Abstract
Purpose
This study aims to define the role of adiponectin (APN) in preventing goblet cell apoptosis and in differentiation of epithelial cells to goblet cell lineage resulting in greater mucus production and hence greater protection from chronic inflammation-induced colon cancer (CICC).
Methods
Six- to eight-week-old male APNKO and C57BL/6 (WT) mice were randomly distributed to three treatment groups: DSS, DMH, DSS+DMH and control. Chronic inflammation was induced in DSS and DSS+ DMH group by administrating 2 % DSS in drinking water for 5 days followed by 5 days of normal drinking water and this constitutes one DSS cycle. Three cycles of DSS were administered to induce chronic inflammation. Cancer was induced in both APNKO and WT mice in DMH and DSS+ DMH groups by intraperitoneal injections of DMH (20 mg/kg body weight) once for DSS+DMH group and once per week for 12 weeks for DMH group. On day 129, the colon tissue was dissected for mucus thickness measurements and for genomic studies. HT29-Cl.16E and Ls174T cells were used for several genomic and siRNA studies.
Results
APNKO mice have more tumors and tumor area in DSS+DMH group than WT mice. APN deficiency down-regulated goblet to epithelial cell ratio and enhanced the colonic mucosal erosion with reduced mucus thickness. APN increases Muc2 production with no affect on Muc1 production. APN abated goblet cell apoptosis, while APN deficiency reduced epithelial to goblet cell differentiation.
Conclusion
APN may be involved in reducing the severity of CICC by preventing goblet cell apoptosis and increasing epithelial to goblet cell differentiation.
Keywords: Mucus, Adiponectin, Colon cancer, Inflammation, Goblet cells
Introduction
Colorectal cancer (CRC) is globally the third most commonly diagnosed cancer [1]. Several epidemiological studies have linked long standing inflammatory bowel disease (IBD) with two- to threefold greater lifetime risk of developing colorectal cancer [2, 3]. The development of chronic inflammation-induced colon cancer (CICC) is thought to be multifaceted, and the repetitive cycles of inflammation, damage, increase rate of epithelial cell proliferation, and regeneration initiate and promote the process of CICC [3].
The intestinal homeostasis is a complex interplay of microbiota, intestinal epithelial cells (IECs), and the host immune system [4–7]. Mucus overlies epithelial cells of colon and acts as a physical barrier that prevents the invasion of colonic bacteria and thus inflammation [7]. The mucus-secreting goblet cells protect the epithelial layer, and the depletion of these cells is a common characteristic of multiple models of murine intestinal inflammation [5, 8], UC patients [9], and colon cancer [10]. The goblet cells are derived from the multipotent stem cells, and the Notch signaling pathway has been shown to play a major role in their differentiation [11]. The basic helix-loop-helix transcription factor Math1 and a transcriptional repressor Hairy and Enhancer of split type 1 protein (Hes-1) are important downstream molecules in the Notch signaling pathway, and their interplay may determine the onset of goblet cell differentiation [12].
The viscoelastic, polymer-like properties of mucus are derived from the major gel-forming glycoprotein components called mucins [13, 14]. Fifteen different mucin genes have so far been described of which Muc1, 2, 3, 12, 13, and 17 are found in the colon [15]. Muc2, the major colonic mucin [16, 17], creates a functional barrier between the host and the luminal contents and any alteration in this could act as a trigger for IBD [18, 19], particularly in UC patients [18].
Mucin has also been linked to colorectal cancer [20], and increased expression of Muc1 is associated with poor prognosis [21], while Muc2 is involved in the suppression of colorectal cancer [9, 22, 23] and has a protective role in IBD [4, 5, 7].
Mounting evidence indicates a positive association between adiposity and colon cancer [24, 25]. Obesity is a chronic sub-inflammatory condition and adipocytokines, biologically active substances produced by the adipocytes, are crucial players in the process of CICC [3, 26]. Obesity is also associated with a heightened inflammatory response in people with CD [27] and could result in a vicious cycle of localized inflammation that can expedite CICC.
Recent data suggest that the adipocytokine adiponectin (APN) has an important role in modulating the pathophysiology of the CICC. An inverse correlation has been established between APN plasma concentrations and body mass index [28] and with the risk of CICC [29]. The role of APN in carcinogenesis is not yet fully understood, but its anti-inflammatory, immune-modulatory, and insulin-sensitizing effects are considered to be beneficial [30]. Several clinical studies have shown lower APN levels being associated with colorectal carcinoma [31]. APNKO mice have greater and larger colon lesions as compared to C57BL/6 WT mice in chronic inflammation-induced colon cancer (CICC) model [3]. APN modulates p53 and Bcl-2 gene expression [32] and provides anti-carcinogenic effects, many of which are mediated through the AMP-activated protein kinase (AMPK) system via two receptors; the AdipoR1 and R2 [30].
Recent studies from our laboratory have shown that APN deficiency contributes to CICC [3]. In extension to our ongoing studies, this paper aims at understanding the role of APN in preventing the DSS-, DMH-, and DSS+DMH- induced mucus depletion. This study also aims at deciphering the role of APN in modulating Muc2 and its associated proteins. To achieve our goal, we used the human colonic goblet cell line HT29-Cl.16E [33], as this clonal derivative of HT29 displays a homogeneous and stable differentiated phenotype of mucus-secreting cells. It is a validated model for determining the regulation of mucin secretion [34]. We also used Ls174T cells, a well-established in vitro model to study mucus production [35]. In addition, the Muc gene expression profile of HT29-Cl.16E and Ls174T cells includes Muc1, Muc2 and Muc3, the major mucins expressed in the human colon [33, 36].
Materials and methods
Animals and experimental groups
Six- to eight-week-old male homozygous APNKO−/− and male C57BL/6 (WT) were housed in conventional animal room and treated in the animal facility at the University of South Carolina. The mice were on a 12:12 h light–dark cycle in a low stress environment (22 °C, 50 % humidity, and low noise) and had access to food (Purina Chow) and water ad libitum. All animal care followed institutional guidelines under a protocol approved by the Institutional Animal Care and Use Committee at the University of South Carolina. APNKO and WT mice were randomly assigned to four different groups of equal number (n=12 mice per group): (1) DMH+DSS; (2) DMH; (3) DSS, and (4) control or no treatment. The body weight of both APNKO and WT mice showed no significant difference at the beginning of the study.
Induction of chronic inflammation and cancer
Chronic inflammation was induced in mice assigned to the DSS treatment group. These mice received 2 % dextran sodium sulfate (DSS) (MP BIOCHEMICALS, MW: 36,000–50,000) dissolved in their drinking water for 5 days, followed by 5 days of regular drinking water; this represented single cycle and DSS was administrated for three cycles on day 8, 23, and 38. To establish chronic inflammation-induced colon cancer, another group of mice received three cycles of DSS and a single injection of DMH intraperitoneally (i.p) (SIGMA ALDRICH) (20 mg/kg body weight) administered at the beginning of the DSS treatment. Cancer was induced in mice assigned to DMH treatment group by administering weekly dose of DMH i.p. for 12 and the mice were sacrificed on day 129.
Tissue collection and tumor quantification
Mice colon were harvested and sectioned (2 mm2) to confirm the mouse histopathology of the entire group (6 mice/group). The remainder of the colons was stored at −80 °C for gene and protein expression studies. The dissected tissues were stained with 0.5 % methylene blue (SIGMA ALDRICH) to count tumors and measured tumor area using a stereomicroscope followed by sectioning for Alcian blue staining. Tumor quantification was done in blinded condition by two individuals.
Mucus thickness
Mucus thickness was measured with micropipettes connected to a micromanipulator (LEITZ) with a digimatic indicator (IDC SERIES 543, Mitutoyo). The degraded luminal mucus layer was removed. Glass tubing (borosilicate tubing with 1.2 mm OD and 0.6 mm ID; Frederick Haer) was pulled with a pipette puller (pp-83; NARISHIGE SCIENTIFIC) to a tip diameter of 1–3 µm and to prevent mucus adhering to glass, the pipettes were siliconized by dipping the tip of the micropipette into a silicone solution followed by drying at 100 °C for 30 min. The luminal surface of the mucus gel was visualized by placing graphite particles (activated charcoal, extra pure, Merck) on the gel, and the colonic epithelial cell surface was visible through the microscope. The micropipette was inserted into the mucus gel at an angle of ~30° (θ) to the surface. The distances traveled by the micropipette from the luminal surface of the mucus gel to the epithelial cell surface were measured with a digimatic indicator connected to the micromanipulator, and a mean value (A) was calculated. The mucus thickness (T) was calculated using the formula T=A (sin θ). Mean of four to five different measurements was taken as one thickness value.
Alcian blue staining
Standard deparaffinization procedure was followed using xylene and gradation of ethanol. Alcian blue solution (1 %) of pH 2.5 in 3 % acetic acid and nuclear fast red in aluminum sulfate was prepared. Tissues were stained with Alcian blue and counterstained with nuclear fast red solution. Goblet to epithelial cell ratio was counted per crypt with ten crypts per section and five sections per group.
Cell culture
HT29-Cl.16E and Ls174T cells (ATCC) were seeded on porous nitrocellulose filters (MILLIPORE filters HAHY, porosity 0.45 µm; 2×106 cells per filter) to provide improved access to basolateral membrane of cells [37]. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) (GIBCO) supplemented with 10 % (v/v) heat-inactivated fetal calf serum (FCS) (GIBCO). HT29-Cl.16E and Ls174T cells form at confluence homogeneous monolayer of differentiated goblet cells, secreting a mucus gel in the culture medium. HT29-Cl.16E and Ls174T cells (106/well) were incubated at 37 °C and 5 % CO2 in the presence of different dosages of recombinant APN (0, 0.25, 0.5, 1.0, 1.5, 2.0 µg/mL) in one experiment and in other experiment APN (2 µg/mL), TNF-α (10 ng/mL), and APN+TNF-α (PROSPEC) for 24 h.
Muc1 and Muc2 measurement
Muc1 and Muc2 production was measured in HT29-Cl.16E and Ls174T cells treated with different dosages of APN for 24 h and cell supernatant was collected. A parallel experiment that was undertaken with the goal to determine the spontaneous production of Muc1 and Muc2 at different time points in the colonic epithelial cells of APNKO (n=40) and WT (n=40) mice each treated with three cycles of DSS on day 8, 23, and 38. Mice were sacrificed at different time points after the start of third DSS cycle on day 38, 44, 54, and 62 (n=10 each time point). Colonic epithelial cells were scraped and cultured in DMEM supplemented with 10 % heat-inactivated FCS for 24 h. Muc1 and Muc2 production was measured both in colonic epithelial cells and in HT29-Cl.16E and Ls174T cell supernatant by ELISA (USCN LIFE SCIENCE) using standard protocol.
Genes knockdown using siRNA
Small interfering RNA (siRNA) (40 nM) targeting MUC2, Bax, APN R1 and R2 (QIAGEN), and Math-1 was transfected in HT29-Cl.16E and Ls174T cells (106) using lipofectamine 2000 reagent (Invitrogen) according to the manufacturer's instructions. Controls were transfected with unrelated siRNA (CsiRNA) (SANTA CRUZ, QIAGEN, and INVITROGEN). Reductions of cell-surface proteins were analyzed with flow cytometry. The efficacy of knockdowns was assessed by conventional semi-quantitative RT-PCR. Assays were performed 2 days after transfection.
Apoptosis detection by TUNEL
Terminal deoxynucleotidyl transferase (TdT)-mediated dUTP-biotin nick-end labeling (TUNEL) technique was used as per manufacturer instructions (R&D systems) to detect DNA strand breaks in situ. After treating cells with APN (2 µg/mL), TNF-α (10 ng/mL), and APN+TNF-α pelleted cells were rinsed with PBS and TUNEL staining procedure was performed. Cells were then counterstained with methyl green. Negative controls were performed by substituting PBS for TdT enzyme, which exhibited no immunostaining. Enumeration of apoptotic nuclei was made on ten slides per treatment, using a Zeiss light microscope with a 40× objective and a 10× eyepiece. All nuclei counted showing a brown labeling. The incidence of apoptotic nuclei was given as the percentage relative to total nuclei (apoptotic ratio). The data are representative of three repeated experiments, all displaying similar results.
Reverse transcription-polymerase chain reaction
Semi-quantitative reverse transcription-polymerase chain reaction (RT-PCR) was used to determine the efficacy of siRNA transfection and relative gene expression in HT29-Cl.16E and Ls174T cells and colonic epithelial cells. Total cellular RNA was isolated using TRIzol reagent (Invitrogen) according to the manufacturer's instructions. A total of 2.5 mg extracted RNA was used as the template for complementary DNA (cDNA) synthesis using the Thermoscript reverse transcription-polymerase chain reaction system (Invitrogen). Semi-quantitative PCR was performed for Muc2, Bax, APN R1 and R2, Hes-1, and Math-1 using the following primer pairs: MUC2 forward primer (FP) (5-GACATTTGTCAT GTACTCGGC-3) and reverse primer (RP) (5-GCAAGGACTGAACAA AGACTC-3), Bcl-2 (FP-59-GACTTCGCCGAGATGTCCAG-39 and RP-5-TCACTTG TGGCT CAGATAGG-3), Bax (FP-59-GGTTTCATCCAGGATCGAGACGG-3 and RP-5-ACAAAGATG GTCACGGTCTGCC-3), GAPDH (HT29-Cl.16E cells) (FP-5-GCAGGGGGGAGCCAAAAGGG-3 and RP- 5-TGCCAGCCCCAGCGTCAAAG-3), Hes1 (FP-CAGCCAGTGTCAACACG ACAC and RPTCGTTCATGCACTCGCTGAG), Math1 (FP-AGTGACGGAGAGTTTTCCCC and RP-CTGCAGCCGTCCGAAGTCAA), and GAPDH (colonic epithelial cells) (FP- GTCATCA TCTCCGCCCCTTCTGC and RP-GATGCCTGCTTCACCACC TTCTTG). The synthesized cDNA was amplified by RT-PCR assay; the PCR cycle consisted of 94 °C for 1 min, 56 °C for 2 min, and 72 °C for 1 min, with final extension at 72 °C for 10 min. Relative mRNA abundance of Math-1, hes-1, and ratio of Bax/Bcl-2 was calculated using semi-quantitative RT-PCR and Image J software (NCBI) for densitometry. GAPDH was used as a loading control and the gels were run thrice using different samples from the same group to calculate significant difference.
Statistical analysis
Two-way and one-way analysis of variance (ANOVA) was used to analyze the data with Tukey post hoc-analyses. A p value<0.05 was considered statistically significant. All the statistical analyses were done by using SigmaStat 3.5 (SPSS).
Results
Promotion of colorectal carcinogenesis in adiponectin-deficient mice
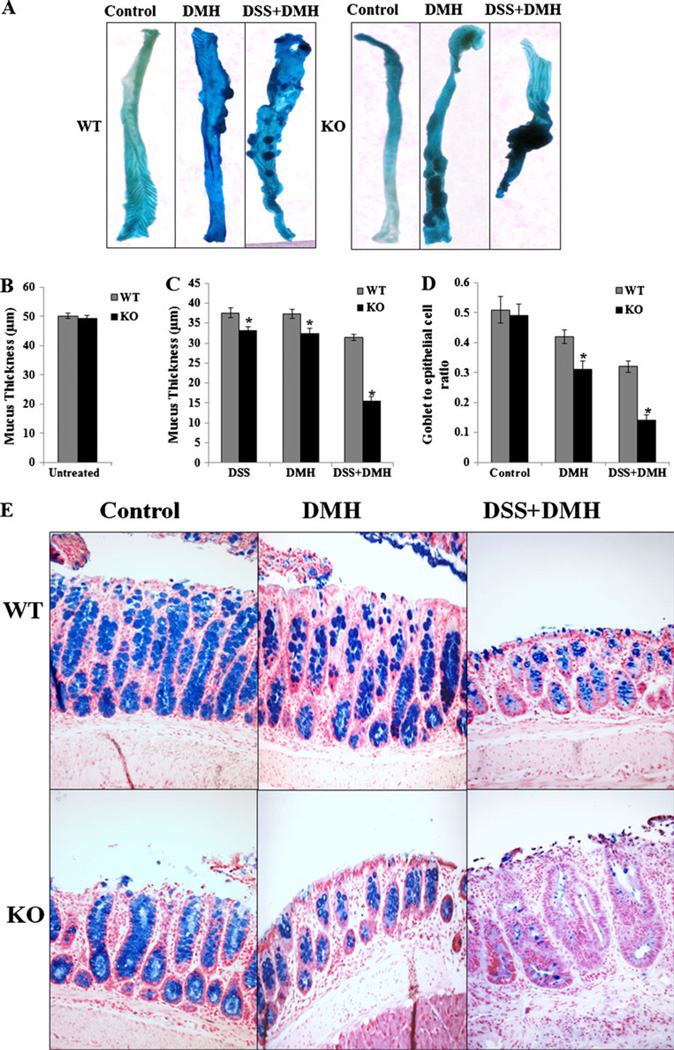
We investigated the role of APN in the progression of CICC. No morphological differences in the colon were observed between the WT and the APNKO control mice (Fig. 1a) and an administration of three cycles of DSS alone did not induce tumors in both WT and APNKO mice. However, APNKO and WT mice receiving DMH and DSS+DMH developed tumors (Fig. 1a), with greater tumor number and size in APNKO mice treated with DMH+DSS when compared to WT mice (Fig. 1a). These were similar to the results obtained in our previous study [3]. The tumors were observed mostly in the descending colon and the rectum. Moreover, shortening of the colon length (one of the macroscopic signs of colitis representing the severity of colitis) was more evident in DSS+DMH-treated APNKO mice when compared to WT mice (Fig. 1a).
Fig. 1.
Tumor incidence and decrease in mucus thickness and goblet to epithelial cell ratio with adiponectin deficiency in different treatment groups: a Representative of methylene blue-stained colonic tissues of WT and APNKO mice treated with DMH, DSS+DMH, and control group. Measurement of mucus thickness in b untreated, c DSS-, DMH-, and DSS+ DMH-treated APNKO and WT mice (n=10). d Graph representing goblet to epithelial cell ratio in control, DMH, and DSS+DMH groups. e Descending colon, 2 mm2 sections of the mice stained with Alcian blue dye representing goblet cells (blue). The data are representative of two independent experiments, all displaying similar results. *p<0.04 (APNKO versus WT in the same group)
APN deficiency enhances colonic mucosal erosions and reduces goblet to epithelial cell ratio
One of the consistent features of both IBD and CICC is the denudation of the mucus layer coating the gastrointestinal tract. To evaluate the role of APN in preventing DSS-, DMH-, and DSS+DMH-induced mucosal erosions; we measured the colon mucosal thickness as well as the goblet to epithelial cell ratio in all the treated groups as described by Petersson et al. [18]. No change in the mucus thickness was observed in untreated APNKO and WT mice (Fig. 1b). All DSS-, DMH-, and DSS+DMH-treated mice had a significant reduction in the mucus thickness as compared to untreated mice (Fig. 1c). APNKO mice treated with DSS, DMH, and DSS+DMH had significantly decreased (p< 0.04) mucus thickness when compared to WT mice in the same treatment group (Fig. 1c). APNKO mice treated with DSS+DMH had the lowest mucosal thickness compared to other groups. Additionally, when compared with the concurrent controls in the WT group, the number of goblet cells was reduced significantly in APNKO mice treated with DMH and DSS+DMH, thereby offering an explanation for the loss of mucus in these animals (Fig. 1d, e). The DSS-treated WT and APNKO mice did not show any difference in the goblet cell number (data not shown).
Effect of APN on in vitro and in vivo Muc2 production and TNF-α induced apoptosis
To elucidate the role of APN in the synthesis of Muc1 and Muc2 production, HT29-Cl.16E and Ls174T cells were cultured for 24 h with media containing various concentrations of APN. The results show a dose-dependent increase in the Muc2 production with an increase in the APN concentration when compared to untreated cells (Fig 2a), suggesting that APN has a direct role in Muc2 production by goblet cells. Similar results were found in Ls174T cells exposed to the same treatment (data not shown). Data also show that APN did not have any effect on Muc1 production (Fig. 2a).
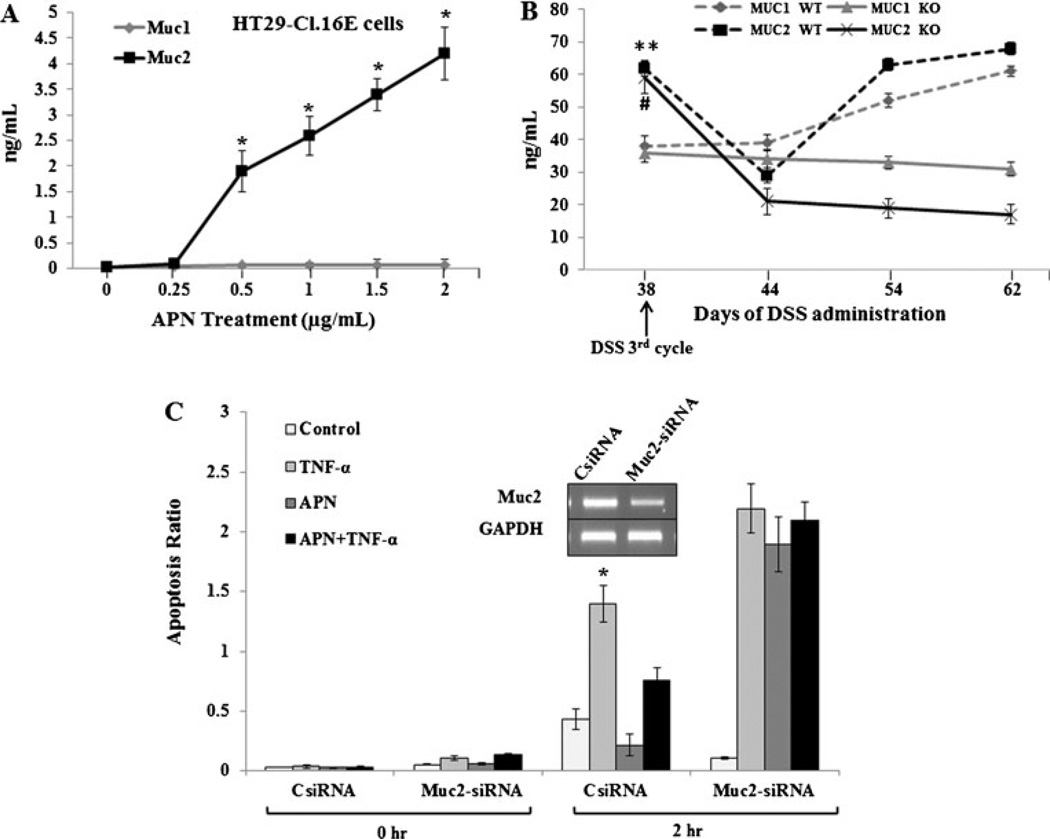
Fig. 2.
Effect of APN on Muc2 production and goblet cell apoptosis. a Graph representing the dose-dependent relationship of Muc1 and 2 productions (nanogram per milliliter) by HT29-CI.16E cells (106) and APN (microgram per milliliter) treatments. b Spontaneous Muc1 and Muc2 production from mucosal tissue obtained from WT and APNKO mice, respectively, and treated with DSS (third cycle). They were measured in different time points. c The gel picture represents the Muc2-siRNA knockdown in HT29-CI.16E cells (106). The cells with CsiRNA and Muc2-siRNA were treated with TNF-α (10 ng/mL) or globular APN (2 µg/mL) or both for 2 h (the peak effect of TNF-α in cell apoptosis), and the apoptosis ratio was determined using TUNEL assay. The data are representative of two independent experiments, all displaying similar results. *p< 0.01 (treated versus untreated cells, TNF-α versus APN+TNF-α at 2 h time point), #p<0.04 (Muc2 levels on day 38 versus 44 in WT mice). **p<0.05 (Muc2 levels on day 38 versus 44, 54, and 62 in KO mice)
To characterize the effect of APN deficiency on Muc1 and Muc2 production by the primary epithelial cells, APNKO and WT mice treated with three cycles of DSS were sacrificed at various time points. The colonic epithelial cells were collected, cultured for 24 h, and measured for Muc1 and Muc2 production by ELISA (Fig. 2b). Muc2 level was significantly reduced on day 44 and was recovered by day 54 and 62 in WT mice without any changes in Muc1 production. However, in APNKO mice, Muc2 level was reduced dramatically and its production was consistent by day 62, indicating that APNKO mice are prone to decreased Muc2 production or fewer goblet cells during and after chronic DSS administration (Fig. 2b).
APNKO mice treated with DSS, DMH, and DSS+DMH had lesser goblet cells as compared to WT mice, indicating that APN has an inhibitory role in goblet cell apoptosis. To validate this observation, the experiment was performed to assess the impact of APN (2 µg/mL) on TNF-α (10 ng/mL) induced apoptosis in cultured HT29-CI.16E and Ls174T cells. The results showed that supplementing the HT29-CI.16E and Ls174T cells for 2 h with media containing APN decreased apoptosis by almost half when compared to untreated controls (no APN or TNF- α). TNF-α increased apoptosis by almost three folds (Fig 2c); however, when these cells are co-treated with APN (APN+TNF-α), the goblet cell apoptosis is reduced, indicating that the APN inhibits TNF- α-induced apoptosis of the goblet cells (Fig. 2c). To test whether APN inhibition of apoptosis is Muc2 dependent, we knocked-down Muc2 expression (~60 % reduction) using Muc2-siRNA [38]. Muc2 knockdown resulted in a fourfold increase in the apoptosis in APN treated group and twofold increase in APN+TNF-α group as compared to siRNA controls (p<0.01) (Fig. 2c). APN plays an important role in inhibiting TNF-α induced apoptosis of goblet cells and is Muc2 mediated. Similar results were found in Ls174T cells exposed to the same treatment (data not shown).
APN inhibits TNF- α induced apoptosis of the goblet cell by modulating Bax/Bcl-2 through the activation of APN receptors
To analyze the Bax and Bcl-2 role in regulating TNF- α induced goblet cell apoptosis by APN, we quantified the Bax/Bcl-2 mRNA levels in cells treated with APN, TNF- α and APN+TNF-α. The results showed down-regulation of Bax expression with up-regulation of Bcl-2 in cells treated with APN alone (Fig. 3a-b). TNF-α treatment resulted in the up-regulation of Bax, while cells co-treated with APN (APN+TNF-α) reduced TNF-α induced up-regulation of Bax with increase in Bcl-2 expression (Fig. 3b).
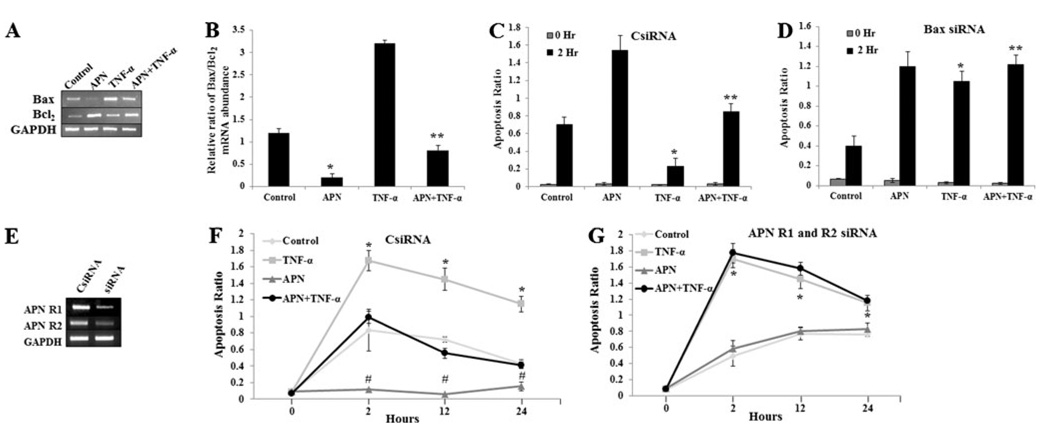
Fig. 3.
APN inhibits goblet cell apoptosis via Bax/Bcl-2 modulation and is APN R1 and R2 dependent. a and b Bax and Bcl-2 expressions were measured in HT29-Cl.16E treated with TNF-α (10ng/mL) or globular APN (2 µg/mL) or both and the ratio of their RNA abundance was calculated. c and d Graph showing Apoptosis ratio in CsiRNA and siRNA-Bax HT29-Cl.16E cells that were treated with TNF-α (10 ng/mL) or globular APN (2 µg/mL) or both. e Gel representing the APN R1 and R2 knocked-down using siRNA. f and g Graph representing Apoptosis ratio in CsiRNA and APN R1 and R2 siRNA injected HT29-Cl.16E cells treated with TNF-α (10 ng/mL) or globular APN (2 µg/mL) or both. The data are representative of two independent experiments, all displaying similar results. *p<0.01 (control versus APN treated), **p<0.03 (APN versus APN+TNF-α treated), #p< 0.04 (APN versus APN+TNF-α treated)
APN alone caused a reduction in Bax/Bcl-2 mRNA levels as compared to untreated controls (p<0.01); also, APN reduced TNF-α induced Bax/Bcl-2 mRNA expression when compared to cells treated with APN+TNF-α (p<0.04) (Fig. 3b). The apoptosis ratio was measured in cells with various treatments, and the results show that APN reduced TNF- α induced apoptosis ratio in control cells (p<0.03), but no significant difference was seen in Bax-depleted cells (siRNA) cells (p>0.05). However, Bax-depleted cells (Bax siRNA) have a higher apoptosis ratio in all treated groups compared to control (CsiRNA) cells (Fig. 3c, d), suggesting that APN inhibits goblet cell apoptosis via Bax/Bcl-2 modulation.
To evaluate the role APN R1 and R2 in mediating APN effect on goblet cell apoptosis by TNF- α, the HT29-Cl.16E and Ls174T cells were treated with APN, TNF-α, and APN+TNF-α in APN R1 and R2-depleted cells. The data show that APN is able to inhibit TNF-α apoptosis ratio in control (CsiRNA) cells (p<0.01) (Fig. 3e, f), while unable to do so in cells deficient of APN receptors APN R1 and R2 (Fig. 3g). Thus, it appears that APN may selectively inhibit TNF-α goblet cell apoptosis through adiponectin receptors.
APN deficiency modulates genes responsible for goblet cell differentiation
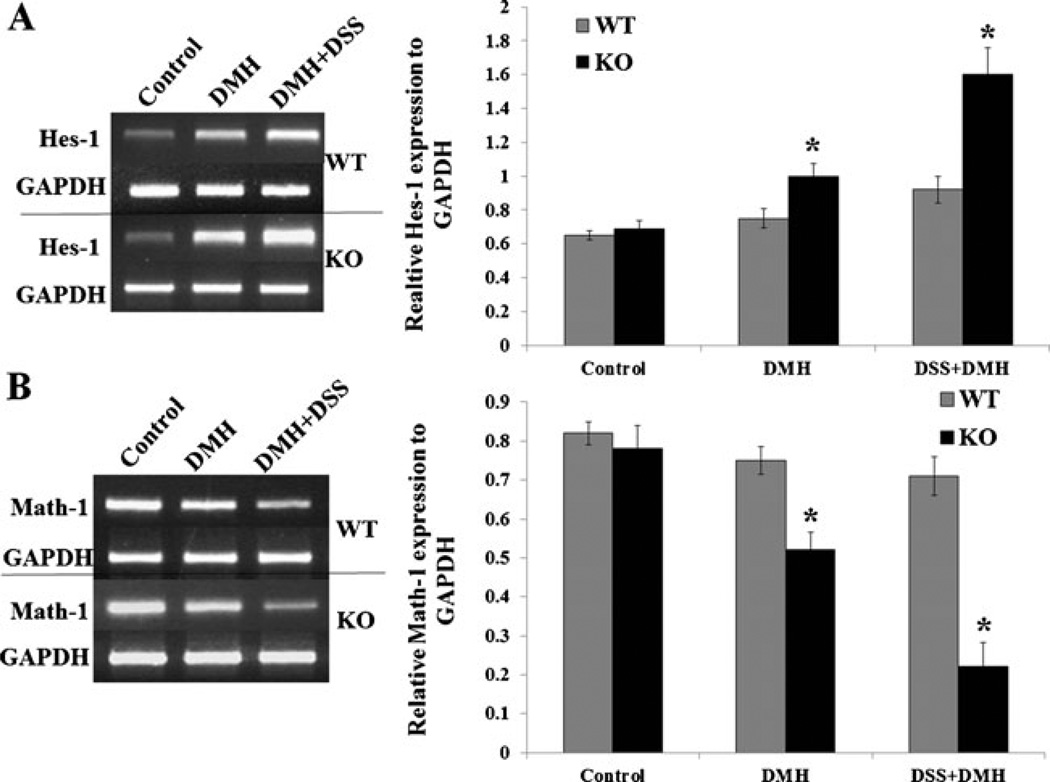
Hes-1 and Math-1 genes play an important role in epithelial to goblet cell differentiation. We evaluated these genes expression in the colonic mucosa of WT and APNKO mice treated with DSS, DMH, and DSS+DMH. Hes-1 gene expression is upregulated in APN deficient mice significantly (Fig. 4a), with concomitant reduction in the expression of Math-1 gene (Fig. 4b) in DMH- and DSS+DMH-treated group (p<0.04), as compared to WT mice, while DSS-treated (data not shown) and -untreated groups did not show any difference in the expression of Hes-1 and Math-1 genes. These results indicate that epithelial cells of the APN deficient mice express genes that reduce epithelial cell differentiation to goblet cells.
Fig. 4.
APNKO mediated alteration in the gene expression leading to the reduction of epithelial to goblet cell differentiation. a Graph and the gel picture showing the relative Hes-1 expression to GAPDH in the non-tumor colonic tissue of WT and APNKO mice treated with DMH and DSS+DMH and control group. b Graph and the gel picture showing the relative Math-1 expression to GAPDH in the non-tumor colonic tissue of WT and APNKO mice treated with DMH and DSS+DMH and control group (n=5 mice/group). *p<0.04 (APNKO versus WT in the same group)
Increase in Muc2 by APN is Math-1 dependent and mediated by APN receptors
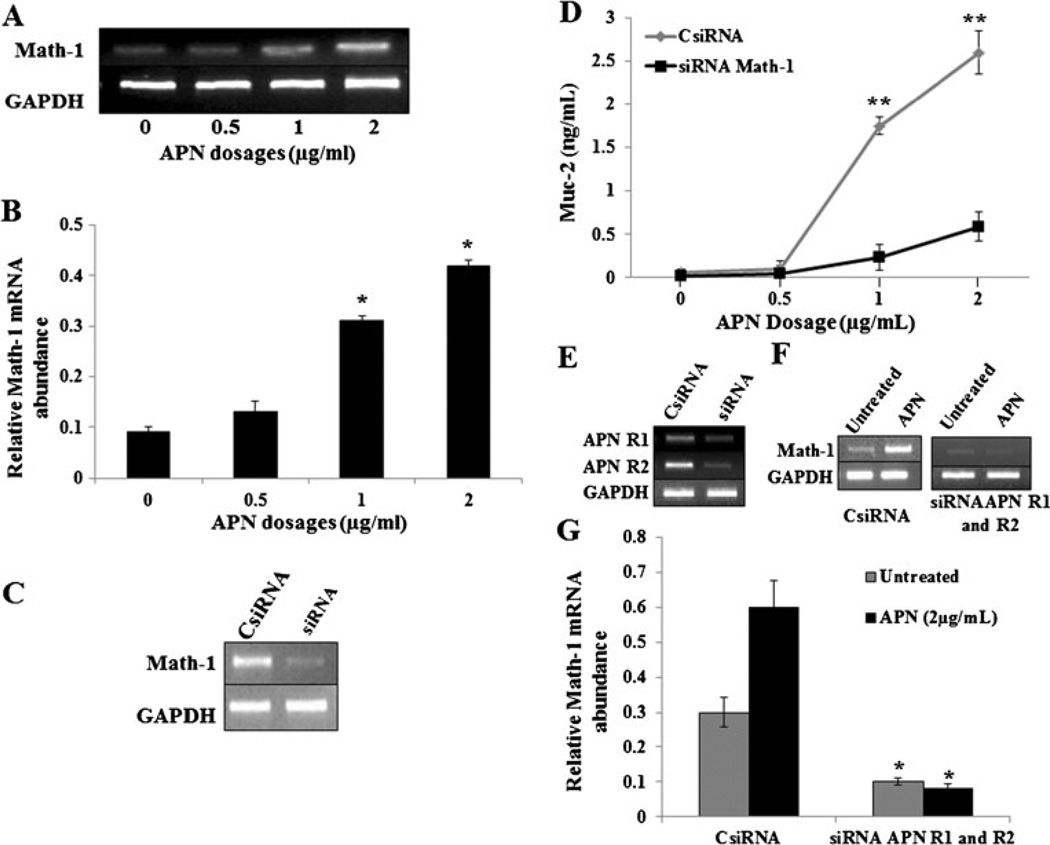
In our previous experiments, it has been shown that APN induces Math-1 expression in vivo. We detected Math-1 expression in HT29-Cl.16E and Ls174T cells treated with various concentrations of APN. The results indicate a dose-dependent increase in the Math-1 expression with an increase in APN concentration (Fig. 5a, b). In order to analyze if Math-1 gene is responsible for APN induced Muc-2 production, various concentrations of APN are treated with Math-1-deficient (by knocking down with Math-1 siRNA) cells. The results show a dose-dependent increase in Muc-2 production with an increase in APN concentration. There was a significant reduction in Muc-2 production in cells deficient of Math-1 gene (Math-1 siRNA), when treated with various concentrations of APN, as compared with control (CsiRNA) group (p<0.01) (Fig. 5c, d), suggesting that APN induced Muc-2 production, is mediated by Math-1 gene expression.
Fig. 5.
APN induces Math-1 expression and an increase of Muc2 is Math-1 dependent which requires APN receptors. a and b Relative Math-1 mRNA abundance in HT29-CI.16E cells (106/ml) incubated with different doses of APN (0, 0.5, 1, and 2 µg/mL). c Gel representing knockdown Math-1 expression using Math-1-siRNA. d Graph showing APN dose-dependent (microgram per milliliter) Muc2 production (nanogram per milliliter) in HT29-CI.16E cells (106/ml) treated with CsiRNA and math-1 siRNA. e Gel showing knockdown expression of APN R1 and R2 receptors. f and g Math-1 expression in HT29-CI.16E cells CsiRNA and siRNA APN R1 and R2 and treated with APN (2 µg/mL). The data are representative of two independent experiments, all displaying similar results. *p<0.01 (0 vs. 1 and 2 µg/mL APN dosage and CsiRNA and siRNA APN R1 and R2 given the same treatment), **p<0.01 (CsiRNA vs. Math-1 siRNA with same dosage)
APN R1 and R2 were knocked down in HT29-Cl.16E (Fig. 5e) and Ls174T cells (data not shown) and were treated with APN and measured for Math-1 expression. The results show a significant reduction in the Math-1 expression in APN R1 and R2 deficient cells compared to control (CsiRNA) cells (p<0.02) (Fig. 5f, g), suggesting that APN R1 and R2 activation is required for Math-1 gene expression.
Discussion
Obesity and IBD are known factors that induce chronic inflammation and contribute to the colorectal carcinogenesis [2]. Deregulation of adipose tissue-derived adipokines may be directly involved in obesity-related carcinogenesis [30]. Adiponectin is arguably one of the highly investigated adipokine as its anti-inflammatory and insulin-sensitizing effects are reported to be beneficial in various health conditions including cancer [30]. The concentration of APN in plasma is reduced in obesity [28], and an inverse relation between serum levels of adiponectin and risk of colorectal cancer has been established [29, 31, 39].
Chronic colonic inflammation is a prerequisite for colitis-associated colorectal carcinogenesis and our earlier studies have shown that APN deficiency contributes to inflammation-induced colon cancer [3]. The present study plans to investigate the role of APN and goblet cells in preventing CICC by emphasizing on the mechanism/s responsible for the protective effects.
Tumor development was found to be similar in all the treatment groups when compared to our previous study [3], with significantly greater number of tumor and tumor size in APNKO mice treated with DSS+DMH in comparison to WT mice indicating that the absence of APN made the mice more vulnerable to DSS induced colitis and DMH-induced colon cancer. This could be explained by the apoptotic effect of APN on tumor cells and decreased neovascularization in T241 mice fibrosarcoma [40]. It has also been found to decrease azoxymethane-induced intestinal carcinogenesis in Apcmin/+ and WT mice [41]. It is quite possible that APN mediates the protective effects possibly by inhibiting chemokine production in intestinal epithelial cells and the following inflammatory responses, including infiltration of macrophages and release of proinflammatory cytokines [3, 42].
The mucus layer has a crucial role in intestinal homeostasis and act as a hallmark of human IBD, particularly UC and in mice lacking the major mucin protein Muc2 that develop spontaneous colitis [43]. The mucus layer produced by the goblet cells is part of the innate immunity and forms a physical barrier against mechanical and chemical insults [5, 8, 44]. We therefore detected the role of APN deficiency in mucin depletion by quantifying mucus thickness in both APNKO and WT mice treated with DSS, DMH, and DSS+DMH. The results indicate significant reduction in mucus thickness in both APNKO and WT mice, which are in agreement with earlier reports for IBD [18, 19, 45] and CICC [46]. The depletion of mucus was significantly pronounced in the APNKO mice, indicating that APN could directly or indirectly modulate mucus production. We also a found a significant reduction in the goblet to epithelial cell ratio in the APNKO mice treated with DMH alone and DSS+DMH when compared to WT counterparts. Therefore, a positive correlation is achieved between APN deficiency and decrease mucus production with a concomitant decrease in goblet cells.
The mucus composed of secretory (Muc2, Mic5AC, Muc5B, and Muc6) and transmembrane proteins (Muc1, Muc3A, Muc3B, Muc4, Muc12, Muc17) that form a semi-permeable barrier between the intestinal lumen and the underlying epithelium [9, 47]. Several lines of evidence point towards a biological role of mucin in preventing colorectal cancer [48] with Muc2 being an active player [22]. Muc2 may act as a trigger for intestinal tumorigenesis as Muc2 deficient mice develop small and large intestinal and rectal tumors [22, 49]. Experiments with cultured HT29-Cl.16E and Ls174T cells have clearly shown that APN increased the synthesis of Muc2, but not of Muc1. Similar results were obtained in the animal model with reduced Muc2 production in APNKO mice when compared to WT mice. Altered Muc2 and Muc1 expression is a hallmark of IBD and colon cancer, and we provided substantial evidence that APN may participate in modulating the expression of these two proteins and might contribute to the reduction in the symptoms associated with CICC.
To further explore the protective role of APN, we hypothesized that APN inhibition of goblet cell apoptosis is Muc2 dependent. To validate this hypothesis, Muc2 expression was knocked down (~60 % reduction) using siRNA [38], and the goblet cells (siRNA against Muc2 and controls) were investigated for the anti-apoptotic effects of APN. We observed that Muc2 knockdown resulted in a significant increase in apoptotic ratio in all the treatment groups with a fourfold increase in APN treated group and twice in APN+TNF-α group when compared to CsiRNA treated cells, thereby strengthening our hypothesis.
To further explore the mechanism of APN-mediated goblet cell protection, we calculated the relative ratio of Bax (pro-apoptotic) and Bcl-2 (anti-apoptotic) mRNA abundance in all the treatment groups (untreated, APN, TNF-α, and APN+TNF-α) and found that administration of APN decreased Bax, but increased Bcl-2 expression and attenuated the pro-apoptotic effect of TNF-α. Additionally, using siRNA-Bax, we were able to observe that APN neither reduced apoptosis nor inhibited the pro-apoptotic effect of TNF-α in partially depleted Bax (siRNA) in HT29-Cl.16E and Ls174T cells (Fig. 5c), which indicates that APN reduces goblet cell apoptosis by regulating Bax/Bcl-2 ratio. To the best of our knowledge, this is the first study that demonstrates APN inhibiting goblet cell apoptosis via Bax/Bcl-2 modulation.
Adiponectin has been shown to exert some of its protective effects through adiponectin receptors (AdipoR1 and AdipoR2) [50, 51]. The up-regulation of both adiponectin receptors in tumor cells may be a cellular response to lower circulating adiponectin levels in patients with colorectal cancer and/or a compensatory response of their malignant cells [39]. To examine whether the anti-apoptotic effect of APN in goblet cells is mediated through adiponectin receptors, we repressed the expression of AdipoR1 or AdipoR2 via siRNA technique. The results indicated that the presence of APN treatment decreased apoptosis ratio in CsiRNA HT29-Cl.16E and Ls174T cells treated with TNF-α, but no change in the apoptotic ratio was observed in the cells deficient in APN R1 and R2. This study provides conclusive evidence that APN may prevent goblet cell apoptosis through the activation of its two receptors R1 and R2 and its downstream effecters, which could further modulate Bax/Bcl-2 ratio and up-regulate Muc2 expression.
Notch signaling pathway is essential in regulating the differentiation of colonic goblet cells and stem cells/progenitor cells [52, 53]. Canonical activation of the Notch signaling leads to Hes1 up-regulation and Atoh1/Hath1/Math1 down-regulation [10]. Suppression of Notch signaling by depletion of Hes-1 was associated with a significant increase in the secretory lineage of intestinal epithelial cells and vice versa [54, 55]. The Hes family of genes functions as transcriptional repressors of further downstream targets like Math-1, which defines the secretory epithelial lineages [56]. Recent studies also indicate that Math1 possess tumor suppressor function in colorectal neoplasia and its function is lost in some patients with colorectal cancer [57].
To further explore the role of APN in goblet cell differentiation, we quantified the expression of Hes-1 and Math-1. We found a significant increase in the expression of Hes-1 and decrease Math-1 expression in mice treated with DMH alone and DSS+DMH when compared to WT counterpart. We further pursue this path by studying the expression of Math-1 with the exogenous APN dose response in HT-29-C1.16 E and Ls174T cells and found an increase in the Math-1 mRNA expression with increasing APN dosage. This was followed by another set of experiments where we demonstrated that the expression of Muc2 by the goblet cell is Math-1 dependent, which could be further regulated by APN. It was also found that the expression of Math-1 is dependent on the presence of APN receptors R1 and R2, which was achieved by silencing receptors expression and quantifying Math-1 mRNA abundance in CsiRNA and siRNA APN R1 and R2-treated groups. These results clearly demonstrate that APN is governing the overall expression of both Muc2 and Math-1 in a dose-dependent manner in the presence of both APN R1 and R2 receptors and directing the pathway of intestinal cell differentiation towards the formation of goblet cells, which secrete mucus to form a protective layer to prevent colonic epithelial cells from luminal factors that may induce inflammation and cancer.
Conclusion
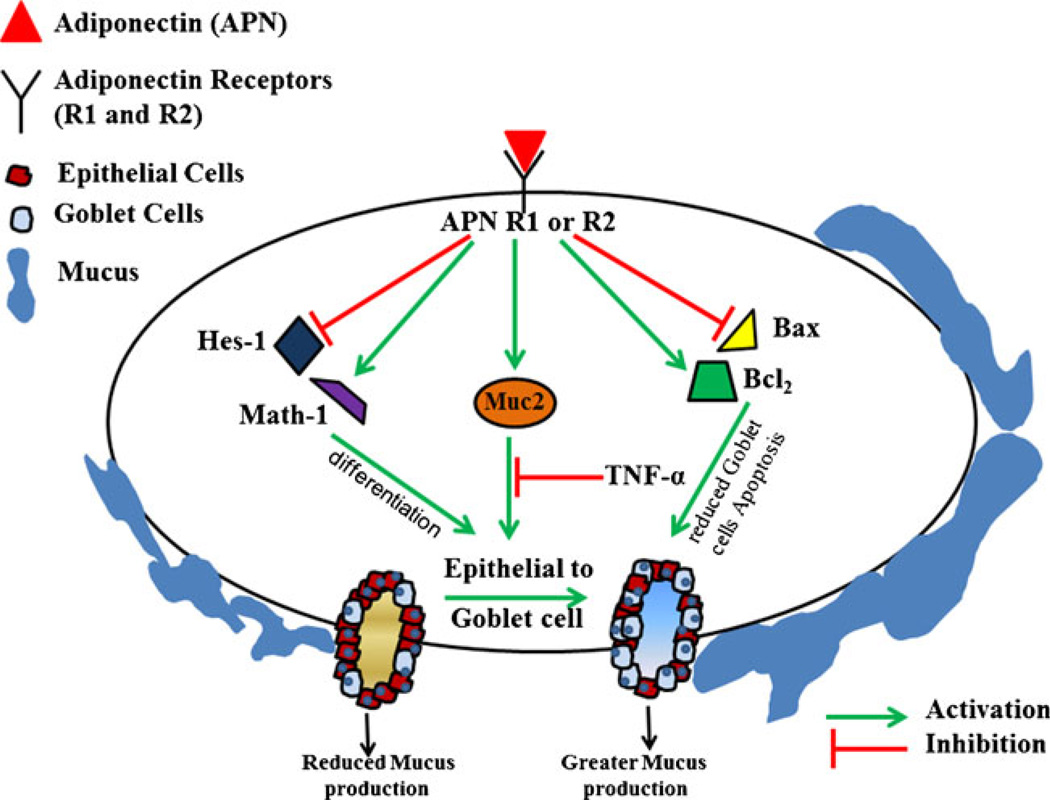
By the means of this study, we have established the protective role of APN and its receptors in CICC that is depicted in our hypothetical model (Fig. 6). APN can down-regulate the expression of Hex-1 with the concomitant up-regulation of Math-1 expression leading to the differentiation of epithelial cell to goblet lineage. Also, APN can modulate the ratio of Bax and Bcl-2, which could be a contributing factor in reducing goblet cell apoptosis, which is Muc2 dependent. APN has also been shown to increase the production of Muc2, which is dependent on Math-1. All these data point towards the common observation of the protective role of APN in reducing the severity of CICC.
Fig. 6.
A hypothetical model showing the effect of APN on epithelial to goblet cell differentiation, goblet cell apoptosis, and Muc-2 production. In CICC, APN may play a protective role by reducing the expression of Hes-1 and Bax with a concomitant increase in the production of Math-1 and Bcl-2 leading to greater epithelial to goblet differentiation and reduction in goblet cell apoptosis. APN also increases the expression of Muc-2 leading which affects goblet cell differentiation. All the above pathways lead to an increase in goblet cell number and hence greater mucus secretion. This enhances the protection from luminal factors that invade the colon epithelium and hence reducing the incidence of CICC
Although this study provided evidences of the protective role of APN in CICC, reconstituting APN in the same model system is crucial in defining the role of APN in mucus production. This will be the framework for our future studies.
Acknowledgment
We would like to acknowledge Dr. Bob Price, Medical School, University of South Carolina for technical support with the microscope and other instrumentation. This work was funded and supported by Grant P20 RR-017698, the National Center for Research Resources, Center for Colon Cancer Research, Center of Biomedical Research Excellence (COBRE) Program, University of South Carolina, Columbia SC.
Footnotes
Conflict of interest None.
Contributor Information
Arpit Saxena, Center for Colon Cancer Research, University of South Carolina, Columbia, SC 29208, USA.
Manjeshwar Shrinath Baliga, Department of Research, Father Muller Medical College, Kankanady, Mangalore, Karnataka 575003, India.
Venkatesh Ponemone, Fortis-Totipotent RX Centre for Cellular Medicine, Delhi, India.
Kamaljeet Kaur, Center for Colon Cancer Research, University of South Carolina, Columbia, SC 29208, USA.
Bianca Larsen, Center for Colon Cancer Research, University of South Carolina, Columbia, SC 29208, USA.
Emma Fletcher, Center for Colon Cancer Research, University of South Carolina, Columbia, SC 29208, USA.
Jennifer Greene, Center for Colon Cancer Research, University of South Carolina, Columbia, SC 29208, USA.
Raja Fayad, Email: rfayad@sc.edu, Center for Colon Cancer Research, University of South Carolina, Columbia, SC 29208, USA; Arnold School of Public Health, Applied Physiology Division, University of South Carolina, 921 Assembly St. room 403A, Columbia, SC 29208, USA.
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA: a cancer journal for clinicians. 2011;61(2):69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Hartnett L, Egan LJ. Inflammation, DNA methylation and colitis-associated cancer. Carcinogenesis. 2012;33(4):723–731. doi: 10.1093/carcin/bgs006. [DOI] [PubMed] [Google Scholar]
- 3.Saxena A, Chumanevich A, Fletcher E, Larsen B, Lattwein K, Kaur K, Fayad R. Adiponectin deficiency: role in chronic inflammation induced colon cancer. Biochim Biophys Acta. 2012;1822(4):527–536. doi: 10.1016/j.bbadis.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Artis D. Epithelial-cell recognition of commensal bacteria and maintenance of immune homeostasis in the gut. Nat Rev Immunol. 2008;8(6):411–420. doi: 10.1038/nri2316. [DOI] [PubMed] [Google Scholar]
- 5.Hooper LV, Macpherson AJ. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10(3):159–169. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 6.Kaser A, Zeissig S, Blumberg RS. Genes and environment: how will our concepts on the pathophysiology of IBD develop in the future? Dig Dis. 2010;28(3):395–405. doi: 10.1159/000320393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kaser A, Zeissig S, Blumberg RS. Inflammatory bowel disease. Annu Rev Immunol. 2010;28:573–621. doi: 10.1146/annurev-immunol-030409-101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Specian RD, Oliver MG. Functional biology of intestinal goblet cells. Am J Physiol. 1991;260(2 Pt 1):C183–C193. doi: 10.1152/ajpcell.1991.260.2.C183. [DOI] [PubMed] [Google Scholar]
- 9.Sheng XZ, Xu GJ, Tang XQ, Zhan WB. Monoclonal antibodies recognizing mucus immunoglobulin and surface immunoglobulin-positive cells of flounder (Paralichthys olivaceus) Vet Immunol Immunopathol. 2012;145(1–2):143–150. doi: 10.1016/j.vetimm.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Leow CC, Polakis P, Gao WQ. A role for Hath1, a bHLH transcription factor, in colon adenocarcinoma. Ann N Y Acad Sci. 2005;1059:174–183. doi: 10.1196/annals.1339.048. [DOI] [PubMed] [Google Scholar]
- 11.Yang Q, Bermingham NA, Finegold MJ, Zoghbi HY. Requirement of Math1 for secretory cell lineage commitment in the mouse intestine. Science. 2001;294(5549):2155–2158. doi: 10.1126/science.1065718. [DOI] [PubMed] [Google Scholar]
- 12.Wong MH. Regulation of intestinal stem cells. J Investig Dermatol Symp Proc. 2004;9(3):224–228. doi: 10.1111/j.1087-0024.2004.09304.x. [DOI] [PubMed] [Google Scholar]
- 13.Lang T, Hansson GC, Samuelsson T. Gel-forming mucins appeared early in metazoan evolution. Proc Natl Acad Sci U S A. 2007;104(41):16209–16214. doi: 10.1073/pnas.0705984104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deplancke B, Gaskins HR. Microbial modulation of innate defense: goblet cells and the intestinal mucus layer. Am J Clin Nutr. 2001;73(6):1131S–1141S. doi: 10.1093/ajcn/73.6.1131S. [DOI] [PubMed] [Google Scholar]
- 15.Saeki N, Saito A, Choi IJ, Matsuo K, Ohnami S, Totsuka H, Chiku S, Kuchiba A, Lee YS, Yoon KA, Kook MC, Park SR, Kim YW, Tanaka H, Tajima K, Hirose H, Tanioka F, Matsuno Y, Sugimura H, Kato S, Nakamura T, Nishina T, Yasui W, Aoyagi K, Sasaki H, Yanagihara K, Katai H, Shimoda T, Yoshida T, Nakamura Y, Hirohashi S, Sakamoto H. A functional single nucleotide polymorphism in mucin 1, at chromosome 1q22, determines susceptibility to diffuse-type gastric cancer. Gastroenterology. 2011;140(3):892–902. doi: 10.1053/j.gastro.2010.10.058. [DOI] [PubMed] [Google Scholar]
- 16.Tytgat KM, Buller HA, Opdam FJ, Kim YS, Einerhand AW, Dekker J. Biosynthesis of human colonic mucin: Muc2 is the prominent secretory mucin. Gastroenterology. 1994;107(5):1352–1363. doi: 10.1016/0016-5085(94)90537-1. [DOI] [PubMed] [Google Scholar]
- 17.Van Klinken BJ, Dekker J, Buller HA, de Bolos C, Einerhand AW. Biosynthesis of mucins (MUC2-6) along the longitudinal axis of the human gastrointestinal tract. Am J Physiol. 1997;273(2 Pt 1):G296–G302. doi: 10.1152/ajpgi.1997.273.2.G296. [DOI] [PubMed] [Google Scholar]
- 18.Petersson J, Schreiber O, Hansson GC, Gendler SJ, Velcich A, Lundberg JO, Roos S, Holm L, Phillipson M. Importance and regulation of the colonic mucus barrier in a mouse model of colitis. Am J Physiol Gastrointest Liver Physiol. 2011;300(2):G327–G333. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johansson ME, Gustafsson JK, Sjoberg KE, Petersson J, Holm L, Sjovall H, Hansson GC. Bacteria penetrate the inner mucus layer before inflammation in the dextran sulfate colitis model. PLoS One. 2010;5(8):e12238. doi: 10.1371/journal.pone.0012238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niv Y, Byrd JC, Ho SB, Dahiya R, Kim YS. Mucin synthesis and secretion in relation to spontaneous differentiation of colon cancer cells in vitro. Int J Cancer. 1992;50(1):147–152. doi: 10.1002/ijc.2910500129. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed FE. Colon cancer: prevalence, screening, gene expression and mutation, and risk factors and assessment. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2003;21(2):65–131. doi: 10.1081/GNC-120026233. [DOI] [PubMed] [Google Scholar]
- 22.Velcich A, Yang W, Heyer J, Fragale A, Nicholas C, Viani S, Kucherlapati R, Lipkin M, Yang K, Augenlicht L. Colorectal cancer in mice genetically deficient in the mucin Muc2. Science. 2002;295(5560):1726–1729. doi: 10.1126/science.1069094. [DOI] [PubMed] [Google Scholar]
- 23.Van der Sluis M, De Koning BA, De Bruijn AC, Velcich A, Meijerink JP, Van Goudoever JB, Buller HA, Dekker J, Van Seuningen I, Renes IB, Einerhand AW. Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology. 2006;131(1):117–129. doi: 10.1053/j.gastro.2006.04.020. [DOI] [PubMed] [Google Scholar]
- 24.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371(9612):569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 25.Paz-Filho G, Lim EL, Wong ML, Licinio J. Associations between adipokines and obesity-related cancer. Front Biosci. 2011;16:1634–1650. doi: 10.2741/3810. [DOI] [PubMed] [Google Scholar]
- 26.Barb D, Williams CJ, Neuwirth AK, Mantzoros CS. Adiponectin in relation to malignancies: a review of existing basic research and clinical evidence. Am J Clin Nutr. 2007;86(3):s858–s866. doi: 10.1093/ajcn/86.3.858S. [DOI] [PubMed] [Google Scholar]
- 27.Blain A, Cattan S, Beaugerie L, Carbonnel F, Gendre JP, Cosnes J. Crohn's disease clinical course and severity in obese patients. Clin Nutr. 2002;21(1):51–57. doi: 10.1054/clnu.2001.0503. [DOI] [PubMed] [Google Scholar]
- 28.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, Hotta K, Shimomura I, Nakamura T, Miyaoka K, Kuriyama H, Nishida M, Yamashita S, Okubo K, Matsubara K, Muraguchi M, Ohmoto Y, Funahashi T, Matsuzawa Y. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257(1):79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 29.Wei EK, Giovannucci E, Fuchs CS, Willett WC, Mantzoros CS. Low plasma adiponectin levels and risk of colorectal cancer in men: a prospective study. Journal of the National Cancer Institute. 2005;97(22):1688–1694. doi: 10.1093/jnci/dji376. [DOI] [PubMed] [Google Scholar]
- 30.van Kruijsdijk RC, van der Wall E, Visseren FL. Obesity and cancer: the role of dysfunctional adipose tissue. Cancer Epidemiol Biomarkers Prev. 2009;18(10):2569–2578. doi: 10.1158/1055-9965.EPI-09-0372. [DOI] [PubMed] [Google Scholar]
- 31.Otake S, Takeda H, Suzuki Y, Fukui T, Watanabe S, Ishihama K, Saito T, Togashi H, Nakamura T, Matsuzawa Y, Kawata S. Association of visceral fat accumulation and plasma adiponectin with colorectal adenoma: evidence for participation of insulin resistance. Clin Cancer Res. 2005;11(10):3642–3646. doi: 10.1158/1078-0432.CCR-04-1868. [DOI] [PubMed] [Google Scholar]
- 32.Mistry T, Digby JE, Desai KM, Randeva HS. Leptin and adiponectin interact in the regulation of prostate cancer cell growth via modulation of p53 and bcl-2 expression. BJU Int. 2008;101(10):1317–1322. doi: 10.1111/j.1464-410X.2008.07512.x. [DOI] [PubMed] [Google Scholar]
- 33.Augeron C, Laboisse CL. Emergence of permanently differentiated cell clones in a human colonic cancer cell line in culture after treatment with sodium butyrate. Cancer Res. 1984;44(9):3961–3969. [PubMed] [Google Scholar]
- 34.Gaudier E, Forestier L, Gouyer V, Huet G, Julien R, Hoebler C. Butyrate regulation of glycosylation-related gene expression: evidence for galectin-1 upregulation in human intestinal epithelial goblet cells. Biochem Biophys Res Commun. 2004;325(3):1044–1051. doi: 10.1016/j.bbrc.2004.10.141. [DOI] [PubMed] [Google Scholar]
- 35.Wright DH, Ford-Hutchinson AW, Chadee K, Metters KM. The human prostanoid DP receptor stimulates mucin secretion in LS174T cells. Br J Pharmacol. 2000;131(8):1537–1545. doi: 10.1038/sj.bjp.0703688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gaudier E, Jarry A, Blottiere HM, de Coppet P, Buisine MP, Aubert JP, Laboisse C, Cherbut C, Hoebler C. Butyrate specifically modulates MUC gene expression in intestinal epithelial goblet cells deprived of glucose. Am J Physiol Gastrointest Liver Physiol. 2004;287(6):G1168–G1174. doi: 10.1152/ajpgi.00219.2004. [DOI] [PubMed] [Google Scholar]
- 37.Augeron C, Voisin T, Maoret JJ, Berthon B, Laburthe M, Laboisse CL. Neurotensin and neuromedin N stimulate mucin output from human goblet cells (Cl.16E) via neurotensin receptors. Am J Physiol. 1992;262(3 Pt 1):G470–G476. doi: 10.1152/ajpgi.1992.262.3.G470. [DOI] [PubMed] [Google Scholar]
- 38.Tai EK, Wong HP, Lam EK, Wu WK, Yu L, Koo MW, Cho CH. Cathelicidin stimulates colonic mucus synthesis by up-regulating MUC1 and MUC2 expression through a mitogen-activated protein kinase pathway. J Cell Biochem. 2008;104(1):251–258. doi: 10.1002/jcb.21615. [DOI] [PubMed] [Google Scholar]
- 39.Williams CJ, Mitsiades N, Sozopoulos E, Hsi A, Wolk A, Nifli AP, Tseleni-Balafouta S, Mantzoros CS. Adiponectin receptor expression is elevated in colorectal carcinomas but not in gastrointestinal stromal tumors. Endocrine-related cancer. 2008;15(1):289–299. doi: 10.1677/ERC-07-0197. [DOI] [PubMed] [Google Scholar]
- 40.Brakenhielm E, Veitonmaki N, Cao R, Kihara S, Matsuzawa Y, Zhivotovsky B, Funahashi T, Cao Y. Adiponectin-induced antiangiogenesis and antitumor activity involve caspase-mediated endothelial cell apoptosis. Proc Natl Acad Sci U S A. 2004;101(8):2476–2481. doi: 10.1073/pnas.0308671100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mutoh M, Teraoka N, Takasu S, Takahashi M, Onuma K, Yamamoto M, Kubota N, Iseki T, Kadowaki T, Sugimura T, Wakabayashi K. Loss of adiponectin promotes intestinal carcinogenesis in Min and wild-type mice. Gastroenterology. 2011;140(7):2000–2008. 2008, e2001–e2002. doi: 10.1053/j.gastro.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 42.Nishihara T, Matsuda M, Araki H, Oshima K, Kihara S, Funahashi T, Shimomura I. Effect of adiponectin on murine colitis induced by dextran sulfate sodium. Gastroenterology. 2006;131(3):853–861. doi: 10.1053/j.gastro.2006.06.015. [DOI] [PubMed] [Google Scholar]
- 43.Maloy KJ, Powrie F. Intestinal homeostasis and its breakdown in inflammatory bowel disease. Nature. 2011;474(7351):298–306. doi: 10.1038/nature10208. [DOI] [PubMed] [Google Scholar]
- 44.Hollingsworth MA, Swanson BJ. Mucins in cancer: protection and control of the cell surface. Nat Rev Cancer. 2004;4(1):45–60. doi: 10.1038/nrc1251. [DOI] [PubMed] [Google Scholar]
- 45.Pelissier MA, Muller C, Hill M, Morfin R. Protection against dextran sodium sulfate-induced colitis by dehydroepiandrosterone and 7alpha-hydroxy-dehydroepiandrosterone in the rat. Steroids. 2006;71(3):240–248. doi: 10.1016/j.steroids.2005.10.009. [DOI] [PubMed] [Google Scholar]
- 46.Femia AP, Dolara P, Luceri C, Salvadori M, Caderni G. Mucin-depleted foci show strong activation of inflammatory markers in 1,2-dimethylhydrazine-induced carcinogenesis and are promoted by the inflammatory agent sodium dextran sulfate. Int J Cancer. 2009;125(3):541–547. doi: 10.1002/ijc.24417. [DOI] [PubMed] [Google Scholar]
- 47.Corfield AP, Carroll D, Myerscough N, Probert CS. Mucins in the gastrointestinal tract in health and disease. Front Biosci. 2001;6:D1321–D1357. doi: 10.2741/corfield. [DOI] [PubMed] [Google Scholar]
- 48.Byrd JC, Bresalier RS. Mucins and mucin binding proteins in colorectal cancer. Cancer Metastasis Rev. 2004;23(1–2):77–99. doi: 10.1023/a:1025815113599. [DOI] [PubMed] [Google Scholar]
- 49.Andrianifahanana M, Moniaux N, Batra SK. Regulation of mucin expression: mechanistic aspects and implications for cancer and inflammatory diseases. Biochim Biophys Acta. 2006;1765(2):189–222. doi: 10.1016/j.bbcan.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Kadowaki T, Yamauchi T. Adiponectin and adiponectin receptors. Endocr Rev. 2005;26(3):439–451. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 51.Kim AY, Lee YS, Kim KH, Lee JH, Lee HK, Jang SH, Kim SE, Lee GY, Lee JW, Jung SA, Chung HY, Jeong S, Kim JB. Adiponectin represses colon cancer cell proliferation via AdipoR1- and -R2-mediated AMPK activation. Mol Endocrinol. 2010;24(7):1441–1452. doi: 10.1210/me.2009-0498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Katoh M. Notch signaling in gastrointestinal tract (review) Int J Oncol. 2007;30(1):247–251. [PubMed] [Google Scholar]
- 53.Qiao L, Wong BC. Role of Notch signaling in colorectal cancer. Carcinogenesis. 2009;30(12):1979–1986. doi: 10.1093/carcin/bgp236. [DOI] [PubMed] [Google Scholar]
- 54.Jensen J, Pedersen EE, Galante P, Hald J, Heller RS, Ishibashi M, Kageyama R, Guillemot F, Serup P, Madsen OD. Control of endodermal endocrine development by Hes-1. Nat Genet. 2000;24(1):36–44. doi: 10.1038/71657. [DOI] [PubMed] [Google Scholar]
- 55.Stanger BZ, Datar R, Murtaugh LC, Melton DA. Direct regulation of intestinal fate by Notch. Proc Natl Acad Sci U S A. 2005;102(35):12443–12448. doi: 10.1073/pnas.0505690102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shroyer NF, Helmrath MA, Wang VY, Antalffy B, Henning SJ, Zoghbi HY. Intestine-specific ablation of mouse atonal homolog 1 (Math1) reveals a role in cellular homeostasis. Gastroenterology. 2007;132(7):2478–2488. doi: 10.1053/j.gastro.2007.03.047. [DOI] [PubMed] [Google Scholar]
- 57.Bossuyt W, Kazanjian A, De Geest N, Van Kelst S, De Hertogh G, Geboes K, Boivin GP, Luciani J, Fuks F, Chuah M, VandenDriessche T, Marynen P, Cools J, Shroyer NF, Hassan BA. Atonal homolog 1 is a tumor suppressor gene. PLoS Biol. 2009;7(2):e39. doi: 10.1371/journal.pbio.1000039. [DOI] [PMC free article] [PubMed] [Google Scholar]