Abstract
Adiponectin (APN), an adipokine, exerts an anti-inflammatory and anti-cancerous activity with its role in glucose and lipid metabolism and its absence related to several obesity related malignancies including colorectal cancer. The aim of this study is to determine the effect of APN deficiency on the chronic inflammation-induced colon cancer. This was achieved by inducing inflammation and colon cancer in both APN knockout (KO) and C57B1/6 wild type (WT) mice. They were divided into four treatment groups (n=6): 1) control (no treatment); 2) treatment with three cycles of dextran sodium sulfate (DSS); 3) weekly doses of 1,2-dimethylhydrazine (DMH) (20 mg/kg of mouse body weight) for twelve weeks; 4) a single dose of DMH followed by 3 cycles of DSS (DMH+DSS). Mice were observed for diarrhea, stool hemoccult, and weight loss and were sacrificed on day 153. Tumor area and number were counted. Colonic tissues were collected for Western blot and immunohistochemistry analyses. APNKO mice were more protected than WT mice from DSS induced colitis during first DSS cycle, but lost this protection during the second and the third DSS cycles. APNKO mice had significantly severe symptoms and showed greater number and larger area of tumors with higher immune cell infiltration and inflammation than WT mice. This result was further confirmed by proteomic study including pSTAT3, pAMPK and Cox-2 by western blot and Immunohistochemistry. Conclusively, APN deficiency contributes to inflammation-induced colon cancer. Hence, APN may play an important role in colorectal cancer prevention by modulating genes involved in chronic inflammation and tumorigenesis.
Keywords: Adiponectin, Inflammation, Colon, Cancer
1. Introduction
Patients with inflammatory bowel disease, such as Crohn's and ulcerative colitis (UC), are at increased risk for developing colorectal cancer [1,2]. UC patients, in particular, have ten to forty fold increased risk of developing cancer, compared with the general population [3,4]. The pathogenesis of colitis-associated colorectal cancer is thought to be related to an increased rate of epithelial cell proliferation associated with the repetitive cycles of inflammation, damage and regeneration [5,6]. In addition, obesity, an established risk factor for colorectal cancer, is characterized by the accumulation of excessive adipose tissue, which is the source of a variety of biologically active substances, collectively referred to as adipokines [7,8]. Adiponectin (APN), an adipokine secreted by the adipose tissue, has been found at low levels in obese subjects [9,10]. Obesity and APN have been linked to colon cancer by various studies [11–15]. Furthermore; obesity is associated with a heightened inflammatory response in Crohn's disease (CD) patients who have typical alterations of mesenteric fat deposits [16].
Adiponectin exerts its activity through its receptors. Two receptors, AdipoR1 and AdipoR2, had been identified for APN and were found to mediate glucose and fatty acid metabolism by Adenosine mono-phosphate kinase (AMPK) activation, although no mechanism has been devised for its activation [17–20]. 1 AMPK is a serine threonine kinase, which is activated by phosphorylation, in the presence of cellular stress [21,22]. AMPK has anti-inflammatory effects and has been considered as a potential therapeutic target for cancer [23–26]. APN increases the rate of phosphorylation of AMPK and suppresses cell growth by inhibiting the mammalian target of rapamycin (mTOR) [23]. AMPK activity also inhibits the activity of Cyclooxygenase (Cox)-2, an enzyme that is upregulated in the presence of various stimuli like inflammation, tumorigenesis, metastasis, and growth factors [24,25]. More importantly, APN has been linked to reduce expression of Cox-2 in APNKO mice treated with azoxymethane [26].
Signal transducer and activator of transcription (STAT)-3 is overexpressed in many cancers and is a potent indicator of inflammation [27]. It plays a critical role in tumor progression and metastasis through transcriptional activation of anti-apoptotic genes like Bcl-xL, Mcl-1 and survivin, cell-cycle regulators (e.g. cyclin D1 and c-Myc) and inducers of angiogenesis (e.g. vascular endothelial growth factor) [28,29].
In this paper, we have used dextran sodium sulphate (DSS), a potent inducer of inflammation and promoter of colorectal carcinogenesis. It was administered in mice for three cycles to induce chronic inflammation, while dimethylhydrazine (DMH) was injected intraperitoneally to induce cancer. APN knockout (KO) and the C57Bl/6 wild type (WT) mice were given different treatments to elucidate the role of APN deficiency in chronic inflammation-induced colon cancer (CICC).
We hypothesized that APNKO mice are more susceptible to CICC as compared to their WT counterpart, which could be deduced by greater weight loss, bloody stools, diarrhea, larger number and size of the tumors. It is also evident by the up-regulation of procancerous and downregulation of anti-cancerous and inflammatory genes.
2. Materials and methods
2.1. Animals and experimental groups
Six to eight week old male APNKO (n=24) and male C57BL/6 (WT, n=24) were housed in conventional animal room and treated in the animal facility at the University of South Carolina. All APNKO mice were homozygous for APN deficiency (−/−). The mice were on a 12:12 h light–dark cycle in a low stress environment (22 °C, 50% humidity and low noise) and had access to food (Purina Chow) and water ad libitum. All animal care followed institutional guidelines under a protocol approved by the Institutional Animal Care and Use Committee at the University of South Carolina. APNKO and WT mice were randomly assigned to 4 different groups of equal number (n=6 mice per group): 1) DMH+DSS; 2) DMH; 3) DSS and 4) Control or no treatment. The body weight of both APNKO and WT mice showed no significant difference at the beginning of the study.
2.2. Induction of chronic inflammation and cancer
Chronic inflammation was induced in the WT and APNKO mice assigned to the DSS treatment group. These mice received 2% dextran sodium sulfate (DSS) (MP Biochemicals, MW: 36,000–50,000) dissolved in their drinking water for five days, followed by five days of regular drinking water, this represented single cycle and DSS was administrated for three cycles on days 4, 27 and 50. To establish chronic inflammation induced colon cancer, another group of mice received 3 cycles of DSS and a single injection of DMH intraperitoneally (i.p) (SIGMA ALDRICH) (20 mg/kg body weight) administered at the beginning of the DSS treatment. Cancer was induced in the WT and APNKO mice assigned to DMH treatment group by administering weekly dose of DMH i.p. for twelve weeks and the mice were sacrificed on day 153.
2.3. Monitoring animal health
Mice were observed daily for clinical signs of disease attributed by weight loss, fecal hemoccult and diarrhea during all treatments and till day 153. Ranking for the weight loss was based on the following scale: 0 = 0–5% weight loss; 1 = 6–10% weight loss; 2 = 11–15% weight loss; 3 = 16–20% weight loss; and 4 = >20% weight loss. The appearance of diarrhea was scored as: 0 = well-formed pellets, 2 = pasty and semi-formed stools that do not adhere to the anus, 4 = liquid stools that adhere to the anus. Appearance of blood in the stools was assessed using a hemoccult kit (BECKMAN COULTER) and scored as: 0 = no blood, 2 = positive hemoccult, 4 = gross bleeding. The clinical score was then determined by totaling the weight loss, hemoccult, and diarrhea scores with the highest score being twelve.
2.4. Tissue collection and tumor quantification
The mice were euthanized by cervical dislocation. The colon was removed and flushed with PBS containing 5000 IU/mL and 5000 IU/mL penicillin and streptomycin (CELLGRO) respectively. Two 2 mm2 sections of the descending colon were dissected and stored at −80 °C for Western blot analysis and at 37 °C, 5% CO2 with overnight incubation for tissue culture. The remaining colon was fixed in 10% formalin (AZER SCIENTIFIC) for a day. It was then stained with 0.2% methylene blue (FISHER SCIENTIFIC) to count tumors and measure tumor area followed by its swiss roll and sectioning it for immunohistochemistry and Hematoxylin and Eosin (H&E) staining. Tumor quantification was done in blinded condition by two individuals.
2.5. Colonic cytokines and serum APN measurement
The secreted concentrations (pg/mg of protein) of IL-6, IL-10, IL-1β and TNF-α in the tissue culture were determined by Enzyme Linked Immunosorbent Assay (ELISA) using BD OptEIA kit (BD BIOSCIENCES). Serum APN was determined in all the treatment groups at the beginning and at the end of the study. This was achieved by diluting the serum samples to 1:2000 and performing ELISA using DuoSet mouse/Acrp30 kit (R&D SYSTEMS).
2.6. Protein determination and western blot
Tissues frozen at −80 °C were thawed and homogenized in RIPA buffer (SIGMA) containing 1:1000 dilution of protease inhibitor solution (SIGMA). Concentration of the total protein in the solution of homogenized tissue was calculated by Bradford protein assay. Protein homogenate from each group was loaded and electrophoresed on 10% SDS-PAGE gels and transferred to a nitrocellulose membrane at constant voltage of 200 mA for 150 min at 4 °C. The membrane was then blocked with 5% non-fat dry milk (BIO-RAD) in DPBS (cellgro)+0.1% Tween 20 (BIO-RAD) for one hour at room temperature followed by overnight incubation at 4 °C with STAT3 and pSTAT3 (1:000) (CELL SIGNALING TECHNOLOGY), AMPK and pAMPK (1:1000) (SANTA CRUZ BIOTECHNOLOGY) and GAPDH (1:3000) (R&D SYSTEM) diluted in DPBS+0.1% Tween 20. Membrane was subsequently washed 5 times for 10 min with DPBS+0.1% Tween 20 with consequent incubation with anti-rabbit or anti goat secondary antibody conjugated with horseradish peroxidase enzyme (1:3000) (SANTA CRUZ BIOTECHNOLOGY) for 1 h. The membrane was washed and incubated for 5 min in Amersham ECL Plus Western blot detection system (GE HEALTHCARE) with subsequent detection on high performance chemiluminescence film (Amersham Hyperfilm ECL, GE HEALTHCARE). GAPDH was used as a loading control to normalize the protein expression by densitometry using Image J software (NCBI). Western blot for each protein was done thrice by using all samples from the same treatment group to determine statistical significance.
2.7. Immunohistochemical staining
Serial tumor and non tumor sections of mouse colon tissues were incubated with antibodies against cyclooxygenase-2 (Cox-2; rabbit polyclonal; diluted 1:5000; CAYMAN CHEMICAL). To ensure even staining and reproducible results, sections were incubated by slow rocking overnight in primary antibodies (4 °C) using the Antibody Amplifier (ProHisto, LLC). Following incubation with primary antibody, sections were processed using EnVision+System-HRP kit (DAKO CYTOMATION). The chromogen was 3,3′-diaminobenzidine and sections were counterstained with 0.5% methyl green. The positive control tissue was colon cancer sections which were highly positive for iNOS, Cox-2, TNF-α, p53, and p53-phospho-Ser15.
Stained tissues were examined for intensity of staining using a method similar to that previously described by Nishihara et al. [30]. Briefly, intensity of staining in colon sections was evaluated independently by two blinded investigators. For each tissue section, the summation of the intensity of staining and the percentage of the positively stained cells was analyzed and quantified on a scale of 4, where 0 represents no staining, 2 — 50–75% of moderately stained cells, and 4 indicates more than 75% of darkly stained cells. All the images were taken in 20X magnification with Nikon e600 microscope. The scale bar represents 120 µm.
2.8. Hematoxylin and Eosin staining and histological scoring
To determine the pathological state of the colon, a standard protocol for H&E staining was used. Quantification of severity of disease including inflammation and immune cell infiltration was done on the scale of 4 for both the parameters, where 0 = no infiltration or no inflammation; 2 = moderate infiltration or inflammation; and 4 = severe inflammation with distorted crypts or infiltration and formation of lymphatic follicles. All the images were taken in 20× magnification with Nikon e600 microscope. The scale bar represents 120 µm.
2.9. Aberrant crypt foci (ACF)
The H&E stained slides for DSS treated groups for APNKO and WT were scanned by two investigators in the blinded conditions for aberrant crypt foci. The ratio of the number of ACF to normal crypts was calculated in the 2 mm2 area and was plotted on a graph. All the images were taken in 40× magnification with Nikon e600 microscope. The scale bar represents 60 µm.
2.10. Statistical analysis
Two-way analysis of variance (ANOVA), Two-way repeated measure ANOVA and One-way ANOVA was used to analyze the data with Tukey post hoc-analyses. A p value<0.05 was considered statistically significant. All the statistical analyses were done by using SigmaStat 3.5 (SPSS, Chicago, IL).
3. Results
3.1. Clinical manifestation of CICC in APNKO and WT mice
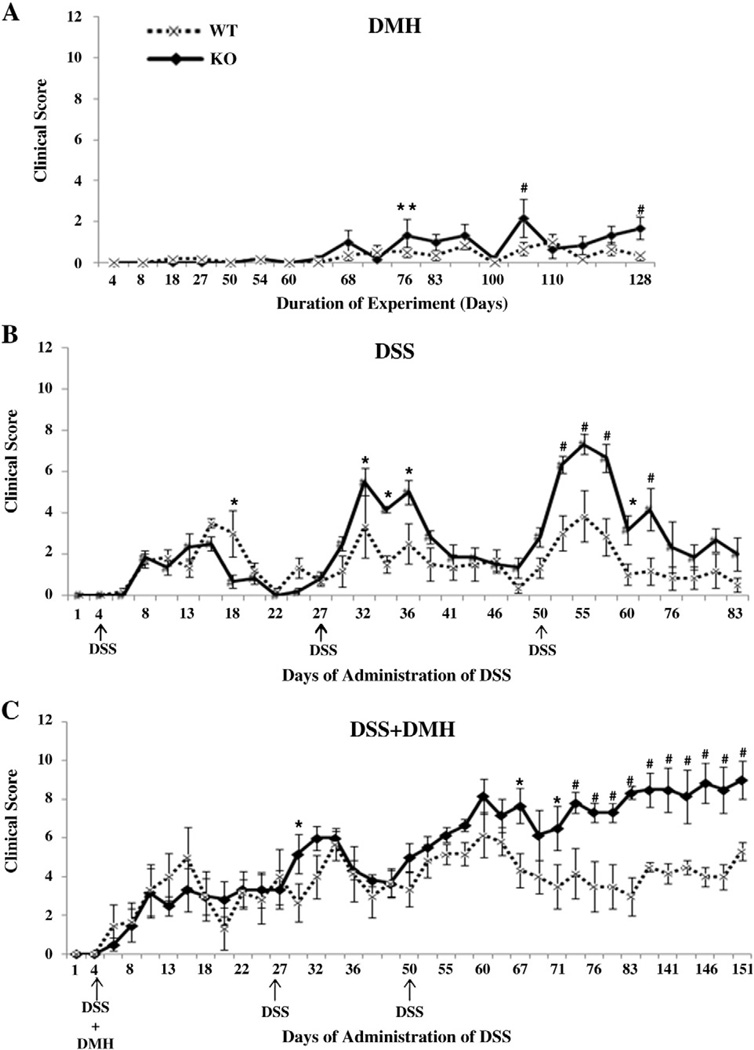
To study the role of APN deficiency in CICC, WT and APNKO mice were given three different treatments and were observed for weight loss, diarrhea and hemoccult for 153 days and during this time clinical score was calculated. After the first DSS cycle in DSS and DSS+DMH group, WT mice lost their protection from DSS in comparison to APNKO mice and showed a significantly higher clinical score on day 18 in DSS group (Fig. 1B and C). However, no significance was observed in DSS+DMH group. After the second DSS cycle, APNKO mice manifested significantly higher clinical score on days 32, 34 and 36 when treated with DSS alone, but in DSS+DMH treatment group, it was observed on day 29 (Fig. 1B and C). Following the third DSS cycle, there was a complete loss of protection from DSS in the DSS and DSS+DMH group resulting in a significantly higher clinical score in APNKO mice as compared to WT mice (Fig. 1B and C). Mice in DSS+DMH group didn't recover from the treatment and the clinical score continued to remain significantly higher in APNKO mice till the date of sacrifice (Fig. 1C). DSS treated mice recovered after every DSS cycle but after the third cycle, APNKO mice showed a comparatively higher clinical score than WT (data not shown). In DMH treatment group, significantly higher clinical score was observed in APNKO mice when compared to WT mice on days 77, 105 and 128, after which the score continued to remain higher till the date of sacrifice (Fig. 1A).
Fig. 1.
Clinical score of DSS and DMH treatments. (A–C) Clinical score for DMH, DSS and DSS+DMH was plotted against the different time point during the study. The arrows represent the days of DSS administration in DSS and DSS+DMH study groups. The score was calculated out of twelve points; four points for each weight loss, diarrhea and hemoccult. Two-way repeated measure analysis of variance (ANOVA) was applied to calculate the significant difference between the clinical score for APNKO and WT mice thought the length of the experiment. **p<0.05, #p<0.001, *p<0.03.
3.2. Tumor development in APNKO and WT mice
Immediately following the sacrifice, mice colon was fixed and stained in methylene blue for tumor quantification (Fig. 2A). APNKO mice had a significantly greater tumor number than WT mice in all the treatment groups (Fig. 2A and B). The degree of significance was higher in DMH group, but the number of tumors was higher in DSS+DMH group as compared to others. In DSS+DMH group, APNKO mice showed a significantly larger tumor area as compared to the WT group (Fig. 2C). Most of the tumors were observed in the descending and the rectal part of the colon.
Fig. 2.
Tumor development in APNKO and WT mice treated with DSS and DMH. (A) Colon of WT and APNKO mice treated with DSS+DMH and stained with 0.2% methylene blue. (B) Average number of tumors counted per mice per group for all the treatments in order of their severity. (C) Average tumor area in mm2 counted for each mouse per group plotted in the order of their severity. One-way ANOVA was used to calculate significant difference between APNKO and WT mice given the same treatment for all the experimental groups. *p<0.05 and # p<0.03.
3.3. Colon morphology
To study the change in the colon morphology in CICC, H&E staining for all the treatment groups was done for both WT and APNKO mice and quantified on a scale of one to four. It was observed that the APNKO mice treated with DSS+DMH showed a complete loss of structural integrity of the colon epithelium. Infiltration of the immune cells was also observed in addition to crypt destruction. Tumor growth was more prominent and the number of tumors was observed to be greater in APNKO mice than WT in DSS+DMH group (Fig. 3A). APNKO mice treated with DSS+DMH had significantly severe immune cell infiltration, inflammation and distorted crypts as compared to WT mice (Fig. 3B). Significance in the severity of inflammation and infiltration was also observed in APNKO mice treated with DMH (H&E not shown). DSS treated group showed no significant difference between the APNKO and WT mice (Fig. 3B). DSS treated group however showed a significantly higher ACF in the APNKO mice as compared to WT mice (Fig. 3C and D). Other treatment groups were observed for ACF, but neither showed ACF as they have developed tumors or the observed difference was not significant between the APNKO and WT mice.
Fig. 3.
Hematoxylin and Eosin staining and aberrant crypt foci. (A) H&E stained sections of the descending colon of KO and WT mice in DSS group with 20× magnification (scale bar=120 µm). (B) Quantification of inflammation and immune cell infiltration in all the treatment groups on a scale of four. (C) H&E stained slides of the descending colon section of the APNKO and WT mice treated with DSS alone and observed and marked for Aberrant crypt foci with 40× magnification (scale bar=60 µm). (D) Quantification of the ACF by calculating the ratio of the aberrant crypts to the normal crypts. Degree of significance was calculated by One-way ANOVA between the KO and WT of the same treatment group. *p<0.05 and # p<0.01.
3.4. Systemic APN in WT mice and secreted cytokine profiling for all the treatment groups
To determine the effect of different treatments on the systemic APN levels, sera were obtained from the WT mice at the beginning of the study or pre-treatment and at the time of sacrifice. It was observed that the serum APN concentration in DSS+DMH treated group was significantly less when compared to the WT mice treated with DSS or DMH alone and with the control group (Fig. 4E). DMH treated mice showed a significantly lower concentration of serum APN than the DSS alone and control group. Significant decrease in the serum APN level was observed in the DSS+DMH and DMH alone treated WT mice on days 153 and 0 (Fig. 4E). However no significant difference was found in the mice treated with DSS and the control group (Fig. 4E).
Fig. 4.
Cytokine secretion from the colon of APNKO and WT mice. (A-D) Amount of IL-6, IL-10, Il-1β and TNF-α secreted respectively from the colon of the APNKO and WT mice subjected to treatment of DSS, DMH and DSS+DMH and to the control group. (E) Serum adiponectin levels (µg/mL) in WT mice treated with DSS+DMH, DMH alone, DSS alone and control group at days 0 and 153. Significant difference was observed in the serum adiponectin level of the WT mice treated with DSS+DMH and DMH alone, DSS alone and control group, and WT mice treated with DMH and DSS alone and control group. Significant difference was also observed within the DSS+DMH and DMH alone group at days 0 and 153. One way ANOVA was applied to calculate the significant difference between the secretion of cytokine from APNKO and WT mice belonging to the same group. *p<0.01,**p<0.04, ***p<0.02, #p<0.05.
The tissue culture media was then used to perform secreted cytokine profiling and the concentration of the secreted IL-6, IL-10, TNF-α and IL-1β was calculated in all the treatment groups by using ELISA. APNKO mice showed a significantly higher colonic IL-6 and TNF-α levels in DSS and DSS+DMH groups when compared to the WT mice (Fig. 4A and D). IL-1β, another pro-inflammatory cytokine was found to be secreted in significantly higher concentrations in APNKO mice in DMH and DSS+DMH group (Fig. 4C). IL-10, grouped as an anti-inflammatory cytokine was quantified and it was found that in all the treatment groups, was a significant decrease in APNKO mice with greater significance in the DSS+DMH group as compared to WT mice. IL-10 level in APNKO mice of DSS+DMH group was comparable to the baseline untreated control group (Fig. 4B).
3.5. Colonic STAT3, AMPK, and Cox-2 expression
We investigated the effect of APN deficiency on various pathways involved in inflammation and colon cancer in our different treatment groups. We observed significantly higher expression levels of pSTAT3Ser727 and Cox-2 in APNKO mice treated with DSS+DMH and DMH in comparison to WT mice. However, no significant difference was observed in the expression level of APNKO and WT mice administered with DSS alone (Fig. 5A, B and D). No to minimum expression of pSTAT3Ser727 was detected in control group (Fig. 5A and B). However, low expression of Cox-2 was observed in APNKO mice in control group while there was no expression in WT mice (Fig. 5A and D). p-AMPK-α ½Thr 172 was downregulated and significantly decreased expression in APNKO mice treated with DSS+DMH and DSS alone in comparison to the WT mice. Comparatively, higher expression of p-AMPK-α ½Thr 172 was observed in control group (Fig. 5A and C). GAPDH was used as a loading control and was used to normalize all the protein density measurements (Fig. 5A). Ratio of the phosphorylated protein to full protein was represented on the graph.
Fig. 5.
Protein expression of STAT3, pSTAT3, AMPK, pAMPK, COX-2 in APNKO and WT mice under different treatment conditions. A) Western blot representing the expression of STAT3, pSTAT3, AMPK, pAMPK, COX-2 and GAPDH. B–D) Graphs showing the ratio of the relative density of the bands for pSTAT3/STAT3 (B), p-AMPK/AMPK (C) and COX-2 (D) being normalized by GAPDH, for control and three other treatments in the order of their severity in WT and APNKO mice. ANOVA was used to determine the significant difference between APNKO and its wild counter parts for all the treatments. * p<0.03, **p<0.05, #p<0.02.
3.6. Cox-2 localization
After proteomic study by Western blot, localization of Cox-2 was determined, by immunohistochemistry for Cox-2 antigen in the tumor and the non tumor area of the descending colon section of both APNKO and WT mice. This study revealed significantly higher expression of Cox-2 in the tumor area than the non tumor area of the mice treated with DSS+DMH, irrespective of mice genotype (Fig. 6A, B and C). APNKO mice also showed significantly higher expression of Cox-2 when compared with WT mice treated with DSS+DMH (Fig. 6A, B and C). Significant difference was also found in APNKO mice of the tumor and the non tumor group and most importantly between the APNKO and the WT mice treated with DSS+DMH in the tumor group (Fig. 6A, B and C). However, the Cox-2 expression level was insignificant between the WT and APNKO mice treated with DSS+DMH in the non tumor sections and other treatment group including DSS alone and DMH alone group with no expression in untreated groups (Fig. 6A, B and C).
Fig. 6.
Cox-2 immunohistochemical staining and quantification. (A) Immunohistochemical staining for Cox-2 (brown) in the non tumor area of the descending colon (2 mm2) section of control, DSS, DMH and DSS+DMH treated groups in both WT and KO mice. (B) Immunohistochemical staining for Cox-2 (brown) in the tumor area of the descending colon (2 mm2) section of DSS+DMH treated groups in both WT and KO mice. (C) Quantification of the Cox-2 expression in the non tumor descending colon 2 mm2 section of the WT and KO mice treated with DSS, DMH, DSS+DMH and control group and DSS+DMH group in the tumor area. Two-way ANOVA was used for statistical significance. # versus ‡ (p<0.002), † and * versus § and ** (p<0.002), § versus **(p<0.002), * versus ** (p<0.002).
4. Discussion
Protective role of APN in obesity, cancer and other disease models has been established but, in this paper, we have tried to delineate its function in the CICC, which is a common condition in patients suffering from IBD and have been the second leading cause of cancer death in the United States [31]. This study was primarily designed to deduce the effect of APN deficiency on the physical and molecular attributes of CICC. Our results showed a significantly severe clinical manifestation in the APNKO mice treated with DSS+DMH after the third DSS cycle. It continued to remain severe till the date of sacrifice. However there was no significant difference observed after the first and second DSS cycles. This observation is in accordance with common diseased state observed during colon cancer and could be explained by the loss of protection in the colonic epithelium of APNKO mice treated with DSS and DMH in chronic conditions. Absence of APN made the mice more vulnerable to DSS induced colitis and DMH induced colon cancer. Our group has shown that in acute phase in WT mice, this effect was not prominent as APN binds to growth factors like HB-EGF, bFGF that prevents the renewal of the colonic epithelium and hence there was no significant difference between the clinical score of APNKO and WT mice treated with DSS+DMH [32]. This paper adds another chapter in APN research on the much-debated topic of APN as an anti-inflammatory or pro-inflammatory adipokine. Our results show a peculiar behavior of APN deficiency that has an anti-inflammatory effect in acute phase and a pro-inflammatory entity in chronic condition and colon cancer. Nishihara et al. [30] showed significant weight loss in the APNKO mice treated with AOM than WT mice; however we followed a more comprehensive approach and combined weight loss with diarrhea and hemoccult.
DSS+DMH treated group was found to show the highest number of tumors and tumor areas. A significant difference was found between the WT and APNKO mice for both area and number of tumors. This observation could be explained by the results of Brakenhielm et al. [33], where they proved APN to induce anti-angiogenesis and to perform anti-tumor activity involving caspase- mediated endothelial cell apoptosis. DSS and DMH alone groups also showed a significant difference in the tumor number between the WT and APNKO. Most of the tumors observed were in the descending and the rectal portion of the colon with greater tumor area in the descending colon. Spleen was also found to be enlarged and more number of tumors was observed in the APNKO mice as compared to WT (data not shown). However, no metastasis was found in the lungs and liver. These data are in agreement with our previous observations and provide an insight about the role of APN in tumor development with absence of APN being a risk factor for chronic colonic inflammation and colitis. It also follows the same pattern as counted by Nishihara et al. [30].
Ferroni et al. [34] had regarded lower serum APN as an adjunctive prognosis for the risk of colorectal cancer. Likewise, we found significantly decreased level of serum APN in the DSS+DMH treated WT mice as compared to control and along the length of the experiment (days 0 and 153). This indicates an inverse relation between the APN and CICC and could be attributed to greater chronic inflammation and cancer growth [35]. A study conducted by Karmiris et al. showed significantly elevated APN levels in UC patients [36]. On the contrary, we observed no significant difference in the APN level of the WT mice treated with DSS and control and also during the length of the experiment. This disparity could be due to that most of the UC patients received 5-aminosalicylic acid, which has been shown to increase PPAR-γ expression, which in turn increases APN production [37]. In addition, the DSS model that we used is not an ideal model for UC. This protective role of APN was further strengthen by our histological evidences which indicates significantly greater formation of ACF in APNKO mice treated with DSS as compared to WT mice, which has been debated by several pathologist as a diagnostic tool for colon cancer. We confirmed the results obtained by Fujisawa et al. [38] indicating significantly higher ACF and aberrant crypt in APNKO under high fat diet condition. However, we obtained the same results under normal diet with DSS treatment. Further exploration was done by quantification of inflammation and immune cell infiltration in DSS+DMH group, which was found to be significantly higher in the APNKO mice. Tumors could be histologically observed in the APNKO mice treated with DSS+DMH. Higher infiltration of the immune cells and greater inflammation, epithelial cell disruption and formation of the primary and secondary lymphatic follicles were common sightings, which is a clear indication of greater colon epithelium insults in APNKO mice when compared to WT.
Adipose tissues have been linked to the secretion of several proinflammatory cytokines and APN has been proved to play an important role in regulating their secretion by downregulating the autocrine release of adipose tissue derived pro-inflammatory cytokines like IL-6, IL-8 from macrophages [39,40]. Our results indicate similar finding with significantly higher secretions of pro-inflammatory cytokines like IL-6, IL-1β and TNF-α and reduced secretion of anti-inflammatory cytokines like IL-10 in the descending colon section of the APNKO mice treated with DSS+DMH as compared to WT mice. IL-6 and TNF-α was also found to show significantly higher level in the APNKO mice treated with DSS while IL-1β was found to show significantly higher level in APNKO mice treated with DMH while IL-10, an anti-inflammatory cytokine, level was significantly downregulated in the same group. Higher secretion of TNF-α was observed in the DSS-treated than DSS+DMH-treated APNKO mice, but the difference was not significant. This could be explained by the multifunctional roles of this cytokine in apoptosis, cell survival and inflammation [41]. Although some investigators argue that the higher TNF-α levels are associated with tumorigenesis but at the same time, TNF-α is a well know pyrogen that induce apoptotic cell death and acts synergistically with cytostatic drugs in cancer treatments [41,42]. Lower levels of TNF-α in DSS+DMH treated APNKO mice directly correlate with greater tumor number and size. Higher TNF-α production in DSS treated APNKO mice could be primarily due to the greater secretion of TNF-α by macrophages and lymphocytes due to chronic inflammation [43,44], delayed recovery from the third DSS cycle and the involvement of the gut microflora that may exacerbate the inflammatory condition [45]. However, these mechanisms are remained to be investigated. These data provide a link between the absence of APN and higher production of pro-inflammatory cytokines and decrease production of anti-inflammatory cytokines under the conditions including cancer, inflammation and CICC. This might be regarded as the first direct influence of the APN deficiency on CICC.
To further investigate the role of the APN deficiency in CICC, we searched deeper in to the protein expression especially targeting the key pathways involved in cancer and inflammation. Our main focus lies on the JAK/STAT signaling pathways, followed by phosphorylation of AMPK, which is directly correlated with the major protein synthesis, mTOR pathway. Phosphorylation of the STAT3 was found to be up-regulated in oppose of AMPK phosphorylation in the APNKO mice treated with DSS+DMH. This indicates higher inflammation and reduction in the energy state milieu and might be the reason for slower recovery. This up-regulation of JAK/STAT3 signaling pathway could be mediated by IL-6 and regulated by SOCS3 signaling [46,47]. However, partial hepatectomy or liver regeneration studies showed reduced expression of phosphorylated STAT3 with the concomitant increase in SOCS3 mRNA in APN null mice [46]. This variability could be accounted to the variable SOCS3 mediated activation pattern of STAT3, during different time points along the length of the study. Another explanation could be the variable roles of APN that may play in different tissues and disease models. Higher STAT3 expression was observed in the control group as compared to DSS treated groups, which could be attributed to the constitutive expression of STAT3. However, only the activated or the phosophorylation of the protein could be affected by the diseased state. This was followed by another investigation of the Cox-2 expression, which plays multiple roles in oncogenesis, metastasis and inflammation related to cancer. Cox-2 was found to be over-expressed in the APNKO mice treated with DSS+DMH and DMH alone. However in heart ischemia model, APN was found to increase the expression of Cox-2 [48]. This provides another evidence of the multifaceted nature of adiponectin. By the means of Cox-2 expression studies, we confirmed the results of Nishihara et al. [30], but in our CICC model we went a step further to investigate localized expression of Cox-2 in tumor and non tumor area by immunohistochemistry. Cox-2 was found to be significantly over-expressed in tumor area of the colon as compared to non tumor area, which could be due to the combined effect of the overexpression of cancer epithelial cells in addition to the localized secretion by inflammatory mononuclear cells, fibroblast and vascular endothelial cells. However the majority of the intensity and the extent of Cox-2 are due to the overexpression by cancer cells [49,50]. In APNKO mice treated with DSS+DMH, Cox-2 expression was found to be the highest and significantly greater than WT mice in the tumor area. This approach was also instrumental in studying the histopathology of the colon, which was found to show a complete disruption of the tissue architecture in the tumor area along with the formation of the primary lymphoid follicle.
Although, our study established a link between the absence of APN and its effects on the molecular and physical attributes of CICC, with its absence linked with higher expression of pro-inflammatory and pro-cancerous markers, it did not illustrate the direct role of APN in CICC. To define the function of APN in our model system, our future work will be to reconstitute APN in all the treatment groups and observe its role during the same timeline. It could also be suggested to further explore the role of APN in apoptotic pathways and establish the interplay of APN in other molecular and cellular pathways by the application of whole genome microarray. Further characterization of APN isoforms could also result in clinical applications.
5. Conclusion
The novelty of this work encompasses several findings including the anti-inflammatory condition in the state of APN deficiency during the first DSS cycle, an acute phase of inflammation. However, in the second and the third DSS cycles, the chronic inflammation, APNKO mice lost their protection and showed a higher clinical score reflecting upon the pro-inflammatory role of APN deficiency. APNKO mice were found to have greater number of tumors and tumor area with marked inflammation and infiltration in CICC as compared to WT mice given the same treatment. APNKO mice treated with DSS+DMH have significantly higher expression of pSTAT3 and Cox-2 and downregulation of pAMPK. Cox-2 was found to be localized in significantly higher amount and intensity in the colon tumor area of the APNKO mice as compared to the WT mice treated with DSS+DMH. In conclusion, we propose a model to define the effects of APN deficiency in CICC (Fig. 7). These findings provide strong evidence for the use of APN or a chemically analogous structure as a therapeutic compound in decreasing the severity of the symptoms caused by CICC and other related diseases.
Fig. 7.
A hypothetical model showing the effect of APN deficiency in chronic inflammation induced colon cancer. In CICC, APN deficiency aggravates the inflammatory condition by increased expression of pro-inflammatory molecule like STAT3 and cytokines like IL-6, TNF-α and IL-1β and reduced production of anti-inflammatory molecules like pAMPK and cytokines like IL-10. It also results in higher ACF presence, which subsequently leads to adenocarcinoma. APN deficiency in CICC increased Cox-2 expression in the epithelial cells with the highest been found in the tumor area. This might be a potential mechanism of Cox-2, which inhibits apoptosis mediated by Bcl-2 that regulates the release of cytochrome c from mitochondria with further inhibition of caspases.
Acknowledgements
The authors would like to acknowledge Alena P. Chumanevich, for her help in immunohistochemical analyses. We would also like to acknowledge Center of Biomedical Research Excellence (COBRE), NIH Center for Colon Cancer Research, USC for funding our study. The study sponsor has no personal and financial interest and involvement in any section of the manuscript.
Footnotes
Abbreviations: AC, Aberrant crypt; ACF, Aberrant crypt foci; AMPK, Adenosine mono-phosphate kinase; ANOVA, Analysis of variance; APN, Adiponectin; APNKO, Adiponectin knockout; CD, Crohn's disease; CICC, Chronic inflammation-induced colon cancer; COX, Cyclooxygenase; DMH, Dimethylhydrazine; DSS, Dextran sodium sulfate; ELISA, Enzyme linked immunosorbent assay; GAPDH, Glyceraldehyde-3-phosphate dehydrogenase; H&E, Hematoxylin and eosin; HRP, Horseradish peroxidase; IL, Interleukin; iNOS, Nitric oxide synthase; mTOR, Mammalian target of rapamycin; SDS-PAGE, Sodium dodecyl sulphate-polyacrylamide gel electrophoresis; STAT, Signal transducer and activator of transcription; TNF, Tumor necrosis factor; UC, Ulcerative colitis; WT, Wild type.
References
- 1.Itzkowitz SH, Yio X. Inflammation and cancer IV. Colorectal cancer in inflammatory bowel disease: the role of inflammation. Am. J. Physiol. Gastrointest. Liver Physiol. 2004;287:G7–G17. doi: 10.1152/ajpgi.00079.2004. [DOI] [PubMed] [Google Scholar]
- 2.Guthrie E, Jackson J, Shaffer J, Thompson D, Tomenson B, Creed F. Psychological disorder and severity of inflammatory bowel disease predict health-related quality of life in ulcerative colitis and Crohn's disease. Am. J. Gastroenterol. 2002;97:1994–1999. doi: 10.1111/j.1572-0241.2002.05842.x. [DOI] [PubMed] [Google Scholar]
- 3.Whelan G. Ulcerative colitis—what is the risk of developing colorectal cancer? Aust. N. Z. J. Med. 1991;21:71–77. doi: 10.1111/j.1445-5994.1991.tb03008.x. [DOI] [PubMed] [Google Scholar]
- 4.Munkholm P. Review article: the incidence and prevalence of colorectal cancer in inflammatory bowel disease. Aliment. Pharmacol. Ther. 2003;18(Suppl. 2):1–5. doi: 10.1046/j.1365-2036.18.s2.2.x. [DOI] [PubMed] [Google Scholar]
- 5.Seril DN, Liao J, Yang GY, Yang CS. Oxidative stress and ulcerative colitis-associated carcinogenesis: studies in humans and animal models. Carcinogenesis. 2003;24:353–362. doi: 10.1093/carcin/24.3.353. [DOI] [PubMed] [Google Scholar]
- 6.Eaden JA, Abrams KR, Mayberry JF. The risk of colorectal cancer in ulcerative colitis: a meta-analysis. Gut. 2001;48:526–535. doi: 10.1136/gut.48.4.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann. Intern. Med. 1995;122:327–334. doi: 10.7326/0003-4819-122-5-199503010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Berg AH, Combs TP, Scherer PE. ACRP30/adiponectin: an adipokine regulating glucose and lipid metabolism. Trends Endocrinol. Metab. 2002;13:84–89. doi: 10.1016/s1043-2760(01)00524-0. [DOI] [PubMed] [Google Scholar]
- 9.Weiss R, Dufour S, Groszmann A, Petersen K, Dziura J, Taksali SE, Shulman G, Caprio S. Low adiponectin levels in adolescent obesity: a marker of increased intramyocellular lipid accumulation. J. Clin. Endocrinol. Metab. 2003;88:2014–2018. doi: 10.1210/jc.2002-021711. [DOI] [PubMed] [Google Scholar]
- 10.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. J. Natl. Cancer Inst. 2005;97:1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 11.Larsson SC, Wolk A. Obesity and colon and rectal cancer risk: a meta-analysis of prospective studies. Am. J. Clin. Nutr. 2007;86:556–565. doi: 10.1093/ajcn/86.3.556. [DOI] [PubMed] [Google Scholar]
- 12.Gunter MJ, Leitzmann MF. Obesity and colorectal cancer: epidemiology, mechanisms and candidate genes. J. Nutr. Biochem. 2006;17:145–156. doi: 10.1016/j.jnutbio.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Calle EE, Kaaks R. Overweight, obesity and cancer: epidemiological evidence and proposed mechanisms. Nat. Rev. Cancer. 2004;4:579–591. doi: 10.1038/nrc1408. [DOI] [PubMed] [Google Scholar]
- 14.Birmingham JM, Busik JV, Hansen-Smith FM, Fenton JI. Novel mechanism for obesity-induced colon cancer progression. Carcinogenesis. 2009;30:690–697. doi: 10.1093/carcin/bgp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bianchini F, Kaaks R, Vainio H. Overweight, obesity, and cancer risk. Lancet Oncol. 2002;3:565–574. doi: 10.1016/s1470-2045(02)00849-5. [DOI] [PubMed] [Google Scholar]
- 16.Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin. Nutr. 2002;21:51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]
- 17.Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, Ito Y, Kamon J, Tsuchida A, Kumagai K, Kozono H, Hada Y, Ogata H, Tokuyama K, Tsunoda M, Ide T, Murakami K, Awazawa M, Takamoto I, Froguel P, Hara K, Tobe K, Nagai R, Ueki K, Kadowaki T. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat. Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, Eto K, Akanuma Y, Froguel P, Foufelle F, Ferre P, Carling D, Kimura S, Nagai R, Kahn BB, Kadowaki T. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat. Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- 19.Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, Murakami K, Ohteki T, Uchida S, Takekawa S, Waki H, Tsuno NH, Shibata Y, Terauchi Y, Froguel P, Tobe K, Koyasu S, Taira K, Kitamura T, Shimizu T, Nagai R, Kadowaki T. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- 20.Kim AY, Lee YS, Kim KH, Lee JH, Lee HK, Jang SH, Kim SE, Lee GY, Lee JW, Jung SA, Chung HY, Jeong S, Kim JB. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol. 2010;24:1441–1452. doi: 10.1210/me.2009-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw RJ, Kosmatka M, Bardeesy N, Hurley RL, Witters LA, DePinho RA, Cantley LC. The tumor suppressor LKB1 kinase directly activates AMP-activated kinase and regulates apoptosis in response to energy stress. Proc. Natl. Acad. Sci. U. S. A. 2004;101:3329–3335. doi: 10.1073/pnas.0308061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carling D. The AMP-activated protein kinase cascade–a unifying system for energy control. Trends Biochem. Sci. 2004;29:18–24. doi: 10.1016/j.tibs.2003.11.005. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J. Biol. Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masferrer JL, Leahy KM, Koki AT, Zweifel BS, Settle SL, Woerner BM, Edwards DA, Flickinger AG, Moore RJ, Seibert K. Antiangiogenic and antitumor activities of cyclooxygenase-2 inhibitors. Cancer Res. 2000;60:1306–1311. [PubMed] [Google Scholar]
- 25.Grossmann ME, Nkhata KJ, Mizuno NK, Ray A, Cleary MP. Effects of adiponectin on breast cancer cell growth and signaling. Br. J. Cancer. 2008;98:370–379. doi: 10.1038/sj.bjc.6604166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hwang JT, Ha J, Park OJ. Combination of 5-fluorouracil and genistein induces apoptosis synergistically in chemo-resistant cancer cells through the modulation of AMPK and COX-2 signaling pathways. Biochem. Biophys. Res. Commun. 2005;332:433–440. doi: 10.1016/j.bbrc.2005.04.143. [DOI] [PubMed] [Google Scholar]
- 27.Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene. 2000;19:2474–2488. doi: 10.1038/sj.onc.1203527. [DOI] [PubMed] [Google Scholar]
- 28.Miyazaki T, Bub JD, Uzuki M, Iwamoto Y. Adiponectin activates c-Jun NH2- terminal kinase and inhibits signal transducer and activator of transcription 3. Biochem. Biophys. Res. Commun. 2005;333:79–87. doi: 10.1016/j.bbrc.2005.05.076. [DOI] [PubMed] [Google Scholar]
- 29.Darnell JE. Validating Stat3 in cancer therapy. Nat. Med. 2005;11:595–596. doi: 10.1038/nm0605-595. [DOI] [PubMed] [Google Scholar]
- 30.Nishihara T, Baba M, Matsuda M, Inoue M, Nishizawa Y, Fukuhara A, Araki H, Kihara S, Funahashi T, Tamura S, Hayashi N, Iishi H, Shimomura I. Adiponectin deficiency enhances colorectal carcinogenesis and liver tumor formation induced by azoxymethane in mice. World J. Gastroenterol. 2008;14:6473–6480. doi: 10.3748/wjg.14.6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics. CA Cancer J. Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 32.Fayad R, Pini M, Sennello JA, Cabay RJ, Chan L, Xu A, Fantuzzi G. Adiponectin deficiency protects mice from chemically induced colonic inflammation. Gastroenterology. 2007;132:601–614. doi: 10.1053/j.gastro.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 33.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2004;101:2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ferroni P, Palmirotta R, Spila A, Martini F, Raparelli V, Fossile E, Mariotti S, Del Monte G, Buonomo O, Roselli M, Guadagni F. Prognostic significance of adiponectin levels in non-metastatic colorectal cancer. Anticancer. Res. 2007;27:483–489. [PubMed] [Google Scholar]
- 35.Ishikawa M, Kitayama J, Kazama S, Hiramatsu T, Hatano K, Nagawa H. Plasma adiponectin and gastric cancer. Clin. Cancer Res. 2005;11:466–472. [PubMed] [Google Scholar]
- 36.Karmiris K, Koutroubakis IE, Xidakis C, Polychronaki M, Voudouri T, Kouroumalis EA. Circulating levels of leptin, adiponectin, resistin, and ghrelin in inflammatory bowel disease. Inflamm. Bowel Dis. 2006;12:100–105. doi: 10.1097/01.MIB.0000200345.38837.46. [DOI] [PubMed] [Google Scholar]
- 37.Westerink J, Visseren FL. Pharmacological and non-pharmacological interventions to influence adipose tissue function. Cardiovasc Diabetol. 2011;10:13. doi: 10.1186/1475-2840-10-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujisawa T, Endo H, Tomimoto A, Sugiyama M, Takahashi H, Saito S, Inamori M, Nakajima N, Watanabe M, Kubota N, Yamauchi T, Kadowaki T, Wada K, Nakagama H, Nakajima A. Adiponectin suppresses colorectal carcinogenesis under the high-fat diet condition. Gut. 2008;57:1531–1538. doi: 10.1136/gut.2008.159293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dietze-Schroeder D, Sell H, Uhlig M, Koenen M, Eckel J. Autocrine action of adiponectin on human fat cells prevents the release of insulin resistance-inducing factors. Diabetes. 2005;54:2003–2011. doi: 10.2337/diabetes.54.7.2003. [DOI] [PubMed] [Google Scholar]
- 40.Bruun JM, Lihn AS, Verdich C, Pedersen SB, Toubro S, Astrup A, Richelsen B. Regulation of adiponectin by adipose tissue-derived cytokines: in vivo and in vitro investigations in humans. Am. J. Physiol. Endocrinol. Metab. 2003;285:E527–E533. doi: 10.1152/ajpendo.00110.2003. [DOI] [PubMed] [Google Scholar]
- 41.van Horssen R, Ten Hagen TL, Eggermont AM. TNF-alpha in cancer treatment: molecular insights, antitumor effects, and clinical utility. Oncologist. 2006;11:397–408. doi: 10.1634/theoncologist.11-4-397. [DOI] [PubMed] [Google Scholar]
- 42.Kapas L, Hong L, Cady AB, Opp MR, Postlethwaite AE, Seyer JM, Krueger JM. Somnogenic, pyrogenic, and anorectic activities of tumor necrosis factor-alpha and TNF-alpha fragments. Am. J. Physiol. 1992;263:R708–R715. doi: 10.1152/ajpregu.1992.263.3.R708. [DOI] [PubMed] [Google Scholar]
- 43.Carswell EA, Old LJ, Kassel RL, Green S, Fiore N, Williamson B. An endotoxininduced serum factor that causes necrosis of tumors. Proc. Natl. Acad. Sci. U. S. A. 1975;72:3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Trayhurn P, Wood IS. Adipokines: inflammation and the pleiotropic role of white adipose tissue. Br. J. Nutr. 2004;92:347–355. doi: 10.1079/bjn20041213. [DOI] [PubMed] [Google Scholar]
- 45.Gordon JI, Hooper LV, McNevin MS, Wong M, Bry L. Epithelial cell growth and differentiation. III. Promoting diversity in the intestine: conversations between the microflora, epithelium, and diffuse GALT. Am. J. Physiol. 1997;273:G565–G570. doi: 10.1152/ajpgi.1997.273.3.G565. [DOI] [PubMed] [Google Scholar]
- 46.Shu RZ, Zhang F, Wang F, Feng DC, Li XH, Ren WH, Wu XL, Yang X, Liao XD, Huang L, Wang ZG. Adiponectin deficiency impairs liver regeneration through attenuating STAT3 phosphorylation in mice. Lab. Invest. 2009;89:1043–1052. doi: 10.1038/labinvest.2009.63. [DOI] [PubMed] [Google Scholar]
- 47.Li Y, de Haar C, Chen M, Deuring J, Gerrits MM, Smits R, Xia B, Kuipers EJ, van der Woude CJ. Disease-related expression of the IL6/STAT3/SOCS3 signalling pathway in ulcerative colitis and ulcerative colitis-related carcinogenesis. Gut. 2010;59:227–235. doi: 10.1136/gut.2009.184176. [DOI] [PubMed] [Google Scholar]
- 48.Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat. Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sano H, Kawahito Y, Wilder RL, Hashiramoto A, Mukai S, Asai K, Kimura S, Kato H, Kondo M, Hla T. Expression of cyclooxygenase-1 and −2 in human colorectal cancer. Cancer Res. 1995;55:3785–3789. [PubMed] [Google Scholar]
- 50.Mikkelsen HB, Rumessen JJ, Qvortrup K. Prostaglandin H synthase immunoreactivity in human gut. An immunohistochemical study. Histochemistry. 1991;96:295–299. doi: 10.1007/BF00271349. [DOI] [PubMed] [Google Scholar]