Abstract
Cyprinid herpesvirus 3 (CyHV-3) is the aetiological agent of a serious and notifiable disease afflicting common and koi carp, Cyprinus carpio L., termed koi herpesvirus disease (KHVD). Significant progress has been achieved in the last 15 years, since the initial reports surfaced from Germany, USA and Israel of the CyHV-3 virus, in terms of pathology and detection. However, relatively few studies have been carried out in understanding viral replication and propagation. Antibody-based affinity has been used for detection of CyHV-3 in enzyme-linked immunosorbent assay and PCR-based techniques, and immunohistological assays have been used to describe a CyHV-3 membrane protein, termed ORF81. In this study, monoclonal antibodies linked to N-hydroxysuccinimide (NHS)-activated spin columns were used to purify CyHV-3 and host proteins from tissue samples originating in either CyHV-3 symptomatic or asymptomatic fish. The samples were next analysed either by polyacrylamide gel electrophoresis (PAGE) and subsequently by electrospray ionization coupled to mass spectrometry (ESI-MS) or by ESI-MS analysis directly after purification. A total of 78 host proteins and five CyHV-3 proteins were identified in the two analyses. These data can be used to develop novel control methods for CyHV-3, based on pathways or proteins identified in this study.
Keywords: electrospray ionization mass spectrometry, immunochemistry, koi herpesvirus, polyacrylamide gel electrophoresis, protein purification
Introduction
Cyprinid herpesvirus 3 (CyHV-3), formerly known as koi herpesvirus (KHV), is the aetiological agent of a serious and notifiable disease afflicting common and koi carp, Cyprinus carpio L., termed koi herpesvirus disease (KHVD), and it has spread via carp trade and koi shows (Pikarsky et al. 2004; Pokorova et al. 2005; Ilouze et al. 2008). CyHV-3 has been isolated from kidney, gill, spleen, intestine, liver and brain of dead fish and causes a high mortality rate, between 80 and 100% (Hedrick et al. 2000). The most common clinical signs of the disease are white patches, sunken eyes, enlargement of the spleen and kidney, and necrosis in the gills in infected fish (Hedrick et al. 2005). CyHV-3 is known to infect carp at temperatures between 15 and 25 °C (Gilad et al. 2003). The genome of CyHV-3 consists of approximately 295 thousand base pairs (kB), coding for 156 novel putative proteins (Aoki et al. 2007).
Several studies have used immunochemistry, which takes advantage of affinity based on antibody avidity to an antigen, in investigations into CyHV-3 (Rosenkranz et al. 2008; Soliman & El-Matbouli 2009). In a recent study, polyclonal antibodies against CyHV-3 were used for viral detection purposes (Soliman & El-Matbouli 2009), and the technique may be more sensitive than other traditional PCR-based methods (Gilad et al. 2002; Bercovier et al. 2005; El-Matbouli, Rucker & Soliman 2007; Bergmann et al. 2010; Soliman & El-Matbouli 2010). A sensitive enzyme-linked immunosorbent assay (ELISA) was developed for the detection of CyHV-3 by Adkinson, Oren & Hendrick (2005), and a different monoclonal antibody against ORF68 has also been developed by Aoki et al. (2011). Another monoclonal antibody, derived against ORF81, localized to cytoplasmic regions of cells, including endoplasmic reticulum or Golgi apparatus probably during protein synthesis, and into the viral envelope in mature virons (Rosenkranz et al. 2008).
Knowledge about protein interactions can be used to understand how viruses enter host cells and propagate during infection (Guo et al. 2011; Blondot et al. 2012). Although some genes in CyHV-3 maintenance and replication have been identified (Fuchs et al. 2011), little is known about the protein interactions in viral propagation and even less is known whether this virus interacts with any endogenous common carp proteins. A recent report (Michel et al. 2010) used polyacrylamide gel electrophoresis (PAGE) and chemical-based protein purification techniques to identify 40 CyHV-3 proteins incorporated into mature virons. Eighteen fish proteins were also identified in the study including one common carp protein (Michel et al. 2010). Although a number of methods for protein purification have been described (de Souza et al. 2008; Moen et al. 2011), purification by antibody-based avidity is a powerful technique to identify in vivo protein binding partners (Tuxworth et al. 2005; Gotesman, Hosein & Gavin 2010; Gotesman, Hosein & Gavin 2011).
During mass spectrometry, samples are ionized and filtered based on mass, charge and shape, and subsequently, a detector measures the sample’s mass-to-charge ratio (Cameron 2012). Analysis of samples containing polymers, peptides and proteins of molecular weights beyond 20 kDa using an electrospray ionization source coupled with a quadruple mass spectrometer (ESI-MS) was first proposed by Fenn et al. (1989). Since then, ESI-MS has developed to be a sensitive tool for detecting samples at femtomolar concentrations in nanomole quantities and a routine method for characterizing non-volatile and thermally labile bio-molecules that are not amenable to analysis by other conventional techniques (Ho et al. 2003). Over the last decade, ESI-MS has emerged as an important technique in proteomics for confirmation of amino acid sequence and for characterization of post-translational modifications (Griffiths et al. 2001). We used antibody purification in conjunction with ESI-MS analysis to identify novel proteins that may be used as potential targets for the inhibition of CyHV-3 replication.
Materials and methods
Fish specimens
Two naturally infected fish, which showed signs of severe necrosis of the gills and skin, and loss of mucus on the skin, were used as CyHV-3 asymptomatic samples. One fish that lacked any signs of CyHV-3 was used as a control.
Tissue preparation
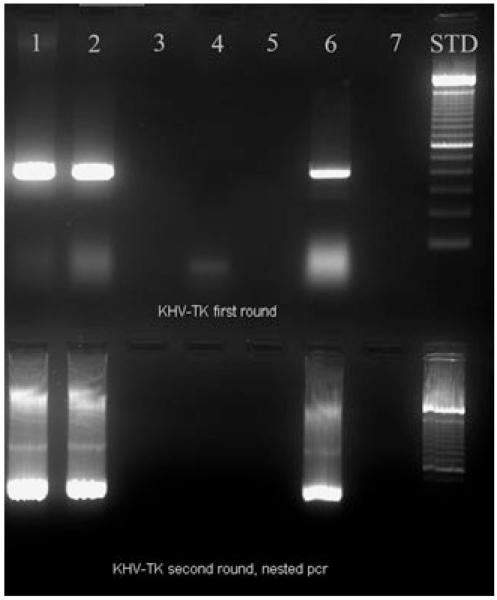
Tissue extracts (pooled from gill, liver, kidney, spleen and brain) were separately sampled aseptically from two different CyHV-3 symptomatic and one asymptomatic koi carp, and the samples were separately homogenized in minimum essential medium. Part of each homogenate was used for DNA extraction using a DNeasy Blood Tissue Kit (Qiagen) in accordance with the manufacturer’s instructions. Another aliquot of the homogenate was used for propagation of CyHV-3 on common carp brain cell line (CCB). Extracted DNA was subjected to conventional PCR according to Bercovier et al. (2005) and real-time PCR according to Gilad et al. (2004). CyHV-3 from symptomatic fish was successfully propagated on CCB cells and subsequently confirmed by PCR amplification of target DNA segments by conventional (Fig. 1) and real-time PCR as previously described. However, neither conventional PCR nor real-time PCR detected CyHV-3 in the asymptomatic fish sample. The remaining aliquots were separately used to prepare tissue lysate for electrospray ionization mass spectrometry (ESI-MS) analysis.
Figure 1.
PCR analysis of infected fish samples. Lane 1: DNA extract from the symptomatic fish that was later used for PAGE and subsequent ESI-MS analysis. Lane 2: DNA extract from the symptomatic fish that was later used directly for ESI-MS analysis. Lane 3: empty. Lane 4: DNA extract from the asymptomatic fish that was later used directly for ESI-MS analysis. Lane 5: empty. Lane 6: DNA extract from CyHV-3 positive control. Lane 7: empty. Fifteen microlitres of sample was added to lanes 1–7. Lane 8: 1 μL of Bio-Rad 1 kb DNA Ladder.
Tissue lysate preparation
Each homogenate from the previous step was separately lysed in a 1:1 ratio with a non-denaturing lysis buffer: 50 mm Tris–HCl (pH 8.0), 150 mm NaCl, 20 mm ethylene diamine tetraacetic acid (EDTA), 1% Na-deoxycholate, 1% Triton X-100 (Williams 2000) and protease inhibitor cocktail (50 μL mL−1 of lysis buffer). Subsequently, each lysate was vigorously vortexed and centrifuged at 16 000 g for 15 min. The supernatant was transferred to a fresh 1.5-mL Eppendorf tube and recentrifuged at 16 000 g for an additional 15 min. Supernatant from the second centrifugation for each fraction was separately used for affinity purification as described in the following sections.
Preparation of monoclonal antibody-linked spin columns
Monoclonal antibodies against CyHV-3 glycoprotein (KHV/10A9 #171103, Insel Riems, Germany) were conjugated to N-hydroxysuccinimide (NHS)-activated 33-mg-capacity agarose spin column (Thermo Fisher Scientific) according to the manufacturer’s instructions. Briefly, 50 μL of CyHV-3 monoclonal antibody was resuspended into 350 μL of phosphate-buffered saline (PBS): 13.7 mm NaCl, 0.27 mm KCl, 10 mm Na2HPO4, 0.2 mm KH2PO4, pH 7.4 to make a total solution of 400 μL, and incubated overnight at 4 °C with mild shaking of 300 rpm in an Eppendorf Thermomixer Comfort. The spin columns were emptied and washed twice with PBS after overnight incubation at 4 °C with mild shaking. Subsequently, the spin columns were quenched with 400 μL of 1 m ethanolamine (pH 7.4) by incubation for 1 h at 4 °C with mild shaking. Finally, the spin columns were emptied by centrifugation at 16 000 g for 30 s and washed six times with PBS, and optical density at 280 (OD280) of the final flow-through was zero.
Protein purification
Non-denatured whole-cell extracts from CyHV-3 tissue samples originating in either symptomatic or asymptomatic fish were separately incubated overnight at 4 °C with mild shaking at 300 rpm in monoclonal antibody-linked spin columns (previously described). Next, the spin columns were cleared, and the flow-through was saved for gel electrophoresis. The spin columns were washed eight times with PBS, and the OD280 of the final flow-through was zero. The spin columns were subsequently eluted with 500 μL of 0.1 m glycine (pH 3.0) by incubation with mild shaking for 1 h at 4 °C, and the pH was immediately neutralized by addition of 50 μL of 1 m Tris base (pH 8.0). The columns were immediately washed with PBS, and both the eluate and the first wash fraction were concentrated, using a dry vacuum concentrator (Eppendorf) to a final volume of 55 μL. Next, the samples were either analysed by ESI-MS or further concentrated to 10 μL and analysed by sodium dodecyl sulphate - polyacrylamide gel electrophoresis (SDS-PAGE).
SDS-PAGE
The 10 μL concentrated sample was mixed with 10 μL of 2× Laemmli sample buffer (100 mm Tris–HCl, pH 6.8, 200 mm 2-mercaptoethanol, 4% SDS, 0.2% bromophenol blue, 20% glycerol) and heated at 99 °C with mild shaking (300 rpm) for 10 min. Proteins were separated in 12% polyacrylamide resolving gel and 5% stacking gel prepared according to Sambrook & Russell 2001). Gels were run using the Mini-Protein Tetra Cell (Bio-Rad laboratories GmbH) at 120 V for 70 min in 1× running buffer (25 mm Tris, 250 mm glycine, pH 8.3, 0.1% SDS). Gel loading was equal for all lysates. Proteins were visualized by staining with Coomassie Brilliant Blue R-250 (Sigma-Aldrich). The broad range (10–200 kDa) ProSieve Unstained Protein Marker II (Lonza), which contain 11 bands, 10, 15, 20,30, 40, 50, 70, 100, 120, 150 and 200 kDa, was used to estimate the molecular weight of the separated proteins.
Electrospray ionization mass spectrometry (ESI-MS) analysis
After staining with Coomassie Blue, the PAGE-gel was cut at sites corresponding to regions where bands of interest were visualized and sent for electrospray ionization coupled to mass spectrometry (ESI-MS) analysis. The other samples, which consisted of the entire antibody-purified products originating in either symptomatic or asymptomatic fish, were also separately analysed by ESI-MS. Electrospray ionization mass spectrometry analysis was performed by the DKFZ, The German Cancer Research Centre in the Helmholtz Association (Heidelberg, Germany).
Results
Protein purification and gel electrophoresis
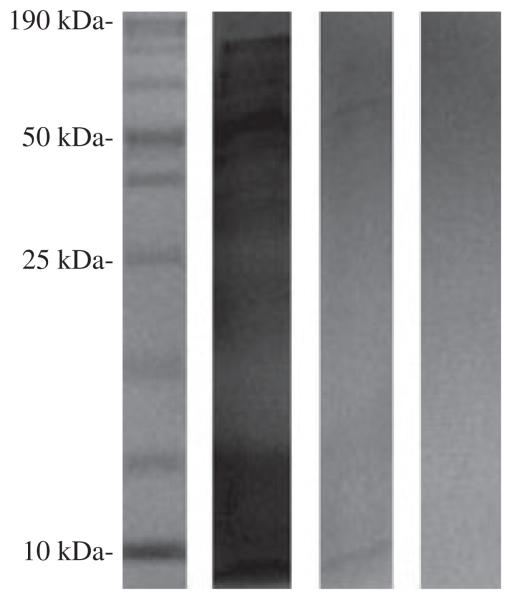
Protein purification by affinity was used to analyse host and viral proteins involved in CyHV-3 infection. Tissue samples originating in CyHV-3 symptomatic and asymptomatic fish were processed by anti-CyHV-3 monoclonal antibody conjugated to spin columns (as described in the Materials and methods). The two symptomatic samples were eluted with respective optical densities of 0.264 and 0.552 at 280 (OD280). After concentration, the OD280 of the samples was above 3.0. The third sample, originating from asymptomatic and PCR-negative fish, was also purified, and the eluate had an OD280 of 0.012. The sample with an original OD of 0.264 was analysed on sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE). One band corresponding to ~10 kDa and another band corresponding to ~60 kDa were visualized (Fig. 2).
Figure 2.
PAGE analysis of immunoprecipitation. Lane 1: 5 μL of the ProSieve Unstained Protein Marker II (By Lonza), 10–200 kDa was used, which consists of 11 bands: 10, 15, 20, 30, 40, 50, 70, 100, 120, 150 and 200 kDa. The 120, 150 and 200 kDa did not separate well and appear as 1 band. Lane 2: Flow-through of non-denatured lysate (see methods). Lane 3: Monoclonal antibody–purified peptides. Two bands are observed: one at ~10 kDa and another band corresponding to ~60 kDa. Lane 4: First phosphate-buffered saline wash after elution with glycine (pH 3.0). Forty microlitres of the respective samples was run in lanes 2–4.
ESI-MS analysis
Two separate samples of anti-CyHV-3 monoclonal antibody–purified CyHV-3 tissue samples originating in symptomatic fish and a tissue sample originating in asymptomatic, and PCR-negative fish were analysed by electrospray ionization coupled to mass spectrometry (ESI-MS) analysis.
The first sample, which had an original OD280 of 0.264 after purification, consisted of two slices that showed bands in Coomassie-stained gel as previously described. The other sample, which had an original OD280 of 0.552 after purification, was analysed without any modifications except for concentration. The two analyses identified a total of five CyHV-3 proteins, two of which were overlapping (40%), and a total of 78 carp proteins, 28 of which were overlapping (36%). In the sample originating from asymptomatic fish, only one CyHV-3 was identified in conjunction with the 12 carp proteins identified, of which eight (66%) were identical to ones identified in the gel separation.
Gel separation
In the gel separation sample, 56 proteins were identified by ESI analysis of anti-CyHV-3 monoclonal antibody–purified tissue samples originating in CyHV-3 symptomatic fish. Two CyHV-3 proteins were identified: the major capsid protein and glycoprotein (Table 1a). The remaining 54 proteins, corresponding to proteins originating from carp were classified into seven different groups (Table 2a). Five of the carp proteins or their homologs were previously identified in Michel et al.’s (2010) report, in conjunction with beta-actin (Kuznetsov, Langford & Weiss 1992), which was grouped with three other cytoskeletal proteins. Also, seven proteins involved in host defence regulation in addition to 12 proteins that are involved in protein modification were identified. Fifteen haem-harbouring proteins in conjunction with three transferrins were also identified, and the remaining eight proteins were unclassified.
Table 1.
CyHV-3 proteins identified by ESI-MS analysis
| Accession no. | Protein description | Score | Mass [Da] | Matches | Coverage [%] |
|---|---|---|---|---|---|
| (a) Two CyHV-3 proteins identified by eiectrospray analysis from gei slices originating in symptomatic fish samples | |||||
| gi|65306693 | Major capsid protein [Cyprinid herpesvirus 3] | 278 | 140437 | 7 | 4.8 |
| gi|129560573 | Glycoprotein [Cyprinid herpesvirus 3] | 43 | 100710 | 2 | 2 |
| (b) Five CyHV-3 proteins identified by electrospray analysis from unmodified lysate solution originating in symptomatic fish samples | |||||
| gi|61696095 | Major capsid protein [Cyprinid herpesvirus 3] | 25 | 140436 | 1 | 0.9 |
| gi|l29560573 | Glycoprotein [Cyprinid herpesvirus 3] | 24 | 100710 | 1 | 0.9 |
| gi|l29560669 | 0RF150 [Cyprinid herpesvirus 3] | 23 | 70515 | 1 | 1.1 |
| gi|l31840052 | 0RF22 [Cyprinid herpesvirus 3] | 22 | 67563 | 1 | 1 |
| gi|l31840053 | ORF23 [Cyprinid herpesvirus 3] | 26 | 38808 | 1 | 2.1 |
| (c) One CyHV-3 proteins identified by electrospray analysis from unmodified lysate solution originating in asymptomatic fish | |||||
| gi|129560573 | Glycoprotein [Cyprinid herpesvirus 3] | 24 | 100710 | 1 | 1.1 |
CyHV-3, Cyprinid herpesvirus 3.
Table 2.
Cyprinus carpio proteins identified by ESI-MS analysis
| Accession no. | Protein description | Score | Mass [Da] | Matches | Coverage [%] |
|---|---|---|---|---|---|
| (a) Fifty-four C. carpio proteins identified by electrospray analysis from gel slices originating in symptomatic fish samples grouped into 7 aforementioned categories | |||||
| Michel et al. 2010 or homolog proteins | |||||
| gi|28628941 | Elongation factor 1-alpha | 128 | 50 325 | 3 | 6.1 |
| gi|l7369826 | Lactate dehydrogenase A chain | 115 | 36 539 | 2 | 5.4 |
| gi|l5628189 | Heat shock protein 90 alpha | 28 | 25 336 | 1 | 5.3 |
| gi|l4388583 | Warm-temperature acclimation-related 65-kDa protein | 78 | 50 640 | 3 | 6.6 |
| gi|218511593 | RAB8A | 23 | 23 823 | 1 | 5.3 |
| Cytoskeletal proteins | |||||
| gi|42560193 | Beta-actin | 496 | 42 068 | 24 | 25.1 |
| gi|1703140 | Alpha-actin-1 | 326 | 42 274 | 14 | 18.8 |
| gi|13365501 | Integrin beta-2 chain | 173 | 87 597 | 5 | 7.3 |
| gi|6226787 | Vimentin | 56 | 52 516 | 4 | 3.5 |
| Host defence proteins | |||||
| gi|4126587 | Complement C3-H1 | 223 | 184 985 | 6 | 4 |
| gi|9453863 | Complement C4-1 | 67 | 193 004 | 1 | 0.8 |
| gi|9453865 | Complement C4-2 | 98 | 194 643 | 3 | 1.9 |
| gi|3399699 | Natural killer cell enhancing factor | 44 | 22 410 | 1 | 5.5 |
| gi|297718575 | Granzyme A/K | 26 | 29 149 | 2 | 4.3 |
| gi|7939556 | Lysozyme C | 49 | 17 241 | 2 | 11.7 |
| gi|2829695 | Granulin-3 | 47 | 6995 | 2 | 24.6 |
| Protein modification enzymes or inhibitors | |||||
| gi|439153 | Serine protease inhibitor | 288 | 45 960 | 12 | 13.4 |
| gi|416561 | Alpha-1-antitrypsin homolog | 372 | 42 044 | 22 | 23.7 |
| gi|56709493 | Putative glyceraldehyde-3-phosphate dehydrogenase | 21 | 33 540 | 2 | 2.2 |
| gi|4027925 | Creatine kinase M1-CK | 37 | 42 980 | 1 | 3.4 |
| gi|73762632 | Carbonic anhydrase | 80 | 28 716 | 3 | 11.9 |
| gi|373503076 | Glutamate dehydrogenase | 910 | 54 750 | 46 | 47.1 |
| gi|112901127 | Glutathione S-transferase rho | 35 | 26 700 | 1 | 6.6 |
| gi|152925970 | Ubiquitin fusion protein | 279 | 14 987 | 19 | 41.4 |
| gi|91798526 | Proteasome activator PA28 subunit | 37 | 28 579 | 1 | 2.8 |
| gi|217272706 | Monoamine oxidase | 442 | 59 621 | 13 | 16.3 |
| gi|6690508 | Janus kinase 3 | 22 | 131 078 | 1 | 0.7 |
| gi|300676317 | Catalase | 21 | 49 187 | 1 | 3.1 |
| Globins | |||||
| gi|2208883 | Alpha-globin | 529 | 15 421 | 35 | 52.4 |
| gi|2208891 | Alpha-globin | 488 | 15 566 | 35 | 54.5 |
| gi|2208889 | Alpha-globin | 390 | 15 574 | 28 | 61.5 |
| gi|122392 | Alpha-globin | 595 | 15 437 | 45 | 62.2 |
| gi|6009727 | Alpha-2-macroglobulin-1 | 631 | 162 274 | 22 | 11.8 |
| gi|6009729 | Alpha-2-macroglobulin-2 | 541 | 157 876 | 18 | 10 |
| gi|6009731 | Alpha-2-macroglobulin-3 | 434 | 88 297 | 14 | 15.1 |
| gi|140368180 | Alpha-2-macroglobulin 4 | 368 | 101 340 | 12 | 11.4 |
| gi|22135548 | Beta-globin | 584 | 16 520 | 41 | 63.3 |
| gi|2208895 | Beta-globin | 732 | 16 617 | 67 | 87.2 |
| gi|1644253 | Beta-globin | 718 | 16 645 | 63 | 87.2 |
| gi|1644251 | Beta-globin | 706 | 16 542 | 68 | 87.2 |
| gi|3953518 | Immunoglobulin heavy chain | 1053 | 50 331 | 54 | 47.6 |
| gi|55700034 | Immunoglobulin heavy chain | 78 | 40 300 | 4 | 6.4 |
| gi|85067845 | Myoglobin isoform 2 | 29 | 16 278 | 1 | 5.4 |
| Transferrins | |||||
| gi|18034630 | Transferrin variant A | 1882 | 75 595 | 100 | 47.1 |
| gi|189473163 | Transferrin variant F | 986 | 75 036 | 45 | 26 |
| gi|189473165 | Transferrin variant G | 1003 | 75 083 | 47 | 26.9 |
| Unclassified | |||||
| gi|29501366 | Fetuin short form | 43 | 34 332 | 2 | 7 |
| gi|13445027 | Apolipoprotein A-I | 83 | 20 797 | 4 | 16.1 |
| gi|435737 | Glial fibrillary acidic protein | 119 | 24 944 | 69 | 9.3 |
| gi|151558991 | Vitellogenin B1 | 2221 | 148 683 | 100 | 39.6 |
| gi|52782167 | Vitellogenin B2 | 964 | 179 640 | 48 | 13.4 |
| gi|14009437 | Mitochondrial ATP synthase | 32 | 59 699 | 1 | 2.2 |
| gi|47605558 | Mitochondrial synthase subunit beta | 71 | 55 327 | 2 | 4.1 |
| gi|10566900 | Myeloid protein-1 | 35 | 17 849 | 1 | 7.5 |
| (b) Fifty-two C. carpio proteins identified by electrospray analysis from unmodified lysate solution originating in symptomatic fish samples grouped into 7 aforementioned categories | |||||
| Michel et al. 2010 or homologs | |||||
| gi|28628941 | Elongation factor 1-alpha | 97 | 50 325 | 5 | 6.7 |
| gi|l7369826 | Lactate dehydrogenase A chain | 110 | 36 539 | 2 | 6.3 |
| gi|55669145 | Lactate dehydrogenase B-type subunit | 150 | 36 669 | 3 | 9 |
| gi|33598990 | Constitutive heat shock protein HSC70-2 | 329 | 70 779 | 12 | 12.3 |
| gi|218511593 | RAB8A | 26 | 23 823 | 1 | 5.3 |
| Cytoskeletal proteins | |||||
| gi|42560193 | Beta-actin | 457 | 42 068 | 15 | 24.8 |
| gi|6226787 | Vimentin | 127 | 52 516 | 6 | 3.5 |
| Host defence proteins | |||||
| gi|4126587 | Complement C3-H1 | 362 | 184 985 | 9 | 5.2 |
| gi|4126593 | Complement C3-S | 179 | 185 561 | 5 | 2.9 |
| gi|3399699 | Natural killer cell enhancing factor | 93 | 22 410 | 3 | 9.5 |
| gi|84569882 | Natural killer cell enhancing factor | 251 | 22 014 | 10 | 47.7 |
| gi|165880803 | MIF | 26 | 12 525 | 1 | 14.8 |
| gi|47117021 | Lysozyme g | 88 | 20 425 | 3 | 10.8 |
| Protein modification enzymes or inhibitors | |||||
| gi|439153 | Serine protease inhibitor | 83 | 45 960 | 2 | 9 |
| gi|416561 | Alpha-1-antitrypsin homolog | 261 | 42 044 | 7 | 15.6 |
| gi|56709493 | Putative glyceraldehyde-3-phosphate dehydrogenase | 146 | 33 540 | 2 | 9.2 |
| gi|4027925 | Creatine kinase M1-CK | 34 | 42 980 | 1 | 1.6 |
| gi|73762632 | Carbonic anhydrase | 285 | 28 716 | 7 | 40.4 |
| gi|373503074 | Glutamate dehydrogenase | 63 | 54 737 | 2 | 3.1 |
| gi|112901127 | Glutathione S-transferase rho | 51 | 26 700 | 1 | 4.4 |
| gi|95832156 | Pi-class glutathione S-transferase | 69 | 23 794 | 2 | 10.1 |
| gi|300676299 | Glutathione peroxidase 1 | 37 | 19 182 | 1 | 10 |
| gi|343481094 | Glutathione synthetase | 22 | 41 342 | 1 | 2.5 |
| gi|353351682 | Trypsin 1 | 114 | 26 940 | 1 | 6.6 |
| gi|117518748 | Enolase | 21 | 16 206 | 1 | 4.1 |
| Globins | |||||
| gi|2208891 | Alpha-globin | 527 | 15 566 | 44 | 73.4 |
| gi|2208883 | Alpha-globin | 494 | 15 421 | 35 | 69.2 |
| gi|2208887 | Alpha-globin | 492 | 15 449 | 36 | 69.2 |
| gi|2208889 | Alpha-globin | 372 | 15 574 | 24 | 69.2 |
| gi|122392 | Alpha-globin | 659 | 15 437 | 56 | 81.1 |
| gi|1644251 | Beta-globin | 652 | 16 542 | 47 | 78.4 |
| gi|2208895 | Beta-globin | 568 | 16 617 | 46 | 70.9 |
| gi|22135548 | Beta-globin | 485 | 16 520 | 26 | 54.4 |
| gi|3953518 | Immunoglobulin heavy chain | 177 | 50 331 | 5 | 12.8 |
| gi|85067845 | Myoglobin isoform 2 | 86 | 16 278 | 2 | 12.9 |
| Transferrins | |||||
| gi|18034630 | Transferrin variant A | 420 | 75 595 | 17 | 12.3 |
| gi|189473165 | Transferrin variant G | 321 | 75 083 | 12 | 10.2 |
| Unclassified | |||||
| gi|29501366 | Fetuin short form | 64 | 34 332 | 3 | 7.3 |
| gi|13445027 | Apolipoprotein A-I | 226 | 20 797 | 7 | 32.2 |
| gi|435737 | Glial fibrillary acidic protein | 417 | 24 944 | 41 | 28 |
| gi|435739 | Glial fibrillary acidic protein | 504 | 24 531 | 44 | 33.2 |
| gi|49356890 | Glia-derived neurotrophic factor | 25 | 11 004 | 1 | 8.6 |
| gi|2252655 | Nephrosin precursor | 340 | 31 629 | 13 | 27.5 |
| gi|281333450 | Heart-type fatty-acid-binding protein | 156 | 14 704 | 5 | 21.8 |
| gi|15778562 | Vitellogenin | 294 | 148 794 | 12 | 7.8 |
| gi|84569880 | Translationally controlled tumour protein | 84 | 19 152 | 2 | 8.2 |
| gi|281429776 | Adipocyte fatty-acid-binding protein | 124 | 15 278 | 2 | 16.4 |
| gi|1173446 | Somatoliberin | 28 | 4976 | 1 | 17.8 |
| gi|585105 | Ependymin | 385 | 24 433 | 67 | 41.9 |
| gi|40549335 | Cytochrome P4501C1 | 24 | 59 583 | 1 | 1.3 |
| gi|219935425 | Mineralocorticoid receptor | 24 | 107 380 | 1 | 0.5 |
| gi|169674670 | Liver-basic fatty-acid-binding protein b | 24 | 14 255 | 1 | 15.9 |
| (c) Twelve C. carpio proteins identified by electrospray analysis from unmodified lysate solution originating in asymptomatic fish samples grouped into 7 aforementioned categories | |||||
| Michel et al. 2010 or homolog proteins | |||||
| gi|32394421 | Muscle-specific heat shock protein Hsc70-1 | 51 | 70 660 | 2 | 6.1 |
| Cytoskeletal proteins | |||||
| gi|42560193 | Beta-actin | 78 | 42 068 | 4 | 8.8 |
| gi|1703140 | Alpha-actin-1 | 77 | 42 274 | 3 | 9 |
| gi|6226787 | Vimentin | 32 | 52 516 | 1 | 1.5 |
| Host defence proteins | |||||
| gi|7939556 | Lysozyme C | 264 | 17 241 | 20 | 43.4 |
| gi|313509543 | Toll-like receptor 22 | 30 | 109 136 | 3 | 0.7 |
| gi|2829694 | Granulin-2 | 43 | 7215 | 1 | 17.5 |
| Protein modification enzymes or inhibitors | |||||
| gi|152925970 | Ubiquitin fusion protein | 99 | 14 987 | 5 | 24.2 |
| Globins | |||||
| gi|122392 | Alpha-globin | 88 | 15 437 | 4 | 16.1 |
| gi|85067845 | Myoglobin isoform 2 | 25 | 16 278 | 1 | 5.4 |
| Unclassified | |||||
| gi|435737 | Glial fibrillary acidic protein | 80 | 24 944 | 30 | 5.1 |
| gi|162423638 | Cyclin B | 27 | 45 068 | 1 | 2 |
MIF, migration inhibitory factor.
The 54 and 52 identified proteins from either gel slices or unmodified samples, respectively, were classified into seven groups that include: Michel et al. 2010, cytoskeletal, host defence, protein modification or inhibitors, globins, transferrins, unclassified. Proteins that showed up in both analyses are in bold.
In solution
The second sample, which had an original OD of 0.552 at 280, was analysed directly by ESI-MS, and 57 proteins were identified. In addition to the two CyHV-3 proteins identified in the previous section, three additional CyHV-3 proteins were identified: ORF22, ORF23 and ORF150 (Table 1b). Additionally 52 carp proteins were identified, which were classified into seven different groups as in the previous section (Table 2b). Similarly, five of the carp proteins or their homologs were previously identified in Michel et al. (2010) in conjunction with beta-actin, which was grouped with vimentin, another cytoskeletal protein. Also, six proteins involved in host defence regulation in addition to 12 proteins that are involved in protein modification were identified. Ten haem-harbouring proteins in conjunction with two transferrins were also identified, and the remaining 15 proteins were unclassified.
Asymptomatic fish group
The third sample, which consisted of anti-CyHV-3 monoclonal antibody–purified tissue samples originating in CyHV-3 asymptomatic fish, identified only one CyHV-3 protein, glycoprotein, ORF56 (Table 1c), and 12 carp proteins that include one homolog to the Michel et al. (2010) study, three cytoskeletal proteins, two host defence–related proteins, one protein involved in protein modification, two haem-harbouring proteins and three unclassified proteins (2c).
Discussion
The importance of understanding proteomics such as protein–protein interactions is fundamental in understanding biological systems (Watson James 2003). In conjunction with the traditional tools used to describe protein interactions, such as immunochemistry and yeast two-hybrid system, analysis by mass spectrometry has become a very useful tool to study protein interactions (Cameron 2012). We used antibody-based purification coupled with mass spectrometry to identify protein targets during Cyprinid herpesvirus 3 infection that could ultimately be used to develop novel methods for CyHV-3 control in koi and in common carp. During this study, 78 host proteins involved in Cyprinid herpesvirus 3 infection or propagation and five potential immunogenic CyHV-3 protein targets were identified in CyHV-3 diseased fish. Additionally, four host proteins were identified in CyHV-3 asymptomatic fish. The major capsid protein identified in this study corresponds to ORF92 previously identified in Michel et al. (2010); however, the remaining four proteins, ORF22, ORF23, glycoprotein (ORF56) and ORF150, were previously undetected. To enhance our understanding of the CyHV-3 proteins, Prosite (Sigrist et al. 2010) and Simple Modular Architect Research Tool (SMART) by Letunic, Doerks & Bork (2012) were used to elucidate important domain structures from the five CyHV-3-identified proteins (Table 3).
Table 3.
Description of CyHV-3 proteins identified. Prosite (Sigrist et al. 2010) and Simple Modular Architect Research Tool (SMART) by Letunic et al. (2012) were used to identify important domains in the 5 CyHV-3 proteins identified in this study. Amino acid number is referred to by the abbreviation aa
| Accession no. | Protein description | Protein length | Domain of interest | Spanning region |
|---|---|---|---|---|
| (a) Glycoprotein and 0RF150 modelled using Prosite | ||||
| gi|129560573 | Glycoprotein | 897 aa | Putative AMP binding | 173-184 aa |
| gi|129560669 | 0RF150 | 628 aa | Zinc finger Ring-type | 24-66 aa |
| (b) The remaining three proteins, 0RF22, 0RF23 and 0RF92 (major capsid protein), modelled by SMART | ||||
| gi|65306693 | Major capsid protein | 1268 aa | MHC II beta | 461-513 aa |
| gi|131840052 | 0RF22 | 588 aa | Transmembrane domain | 22-217 aa |
| gi|131840053 | 0RF23 | 335 aa | Interleukin-10 (IL-10) | 24-163 aa |
We compared proteins identified in this purification to the ones identified by Michel et al. (2010) to assess the validity of our assay. The comparison is complicated because the data set used in the previous study was composed before the entire genome for C. carpio was available, and therefore, all but one of the proteins (elongation factor) listed in the previous report come from either zebra fish, Danio rerio, or Atlantic salmon, Salmo salar. However, two proteins (elongation factor, and lactate dehydrogenase) were identical matches for proteins identified in the aforementioned studies. Rab8a was also included in as a match because of the high sequence homology amongst the Rab family of proteins (Bright et al. 2010). Similar logic was used to include heat shock proteins amongst the proteins also identified in the Michel et al.’s (2010) study. Although glutathione S-transferase rho was identified in both isolations and is a ras-related protein, it was not included in the Michel et al. (2010) list because ClustalW alignment did not show significant homology between the two proteins. Interestingly, our study identified several cytoskeletal proteins including actin (Hosein et al. 2003; Williams et al. 2006) and vimentin, and the Michel et al. (2010) study also identified cytoskeleton proteins that include two of the ubiquitous dynamic cytoskeletal proteins tubulin and actin (Gavin 1997), and a cofilin-like protein that may act as a regulator of actin dynamics (De La Cruz 2009; Shiozaki et al. 2009).
Electrospray analysis of samples originating in symptomatic fish identified seven proteins from the in-gel analysis and six proteins from the in-solution analysis that are involved in the host defence pathway. Two of the proteins, natural killer cell enhancing factor (Fujiki et al. 1999) and complement C3-H1 (Nakao et al. 2000), were observed in each of the two samples originating in symptomatic fish; therefore, a total of 11 proteins were identified. In addition to the C3-H1 protein, three other proteins (complements C3-S, C4-1 and C4-2) were identified in the complement host defence pathway (Kato et al. 2004). Of the remaining five host defence proteins, granzyme A/K is a member of the serine protease family (Huang et al. 2010) along with natural killer cell enhancing factor, which is a component of natural kill cells, and macrophage migration inhibitory factor (MIF) is a member of the cytokine signalling pathway (Bernhagen et al. 1993). Belcourt et al. (1995) demonstrated that granulin-1 localizes to macrophages in common carp and gold fish, Carassius auratus auratus, and granulin-3 which was identified in the gel analysis also is likely part of the host defence pathway. Lysozyme C and lysozyme G were also identified in this analysis; ClustalW alignment did not show significant homology between the two proteins and are phylogenetically unlinked (Savan, Aman & Sakai 2003). Overexpression of endogenous lysozyme C has antimicrobial and antiviral activities in grouper, Epinephelus coioides, spleen cells (Wei et al. 2012). Although lysozyme C and lysozyme G share very low sequence homology in the grass carp, Ctenopharyngodon idellus, they share similar expression profiles and were both shown to have antimicrobial activities, which may indicate that lysozyme G also has antiviral activities consistent with the previous study (Ye et al. 2010).
Protein modification is the final method in protein regulation, such as in the modifying gene regulation via the histone code (Jenuwein & Allis 2001); the ubiquitin code and other proteins modifiers are used to activate, repress, target, stabilize or degrade proteins (Sims & Reinberg 2008; Harper & Schulman 2006; Williams & Schwarzbauer 2009). A total of 17 proteins involved in protein modification were identified in this study. Seven of the proteins overlapped in both the gel and unmodified solution analysis. Two members of the protein degradation pathway, an ubiquitin fusion protein along with a proteasome activator subunit, were identified in the gel solution (Table 2a), and accordingly, one of the CyHV-3 proteins identified (Table 3a) has a RING-finger domain, which is known to mediate E3 ubiquitin-ligase activity (Joazeiro & Weissman 2000). Both analyses identified glutathione S-transferase rho; in addition, four other glutathione-related proteins were also identified in the unmodified solution (Table 2b), and these proteins are known to regulate inflammatory response and modulate cytoskeletal proteins (Zhang et al. 1995). Interestingly, alpha-1-antitrypsin homolog was identified in both analyses, and trypsin 1 was identified in the untreated sample; one may wonder whether interaction of these proteins is modulated during CyHV-3 disease.
Cyprinid herpesvirus 3 is also known as KHV, which was once called carp interstitial nephritis and gill necrosis virus (CNGV) because the disease was initially described to affect the kidneys and gills (Hutoran et al. 2005; Pokorova et al. 2005; Ilouze et al. 2008). Recently, there has been debate as to whether the skin, especially at points of abrasion, is the site for viral entry (Costes et al. 2009; Raj et al. 2011; Fournier et al. 2012). However, we are unaware of a study that describes how the virus spreads to other major organs such as liver, spleen and brain (Gilad et al. 2004; Dishon et al. 2007). Abundance of blood-related proteins such as the globins and transferrins may lead one to speculate whether CyHV-3 propagates via the blood transport system.
Amongst the unclassified list, a total of 21 proteins were grouped. Three proteins were identified in both analyses, the gel solution contained an additional six proteins, and the unmodified solution contained an additional 12 proteins (Table 3). CyHV-3 is known to affect kidneys, spleen and brain of carp (Ronen et al. 2003; Pikarsky et al. 2004; Miyazaki et al. 2008); several proteins that localize to CyHV-3-affected areas were identified including nephrosis precursor, fetuin short form and three other glial-associated proteins (Kálmán 1998; Tsai et al. 2004).
Initial attempts to control CyHV-3 outbreaks were carried out by a short exposure to CyHV-3 at permissible temperature (i.e. 15–25 °C) and subsequent treatment at non-permissible temperature (30 °C) to stimulate an immunogenic response against CyHV-3 (Ronen et al. 2003). However, several studies indicate that CyHV-3 stays dormant in survivors of CyHV-3 disease (Bretzinger et al. 1999; Bergmann et al. 2009; Eidea et al. 2011) and that CyHV-3 can even resurface to propagate and infect naïve fish (St-Hilaire et al. 2005; St-Hilaire et al. 2009). The third sample that was analysed came from a fish with no signs of CyHV-3 disease and was free of CyHV-3 according to analysis by conventional PCR (Bercovier et al. 2005) and real-time PCR (Gilad et al., 2004); however, ESI-MS detected glycoprotein (ORF56), indicating that the sample may have been a latent carrier for CyHV-3 disease (Table 1c). Interestingly, in the asymptomatic carrier, four novel proteins were identified in conjunction with the 8 proteins previously identified. The four novel proteins include an additional homolog to heat shock proteins, termed Hsc70-1 (Wu et al. 2011), and a cell cycle regulator cyclin B in addition to the two host defence proteins. Granulins share very high sequence homology (Belcourt et al. 1995), and therefore, granulin-2 may not be a novel finding in the asymptomatic study. However, Toll-like receptor 22 (TLR-22) was not detected in the other two analyses and has been demonstrated to have antiviral function (Lv et al. 2012).
A few regions of the world are relatively free of the Cyprinid herpesvirus such as India and Australia (McColl et al. 2007; Rathore et al. 2009); however, CyHV-3 has become an epidemic threat to the world carp industry, especially in Europe, Israel, Indonesia and the largest aquaculture carp producer, China (Bergmann et al. 2006; Bondad-Reantaso, Sunarto & Subasinghe 2007; Dishon et al. 2007; Sunarto et al. 2007; Dong et al. 2011). Several studies have explored the method of infection, latency and detection of CyHV-3 (Bergmann et al. 2009, 2010; Costes et al. 2009). However, relatively few studies have explored CyHV-3 on a molecular level (Michel et al. 2010; Fuchs et al. 2011). We used anti-body-based purification followed by electrospray ionization analysis to investigate molecular interactions during CyHV-3 infections. Seventy-eight endogenous host proteins along with five putative immunogenic CyHV-3 proteins involved in CyHV-3 replication and propagation were identified in this investigation. Additionally, four endogenous proteins were identified in samples originating from CyHV-3 asymptomatic fish. CyHV-3 infection has been recently shown to modulate gene expression in the proteasome degradation pathway, granulin, and other protein modifying enzymes identified in this report (Rakus et al. 2012). The antibody against glycoprotein (ORF56) was able to isolate CyHV-3 proteins in a sample that was KHV negative according to PCR methods detecting the TK gene. The glycoprotein may also be highly immunogenic and a good target for detection of CyHV-3 in asymptomatic fish by ELISA or even by PCR analysis. The diversity of proteins identified in this study opens the door for further analysis of host proteins involved in CyHV-3 replication, propagation and repression. For example, Toll-like receptor 22 appears to be involved in suppressing CyHV-3 disease in infected fish. Therefore, stimulating the overexpression of TLR-22 may be an alternative method of controlling CyHV-3 disease.
Acknowledgements
Monoclonal antibodies were kindly provided by Dr Bergmann and Dr Fischtner; Friedrich Loeffler Institute (FLI), the National Research Centre for Animal Health in Greifswald, Germany. We would like to thank the Proteomics Core Facility of the German Cancer Research Centre, Heidelberg, for their excellent work regarding protein identification by ESI-MS. Technical assistance was provided by Dr Andrea Dressler.
Funding for this project was provided by the Austrian Science Fund (FWF), grant no. P 23550.
References
- Adkinson M, Oren G, Hendrick RP. An enzyme linked immunosorbent assay (ELISA) for detection of antibodies to the koi herpesvirus (KHV) in the serum of koi Cyprinus carpio. Fish Pathology. 2005;40:53–62. [Google Scholar]
- Aoki T, Hirono I, Kurokawa K, Fukuda H, Nahary R, Eldar A, Davison AJ, Waltzek TB, Bercovie H, Hedrick RP. Genome sequences of three koi herpesvirus isolates representing the expanding distribution of an emerging disease threatening koi and common carp worldwide. Journal of Virology. 2007;81:5058–5065. doi: 10.1128/JVI.00146-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki T, Takano T, Unajak S, Takagi M, Kim YR, Park SB, Kondo H, Hirono I, Saito-Taki T, Hikima J, Jung TS. Generation of monoclonal antibodies specific for ORF68 of koi herpesvirus. Comparative Immunology, Microbiology & Infectious Diseases. 2011;34:209–216. doi: 10.1016/j.cimid.2010.11.004. [DOI] [PubMed] [Google Scholar]
- Belcourt DR, Okawara Y, Fryer JN, Bennett HP. Immunocytochemical localization of granulin-1 to mononuclear phagocytic cells of the teleost fish Cyprinus carpio and Carassius auratus. Journal of Leukocyte Biology. 1995;57:94–100. doi: 10.1002/jlb.57.1.94. [DOI] [PubMed] [Google Scholar]
- Bercovier H, Fishman Y, Nahary R, Sinai S, Zlotkin A, Eyngor M, Gilad O, Eldar A, Hedrick RP. Cloning of the koi herpesvirus (KHV) gene encoding thymidine kinase and its use for a highly sensitive PCR-based diagnosis. BMC Microbiology. 2005;5:13–22. doi: 10.1186/1471-2180-5-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergmann SM, Kempter J. Detection of koi herpesvirus (KHV) after re-activation in persistently infected common carp (Cyprinus carpio L.) using non-lethal sampling methods. Bulletin of the European Association of Fish Pathologists. 2011;30:74. [Google Scholar]
- Bergmann SM, Kempter J, Sadowski J, Fichtner D. First detection, confirmation and isolation of koi herpesvirus (KHV) in cultured common carp (Cyprinus carpio L.) in Poland. Bulletin of the European Association of Fish Pathologists. 2006;26:97–104. [Google Scholar]
- Bergmann SM, Schütze H, Fischer U, Fichtner D, Riechardt M, Meyer K, Schrudde D, Kempter J. Detection of koi herpes virus (KHV) genome in apparently healthy fish. Bulletin of the European Association of Fish Pathologists. 2009;31:92–100. [Google Scholar]
- Bergmann SM, Riechart M, Fichtner D, Leeb P, Kempter J. Investigation on the diagnostic sensitivity of molecular tools used for detection of koi herpesvirus. Journal of Virological Methods. 2010;163:229–233. doi: 10.1016/j.jviromet.2009.09.025. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Calandra T, Mitchell RA, Martin SB, Tracey KJ, Voelter W, Manogue KR, Cerami A, Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993;365:756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Blondot ML, Dubosclard V, Fix J, Lassoued S, Aumont-Nicaise M, Bontems F, Eléouët JF, Sizun C. Structure and functional analysis of the rna- and viral phosphoprotein-binding domain of respiratory syncytial virus M2-1 protein. PLoS Pathogen. 2012;8:e1002734. doi: 10.1371/journal.ppat.1002734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondad-Reantaso MG, Sunarto A, Subasinghe RP. Managing the koi herpesvirus disease outbreak in Indonesia and the lessons learned. Developments in Biologicals. 2007;129:21–28. [PubMed] [Google Scholar]
- Bretzinger A, Fischer-Scherl T, Oumouna M, Hoffmann R, Truyen U. Mass mortality in koi carp, Cyprinus carpio, associated with gill and skin disease. Bulletin of the European Association of Fish Pathologists. 1999;19:182–185. [Google Scholar]
- Bright LJ, Kambesis N, Nelson SB, Jeong B, Turkewitz AP. Comprehensive analysis reveals dynamic and evolutionary plasticity of Rab GTPases and membrane traffic in Tetrahymena thermophila. PLoS Genetics. 2010;6:e1001155. doi: 10.1371/journal.pgen.1001155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron LC. Mass spectrometry imaging: facts and perspectives from a non-mass spectrometrist point of view. Methods. 2012;57:417–422. doi: 10.1016/j.ymeth.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Costes B, Raj VS, Michel B, Fournier G, Thirion M, Gillet L, Mast J, Lieffrig F, Bremont M, Vanderplasschen A. The major portal of entry of koi herpesvirus in Cyprinus carpio is the skin. Journal of Virology. 2009;83:2819–2830. doi: 10.1128/JVI.02305-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De La Cruz EM. How cofilin severs an actin filament. Biophysical Reviews. 2009;1:51–59. doi: 10.1007/s12551-009-0008-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dishon A, Davidovich M, Ilouze M, Kotler M. Persistence of cyprinid herpesvirus 3 in infected cultured carp cells. Journal of Virology. 2007;9:4828–4836. doi: 10.1128/JVI.02188-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Weng S, Li W, Li X, Yi Y, Liang Q, He J. Characterization of a new cell line from caudal fin of koi, Cyprinus carpio koi, and first isolation of cyprinid herpesvirus 3 in China. Virus Research. 2011;161:140–149. doi: 10.1016/j.virusres.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Eidea K, Miller-Morgana T, Heidela JR, Kent ML, Bildfella RJ, LaPatra S, Watson G, Jin L. Investigation of koi herpesvirus latency in koi. Journal of Virology. 2011;85:4954–4962. doi: 10.1128/JVI.01384-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Matbouli M, Rucker U, Soliman H. Detection of Cyprinid herpesvirus-3 (CyHV-3) DNA in infected fish tissues by nested polymerase chain reaction. Diseases of Aquatic Organisms. 2007;78:23–28. doi: 10.3354/dao01858. [DOI] [PubMed] [Google Scholar]
- FAO . The State of World Fisheries and Aquaculture 2010. FAO; Rome: 2010. p. 197. [Google Scholar]
- Fenn JB, Mann M, Meng CK, Wong SF, Whitehouse CM. Electrospray ionization for mass spectrometry of large biomolecules. Science. 1989;246:64–71. doi: 10.1126/science.2675315. [DOI] [PubMed] [Google Scholar]
- Fournier G, Boutier M, Raj VS, Mast J, Parmentier E, Vanderwalle P, Peeters D, Lieffrig F, Farnir F, Gillet L, Vanderplasschen A. Feeding Cyprinus carpio with infectious materials mediates cyprinid herpesvirus 3 entry through infection of pharyngeal periodontal mucosa. Veterinary Research. 2012;43:6. doi: 10.1186/1297-9716-43-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs W, Fichtner D, Bergmann SM, Mettenleiter TC. Generation and characterization of koi herpesvirus recombinants lacking viral enzymes of nucleotide metabolism. Archives of Virology. 2011;156:1059–1063. doi: 10.1007/s00705-011-0953-8. [DOI] [PubMed] [Google Scholar]
- Fujiki K, Shin DH, Nakao M, Yano T. Molecular cloning of carp (Cyprinus carpio) CC chemokine, CXC chemokine receptors, allograft inflammatory factor-1, and natural killer cell enhancing factor by use of suppression subtractive hybridization. Immunogenetics. 1999;49:909–914. doi: 10.1007/s002510050573. [DOI] [PubMed] [Google Scholar]
- Gavin RH. Microtubule-microfilament synergy in the cytoskeleton. International Review of Cytology. 1997;173:207–242. doi: 10.1016/s0074-7696(08)62478-x. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Andree KB, Adkison MA, Zlotkin A, Bercovier H, Eldar A, Hedrick RP. Initial characteristics of koi herpesvirus and development of a polymerase chain reaction assay to detect the virus in koi, Cyprinus carpio koi. Diseases of Aquatic Organisms. 2002;48:101–108. doi: 10.3354/dao048101. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Andree KB, Adkison MA, Way K, Willits NH, Bercovier H, Hedrick RP. Molecular comparison of isolates of an emerging fish pathogen, the koi herpesvirus, and the effect of water temperature on mortality of experimentally infected koi. Journal of General Virology. 2003;84:1–8. doi: 10.1099/vir.0.19323-0. [DOI] [PubMed] [Google Scholar]
- Gilad O, Yun S, Zagmutt-Vergara FJ, Leutenegger CM, Bercovier HV, Hedrick RP. Concentrations of a koi herpesvirus (KHV) tissues of experimentally infected Cyprinus carpio koi as assessed by real-time TaqMan PCR. Diseases of Aquatic Organisms. 2004;60:179–187. doi: 10.3354/dao060179. [DOI] [PubMed] [Google Scholar]
- Gotesman M, Hosein RE, Gavin RH. A FERM domain in a class XIV myosin interacts with actin and tubulin and localizes to the cytoskeleton, phagosomes, and nucleus in Tetrahymena thermophila. Cytoskeleton. 2010;67:90–101. doi: 10.1002/cm.20426. [DOI] [PubMed] [Google Scholar]
- Gotesman M, Hosein RE, Gavin RH. MyTH4, independent of its companion FERM domain, affects the organization of an intramacronuclear microtubule array and is involved in elongation of the macronucleus in Tetrahymena thermophila. Cytoskeleton. 2011;68:220–236. doi: 10.1002/cm.20506. [DOI] [PubMed] [Google Scholar]
- Griffiths WJ, Jonsson AP, Liu S, Rai DK, Wang Y. Electrospray and tandem mass spectrometry in biochemistry. Biochemical Journal. 2001;355:545–561. doi: 10.1042/bj3550545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo KK, Tang QH, Zhang YM, Kang K, He L. Identification of two internal signal peptide sequences: critical for classical swine fever virus non-structural protein 2 to trans-localize to the endoplasmic reticulum. Virology Journal. 2011;18:236–242. doi: 10.1186/1743-422X-8-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper JW, Schulman BA. Structural complexity in ubiquitin recognition. Cell. 2006;124:1133–1136. doi: 10.1016/j.cell.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, Gilad O, Yun S, Spangenberg JV, Marty GD, Nordhausen RW, Kebus MJ, Bercovier H, Eldar A. A herpesvirus associated with mass mortality of juvenile and adult koi, a strain of common carp. Journal of Aquatic Animal Health. 2000;12:44–57. doi: 10.1577/1548-8667(2000)012<0044:AHAWMM>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Hedrick RP, Gilad O, Yun SC, Mcdowell TS, Waltzek TB, Kelley GO, Adkison MA. Initial isolation and characterization of a herpes-like virus (KHV) from koi and common carp. Bulletin of Fisheries Research Agency. 2005;2:1–7. [Google Scholar]
- Ho CS, Lam CW, Chan MH, Cheung RC, Law LK, Lit LC, Ng KF, Suen MW, Tai HL. Electrospray ionisation mass spectrometry: principles and clinical applications. The Clinical Biochemist Reviews. 2003;24:3–12. [PMC free article] [PubMed] [Google Scholar]
- Hosein RE, Williams SA, Haye K, Gavin RH. Expression of GFP-actin leads to failure of nuclear elongation and cytokinesis in Tetrahymena thermophila. Journal of Eukaryotic Microbiology. 2003;6:403–408. doi: 10.1111/j.1550-7408.2003.tb00261.x. [DOI] [PubMed] [Google Scholar]
- Huang R, Zhong S, Liu H, Kong R, Wang Y, Hu W, Guo Q. Identification and characterization of common carp (Cyprinus carpio L.) granzyme A/K, a cytotoxic cell granule-associated serine protease. Fish and Shellfish Immunology. 2010;29:388–398. doi: 10.1016/j.fsi.2010.04.002. [DOI] [PubMed] [Google Scholar]
- Hutoran M, Ronen A, Perelberg A, Ilouze M, Dishon A, Bejerano I, Chen N, Kotler M. Description of an as yet unclassified DNA virus from diseased Cyprinus carpio species. Journal of Virology. 2005;79:1983–1991. doi: 10.1128/JVI.79.4.1983-1991.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilouze M, Dishon A, Davidovich M, Perelberg A, Kotler M. KHV, CNGV or CyHV-3, which is the koi/carp killer? Diseases in Asian Aquaculture. 2008;VI:115–128. [Google Scholar]
- Jenuwein T, Allis CD. Translating the histone code. Science. 2001;293:1074–1080. doi: 10.1126/science.1063127. [DOI] [PubMed] [Google Scholar]
- Joazeiro CA, Weissman AM. RING finger proteins: mediators of ubiquitin ligase activity. Cell. 2000;102:549–552. doi: 10.1016/s0092-8674(00)00077-5. [DOI] [PubMed] [Google Scholar]
- Kálmán M. Astroglial architecture of the carp (Cyprinus carpio) brain as revealed by immunohistochemical staining against glial fibrillary acidic protein (GFAP) Anatomy and Embryology. 1998;198:409–433. doi: 10.1007/s004290050193. [DOI] [PubMed] [Google Scholar]
- Kato Y, Nakao M, Shimizu M, Wariishi H, Yano T. Purification and functional assessment of C3a, C4a and C5a of the common carp (Cyprinus carpio) complement. Developmental & Comparative Immunology. 2004;28:901–910. doi: 10.1016/j.dci.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Kuznetsov SA, Langford GM, Weiss DG. Actin-dependent organelle movement in squid axoplasm. Nature. 1992;356:722–725. doi: 10.1038/356722a0. [DOI] [PubMed] [Google Scholar]
- Letunic I, Doerks T, Bork P. SMART 7: recent updates to the protein domain annotation resource. Nucleic Acid Research. 2012;40:D302–D305. doi: 10.1093/nar/gkr931. Epub 2011 Nov 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lv J, Huang R, Li H, Luo D, Liao L, Zhu Z, Wang Y. Cloning and characterization of the grass carp (Ctenopharyngodon idella) Toll-like receptor 22 gene, a fish-specific gene. Fish and Shellfish Immunology. 2012;32:1022–1031. doi: 10.1016/j.fsi.2012.02.024. [DOI] [PubMed] [Google Scholar]
- McColl K, Sunarto A, Williams LM, Crane MSTJ. Koi herpes virus: dreaded pathogen or white knight? Aquaculture Health International. 2007;9:4–6. [Google Scholar]
- Michel B, Leroy B, Stalin Raj V, Lieffrig F, Mast J, Wattiez R, Vanderplasschen AF, Costes B. The genome of cyprinid herpesvirus 3 encodes 40 proteins incorporated in mature virions. Journal of General Virology. 2010;91:452–462. doi: 10.1099/vir.0.015198-0. [DOI] [PubMed] [Google Scholar]
- Miyazaki T, Kuzuya Y, Yasumoto S, Yasuda M, Kobayashi T. Histopathological and ultrastructural features of koi herpesvirus (KHV)-infected carp Cyprinus carpio, and the morphology and morphogenesis of KHV. Diseases of Aquatic Organisms. 2008;80:1–11. doi: 10.3354/dao01929. [DOI] [PubMed] [Google Scholar]
- Moen RJ, Johnsrud DO, Thomas DD, Titus MA. Characterization of a myosin VII MyTH/FERM domain. Journal of Molecular Biology. 2011;413:17–23. doi: 10.1016/j.jmb.2011.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M, Mutsuro J, Obo R, Fujiki K, Nonaka M, Yano T. Molecular cloning and protein analysis of divergent forms of the complement component C3 from a bony fish, the common carp (Cyprinus carpio): presence of variants lacking the catalytic histidine. European Journal of Immunology. 2000;30:858–866. doi: 10.1002/1521-4141(200003)30:3<858::AID-IMMU858>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Pikarsky E, Ronen A, Abramowitz J, Levavi-Sivan B, Hutoran M, Shapira Y, Steinitz M, Perelberg A, Soffer D, Kotler M. The pathogenesis of the acute viral disease in fish induced by the carp interstitial nephritis and gill necrosis virus. Journal of Virology. 2004;78:9544–9551. doi: 10.1128/JVI.78.17.9544-9551.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokorova D, Vesely T, Piackova V, Reschova S, Hulova J. Current knowledge on koi herpesvirus (KHV): a review. Veterinary Medicine. – Czech. 2005;50:139–147. [Google Scholar]
- Raj VS, Fournier G, Rakus K, Ronsmans M, Ouyang P, Michel B, Delforges C, Costes B, Farnir F, Leroy B, Wattiez R, Melard C, Mast J, Lieffrig F, Vanderplasschen A. Skin mucus of Cyprinus carpio inhibits cyprinid herpesvirus 3 binding to epidermal cells. Veterinary Research. 2011;42:92–100. doi: 10.1186/1297-9716-42-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakus K, Irnazarow I, Adamek M, Palmeira L, Kawana Y, Hirono I, Kondo H, Matras M, Steinhagen D, Flasz B, Brogden G, Vanderplasschen A, Aoki T. Gene expression analysis of common carp (Cyprinus carpio L.) lines during Cyprinid herpesvirus 3 infection yields insights into differential immune responses. Developmental and Comparative Immunology. 2012;37:65–76. doi: 10.1016/j.dci.2011.12.006. [DOI] [PubMed] [Google Scholar]
- Rathore G, Kumar G, Swain P, Lakra WS. Development of new PCR primers for detection of koi herpes virus (KHV) Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases. 2009;30:52–53. [Google Scholar]
- Ronen A, Perelberg A, Abramowitz J, Hutoran M, Tinman S, Bejerano Y, Steinitz M, Kotler M. Efficient vaccine against the virus causing a lethal disease in cultured Cyprinus carpio. Vaccine. 2003;21:4677–4684. doi: 10.1016/s0264-410x(03)00523-1. [DOI] [PubMed] [Google Scholar]
- Rosenkranz D, Klupp BG, Teifke JP, Granzow H, Fichtner D, Mettenleiter TC, Fuchs W. Identification of envelope protein pORF81 of koi herpesvirus. Journal of General Virology. 2008;89:896–900. doi: 10.1099/vir.0.83565-0. [DOI] [PubMed] [Google Scholar]
- Sambrook J, Russell D. Molecular Cloning, A Laboratory Manual. 3rd edn. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 2001. [Google Scholar]
- Savan R, Aman A, Sakai M. Molecular cloning of G type lysozyme cDNA in common carp (Cyprinus carpio L.) Fish and Shellfish Immunology. 2003;15:263–268. doi: 10.1016/s1050-4648(02)00159-6. [DOI] [PubMed] [Google Scholar]
- Shiozaki N, Nakano K, Takaine M, Abe H, Numata O. Usual and unusual biochemical properties of ADF/cofilin-like protein Adf73p in ciliate Tetrahymena thermophila. Biochemical and Biophysical Research Communications. 2009;390:54–59. doi: 10.1016/j.bbrc.2009.09.063. [DOI] [PubMed] [Google Scholar]
- Sigrist CJA, Cerutti L, de Castro E, Langendijk-Genevaux PS, Bulliard V, Bairoch A, Hulo N. PROSITE, a protein domain database for functional characterization and annotation. Nucleic Acids Research. 2010;38:161–166. doi: 10.1093/nar/gkp885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims RJ, 3rd, Reinberg D. Is there a code embedded in proteins that is based on post-translational modifications? Nature Reviews Molecular Cell Biology. 2008;9:815–820. doi: 10.1038/nrm2502. [DOI] [PubMed] [Google Scholar]
- Soliman H, El-Matbouli M. An inexpensive and rapid diagnostic method of koi herpesvirus (KHV) infection by loop-mediated isothermal amplification. Virology Journal. 2005;2:83. doi: 10.1186/1743-422X-2-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soliman H, El-Matbouli M. Immunocapture and direct binding loop mediated isothermal amplification simplify molecular diagnosis of Cyprinid herpesvirus-3. Journal of Virological Methods. 2009;162:91–95. doi: 10.1016/j.jviromet.2009.07.021. [DOI] [PubMed] [Google Scholar]
- Soliman H, El-Matbouli M. Loop mediated isothermal amplification combined with nucleic acid lateral flow strip for diagnosis of cyprinid herpes virus-3. Molecular and Cellular Probes. 2010;1:38–43. doi: 10.1016/j.mcp.2009.09.002. [DOI] [PubMed] [Google Scholar]
- de Souza MG, Grossi AL, Pereira EL, da Cruz CO, Mendes FM, Cameron LC, Paiva CL. Actin immobilization on chitin for purifying myosin II: a laboratory exercise that integrates concepts of molecular cell biology and protein chemistry. Biochemistry and Molecular Biology Education. 2008;36:55–60. doi: 10.1002/bmb.122. [DOI] [PubMed] [Google Scholar]
- St-Hilaire S, Beevers N, Way K, Le Deuff RM, Martin P, Joiner C. Reactivation of koi herpesvirus infections in common carp Cyprinus carpio. Diseases of Aquatic Organisms. 2005;67:15–23. doi: 10.3354/dao067015. [DOI] [PubMed] [Google Scholar]
- St-Hilaire S, Beevers N, Joiner C, Hedrick RP, Way K. Antibody response of two populations of common carp, Cyprinus carpio L., exposed to koi herpesvirus. Journal of Fish Diseases. 2009;32:311–320. doi: 10.1111/j.1365-2761.2008.00993.x. [DOI] [PubMed] [Google Scholar]
- Sunarto A, McColl KA, Crane MSTJ, Sumiati T, Hyatt AD, Barnes AC, Walker PJ. Isolation and characterization of koi herpesvirus (KHV) from Indonesia: identification of a new genetic lineage. Journal of Fish Diseases. 2007;34:87–101. doi: 10.1111/j.1365-2761.2010.01216.x. [DOI] [PubMed] [Google Scholar]
- Tsai PL, Chen CH, Huang CJ, Chou CM, Chang GD. Purification and cloning of an endogenous protein inhibitor of carp nephrosin, an astacin metalloproteinase. The Journal of Biological Chemistry. 2004;279:11146–11155. doi: 10.1074/jbc.M310423200. [DOI] [PubMed] [Google Scholar]
- Tuxworth RI, Stephens S, Ryan ZC, Titus MA. Identification of a myosin VII-talin complex. The Journal of Biological Chemistry. 2005;28:26557–26564. doi: 10.1074/jbc.M503699200. [DOI] [PubMed] [Google Scholar]
- Watson James D. DNA: The Secrets of Life. Knopf. Print; New York City, NY: 2003. pp. 220–222.pp. 226–228. [Google Scholar]
- Wei S, Huang Y, Cai J, Huang X, Fu J, Qin Q. Molecular cloning and characterization of c-type lysozyme gene in orange-spotted grouper, Epinephelus coioides. Fish and Shellfish Immunology. 2012;33:186–196. doi: 10.1016/j.fsi.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Williams NE. Immunoprecipitation procedures. In: Asai DJ, Forney JD, editors. Methods in Cell Biology: Tetrahymena thermophila. Vol. 62. Academic Press; New York, NY: 2000. pp. 449–453. [PubMed] [Google Scholar]
- Williams SA, Schwarzbauer JE. A shared mechanism of adhesion modulation for tenascin-C and fibulin-1. Molecular Biology of the Cell. 2009;20:1141–1149. doi: 10.1091/mbc.E08-06-0621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NE, Tsao CC, Bowen J, Hehman GL, Williams RJ, Frankel J. The actin gene ACT1 is required for phagocytosis, motility, and cell separation of Tetrahymena thermophila. Eukaryotic Cell. 2006;5:555–567. doi: 10.1128/EC.5.3.555-567.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CL, Lin TH, Chang TL, Sun HW, Hui CF, Wu JL. Zebrafish HSC70 promoter to express carp muscle-specific creatine kinase for acclimation under cold condition. Transgenic Research. 2011;20:1217–1226. doi: 10.1007/s11248-011-9488-8. [DOI] [PubMed] [Google Scholar]
- Ye X, Zhang L, Tian Y, Tan A, Bai J, Li S. Identification and expression analysis of the g-type and c-type lysozymes in grass carp Ctenopharyngodon idellus. Developmental and Comparative Immunology. 2010;34:501–509. doi: 10.1016/j.dci.2009.12.009. [DOI] [PubMed] [Google Scholar]
- Zhang S, Han J, Sells MA, Chernoff J, Knaus UG, Ulevitch RJ, Bokoch GM. Rho family GTPases regulate p38 mitogen-activated protein kinase through the downstream mediator Pak1. The Journal of Biological Chemistry. 1995;270:23934–23936. doi: 10.1074/jbc.270.41.23934. [DOI] [PubMed] [Google Scholar]