Abstract
In Escherichia coli, the bifunctional penicillin-binding proteins (PBPs), PBP1A and PBP1B, play critical roles in the final stage of peptidoglycan (PG) biosynthesis. These synthetic enzymes each possess a PG glycosyltransferase (PGT) domain and a transpeptidase (TP) domain. Recent genetic experiments have shown that PBP1A and PBP1B each require an outer membrane lipoprotein, LpoA and LpoB respectively, to function properly in vivo. Here, we use complementary assays to show that LpoA and LpoB each increase the PGT and TP activities of their cognate PBPs, albeit by different mechanisms. LpoA directly increases the rate of the PBP1A TP reaction, which also results in enhanced PGT activity; in contrast, LpoB directly affects PGT domain activity, resulting in enhanced TP activity. These studies demonstrate bidirectional coupling of PGT and TP domain function. Additionally, the transpeptidation assay described here can be applied to study other activators or inhibitors of the TP domain of PBPs, which are validated drug targets.
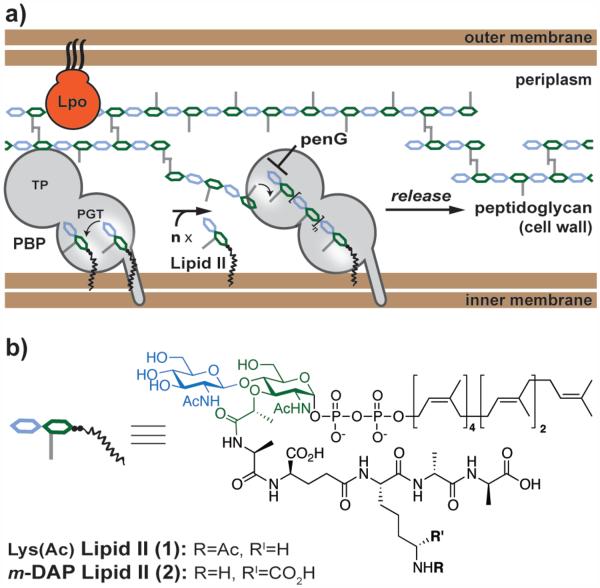
Peptidoglycan (PG) is an essential crosslinked polymer that surrounds bacterial cells and prevents lysis due to high internal osmotic pressures.1 Since PG is required for survival and is unique to bacteria, it is a target for antibiotics. Understanding PG biosynthesis is therefore crucial for developing strategies to overcome antibiotic resistance.1b, 2 PG is synthesized from a membrane-anchored disaccharide-peptide substrate, Lipid II, by bifunctional penicillin-binding proteins (PBPs) that contain two domains: a PG glycosyltransferase (PGT) domain that assembles the glycan polymer chains and a transpeptidase (TP) domain that forms peptide crosslinks between these chains (Figure 1).3 In Escherichia coli, two bifunctional PBPs, PBP1A and PBP1B, play important roles in PG synthesis. Genetic studies have established that each PBP requires an outer membrane lipoprotein to function in cells.1d,4
Figure 1.
E. coli outer membrane Lpo proteins are required in vivo for synthesis of peptidoglycan (PG) by penicillin-binding proteins (PBPs). (a) Schematic of bifunctional PBP-catalyzed PG synthesis by the PG glycosyltransferase (PGT) and transpeptidase (TP) domains. Penicillin G (penG) inhibits the TP step. Lpo proteins effect PG synthesis by an unknown mechanism. (b) Structures of Lipid II analogs.
These lipoprotein cofactors, LpoA and LpoB, are essential for the in vivo function of the bifunctional PBPs, but their specific functions remain unclear. Here we characterize the effects of LpoA and LpoB on the TP and PGT activities of PBP1A and PBP1B. We show that LpoA and LpoB stimulate the activity of their cognate PBPs by affecting different domains. Surprisingly, activation of one domain leads to enhanced activity of the other domain, demonstrating that the activities of the domains are coupled. Disruption of domain coupling or activation provides a possible alternative strategy to disable essential cellular PG synthesis machinery.
Assays to quantify PGT activity have previously been established,5 but monitoring TP activity is more difficult.5o-q,6 TP domains can catalyze several different reactions, which proceed through a common acyl-enzyme intermediate formed by attack of a catalytic serine on a substrate D-Ala-D-Ala amide bond.6b-d Deacylation can occur via attack by water to release a tetrapeptide or through attack by an amine.7 If the amine is on the side chain of a peptide from another glycan strand, a crosslink results (Figure 1a), but it is also possible to incorporate a number of different D-amino acids (Figure 2a).6f,8 Rate analysis based on peptide crosslinking is challenging because the products are heterogeneous polymers.5o-q Therefore, we decided to quantify transpeptidation activity by following incorporation of radiolabeled D-Ala into newly synthesized PG prepared from Lys-Lipid II5b,9 acetylated on the ε-amine (Figure 1b, 1).5f,6f We have previously established that glycan chains made from this substrate form acyl enzyme intermediates with TP domains, but they are not crosslinked because they do not contain free peptide side chain amines.6f-g,10 In this way, we used incorporation of D-amino acid to directly report on the activity of the TP domain.
Figure 2.
Lpo proteins enhance the TP activities of their cognate PBPs. (a) Reaction scheme showing attack on a PG peptide side chain by a TP domain’s catalytic serine to form an acyl enzyme intermediate followed by addition of D-amino acid, producing a modified peptide side chain. (b) Rate analysis of DAla incorporation into PG polymers produced by PBP1A +/− LpoA or LpoB (200 nM each). (c) Rate analysis of D-Ala incorporation into PG polymers produced by PBP1B +/− LpoA or LpoB (50 nM each). For all experiments, 40 μM Lys(Ac)-Lipid II (1) and 40 μM [14C]-D-Ala were used. Error bars indicate the standard deviation for duplicate experiments.
E. coli PBP1A was incubated with a 1:1 mixture of Lys(Ac)-Lipid II (1) and [14C]-D-Ala, and the radioactivity incorporated into PG polymers was plotted as a function of time (Figure 2b).5a,6f After an initial lag, D-Ala incorporation into PG proceeded at a steady rate before plateauing at ~15% conversion.11 D-Ala incorporation was not detected in the presence of penicillin G (penG), confirming that the process depends on the TP domain (see Supplemental Information, Figure S1). The plateau occurred at a time point coinciding with complete conversion of Lipid II to product (see Figure 4a), suggesting that amino acid incorporation requires ongoing PGT domain activity. Consistent with this hypothesis, a PBP1A variant in which an essential catalytic glutamate in the PGT domain is replaced with glutamine did not incorporate D-amino acids into previously prepared glycan polymers (Figure S2).12
Figure 4.
Enhancement of the PGT activity of PBP1A by LpoA requires an active TP domain, while LpoB activation of the PGT activity of PBP1B does not. (a-b) Rate analysis of glycan polymerization by PBP1A +/− equimolar LpoA without (a) and with (b) the addition of penG, which inhibits TP activity. (c-d) Rate analysis of glycan polymerization by PBP1B +/− equimolar LpoB without (c) and with (d) the addition of penG. Indicated concentrations of PBP and Lpo were incubated with 40 μM Lys(Ac) [14C]-Lipid II (1, LPII) with 40 μM D-Ala or 1 kU/ml penG for the indicated time points (see Figure S6). Error bars indicate the standard deviation for duplicate experiments.
Having established conditions to monitor D-Ala incorporation into PG polymers, we examined the effect of LpoA and LpoB on PBP1A TP activity. Whereas LpoB did not affect D-Ala incorporation, one equivalent of LpoA increased the rate of incorporation by 4.5-fold (Figure 2b). The rate enhancement reached a maximum of 6-fold at a ratio of 1:2 PBP1A:LpoA (Figure S3a), indicating that LpoA activates PBP1A in a stoichiometric rather than catalytic manner. An analogous series of experiments was performed using PBP1B, and in this case we observed that LpoB, but not LpoA, affected the rate of D-Ala incorporation. The rate enhancement was modest, reaching a maximum of only 1.5-fold (Figure 2c; Figure S3b). Nevertheless, the results showed that each lipoprotein affects the TP activity only of its cognate PBP.
We next analyzed the products formed by PBP1A in the presence and absence of LpoA using the native E. coli substrate, m-DAP Lipid II (2).6g PG polymers containing m-DAP can undergo crosslinking (Figure 1a) as well as D-Ala incorporation (Figure 2a). For product analysis, we used a previously described LC/MS assay that allows us to identify different transpeptidation products following post-reaction degradation of PG (Figure 3a).6g,13 Incubation of E. coli PBP1A with 2 for 15 minutes followed by degradation produced the pentapeptide-containing fragment A, the tetrapeptide-containing fragment B, and the crosslinked muropeptide fragment C (Figure 3b, trace i). When LpoA was added to the reaction, hydrolysis product B increased slightly and a small amount of hydrolyzed cross-linked product was also observed, consistent with increased TP activity (Figure S4). In order to detect changes in amino acid incorporation as well as cross-linking, deuterated D-Ala was added to PBP1A reactions with and without LpoA (compare traces ii and iii). Upon addition of LpoA, we observed a dramatic increase in transpeptidation products, comprising crosslinked peak C, deuterated pentapeptide peak A’ and crosslinked deuterated peak C’ (Figure 3c). Consistent with previous work,4b the total cross-linked material increased from 19% to 29% of detected products. These studies show that LpoA substantially increases the transpeptidation activity of PBP1A, whereas analogous experiments with PBP1B show that LpoB has a much smaller effect on TP activity (Figure S5).
Figure 3.
LpoA increases transpeptidation during PG synthesis by PBP1A. (a) Schematic of method for analyzing PG synthesis by PBPs. (b) LC/MS extracted chromatograms of PBP1A (400 nM) and m-DAP Lipid II (2, 20 μM) reactions (t = 15 min) produce A (representing unmodified peptide side chain), B (representing hydrolyzed peptide), and C (representing crosslinked peptides) (i). Reactions containing D-Ala-d3 (60 μM) result in deuterated peaks, pentapeptide A’ and cross-linked C’ (ii). Upon addition of LpoA (400 nM), A’ and C’ increase in intensity (iii).14 (c) Quantification of percent transpeptidation and cross-linking. % transpeptidation = (A’+C+ C’)/(A+A’+C+C’); % cross-links = (C+C’)/(A+A’+C+C’).
We next examined PGT activity in the presence of the Lpo proteins under the same conditions used to monitor TP activity. PBP1A and PBP1B were incubated with a 1:1 mixture of [14C]-GlcNAc-labeled Lys(Ac) Lipid II (1)5f,6f and unlabeled D-Ala in the presence and absence of their cognate lipoproteins, and the reactions were analyzed by paper chromatography to separate polymer from unreacted starting material.5a-n LpoA increased the rate of PBP1A-catalyzed glycan polymer synthesis approximately 1.5-fold compared to reactions lacking LpoA (Figure 4a).15 This effect was not due to the added D-Ala because reactions lacking D-Ala showed a similar increase in PGT activity in the presence of LpoA (Figure S6). To determine whether the enhanced PGT activity was dependent on TP activity, we monitored the reaction in the presence of penG, which covalently inactivates the TP domain.6b As shown in Figure 4b, addition of penG obliterated the rate enhancement due to LpoA. Similar experiments carried out with PBP1B showed that LpoB also increased the rate of glycan polymer synthesis by ~1.5 fold.4a However, inactivation of the TP domain with penG did not attenuate this rate enhancement (compare Figures 4c and 4d) and may even have increased it.
LpoA and LpoB were recently identified as essential cofactors that “activate” E. coli PBP1A and PBP1B so that these enzymes can perform the essential function of making cross-linked PG.4 It was proposed that each lipoprotein stimulates the transpeptidase activity of its cognate PBP,4b thereby facilitating attachment of new PG to the cell wall. In this paper we show that the lipoproteins have very different effects on their cognate PBPs. Both Lpo proteins increase the rate of glycan polymerization, but in the case of LpoA the rate enhancement depends on TP activity whereas in the case of LpoB it does not. Since LpoA enhances D-amino acid incorporation (Figure 2) as well as crosslinking (Figure 3), its likely function is to promote formation of the covalent intermediate, i.e., substrate acylation (Figure 2a), rather than to bring substrates in close proximity. LpoB’s primary effect appears to be on PGT domain activity1d,4 because its addition to PBP1B reactions not only increases the rate of polymerization, it also substantially reduces the average length of the glycan strands that are made.4a LpoA does not affect the length of polymers produced by PBP1A (Figure S8). Hence, each Lpo protein has a dominant effect on a different domain: LpoA on the TP domain and LpoB on the PGT domain. While these studies clearly show that each Lpo protein primarily affects a different domain of its cognate PBP, the kinetic effects are less than ten-fold in vitro, which amounts to less than 1 kcal/mole on the energetic profiles of the enzymes. Nevertheless, the phenotypic consequences are significant because each Lpo protein is essential for the biological function of its cognate PBP. In the case of LpoB, the dramatic effect on glycan length may affect resulting PG structure. We note that in E. coli, PBP1B is believed to be responsible for making septal PG whereas PBP1A is thought to make PG during cell elongation.1c-d,3,16 It would not be surprising if the differences in PBP activity caused by the Lpo proteins were related to differences in both the rates of synthesis and optimal PG structure formed by the elongation and cell division complexes.
One last notable feature of LpoA and LpoB behavior is that while each acts predominantly on one domain of its cognate PBP, both domains are affected. There are two possible explanations for how increased glycan polymerization due to LpoB could affect transpeptidation activity. First, some TP domains may only recognize polymeric substrates.1c-d,3,5q,t In such cases, increasing PGT activity would make more polymeric substrate available for crosslinking. Alternatively, or in addition, a conformational change may be transmitted from an actively polymerizing PGT domain to the TP domain, activating it in turn. While it has previously been noted that TP activity may require PGT activity,1c-d,3,5o-r the converse had not been observed, yet our results show that LpoA enhances PGT activity in a manner that depends on having a functional TP domain. Therefore, we suggest that the active states of the PGT and TP domains of bifunctional PBPs are conformationally coupled in a bidirectional fashion. Efforts to elucidate the molecular basis for the cooperative functioning of the two enzymatic activities of bifunctional PBPs are underway.
Supplementary Material
ACKNOWLEDGMENT
This research was supported by the National Institutes of Health (GM76710; GM066174; AI083365; GM103056) and NERCE (AI057159).
Footnotes
Supporting Information. Experimental procedures, protein purification protocols, rate analysis of PBP reactions, and LC/MS and SDS-PAGE analysis of PG polymers. This material is available free of charge via the Internet at http://pubs.acs.org.
REFERENCES
- 1.(a) van Heijenoort J. Glycobiology. 2001;11:25R. doi: 10.1093/glycob/11.3.25r. [DOI] [PubMed] [Google Scholar]; (b) Walsh C. Antibiotics: actions, origins, resistance. ASM Press; Washington, D.C.: 2003. [Google Scholar]; (c) Vollmer W, Bertsche U. Biochim. Biophys. Acta. 2008;1778:1714. doi: 10.1016/j.bbamem.2007.06.007. [DOI] [PubMed] [Google Scholar]; (d) Typas A, Banzhaf M, Gross CA, Vollmer W. Nat. Rev. Microbiol. 2012;10:123. doi: 10.1038/nrmicro2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.(a) Tam PH, Lowary TL. Curr. Opin. Chem. Biol. 2009;13:618. doi: 10.1016/j.cbpa.2009.09.012. [DOI] [PubMed] [Google Scholar]; (b) Llarrull LI, Testero SA, Fisher JF, Mobashery S. Curr. Opin. Microbiol. 2010;13:551. doi: 10.1016/j.mib.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Carlson EE. ACS Chem. Biol. 2010;5:639. doi: 10.1021/cb100105c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sauvage E, Kerff F, Terrak M, Ayala JA, Charlier P. FEMS Microbiol. Rev. 2008;32:234. doi: 10.1111/j.1574-6976.2008.00105.x. [DOI] [PubMed] [Google Scholar]
- 4.(a) Paradis-Bleau C, Markovski M, Uehara T, Lupoli TJ, Walker S, Kahne DE, Bernhardt TG. Cell. 2010;143:1110. doi: 10.1016/j.cell.2010.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Typas A, Banzhaf M, van den Berg van Saparoea B, Verheul J, Biboy J, Nichols RJ, Zietek M, Beilharz K, Kannenberg K, von Rechenberg M, Breukink E, den Blaauwen T, Gross CA, Vollmer W. Cell. 2010;143:1097. doi: 10.1016/j.cell.2010.11.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.(a) Anderson JS, Matsuhashi M, Haskin MA, Strominger JL. Proc. Natl. Acad. Sci. U.S.A. 1965;53:881. doi: 10.1073/pnas.53.4.881. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Ye XY, Lo MC, Brunner L, Walker D, Kahne D, Walker S. J. Am. Chem. Soc. 2001;123:3155. doi: 10.1021/ja010028q. [DOI] [PubMed] [Google Scholar]; (c) Chen L, Walker D, Sun B, Hu Y, Walker S, Kahne D. Proc. Natl. Acad. Sci. U.S.A. 2003;100:5658. doi: 10.1073/pnas.0931492100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Barrett DS, Chen L, Litterman NK, Walker S. Biochemistry. 2004;43:12375. doi: 10.1021/bi049142m. [DOI] [PubMed] [Google Scholar]; (e) Barrett D, Leimkuhler C, Chen L, Walker D, Kahne D, Walker S. J. Bacteriol. 2005;187:2215. doi: 10.1128/JB.187.6.2215-2217.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Barrett D, Wang TS, Yuan Y, Zhang Y, Kahne D, Walker S. J. Biol. Chem. 2007;282:31964. doi: 10.1074/jbc.M705440200. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Yuan Y, Barrett D, Zhang Y, Kahne D, Sliz P, Walker S. Proc. Natl. Acad. Sci. U.S.A. 2007;104:5348. doi: 10.1073/pnas.0701160104. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Perlstein DL, Zhang Y, Wang TS, Kahne DE, Walker S. J. Am. Chem. Soc. 2007;129:12674. doi: 10.1021/ja075965y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Wang TS, Manning SA, Walker S, Kahne D. J. Am. Chem. Soc. 2008;130:14068. doi: 10.1021/ja806016y. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Perlstein DL, Wang TS, Doud EH, Kahne D, Walker S. J. Am. Chem. Soc. 2010;132:48. doi: 10.1021/ja909325m. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Wang TS, Lupoli TJ, Sumida Y, Tsukamoto H, Wu Y, Rebets Y, Kahne DE, Walker S. J. Am. Chem. Soc. 2011;133:8528. doi: 10.1021/ja2028712. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Oman TJ, Lupoli TJ, Wang TS, Kahne D, Walker S, van der Donk WA. J. Am. Chem. Soc. 2011;133:17544. doi: 10.1021/ja206281k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Knerr PJ, Oman TJ, Garcia De Gonzalo CV, Lupoli TJ, Walker S, van der Donk WA. ACS Chem. Biol. 2012;7:1791. doi: 10.1021/cb300372b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Gampe CM, Tsukamoto H, Doud EH, Walker S, Kahne D. J. Am. Chem. Soc. 2013;135:3776. doi: 10.1021/ja4000933. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Schwartz B, Markwalder JA, Seitz SP, Wang Y, Stein RL. Biochemistry. 2002;41:12552. doi: 10.1021/bi026205x. [DOI] [PubMed] [Google Scholar]; (p) Bertsche U, Breukink E, Kast T, Vollmer W. J. Biol. Chem. 2005;280:38096. doi: 10.1074/jbc.M508646200. [DOI] [PubMed] [Google Scholar]; (q) Born P, Breukink E, Vollmer W. J. Biol. Chem. 2006;281:26985. doi: 10.1074/jbc.M604083200. [DOI] [PubMed] [Google Scholar]; (r) Sung MT, Lai YT, Huang CY, Chou LY, Shih HW, Cheng WC, Wong CH, Ma C. Proc. Natl. Acad. Sci. U.S.A. 2009;106:8824. doi: 10.1073/pnas.0904030106. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Liu CY, Guo CW, Chang YF, Wang JT, Shih HW, Hsu YF, Chen CW, Chen SK, Wang YC, Cheng TJ, Ma C, Wong CH, Fang JM, Cheng WC. Org. Lett. 2010;12:1608. doi: 10.1021/ol100338v. [DOI] [PubMed] [Google Scholar]; (t) Banzhaf M, van den Berg van Saparoea B, Terrak M, Fraipont C, Egan A, Philippe J, Zapun A, Breukink E, Nguyen-Distèche M, den Blaauwen T, Vollmer W. Mol. Microbiol. 2012;85:179. doi: 10.1111/j.1365-2958.2012.08103.x. [DOI] [PubMed] [Google Scholar]
- 6.(a) Waxman DJ, Yu W, Strominger JL. J. Biol. Chem. 1980;255:11577. [PubMed] [Google Scholar]; (b) Goffin C, Ghuysen JM. Microbiol. Mol. Biol. Rev. 2002;66:702. doi: 10.1128/MMBR.66.4.702-738.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Shi Q, Meroueh SO, Fisher JF, Mobashery S. J. Am. Chem. Soc. 2008;130:9293. doi: 10.1021/ja801727k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Shi Q, Meroueh SO, Fisher JF, Mobashery S. J. Am. Chem. Soc. 2011;133:5274. doi: 10.1021/ja1074739. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Schwartz B, Markwalder JA, Wang Y. J. Am. Chem. Soc. 2001;123:11638. doi: 10.1021/ja0166848. [DOI] [PubMed] [Google Scholar]; (f) Lupoli TJ, Tsukamoto H, Doud EH, Wang TS, Walker S, Kahne D. J. Am. Chem. Soc. 2011;133:10748. doi: 10.1021/ja2040656. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Lebar MD, Lupoli TJ, Tsukamoto H, May JM, Walker S, Kahne D. J. Am. Chem. Soc. 2013;135:4632. doi: 10.1021/ja312510m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee M, Hesek D, Suvorov M, Lee W, Vakulenko S, Mobashery S. J. Am. Chem. Soc. 2003;125:16322. doi: 10.1021/ja038445l. [DOI] [PubMed] [Google Scholar]
- 8.(a) Pollock JJ, Ghuysen JM, Linder R, Salton MR, Perkins HR, Nieto M, Leyh-Bouille M, Frere JM, Johnson K. Proc. Natl. Acad. Sci. U.S.A. 1972;69:662. doi: 10.1073/pnas.69.3.662. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Adam M, Damblon C, Jamin M, Zorzi W, Dusart V, Galleni M, el Kharroubi A, Piras G, Spratt BG, Keck W. Biochem. J. 1991;279:601. doi: 10.1042/bj2790601. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Nemmara VV, Adediran SA, Dave K, Duez C, Pratt RF. Biochemistry. 2013;52:2627. doi: 10.1021/bi400211q. [DOI] [PubMed] [Google Scholar]
- 9.(a) Men H, Park P, Ge M, Walker S. J. Am. Chem. Soc. 1998;120:2484. [Google Scholar]; (b) Ha S, Chang E, Lo M, Men H, Park P, Ge M, Walker S. J. Am. Chem. Soc. 1999;121:8415. [Google Scholar]
- 10.(a) Mengin-Lecreulx D, Michaud C, Richaud C, Blanot D, van Heijenoort J. J. Bacteriol. 1988;170:2031. doi: 10.1128/jb.170.5.2031-2039.1988. In vivo work also shows that L-Lys-containing PG is not incorporated into peptide crosslinks, see. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Mengin-Lecreulx D, Falla T, Blanot D, van Heijenoort J, Adams DJ, Chopra I. J. Bacteriol. 1999;181:5909. doi: 10.1128/jb.181.19.5909-5914.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. There is also a lag phase for PBP1A-catalyzed glycan polymerization seen in Figure 4a (and ref. 5k,q)
- 12. These observations are consistent with previous in vitro work with E. coli PBP1B (see ref. 5p), E. coli PBP1A (see ref. 5q), and Streptococcus pneumonia PBP1b (see ref. 5o), which suggest that cross-linking and glycosyltransfer reactions occur simultaneously.
- 13.Glauner B, Höltje JV, Schwarz U. J. Biol. Chem. 1988;263:10088. [PubMed] [Google Scholar]
- 14. (M+2)/2 ions were extracted: A: 507.2; A’: 508.7; B: 471.7; C: 968.9; C’: 970.4. A and A’, as well as C and C’, elute at identical retention times and are offset for clarity.
- 15. The effect of LpoA on the PGT activity of PBP1A is only observed at high Lipid II concentration, but not at low concentrations of Lipid II (see Figure S7 and ref. 4a)
- 16.Cava F, Kuru E, Brun YV, de Pedro MA. Curr. Opin. Microbiol. 2013;16:731. doi: 10.1016/j.mib.2013.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.