Abstract
In this study, we had 3 major goals. The 1st goal was to establish a link between behavioral and event-related potential (ERP) measures of infant attention and recognition memory. To assess the distribution of infant visual preferences throughout ERP testing, we designed a new experimental procedure that embeds a behavioral measure (paired comparison trials) in the modified-oddball ERP procedure. The 2nd goal was to measure infant ERPs during the paired comparison trials. Independent component analysis (ICA) was used to identify and to remove eye-movement components from the electroencephalographic data, thus allowing for the analysis of ERP components during paired comparison trials. The 3rd goal was to localize the cortical sources of infant visual preferences. Equivalent current dipole analysis was performed on the ICA components related to experimental events. Infants who demonstrated novelty preferences in paired comparison trials demonstrated greater amplitude Negative central ERP components across tasks than infants who did not demonstrate novelty preferences. Visual preference also interacted with attention and stimulus type. The cortical sources of infant visual preferences were localized to inferior and superior prefrontal cortex and to the anterior cingulate cortex.
Keywords: visual attention, infancy, visual recognition memory, event-related potentials, cortical source localization
Infant Attention and Visual Preferences
Several decades of research on infants’ visual attention have provided insights into the early development of visual recognition memory and other cognitive processes. A common procedure used to measure visual recognition memory in infant participants is the paired comparison procedure in which their preferential looking behavior (look duration) to novel and familiar stimuli is measured. The preference for a novel stimulus is commonly interpreted to indicate the infant’s recognition of the familiar stimulus. Collectively, the results of this research have revealed that encoding, storage, and retrieval processes in preverbal children could be examined through their visual attention to stimuli; that even very young infants could recognize faces, patterns, and forms seen previously; and that many of the processes and variables that were known to affect recognition memory in older children and adults (e.g., interference, reinstatement) were integral to infant memory processes as well (for reviews, see Fagan, 1990; Rose, Feldman, & Jankowski, 2004, 2007).
Although measures of preferential-looking have been at the forefront of behavioral research on recognition memory, a parallel line of inquiry in which event-related potentials (ERPs) are used to investigate the electrophysiologicial correlates of infant recognition memory has emerged (e.g., Nelson & Collins, 1991, 1992; Reynolds & Richards, 2005; Richards, 2003a, 2003b). ERPs are voltage oscillations in scalp-recorded electroencephalographic (EEG) data that are time-locked with a perceptual or cognitive event of interest (Fabiani, Gratton, & Coles, 2000; Picton et al., 2000). Stimuli are presented briefly and repeatedly to each participant, and then all trials for a particular group or stimulus type are averaged together to identify the ERP. This averaging serves to increase the signal-to-noise ratio in the EEG so that waveform components associated with a particular stage or type of processing in the event (e.g., stimulus orienting, stimulus encoding) can be identified. For example, the Negative central (Nc) component has been the focus of interest in studies in which recognition of faces, objects, and events has been examined (e.g., Carver, Bauer, & Nelson, 2000; Courchesne, 1977; Courchesne, Ganz, & Norcia, 1981; de Haan & Nelson, 1997, 1999; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995; Nikkel & Karrer, 1994; Reynolds & Richards, 2005; Richards, 2003a; Webb, Long, & Nelson, 2005). The Nc is a component of negative polarity located over frontal and central electrodes with a peak latency of between 400 and 800 ms following stimulus onset. One of the most important questions in this research has been the nature of the cognitive and brain processes (e.g., attention, recognition memory) that generate the Nc component (for discussions, see Ackles & Cook, 2007; Reynolds & Richards, 2005).
The measures used in the behavioral and electrophysiological procedures are very different, but the constructs of interest (e.g., attention, recognition memory) and stimuli used (e.g., visual patterns, faces, objects) are often the same. However, these two research areas have generally been conducted independently and have sometimes yielded inconsistent findings (e.g., de Haan & Nelson, 1997; Nelson & Collins, 1991). In the research reported here, these methodologies have been integrated in a developmental study of attention and recognition in which infants’ behavioral (i.e., preferential-looking) and electrophysiological (ERP) responses are compared concurrently. Cortical source localization analyses of the ERP data were also conducted to establish whether these two different measures tap into activity within the same areas of the brain.
This integration of behavioral and brain processes is essential if certain long-standing questions about the nature of recognition memory in infants are to be resolved. These questions include the type of memory processes that recognition represents (e.g., explicit or implicit); the interpretation of the novelty, familiarity, and null preferences that infants show in the paired comparison procedure; and the nature of the interaction between recognition memory and attention processes (for discussions, see Rose et al., 2007; Snyder, 2007). To date, the brain-behavior relationships that underlie the development of recognition memory have been inferred from overt behavior putatively linked to brain areas (i.e., marker tasks) from clinical research findings with amnesic patients and from experimental research on juvenile and adult nonhuman primates. However, the direct application of these to developmental processes in human infants is potentially problematic (see Reynolds & Richards, 2008; Snyder, 2007). A major strength of EEG/ERP techniques is that they allow the measurement of infants’ brain activity while they participate in attention and recognition tasks. Conducting cortical source analysis on the EEG/ERP data enables identification of the brain areas from which attention and recognition processes emanate (Reynolds & Richards, 2005, 2009; Richards, Reynolds, & Courage, 2010).
Methodology and Background Issues in the Interpretation of Nc
Early ERP studies of recognition memory used an oddball procedure in which two unfamiliar stimuli were presented briefly and with unequal frequency (Courchesne, 1977; Karrer & Ackles, 1987, 1988; Karrer & Monti, 1995; Nikkel & Karrer, 1994). The consistent finding was a larger Nc component to the infrequently presented “oddball” stimulus than to the frequently presented “standard” stimulus. Courchesne et al. (1981) concluded that Nc was associated with novelty detection. However, the greater Nc amplitude following infrequent stimulus presentations in this procedure could also be related to the detection of a low probability event rather than to the detection of a novel stimulus per se. Nelson and Collins (1991, 1992) developed a modified-oddball procedure to address this confound. Infants were first exposed to repeated presentations of two different face stimuli. They were then exposed to one of the familiar stimuli on 60% of the trials (frequent familiar), to the other familiar stimulus on 20% of the trials (infrequent familiar), and to novel stimulus presentations on the remaining 20% of the trials (infrequent novel). The use of the three stimulus types permitted the assessment of infants’ responses to presentation probability (frequent familiar vs. infrequent familiar) and stimulus novelty (infrequent familiar vs. infrequent novel) separately. They found no differences in the Nc component for any of the stimuli for 4-, 6-, and 8-month-olds and concluded that the Nc reflected a general orienting response rather than the detection of a novel stimulus.
Richards (2003a) substantiated the relationship between the Nc component and attention processes in a modified-oddball procedure. Infants of 4.5, 6, or 7.5 months of age were presented with a video of a Sesame Street movie as a background stimulus. Heart rate (HR) changes elicited by the video were used to distinguish periods of time before attention was engaged (before HR deceleration), during sustained attentiveness (during HR deceleration), and during inattentiveness (when HR had returned to prestimulus levels; e.g., Casey & Richards, 1988; Reynolds & Richards, 2007; Richards, 1997, 2001; Richards & Casey, 1992). The Nc component did not differ for the three stimuli. Richards proposed that Nc may be more sensitive to contextual change than novelty or familiarity. Nc amplitude was greater during periods of attention than inattention and also increased in magnitude across age, but only during periods of attentiveness. This association between attention and the Nc component provided further evidence that the processes underlying Nc are related to the orienting of attention rather than to recognition memory. However, questions still remained about the conflicting interpretations of Nc in the literature and in particular about the role that familiarization might have played in these.
Reynolds and Richards (2005) manipulated the type of familiarization that infants experienced in a modified-oddball procedure. Infants at 4.5, 6, and 7.5 months of age were assigned to a preexposure condition in which two stimuli were presented for familiarization (i.e., the typical modified-oddball procedure) or to a control condition in which the infants were familiarized with two stimuli that were not seen later in the oddball procedure. An important addition to this study was the use of a high-density, 128-channel EEG recording system. This enabled the application of cortical source analysis on the ERP data for identification of locations in the cortex that could be potential generators of Nc (Reynolds & Richards, 2009; Richards, 2003b, 2004b, 2005). The results of the cortical source analyses identified areas of prefrontal cortex including the anterior cingulate as likely sources of the Nc component.
There was a significant effect of the familiarization manipulation on the Nc response to the familiar and novel stimuli. The preexposure group had a larger amplitude Nc to the novel stimulus presentations than to either the frequent-familiar or infrequent-familiar stimulus presentations. The control group showed equivalent Nc responses to the three stimulus types. This finding is consistent with a novelty detection function for the processes that generate Nc but is inconsistent with other research in which Nc appeared to be a general orienting response that was insensitive to stimulus novelty or probability (e.g., Nelson & Collins, 1991; Richards, 2003a). No age differences were found in Nc amplitude. This indicated that by 4.5 months of age, infants responded to novelty at the cortical level with 20 s of preexposure to a familiar stimulus. This is consistent with behavioral findings that indicate that between 3.5 and 6 months of age, 20 s of familiarization is an adequate amount of stimulus exposure for infants to demonstrate novelty preferences (e.g., Courage & Howe, 2001; Richards, 1997; Rose, 1983; Rose, Gottfried, Melloy-Carminar, & Bridger, 1982). However, the modified-oddball procedure is designed for measuring ERP correlates of recognition memory, and any conclusions regarding the consistency between ERP correlates and behavioral correlates of recognition memory remain speculative.
The Integration of Behavioral and Electrophysiological Measures of Recognition
Several studies have attempted to establish a link between ERP correlates and behavioral measures of recognition memory. For example, Nelson and Collins (1991) followed up the modified-oddball procedure with 16 paired comparison choice trials, and they found a lack of consistency between results of the two different levels of analysis. However, the looking-time analyses were conducted 5 min following the ERP phase of the experiment. This timing protracted the retention interval for the behavioral measures such that forgetting and infant fatigue might have obscured evidence of recognition.
Subsequently, de Haan and Nelson (1997) compared 6-month-olds’ ERPs and look durations following presentation of the mother’s face paired with a similar or dissimilar looking stranger’s face. The Nc component was found to be greater in amplitude to the mother’s face than to a dissimilar looking stranger’s face but not to a similar looking stranger’s face. In contrast to the ERP data, no behavioral differences were found in looking times to any of the face types. They concluded that ERP correlates of recognition memory were more sensitive than looking-time measures. However, findings from previous studies indicate that infants even younger than 6 months can demonstrate recognition of their mother’s face at the behavioral level (Bushnell, Sai, & Mullin, 1989; Field, Cohen, Garcia, & Greenberg, 1984; Pascalis, de Schonen, Morton, Deruelle, & Fabre-Grenet, 1995). Several methodological issues might have accounted for the differences between ERP and behavioral measures across these studies. Infants in de Haan and Nelson’s study were tested with sequential presentations of the face stimuli rather than with simultaneous presentations (i.e., paired comparison trials) more typical in behavioral studies. In addition, they assessed ERPs and look durations separately rather than simultaneously. To date, simultaneous measurement of ERPs and paired comparisons has not been done, in part because of significant artifacts produced in the EEG by eye movements. However, quantitative techniques are currently available to remove those artifacts from EEG data, and these could be applied to data obtained from infants (Jung et al., 1998, 2000).
In another series of studies, Ackles and colleagues (Ackles, 2008; Ackles & Cook, 1998, 2007; Karrer & Ackles, 1987) examined the relationship between 6-month-olds’ look durations to novel (oddball) and familiar (frequent) stimuli concurrently with ERPs measured during those looks. Their general findings were that fixations to oddball stimuli were reliably longer than those to familiar stimuli and that there was some relationship between longer looks and larger amplitude Nc responses. However, there were methodological limitations in these studies that left the relationship between look duration and Nc unclear, especially at the level of the individual participant (we expand on this point in the Discussion section).
In the current study, we tested infants at 4.5, 6, or 7.5 months of age. These ages were chosen because past research on the development of visual attention has demonstrated that a developmental transition occurs around 6 months of age associated with gains in voluntary attention potentially driven by further development of frontal brain areas (e.g., Colombo, Richman, Shaddy, Maikranz, & Blaga, 2004; Courage, Reynolds, & Richards, 2006). We predicted that Nc would increase in amplitude across these ages reflecting greater involvement of frontal areas in visual attention from 6 months on. This study was designed to address three major goals. The first goal was to examine the relationship between ERP and behavioral correlates of infant attention and recognition memory. To accomplish this, we embedded paired comparisons within the modified-oddball ERP procedure, and we examined the distribution of infant visual preferences in relationship to individual infant’s ERP responding. On the basis of the existing literature indicating that the magnitude of the Nc response is related to stimulus salience, we predicted that Nc amplitude would be greatest to the preferred stimulus. The second goal was to simultaneously measure ERPs and infant visual preferences by processing and analyzing the EEG measured during paired comparison trials. Independent component analysis (ICA) was used to identify eye movement components in the EEG data and to remove them from the paired comparison trials (see Jung et al., 1998, 2000). The second goal was somewhat exploratory in nature given that no study to date has examined ERPs during paired comparison trials, thus we made no specific predictions regarding the ERP response during the paired comparison procedure. The third goal was to identify the cortical sources of infant visual preferences. The cortical sources of the ICA components associated with experimental effects were localized with equivalent current dipole (ECD) analysis. This was done for the standard ERP trials surrounding the paired comparison trials and also for the EEG measured during the paired comparison trials. We were particularly interested in determining the brain areas that demonstrated common activation across tasks. A lack of common activation in specific brain areas across tasks would indicate that these two tasks tap into different cognitive processes. However, we expected substantial overlap between brain areas involved in processing ERP trials and paired comparison trials. On the basis of the findings of Reynolds and Richards (2005), we predicted that areas of prefrontal cortex would be involved in infant visual preferences.
Method
Participants
Forty-seven infants were tested in a cross-sectional design at 4.5 (M = 144.78 days, SD = 3.78; eight female/nine male infants), 6 (M = 188.0 days, SD = 5.27; eight female/seven male infants), or 7.5 (M = 225.76 days, SD = 5.76; four female/11 male infants) months of age. An additional 24 infants were tested who did not provide useable data because of fussiness, inattentiveness, excessive artifact, or technical problems. The infants were born full-term (gestational age of 38 weeks or greater), weighed greater than 2,500 g at birth, and were without pre- or perinatal medical complications. Only infants who maintained an alert, awake state throughout the entire procedure were retained in the study. The participants were solicited by contacting parents whose names appeared in commercial mailing lists. Parents were paid $30 for their infant’s participation in the study. Detailed demographic information (e.g., education, occupation, income) was not collected from parents, but the participants were solicited without regard to minority or ethnic group. This resulted in minority group participation representative of the local population of Columbia, South Carolina (the majority of participants were non-Hispanic and of Caucasian or African American descent).
Apparatus and Stimuli
A 29-in. (73.66-cm) color video monitor (NEC Multisync XM29) was used. The display was set to 1,280 horizontal and 1,024 vertical pixels. The center of the monitor was located approximately 55 cm from the infant’s eyes.
Camera and participant monitor
A video camera was located above the monitor for the purpose of judging infant visual fixation. Fixations were judged online using a TV monitor in a room adjacent to the testing room. The video was recorded with the use of a Broadway digital video card installed on a Dell Workstation 610 computer. The Broadway video card digitized video and audio signals, saving them in an AVI format. Video resolution was limited to a single video frame scan (30 frames per second, one frame = ~33 ms). A time code based on the frame number of the digitized video was read by the computer controlling the experiment; this time code was used to synchronize physiological recordings, video information, and experimental events.
Visual Stimuli
Computer-generated patterns
The memory stimuli consisted of achromatic computer-generated visual patterns. These stimuli took the form of simple shapes or patterns (examples are shown in Figure 1). The experimental stimuli covered a 17° visual angle.
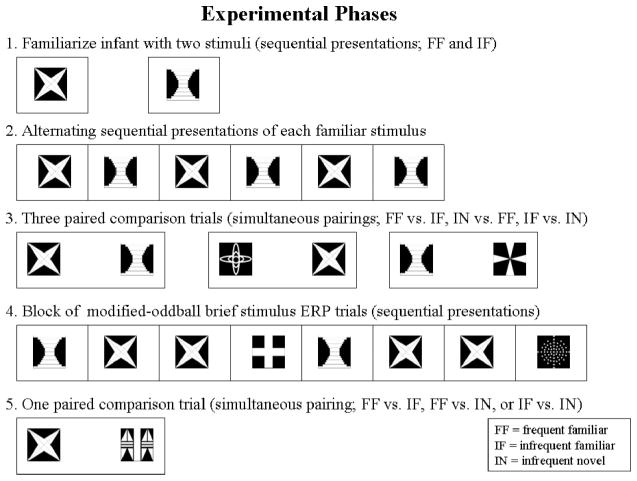
Figure 1.
Schematic diagram of the experimental procedure. Examples of stimuli used in the experiment are shown. Phases 1–3 were nonrepeated. After completion of the initial round of Phases 1–5, Phases 4 and 5 were repeated in an alternating sequence until the infant was no longer on task, allowing collection of as much event-related potential (ERP) and visual preference data as possible.
Sesame street characters
Videos of Sesame Street characters were used to attract initial fixation to the monitor before the onset of testing trials and to regain the fixation of distracted infants. This stimulus covered a 2° × 3° rectangular area. The character was placed at the center of the monitor to attract infant fixation; once the infant shifted fixation on the character, the experimental presentations were resumed following a random delay of 300–800 ms.
Procedure
An overview of the procedure is provided in Figure 1. Infants were held on a parent’s lap during testing approximately 55 cm from the center of the monitor (as described above). There were five phases of the experimental trials. The first phase was the familiarization phase. Infants were given 20 s of exposure to two stimuli. One stimulus was designated as the frequent familiar stimulus, and the other was designated the infrequent familiar stimulus. One stimulus was presented on the monitor. The stimulus remained on the monitor until 5 s of looking time accumulated for the infant. The other stimulus was similarly presented until 5 s of accumulated looking time. This procedure was repeated four times for each stimulus, resulting in a total accumulated looking time of 20 s per stimulus. The familiarization phase lasted 75 s on average.
The second phase of the experiment was a presentation period of the frequent familiar and infrequent familiar stimuli. The stimuli were presented alternatively for five presentations of 500 ms each. In this phase, a Sesame Street character video was placed on the monitor, and the familiar stimuli were only presented when the infant was judged to be fixated on the monitor. If the infant was judged not to be looking at the screen by an online observer, then a delay of up to 10 s was given until the infant attended to the character. There was an interstimulus interval of 1.5–2.0 s between presentations of the familiar stimuli. The ERP was analyzed from this phase as a manipulation check to ensure equivalent responses to each of the familiar stimuli.1
The third phase was made up of three visual paired comparison trials. Paired comparison trials involve the simultaneous presentation of two stimuli. The stimuli were not presented until the infant was judged to be fixated on the Sesame Street character in the center of the screen. The center of each stimulus was presented 30° from midline (one to the left of midline, the other to the right of midline). Each stimulus subtended a 17° visual angle. Paired comparison trials were given comparing the frequent familiar versus infrequent familiar stimulus, the frequent familiar versus an infrequent novel stimulus, and the infrequent familiar versus an infrequent novel stimulus. The length of the paired comparison trial was 5 s of accumulated looking time. On average, infants required 7.69 s of exposure to reach 5 s of accumulated looking. The order of the paired comparisons was randomly selected, and the assignment of the lateralized position of the stimuli was counterbalanced across trials.
The fourth phase consisted of brief presentations of the frequent familiar stimulus, the infrequent familiar stimulus, and infrequent novel stimuli (i.e., the standard ERP phase from the modified-oddball procedure). Different stimuli were used for the infrequent novel in each phase of the experiment. Each trial began with the presentation of a Sesame Street character to elicit the infant’s attention. Once the infant was fixated on the Sesame Street character, one of the three memory stimuli was presented following a 300–800 ms random delay. The stimulus was presented for 500 ms followed by a blank screen for a period of 1.5–2 s. Throughout the remainder of this phase, the Sesame Street character was only used if the infant looked away from the monitor. The first memory stimulus presented for each trial was divided equally among the three types of memory stimuli. Throughout each trial, the presentations followed the oddball procedure, with the frequent familiar stimulus being presented on 60% of the presentations, the infrequent familiar presented on 20% of the presentations, and the infrequent novel stimuli presented on the remaining 20% of the presentations. The sequence was randomly ordered until the completion of 10 stimulus presentations.
The fifth phase consisted of a single paired comparison trial with 5 s of accumulated looking. Comparisons between frequent familiar versus infrequent familiar, frequent familiar versus infrequent novel, or infrequent familiar versus infrequent novel were evenly distributed throughout testing. After completion of the initial round of Phases 1–5, the fourth and fifth phases of the experiment were repeated in an alternating sequence for as long as the infant was not fussy or tired to obtain as much ERP (Phase 4) and visual preference (Phase 5) data as possible. Phases 1–3 were nonrepeated phases. The average session lasted approximately 10 min.
Fixation Judgments and Interobserver Reliability Assessment
Infant fixations were judged online by an observer in an adjacent room to determine the timing of stimulus presentations. Offline judgments were completed following the testing procedure. If the offline observer judged that the infant was visually fixated on the stimulus, then HR, EEG, and ERP were analyzed for that stimulus presentation. Those occurrences in which the offline observer judged that the infant was not fixated on the presented stimulus were not included in the analysis.
Fixation direction
For paired comparison trials, two observers judged fixation direction offline for a selected number of participants (26; about eight per age). Observers were blind to the experimental conditions for each trial. The observers judged each look as looking to the right stimulus, looking to the left stimulus, or looking away. Interrater reliabilities were computed between the ratings of the two observers. The average agreement between observers, across the participants, that a look occurred (right, left, away) was 90%. The average Cohen’s kappa was .79, which indicates substantial agreement between the two observers (Landis & Koch, 1977). The correlation between the two observers for the duration of the looks was .86. Novelty preferences were calculated by dividing the total time looking toward the novel stimulus by the total time of accumulated looking.
Measurement and Quantification of HR
Ag-AgCl electrodes were placed on the infant’s chest with disposable electrode collars for the recording of the electrocardiogram (ECG). The Electrical Geodesics Incorporated (EGI; Eugene, OR) system was used to amplify and to digitize the ECG. The ECG was sampled at 250 Hz. The R − R intervals were identified in the ECG and used to compute the interbear intervals (IBIs).
HR-defined attention phases
Each part of the experiment was classified by HR changes into attentive and inattentive. The attentive periods were defined by the onset of a deceleration in HR (lengthening of the IBI) continuing until the HR returned to predeceleration level. HR decelerations were defined as five successive beats with IBIs longer than the median of the five beats preceding stimulus presentation. A return of HR to its prestimulus level was defined as five successive beats with IBIs shorter than the median IBI of the five prestimulus beats, following a deceleration. Any period of time from when the infant looked at the stimulus before a HR deceleration began was defined as inattentive. Periods of time in between the return of HR to predeceleration levels and the onset of a subsequent HR deceleration were also defined as inattentive.
Measurement and Quantification of EEG
A high-density, 128-channel EEG recording system produced by EGI was used. The 128-channel Geodesic sensor net used for infant recordings consists of 124 electrodes mounted in a geodesic configuration of pedestals held in place with elastic connections. The EGI system utilizes high-impedance amplifiers connected to a PowerPC-based computer system. The Netstation program included with the EGI system was used for the A/D sampling, data storage, zero and gain calibration for each channel, and impedance measurement. The Netstation program received serial communication from a Dell Workstation used to control the experimental protocol. Further details of the equipment and procedures may be found in Johnson et al. (2001) and Reynolds and Richards (2005, 2009).
The electrode net was placed on the infant’s head, and impedances were assessed until below 100 kΩ. The sampling rate of the EEG was 250 Hz, and band-pass filters were set from 0.1 to 100 Hz, with 20-K amplification. The EEG recordings were inspected for artifacts, poor recordings, or blinks. Individual channels or locations within trials were eliminated from the analyses if these occurred. Blinks were defined on the basis of a difference between the two electrodes on the sensor net on the outside canthii of the eye and the two electrodes above the eye and were defined as electrooculogram (EOG) changes > 150 μV in the vertical direction.
Quantification of ERP and ICA
The ERP averages for the brief stimulus presentations used in Phases 2 and 4 were calculated from 50 ms before stimulus onset through 2 s after onset. The ERP averages for the paired comparison trials were segmented from 50 ms prior to the completion of a saccade toward one of the visual stimuli (i.e., stimulus localization) for up to 2 s following stimulus localization (depending on the length of the look). Saccades were identified with an algorithm on the basis of a third-order differentiation of the raw EOG signal (Matsuoka & Harato, 1983; Matsuoka & Ueda, 1986; Richards, 2000, 2001; Richards & Hunter, 1997). Saccades representing shifts between stimuli on paired comparison trials were identified by synchronizing video-based fixation judgments with EOG data. The completion of a saccade was defined as the point at which the vertical change in the EOG terminated. Topographical ERP scalp potential maps were calculated for designated experimental effects using the interpolations from a third-order spherical spline technique (Nunez, 1990; Perrin, Bertrand, & Pernier, 1987; Perrin, Pernier, Bertrand, & Echallier, 1989).
The Nc component is typically located at frontal and central electrodes (i.e., Fz and Cz). We analyzed the mean data from clusters of electrodes of the EGI sensor net that corresponded to these regions. Nc peak and mean amplitude were analyzed from the intervals from 400 ms to 800 ms following stimulus onset from midline frontal (4, 10, 11, 16, 19, and 20; “FrontalZ”) and central (7, 32, 55, 81, and 107; “CentralZ”) electrode locations. Because the ERP analysis of the paired comparison trials was somewhat exploratory in nature, we inspected the topographical plots of the ERP grand averages during these trials to identify potentially significant components of activity. In addition to identifying activity resembling the Nc component at FrontalZ and CentralZ sites, midline parietal sites demonstrated substantial, negatively charged electrical activity with a latency similar to Nc. Thus, for the paired comparison trials, we also analyzed a cluster of electrodes from midline parietal (61, 62, 68, and 79; “ParietalZ”) locations.
A spatial ICA was done following the procedures outlined by Makeig, Sejnowski, and their colleagues (DeLorme, Makeig, Fabre-Thorpe, & Sejnowski, 2002; Jung, Makeig, Fabre-Thorpe, & Sejnowski, 2001; Makeig, Bell, Jung, & Sejnowski, 1996; Makeig, Jung, Bell, Ghahremani, & Sejnowski, 1997; also see Reynolds & Richards, 2009; Richards, 2005). The analysis was done on the raw EEG data. All EEG segments from a single participant were concatenated. The variables for the ICA were the EEG channels, and the observations were the millisecond intervals for which the EEG was sampled. The weights were calculated using the extended-ICA algorithm of Lee, Girolami, and Sejnowski (1999), using sphering of the input matrix to aid in convergence, with an initial learning rate of .003. The ICAs were done separately on each participant’s data. The ICA components from all participants were clustered according to the similarity of the component loading weights.
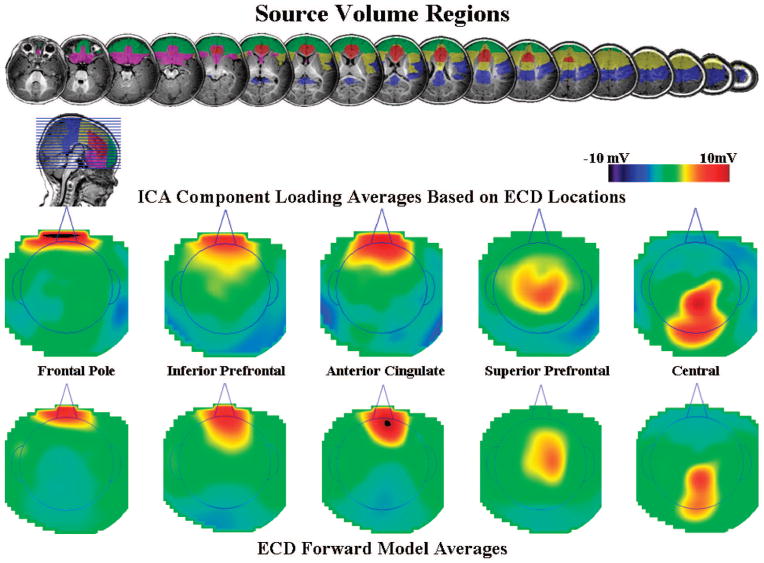
Figure 2 shows the topographic maps of the clusters. Each topographical map represents the average loadings for the ICA components that were put in each cluster. Four clusters were identified that could be related to the Nc that were located over the frontal pole electrodes, anterior-central electrodes, central electrodes, and parietal electrodes. The other components included loadings near the eyes, over the occipital electrodes, and over temporal electrodes. These clusters accounted for about 70% of the variance of the ICA projections. The rest of the components did not cluster together well or had idiosyncratic topographical patterns in the loading weights.
Figure 2.
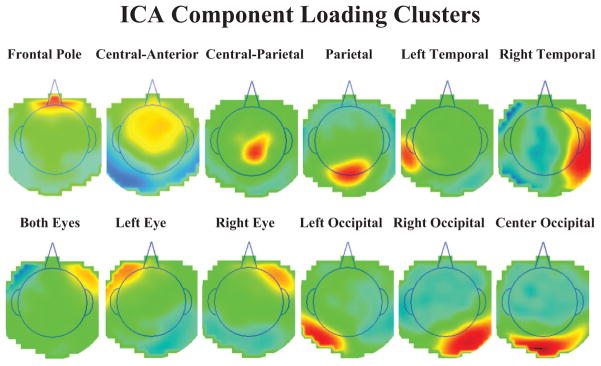
The independent component analysis (ICA) component loading clusters. The graphs represent topographical scalp potential maps with the component loadings as the values; each graph represents the average the ICA components in each cluster. The area inside the line indicating the head is above the meridian of the head, and outside the line is below the meridian. The titles for each cluster represent electrode names on the scalp (e.g., FP, central, anterior) and do not refer to brain areas. The four clusters with loadings centered on the scalp were used in the source analysis, whereas the lateralized eye components, and central and lateral occipital components, were used in the eye movement correction technique.
We wished to analyze the ERP data in the paired comparison procedure following an eye movement to one or the other stimulus. To do this, we first removed the electrical activity due to the eye movements from the EEG activity following procedures outlined by Jung et al. (1998, 2000). The ICA component activations were examined at the point of an identified eye movement in the EOG record. Components were identified whose activations occurred primarily around the eye movement. Figure 2 (three bottom left figures) shows components with topographical scalp maps that appear to have components in both eyes or the right or left eye. We also used source analysis (see the next section for further details) to confirm that an ECD placed in the eye area accounted for a substantial amount of variance for that component. We then used the activation, scalp topography, and ECD source analysis to identify those ICA components that were primarily eye movement components. Only the remaining ICA components were used with loadings/activations to project back into the temporal EEG space. This resulted in EEG data with the eye movement artifacts removed.
Cortical Source Analysis
Source analysis was done to determine the cortical sources of the electrical activity measured with the EEG and ERP (brain electrical source analysis, ECD; Huizenga & Molenaar, 1994; Scherg, 1990, 1992; Scherg & Picton, 1991; Richards, 2004, 2005, 2006). Only an overview of the source analysis is given, details are presented in other sources (Reynolds & Richards, 2005, 2009; Richards, 2003b, 2006). The component weights resulting from the spatial ICA represent the topographical information in the EEG and are similar to a set of topographic scalp maps. Cortical source analysis can be conducted on these component weights to identify cortical sources of the component weights. We estimated the location of cortical sources from the ICA weights with ECD analysis. This analysis estimates a dipole (or set of dipoles) located in the cortex representing a hypothetical current source that could potentially generate the observed component weights. A forward model is used to produce a simulated scalp current originating from the hypothetical dipole. Simulated scalp currents are compared with the observed data in an iterative manner until the best fitting dipole is identified. The cortical source models used realistic head model and a finite-element model (FEM) mapping of the electrical conductivity of the head to calculate the forward model (Reynolds & Richards, 2009).2
The cortical source models also used source locations and FEM models based on anatomical MRIs from infant participants.3 We had 11 anatomical MRIs from participants at ages 4.5 months (n = 2), 6.0 months (n = 7), and 7.5 months (n = 2). The MRIs were segmented into component materials (e.g., CSF, white matter, gray matter, scalp, eyes, skull) with the FSL computer programs (BETSURF: Jenkinson, Pechaud, & Smith, 2005; BET2: Smith, 2002; Smith et al., 1999). The segmented MRIs were transformed to wire frames with the MRI Viewer program (Source Signal, Inc.), and complete models for these 11 infants were developed. We also did the source analysis using source locations based on the registration of the MRI volumes with stereotaxic atlases from the Montreal Neurological Institute (MNI) brain using the FSL FLIRT program (Jenkinson & Smith, 2001). The source locations for the analyses were Brodmann and anatomical brain areas that were determined by reference to past work in this area (Reynolds & Richards, 2005). The realistic head model for each participant was chosen by using external head measurements from the participant and the 11 infant MRIs and by using the MRI of the infant whose head measurements most closely matched that of the participant. The MR Viewer (Signal Source Imaging, Inc) and the MRIcron program (Rorden, http://www.sph.sc.edu/comd/rorden/mricron/) were used to display the MRIs.
Design for Statistical Analysis
The design for the study included the experimental factors of testing age (3: 4.5, 6, 7.5 months) and preference (2: novelty preference, nonpreference) as between-subjects factors, and attention phase (2: attention, inattention) and stimulus type (3: frequent familiar, infrequent familiar, infrequent novel) as repeated measures factors. The analysis focused on the Nc component. Because of the unequal distribution of the number of trials in the cells of the factorial design, the analyses of variance (ANOVAs) for the analyses were done with a general linear models approach using nonorthogonal design (see Hocking, 1985; Searle, 1971, 1987). This was computed using the Proc GLM of SAS. The statistical tests used the error terms derived from the related intervals effects analyses and Scheffe-type methods to control for inflation of test wise error rate; all significant tests are reported at p < .05. Effect sizes ( ) are reported on all significant effects.4
Results
Grand Average ERP Overview for Brief Stimulus and Paired Comparison Procedures
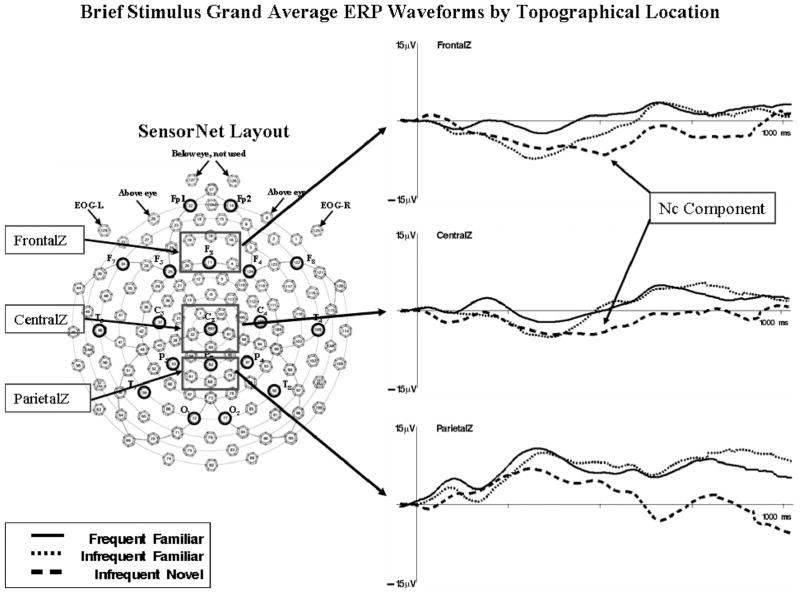
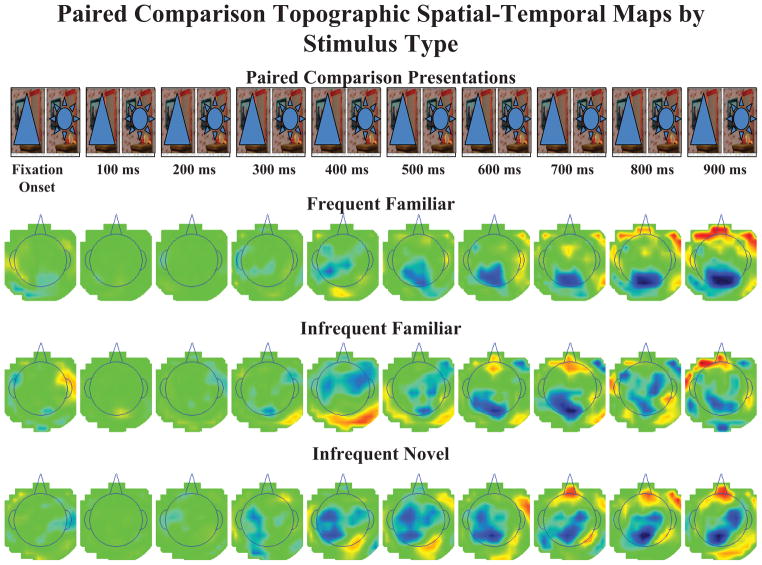
The ERP data were analyzed from the brief stimulus exposures and paired comparisons. Figure 3 displays the grand average ERP waveforms for the brief stimulus exposure trials (on the basis of 749 trials) separately for the FrontalZ, CentralZ, and ParietalZ electrode clusters, and separately for the three stimulus types. Figure 4 displays topographical scalp potential maps from these same trials. The Nc component occurred as a large negative ERP change located primarily in the frontal and central electrodes.
Figure 3.
The grand average event-related potential (ERP) waveforms for the brief stimulus presentations. Left panel: a schematic diagram of the 128-channel Electrical Geodesics Incorporated sensor net. The boxes indicate clusters of electrodes that were used in the analysis of the Negative central (Nc) ERP component. Right panel: the grand average ERP waveforms for each topographical location analyzed in the experimental analysis. The Nc component is indicated. The y-axis represents change in electrical potential relative to baseline, and the x-axis represents time following stimulus onset. EOG = electrooculogram.
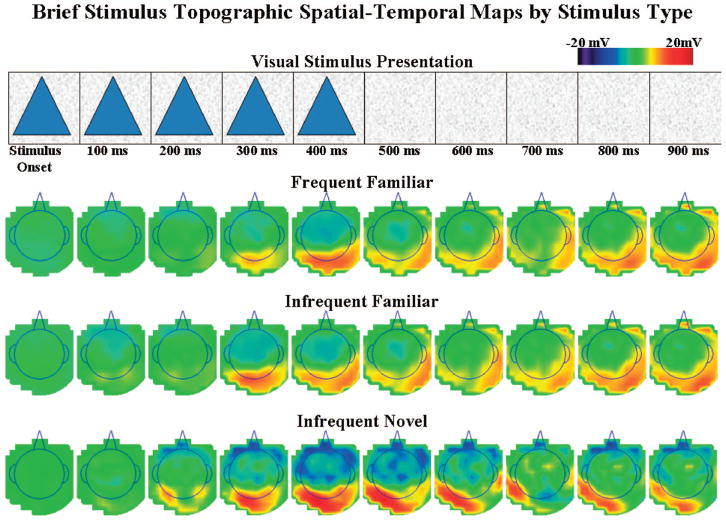
Figure 4.
The event-related potential (ERP) recording for 1 s following stimulus onset is shown by stimulus type. Each row of topographical scalp potential maps represents a 1-s sequence of 100-ms averages of the ERP data for each stimulus type. The top panel displays the corresponding time-course of the stimulus presentations, with stimulus offset occurring 500 ms after stimulus onset.
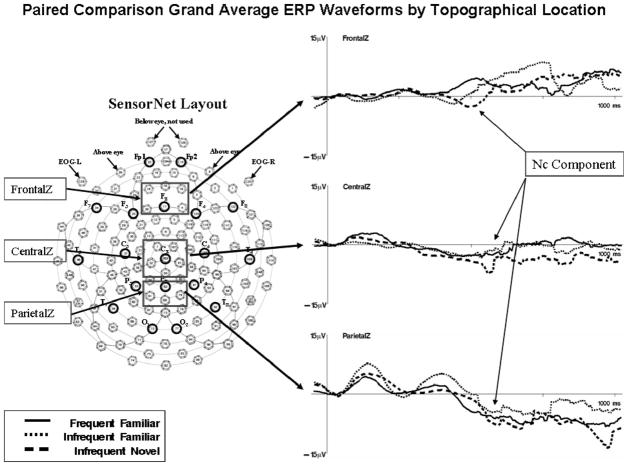
Figure 5 shows the grand average ERP waveforms from the paired comparison trials (on the basis of 274 looks), and Figure 6 shows the analogous topographical scalp potential maps. The averages for the paired comparison trials were done beginning with the end of the saccade toward a stimulus, and thus represent a postsaccadic ERP. The ERP pattern for these trials differed somewhat from the pattern on the brief stimulus exposures. A large negative deflection may be seen in the CentralZ electrodes occurring at about the same time as the ERP in the brief stimulus exposures, and the overall topographical pattern was similar (cf. Figures 4 and 6). However, a large negative deflection occurred in the ParietalZ electrode clusters as well, and the difference between the three stimulus types was not as distinct.
Figure 5.
The grand average event-related potential (ERP) waveforms for the paired comparison presentations. Left panel: a schematic diagram of the 128-channel Electrical Geodesics Incorporated sensor net. The boxes indicate clusters of electrodes that were used in the analysis of the Negative central (Nc) ERP component. Right panel: the grand average ERP waveforms for each topographical location analyzed in the experimental analysis. The Nc component is indicated. The y-axis represents change in electrical potential relative to baseline, and the x-axis represents time following stimulus onset. EOG = electrooculogram.
Figure 6.
The event-related potential (ERP) recording from single looks within paired comparison trials for 1 s following stimulus fixation is shown by stimulus type. Each row of topographical scalp potential maps represents a 1-s sequence of 100-ms averages of the ERP data for each stimulus type. The top panel displays the corresponding time-course of the paired comparison trials, with continuous display of the paired stimuli.
Brief stimulus procedure
The ERP data from the intervals from 400 to 800 ms following stimulus onset were analyzed. The peak (maximum) amplitude and mean amplitude during this time interval were examined for experimental effects at FrontalZ and CentralZ electrode clusters.
To address the first major aim of identifying the amount of consistency between behavioral correlates and ERP correlates of infant attention, we divided infants into groups post hoc on the basis of preference scores during paired comparisons. Paired comparisons of familiar (frequent or infrequent) versus novel stimuli were used to determine groups on the basis of preference. A novelty preference was defined as total proportion of looking time toward the novel stimulus ≥ .55. Proportion of looking toward the novel stimulus < .55 was defined as a nonpreference. ANOVAs were run using Age (3: 4.5, 6, and 7.5 months) and Preference (2: novelty preference and nonpreference) as between-subjects factors and Attention (2: attention and inattention) and Stimulus Type (3: frequent familiar, infrequent familiar, and infrequent novel) as within-subjects factors.
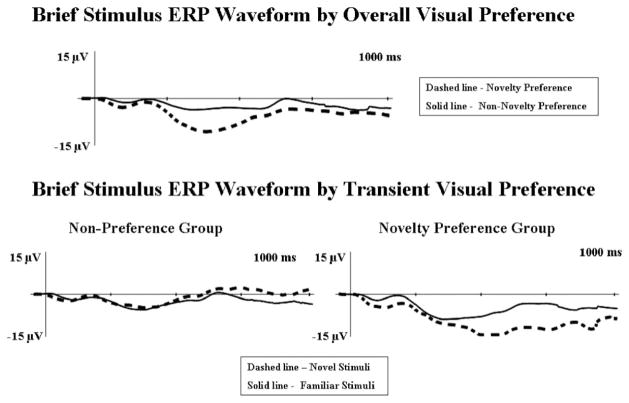
We approached the analysis of the visual preference factor in two different ways. Our first analysis was on “overall preference.” We took the individual’s average proportion of looking during all familiar versus novel paired comparison trials, and we used this overall preference score when splitting infants into preference groups. We then compared preference groups on their averaged ERP responses during the entire testing phase (i.e., averaged across all modified-oddball ERP phases). There was a main effect for preference at CentralZ, F(1, 51) = 9.95, p = .003, . It can be seen in Figure 7 (top panel) that infants who demonstrated an overall novelty preference (n = 25) showed significantly greater amplitude Nc than infants who did not demonstrate novelty preferences overall (n = 22). There was also a main effect for age, F(2, 51) = 6.56, p = .003, . Nc amplitude increased with age (4.5 months: M = −12.00 μV; 6 months: M = −15.56 μV; 7.5 months: M = −23.24 μV).
Figure 7.
The brief stimulus event-related potential (ERP) waveforms by visual preference. The data are shown through 1 s following fixation onset. The y-axes represent change in electrical potential relative to baseline, and the x-axes represent time following stimulus onset. Top panel: display of differences in responding for group displaying an overall novelty preference (dashed line) versus group that did not demonstrate an overall novelty preference (solid line). Bottom panel: display of responses to familiar (solid line) versus novel (dashed line) stimuli for the transient nonpreference group (left graph) and the transient novelty preference group (right graph).
In addition to examining overall preference, we examined “transient” preferences by examining brief presentation ERP trials that surrounded specific individual paired comparison trials. That is, we only examined the two blocks of ERP trials that either immediately preceded or followed a specific paired comparison trial. Individual participant’s preference scores on the specific paired comparison trial embedded within these two blocks of ERP trials were used to determine preference groups on the transient analysis. This served as a more real-time analysis of the relationship between visual preference and Nc amplitude. There was an interaction between transient preference and stimulus type, F(2, 34) = 4.32, p = .021, . The group showing a transient novelty preference demonstrated greater amplitude Nc to novel stimuli, whereas minimal differences on the basis of stimulus type were found for the infants who did not demonstrate a transient novelty preference (nonpreference group; see bottom panel of Figure 7).
To summarize the findings from the brief stimulus procedure, there was a main effect for overall visual preference with infants who demonstrated an overall novelty preference showing greater amplitude Nc than infants who did not demonstrate an overall novelty preference. There was also a main effect for age. Nc amplitude increased with age. Transient visual preference interacted with stimulus type. Infants who demonstrated a novelty preference on a single paired comparison trial had greater amplitude Nc to novel stimuli on ERP trials occurring immediately before and after that specific paired comparison trial.
Paired comparison procedure
The ERP data were also analyzed during the paired comparison procedure. The ERP data were gathered from the end of a look toward a stimulus, defined as the end of the saccade. The major focus of this analysis was to identify differences in the ERP related to visual preferences simultaneously displayed at the behavioral level. Thus, ANOVAs were once again run using Age (3: 4.5, 6, and 7.5 months) and Preference (2: preference and nonpreference) as between-subjects factors and Attention (2: attention and inattention) and Stimulus Type (2: familiar [frequent and infrequent combined] and infrequent novel) as within-subjects factors.
We conducted three separate analyses using “transient preference,” “transient novelty preference,” and “overall novelty preference” as the Preference factor. First, we split up participants on individual paired comparison trials on the basis of whether they displayed a preference (i.e., looking proportion ≥ .55 for a given stimulus). With this approach we simply analyzed transient preference regardless of preferred stimulus type (familiar or novel), and we looked at differences in ERP responding to the preferred versus nonpreferred stimulus. We then examined transient novelty preference, and we defined a novelty preference as proportion of looking to the novel over familiar stimulus ≥ .55. In both of the transient analyses, the ERP was only analyzed on the actual paired comparison trial that was used to define preference groups (i.e., simultaneous measurement). For overall novelty preference, we split up infants on the basis of their average novelty preference score across all paired comparison trials, and then we compared group differences on ERPs averaged across all paired comparison trials.
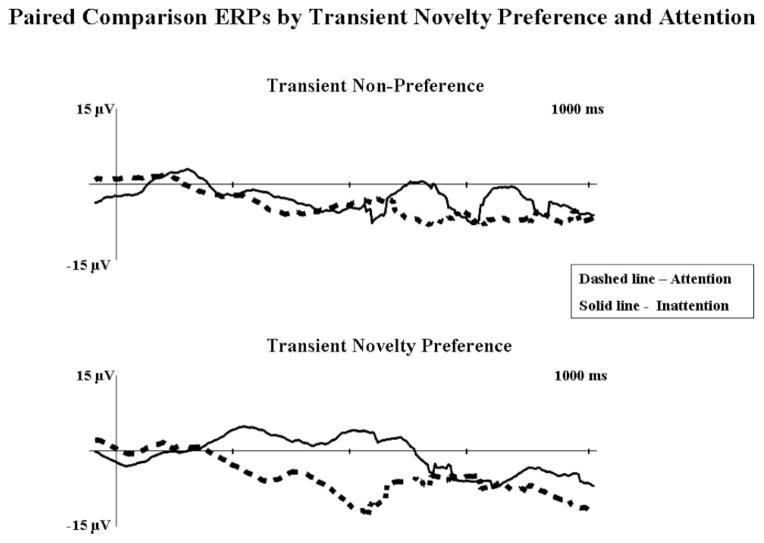
For the transient preference analysis, there was a main effect for preference at ParietalZ electrodes, F(1, 53) = 6.43, p = .0142, . Infants demonstrated greater amplitude Nc to the preferred stimulus (M = −32.49 μV) than to the nonpreferred stimulus (M = −26.98 μV). On the transient novelty preference analysis, significant effects were found at CentralZ. There was a main effect for attention, F(1, 8) = 7.58, p = .025, . Infants showed greater average amplitude Nc during attention (M = −5.49 μV) than inattention (M = − 0.17 μV). There was also a significant interaction between attention and preference, F(1, 8) = 7.36, p = .027, (see Figure 8). Infants who demonstrated novelty preferences showed greater mean amplitude Nc during attention (M = −7.99 μV) than inattention (M = 4.96 μV); infants in the nonpreference group showed little difference in amplitude for attentive (M = − 4.83 μV) and inattentive (M = −2.56 μV) trials.
Figure 8.
Postsaccade paired comparison trial event-related potentials (ERPs) at CentralZ for “transient” visual preference by attention. The y-axes represent change in electrical potential relative to baseline, and the x-axes represent time following stimulus onset. The top graph shows the response of the transient familiarity preference group. The bottom graph shows the response of the transient novelty preference group. Solid lines represent inattentive trials, and dashed lines represent attentive trials.
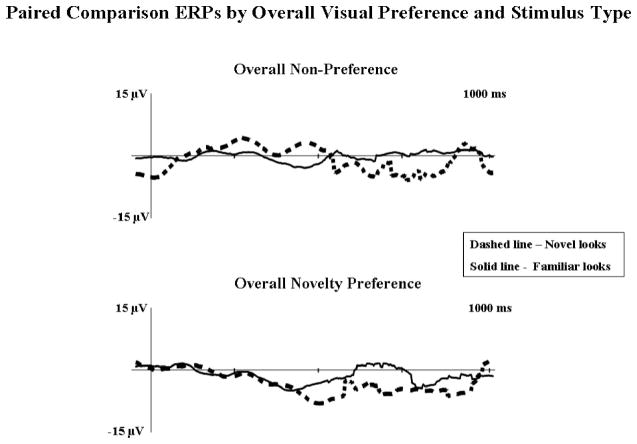
For the overall novelty preference analysis, there was an interaction of attention and stimulus type on peak amplitude Nc at ParietalZ, F(1, 13) = 4.99, p = .0164, . Infants demonstrated greater amplitude Nc during attention (M = −30.68 μV) than inattention (M = −25.34 μV) on novel looks, whereas no differences (M = −30.17 μV, and M = −30.44 μV, respectively) were found during familiar looks. At CentralZ, there was a main effect for attention, F(1, 13) = 4.99, p = .0437, that was qualified by an interaction of attention and preference, F(1, 13) = 14.92, p = .002, . Infants who demonstrated overall novelty preferences showed greater amplitude Nc during attention (M = −26.29 μV) than inattention (M = −18.73 μV). The trend was reversed for infants who did not demonstrate an overall novelty preference (M = −20.26 μV, and M = −26.50 μV, respectively). Finally, there was a Preference × Stimulus Type interaction, F(1, 13) = 5.31, p = .038, . Infants who preferred the novel stimulus demonstrated greater amplitude Nc during novel looks, whereas the nonpreference group demonstrated no differences in Nc amplitude on the basis of stimulus type (see Figure 9).
Figure 9.
Postsaccade event-related potentials (ERPs) at CentralZ from paired comparison trials for overall visual preference and stimulus type. The y-axes represent change in electrical potential relative to baseline, and the x-axes represent time following stimulus onset. The top graph shows the response of the overall nonpreference group. The bottom graph shows the response of the overall novelty preference group. Solid lines represent looks to familiar stimuli, and dashed lines represent looks to novel stimuli.
To summarize the ERP findings from paired comparison trials, there was a main effect for transient preference. On a specific paired comparison trial, infants demonstrated greater amplitude Nc to their preferred stimulus (regardless of novelty or familiarity). There was also a main effect for attention with infants displaying greater amplitude Nc during attention. Attention interacted with transient novelty preference. Infants who demonstrated a novelty preference showed greater amplitude Nc during looks when attentive than when inattentive. Attention interacted with stimulus type with infants showing differences in Nc amplitude on the basis of attention, but only during novel trials. Finally, overall novelty preference interacted with stimulus type. Infants who demonstrated an overall novelty preference showed greater amplitude Nc to novel stimuli; the trend was reversed for those infants who did not demonstrate an overall novelty preference.
Cortical Source Analysis
To address the third goal, we conducted cortical source analysis on the ICA components identified in the analysis of the EEG data. The components that clustered in the center part of the scalp (see Figure 3: “frontal pole,” “anterior-central,” “central,” and “parietal”) were analyzed. The source volumes that were chosen to restrict ECD locations were the frontal pole, inferior prefrontal cortex, anterior cingulate, superior and posterior prefrontal cortex, central brain areas, and a MRI volume(s) representing the rest of the brain.5 Figure 10 shows the regions of interest located on a 6-month-old participant. The regions were chosen on the basis of ECDs for the Nc component found in a prior study (Reynolds & Richards, 2005) and expected source locations for the four component clusters. Single dipole models were fit for each of these areas, and the best fitting dipole model was chosen. Table 1 shows the mean MNI and stereotaxic atlas locations (Talairach & Tournoux, 1988) for these ECDs grouped by region. The fits of the model ranged from 0.70 to 0.97 (M = 0.86).
Figure 10.
Regions of interest (ROIs) for the source analysis shown for one 6-month-old infant. For the equivalent current dipoles (ECDs), ROIs were designated for the location of the dipoles. The ROIs came from the Harvard–Oxford cortical areas established on the Montreal Neurological Institute (MNI) MRI. The independent component analysis (ICA) in each cluster was fit to a ROI specific to that cluster, and each had a non-ROI region that was the rest of the brain. The ECDs were restricted to gray matter likely composed of cell bodies and were excluded from white matter (identified on infant MRI) and putative nonmyelinated axons (estimated from white matter of MNI brain warped to infant). For the source volume regions displayed on the MRI images, violet represents inferior prefrontal cortex, green represents frontal pole, red represents anterior cingulate and anterior portion of cingulate gyrus, yellow represents superior and posterior portions of prefrontal cortex, blue represents central (pre- and postcentral gyri), and aqua represents parietal cortex.
Table 1.
MNI and Talairach Coordinates (in Millimeters) and Cortical Areas of the Equivalent Current Dipole Locations
| N | Positive anterior and negative central
|
|||||
|---|---|---|---|---|---|---|
| Saggital | Coronal | Axial | Magnitude SDa | Brodmann area | Cortical area | |
| 70 | 2.0 | 26.9 | 16.8 | 10.2 | 24, 32 | Anterior cingulate |
| 1.9 | 26.3 | 15.3 | 9.3 | |||
| 153 | −4.6 | −35.0 | 24.4 | 17.1 | 2, 3, 4 | Central |
| −4.6 | −32.8 | 24.3 | 16.2 | |||
| 69 | 1.6 | 44.3 | −13.3 | 12.1 | 10 | Frontal pole |
| 1.6 | 42.3 | −13.2 | 11.6 | |||
| 197 | −3.2 | 17.4 | 9.8 | 14.0 | 11, 25, 34 | Inferior prefrontal |
| −3.2 | 16.1 | 9.6 | 13.3 | |||
| 94 | 4.2 | −3.2 | 17.9 | 11.0 | 6, 8, 9, 24, 46 | Superior prefrontal |
| 4.2 | −2.1 | 17.6 | 10.3 | |||
Note. The coordinates represent a translation from the infant participant MRIs to the stereotaxic coordinates of the Montreal Neurological Institute (MNI) standard brain (first row of each table cell) and a translation from the MNI coordinates to Talairach stereotaxic coordinates.
Magnitude SD, defined as the standard deviation of the length of a vector from the average equivalent current dipole location to a dipole location, indicates the approximate radius of a sphere for locations within 1 SD of the centroid.
The ICA components were grouped by the source volume in which the best fitting ECD was found. Figure 10 shows the average loadings from the ICA components for these five groups. The component averages for the anterior cingulate, inferior prefrontal, and superior prefrontal regions have the bipolar-like configuration similar to the Nc component. Note that the component loadings are in the opposite orientation (positive–negative) from the ERP activity. This is accounted for by negative values on the component activations. Figure 10 also shows the forward model of the projection from the ECD to the scalp, averaged across ICA components, separately for these regions. These forward model projections are made during the ECD minimization procedures. They represent the electrical activity on the scalp computed from the dipole location-moment and the realistic electrical properties of the MRI volume in the cortical source model.
Cortical sources of ERP
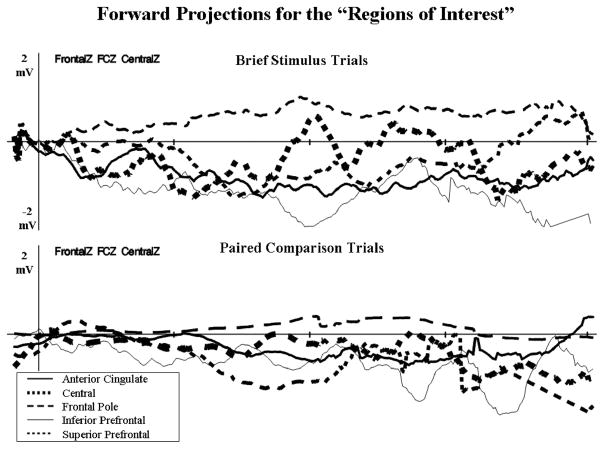
The data were examined to determine the similarity of the cortical sources of the ERP for the brief stimulus and the paired comparison procedures. We did not do a full factorial analysis of the ERP sources because we have reported on similar analyses in other places (Reynolds & Richards, 2005, 2009). The cortical sources for the five regions shown in Figure 2 and Table 1 were used to calculate projections of the electrical activity on the scalp expected from a dipole in that location and moment (e.g., forward model). This was done on a 4-ms × 4-ms basis in the same temporal domain as the ERP data, averaged over the electrodes from the frontal to the central scalp locations (i.e., FrontalZ, CentralZ), separately for the five brain regions. Figure 11 shows the results of this calculation. The inferior prefrontal, superior prefrontal, and anterior cingulate areas showed a pattern of response most similar to the Nc ERP component, that is, a large negative deflection with a peak at about 500 ms following stimulus onset (see Figure 11, top graph, thin and thick solid lines, thin dashed line). The forward model projection from the same brain regions showed a similar pattern of amplitude and response for the data from the paired comparison trials (see Figure 11, bottom graph). In addition to the peak at 500 ms, these three brain areas had a sustained response for several ms following the peak area of the Nc. The projections from the central and frontal pole regions were dissimilar to the pattern of the Nc ERP component for the brief stimulus and paired comparison procedures.
Figure 11.
The forward projections for the five regions of interest areas. These represent the 4-ms × 4-ms activity of these cortical areas, projected from the dipole through the realistic cortical model to the scalp, and then averaged over the FrontalZ, CentralZ, and FrontalCentralZ electrodes. The top graph shows the projections from the brief stimulus trials, and the bottom graph displays the projections from paired comparison trials. The projections from the anterior cingulate, inferior prefrontal, and superior prefrontal brain areas had temporal activity in the projected data that was similar to that recorded in the Negative central event-related potential component.
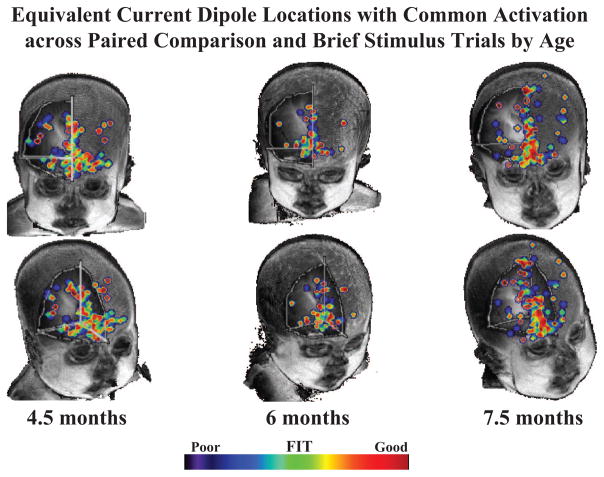
We also examined the location of functionally active brain areas with the projections of the ECDs. The ECD projections shown in Figure 11 were compared with the grand average waveform from the ERP. The similarity of the ERP and ECD projections was calculated by multiplying the millisecond × millisecond values from each and summing over the interval from +100 to +700 ms poststimulus (brief stimulus) or postsaccade (paired comparison). Large values of this represent waveforms from the ECD values that match the temporal changes, scalp location, and electrical direction of the ERP. We then found those ECD locations where the values for the paired comparison and brief stimulus procedures were the same. Figure 12 shows the fit of the ERP data and the projected data for the locations from the cortical sources. This figure shows the common ECDs that were activated in both procedures. The best fitting areas in common between the brief stimulus and paired comparison procedures were in the inferior prefrontal regions (e.g., Brodmann Areas 11, 25, 34). The cortical sources for the 20-week-olds were scattered across the medial-lateral aspects of the basal prefrontal cortex, well into the lateral aspects (Brodmann Area 34). There was an increasing trend from 4.5 to 7.5 months to show a larger proportion of active cortical areas more along the midline. By 7.5 months, this activity reached into the superior and posterior regions of the prefrontal cortex.
Figure 12.
Common equivalent current dipoles that were activated across tasks. Age groups are divided into separate columns. The best fitting areas in common between the brief stimulus and paired comparison procedures were in the inferior prefrontal regions (e.g., Areas 11, 25, 34).
Discussion
There were three major goals of the current study. The first goal was to examine the relationship between ERP and behavioral correlates of infant attention and recognition memory. The second goal was to simultaneously measure ERPs and infant visual preferences by processing and analyzing the EEG measured during paired comparison trials. The third goal was to identify the cortical sources of infant visual preferences through ECD analysis of the ICA components occurring during both ERP and paired comparison trials.
Relationship Between Behavioral and ERP Measures
Our approach to addressing goal one was to examine the Nc ERP component occurring during ERP trials in relationship to preference scores demonstrated during paired comparison trials. Consistent with past work (e.g., Richards, 2003a; Webb et al., 2005), there was a main effect for age with Nc increasing in amplitude with age. This may reflect the increasing involvement of prefrontal cortical areas in visual attention across infancy. There was also a main effect for preference that was qualified by an interaction of preference with stimulus type. Infants who demonstrated an overall novelty preference also demonstrated greater amplitude Nc in response to novel rather than familiar stimuli.6 Similarly, infants who demonstrated a transient preference for the novel stimulus on a single paired comparison trial showed greater amplitude Nc to the novel stimulus on ERP trials immediately preceding and following that specific paired comparison trial (see Figure 7).
This study is the first to demonstrate clear consistency between visual preference behavior on the paired comparison task and Nc amplitude. Studies that have utilized paired comparison trials or infant-controlled serial looking tasks following ERP testing have yielded inconsistent results in look duration and Nc amplitude (de Haan & Nelson, 1997; Karrer & Monti, 1995; Nelson & Collins, 1991, 1992). In these studies, differences were found in the ERP data on the basis of stimulus-type, but no analogous effects were found in the looking-time data. This may be due to the fact that the length of time following familiarization was longer for the behavioral phase of these experiments than for the ERP phase, and the infants may have been fatigued or off-task for the behavioral phase following ERP testing. By embedding paired comparison trials within the ERP phase of testing, we were able to control for the confounding effects of time and fatigue.
Past studies that have measured fixation duration throughout ERP testing have found some consistency between looking duration and Nc amplitude with looks to oddball or novel stimuli being longer than looks to standard or familiar stimuli (Ackles, 2008; Ackles & Cook, 1998; Hill-Karrer, Karrer, Bloom, Chaney, & Davis, 1998; Karrer & Ackles, 1987). For example, in a recent study conducted by Ackles (2008), 6–7-month-old infants demonstrated longer looking to novel or infrequent stimuli when compared with frequently presented stimuli; infants also demonstrated greater amplitude Nc to novel and infrequent stimuli. However, the measure of look duration used was unconventional. Looks were allowed to continue through multiple stimulus presentations that possibly contained more than one stimulus type. Blinking lights were also shown during interstimulus intervals when look duration was allowed to continue accumulating. Thus, length of a look did not reflect processing of a single stimulus or in some instances processing of a particular stimulus type. Additionally, the looking time data and ERP data were examined in separate analyses. Thus, the relationship between looking time and Nc amplitude was only examined at the group level and not within individual participants.
The current finding that infants demonstrate greater amplitude Nc to their “transiently” preferred stimulus is informative for the current debate on the functional significance of Nc. On the basis of earlier studies demonstrating a lack of stimulus type effects on Nc, it has been proposed that Nc reflects a general orienting response or processing of a contextual shift (e.g., Nelson, 1994; Richards, 2003a). According to these proposals, Nc is insensitive to stimulus characteristics. The current findings and others clearly indicate that Nc amplitude is impacted by stimulus characteristics as well as previous experience (e.g., Ackles, 2008; Ackles & Cook, 1998, 2007; Courchesne et al., 1981; Karrer & Ackles, 1987, 1988; Reynolds & Richards, 2005).
ERP Analysis of Paired Comparison Trials
To address the second goal, we conducted an ERP analysis of the EEG measured during paired comparison trials. This was done by segmenting the ERP trials on the basis of fixation onset as opposed to stimulus onset and through processing and removing the eye movement artifacts produced during shifts between stimuli. There were several interesting findings. First, there was a main effect of attention at central and parietal sites, with infants demonstrating greater average Nc amplitude when engaged in attention than when inattentive. This main effect was qualified by two interactions. Attention interacted with stimulus type; infants demonstrated greater Nc amplitude during attention but only on novel trials. Attention also interacted with preference; participants who demonstrated an overall novelty preference showed greater amplitude Nc during attention, whereas Nc amplitude was not affected by attention for infants who did not demonstrate an overall novelty preference. This effect also occurred in infants demonstrating a “transient novelty preference” on a single paired comparison trial (see Figure 8). Furthermore, there was a main effect for preference. Infants demonstrated greater amplitude Nc to their preferred stimulus, regardless of stimulus type (novel or familiar). Finally, there was an interaction of preference by stimulus type. Infants who demonstrated an “overall novelty preference” also demonstrated greater amplitude Nc on looks to novel stimuli. The group showing a “nonpreference overall” did not demonstrate differences on the basis of stimulus type (see Figure 9).
Similar to the analysis of the brief stimulus ERP data, these findings clearly demonstrate consistency between visual preference behavior and Nc amplitude. Additionally, the current findings indicate that Nc does not simply reflect detection of an improbable or novel event. Infants displayed greater amplitude Nc to their preferred stimulus regardless of stimulus type (novel or familiar). Thus, Nc amplitude is related to stimulus salience or what Cohen (1972) referred to as the “attention-getting” properties of the stimulus, and Nc is most likely functionally related to the onset of sustained attention (Reynolds & Richards, 2005). The main effect of attention on Nc amplitude replicating Richards (2003a) is consistent with this proposal. The relationship between attention and Nc was clarified in this analysis. Infants demonstrated greater amplitude Nc during attention but only during novel looks. During familiar looks, no differences were found in Nc amplitude for attention versus inattention. This further indicates that Nc reflects the onset of sustained attention as opposed to a general orienting response. Recognition of a stimulus as familiar or novel must occur prior to the peak of Nc during the early stages of visual processing (Ackles, 2008). Familiar stimulus presentations may have only elicited an orienting response and not led to further attention and information processing.
These results also indicate that individual differences may play a role in responsiveness of the Nc component to stimulus characteristics. Infants who demonstrated an overall novelty preference displayed differences in Nc amplitude on the basis of attention. However, those infants who demonstrated a nonpreference overall did not demonstrate differences in Nc amplitude on the basis of attention. This finding coupled with longitudinal research demonstrating that Nc amplitude increases with age within individual infant participants (Webb et al., 2005) warrants future investigation of individual differences in attention on the basis of look duration and ERP measures.
Cortical Source Localization of Visual Preferences
The third goal of the study addressed whether these two different measures tap into activity within the same areas of the brain. Ultimately, this goal was also concerned with whether these two measures involve the same (or similar) cognitive processes. We conducted ECD analysis of the ICA components most similar to the Nc ERP component. This analysis was done separately on the EEG from brief stimulus trials and paired comparison trials. We then compared the components from each data set on experimental effects and cortical sources to analyze the consistency between brain activity on paired comparison and brief stimulus trials. Three components were extracted from the ERP trials that contribute to Nc. These components were found to be active during ERP and paired comparison trials. In addition to demonstrating spatial distributions consistent with the Nc ERP component, these ICA components demonstrated temporal activation and experimental effects consistent with Nc. The cortical sources for these components were located in inferior prefrontal areas (Brodmann Areas 11, 25, 34), superior prefrontal areas (Brodmann Areas 6, 8, 9, 46), and the anterior cingulate (Brodmann Areas 24 and 32). The inferior prefrontal component demonstrated the greatest consistency in activation across brief stimulus ERP trials and paired comparison trials. There were some notable differences in the scalp distribution of Nc during brief presentations versus paired comparisons, indicating that there are additional brain areas that make unique contributions to each task; however, our analysis focused on areas that were active across tasks.
The results of our source analysis are consistent with the findings in the adult literature that bilateral activation of dorsolateral prefrontal cortex, inferior prefrontal cortex, and dorsal anterior cingulate cortex occurs across a wide variety of tasks requiring diverse cognitive demands (for a review, see Duncan & Owen, 2000). Researchers have proposed that this common activation of these specific prefrontal cortical areas is due to their role in a variety of cognitive functions, including executive attention, recognition memory, working memory, response inhibition, shifts of attention, suppression of saccades, integration of events across time, and executive control (e.g., Aron, Robbins, & Poldrack, 2004; Duncan & Owen, 2000; Fuster, 2001). There is a body of literature indicating that areas within the medial temporal lobe (MTL) play a pivotal role in recognition memory for nonhuman primates and adults (for reviews, see Eichenbaum, Yonelinas, & Ranganath, 2007; Snyder, 2007). The MTL is most likely involved in infant recognition memory as well and, thus, most likely influences infant visual preferences. It is plausible that MTL activity is associated with the late slow waves (LSWs) proposed to reflect recognition memory processes in human infants, as these ERP components often occur over temporal leads. However, because the LSWs begin approximately 1 s after stimulus onset and continue for up to 2 s after stimulus onset, many of the looks that occurred during paired comparison trials were not long enough to properly examine LSWs. For this and other practical reasons, we chose to focus our analysis exclusively on the Nc component.
ICA components localized to inferior prefrontal cortex, superior prefrontal cortex, and anterior cingulate cortex demonstrated spatial localization, temporal activation, and experimental effects similar to that of the Nc ERP component. This replicates our previous source localization work with human infants (Reynolds & Richards, 2005) and supports our proposal that these areas are involved in sustained attention and are strongly related to recognition memory. The ICA component with dipoles located in inferior prefrontal cortex demonstrated the most common activation across tasks. This finding combined with the visual preference effects found across tasks indicates that these areas of the brain are involved in the allocation of attention toward a given stimulus. Thus, Nc is likely a component of a general arousal system involved in attention. Activation of this system leads to decreased HR through parasympathetic outflow from the brain stem to the heart via the vagus nerve. This explains the relationship between the HR phases of attention and Nc amplitude. The general arousal system also involves enhanced processing throughout the cortex through the influence of the noradrenergic and dopaminergic neurochemical systems. Our results show that areas of the cortex involved in this attention system include inferior and superior prefrontal cortex and the anterior cingulate cortex.
Conclusion
The current findings as a whole demonstrate the increased level of information gained by measuring behavior and ERPs simultaneously in combination with the application of cortical source localization techniques. This is the first study to date to simultaneously measure visual preference behavior and ERPs during paired comparison trials. The complicated nature of brain-behavior relations in infancy was illustrated by multiple interaction effects. These results indicate that these approaches (paired comparison and ERPs) tap into the same underlying cognitive processes, thus eliciting similar cortical activity. This is an initial step in demonstrating convergent validity across these two commonly used behavioral and electrophysiological measures of visual attention and recognition memory. More work is needed in the area, and future research should be aimed at continued development of techniques for simultaneously measuring behavior and brain activity in human infants.
Acknowledgments
The research reported in this article and the writing of this article were supported by National Institute of Child Health and Human Development Grants R03-HD05600 (to Greg D. Reynolds) and R01-HD18942 (to John E. Richards) and by Natural Sciences and Research Council of Canada Grant OPG0093057 (to Mary L. Courage).
Footnotes
On all analyses, p > .05 for all channel clusters.
The ECD procedure hypothesizes a dipole with location and amplitude (moment) that generates a current on the scalp. The FEM model represents the electrical conductivity of the varying segments of the head, and the dipole moment generates a current through this media that results in a hypothesized current on the head, the so-called “forward model.” The forward model is compared with the empirical current data, and the dipole moment is iteratively adjusted to minimize the difference between the forward model and the empirical data (see Reynolds & Richards, 2009).
The traditional model for cortical source analysis is based on impedance values for cortical matter, skull, and scalp of adult participants. The use of adult impedance values with infant participants may affect the accuracy of cortical source localization with infant participants. In this study, we used structural MRIs from 11 infant participants and generated electrode placement maps on the basis of these individuals’ head measurements. One of these placement maps was then transformed to match the head measurements of each participant, and these transformed placement maps were used for each participant’s ECD analysis. This constrained the dipole localization to a somewhat realistic topography on the basis of the anatomy of each participant. A stronger but less practical approach would be to obtain structural MRIs for each individual infant. Additionally, a precise relation between the adult-based stereotaxic atlases and infant coordinate space is undetermined, thus the MNI atlas may be problematic for use in infant studies at this time. Richards is currently developing a realistic head modeling technique for use with infant participants that addresses these current limitations (for a discussion, see Reynolds & Richards, 2009). However, the fact that the current findings replicate our previous work using infant cortical source localization provides support for the utility of this approach with infant participants (Reynolds & Richards, 2005).
The ANOVAs used PROC GLM from SAS. Analyses of the intervals effect (4-ms intervals) were done but not reported because they are not relevant to the goals of the article. Additionally, we used a procedure to estimate any missing data using orthogonal polynomial components to reconstruct the missing data, which has the effect of doing a mean over the interval to be considered. This allowed the paired comparison ERP data, which on some trials had only 500 ms of data, to be directly compared with the brief stimulus ERP data. Also, the paired comparison trials were analyzed only when an infrequent novel stimulus was paired with one of the other stimuli (i.e., infrequent novel vs. frequent familiar; infrequent novel vs. infrequent familiar) and not when the two familiar stimuli were presented (frequent vs. infrequent familiar).
The regions chosen for the source volumes of relevance for the analyses include the anterior cingulate, central region, frontal pole, inferior prefrontal cortex, and superior–posterior prefrontal cortex. We also included the parietal cortex, occipital cortex, and a region consisting of the rest of the brain. These latter sections were included so that ECDs with a location in these regions were not included in the five relevant regions for the analysis.
It is interesting that approximately half of the infants did not demonstrate an overall novelty preference. In the classic paired comparison paradigm, 20 s of familiarization with achromatic visual patterns would be sufficient for the majority of participants in this age range to demonstrate a novelty preference. However, we believe that embedding paired comparison trials in the modified-oddball procedure resulted in a more complex task with greater levels of interference, thus accounting for a lower proportion of participants demonstrating overall novelty preferences (see Fagan, 1990, for a discussion of variables that may affect paired comparison performance).
Contributor Information
Greg D. Reynolds, University of Tennessee
Mary L. Courage, Memorial University
John E. Richards, University of South Carolina
References
- Ackles PK. Stimulus novelty and cognitive-related ERP components of the infant brain. Perceptual and Motor Skills. 2008;106:3–20. doi: 10.2466/pms.106.1.3-20. [DOI] [PubMed] [Google Scholar]
- Ackles PK, Cook KG. Stimulus probability and event-related potentials of the brain in 6-month-old human infants: A parametric study. International Journal of Psychophysiology. 1998;29:115–143. doi: 10.1016/s0167-8760(98)00013-0. [DOI] [PubMed] [Google Scholar]
- Ackles PK, Cook KG. Attention or memory? Effects of familiarity and novelty on the Nc component in event-related brain potentials in six-month-old infants. The International Journal of Neuroscience. 2007;117:837–867. doi: 10.1080/00207450600909970. [DOI] [PubMed] [Google Scholar]
- Aron AR, Robbins TW, Poldrack RA. Inhibition and the right inferior frontal cortex. Trends in Cognitive Neuroscience. 2004;8:170–177. doi: 10.1016/j.tics.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Bushnell IWR, Sai F, Mullin JT. Neonatal recognition of the mother’s face. British Journal of Developmental Psychology. 1989;7:3–15. [Google Scholar]
- Carver LJ, Bauer PJ, Nelson CA. Associations between infant brain activity and recall memory. Developmental Science. 2000;3:234–246. [Google Scholar]
- Casey BJ, Richards JE. Sustained visual attention measured with an adapted version of the visual preference paradigm. Child Development. 1988;59:1514–1521. [PubMed] [Google Scholar]
- Cohen LB. Attention-getting and attention-holding processes of infant visual preferences. Child Development. 1972;43:869–879. [PubMed] [Google Scholar]
- Colombo J, Richman WA, Shaddy DJ, Maikranz JM, Blaga O. Developmental course of visual habituation and preschool cognitive and language outcome. Infancy. 2004;5:1–38. [Google Scholar]
- Courage ML, Howe ML. Long-term retention in 3.5-month-olds: Familiarization time and individual differences in attentional style. Journal of Experimental Child Psychology. 2001;79:271–293. doi: 10.1006/jecp.2000.2606. [DOI] [PubMed] [Google Scholar]
- Courage ML, Reynolds GD, Richards JE. Infants’ visual attention to patterned stimuli: Developmental change from 3- to 12-months of age. Child Development. 2006;77:680–695. doi: 10.1111/j.1467-8624.2006.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courchesne E. Event-related brain potentials: Comparison between children and adults. Science. 1977 Aug 5;197:589–592. doi: 10.1126/science.877575. [DOI] [PubMed] [Google Scholar]
- Courchesne E, Ganz L, Norcia AM. Event-related brain potentials to human faces in infants. Child Development. 1981;52:804–811. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Recognition of the mother’s face by six-month-old infants: A neurobehavioral study. Child Development. 1997;68:187–210. [PubMed] [Google Scholar]
- de Haan M, Nelson CA. Brain activity differentiates face and object processing in 6-month-old infants. Developmental Psychology. 1999;35:1113–1121. doi: 10.1037//0012-1649.35.4.1113. [DOI] [PubMed] [Google Scholar]
- DeLorme A, Makeig S, Fabre-Thorpe M, Sejnowski T. From single-trial EEG to brain area dynamics. Neurocomputing. 2002;44–46:1057–1064. [Google Scholar]
- Duncan J, Owen AM. Common regions of the human frontal lobe recruited by diverse cognitive demands. Trends in Neurosciences. 2000;23:475–483. doi: 10.1016/s0166-2236(00)01633-7. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas A, Ranganath C. The medial temporal lobe and recognition memory. Annual Review of Neuroscience. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabiani M, Gratton G, Coles MGH. Event-related brain potentials: Methods, theory, and applications. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. New York, NY: Cambridge University Press; 2000. pp. 53–84. [Google Scholar]
- Fagan JF. The paired-comparison procedure and infant intelligence. In: Diamond A, editor. Annals of the New York Academy of Sciences: Vol. 68. The development and neural bases of higher cognitive functions. New York, NY: New York Academy of Sciences; 1990. pp. 337–364. [Google Scholar]
- Field TM, Cohen D, Garcia R, Greenberg R. Mother–stranger face discrimination by the newborn. Infant Behavior and Development. 1984;7:19–25. [Google Scholar]
- Fuster JM. The prefrontal cortex—An update. Time is of the essence. Neuron. 2001;36:319–333. doi: 10.1016/s0896-6273(01)00285-9. [DOI] [PubMed] [Google Scholar]
- Hill-Karrer J, Karrer R, Bloom D, Chaney L, Davis R. Event-related brain potentials during an extended visual recognition memory task depict delayed development of cerebral inhibitory processes among 6-month-olds with Down syndrome. International Journal of Psychophysiology. 1998;29:167–200. doi: 10.1016/s0167-8760(98)00015-4. [DOI] [PubMed] [Google Scholar]
- Hocking RR. The analysis of linear models. Monterey, CA: Brooks/Cole; 1985. [Google Scholar]
- Huizenga HM, Molenaar PCM. Estimating and testing the sources of evoked potentials in the brain. Multivariate Behavioral Research. 1994;29:237–262. doi: 10.1207/s15327906mbr2903_3. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Pechaud M, Smith S. BET2: MR-based estimation of brain, skull, and scalp surfaces. Paper presented at the 11th Annual Meeting of the Organization for Human Brain Mapping; Toronto, Ontario, Canada. 2005. Jun, [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Medical Image Analysis. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Johnson MH, de Haan M, Oliver A, Smith W, Hatzakis H, Tucker LA, Csibra G. Recording and analyzing high-density event-related potentials with infants using the Geodesic Sensor Net. Developmental Neuropsychology. 2001;19:295–323. doi: 10.1207/S15326942DN1903_4. [DOI] [PubMed] [Google Scholar]
- Jung TP, Humphries C, Lee TW, Makeig S, McKeown MJ, Iragui V, Sejnowski TJ. Extended ICA removes artifacts from electroencephalographic recordings. Advances in Neural Information Processing Systems. 1998;10:854–890. [Google Scholar]
- Jung TP, Makeig S, Fabre-Thorpe M, Sejnowksi TJ. Analysis and visualization of single-trial event-related potentials. Human Brain Mapping. 2001;14:166–185. doi: 10.1002/hbm.1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000;37:163–178. [PubMed] [Google Scholar]
- Karrer R, Ackles PK. Visual event-related potentials of infants during a modified oddball procedure. In: Johnson R, Rohrbaugh JW, Parasuraman R, editors. Current trends in event-related potential research. Amsterdam, the Netherlands: Elsevier Science; 1987. pp. 603–608. [PubMed] [Google Scholar]
- Karrer R, Ackles PK. Brain organization and perceptual/cognitive development in normal and Down syndrome infants: A research program. In: Vietze P, Vaughan HG Jr, editors. The early identification of infants with developmental disabilities. Philadelphia, PA: Grune & Stratton; 1988. pp. 210–234. [Google Scholar]
- Karrer R, Monti LA. Event-related potentials of 4–7 week-old infants in a visual recognition memory task. Electroencephalography and Clinical Neurophysiology. 1995;94:414–424. doi: 10.1016/0013-4694(94)00313-a. [DOI] [PubMed] [Google Scholar]
- Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics. 1977;33:159–174. [PubMed] [Google Scholar]
- Lee TW, Girolami M, Sejnowski TJ. Independent component analysis using an extended infomax algorithm for mixed subgaussian and supergaussian sources. Neural Computing. 1999;11:417–441. doi: 10.1162/089976699300016719. [DOI] [PubMed] [Google Scholar]
- Makeig S, Bell AH, Jung TP, Sejnowksi TJ. Independent component analysis of electroencephalographic data. Advances in Neural Information Processing Systems. 1996;8:145–151. [Google Scholar]
- Makeig S, Jung TP, Bell AH, Ghahremani D, Sejnowski TJ. Blind separation of auditory event-related brain responses into independent components. Proceedings of the National Academy of Sciences, USA. 1997;94:10979–10984. doi: 10.1073/pnas.94.20.10979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K, Harato H. Detection of rapid phases of eye movements using third-order derivatives. Japanese Journal of Ergonomics. 1983;19:147–153. [Google Scholar]
- Matsuoka K, Ueda Y. Frequency characteristics of the smooth pursuit component in tracking eye movements. Ergonomics. 1986;29:197–214. doi: 10.1080/00140138608968260. [DOI] [PubMed] [Google Scholar]
- Nelson CA. Neural correlates of recognition memory in the first postnatal year of life. In: Dawson G, Fischer K, editors. Human behavior and the developing brain. New York, NY: Guilford Press; 1994. pp. 269–313. [Google Scholar]
- Nelson CA, Collins PF. Event-related potential and looking-time analysis of infants’ responses to familiar and novel events: Implications for visual recognition memory. Developmental Psychology. 1991;27:50–58. [Google Scholar]
- Nelson CA, Collins PF. Neural and behavioral correlates of visual recognition memory in 4- and 8-month-old infants. Brain and Cognition. 1992;19:105–121. doi: 10.1016/0278-2626(92)90039-o. [DOI] [PubMed] [Google Scholar]
- Nikkel L, Karrer R. Differential effects of experience on the ERP and behavior of 6-month-old infants: Trends during repeated stimulus presentation. Developmental Neuropsychology. 1994;10:1–11. [Google Scholar]
- Nunez PL. Localization of brain activity with electroencephalography. Advances in Neurology. 1990;54:39–65. [PubMed] [Google Scholar]
- Pascalis O, de Schonen S, Morton J, Deruelle C, Fabre-Grenet M. Mother’s face recognition by neonates: A replication and extension. Infant Behavior and Development. 1995;18:79–95. [Google Scholar]
- Perrin F, Bertrand O, Pernier J. Scalp current density mapping: Value and estimation from brain data. IEEE Transactions on Biomedical Engineering. 1987;34:283–288. doi: 10.1109/tbme.1987.326089. [DOI] [PubMed] [Google Scholar]
- Perrin F, Pernier J, Bertrand O, Echallier JF. Spherical splines for scalp potential and current density mapping. Electroencephalography and Clinical Neurophysiology. 1989;72:184–187. doi: 10.1016/0013-4694(89)90180-6. [DOI] [PubMed] [Google Scholar]
- Picton TW, Bentin S, Berg P, Donchin E, Hillyard SA, Johnson R, Taylor MJ. Guidelines for using human event-related potentials to study cognition: Recording standards and publication criteria. Psychophysiology. 2000;37:127–152. [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Familiarization, attention, and recognition memory in infancy: An ERP and cortical source localization study. Developmental Psychology. 2005;41:598–615. doi: 10.1037/0012-1649.41.4.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds GD, Richards JE. Infant heart rate: A developmental psychophysiological perspective. In: Schmidt LA, Segalowitz SJ, editors. Developmental psychophysiology: Theory, systems, and applications. Cambridge, England: Cambridge University Press; 2007. pp. 173–212. [Google Scholar]
- Reynolds GD, Richards JE. Attention and early brain development. In: Tremblay RE, Barr RG, DeV Peters R, Boivin M, editors. Encyclopedia on early childhood development. Montreal, Quebec, Canada: Centre of Excellence for Early Childhood Development; 2008. pp. 1–5.pp. 1–5. Available from http://www.child-encyclopedia.com/documents/Reynolds-RichardsANGxp.pdf. [Google Scholar]
- Reynolds GD, Richards JE. Cortical source localization of infant cognition. Developmental Neuropsychology. 2009;34:312–329. doi: 10.1080/87565640902801890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Effects of attention on infants’ preference for briefly exposed visual stimuli in the paired-comparison recognition-memory paradigm. Developmental Psychology. 1997;33:22–31. doi: 10.1037//0012-1649.33.1.22. [DOI] [PubMed] [Google Scholar]
- Richards JE. Localizing the development of covert attention in infants using scalp event-related-potentials. Developmental Psychology. 2000;36:91–108. [PubMed] [Google Scholar]
- Richards JE. Cortical indices of saccade planning following covert orienting in 20-week-old infants. Infancy. 2001;2:135–157. [Google Scholar]
- Richards JE. Attention affects the recognition of briefly presented visual stimuli in infants: An ERP study. Developmental Science. 2003a;6:312–328. doi: 10.1111/1467-7687.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Cortical sources of event-related-potentials in the prosaccade and antisaccade task. Psychophysiology. 2003b;40:878–894. doi: 10.1111/1469-8986.00106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Recovering cortical dipole sources from scalp-recorded event-related-potentials using component analysis: Principal component analysis and independent component analysis. International Journal of Psychophysiology. 2004;54:201–220. doi: 10.1016/j.ijpsycho.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Richards JE. Localizing cortical sources of event-related potentials in infants’ covert orienting. Developmental Science. 2005;8:255–278. doi: 10.1111/j.1467-7687.2005.00414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards JE. Realistic cortical source models of ERP. 2006 Unpublished manuscript. Retrieved from http://jerlab.psych.sc.edu./PDF%20Files/RealisticSourceModels.pdf.
- Richards JE, Casey BJ. Development of sustained visual attention in the human infant. In: Campbell BA, Hayne H, Richardson R, editors. Attention and information processing in infants and adults. Mahwah, NJ: Erlbaum; 1992. pp. 30–60. [Google Scholar]
- Richards JE, Hunter SK. Peripheral stimulus localization by infants with eye and head movements during visual attention. Vision Research. 1997;37:3021–3035. doi: 10.1016/s0042-6989(97)00082-5. [DOI] [PubMed] [Google Scholar]
- Richards JE, Reynolds GD, Courage ML. The neural bases of infant attention. Current Directions in Psychological Science. 2010;19:41–46. doi: 10.1177/0963721409360003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose SA. Differential rates of visual information processing in full-term and preterm infants. Child Development. 1983;54:1189–1198. [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Infant visual recognition memory. Developmental Review. 2004;24:74–100. doi: 10.1037/0012-1649.39.3.563. [DOI] [PubMed] [Google Scholar]
- Rose SA, Feldman JF, Jankowski JJ. Developmental aspects of visual recognition memory in infancy. In: Oakes LM, Bauer PJ, editors. Short- and long-term memory in infancy and early childhood. New York, NY: Oxford University Press; 2007. pp. 153–178. [Google Scholar]
- Rose SA, Gottfried AW, Melloy-Carminar PM, Bridger WH. Familiarity and novelty preferences in infant recognition memory: Implications for information processing. Developmental Psychology. 1982;18:704–713. [Google Scholar]
- Scherg M. Fundamentals of dipole source potential analysis. In: Grandon F, Hoke M, Romani GL, editors. Auditory evoked magnetic fields and potentials. Vol. 6. Basel, Switzerland: Karger; 1990. pp. 40–69. [Google Scholar]
- Scherg M. Functional imaging and localization of electromagnetic brain activity. Brain Topography. 1992;5:103–111. doi: 10.1007/BF01129037. [DOI] [PubMed] [Google Scholar]
- Scherg M, Picton TW. Separation and identification of event-related potential components by brain electrical source analysis. In: Brunia CHM, Mulder G, Verbaten MN, editors. Event-related brain research. Amsterdam, the Netherlands: Elsevier; 1991. pp. 24–37. [PubMed] [Google Scholar]
- Searle SR. Linear models. New York, NY: Wiley; 1971. [Google Scholar]
- Searle SR. Linear models for unbalanced data. New York, NY: Wiley; 1987. [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Human Brain Mapping. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage. 1999;23:208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Snyder K. Neural mechanisms of attention and memory in preferential looking tasks. In: Oakes LM, Bauer PJ, editors. Short- and long-term memory in infancy and early childhood. New York, NY: Oxford University Press; 2007. pp. 179–208. [Google Scholar]
- Talairach J, Tournoux P. Co-planar stereotaxic atlas of the human brain. New York, NY: Thieme Medical; 1988. [Google Scholar]
- Webb SJ, Long JD, Nelson CA. A longitudinal investigation of visual event-related potentials in the first year of life. Developmental Science. 2005;8:605–616. doi: 10.1111/j.1467-7687.2005.00452.x. [DOI] [PubMed] [Google Scholar]