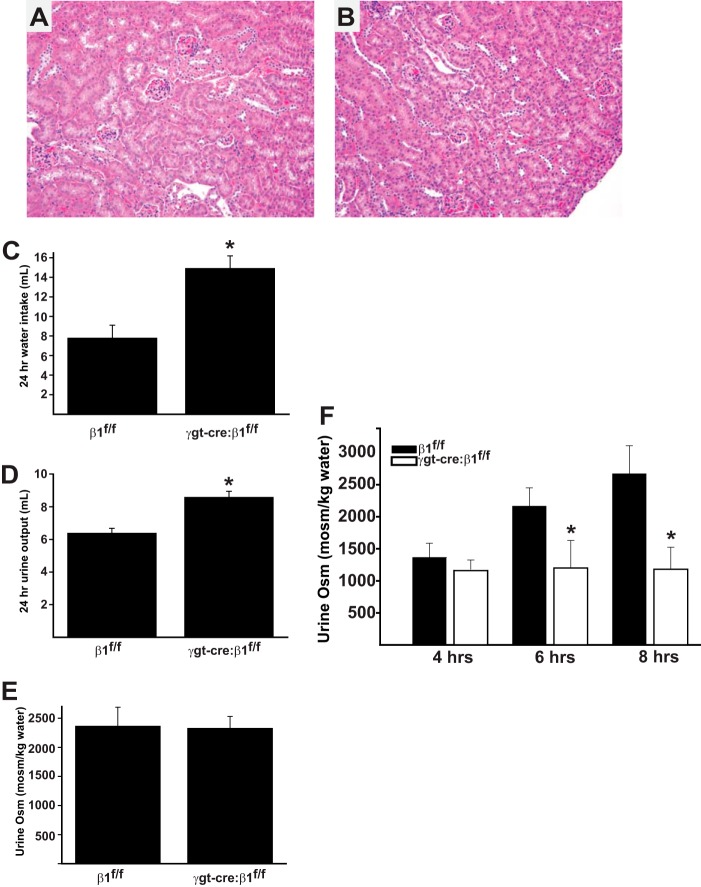
The in vitro data from the β1−/− PTCs suggested that β1 integrin expression is required for filtrate reabsorption by the proximal tubule. We therefore generated mice deficient of the β1 integrin subunit in the proximal tubule by crossing the β1flox/flox (19) and the γgt-cre (expressed in the proximal tubules at P10) mice (11, 20). Surprisingly, γgt-cre:β1flox/flox mice are born in the normal Mendelian ratio, have a normal lifespan, and do not exhibit any gross morphological abnormalities of their kidneys when compared with β1flox/flox controls (Fig. 8, A and B). We next defined whether the γgt-cre:β1flox/flox mice displayed any alterations in renal physiology. When placed in metabolic cages, the γgt-cre:β1flox/flox mice drank significantly more water than the β1flox/flox mice (14.9 versus 7.8 ml/24 h.) (Fig. 8C). Consistent with this observation, the γgt-cre:β1flox/flox mice passed significantly more urine than the controls (8.4 versus 6.2 ml/24 h) (Fig. 8D). The spot urine osmolality was similar between the genotypes (Fig. 8D), suggesting that the γgt-cre:β1flox/flox mice had an isosmolar diuresis. We then defined whether the γgt-cre:β1flox/flox mice were able to dilute and concentrate their urine normally by performing a water loading followed by deprivation study. After water loading, both genotypes were able to dilute their urines; however, 6 and 8 h following water deprivation, the γgt-cre:β1flox/flox continued to pass dilute urines (Fig. 8E). Thus these data demonstrate that deleting the β1 integrin subunit in the proximal tubules of the kidney results in an isosmolar diuresis under basal conditions and an inability to concentrate urine following water deprivation.
FIGURE 8.
Kidneys of γgt-cre:β1flox/flox mice have morphologically normal kidneys but have an isosmolar diuresis and an inability to concentrate urine after water loading and subsequent deprivation. A and B, histology of kidney cortex of 6 week β1flox/flox (A) and γgt-cre:β1flox/flox (B) mice is normal (200×). C, 24 h water intake (*, p < 0.01) and (D) 24 h urine output is increased in γgt-cre:β1flox/flox mice (*, p < 0.01). E, baseline urine osmolality is similar in β1flox/flox and γgt-cre:β1flox/flox mice. F, mice (6 in each group) were water loaded with an intraperitoneal injection of 2ml of water 18 h prior to and at the commencement of the experiment. At 4 h, γgt-cre:β1flox/flox and β1flox/flox mice diluted their urines appropriately; however, γgt-cre:β1flox/flox mice were unable to concentrate their urines 6 and 8 h after water deprivation (*, p < 0.01).
DISCUSSION
One of the defining characteristics of the proximal tubule is that it allows paracellular and transcellular bulk transport of water and solutes because of the loose cell-cell junctions between the epithelial cells. This contrasts with the tight epithelium in the distal nephron where transport is predominantly transcellular. The mechanism whereby the “tightness” of terminally differentiated renal tubular epithelium is regulated is poorly defined. In this study, we show that deleting the integrin β1 subunit in PTCs converts them from a loose epithelium, characterized by low expression of E-cadherin and claudin-7 and high expression of claudin-2, to a very tight epithelium with increased E-cadherin and claudin-7 expression and decreased claudin-2 and AQP1 expression. This effect is mediated by the integrin β1 cytoplasmic tail and does not require integrin αβ1 heterodimerization or localization of integrin β1 to the cell surface. The membrane proximal 18 amino acids of the β1 cytoplasmic tail regulates E-cadherin expression, while the distal 29 amino acids that include the NPXY motifs regulate claudin-2 expression (see Fig. 7 for details). Deleting the β1 integrin subunit in the proximal tubules of the kidney in vivo results in an isosmolar diuresis under basal conditions and an inability to concentrate urine normally following water deprivation. Thus we conclude that the integrin β1 tail is a critical regulator of the loose epithelial characteristics of PTCs, and this is mediated by mechanisms that are independent of integrin αβ1 heterodimerization or localization to the cell surface membrane. Taken together with the in vivo data, we propose that integrin β1 plays a role in regulating the absorptive characteristics of the proximal tubule by modulating its terminal differentiation during development.
One of the key players in regulating proximal tubule leakiness with respect to water and solute transport is claudin-2. There is in vitro evidence that claudin-2 expression levels are regulated by specific signaling pathways activated by extracellular cues in polarized epithelial kidney cells. Claudin-2 expression was shown to be decreased by ERK1/2 activation in Marbin-Darby canine kidney (MDCK) cells (21). Furthermore, EGF treatment of MDCK-II cells decreased claudin-2 expression and increased TER, which was prevented by inhibiting ERK1/2 signaling (22). In addition to the ERK pathway, the Rho GTPases were shown to regulate distribution of claudin-2 in MDCK cells. Specifically overexpression of activated forms of RhoA and Cdc42 changed the localization of numerous AJ proteins including claudin-2 by altering the actin cytoskeleton (23). As β1 integrins are well established regulators of both ERK and Rho GTPase signaling (24, 25), it is possible that dysregulation of these signaling pathways accounts for the decreased claudin-2 expression observed in β1−/− PTCs.
One of the key findings from our study is that claudin-7 and E-cadherin expression increased concomitant with the decreased claudin-2 expression in the β1−/− PTCs. Consistent with our data, overexpression of claudin-7 in porcine PTCs markedly increases TER, decreases paracellular chloride conductance and increases paracellular sodium conductance (26). E-cadherin expression has also been shown to regulate TER in MDCK cells (27, 28). These previous studies support our hypothesis that the increase in TER in the β1−/− PTCs is in part due to the increased expression of claudin-7 and E-cadherin. Our data that the Glu-769 and YY/AA mutants, which decrease claudin-7 and E-cadherin expression but do not alter claudin-2 expression and only partially reconstitute the low TER found in PTCs, further suggest that both claudins and E-cadherin play an important role in regulating PTC TER.
To the best of our knowledge there are no reported data on how integrins regulate claudin expression. It has however been shown that expression of integrin β1 into embryonic integrin β1-null GE11 epithelial cells increases cell scattering that is accompanied by the disruption of both cadherin-based intercellular adhesions and TJs (29). The loss of cell-cell adhesions in the GE11 cells only required the integrin β1 tail, which is consistent with our findings that decreased E-cadherin and increased claudin-2 expression in β1−/− PTCs could be reconstituted with Tac β1 (29). The small Rho GTPases were shown to play a role in integrin β1-dependent scattering of GE11 cells; however, no specific mechanism was identified. Integrin β1 has been shown to regulate common cytoskeletal components such as α-actinin and vinculin (30) as well as signaling molecules such as Rap1, focal adhesion kinase, and Src (30–32), which bind and regulate the function of both cell-cell junctions as well as focal adhesions. Integrin β1 interactions with any of these proteins might be a mechanism whereby this integrin subunit regulates AJs and TJs in PTCs.
Our data that PTC TER as well as claudin and E-cadherin expression are regulated by the integrin β1 cytoplasmic tail that remains within the cytoplasm and does not cross into the nucleus strongly suggest the tail binds to yet to be defined cytoplasmic effector protein(s). The transcription factors that control the loose epithelial phenotype of PTCs, and how they are regulated are poorly understood. Potential candidates include the snail proteins, which play a key role in regulating epithelial to mesenchymal transitions as well as claudin and E-cadherin expression (33); GATA-4, which has been implicated in regulating claudin-2 expression (33), and TCF4, which can regulate E-cadherin expression (34). Multiple integrin-binding proteins such as integrin-linked kinase (30) and the kindlins, which bind to the NPXY motifs of the integrin β1 tail (35) have been shown to regulate these transcription factors under specific conditions in different cell types. Although there are no data on PTCs, we suggest that binding of specific cytosolic proteins to integrin β1 tails within the PTCs regulate transcription factors that will define these cells as a loose epithelium in the terminally differentiated proximal tubule. This hypothesis is supported by the fact that wild type loose PTCs and tight collecting duct cells express similar amounts of integrin β1 on the cell surface (data not shown). It is important to note that the proximal tubule and the collecting duct are derived from embryologically distinct precursors, which might result in them expressing distinct integrin β1 tail-binding proteins.
The molecular basis of nephron segmentation is very poorly understood, especially with reference to the proximal tubule. There is evidence that certain transcription factors such as Hnf1b are critical for nephron patterning, and Hnf1b-null mice do not develop a differentiated tubule (36, 37). Notch signaling is also required for proximal tubule differentiation, and mice with deficiencies in this pathway have abnormal tubule differentiation (38–44). Very recently, snail 2 was implicated in the ability of stem cells to form renal tubules, where it functions to suppress E-cadherin expression (45). Therefore, based on our data it is possible that transcription factors such as GATA4 and snail that can regulate claudin and E-cadherin expression are modulated by integrin β1 expression in the proximal tubule in vivo by currently undefined mechanisms.
Our data also show that expression of the integrin β1 cytoplasmic domain regulates AQP1 expression in PTCs. These data complement studies showing that deleting integrin β1 in the developing collecting system in vivo resulted in significantly decreased AQP2 and vasopressin 2 receptor expression and increased expression of AQP1 (46). Thus it is highly likely that that β1 expression will regulate expression of different AQPs in the proximal tubule in vivo. Interestingly, AQP1 has been shown to regulate cell migration in PTCs by unknown mechanisms (47); and AQP2 has been demonstrated to bind to the extracellular domain of the integrin β1, and this interaction promotes cell migration and epithelial morphogenesis (48). Our study provides new evidence consolidating the link between integrin β1 and AQPs, which are specifically expressed in different terminally differentiated renal tubule epithelial cells.
The principal function of the proximal tubule is to isosmotically reabsorb ∼70% of glomerular filtrate. We show that the γgt-cre:β1flox/flox mice have an isosmolar diuresis under basal conditions and an inability to concentrate urine after water loading and subsequent deprivation. These abnormalities are consistent with the phenotype of the β1−/− PTCs, which behave like tight epithelial cells that do not express AQP1. We propose that in γgt-cre:β1flox/flox mice, the inability of PTCs to allow paracellular transport results in a severe decrease in reabsorption of the isosmolar proximal filtrate, and the excess water and electrolytes overwhelm the transport mechanisms of the distal nephron resulting in large amounts of isosmotic urine. Under basal conditions, the mice compensate by eating and drinking more (Fig. 8, C–E). The concentrating defect of the γgt-cre:β1flox/flox mice in the setting of water restriction following water loading is probably due to the inability of PTCs to reabsorb the hypotonic filtrate presented to the proximal tubule.
In conclusion, we have presented convincing evidence that integrin β1 regulates the terminal differentiation of PTCs in vitro by a unique mechanism that is independent of integrin αβ1 heterodimerization or localization to the cell surface membrane. Consistent with these observations, we demonstrated that deleting the integrin β1 subunit in the proximal tubule in vivo results in abnormalities of isosmotic urine reabsorption in the proximal tubule. These results, combined with the previous report showing that deletion of integrin β1 resulted in abnormal expression of the AQP transporters in the developing collecting system of the kidney (46), suggest that integrin β1 expression plays a crucial role in the terminal differentiation of polarized epithelial cells in kidney tubules.
Acknowledgments
We thank Leslie Gewin for technical support and Reinhard Fassler for constructive criticism of the project. Catherine Alford provided invaluable help with cell sorting and flow cytometry. We thank Eric Neilson for the γct cre mice and Elaine Fuchs for the β1flox/flox mice.
This work was supported by VA Merit Review 1I01BX002025 (to A. P.), 2I01BX000320 (to R. C. H.), and 1I01BX002196 (to R. Z.); the National Institutes of Health Grants R01-CA162433 (to A. P.), R01-DK095761 (to A. P.), R01-DK083187 (to R. Z.), R01-DK075594 (to R. Z.), R01-DK383069221 (to R. Z.), R01-DK51265 (to R. C. H.), R01-DK62794 (to R. C. H.), R01-DK95785 (to R. C. H.), and R01-DK088902 (to A. B. S.).
- AJ
- adherens junction
- TER
- transepithelial resistance
- TJ
- tight junction
- MDCK
- Madin-Darby Canine Kidney
- ECM
- extracellular matrix
- PTC
- proximal tubule cell
- FACS
- fluorescence-activated cell sorting
- AQP
- aquaporin.
REFERENCES
- 1. Shin K., Fogg V. C., Margolis B. (2006) Tight junctions and cell polarity. Annu. Rev. Cell Dev. Biol. 22, 207–235 [DOI] [PubMed] [Google Scholar]
- 2. Li J., Ananthapanyasut W., Yu A. S. (2011) Claudins in renal physiology and disease. Pediatric Nephrol. 26, 2133–2142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niessen C. M., Gottardi C. J. (2008) Molecular components of the adherens junction. Biochim. Biophys. Acta 1778, 562–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Epifano C., Perez-Moreno M. (2012) Crossroads of integrins and cadherins in epithelia and stroma remodeling. Cell Adh. Migr. 6, 261–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Saito M., Tucker D. K., Kohlhorst D., Niessen C. M., Kowalczyk A. P. (2012) Classical and desmosomal cadherins at a glance. J. Cell Sci. 125, 2547–2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Perantoni A. O. (1999) Cell adhesion molecules in the kidney: from embryo to adult. Exp. Nephrol. 7, 80–102 [DOI] [PubMed] [Google Scholar]
- 7. Prozialeck W. C., Lamar P. C., Appelt D. M. (2004) Differential expression of E-cadherin, N-cadherin, and β-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 4, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Pozzi A., Zent R. (2011) Extracellular matrix receptors in branched organs. Curr. Opin. Cell Biol. 23, 547–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pozzi A., Zent R. (2003) Integrins: sensors of extracellular matrix and modulators of cell function. Nephron Exp. Nephrol. 94, e77–84 [DOI] [PubMed] [Google Scholar]
- 10. Moser M., Legate K. R., Zent R., Fässler R. (2009) The tail of integrins, talin, and kindlins. Science 324, 895–899 [DOI] [PubMed] [Google Scholar]
- 11. Gewin L., Vadivelu S., Neelisetty S., Srichai M. B., Paueksakon P., Pozzi A., Harris R. C., Zent R. (2012) Deleting the TGF-β receptor attenuates acute proximal tubule injury. J. Am. Soc. Nephrol. 23, 2001–2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Zhang X., Mernaugh G., Yang D. H., Gewin L., Srichai M. B., Harris R. C., Iturregui J. M., Nelson R. D., Kohan D. E., Abrahamson D., Fässler R., Yurchenco P., Pozzi A., Zent R. (2009) β1 integrin is necessary for ureteric bud branching morphogenesis and maintenance of collecting duct structural integrity. Development 136, 3357–3366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mathew S., Lu Z., Palamuttam R. J., Mernaugh G., Hadziselimovic A., Chen J., Bulus N., Gewin L. S., Voehler M., Meves A., Ballestrem C., Fässler R., Pozzi A., Sanders C. R., Zent R. (2012) β1 Integrin NPxY Motifs Regulate Kidney Collecting Duct Development and Maintenance by Induced-Fit Interactions with Cytosolic Proteins. Mol. Cell. Biol. 32, 4080–4091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. LaFlamme S. E., Thomas L. A., Yamada S. S., Yamada K. M. (1994) Single subunit chimeric integrins as mimics and inhibitors of endogenous integrin functions in receptor localization, cell spreading and migration, and matrix assembly. J. Cell Biol. 126, 1287–1298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yamashita S., Maeshima A., Kojima I., Nojima Y. (2004) Activin A is a potent activator of renal interstitial fibroblasts. J. Am. Soc. Nephrol. 15, 91–101 [DOI] [PubMed] [Google Scholar]
- 16. Basuroy S., Sheth P., Kuppuswamy D., Balasubramanian S., Ray R. M., Rao R. K. (2003) Expression of kinase-inactive c-Src delays oxidative stress-induced disassembly and accelerates calcium-mediated reassembly of tight junctions in the Caco-2 cell monolayer. J. Biol. Chem. 278, 11916–11924 [DOI] [PubMed] [Google Scholar]
- 17. Seth A., Sheth P., Elias B. C., Rao R. (2007) Protein phosphatases 2A and 1 interact with occludin and negatively regulate the assembly of tight junctions in the CACO-2 cell monolayer. J. Biol. Chem. 282, 11487–11498 [DOI] [PubMed] [Google Scholar]
- 18. Jain S., Suzuki T., Seth A., Samak G., Rao R. (2011) Protein kinase Cζ phosphorylates occludin and promotes assembly of epithelial tight junctions. Biochem. J. 437, 289–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Raghavan S., Bauer C., Mundschau G., Li Q., Fuchs E. (2000) Conditional ablation of beta1 integrin in skin. Severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 150, 1149–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Iwano M., Plieth D., Danoff T. M., Xue C., Okada H., Neilson E. G. (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Invest. 110, 341–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lipschutz J. H., Li S., Arisco A., Balkovetz D. F. (2005) Extracellular signal-regulated kinases 1/2 control claudin-2 expression in Madin-Darby canine kidney strain I and II cells. J. Biol. Chem. 280, 3780–3788 [DOI] [PubMed] [Google Scholar]
- 22. Singh A. B., Harris R. C. (2004) Epidermal growth factor receptor activation differentially regulates claudin expression and enhances transepithelial resistance in Madin-Darby canine kidney cells. J. Biol. Chem. 279, 3543–3552 [DOI] [PubMed] [Google Scholar]
- 23. Bruewer M., Hopkins A. M., Hobert M. E., Nusrat A., Madara J. L. (2004) RhoA, Rac1, and Cdc42 exert distinct effects on epithelial barrier via selective structural and biochemical modulation of junctional proteins and F-actin. Am. J. Physiol. 287, C327–335 [DOI] [PubMed] [Google Scholar]
- 24. Del Pozo M. A., Schwartz M. A. (2007) Rac, membrane heterogeneity, caveolin, and regulation of growth by integrins. Trends Cell Biol. 17, 246–250 [DOI] [PubMed] [Google Scholar]
- 25. Canel M., Serrels A., Frame M. C., Brunton V. G. (2013) E-cadherin-integrin crosstalk in cancer invasion and metastasis. J. Cell Sci. 126, 393–401 [DOI] [PubMed] [Google Scholar]
- 26. Alexandre M. D., Lu Q., Chen Y. H. (2005) Overexpression of claudin-7 decreases the paracellular Cl- conductance and increases the paracellular Na+ conductance in LLC-PK1 cells. J. Cell Sci. 118, 2683–2693 [DOI] [PubMed] [Google Scholar]
- 27. Winter M. C., Shasby S., Shasby D. M. (2008) Compromised E-cadherin adhesion and epithelial barrier function with activation of G protein-coupled receptors is rescued by Y-to-F mutations in β-catenin. Am. J. Physiol. Lung Cell Mol. Physiol. 294, L442–L448 [DOI] [PubMed] [Google Scholar]
- 28. Contreras R. G., Shoshani L., Flores-Maldonado C., Lazaro A., Monroy A. O., Roldan M. L., Fiorentino R., Cereijido M. (2002) E-Cadherin and tight junctions between epithelial cells of different animal species. Pflugers Archiv : Eur. J. Physiol. 444, 467–475 [DOI] [PubMed] [Google Scholar]
- 29. Gimond C., van Der Flier A., van Delft S., Brakebusch C., Kuikman I., Collard J. G., Fässler R., Sonnenberg A. (1999) Induction of cell scattering by expression of β1 integrins in β1-deficient epithelial cells requires activation of members of the rho family of GTPases and down-regulation of cadherin and catenin function. J. Cell Biol. 147, 1325–1340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Legate K. R., Montañez E., Kudlacek O., Fässler R. (2006) ILK, PINCH, and parvin: the tIPP of integrin signalling. Nat. Rev. Mol. Cell Biol. 7, 20–31 [DOI] [PubMed] [Google Scholar]
- 31. Kim C., Ye F., Ginsberg M. H. (2011) Regulation of integrin activation. Annu. Rev. Cell Dev. Biol. 27, 321–345 [DOI] [PubMed] [Google Scholar]
- 32. Shattil S. J., Kim C., Ginsberg M. H. (2010) The final steps of integrin activation: the end game. Nat. Rev. Mol. Cell Biol. 11, 288–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Carrozzino F., Soulie P., Huber D., Mensi N., Orci L., Cano A., Feraille E., Montesano R. (2005) Inducible expression of Snail selectively increases paracellular ion permeability and differentially modulates tight junction proteins. Am. J. Physiol. 289, C1002–C1014 [DOI] [PubMed] [Google Scholar]
- 34. Wang Q., Sun Z. X., Allgayer H., Yang H. S. (2010) Downregulation of E-cadherin is an essential event in activating β-catenin/Tcf-dependent transcription and expression of its target genes in Pdcd4 knockdown cells. Oncogene 29, 128–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Meves A., Stremmel C., Gottschalk K., Fässler R. (2009) The Kindlin protein family: new members to the club of focal adhesion proteins. Trends Cell Biol. 19, 504–513 [DOI] [PubMed] [Google Scholar]
- 36. Heliot C., Desgrange A., Buisson I., Prunskaite-Hyyryläinen R., Shan J., Vainio S., Umbhauer M., Cereghini S. (2013) HNF1B controls proximal-intermediate nephron segment identity in vertebrates by regulating Notch signalling components and Irx1/2. Development 140, 873–885 [DOI] [PubMed] [Google Scholar]
- 37. Massa F., Garbay S., Bouvier R., Sugitani Y., Noda T., Gubler M. C., Heidet L., Pontoglio M., Fischer E. (2013) Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140, 886–896 [DOI] [PubMed] [Google Scholar]
- 38. Boyle S. C., Kim M., Valerius M. T., McMahon A. P., Kopan R. (2011) Notch pathway activation can replace the requirement for Wnt4 and Wnt9b in mesenchymal-to-epithelial transition of nephron stem cells. Development 138, 4245–4254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cheng H. T., Kim M., Valerius M. T., Surendran K., Schuster-Gossler K., Gossler A., McMahon A. P., Kopan R. (2007) Notch2, but not Notch1, is required for proximal fate acquisition in the mammalian nephron. Development 134, 801–811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Cheng H. T., Kopan R. (2005) The role of Notch signaling in specification of podocyte and proximal tubules within the developing mouse kidney. Kidney Int. 68, 1951–1952 [DOI] [PubMed] [Google Scholar]
- 41. Costantini F., Kopan R. (2010) Patterning a complex organ: branching morphogenesis and nephron segmentation in kidney development. Dev. Cell 18, 698–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kopan R., Cheng H. T., Surendran K. (2007) Molecular insights into segmentation along the proximal-distal axis of the nephron. J. Am. Soc. Nephrol. 18, 2014–2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Surendran K., Selassie M., Liapis H., Krigman H., Kopan R. (2010) Reduced Notch signaling leads to renal cysts and papillary microadenomas. J. Am. Soc. Nephrol. 21, 819–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bonegio R. G., Beck L. H., Kahlon R. K., Lu W., Salant D. J. (2011) The fate of Notch-deficient nephrogenic progenitor cells during metanephric kidney development. Kidney Int. 79, 1099–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hendry C. E., Vanslambrouck J. M., Ineson J., Suhaimi N., Takasato M., Rae F., Little M. H. (2013) Direct transcriptional reprogramming of adult cells to embryonic nephron progenitors. J. Am. Soc. Nephrol. 24, 1424–1434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wu W., Kitamura S., Truong D. M., Rieg T., Vallon V., Sakurai H., Bush K. T., Vera D. R., Ross R. S., Nigam S. K. (2009) β1-Integrin is required for kidney collecting duct morphogenesis and maintenance of renal function. Am. J. Physiol. Renal Physiol. 297, F210–F217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Hara-Chikuma M., Verkman A. S. (2006) Aquaporin-1 facilitates epithelial cell migration in kidney proximal tubule. J. Am. Soc. Nephrol 17, 39–45 [DOI] [PubMed] [Google Scholar]
- 48. Chen Y., Rice W., Gu Z., Li J., Huang J., Brenner M. B., Van Hoek A., Xiong J., Gundersen G. G., Norman J. C., Hsu V. W., Fenton R. A., Brown D., Lu H. A. (2012) Aquaporin 2 promotes cell migration and epithelial morphogenesis. J. Am. Soc. Nephrol 23, 1506–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]