Abstract
Uremic toxins are mainly represented by blood urine nitrogen (BUN) and creatinine (Crea) whose removal is critically important in hemodialysis (HD) for kidney disease. Patients undergoing HD have a complex illness, resulting from: inadequate removal of organic waste, dialysis-induced oxidative stress and membrane-induced inflammation. Here we report innovative breakthroughs for efficient and safe HD by using a plasmon-induced dialysate comprising Au nanoparticles (NPs)-treated (AuNT) water that is distinguishable from conventional deionized (DI) water. The diffusion coefficient of K3Fe(CN)6 in saline solution can be significantly increased from 2.76, to 4.62 × 10−6 cm s−1, by using AuNT water prepared under illumination by green light-emitting diodes (LED). In vitro HD experiments suggest that the treatment times for the removals of 70% BUN and Crea are reduced by 47 and 59%, respectively, using AuNT water instead of DI water in dialysate, while additionally suppressing NO release from lipopolysaccharide (LPS)-induced inflammatory cells.
Hemodialysis (HD) treatment for patients with limited (or no) kidney function has been used for more than fifty years. Patients undergoing HD have a complex illness, resulting from: inadequate removal of organic waste1, dialysis-induced oxidative stress2 and membrane-induced inflammation3. Thus, technical improvements in HD have primarily focused on the development of biocompatible antioxidant dialyzer membranes4,5, and the modification of dialysates6,7. Also, efforts have been done to increase the efficiency and safety of HD using convective therapies8, ultrapure dialysate9 and intelligent therapy control with advanced dialysis machines10. Current renal substitution therapy with HD or home-based peritoneal dialysis (PD) has been the only successful long-term ex vivo organ substitution therapy for sustaining life11, while technical advances directed at improving clinical outcomes in both HD and PD have been limited. Compared to bulk water, the unusual property of liquid water confined inside carbon nanotubes has been widely investigated using molecular dynamics simulation12,13. However, the potential application of confined water is limited in its confinement environment and unavailable mass-production is its other disadvantage for wider application. The standard schedule for HD is three sessions per week (3 ~ 4 h per treatment) largely due to logistic and cost concerns7, while alterations to these schedules remain controversial7,14,15. The steady improvement in procedure-focused, and process-related measures, has led to a noticeable improvement in patient survival7,16. However, to date, less effort has been directed towards the improvement of HD efficiency, i.e. to clear uremic toxins, due to the limitations of dialyzers and dialysates. In the literature17,18, Ag NP-treated catheters have been prepared for use in HD to prevent bacterial adhesion and to act as antibacterial coatings. Xia et al.19, established in vitro that elevated levels of parathyroid hormone can be reduced to normal levels within a typical dialysis session by using an immunosorptive packed bed in conjunction with HD. As reported20, reducing the dialysate sodium level can lower blood pressure for older patients and women. Also, advances in dialysis membrane technology have refocused attention on water quality and its potential role in the bio-incompatibility of HD circuits and adverse patient outcomes21.
Au NPs with well-defined localized surface plasmon resonance (LSPR) bands in the UV-near IR regions are often employed in studies focused on surface-enhanced Raman scattering (SERS)22 and the photothermal ablation of tumors23. Also, supported Au NPs demonstrate catalytic activity for the oxidation of CO at, or below, room temperature24. Recently, light-induced vapor generation on water-immersed Au NPs was enabled when Au NPs were illuminated with solar energy, or resonant light of sufficient intensity25,26. In HD the clearance of uremic toxins, namely BUN (a metabolite from protein) and Crea (a breakdown product of creatine phosphate in muscle), is quantified to denote treatment efficiency. In this work, we report an innovative method for preparing AuNT water, resulting from reduced hydrogen bonding, by letting bulk deionized (DI) water flow through supported Au NPs under resonant illumination [denoted Au NPs-treated (AuNT) water and for illumination under fluorescent lamps, while giving ‘super' AuNT (sAuNT) water, e.g. using illumination from green light-emitting diodes (LED)]. Unprecedented HD efficacies found using AuNT water with weak hydrogen bonds high diffusion coefficients and anti-oxidative activities are reported for the first time.
Results and discussion
Plasmon-induced water with reduced hydrogen bonding
As shown in Fig. S1, the supported Au NPs exhibited a broad distinct surface plasmon absorption band, centered at 540 nm, that extends over the whole visible light region. This characteristic LSPR of Au NPs indicates that light-to-heat conversion for breaking the hydrogen bonds of bulk water can be achieved under illumination with full-wavelength visible light and further enhanced using ‘wavelength optimized resonant light' (for example, green LED light with the wavelength maxima centered at 530 nm as used in this work).
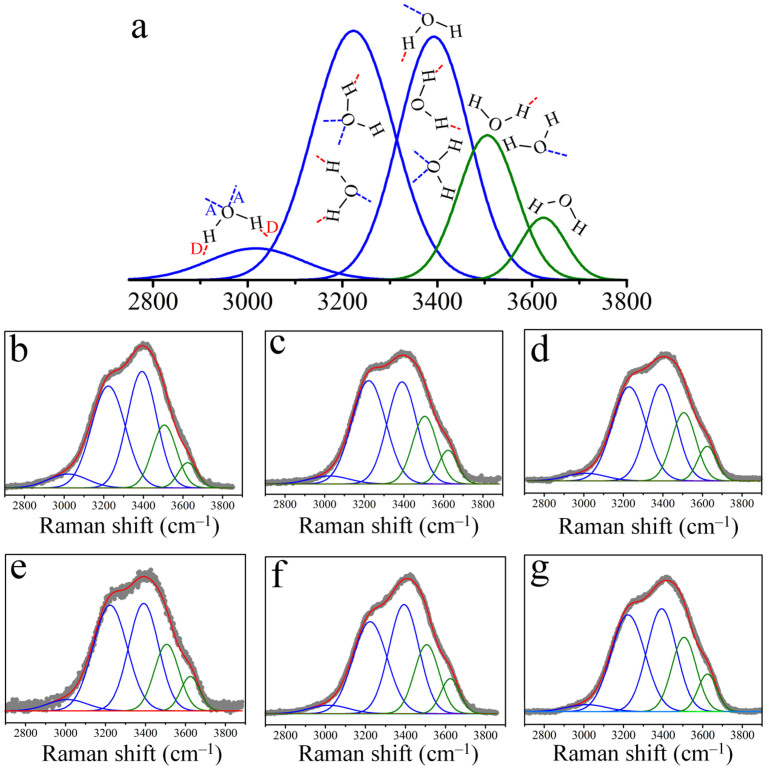
Figure 1 shows the assignments of five-Gaussian components of OH stretching Raman bands and the OH-stretching Raman spectra observed with various pure water samples. These Raman spectra were further de-convoluted into five Gaussian sub-bands based using established literature methods - see Supplementary Methods (SM). Although the exact band assignments are slightly different in the literature27,28,29,30, the consistent idea is that the bands on the low and high frequency sides are related to strong and weak hydrogen-bonded OH features, respectively. In this work, the three components on the low frequency side are assigned to hydrogen-bonded water, while the remaining two high frequency side components are assigned to non-hydrogen-bonded water. The DNHBW is defined as the ratio of the areas of the non-hydrogen-bonded OH stretching bands to the total stretching band areas.
Figure 1. Assignments of five-Gaussian components of OH stretching Raman bands and Raman spectra of OH stretching of various pure water and saline solutions.
(a), In deconvolution the five-Gaussian components with the center wavenumbers at 3018, 3223, 3393, 3506 and 3624 cm−1 were adopted for all samples. The three components on the low frequency side are assigned to hydrogen-bonded water, while the remaining two high frequency side components are assigned to non-hydrogen-bonded water. The full width at half maximum (FWHM) of the individual component in the five-Gaussian fit was equal for all samples. These values are 234, 201, 176, 154 and 112 cm−1 for bands at 3018, 3223, 3393, 3506 and 3624 cm−1, respectively. Red capital letter D and blue capital letter A are noted as donor and acceptor of proton, respectively. (b), DI water for reference. (c), AuNT water under illumination with fluorescent lamps in preparation. (d), sAuNT water under illumination with green LED in preparation. (e), DI water with 0.9 wt% NaCl. (f), AuNT water with 0.9 wt% NaCl. (g), sAuNT water with 0.9 wt% NaCl.
As shown in Table S1, the DNHBW for DI, AuNT and sAuNT derived water are 21.29 (21.37% for stored DI water for 3 weeks), 25.07 and 26.78%, respectively. The low difference between the values for fresh and stored DI water suggests 21.29% is a reliable reference value for bulk water used in DNHBW. Encouragingly, the DNHBW can be significantly increased, from 21.29 to 25.07%, by using the fluorescent lamp-irradiated LSPR effect from the supported Au NPs. This is an increase of 18% for the DNHBW, which can be enhanced (to 26%) by using the green LED-irradiated LSPR effect. As shown in Table S1, the DNHBW values for DI, AuNT and sAuNT water with 0.9 wt% NaCl, noted as saline solution (DI), saline solution (AuNT) and saline solution (sAuNT), are 23.98, 26.00 and 27.66%, respectively. Comparing pure water with saline solutions, it was found that the DNHBW is significantly increased by 13% for DI water, but only by 2.0 and 3.2% for saline solutions based on AuNT and sAuNT water, respectively. Nevertheless, the DNHBW of saline solution (AuNT and sAuNT) is still markedly higher than that of saline solution (DI). Water's almost tetrahedral structure, with two O-H bonds, is disrupted by the dissolution of NaCl31,32. Different water treatments confirm the LSPR effects with respect to the corresponding AuNT water having different DNHBWs, as shown in Table S1 and in the Supplementary Discussion (SD). The energy efficiency for preparing AuNT water under the illumination of green LED is ca. 19% (See SD). A mechanism for the formation of AuNT water and its persistence in liquid water and in saline solutions is shown in SD.
Plasmon-induced water with novel property
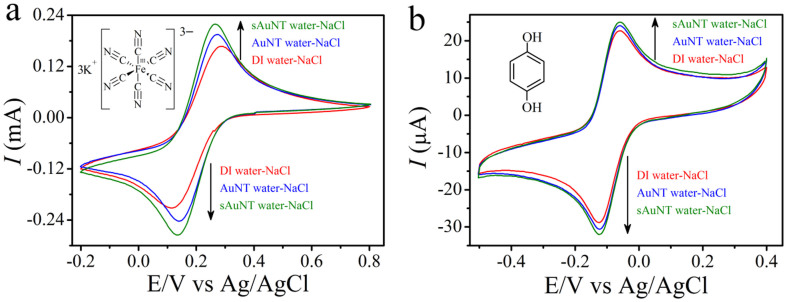
The AuNT water's weak hydrogen-bonding is responsible for its novel diffusion properties, which are critical for HD efficiency. Fig. 2 shows cyclic voltammograms in different water-based saline solutions for K3Fe(CN)6 and hydroquinone (HQ), from which the diffusion coefficients of K3Fe(CN)6 and HQ in saline solution can be obtained (See SD). Encouragingly, the calculated diffusion coefficient of K3Fe(CN)6 increased from 2.76 (1.78 for HQ) to 3.59 (2.00 for HQ) × 10−6 cm s−1 when using AuNT water instead of conventional DI water. This is an increase of 30% (12% for HQ) for the diffusion coefficient. This increased to 67% (24% for HQ) using AuNT water prepared using green LED illumination (4.62 × 10−6 cm s−1; 2.20 × 10−6 cm s−1 for HQ).
Figure 2. Voltammetric data at a scan rate of 0.1 V s−1 recorded in different water-based saline solutions at a 3 mm diameter planar Pt electrode for different systems.
(a), 30 mM K3Fe(CN)6 for one electron participating in the reaction. (b), 1 mM HQ for two electrons participating in the reaction. Red, blue and green lines represent experiments performed in saline solutions based on DI water, AuNT water and sAuNT water, respectively.
The chemical activity of the sAuNT water, compared to DI water, was examined by the reduction of an Au-containing complex to Au NPs at room temperature, as shown in Figs. S2 and S3 (the corresponding discussion is given in the SD). The experimental results indicate that the Au NPs can be successfully prepared with sAuNT water as the reducing agent: this intriguing finding opens a new ‘green pathway' in chemical reduction. It is well-known that hydroxyl radicals can directly or indirectly damage DNA33. The chemical activity of sAuNT water as a reducing agent may offer a new strategy to scavenge free radicals. As shown in Fig. S4 and SI, the Fenton reaction-produced hydroxyl radicals were reduced in the presence of AuNT water in saline solution, as compared to saline solution (DI). The corresponding ESR intensities were decreased by ca. 7.3 and 9.4% in saline solution (AuNT) and saline solution (sAuNT), respectively, compared to the value found from an experiment performed in saline solution (DI). To the best of our knowledge, this anti-oxidative activity by scavenging free radicals from AuNT or sAuNT derived water is the first report in the literature.
Plasmon-induced dialysate with high efficiency and safety
Biological studies focused on anti-inflammatory effects are generally performed by evaluating inhibiting abilities using a LPS-activated monocytes/macrophages nitric oxide (NO) system34,35. Fig. 3 shows the inflammation-preventive effects of AuNT water and sAuNT water, compared to DI water, with respect to the reduction of lipopolysaccharide (LPS)-induced NO release (See SD). The elevated NO production levels were significantly decreased (p < 0.05) in AuNT water (especially for sAuNT water prepared under LED illumination)-DMEM (Dulbecco's Modified Essential Medium) in the presence of LPS from 10 to 100 ng mL−1. Incubation with prepared AuNT water-DMEM gives rise to the suppression of NO release in LPS-activated macrophage cells.
Figure 3. Anti-oxidative activity of AuNT water (blue block) and sAuNT water (green block) compared to DI water (red block) on reduction of lipopolysaccharide (LPS)-induced NO release with dose of LPS.
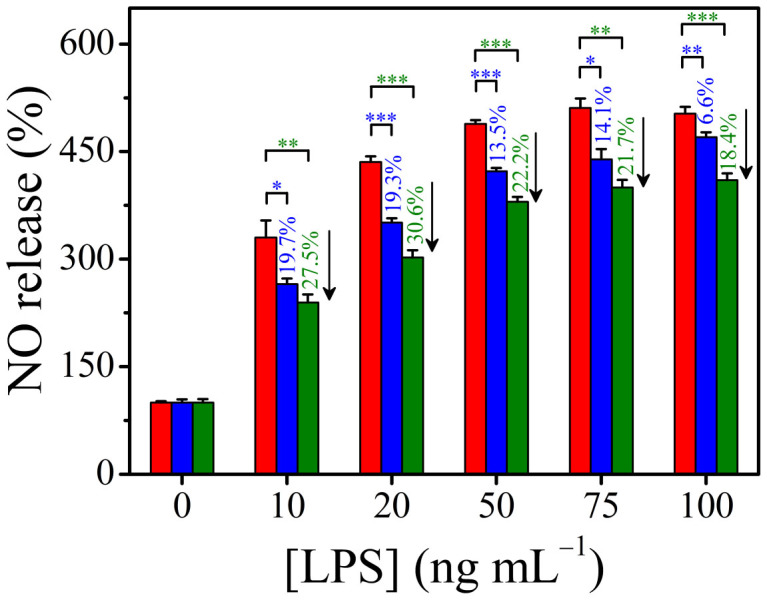
Determination of nitric oxide (NO) production was made following the method shown in the literature (See SD). DI water, AuNT water and sAuNT water were used for medium preparation. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Correspondingly, the proposed AuNT water, or sAuNT water, when compared to DI water, exhibits a high diffusion coefficient and anti-oxidative activities, due to the scavenging of hydroxyl radicals and an anti-inflammatory effect. The high diffusion coefficient of AuNT water was first seen with the fast diffusion of aqueous methylene blue (MB) through a dialysis membrane (See Fig. S5 and SD). Our experimental results indicated that the diffusion efficiency of MB in water can be significantly increased by using AuNT water, rather than DI water. This raised efficiency can be further enhanced by using sAuNT water.
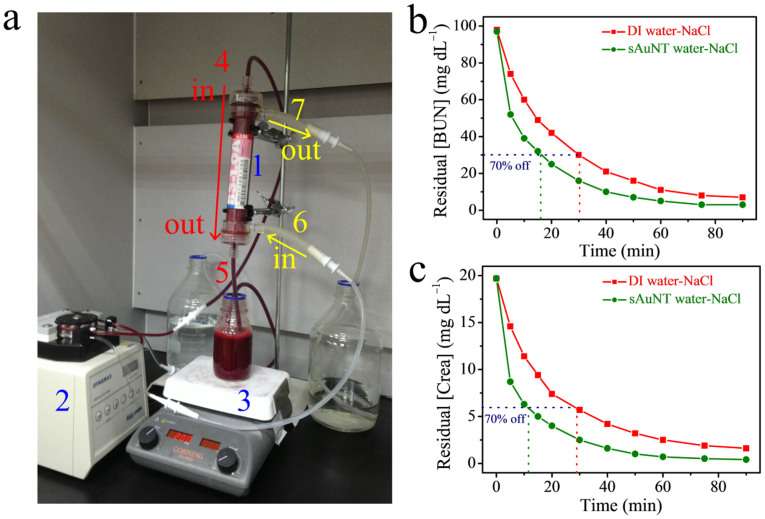
Figure 4 (a) shows the experimental setup used for simulated HD. The flow directions of the treated bloods and dialysates are opposite. Both the flow rates of blood and dialysate were kept at 20 mL min−1 by variable-speed tubing pump. The 250 mL sample of blood was stirred at 200 rpm in the whole experiment to homogenize the concentration of sample. The treatment time was recorded after the first droplet of dialysate appeared at dialysate output. As shown in Figs. 4 (b) and (c), the treatment times for the removal of 70% BUN (100 mg dL−1) in blood are ca. 30 and 16 min using saline solution (DI) and saline solution (sAuNT), respectively. The treatment times for the removal of 70% Crea (20 mg dL−1) in blood are ca. 29 and 12 min using saline solution (DI) and saline solution (sAuNT), respectively. These results suggest that the treatment times for the removal of 70% BUN and Crea can be reduced by 47 and 59%, respectively using AuNT water instead of DI water in dialysate of saline solution. The treatment times for removing BUN to a maximum normal level i.e. 20 mg dL−1 (1.5 mg dL−1 for Crea) in blood are ca. 43 (88 for Crea) and 26 (40 for Crea) min using saline solution (DI) and using saline solution (sAuNT), respectively. Another two HD experiments, based on two different blood samples, and one HD experiment using DI water also supported the innovative contribution of saline solution (sAuNT or AuNT) to more efficient HD (See Figs. S6 ~ 8 and SD). Moreover, additional benefits by using the experimental setup, as shown in Fig. 4 (a), are the efficiency of cholesterol (Chol) removal from blood (ca. 128 mg dL−1) which is enhanced by ca. 80%, using sAuNT water instead of DI water in a dialysate of saline solution, see Fig. S9. Also, Mg ions, uric acid and phosphate in bloods were simultaneously monitored. Experimental results indicate that the corresponding removal efficiencies of these species are similar by using treated water-based and DI water-based saline solutions.
Figure 4. Equipment in HD experiments and removal efficiencies of BUN and Crea by using saline solutions based on different water.
(a), Equipment in HD experiments. 1: hollow fiber dialyzer; 2: tubing pump; 3: magnetic stirrer; 4: treated blood input; 5: treated blood output; 6: dialysate input; 7: dialysate output. Both the flow rates of blood and dialysate were kept at 20 mL min−1 by variable-speed tubing pump. The 250 mL sample of blood was stirred at 200 rpm in the whole experiment to homogenize the concentration of sample. (b), Removal efficiencies of BUN by using different saline solutions. Treatment times for removals of 70% BUN (initially ca. 100 mg dL−1) are ca. 30 and 16 min by using saline solution (DI) and using saline solution (sAuNT), respectively. (c), Removal efficiencies of Crea by using different saline solutions. Treatment times for removals of 70% Crea (initially ca. 20 mg dL−1) are ca. 29 and 12 min by using saline solution (DI) and using saline solution (sAuNT), respectively.
Moreover, the cytotoxicities of DI water-based and sAuNT water-based medium to CCD-966SK cells were derived by the MTT assay method. Experimental result indicates that the cell viability within 6 days under sAuNT water-based medium is similar to that under the DI water-based medium. It suggests that the sAuNT water is possessed of good biocompatibility and low toxicity to cell. Also, in safety tests, the in vivo experiments had been already performed on mice (n = 20) at the age of ca. 8 weeks for drinking DI water and sAuNT water individually. After experiments for three months, the weight and activity of mice drinking sAuNT water are comparable to those drinking DI water. This result suggests that the treated water is biocompatible even it exists in body for a long time.
We have innovatively utilized the LSPR of Au NPs to prepare AuNT water with weak hydrogen bonds, high diffusion coefficients and anti-oxidative activities. The close values of pH for DI water and treated water suggest that the treated water has less influence on the pH of prepared dialysate. The close values of osmolarities for DI water and treated water suggest that the treated water has less influence on the equilibrium of osmotic pressure when it enters the blood stream. The potential to offer more efficient and safer HD by using a plasmon-induced dialysate with AuNT water establishes a new vista in both HD and PD. We believe these innovative approaches will lead to a variety of applications, in medicine, biology and chemistry. Efficient removal of other large-molecular protein-bound uremic toxins based on AuNT water by using dialyzer with larger pore size is underway.
Methods
HD experiments
In experiments, hollow fiber dialyzers with polymethylmethacrylate (PMMA) membranes (model: B3-1.0A, Toray Filtryzer, Japan) were employed. The blood with high concentrations of BUN (ca. 100 mg dL−1) and Crea (ca. 20 mg dL−1) flowed into the dialyzer from the top entrance and out from the bottom exit. The dialysate based on sAuNT water (or based on DI water) flowed into the dialyzer from the bottom entrance and out from the top exit. The flowing mode of these two streams is a countercurrent. Before the experiments, suitable quantities of BUN and Crea (estimation by deducting the originally normal values in blood) were added in blood to prepare blood sample with required 100 mg dL−1 BUN and 20 mg dL−1 Crea under stirring without destroying the structure of blood.
Full Methods and any associated references are available in the online version of the paper.
Author Contributions
Y.C.L. conceived the idea of the project and wrote the manuscript. H.C.C., H.C.L. and H.H.C. designed the experiments. H.C.C., H.Y.T. and C.P.Y. performed the experiments. C.M.L. performed the NO release experiment. H.C.C., H.C.L. and F.D.M. analyzed the experimental data. Y.C.L., H.C.C., H.C.L., H.H.C., F.D.M., C.M.L. and C.C.C. discussed the results and commented on the paper.
Supplementary Material
Innovative strategy with potential to increase hemodialysis efficiency and safety
Innovative strategy with potential to increase hemodialysis efficiency and safety
Acknowledgments
The authors thank the National Science Council of ROC and Taipei Medical University for their financial support.
References
- Meyer T. W. & Hostetter T. H. Uremia. N. Engl. J. Med. 357, 1316–1325 (2007). [DOI] [PubMed] [Google Scholar]
- Turi S. et al. Oxidative stress and antioxidant defense mechanism in glomerular diseases. Free Radical Biol. Med. 22, 161–168 (1997). [DOI] [PubMed] [Google Scholar]
- Herlebin A., Nguyen A. T., Zingraff J., Urena P. & Descamps-Latscha B. Influence of uremia and hemodialysis on circulating interleukin-1 and tumor necrosis factor alpha. Kidney Int. 37, 116–125 (1990). [DOI] [PubMed] [Google Scholar]
- Neelakandan C. et al. In vitro evaluation of antioxidant and anti-inflammatory properties of genistein-modified hemodialysis membranes. Biomacromolecules 12, 2447–2455 (2011). [DOI] [PubMed] [Google Scholar]
- Senthilkumar S., Rajesh S., Jayalakshmi A. & Mohan D. Biocompatibility studies of polyacrylonitrile membranes modified with carboxylated polyetherimide. Mater. Sci. Eng. C 33, 3615–3626 (2013). [DOI] [PubMed] [Google Scholar]
- García-López E., Lindholm B. & Davies S. An update on peritoneal dialysis solutions. Nat. Rev. Nephrol. 8, 224–233 (2012). [DOI] [PubMed] [Google Scholar]
- Himmelfarb J. & Alplkizler T. Hemodialysis. N. Engl. J. Med. 363, 1833–1845 (2010). [DOI] [PubMed] [Google Scholar]
- Fischbach M., Fothergill H., Zaloszyc A. & Seuge L. Hemodiafiltration: the addition of convective flow to hemodialysis. Pediatr Nephrol. 27, 351–356 (2012). [DOI] [PubMed] [Google Scholar]
- Mineshima M. & Eguchi K. Development of intermittent infusion hemodiafiltration using ultrapure dialysis fluid with an automated dialysis machine. Blood Purif. 35, 55–58 (2013). [DOI] [PubMed] [Google Scholar]
- Davenport A. Using dialysis machine technology to reduce intradialytic hypotension. Hemodial. Int. 15, S37–S42 (2011). [DOI] [PubMed] [Google Scholar]
- Humes H. D., Buffington D. A., MacKay S. M., Funke A. J. & Weitzel W. F. Replacement of renal function in uremic animals with a tissue-engineered kidney. Nat. Biotech. 17, 451–455 (1999). [DOI] [PubMed] [Google Scholar]
- Chaban V. V. & Prezhdo O. V. Water boiling inside carbon nanotubes: toward efficient drug release. ACS Nano 5, 5647–5655 (2011). [DOI] [PubMed] [Google Scholar]
- Hummer G. Rasalah J. C. & Noworyta J. P. Water conduction through the hydrophobic channel of a carbon nanotube. Nature 414, 188–190 (2001). [DOI] [PubMed] [Google Scholar]
- Held P. J., Levin N. W., Bovbjerg R. R., Pauly M. V. & Diamond L. H. Mortality and duration of hemodialysis treatment. J. Am. Med. Assoc. 265, 871–875 (1997). [PubMed] [Google Scholar]
- Marshall M. R., Byrne B. G., Kerr P. G. & Mc-Donald S. P. Associations of hemodialysis dose and session length with mortality risk in Australian and New Zealand patients. Kidney Int. 69, 1229–1236 (2006). [DOI] [PubMed] [Google Scholar]
- Himmelfarb J. & Kliger A. S. End-stage renal disease measures of quality. Annu. Rev. Med. 58, 387–399 (2007). [DOI] [PubMed] [Google Scholar]
- Paladini F., Pollini M., Tala A., Alifano P. & Sannino A. Efficacy of silver treated catheters for haemodialysis in preventing bacterial adhesion. J. Mater. Sci: Mater Med. 23, 1983–1990 (2012). [DOI] [PubMed] [Google Scholar]
- Pollini M. et al. Antibacterial coatings on haemodialysis catheters by photochemical deposition of silver nanoparticles. J. Mater. Sci: Mater Med. 22, 2005–2012 (2011). [DOI] [PubMed] [Google Scholar]
- Xia S., Hodge N., Laski M. & Wiesner T. F. Middle-molecule uremic toxin removal via hemodialysis augmented with an immunosorbent packed bed. Ind. Eng. Chem. Res. 49, 1359–1369 (2010). [Google Scholar]
- Ireland R. Does reducing dialysate sodium level lower blood pressure? Nat. Rev. Nephrol. 8, 192–192 (2012). [DOI] [PubMed] [Google Scholar]
- Damasiewicz M. J., Polkinghorne K. R. & Kerr P. G. Water quality in conventional and home haemodialysis. Nat. Rev. Nephrol. 8, 725–734 (2012). [DOI] [PubMed] [Google Scholar]
- Li J. F. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 464, 392–395 (2010). [DOI] [PubMed] [Google Scholar]
- Tsai M. F. et al. Au nanorod design as light-absorber in the first and second biological near-infrared windows for in vivo photothermal therapy. ACS Nano 7, 5330–5342 (2013). [DOI] [PubMed] [Google Scholar]
- Yoshida H. et al. Visualizing gas molecules interacting with supported nanoparticulate catalysts at reaction conditions. Science 335, 317–319 (2012). [DOI] [PubMed] [Google Scholar]
- Neumann O. et al. Solar vapor generation enabled by nanoparticles. ACS Nano 7, 42–49 (2013). [DOI] [PubMed] [Google Scholar]
- Fang Z. et al. Evolution of light-induced vapor generation at a liquid-immersed metallic nanoparticle. Nano Lett. 13, 1736–1742 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J. G., Gierszal K. P., Wang P. & Ben-Amotz D. Water structural transformation at molecular hydrophobic interfaces. Nature 491, 582–585 (2012). [DOI] [PubMed] [Google Scholar]
- Li R., Jiang Z., Guan Y., Yang H. & Liu B. Effects of metal ion on the water structure studied by the Raman O[BOND]H stretching spectrum. J. Raman Spectrosc. 40, 1200–1204 (2009). [Google Scholar]
- Carey D. M. & Korenowski G. M. Measurement of the Raman spectrum of liquid water. J. Chem. Phys. 108, 2669–2775 (1998). [Google Scholar]
- Tomlinson-Phillips J., Davis J. & Ben-Amotz D. Structure and dynamics of water dangling OH bonds in hydrophobic hydration shells. Comparison of simulation and experiment. J. Phys. Chem. A 115, 6177–6183 (2011). [DOI] [PubMed] [Google Scholar]
- Leberman R. & Soper A. K. Effect of high salt concentrations on water structure. Nature 378, 364–366 (1995). [DOI] [PubMed] [Google Scholar]
- Chumaevskii N. A., Rodnikova M. N. & Sirotkin D. A. Cationic effect in aqueous solutions of 1:1 electrolytes by Raman spectral data. J. Mol. Liq. 91, 81–91 (2001). [Google Scholar]
- Imlay J. A. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 77, 755–756 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. S. & Chau L. Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 8, 240–246 (2002). [DOI] [PubMed] [Google Scholar]
- Meng X. L. et al. RV09, a novel resveratrol analogue, inhibits NO and TNF-α production by LPS-activated microglia. Int. Immunopharmacol. 8, 1074–1082 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Innovative strategy with potential to increase hemodialysis efficiency and safety
Innovative strategy with potential to increase hemodialysis efficiency and safety